Abstract
Humoral immune responses are crucial for protection against invading pathogens and are the underlying mechanism of protection for most successful vaccines. Our understanding of how humoral immunity develops is largely based upon animal models utilizing experimental immunization systems. While these studies have made enormous progress for the field and have defined many of the fundamental principles of B cell differentiation and function, we are only now beginning to appreciate the complexities of humoral immune responses induced by infection. Co-evolution of the adaptive immune system and the pathogenic world has created a diverse array of B cell responses to infections, with both shared and unique strategies. In this review, we consider the common mechanisms that regulate the development of humoral immune responses during infection and highlight recent findings demonstrating the evolution of unique strategies used by either host or pathogen for survival.
Introduction
Successful vaccination strategies against a number of pathogens including viruses and pathogenic bacteria depend upon the humoral immune response [1]. In addition, neutralising antibodies induced during infection with highly mutating viruses such as HIV, HCV and influenza have shaped current strategies for vaccine design [2-4]. B cell activation through binding of the B cell receptor (BCR) to a cognate antigen in the context of various additional signals drives both proliferative and differentiation programs. These processes result in expanded populations of both early effector cells that can secrete copious amounts of antibody as well as long-lived populations of B cells that can protect against secondary infections (Figure 1). In recent years, we have made considerable advances in our knowledge of the molecular regulation of the generation, function and maintenance of humoral immune responses induced by immunization. We have a better understanding of the critical interactions between CD4+ T cells and B cells and the key transcriptional regulators that are important for germinal center (GC) responses, and the heterogeneous populations of memory cells that emerge from the GC (both long-lived plasma cells (LLPCs) and memory B cells (MBCs)) [5,6]. In an effort to generate better vaccines however, we now need to understand how specific B cell populations can be optimally protective against specific microbial infections, taking into account unique inflammatory signatures, antigen loads, tropisms or immune evasion mechanisms. We propose that the evolution of host-pathogen interactions over time has led to a greater heterogeneity in the development and function of humoral immune responses than perhaps revealed by protein immunization models. Recent studies in this review illuminate both the common mechanisms shared by infection-specific humoral responses as well as highlighting unique characteristics of pathogen-specific responses to counteract immune evasion strategies. Since innate-like CD5+ B1 B-cells are not thought to form memory and their role in infection has recently been extensively reviewed [7], this review will only focus on B2 B cells.
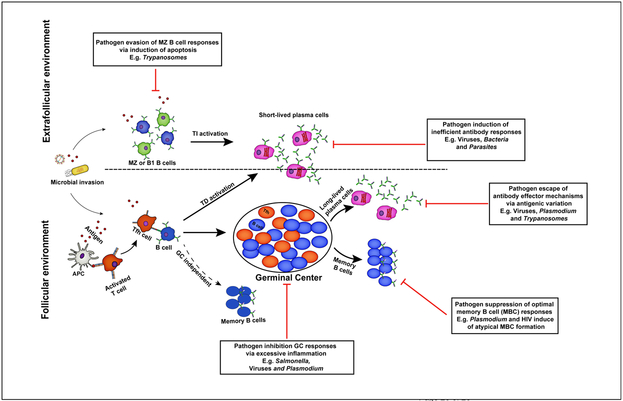
Figure 1. A schematic view of humoral immune responses to infection.
Extrafollicular and follicular antibody responses contribute to protection against invading microbial pathogens. B cells activated within the extrafollicular environment in the presence or absence of T cell help differentiate into short-lived antibody secreting cells that mediate early protection against infection. However, the formation of germinal center dependent or independent memory B cells and long-lived plasma cells in the B cell follicles facilitates complete resolution of primary infections and long-term protection against reinfection. For their survival, pathogens have evolved strategies that enable them to evade specific antibody-dependent killing mechanisms.
Kinetics of the B2 B cell response to infection
B2 B cells can be divided into distinct sub-populations based on their activation requirements, phenotype and localization [8-10]. The first B2 B cells to respond to infection are the innate-like CD21+ marginal zone (MZ) B-cells, located primarily in the splenic MZ. The MZ separates the follicle from the red pulp and provides a unique environment in which resident lymphocytes can sample antigens in the blood. Marginal zone B cells have been shown to be critical early responders to bacterial [11,12], viral [13,14] and parasitic infections [15,16]. Furthermore, MZ B cells can respond to antigen in a T cell-independent manner to rapidly express antibodies and also present captured antigens to CD4+ T cells [17-20], (Figure 1). Upon activation MZ B cells have also been shown to traffic into the B cell follicle where they can deliver antigen to follicular dendritic cells, and facilitate follicular B cell activation [21]. Follicular B cells localized to follicles within the spleen and lymph nodes, require additional time and signals for differentiation [22]. Follicular B-cells respond in a largely T-dependent manner to form either plasmablasts or GC B cells (Figure 1). Plasmablasts are short-lived effector cells that readily secrete antibodies that are critical for controlling a primary infection [23•,24]. Cells that enter the GC undergo mutations within their BCRs that are tested on antigen presented on follicular dendritic cells, resulting in both diversified and higher affinity BCRs. Germinal center-derived memory cells can persist either as long-lived, quiescent, circulating MBCs that remain responsive to reinvading pathogens or sessile long-lived plasma cells (LLPCs) in the bone marrow and spleen [21,25-28]. LLPCs secrete antibodies without requiring further antigenic stimulation [5], but are not thought to respond to a subsequent infection due to their low levels of BCR [25].
The early primary B cell response:
MZ B cells are rapid, T-independent responders to infections of the blood including encapsulated bacteria, parasites such as Plasmodium and some viruses (Figure 1). MZ B cells are able to recognize capsular polysaccharides on bacterial pathogens, microbial CPG DNA and highly repetitive viral motifs, which stimulate TLR and BCR signalling. This enables MZ B cells to rapidly differentiate into plasmablasts, important for early protection against some bacterial and viral pathogens. Indeed, splenectomised individuals and those with disrupted splenic MZ B cells are highly susceptible to encapsulated bacterial pathogens, including Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitis [29,30]. These individuals have reduced levels of serum IgM and IgG. Moreover, serum antibodies detected in these individuals exhibit limited capacity to opsonise encapsulated bacterial antigens. In a more recent study, stroke-induced loss of splenic MZ B cells in mice associated with significant reduction in IgM levels and a spontaneous increase in bacterial infection in the lungs of these mice, suggesting a potential role for MZ B cells in limiting infections throughout the body following a stroke episode [12]. Together, these data support a role for MZ B cell-derived antibodies in early protection against invading pathogens. However, despite their positive roles in immunity to blood-borne pathogens antigens, MZ B cells have also been associated with detrimental effects during some infections. For instance it has been suggested that MZ B cells may obstruct protective T cell responses during early Leishmania donovani infection [31]. In this report, MZ B cell deficiency was associated with improved T cell responses and reduced parasite burden in vivo. Therefore, these data suggest that MZ B cells may also drive pathology during some infections. Understanding how MZ B cells support critical early antibody responses during a specific infection will be important for understanding optimal control of acute infection.
Next phase: activation of the follicular B cell response
Follicular B cell responses are initiated when naïve B-cells encounter antigen situated within primary lymphoid follicles. Activated B-cells migrate via chemokine-sensing towards the T cell zone where they are able to interact with CD4+ T cells, previously activated by dendritic cells [22,32], (Figure 1). Activated B cells present cognate peptides on MHC Class II to CD4+ T cells in the context of co-stimulatory signals and cytokines [33,34]. As CD4+ T follicular helper (Tfh) cells and B cells exchange signals, an extended program of differentiation and expansion begins. CD4+ Tfh cells express co-stimulatory molecules including CD40 ligand and ICOS as well as cytokines essential for initiation of the GC response and the formation of MBC populations [35-39]. Multiple factors including antigen availability, the strength of BCR-signaling and the types of cytokines secreted by the CD4+ T cells influence B cell fate and the decision to become either extrafollicular plasmablasts or GC B cells [5,40-42]. T-dependent plasmablasts can class-switch and can undergo somatic hypermutation and affinity maturation [43••]. In addition, while extra-follicular plasma cells are generally believed to be short-lived, there is some evidence that this is not always the case [44-46]. By immunizing T cell deficient mice with haptenated LPS, a model T-independent antigen, a recent study demonstrated that long-lived GC-independent plasma cells are readily formed and maintained both in the spleen and bone marrow, suggesting that some plasmablast populations may be longer lived than previously appreciated [45]. B cells that receive the appropriate differentiation signals can alternatively be recruited into the GC and undergo somatic hypermutation, class-switch recombination and affinity maturation, giving rise to MBCs and LLPCs [47]. The resulting MBCs and LLPCs, express a diverse array of high affinity BCRs and recent evidence has even demonstrated that non-templated mutations contribute to this process [23•,48]. Interestingly, the post-GC decision to become a LLPC or MBC also appears to be binary and is tightly regulated by a network of transcription factors [5,49,50]. The development and function of LLPCs was recently attributed to three key transcription factors, IRF4, Blimp-1 and XBP-1 [51•]. By using a tamoxifen-driven Cre-recombinase depletion system to IRF4 in established plasma cells, this study demonstrated an essential role for IRF4 in the survival of LLPCs, as measured by upregulation of CD138 and Blimp1 on B220lo cells in the spleen and bone marrow [51•]. Using a similar approach, it was further demonstrated that Blimp-1 and XBP-1 were required for LLPC maturation and production of antibodies, but were not essential for LLPC survival, both in the steady state and after protein immunization. Unlike LLPC fate, transcription factors critical for MBC fate are still being elucidated. Nonetheless, it has been suggested that MBC formation and maintenance is dependent on transcription factors such as PAX5, Bach2 and BCL-6, which are believed to insulate mature B cells against plasma cell differentiation [5,52-54]. For example, in a recent study employing a protein immunization system, genetic ablation of Bach2 in pre-existing MBCs associated with their increased differentiation into CD138-expressing plasma cells [54]. These findings were further corroborated by a more recent report in mice, which established a requirement for Bach2 in the generation of MBCs within the light-zone of the GC following protein immunization [53]. However, in driving the selection of light-zone GC B-cells into MBCs, Bach2 acted in a Blimp1-independent manner, suggesting a more complex interplay among transcription factors in controlling MBC and LLPC fate. Identification of the signals important for the formation, function and maintenance of MBCs and LLPCs during infection is an ongoing area of investigation that will have important implications for vaccine development.
Memory B cells respond to a secondary infection
While it is clear that the presence of continuous antibody production from LLPCs is critical for protection against many subsequent infections (reviewed by Amanna and Slifka) [55], we are now just beginning to understand the contributions that MBCs provide during a homologous secondary infection [56•]. Interestingly, heterogeneous populations of CD27+-expressing MBCs that express either class-switched or unswitched BCRs have been described in humans for many years [57-59]. Yet due to a lack of phenotypic markers to identify small populations of antigen-experienced MBCs in mice, relatively few mechanistic comparative studies have been performed on these cells [23•,60,61]. The advent of both antigen-specific B cell enrichment strategies as well as single cell RNA-seq have been pivotal to renewed efforts to understand the nature and function of endogenous MBCs after infection or immunization [23•,56••,60]. For example, studies from our lab have demonstrated that fluorescently-labeled B cell tetramers containing various Plasmodium-specific proteins can be used to study the development of the B cell response to malaria in both mice and humans and the panoply of different B cell subsets that can form at different times throughout the infection [56,62•]. These studies have revealed both interesting biology of MBCs (reviewed in [63]) and the unique immune evasion strategies that the parasite uses (discussed below). For example, we found that in mice, three different Plasmodium-specific MBC subsets persist in the spleen after a primary infection, exhibiting different phenotypic and functional qualities. The largest population was comprised of cells expressing high-levels of the IgD isotype that resembled naïve follicular B cells. The next prominent population resembled classically defined, somatically hypermutated IgG-expressing MBCs while the smallest population consisted of somatically hypermutated, IgM+ MBC population. Remarkably, despite this difference in number, the IgM+ MBCs responded fastest to a secondary infection and could generate both IgM- and IgG-antibody secreting plasmablasts. These findings suggest that heterogeneity in MBC function may have evolved to control different types of infections that can occur in different regions of the body or require the unique functional attributes of distinct isotypes. Interestingly, IgM-like antibodies exist even in the earliest immune systems including the lamprey [64], suggesting a critical role for these cells throughout evolution. These often over-looked IgM+ MBCs may be important targets for improved vaccine-induced immunity to certain infections and we are currently investigating the cues that lead to their differentiation.
Unique challenges of and responses to specific infections
We believe that heterogeneous populations of MBCs have evolved to control various types of infections as they provide an arsenal to prevent immune evasion. In the following section, we provide examples of some of the unique challenges to humoral immunity that can be ascribed to various infections.
a). Bacterial infection:
Many of the common mechanisms of B cell activation and function described above have been targeted by pathogen immune evasion mechanisms (Figure 1). For instance, antibodies acting via opsonic and complement fixation killing mechanisms have been associated with protection to Salmonella infection, while it exists in the extracellular environment [43••,65-67]. Yet studies have also demonstrated that the bacteria have developed immune evasion strategies to modify the quality of Salmonella-specific B cell responses through the production of inefficient antibodies [66,68•]. One strategy that Salmonella uses is evasion of the GC response as demonstrated in a recent report employing transgenic ovalbumin-expressing Salmonella strains to study B cell responses during Salmonella infection [68•]. In this report, primary Salmonella infection significantly impaired the expansion of endogenous Salmonella-specific B cells and the formation of GCs [68•]. These defects were mediated by factors within the Salmonella Pathogenicity Island 2 (SPI2) since normal B-cell expansion and GC formation was restored in mice infected with SPI2-deficient mutant bacteria. Therefore, targeting bacterial-associated virulence factors such as SPI2 in attenuated bacteria may be a useful vaccine strategy for boosting humoral immunity to Salmonella.
Perhaps as a counter-strategy, the host mounts a robust extrafollicular plasmablast response against Salmonella that can control infection [69,70]. A previous study in mice infected with attenuated enteric Salmonella typhimurium suggested a role for a robust extrafollicular B cell response in limiting bacterial burdens within the extracellular environment in the absence of a GC response [69]. This supports the idea that potent extrafollicular B cell responses may compensate for loss of optimal GC B cell responses during infection. Although it was previously thought that these extrafollicular responses were largely polyclonal and non-specific, a more recent study in mice infected with Salmonella typhimurium demonstrated that they were indeed specific for the bacteria [43••]. Importantly, this study demonstrated that B-cells activated by Salmonella infection are capable of undergoing somatic hypermutation within the extra-follicular environment, boosting affinity maturation and production of isotype-switched antibodies, which was previously thought to primarily occur within the GC. These data highlight the complexity of the humoral response during infection and highlight our need to understand B cell responses to specific pathogens.
b). Viral infection:
Vaccine-mediated humoral immunity has led to the eradication of several devastating infections including Small pox, Measles and Polio. One potential evolutionary mechanism that some viruses may have adapted in subverting humoral immune-mediated killing is the induction of strong inflammatory responses, which suppress B cell differentiation and antibody production [71,72]. Recent evidence in human studies using RNA-seq-based technologies showed a negative correlation between highly upregulated inflammatory transcripts and responses to hepatitis B vaccination (HBV) [71]. However, upregulation of genes associated with B cell signalling positively correlated with heightened responses to HBV, suggesting a potential interplay between inflammation and B cell signalling in regulating B cell responses to infection and vaccination. Indeed, reports in mice demonstrate roles for inflammation-induced disruption of the lymphoid organ architecture, which can also suppress GC formation [72, 73]. For example, a recent study, using influenza and vaccinia viral models demonstrated that infection-induced inflammation disrupts the organisation of sub-capsular macrophages within sub-capsular spaces and inter-follicular regions, impairing GC B-cell and plasma cell formation during secondary viral challenge [72]. As a counter-measure to this evasion mechanism, host regulatory mechanisms that limit excessive inflammation to viral infection have evolved. For instance, a recent report demonstrated a T cell-intrinsic requirement for TGFβ-signalling in the formation of influenza-specific GC B cells and the production of class-switched antibodies [74•]. TGFβ-signalling in T-cells acted by limiting IL-2-induced signals and the formation of virus-specific inflammatory-like Th1 precursor cells, which enhanced Tfh and B cell responses. Similarly, Laidlaw and colleagues more recently demonstrated a role for follicular regulatory T cell (Tfr)-derived IL-10 in promoting GC B cell responses in mice with acute LCMV infection [75•]. Genetic depletion of IL-10 in Tfr cells was associated with reduced frequencies of GC B cells, in particular, those within the dark zone, suggesting that Tfr-derived IL-10 may support dark zone GC responses. Taken together, these data suggest that regulatory mechanisms within the host may not only serve to limit infection-induced immunopathology but also boost immunity against invading pathogens. Therefore, targeting infection-induced inflammatory pathways may be an important avenue for improving humoral immunity to infection.
c). Parasitic infection:
Antibodies have also been shown to play important roles in several parasitic infections including Trypanosomes [15, 76], Helminths (reviewed in [77]) and Plasmodium [78•-82]. Trypanosomes have long been studied as an example of humoral immune evasion as they have developed a robust antigenic variation system that enables them to evade antibody-mediated killing [83]. However, recent data further suggests that Trypanosomes can also directly modulate B cell differentiation and function during infection [15]. Using a mouse model of Trypanosoma brucei, Radwanska and colleagues illustrated that Trypanosome-infection induces apoptosis in MZ B cells, reducing antibody production and parasite control [15]. However, it remained unclear from this report whether cell-death was restricted to MZ B cells alone or affected other B cell subsets, since the latter were not directly examined in this study.
B cells are also critical for control of Plasmodium infections in both mice and humans. B cell deficient mice are unable to clear non-lethal blood-stage Plasmodium infections [84], while passively transferred antibodies are protective in both mice and humans [78•,85]. Plasmodium parasites have also developed strategies to evade these humoral immune responses, including antigenic variation [86], and repression of optimal B cell differentiation and antibody production during infection [62•,73,87]. Our work and that of others has shown that the blood-stage of Plasmodium infection can impinge upon the humoral immune response to the proceeding liver-stage parasites. An examination of the circumsporozoite protein (CSP)-specific B cell response in genetically attenuated parasites (Pyfabb/f) that are unable to establish blood-stage infection compared to wild type parasites, which establish a blood-stage infection [88] showed a direct effect of the blood stage on liver stage GC development [62•]. Moreover, this diminished GC response in the presence of a blood-stage infection alters the quality of CSP-specific MBCs and their ability to respond to a secondary challenge. These data highlight how immunization with attenuated parasites may drive optimal immunity to malaria and suggest further studies on how ongoing blood stage infections may alter immune memory. In addition, Plasmodium parasites may also modify optimal MBC formation and function during infection. Recent studies in humans have identified a unique subset of MBCs, ‘atypical’ MBCs, which develop during chronic Plasmodium infection [89-91]. When compared to classical MBCs, atypical MBCs display increased expression of inhibitory receptors, exhibit reduced BCR-signalling and are unable to differentiate into antibody secreting cells [89,90]. This suggests that these MBCs may be dysfunctional. Although their sources remain unclear, immunoglobulin gene sequencing techniques have predicted a shared developmental history between these atypical and classical MBCs [89].
d). Fungal infection:
Humoral immune responses are necessary for resistance against various fungal infections largely via antibody-mediated activation of the complement system (reviewed in [92,93]). For instance, complement-deficiency in mice has been associated with increased susceptibility to Candida [94], Aspergillus [95] and Cryptococcal [96] infections. This was associated with reduced opsonization and complement-mediated lysis of pathogenic fungi and decreased recruitment of phagocytic cells during infection. In order to subvert complement-mediated killing and establish infection, pathogenic fungi have adapted multiple survival strategies [92,93,97-99]. For example, Candida albicans may evade the complement system by expressing decoy inhibitory ligands such as phosphoglycerate mutase (Gmp1) and the pH-regulated antigen 1 (Pra1) that bind Factor H and Factor H-like protein 1, which are key regulatory proteins in the alternative pathway [97-99]. These ligands have also been implicated in inhibiting the classical and lectin pathways during Candida and Aspergillus infections by binding the regulatory C4BP and thus restricting C3b and CD4b deposition on the fungal surface [97-100]. Pathogenic fungi may also secrete proteolytic enzymes that degrade effector components of the complement pathway hence inhibiting opsonization and phagocytosis [101,102]. For instance, Aspergillus fumigatus (A. fumigatus) secretion of the proteolytic enzyme, alkaline protease Alp1 has been associated with increased degradation of C3, C4, C5 and C1q complement proteins purified from human sera [101] and reduced expression of complement receptor 3 on phagocytic cells in cultured cerebral spinal fluids from A. fumigatus-infected individuals [102]. Together, these evasive strategies may contribute to enhanced fungal infection. Therefore, these data suggest that infection-induced complement inhibitory pathways may be targeted for improved immunity to pathogenic fungal infections.
Conclusions
The ongoing co-evolution of pathogens and host immune responses has introduced critical diversity associated with survival of both. Whereas some responses may be protective to specific infections, they may alternatively be detrimental to others. Therefore, a more comprehensive understanding of the function and generation of heterogeneous humoral immune responses to specific microbial infections is required to lead to more efficacious vaccine strategies. This more comprehensive approach to humoral immunity may reveal B cell strategies that are not induced by current protein immunization strategies. The introduction of new analytical methods including tools to analyze small populations of polyclonal, antigen-specific B cells, improved DNA-sequencing and single cell RNAseq platforms have ushered in a new era of understanding for B cell immunology. It will next be important to develop vaccine platforms that can induce heterogeneous responses or even direct a specific MBC population. It will soon be possible to develop the types of truly rationale-based vaccine design strategies that will be necessary for generating immunity against some of our oldest foes.
Highlights.
Humoral immune responses are crucial for protection against infections
Current paradigms of humoral responses are based on protein immunization models
Pathogens have evolved an array of strategies to evade humoral immunity
Diverse B cell responses have evolved to ensure host survival
Acknowledgements
This work was supported by the National Institutes of Health [grant number RO1 AI118803] and Burroughs Wellcome Fund [grant number 1016766] grants to MP.
Footnotes
Conflict of interest
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest have been highlighted as:
• Of special interest
•• Of Outstanding interest
- 1.Amanna IJ, Carlson NE, Slifka MK: Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med 2007, 357:1903–1915. [DOI] [PubMed] [Google Scholar]
- 2.Burton DR, Hangartner L: Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design. Annu Rev Immunol 2016, 34:635–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball JK, Tarr AW, McKeating JA: The past, present and future of neutralizing antibodies for hepatitis C virus. Antiviral Res 2014, 105:100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joyce MG, Wheatley AK, Thomas PV, Chuang GY, Soto C, Bailer RT, Druz A, Georgiev IS, Gillespie RA, Kanekiyo M, et al. : Vaccine-Induced Antibodies that Neutralize Group 1 and Group 2 Influenza A Viruses. Cell 2016, 166:609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM: The generation of antibody-secreting plasma cells. Nat Rev Immunol 2015, 15:160–171. [DOI] [PubMed] [Google Scholar]
- 6.Shlomchik MJ, Weisel F: Germinal center selection and the development of memory B and plasma cells. Immunol Rev 2012, 247:52–63. [DOI] [PubMed] [Google Scholar]
- 7.Baumgarth N: B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front Immunol 2016, 7:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pillai S, Cariappa A: The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol 2009, 9:767–777. [DOI] [PubMed] [Google Scholar]
- 9.De Silva NS, Klein U: Dynamics of B cells in germinal centres. Nat Rev Immunol 2015, 15:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurosaki T, Kometani K, Ise W: Memory B cells. Nat Rev Immunol 2015, 15:149–159. [DOI] [PubMed] [Google Scholar]
- 11.Chen TT, Tsai MH, Kung JT, Lin KI, Decker T, Lee CK: STAT1 regulates marginal zone B cell differentiation in response to inflammation and infection with blood-borne bacteria. J Exp Med 2016, 213:3025–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCulloch L, Smith CJ, McColl BW: Adrenergic-mediated loss of splenic marginal zone B cells contributes to infection susceptibility after stroke. Nat Commun 2017, 8:15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demberg T, Mohanram V, Musich T, Brocca-Cofano E, McKinnon KM, Venzon D, Robert-Guroff M: Loss of marginal zone B-cells in SHIVSF162P4 challenged rhesus macaques despite control of viremia to low or undetectable levels in chronic infection. Virology 2015, 484:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gatto D, Ruedl C, Odermatt B, Bachmann MF: Rapid response of marginal zone B cells to viral particles. J Immunol 2004, 173:4308–4316. [DOI] [PubMed] [Google Scholar]
- 15.Radwanska M, Guirnalda P, De Trez C, Ryffel B, Black S, Magez S: Trypanosomiasis-induced B cell apoptosis results in loss of protective anti-parasite antibody responses and abolishment of vaccine-induced memory responses. PLoS Pathog 2008, 4:e1000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens R, Ndungu FM, Langhorne J: Germinal centre and marginal zone B cells expand quickly in a second Plasmodium chabaudi malaria infection producing mature plasma cells. Parasite Immunol 2009, 31:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balazs M, Martin F, Zhou T, Kearney J: Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 2002, 17:341–352. [DOI] [PubMed] [Google Scholar]
- 18.Hsu MC, Toellner KM, Vinuesa CG, Maclennan IC: B cell clones that sustain long-term plasmablast growth in T-independent extrafollicular antibody responses. Proc Natl Acad Sci U S A 2006, 103:5905–5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin F, Oliver AM, Kearney JF: Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 2001, 14:617–629. [DOI] [PubMed] [Google Scholar]
- 20.Attanavanich K, Kearney JF: Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol 2004, 172:803–811. [DOI] [PubMed] [Google Scholar]
- 21.Cinamon G, Zachariah MA, Lam OM, Foss FW Jr., Cyster JG: Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol 2008, 9:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi H: T follicular helper cells in space-time. Nat Rev Immunol 2016, 16:612–625. [DOI] [PubMed] [Google Scholar]
- 23.•.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK: Different B cell populations mediate early and late memory during an endogenous immune response. Science 2011, 331:1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used PE-labelled cells to demonstrate the formation of IgM and switched memory B cells following protein immunization.
- 24.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F: Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol 2006, 6:741–750. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida T, Mei H, Dorner T, Hiepe F, Radbruch A, Fillatreau S, Hoyer BF: Memory B and memory plasma cells. Immunol Rev 2010, 237:117–139. [DOI] [PubMed] [Google Scholar]
- 26.Tangye SG, Tarlinton DM: Memory B cells: effectors of long-lived immune responses. Eur J Immunol 2009, 39:2065–2075. [DOI] [PubMed] [Google Scholar]
- 27.Anderson SM, Tomayko MM, Shlomchik MJ: Intrinsic properties of human and murine memory B cells. Immunol Rev 2006, 211:280–294. [DOI] [PubMed] [Google Scholar]
- 28.Deenick EK, Avery DT, Chan A, Berglund LJ, Ives ML, Moens L, Stoddard JL, Bustamante J, Boisson-Dupuis S, Tsumura M, et al. : Naive and memory human B cells have distinct requirements for STAT3 activation to differentiate into antibody-secreting plasma cells. J Exp Med 2013, 210:2739–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amlot PL, Hayes AE: Impaired human antibody response to the thymus-independent antigen, DNP-Ficoll, after splenectomy. Implications for post-splenectomy infections. Lancet 1985, 1:1008–1011. [DOI] [PubMed] [Google Scholar]
- 30.Cerutti A, Cols M, Puga I: Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol 2013, 13:118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bankoti R, Gupta K, Levchenko A, Stager S: Marginal zone B cells regulate antigen-specific T cell responses during infection. J Immunol 2012, 188:3961–3971. [DOI] [PubMed] [Google Scholar]
- 32.Corcoran LM, Tarlinton DM: Regulation of germinal center responses, memory B cells and plasma cell formation-an update. Curr Opin Immunol 2016, 39:59–67. [DOI] [PubMed] [Google Scholar]
- 33.Barroso M, Tucker H, Drake L, Nichol K, Drake JR: Antigen-B Cell Receptor Complexes Associate with Intracellular major histocompatibility complex (MHC) Class II Molecules. J Biol Chem 2015, 290:27101–27112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinstein JS, Bertino SA, Hernandez SG, Poholek AC, Teplitzky TB, Nowyhed HN, Craft J: B cells in T follicular helper cell development and function: separable roles in delivery of ICOS ligand and antigen. J Immunol 2014, 192:3166–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F: Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity 2013, 38:596–605. [DOI] [PubMed] [Google Scholar]
- 36.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, et al. : Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008, 29:138–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crotty S: T follicular helper cell differentiation, function, and roles in disease. Immunity 2014, 41:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W, et al. : Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 2013, 496:523–527. [DOI] [PubMed] [Google Scholar]
- 39.Weinstein JS, Herman EI, Lainez B, Licona-Limon P, Esplugues E, Flavell R, Craft J: TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol 2016, 17:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A, Brink R: High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med 2006, 203:2419–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R: Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med 2006, 203:1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krautler NJ, Suan D, Butt D, Bourne K, Hermes JR, Chan TD, Sundling C, Kaplan W, Schofield P, Jackson J, et al. : Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J Exp Med 2017, 214:1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.••.Di Niro R, Lee SJ, Vander Heiden JA, Elsner RA, Trivedi N, Bannock JM, Gupta NT, Kleinstein SH, Vigneault F, Gilbert TJ, et al. : Salmonella Infection Drives Promiscuous B Cell Activation Followed by Extrafollicular Affinity Maturation. Immunity 2015, 43:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that somatic hypermutation and affinity maturation can occur within the extrafollicular environment and described the importance of these processes during infection.
- 44.Bortnick A, Allman D: What is and what should always have been: long-lived plasma cells induced by T cell-independent antigens. J Immunol 2013, 190:5913–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bortnick A, Chernova I, Quinn WJ 3rd, Mugnier M, Cancro MP, Allman D: Long-lived bone marrow plasma cells are induced early in response to T cell-independent or T cell-dependent antigens. J Immunol 2012, 188:5389–5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foote JB, Mahmoud TI, Vale AM, Kearney JF: Long-term maintenance of polysaccharide-specific antibodies by IgM-secreting cells. J Immunol 2012, 188:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, Haberman AM: Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity 2011, 34:947–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pieper K, Tan J, Piccoli L, Foglierini M, Barbieri S, Chen Y, Silacci-Fregni C, Wolf T, Jarrossay D, Anderle M, et al. : Public antibodies to malaria antigens generated by two LAIR1 insertion modalities. Nature 2017, 548:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kallies A, Hasbold J, Tarlinton DM, Dietrich W, Corcoran LM, Hodgkin PD, Nutt SL: Plasma cell ontogeny defined by quantitative changes in blimp-1 expression. J Exp Med 2004, 200:967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nutt SL, Heavey B, Rolink AG, Busslinger M: Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature 1999, 401:556–562. [DOI] [PubMed] [Google Scholar]
- 51.•.Tellier J, Shi W, Minnich M, Liao Y, Crawford S, Smyth GK, Kallies A, Busslinger M, Nutt SL: Blimp-1 controls plasma cell function through the regulation of immunoglobulin secretion and the unfolded protein response. Nat Immunol 2016, 17:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study in mice described the essential roles of transcription factors IRF4, Blimp1 and XBP1 in the formation and function of long-lived plasma cells.
- 52.Cobaleda C, Schebesta A, Delogu A, Busslinger M: Pax5: the guardian of B cell identity and function. Nat Immunol 2007, 8:463–470. [DOI] [PubMed] [Google Scholar]
- 53.Shinnakasu R, Inoue T, Kometani K, Moriyama S, Adachi Y, Nakayama M, Takahashi Y, Fukuyama H, Okada T, Kurosaki T: Regulated selection of germinal-center cells into the memory B cell compartment. Nat Immunol 2016, 17:861–869. [DOI] [PubMed] [Google Scholar]
- 54.Kometani K, Nakagawa R, Shinnakasu R, Kaji T, Rybouchkin A, Moriyama S, Furukawa K, Koseki H, Takemori T, Kurosaki T: Repression of the transcription factor Bach2 contributes to predisposition of IgG1 memory B cells toward plasma cell differentiation. Immunity 2013, 39:136–147. [DOI] [PubMed] [Google Scholar]
- 55.Amanna IJ, Slifka MK: Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev 2010, 236:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.•.Krishnamurty AT, Thouvenel CD, Portugal S, Keitany GJ, Kim KS, Holder A, Crompton PD, Rawlings DJ, Pepper M: Somatically Hypermutated Plasmodium-Specific IgM(+) Memory B Cells Are Rapid, Plastic, Early Responders upon Malaria Rechallenge. Immunity 2016, 45:402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study revealed antigen-specific, somatically hypermutated IgM memory B cells as rapid responders to a secondary malaria infection.
- 57.Klein U, Rajewsky K, Kuppers R: Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med 1998, 188:1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seifert M, Przekopowitz M, Taudien S, Lollies A, Ronge V, Drees B, Lindemann M, Hillen U, Engler H, Singer BB, et al. : Functional capacities of human IgM memory B cells in early inflammatory responses and secondary germinal center reactions. Proc Natl Acad Sci U S A 2015, 112:E546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weller S, Mamani-Matsuda M, Picard C, Cordier C, Lecoeuche D, Gauthier F, Weill JC, Reynaud CA: Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J Exp Med 2008, 205:1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zuccarino-Catania GV, Sadanand S, Weisel FJ, Tomayko MM, Meng H, Kleinstein SH, Good-Jacobson KL, Shlomchik MJ: CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol 2014, 15:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud CA, Weill JC: Multiple layers of B cell memory with different effector functions. Nat Immunol 2009, 10:1292–1299. [DOI] [PubMed] [Google Scholar]
- 62.•.Keitany GJ, Kim KS, Krishnamurty AT, Hondowicz BD, Hahn WO, Dambrauskas N, Sather DN, Vaughan AM, Kappe SH, Pepper M: Blood Stage Malaria Disrupts Humoral Immunity to the Pre-erythrocytic Stage Circumsporozoite Protein. Cell Rep 2016, 17:3193–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that blood-stage Plasmodium infection hinders the development and maintenance of humoral immunity against liver-stage Plasmodium parasites.
- 63.Harms Pritchard G, Pepper M: Memory B cell heterogeneity: Remembrance of things past. J Leukoc Biol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Herrin BR, Alder MN, Roux KH, Sina C, Ehrhardt GR, Boydston JA, Turnbough CL Jr., Cooper MD: Structure and specificity of lamprey monoclonal antibodies. Proc Natl Acad Sci U S A 2008, 105:2040–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gondwe EN, Molyneux ME, Goodall M, Graham SM, Mastroeni P, Drayson MT, MacLennan CA: Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci U S A 2010, 107:3070–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacLennan CA, Gilchrist JJ, Gordon MA, Cunningham AF, Cobbold M, Goodall M, Kingsley RA, van Oosterhout JJ, Msefula CL, Mandala WL, et al. : Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science 2010, 328:508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.MacLennan CA, Gondwe EN, Msefula CL, Kingsley RA, Thomson NR, White SA, Goodall M, Pickard DJ, Graham SM, Dougan G, et al. : The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest 2008, 118:1553–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.•.Nanton MR, Lee SJ, Atif SM, Nuccio SP, Taylor JJ, Baumler AJ, Way SS, McSorley SJ: Direct visualization of endogenous Salmonella-specific B cells reveals a marked delay in clonal expansion and germinal center development. Eur J Immunol 2015, 45:428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provided some insight into the molecular mechanisms through which pathogens such as Salmonella evade germinal center B cell responses during infection.
- 69.Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, et al. : Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol 2007, 178:6200–6207. [DOI] [PubMed] [Google Scholar]
- 70.Gil-Cruz C, Bobat S, Marshall JL, Kingsley RA, Ross EA, Henderson IR, Leyton DL, Coughlan RE, Khan M, Jensen KT, et al. : The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc Natl Acad Sci U S A 2009, 106:9803–9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fourati S, Cristescu R, Loboda A, Talla A, Filali A, Railkar R, Schaeffer AK, Favre D, Gagnon D, Peretz Y, et al. : Pre-vaccination inflammation and B-cell signalling predict age-related hyporesponse to hepatitis B vaccination. Nat Commun 2016, 7:10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaya M, Castello A, Montaner B, Rogers N, Reis e Sousa C, Bruckbauer A, Batista FD: Host response. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science 2015, 347:667–672. [DOI] [PubMed] [Google Scholar]
- 73.Ryg-Cornejo V, Ioannidis LJ, Ly A, Chiu CY, Tellier J, Hill DL, Preston SP, Pellegrini M, Yu D, Nutt SL, et al. : Severe Malaria Infections Impair Germinal Center Responses by Inhibiting T Follicular Helper Cell Differentiation. Cell Rep 2016, 14:68–81. [DOI] [PubMed] [Google Scholar]
- 74.•.Marshall HD, Ray JP, Laidlaw BJ, Zhang N, Gawande D, Staron MM, Craft J, Kaech SM: The transforming growth factor beta signaling pathway is critical for the formation of CD4 T follicular helper cells and isotype-switched antibody responses in the lung mucosa. Elife 2015, 4:e04851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.•.Laidlaw BJ, Lu Y, Amezquita RA, Weinstein JS, Vander Heiden JA, Gupta NT, Kleinstein SH, Kaech SM, Craft J: Interleukin-10 from CD4+ follicular regulatory T cells promotes the germinal center response. Sci Immunol 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]; These two papers highlighted unexpected roles for anti-inflammatory cytokines in supporting optimal development and maintenance of humoral immune responses during infection.
- 76.Bermejo DA, Amezcua Vesely MC, Khan M, Acosta Rodriguez EV, Montes CL, Merino MC, Toellner KM, Mohr E, Taylor D, Cunningham AF, et al. : Trypanosoma cruzi infection induces a massive extrafollicular and follicular splenic B-cell response which is a high source of non-parasite-specific antibodies. Immunology 2011, 132:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harris N, Gause WC: To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol 2011, 32:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.•.Cohen S, Mc GI, Carrington S: Gamma-globulin and acquired immunity to human malaria. Nature 1961, 192:733–737. [DOI] [PubMed] [Google Scholar]; This was the first description of the critical role of antibodies in control of blood-stage malaria infection.
- 79.Douglas AD, Williams AR, Knuepfer E, Illingworth JJ, Furze JM, Crosnier C, Choudhary P, Bustamante LY, Zakutansky SE, Awuah DK, et al. : Neutralization of Plasmodium falciparum merozoites by antibodies against PfRH5. J Immunol 2014, 192:245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boyle MJ, Reiling L, Feng G, Langer C, Osier FH, Aspeling-Jones H, Cheng YS, Stubbs J, Tetteh KK, Conway DJ, et al. : Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 2015, 42:580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raj DK, Nixon CP, Nixon CE, Dvorin JD, DiPetrillo CG, Pond-Tor S, Wu HW, Jolly G, Pischel L, Lu A, et al. : Antibodies to PfSEA-1 block parasite egress from RBCs and protect against malaria infection. Science 2014, 344:871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.White MT, Griffin JT, Akpogheneta O, Conway DJ, Koram KA, Riley EM, Ghani AC: Dynamics of the Antibody Response to Plasmodium falciparum Infection in African Children. J Infect Dis 2014. [DOI] [PubMed] [Google Scholar]
- 83.Stijlemans B, Caljon G, Van Den Abbeele J, Van Ginderachter JA, Magez S, De Trez C: Immune Evasion Strategies of Trypanosoma brucei within the Mammalian Host: Progression to Pathogenicity. Front Immunol 2016, 7:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matar CG, Anthony NR, O'Flaherty BM, Jacobs NT, Priyamvada L, Engwerda CR, Speck SH, Lamb TJ: Gammaherpesvirus Co-infection with Malaria Suppresses Anti-parasitic Humoral Immunity. PLoS Pathog 2015, 11:e1004858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT: Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 2012, 13:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riley EM, Stewart VA: Immune mechanisms in malaria: new insights in vaccine development. Nat Med 2013, 19:168–178. [DOI] [PubMed] [Google Scholar]
- 87.Sebina I, James KR, Soon MS, Fogg LG, Best SE, Labastida Rivera F, Montes de Oca M, Amante FH, Thomas BS, Beattie L, et al. : IFNAR1-Signalling Obstructs ICOS-mediated Humoral Immunity during Non-lethal Blood-Stage Plasmodium Infection. PLoS Pathog 2016, 12:e1005999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vaughan AM, O'Neill MT, Tarun AS, Camargo N, Phuong TM, Aly AS, Cowman AF, Kappe SH: Type II fatty acid synthesis is essential only for malaria parasite late liver stage development. Cell Microbiol 2009, 11:506–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Portugal S, Tipton CM, Sohn H, Kone Y, Wang J, Li S, Skinner J, Virtaneva K, Sturdevant DE, Porcella SF, et al. : Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sullivan RT, Kim CC, Fontana MF, Feeney ME, Jagannathan P, Boyle MJ, Drakeley CJ, Ssewanyana I, Nankya F, Mayanja-Kizza H, et al. : FCRL5 Delineates Functionally Impaired Memory B Cells Associated with Plasmodium falciparum Exposure. PLoS Pathog 2015, 11:e1004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weiss GE, Crompton PD, Li S, Walsh LA, Moir S, Traore B, Kayentao K, Ongoiba A, Doumbo OK, Pierce SK: Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol 2009, 183:2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marcos CM, de Oliveira HC, de Melo WC, da Silva JF, Assato PA, Scorzoni L, Rossi SA, de Paula ESAC, Mendes-Giannini MJ, Fusco-Almeida AM: Anti-Immune Strategies of Pathogenic Fungi. Front Cell Infect Microbiol 2016, 6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Speth C, Rambach G, Wurzner R, Lass-Florl C: Complement and fungal pathogens: an update. Mycoses 2008, 51:477–496. [DOI] [PubMed] [Google Scholar]
- 94.Mullick A, Elias M, Picard S, Bourget L, Jovcevski O, Gauthier S, Tuite A, Harakidas P, Bihun C, Massie B, et al. : Dysregulated inflammatory response to Candida albicans in a C5-deficient mouse strain. Infect Immun 2004, 72:5868–5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hector RF, Yee E, Collins MS: Use of DBA/2N mice in models of systemic candidiasis and pulmonary and systemic aspergillosis. Infect Immun 1990, 58:1476–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shapiro S, Beenhouwer DO, Feldmesser M, Taborda C, Carroll MC, Casadevall A, Scharff MD: Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect Immun 2002, 70:2598–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo S, Poltermann S, Kunert A, Rupp S, Zipfel PF: Immune evasion of the human pathogenic yeast Candida albicans: Pra1 is a Factor H, FHL-1 and plasminogen binding surface protein. Mol Immunol 2009, 47:541–550. [DOI] [PubMed] [Google Scholar]
- 98.Poltermann S, Kunert A, von der Heide M, Eck R, Hartmann A, Zipfel PF: Gpm1p is a factor H-, FHL-1-, and plasminogen-binding surface protein of Candida albicans. J Biol Chem 2007, 282:37537–37544. [DOI] [PubMed] [Google Scholar]
- 99.Zipfel PF, Hallstrom T, Riesbeck K: Human complement control and complement evasion by pathogenic microbes--tipping the balance. Mol Immunol 2013, 56:152–160. [DOI] [PubMed] [Google Scholar]
- 100.Luo S, Blom AM, Rupp S, Hipler UC, Hube B, Skerka C, Zipfel PF: The pH-regulated antigen 1 of Candida albicans binds the human complement inhibitor C4b-binding protein and mediates fungal complement evasion. J Biol Chem 2011, 286:8021–8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Behnsen J, Lessing F, Schindler S, Wartenberg D, Jacobsen ID, Thoen M, Zipfel PF, Brakhage AA: Secreted Aspergillus fumigatus protease Alp1 degrades human complement proteins C3, C4, and C5. Infect Immun 2010, 78:3585–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rambach G, Dum D, Mohsenipour I, Hagleitner M, Wurzner R, Lass-Florl C, Speth C: Secretion of a fungal protease represents a complement evasion mechanism in cerebral aspergillosis. Mol Immunol 2010, 47:1438–1449. [DOI] [PubMed] [Google Scholar]