Abstract
Theaflavin-3, 3’-digallate (TF3) is a phenolic compound extracted from black tea. We previously demonstrated that TF3 selectively inhibited ovarian cancer cells. Ovarian cancer has high death rate because of acquired cisplatin resistance. We aimed to investigate the synergistic effect of TF3 and cisplatin (CDDP) against cisplatin resistant ovarian cancer cells. In the present study, combination treatment with TF3 and CDDP showed a synergistic cytotoxic effect in A2780/CP70 and OVCAR3 cells. Combination treatment showed a synergistic pro-apoptotic effect and synergistically induced G1/S phase cell cycle arrest. Synergistic apoptosis was accompanied by regulating protein expression of cleaved caspase 3/7, cytochrome c, Bax and Bcl-2. Combination treatment induced G1/S phase cell cycle arrest via regulating protein expression of cyclin A2, cyclin D1, cyclin E1 and CDK2/4. Combination treatment could synergistically down-regulate Akt phosphorylation in both cell lines. TF3 may be used as an adjuvant for the treatment of advanced ovarian cancer.
Keywords: Theaflavin-3, 3’-digallate, Cisplatin, Ovarian cancer, Synergism
1. Introduction
Black tea is one of the most widely consumed beverages in the world and is second only to water in popularity. Black tea was a main dietary source of flavonols for US women, and black tea consumption was associated with a linear decline in ovarian cancer risk (Baker, et al., 2007; Cassidy, Huang, Rice, Rimm, & Tworoger, 2014). Theaflavin-3, 3’-digallate (TF3) is one of the major bioactive components in black tea which contributes to the characteristic color and flavor of black tea. It’s orange-red in color and possesses a benzotropolone skeleton that is formed from the co-oxidation of (–)-epicatechin gallate and (–)-epigallocatechin gallate (EGCG) during black tea production (Finger, 1994). TF3 has been demonstrated to inhibit human prostate cancer cells (Lee, Ho, & Lin, 2004; Sun, et al., 2013), liver cancer cells, gastric cancer cells and lung cancer cells (K. Wang, et al., 2011). TF3 exerted antitumor effects in breast cancer cells through suppressing proteasomal activities (Lin, Chen, & Lin-Shiau, 2006). We have previously reported that TF3 could induce apoptosis, cell cycle arrest (Tu, et al., 2016) and angiogenesis (Gao, Rankin, Tu, & Chen, 2016) in human ovarian cancer cells.
Ovarian cancer ranks fifth in cancer deaths among women in the United States, accounting for approximately 5% of all cancer deaths diagnosed among women (Siegel, Miller, & Jemal, 2016). Ovarian cancer has the highest rate of deaths among the gynecologic cancers (uterine, cervical, and ovarian). The conventional course of therapy is maximal surgical resection of the tumor mass followed by a combination treatment of taxane and platinum-based chemotherapy. In spite of 70% of patients responding well to first-line chemical-based therapy, the emergence of side effects and drug resistance has rendered a variety of the currently available chemotherapeutic agents ineffective (Limtrakul, Pitchakarn, & Suzuki, 2013). The 5-year survival rate for patients with advanced ovarian cancer remains less than 40% because of adverse side effects and acquired drug resistance (Al Rawahi, et al., 2013). Hence, there is an urgent need to explore novel therapeutic interventions and agents to overcome drug resistance for ovarian cancer.
TF3 was a potential agent to reduce the dosage of chemotherapeutic agents for ovarian cancer therapy which could reduce their side effects and overcome the drug resistance of ovarian cancer cells. In the present study, we investigated whether TF3 would synergistically potentiate the antitumor effect of CDDP in cisplatin-resistant human ovarian cancer cell lines. The possible molecular mechanisms underlying the synergistic effect were also studied.
2. Materials and Methods
2.1. Cell culture and reagents
Platinum-resistant human ovarian cancer cell lines A2780/CP70 and OVCAR-3 were kindly provided by Dr. Jiang at West Virginia University. The cells were cultured in RPMI-1640 medium (Sigma, St Louis, MO, USA) supplemented with 10% fetal bovine serum (Invitrogen, Rockford, IL, USA) at 37°C in a humidified incubator with 5% CO2. TF3 monomer was isolated and purified using a previously established method (Xu, Jin, Wu, & Tu, 2010). The purity of TF3 was 93.76%. Cisplatin was purchased from Sigma-Aldrich. TF3 and CDDP were prepared in distilled water and stored at −20 °C. Primary antibodies to cleaved caspase-3 (Asp175), cleaved caspase-7 (Asp198), cyclin A2 (BF683), cyclin D1, cyclin E1 (D7T3U), CDK2 (78B2), CDK4 (D9G3E), Akt, p-Akt (Ser473), Bcl-2 were purchased from Cell Signaling Technology (Danvers, MA, USA). Primary antibodies to cytochrome c, Bad (c-7), Bax, GAPDH (0411) and the secondary antibodies were purchased from Santa Cruz Biotechnology (Mariposa, CA, USA).
2.2. Cell viability assay
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfo phenyl)-2H-tetrazolium (MTS) assay was used to assess the cell viability. Cells were seeded into 96-well plates at a density of 2×104 cells per well and incubated overnight. Then cells were treated with TF3, CDDP or the combination for 24 h. Cell viability was measured using CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega, St Louis, MO, USA), according to the manufacturer’s instructions. Cell viability was expressed as a percentage compared to that of control cells.
2.3. Synergism Determination
The synergistic effect of TF3 and CDDP was determined based on the combination index (CI) using CalcuSyn Version 2.0 (Biosoft, Great Shelford, Cambridge, UK). The CI values indicated synergism at less than 1.0.
2.4. Hoechst 33342 staining
Cells were seeded in 96-well plates at 2 × 104 cells/well and incubated overnight. Cells were treated with TF3, CDDP or the combination for 24 h. After treatment, cells were stained with 10 μg/mL Hoechst 33342 (Sigma, St Louis, MO, USA) in PBS for 10 min in the dark at 37 °C. Cell apoptosis was examined under a fluorescence microscope (ZEISS).
2.5. Colony formation
To determine the long-term effect of TF3 and CDDP combination, colony formation assay was carried out. Cells were seeded in 6-well plates at 5×104 cells/well and incubated overnight. Cells were treated with TF3, CDDP or the combination for 24 h. After treatment, the cells were collected and further cultured for 7 days in six-well plates containing drug-free medium at a cell density of 2×103 per well. Clonogenic cells were determined as those able to form a colony consisting of at least 50 cells. Colonies were then fixed with ice-cold methanol for 10 min and stained with 0.5% crystal violet solution in 25% methanol for 10 min. The stained 6-well plates were carefully rinsed with distilled water several times and photographed.
2.6. Flow cytometric analysis of apoptotic cells
The cell apoptosis was determined using an Alexa Fluor® 488 Annexin V/ Dead Cell Apoptosis kit (Invitrogen, Rockford, IL, USA). After exposure to TF3, CDDP or the combination for 24 h, cells were harvested, centrifuged for 10 min at 1500 rpm and then washed twice with PBS. Cells were suspended in binding buffer with Alexa Fluor 488Annexin V and propidium iodide (PI) for 15 min. The stained cells were analyzed by flow cytometry (FACSCalibur system, BD Biosciences), measuring the fluorescence emission at 530 and 575 nm using 488 nm excitation.
2.7. Flow cytometry analysis of the cell cycle
Cells treated with TF3, CDDP or the combination for 24 h were digested with trypsin and harvested by 1500 rpm centrifugation for 10 min. The cell pellet was suspended with 70% ethanol at -20°C overnight, washed with PBS, and then incubated with 180 μg/ml RNase (Invitrogen, Rockford, IL, USA) at 37°C for 15 min. After 15 min staining with 50 μg/ml PI (Sigma, St Louis, MO, USA) in the dark at 37°C, the samples were analyzed by flow cytometry (FACSCalibur system, BD Biosciences). Data were plotted and analyzed by using FCS Software (De Novo Software, Los Angeles, CA, USA).
2.8. Western blotting
Cells were seeded in 60-mm dishes at 1×106 cells/dish, incubated overnight, and treated with TF3, CDDP or the combination for 24 h. Then cells were harvested with M-PER Mammalian Protein Extraction Reagent (Pierce, St Louis, MO, USA) supplemented with Halt™ Protease and Phosphatase Inhibitor Single-Use Cocktail (Life Technologies, Grand Island, NY, USA). The total protein levels were detected with BCA Protein assay kit (Pierce, St Louis, MO, USA). Equal amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. The membrane was blocked with 5% skim milk in Tris-buffer saline containing 0.1% Tween-20 for 1 h at room temperature, and then incubated with specific primary antibodies and appropriate secondary antibodies conjugated with horseradish peroxidase. The antigen-antibody complex was visualized with Super Signal West Dura Extended Duration Substrate (Life technologies, Grand Island, NY, USA) and ChemiDoc™ MP System (Bio-Rad, Hercules, CA, USA). Protein bands were quantitated with NIH Image J software and normalized by GAPDH bands for analysis.
2.9. Statistical analysis
In this study, all samples were prepared and analyzed in triplicate. The data are presented as means ± standard deviations (SD). Multiple comparisons were performed by one-way analysis of variance followed with least significant difference test. Significant differences among multiple groups are indicated by different letters (p < 0.05). Statistical differences between two groups were evaluated using the Student’s t-test. Significant differences between two groups are indicated by double asterisks (**p< 0.01). All statistical analyses of data were performed using Statistical Analysis System for windows V8. p<0.05 was considered statistically significant, and p<0.01 was considered statistically highly significant. OriginPro 9.1 software was used to create the artwork.
3. Results
3.1. The synergistic anti-proliferative effect of TF3 and CDDP against ovarian cancer cell lines
To evaluate the synergistic anti-proliferative effect of TF3 and CDDP against ovarian cancer cell lines, we determined the effect of TF3, CDDP and the combination on the viability of A2780/CP70 and OVCAR3 cells using the MTS assay. The viability of A2780/CP70 and OVCAR3 cells was decreased by treatment with TF3 alone, CDDP alone or the combination in a dose dependent manner (Fig. 1A-D). The IC50 values of TF3 against A2780/CP70 and OVCAR3 cells were estimated to be 19.04 and 14.64 μM, respectively. The IC50 values of CDDP against A2780/CP70 and OVCAR3 cells were estimated to be 18.19 and 13.96 μM, respectively. We assessed the anti-proliferative effect of the combination treatment with TF3 and CDDP at two constant combination ratios which were set at 1:1 (μM: μM) and 1:2 (μM: μM). The total IC50 values for combined TF3 and CDDP (1:1, μM: μM) against A2780/CP70 and OVCAR3 cells were estimated to be 12.02 and 10.70 μM, respectively. The total IC50 values for combined TF3 and CDDP (1:2, μM: μM) against A2780/CP70 and OVCAR3 cells were estimated to be 13.12 and 10.80 μM, respectively. Combination treatment with 7 μM TF3 and 7 μM CDDP showed significantly greater growth inhibition than either agent alone. The synergistic effect of TF3 and CDDP against ovarian cancer cells was calculated using CalcuSyn. The combination treatment CI values were less than 1.0 (Fig. 1E-F), indicating that the antitumor effect was synergistic.
Fig. 1.
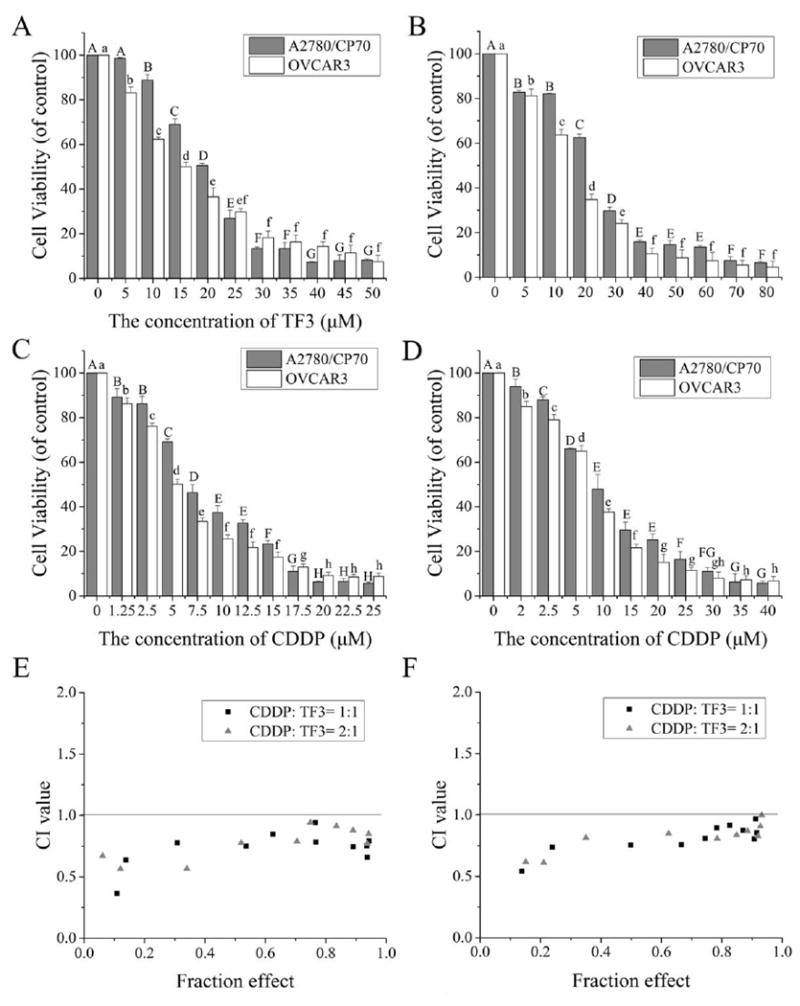
TF3 alone, CDDP alone or the combination treatment effect on A2780/CP70 and OVCAR3 cell viability. (A) Effect of TF3 at various concentrations on cell viability of both cancer cells. (B) Effect of CDDP at various concentrations on cell viability of both cancer cells. (C) Effect of combined TF3 and CDDP at the constant ratio of 1:1 on cell viability of both cancer cells. (D) Effect of combined TF3 and CDDP at the constant ratio of 1:2 on cell viability of both cancer cells. (E) The CI values of combined TF3 and CDDP at the ratio of 1:1 and 1:2 against A2780/CP70 cells were less than 1.0, revealing synergism. (F) The CI values of combined TF3 and CDDP at the ratio of 1:1 and 1:2 against OVCAR3 cells were less than 1.0, revealing synergism. All measurements were done in triplicates. Data represent means ± SD from three independent experiments. Significant differences among different treatments are indicated by different letters (p < 0.05).
Colony formation assay is an effective method to determine single cell proliferation capacity. Thus, colony formation assay was carried out to further examine the anti-proliferation effect of TF3, CDDP and the combination on A2780/CP70 and OVCAR3 cells. As showed in Fig. 2, the number of A2780/CP70 and OVCAR3 cell colonies was significantly lower for cells subjected to treatment with 7.0 μM TF3 alone or 7.0 μM CDDP alone than for control cell. In addition, the reduction in colony formation was greater for cells treated with combined TF3 and CDDP than for cells treated with either agent alone. These results show that TF3 significantly enhanced the anti-proliferation effect induced by CDDP in ovarian cancer A2780/CP70 and OVCAR3 cells.
Fig. 2.
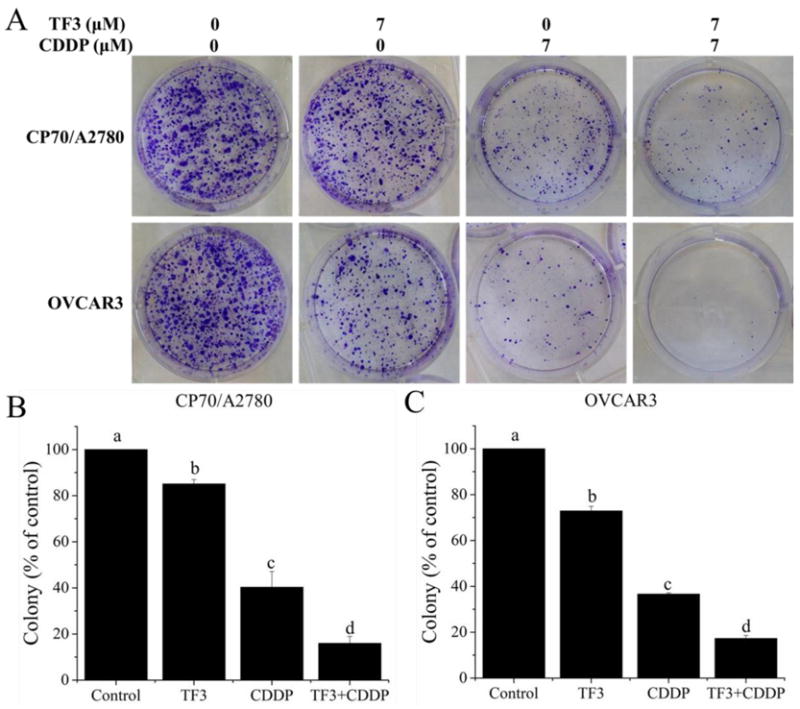
Colony formation assay was used to confirm the antitumor effect against ovarian cancer cells treated with 7 μM TF3 alone, 7 μM CDDP alone or the combination. (A) The cells were treated for 24h and cultured at a cell density of 2×103 per well for 7 days to form colony. (B) The numbers of A2780/CP70 cell colonies were expressed as quantification histograms with error bars. (C) The numbers of OVCAR3 cell colonies were expressed as quantification histograms with error bars. All measurements were done in triplicates. Data represent means ± SD from three independent experiments. Significant differences among different treatments are indicated by different letters (p < 0.05).
3.2. TF3 and CDDP synergistically induced apoptosis in ovarian cancer cell lines
To investigate whether TF3 and CDDP synergistically inhibited ovarian cancer cells by inducing apoptosis, the changes of nuclear morphology in cells treated with TF3, CDDP and the combination for 24 h were analyzed under a fluorescence microscope by Hoechst 33342 DNA staining. As showed in Fig. 3A, the nuclei were stained less bright and intact in the control group. With treatment of TF3 alone, CDDP alone or the combination, the cells exhibited numerous apoptotic cells with condensed or fragmented nuclei, which were much brighter. The percentages of apoptotic cells were determined after counting 500–1000 cells/well in randomly selected fields. The percentage of apoptotic cells was greater for cells treated with combined TF3 and CDDP than the portion sum for cells treated with either agent alone (Fig. 3B-C). These results show that TF3 and CDDP combination treatment could synergistically induce apoptosis in ovarian cancer A2780/CP70 and OVCAR3 cells.
Fig. 3.
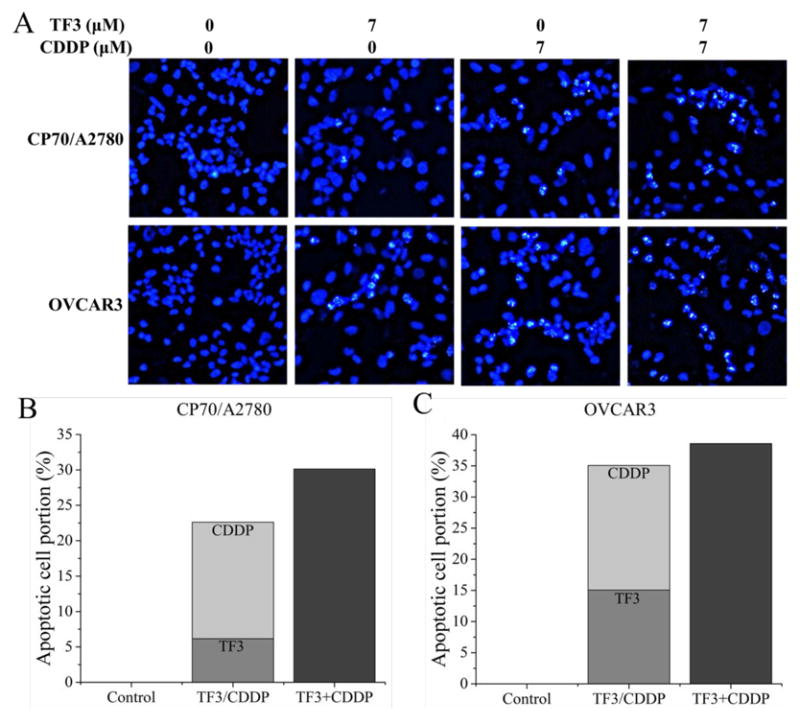
Hoechst 33342 staining of ovarian cancer cells was detected by fluorescent microscopy (magnification, x400) after treatment with 7 μM TF3 alone, 7 μM CDDP alone or the combination for 24 h. (A) The stained cells were detected by fluorescent microscopy (magnification, x400). Highly condensed or fragmented nuclei represent apoptotic cells. Intact nuclei represent viable cells. (B) The portion of apoptotic A2780/CP70 cells was expressed as quantification histograms. (C) The portion of apoptotic OVCAR3 cells was expressed as quantification histograms.
Double staining with Annexin V FITC and PI followed by flow cytometry analysis was also used to investigate the apoptosis induced by TF3, CDDP and the combination. As shown in Fig. 4A-C, the treatment of TF3, CDDP and the combination significantly decreased the percentage of alive cells and increased the percentage of total apoptotic cells (p< 0.05). The cell population of total apoptotic cells increased from 9.14% to 13.61%, 15.36% and 29.98% for A2780/CP70 cells treated with TF3, CDDP and the combination, respectively (Fig. 4B). The cell population of total apoptotic cells increased from 8.52% to 28.46%, 26.74% and 46.28% for OVCAR3 cells treated with TF3, CDDP and the combination, respectively (Fig. 4C). The increased portion of total apoptotic cells was greater for cells treated with combined TF3 and CDDP than the increased portion for total apoptotic cells treated with either agent alone in both ovarian cancer A2780/CP70 and OVCAR3 cells (Fig. 4D-E). The increased portion of total apoptotic cells was greater for A2780/CP70 cells treated with combined TF3 and CDDP than the increased portion sum of total apoptotic cells for A2780/CP70 cells treated with either agent alone (p< 0.05) (Fig. 4D). The increased portion of total apoptotic cells in OVCAR3 cells treated with combined TF3 and CDDP had no significant difference with the increased portion sum of total apoptotic cells in OVCAR3 cells treated with either agent alone (p> 0.05) (Fig. 4E). These flow cytometry analysis results indicated that TF3 and CDDP exerted synergistically apoptotic effect in A2780/CP70 cells and additively apoptotic effect in OVCAR3 cells.
Fig.4.
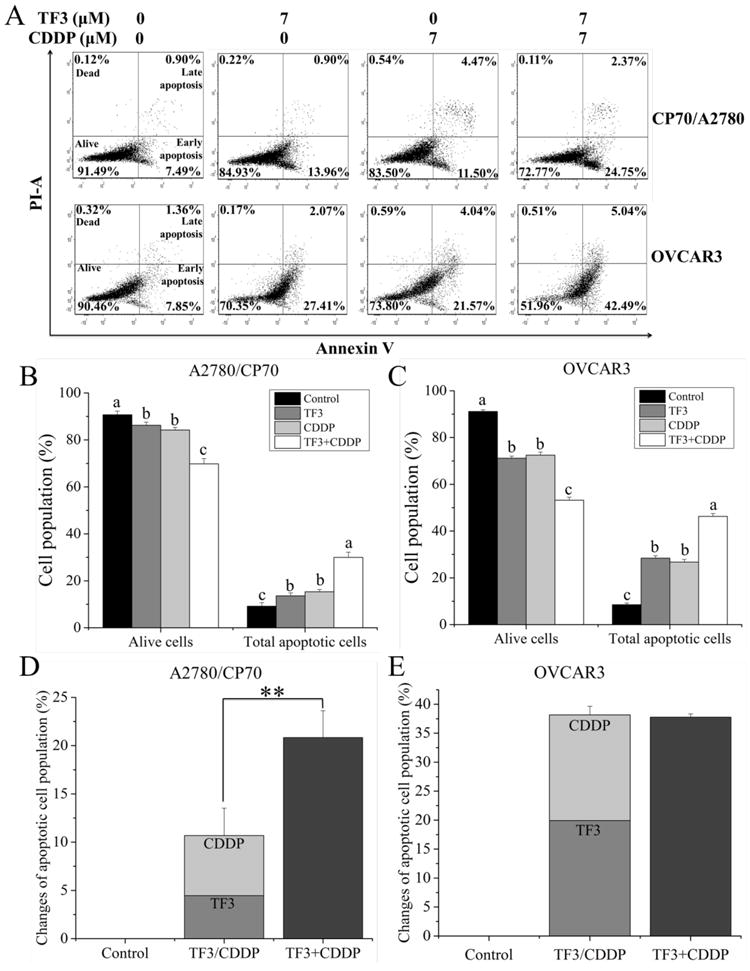
Flow cytometric analysis of ovarian cancer cells after treatment with 7 μM TF3 alone, 7 μM CDDP alone or the combination for 24 h. Cells were stained with Annexin V-FITC and PI solution and analyzed with flow cytometry. (A) The upper left and low left quadrant indicate the percentage of dead and live cells, respectively. The upper right and low right quadrants indicate the percentage of late and early apoptotic cells, respectively. (B) The percentage of alive and total apoptotic A2780/CP70 cells was expressed as quantification histograms with error bars. (C) The percentage of alive and total apoptotic OVCAR3 cells was expressed as quantification histograms with error bars. (D) The percentage change of apoptotic A2780/CP70 cells induced by treatment with 7 μM TF3 alone, 7 μM CDDP alone or the combination was expressed as quantification histograms with error bars. (E) The percentage change of apoptotic OVCAR3 cells induced by treatment with 7 μM TF3 alone, 7 μM CDDP alone or the combination was expressed as quantification histograms with error bars. All measurements were done in triplicates. Data represent means ± SD from three independent experiments. Significant differences among different treatments are indicated by different letters (p < 0.05). Significant differences between two groups are indicated by double asterisks (**p< 0.01).
3.3. TF3 and CDDP induced cell cycle arrest in ovarian cancer cell lines
To examine the mechanism involved in TF3 and CDDP mediated cell growth inhibition, cell cycle phase distribution of cells treated with TF3, CDDP and the combination for 24 h was analyzed by flow cytometry after PI staining. As showed in Fig. 5, TF3 (7 μM) alone induced G1 phase arrest in both A2780/CP70 and OVCAR3 cells. TF3 significantly increased the portion of cells in G1 phase from 59.0% to 75.9% for A2780/CP70 cells and from 61.6% to 71.7% for OVCAR3 cells (p< 0.01). CDDP (7 μM) alone induced S phase arrest in both A2780/CP70 and OVCAR3 cells. CDDP significantly increased the portion of cells in S phase from 24.3% to 65.9% for A2780/CP70 cells and from 24.8% to 66.9% for OVCAR3 cells (p< 0.01).
Fig. 5.
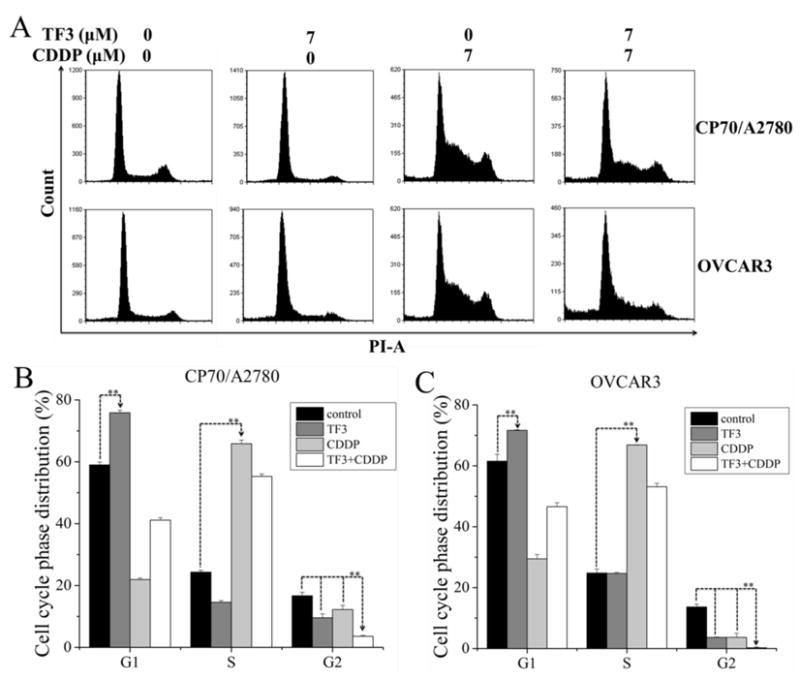
Treatment with 7 μM TF3 alone, 7 μM CDDP alone or the combination induced cell cycle arrest in ovarian cancer cells. The cells were treated for 24 h, fixed in 70% ethanol, and stained with propidium iodide. DNA contents were determined by flow cytometry. (A) Cell cycle phase distributions of both cells were affected by treatment with 7 μM TF3 alone, 7 μM CDDP alone or the combination. (B) The cell cycle phase distribution of A2780/CP70 cells was expressed as quantification histograms with error bars. (C) The cell cycle phase distribution of OVCAR3 cells were expressed as quantification histograms with error bars. p< 0.01. All measurements were done in triplicates. Data represent means ± SD of three independent experiments. Significant differences between two groups at both ends of lines with arrows are indicated by double asterisks (**) (p < 0.01).
The percent of cells in G2 phase was significantly decreased by either agent alone in A2780/CP70 and OVCAR3 cells. TF3 significantly decreased the portion of cells in G2 phase from 16.7% to 9.6% for A2780/CP70 cells and from 13.6% to 3.7% for OVCAR3 cells (p< 0.01). CDDP significantly decreased the portion of cells in G2 phase from 16.7% to 12.2% for A2780/CP70 cells and from 13.6% to 3.6% for OVCAR3 cells (p< 0.01). Both cells subjected to combination treatment showed a significant decrease in the percent of cells in G2 phase than untreated controls and cells treated with either agent alone. The combination treatment significantly decreased the portion of cells in G2 phase to 3.6% for A2780/CP70 cells and to 0.3% for OVCAR3 cells (p< 0.01). The percent of cells in the G1 and S phase of cells treated with combined TF3 and CDDP was significantly higher than that of cells treated with 7 μM TF3 alone and 7 μM CDDP alone (p< 0.01). These cell cycle analysis results indicated that TF3 and CDDP combination treatment could synergistically lead to G1 and S phase accumulation in ovarian cancer A2780/CP70 and OVCAR3 cells.
3.4. TF3 and CDDP synergistically regulate apoptosis and cell cycle related protein expression
Furthermore, the expression of proteins related to apoptosis and cell cycle in A2780/CP70 and OVCAR3 cells was evaluated by western blot analysis. As showed in Fig. 6, the protein level changes of apoptosis related proteins including cleaved caspase 3, cleaved caspase 7, cytochrome c, Bad, Bax and Bcl-2 were evaluated. TF3 had no significant effect on the protein levels of the apoptosis related proteins in A2780/CP70 and OVCAR3 cells (p> 0.05). The pro-apoptotic protein levels of cleaved caspase 3, cleaved caspase 7 and cytochrome c were higher in A2780/CP70 and OVCAR3 cells treated with 7 μM CDDP than in untreated controls (p< 0.05). The pro-apoptotic protein levels of cleaved caspase 3, cleaved caspase 7 and cytochrome c were higher in A2780/CP70 and OVCAR3 cells subjected to combination treatment than in untreated control cells and cells treated with either agent alone (p< 0.05). CDDP and combination treatment had no significant effect on the protein level of Bad in A2780/CP70 and OVCAR3 cells (p> 0.05). The protein level of Bax was higher in A2780/CP70 cells treated with 7 μM CDDP than in untreated controls (p< 0.05)and did not change in OVCAR3 cells (p>; 0.05). The protein level of Bax was higher in A2780/CP70 cells subjected to combination treatment than in untreated control cells and cells treated with either agent alone (p< 0.05). The protein level of Bax in OVCAR3 cells subjected to combination treatment was higher than in untreated control cells (p< 0.05) and did not change compared to OVCAR3 cells treated with either agent alone (p> 0.05). CDDP had no significant effect on the anti-apoptotic protein level of Bcl-2 (p> 0.05). The anti-apoptotic protein level of Bcl-2 was lower in cells subjected to combination treatment than in untreated control cells and cells treated with either agent alone (p< 0.05). These results indicate that the caspase and the Bcl-2 family involved apoptotic pathway are important mechanisms by which TF3 and CDDP combination treatment exerted the synergistic anti-proliferative effect in ovarian cancer A2780/CP70 and OVCAR3 cells.
Fig. 6.
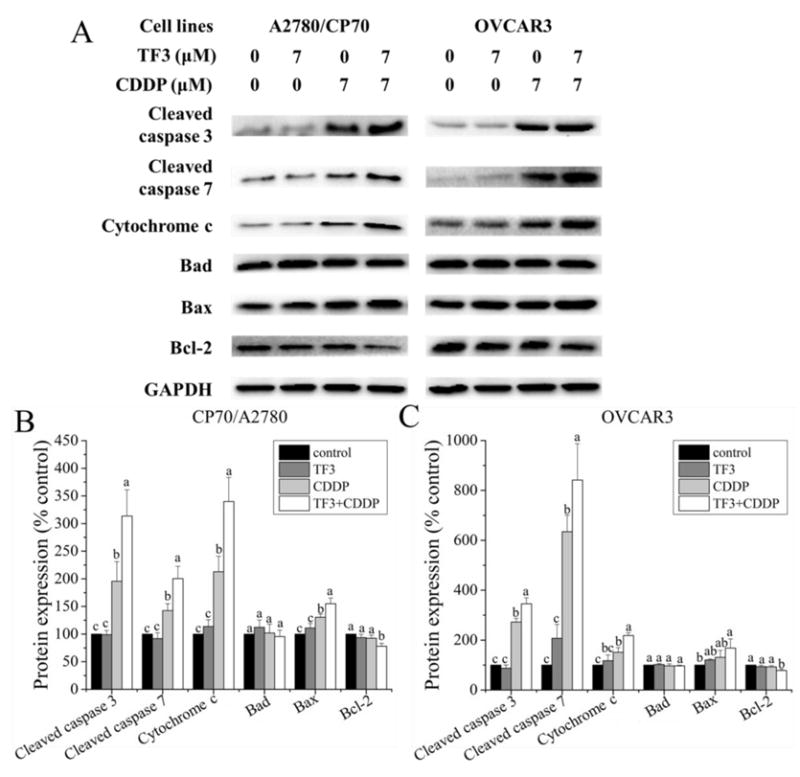
Effect of treatment with 7 μM TF3 alone, 7 μM CDDP alone or the combination on apoptosis related protein expression in ovarian cancer cells. (A)Protein expression levels of cleaved caspase 3, cleaved caspase 7, cytochrome c, Bad, Bax and Bcl-2 were analyzed by western blotting. (B) The expression levels of apoptosis related protein in A2780/CP70 cells were expressed as quantification histograms with error bars. (B) The expression levels of apoptosis related protein in OVCAR3 cells were expressed as quantification histograms with error bars. All measurements were done in triplicates. Data represent means ± SD of three independent experiments. Significant differences among different treatments are indicated by different letters (p< 0.05).
To identify the specific regulatory proteins related to the induction of cell cycle arrest in response to TF3, CDDP and combination treatment, the protein expression of cyclin A2, D1, E1, CDK2 and CDK4 was detected by western blot analysis. As showed in Fig. 7, TF3 treatment significantly decreased the protein expression of cyclin D1 and CDK4 in A2780/CP70 and OVCAR3 cells (p< 0.05). TF3 treatment had no significant effect on the protein expression of cyclin E1 and CDK2 in A2780/CP70 and OVCAR3 cells (p> 0.05). CDDP treatment significantly decreased the protein expression of cyclin E1, CDK2 and CDK4 in A2780/CP70 cells and cyclin D1 and E1 in OVCAR3 cells (p< 0.05). CDDP treatment had no significant effect on the protein expression of cyclin D1 in A2780/CP70 cells and CDK2 and CDK4 in OVCAR3 cells (p> 0.05). The protein level of cyclin D1 in A2780/CP70 cells treated with combined TF3 and CDDP had no significant difference with the cells treated with TF3 alone (p> 0.05). The protein levels of cyclin E1 and CDK2 in A2780/CP70 cells treated with combined TF3 and CDDP had no significant difference with the cells treated with CDDP alone (p> 0.05). The protein level of CDK4 was lower in A2780/CP70 cells subjected to combination treatment than in untreated control cells and cells treated with either agent alone (p< 0.05). The protein levels of cyclin D1 and E1 in OVCAR3 cells treated with combined TF3 and CDDP was higher than the cells treated with TF3 alone (p< 0.05), but had no significant difference with the cells treated with CDDP alone (p> 0.05). TF3, CDDP and combination treatment did not influence the expression of CDK2 protein in OVCAR3 cells (p> 0.05). The protein level CDK4 in OVCAR3 cells treated with combined TF3 and CDDP had no significant difference with the cells treated with TF3 alone (p> 0.05). TF3 and CDDP combination treatment decreased the protein expression of cyclin D1, E1, CDK2 and CDK4 in A2780/CP70 cells and cyclin D1, E1 and CDK4 in OVCAR3 cells, which resulted in the cell accumulation in G1 and S phase.
Fig. 7.
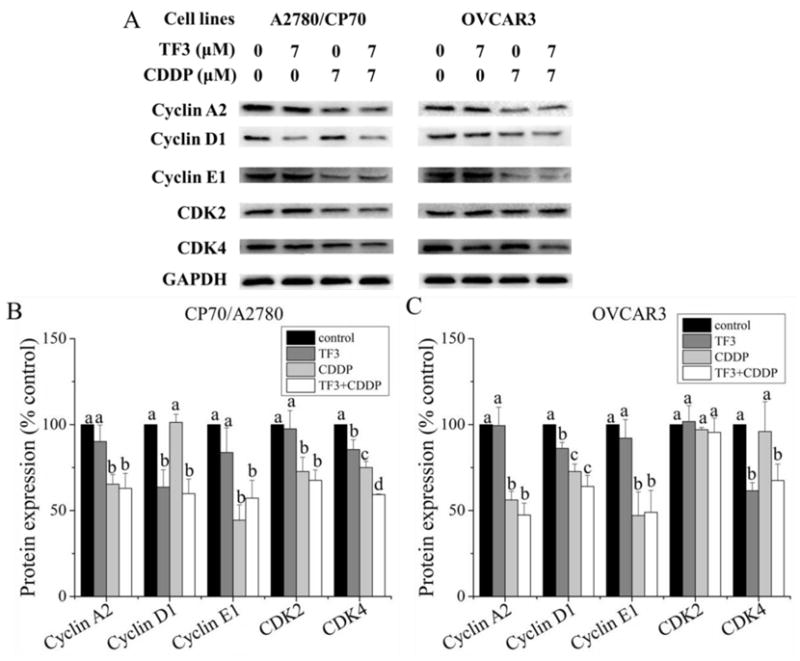
Effect of treatment with 7 μM TF3 alone, 7 μM CDDP alone or the combination on cell cycle related protein expression in ovarian cancer cells. (A) Protein expression levels of cyclin A2, D1, E1, CDK2 and CDK4 were analyzed by western blotting. (B) The expression levels of apoptosis related protein in A2780/CP70 cells were expressed as quantification histograms with error bars. (B) The expression levels of apoptosis related protein in OVCAR3 cells were expressed as quantification histograms with error bars. All measurements were done in triplicates. Data represent means ± SD of three independent experiments. Significant differences among different treatments are indicated by different letters (p< 0.05).
3.5. The role of Akt in the synergistic anti-tumor effect of TF3 and CDDP
Akt plays an important role in the development and progression of cancers. To determine whether the protein levels of Akt changed after treatment with combined treatment with TF3 and CDDP, we examined Akt and its phosphorylation in A2780/CP70 and OVCAR3 cells by Western blot analysis. As showed in Fig. 8, the protein level of p-Akt was attenuated in OVCAR3 cells treated with 7 μM TF3 and had no significant difference in A2780/CP70 cells treated with 7 μM TF3. The protein level of p-Akt was attenuated in both cells treated with 7 μM CDDP. The protein level of p-Akt was significantly lower in both cells subjected to combination treatment than in those treated with TF3 or CDDP alone. These results show that the TF3 and CDDP combination exhibited synergistic antitumor effect in ovarian cancer cells by down-regulating Akt phosphorylation in A2780/CP70 and OVCAR3 cells.
Fig. 8.
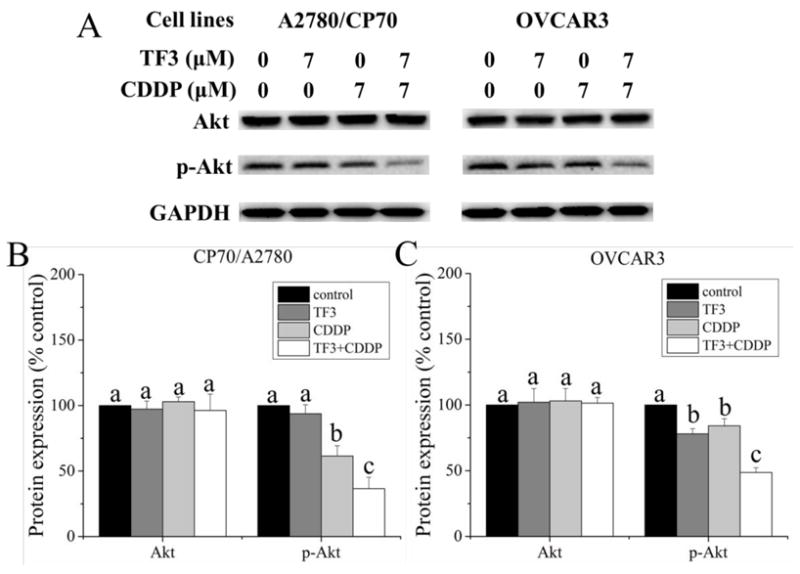
The effect of treatment with 7 μM TF3 alone, 7 μM CDDP alone and the combination on the protein phosphorylation of Akt in ovarian cancer cells. (A) Protein levels of Akt and p-Akt were analyzed by western blotting. (B) The protein levels of Akt and p-Akt in A2780/CP70 cells were expressed as quantification histograms with error bars. (B) The protein levels of Akt and p-Akt in OVCAR3 cells were expressed as quantification histograms with error bars. Results are expressed as mean ± SD from three independent experiments. Significant differences among different treatments are marked with different letters (p < 0.05).
4. Discussion
CDDP and its derivatives are first-line chemotherapeutic agents in the treatment of ovarian cancer. CDDP is a platinum based, alkylating-like drug that crosslinks DNA to induce apoptosis. Ovarian cancer is the most lethal gynecological malignancy which responds well to first-line platinum-based therapy and then easily relapses followed with CDDP resistance. Resistance to CDDP results in defects in apoptosis in ovarian cancer as well as critical steps in tumorigenesis (Li-Weber, 2013). Adverse side effects are also major hurdles in successful ovarian cancer treatment. Thus, combination therapy based on the synergistic antitumor effect between drugs has been used to overcome drug resistance and weaken the adverse side effects.
Combination treatment of chemotherapeutic agents and natural compounds is a good way to overcome drug resistance and weaken its adverse side effects. The synergistic anticancer effects of chemotherapeutic agents and natural compounds have been reported. It was reported that genistein could enhanced the anti-proliferative and cytotoxic effects of CDDP in human medulloblastoma cells (Khoshyomn, Manske, Lew, Wald, & Penar, 2000). Combination treatment with chemotherapeutic agents and the natural compound EGCG offers a clinical benefit in patients with non-small-cell lung cancer (Zhao, et al., 2014). Triptolide could synergistically enhance the antitumor effect of CDDP in human bladder cancer cells through promoting apoptosis and inducing cell cycle arrest (Ho, et al., 2015). EGCG could enhance CDDP sensitivity of ovarian cancer by regulating expression of the copper and cisplatin influx transporter CTR1 (X. Wang, et al., 2015). EGCG showed potential synergism with CDDP in biliary tract cancer cells by inducing cell cycle arrest synergistically (Mayr, et al., 2015). TF3 derives from EGCG, but the synergistic anticancer effects of TF3 and CDDP has not been reported. Our previous study showed that TF3 was less cytotoxic to normal ovarian IOSE-364 cells than ovarian cancer cells (Tu, et al., 2016) which indicated that TF3 was a potential agent to reduce the side effect of CDDP and overcome the CDDP resistance of cancer cells. To our knowledge, the present study is the first to show that TF3 synergistically enhanced the antitumor effects of CDDP in human ovarian cancer cell lines.
In the present study, we first evaluated the synergistic anti-proliferative effect of TF3 and CDDP against ovarian cancer A2780/CP70 and OVCAR3 cells using MTS assay. The combination treatment CI values were less than 1.0, indicating that the antitumor effect was synergistic. When the ovarian cancer cells were treated with combined TF3 and CDDP at the constant combination ratio (1:1, μM: μM), the concentration of CDDP was reduced by 12.18 μM for A2780/CP70 cells and 8.61 μM for OVCAR3 cells to inhibit 50% cell viability. When the ovarian cancer cells were treated with combined TF3 and CDDP at the constant combination ratio (1:2, μM: μM), the concentration of CDDP was reduced by 9.24 μM for A2780/CP70 cells and 6.76 μM for OVCAR3 cells to inhibit 50% cell viability. Since the concentration of CDDP was lower at the constant combination ratio of 1:1 than that of 1:2 and treatment with combined 7 μM TF3 and 7 μM CDDP inhibited about 50% cell viability, we treated the ovarian cancer cells with 7 μM TF3 and/or 7 μM CDDP for further study.
Colony formation assay is an effective method to determine the long term effectiveness of cytotoxic agents against cancer cells (Franken, Rodermond, Stap, Haveman, & Van Bree, 2006). In the current study, the reductions in colony formation for A2780/CP70 cells treated with TF3, CDDP and combined TF3 and CDDP were 19.3%, 61.4% and 84.0%, respectively. The reductions in colony formation for OVCAR3 cells treated with TF3, CDDP and combined TF3 and CDDP were 27.1%, 63.4% and 82.7%, respectively. It was found that the reduction in colony formation was greater for both cells treated with combined TF3 and CDDP than for cells treated with either agent alone. It indicated that TF3 significantly enhanced the anti-proliferation effect induced by CDDP in ovarian cancer A2780/CP70 and OVCAR3 cells.
To further verify the synergistic effect of TF3 and CDDP against ovarian cancer cells, the changes of nuclear morphology in cells treated with TF3, CDDP or the combination were analyzed by Hoechst 33342 DNA staining. Morphological hallmarks of apoptosis in the nucleus are chromatin condensation and nuclear fragmentation (Wong, 2011). The apoptotic cells with condensed or fragmented nuclei look much brighter. In the current study, the portion of apoptotic cells was greater for both cells treated with combined TF3 and CDDP than the portion sum for both cells treated with either agent alone. It indicated that TF3 and CDDP combination treatment could synergistically induce apoptosis in ovarian cancer A2780/CP70 and OVCAR3 cells.
Double staining with Annexin V FITC and PI followed by flow cytometry analysis was also used to investigate the loss of plasma membrane asymmetry which is another morphological hallmark of apoptosis (Hassan, Watari, AbuAlmaaty, Ohba, & Sakuragi, 2014). It was found that the treatment of TF3, CDDP and the combination significantly increased the percentage of total apoptotic cells (p< 0.05). The increased portion of total apoptotic cells was significantly greater for A2780/CP70 cells treated with combined TF3 and CDDP than the increased portion sum of total apoptotic cells for A2780/CP70 cells treated with either agent alone (p< 0.05). The increased portion of total apoptotic cells in OVCAR3 cells treated with combined TF3 and CDDP had no significant difference with the increased portion sum of total apoptotic cells in OVCAR3 cells treated with either agent alone (p> 0.05). It indicated that TF3 could enhance the pro-apoptotic effect induced by CDDP in ovarian cancer A2780/CP70 cells.
Apoptosis can be initiated via both intrinsic and extrinsic pathways, which ultimately activate caspases 3/7 and apoptotic effector molecules (Fulda & Debatin, 2006). The intrinsic apoptosis pathway is triggered by intracellular signals such as cellular and DNA damage. CDDP is a platinum based, alkylating-like drug that by binds to and blocks the duplication of DNA to induce apoptosis mainly through intrinsic pathway. The key events of intrinsic pathway are the depolarization of the mitochondrial membrane potential and the release of mitochondrial factors such as cytochrome c into the cytosol. Mitochondrial activation is critically controlled by the family of Bcl-2 proteins which consists of pro-and anti-apoptotic members (Brunelle & Letai, 2009). Resistance to cisplatin might develop by decreasing the expression of pro-apoptotic proteins, or increasing the expression of anti-apoptotic proteins (Köberle, Tomicic, Usanova, & Kaina, 2010). It was reported that treatment with TF3 induced apoptosis in ovarian cancer cells by regulating the protein expression of Bcl-2 family proteins (Tu, et al., 2016). Caspases are central to the mechanism of apoptosis as they are both the initiators and executioners. Caspases involved in apoptosis have been classified by their mechanism of action and are either initiator caspases (caspase 8/9) or executioner caspases (caspase 3/7) (McIlwain, Berger, & Mak, 2015). Activation of caspase 3/7 requires proteolytic processing of the inactive zymogen into activated fragments. Therefore, we analyzed the levels of the apoptosis related proteins, including cleaved caspase 3, cleaved caspase 7, cytochrome c, Bad, Bax and Bcl-2. Treatment with TF3 alone had no significant effect on the protein levels of the apoptosis related proteins in A2780/CP70 and OVCAR3 cells (p< 0.05). But treatment with TF3 increased the percentage of apoptotic cells in both cell lines. Treatment with 7 μM TF3 only induced early apoptosis in both cell lines. The early apoptotic phase was quite rapid (Henslee, et al., 2016). The expression of proteins related to apoptosis by western blotting often remained unchanged at early apoptotic phase and changed at late apoptotic phase. Treatment with TF3 could enhance the increase of pro-apoptotic protein levels induced by CDDP and enhance the reduction of anti-apoptotic protein level induced by CDDP. TF3 and CDDP combination treatment could synergistically induce apoptosis in ovarian cancer A2780/CP70 and OVCAR3 cells by regulating the protein levels of cleaved caspase 3, cleaved caspase 7, cytochrome c, Bax and Bcl-2.
Most genetic mutates that promote tumorigenesis relate to dysregulation of cell cycle progression (Foster, Yellen, Xu, & Saqcena, 2010). Cells that progress through the cell cycle unchecked may eventually form malignant tumors, where masses of cells grow and divide uncontrollably. It’s an effective target to block cell cycle for improved cancer therapies. A previous report showed that theaflavins could induce G2 cell cycle arrest in human prostate carcinoma PC-3 cells (Prasad, Kaur, Roy, Kalra, & Shukla, 2007). It was reported that TF3 induced G1 cell cycle arrest in human lung adenocarcinoma SPC-A-1 cells (Li, Wu, & Tu, 2010). CDDP could induce G1 cell cycle arrest in MCF-7 breast cancer cells (Otto, Paddenberg, Schubert, & Mannherz, 1996). In the current study, both A2780/CP70 and OVCAR3 cells accumulated in cell cycle G1 phase after exposure to 7 μM TF3 alone. Both cells accumulated in cell cycle S phase after exposure to 7 μM CDDP alone. The percentage of cells accumulating in cell cycle G2 phase was significantly decreased by treatment with TF3 alone and CDDP alone in A2780/CP70 and OVCAR3 cells (p< 0.05). Both cells accumulated in cell cycle G1 and S phase after treated with combined TF3 and CDDP which resulted in a significant decrease in the percent of cells in G2 phase than untreated controls and cells treated with either agent alone. TF3 and CDDP combination treatment could synergistically induce cell cycle arrest in ovarian cancer A2780/CP70 and OVCAR3 cells by accumulating the cells in cell cycle G1 and S phase.
Key regulators of cell cycle progression are the cyclins which interact with and activate specific cyclin-dependent kinases (CDKs). There are 2 major G1/S checkpoint-related cyclins: cyclin D and cyclin E. Cyclin D interacts with CDK4, and cyclin E partners with CDK2 (Foster, et al., 2010). Cyclin A resides in the nucleus during S phase where it is involved in the initiation and completion of DNA replication (Bendris, Lemmers, Blanchard, & Arsic, 2011; Pagano, Pepperkok, Verde, Ansorge, & Draetta, 1992; Soucek, Pusch, Hengstschläger-Ottnad, Adams, & Hengstschläger, 1997). As the cell passes from G1 into S phase, cyclin A associates with CDK2 regulating the cell cycle S phase progression. Cyclin A-CDK2 complex is important and tightly regulated in S phase. To examine the synergistic effect of TF3 and CDDP combination treatment on the cell cycle arrest, we analyzed the protein expression of the G1/S-phase regulators cyclin A2, cyclin D1, cyclin E1, CDK2 and CDK4. In the current study, we found that TF3 induced G1 cell cycle arrest by downregulating cyclin D1 and CDK4 protein in A2780/CP70 and OVCAR3 cells. CDDP induced S cell cycle arrest by downregulating cyclin A2, cyclin E1, CDK2 and CDK4 in A2780/CP70 cells and by downregulating cyclin A2, cyclin D1 and cyclin E1 in OVCAR3 cells. Combination treatment with TF3 and CDDP decreased the protein expression of cyclin A2, cyclin D1, cyclin E1, CDK2 and CDK4 in A2780/CP70 cells and cyclin A2, cyclin D1, cyclin E1 and CDK4 in OVCAR3 cells which resulted in cell accumulation in cell cycle G1 and S phase.
Akt is a serine/threonine kinase that plays an important role in the development and progression of cancers (Hennessy, Smith, Ram, Lu, & Mills, 2005; Vara, et al., 2004). This protein kinase is activated by insulin and various growth and survival factors. The Akt pathway is one of the most frequently hyperactivated signaling pathways in human cancers. Akt is involved in cell cycle regulation by preventing GSK-3β-mediated phosphorylation and degradation of cyclin D1 (Diehl, Cheng, Roussel, & Sherr, 1998). Inhibition of Akt pathway may be a helpful and promising strategy for cancer therapy and can also be useful for overcoming chemotherapy resistance. It was reported that suppression of Akt sensitized cisplatin resistant cells to cisplatin in a p53-dependent manner (Kim, et al., 2013). A previous report showed that TF3 induced apoptosis and cell cycle arrest through the Akt/MDM2/p53 pathway in ovarian cancer cells (Tu, et al., 2016). CDDP could down-regulate the expression of p-Akt protein in human bladder cancer cells (Ho, et al., 2015) and esophageal squamous cell carcinoma cells (Ui, et al., 2014). In the current study, we found that combination treatment with TF3 and cisplatin led to a synergistic decrease in the expression of p-Akt relative to the levels of p-Akt in both cells treated with TF3 or CDDP alone.
5. Conclusion
In summary, our study provided the first evidence that TF3 could inhibit cisplatin-resistant ovarian cancer cells synergistically with CDDP. TF3 and CDDP exerted synergistic cytotoxicity against cisplatin resistant ovarian cancer cells. TF3 and CDDP synergistically induced apoptosis and G1/S cell cycle arrest in ovarian cancer cells. Combination treatment with TF3 and CDDP synergistically down-regulated Akt phosphorylation in ovarian cancer cells. TF3 was a potential agent to reduce the side effect of CDDP and overcome the CDDP resistance of ovarian cancer cells. TF3 may be used as an adjuvant for the treatment of advanced ovarian cancer.
Highlights.
TF3 and CDDP could synergistically inhibit ovarian cancer cells.
TF3 and CDDP showed a synergistic pro-apoptotic effect.
TF3 and CDDP synergistically induced G1/S phase cell cycle arrest.
TF3 and CDDP synergistically regulated apoptosis related protein expression.
TF3 and CDDP synergistically regulated cell cycle related protein expression.
Acknowledgments
We thank Dr. Kathy Brundage from the Flow Cytometry Core at the West Virginia University for providing technical help on apoptosis analysis. This research was supported by NIH grants P20RR016477 from the National Center for Research Resources and P20GM103434 from the National Institute for General Medical Sciences (NIGMS) awarded to the West Virginia IDeA Network of Biomedical Research Excellence. This study was also supported by Grant Number P20GM104932 from NIGMS, a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIGMS or NIH. This study was also supported by COBRE grant GM102488/RR032138, ARIA S10 grant RR020866, FORTESSA S10 grant OD016165 and INBRE grant GM103434. This research was also supported by the Natural Science Foundation of Zhejiang Province (grant no. LY15C200007), the National Natural Science Foundation of China (grant no. 31501474), and Oolong Tea Industry Collaborative Innovation Center.
Footnotes
Author Contributions
H.P. designed and preformed the experiments. H.P. performed the statistical analysis. H.P. wrote the manuscript. J.L., E.K., G.O.R., Y.R., Y.T. and Y.C.C. coordinated and helped to draft the manuscript. All authors read and approved the final manuscript.
Conflicts of interests
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Rawahi T, Lopes AD, Bristow RE, Bryant A, Elattar A, Chattopadhyay S, Galaal K. Surgical cytoreduction for recurrent epithelial ovarian cancer. The Cochrane Library. 2013 doi: 10.1002/14651858.CD008765.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J, Boakye K, McCann S, Beehler G, Rodabaugh KJ, Villella J, Moysich K. Consumption of black tea or coffee and risk of ovarian cancer. International Journal of Gynecological Cancer. 2007;17:50–54. doi: 10.1111/j.1525-1438.2006.00773.x. [DOI] [PubMed] [Google Scholar]
- Bendris N, Lemmers B, Blanchard J-M, Arsic N. Cyclin A2 mutagenesis analysis: a new insight into CDK activation and cellular localization requirements. PloS one. 2011;6:e22879. doi: 10.1371/journal.pone.0022879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle JK, Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. Journal of cell science. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy A, Huang T, Rice MS, Rimm EB, Tworoger SS. Intake of dietary flavonoids and risk of epithelial ovarian cancer. The American journal of clinical nutrition. 2014 doi: 10.3945/ajcn.114.088708. ajcn.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes & development. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger A. In-vitro studies on the effect of polyphenol oxidase and peroxidase on the formation of polyphenolic black tea constituents. Journal of the Science of Food and Agriculture. 1994;66:293–305. [Google Scholar]
- Foster DA, Yellen P, Xu L, Saqcena M. Regulation of G1 cell cycle progression: distinguishing the restriction point from a nutrient-sensing cell growth checkpoint (s) Genes & cancer. 2010;1:1124–1131. doi: 10.1177/1947601910392989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken NA, Rodermond HM, Stap J, Haveman J, Van Bree C. Clonogenic assay of cells in vitro. Nature protocols. 2006;1:2315. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin K. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- Gao Y, Rankin GO, Tu Y, Chen YC. Theaflavin-3, 3’-digallate decreases human ovarian carcinoma OVCAR-3 cell-induced angiogenesis via Akt and Notch-1 pathways, not via MAPK pathways. International journal of oncology. 2016;48:281–292. doi: 10.3892/ijo.2015.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. BioMed research international. 2014;2014 doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nature reviews Drug discovery. 2005;4:988. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- Henslee EA, Serrano RMT, Labeed FH, Jabr RI, Fry CH, Hughes MP, Hoettges KF. Accurate quantification of apoptosis progression and toxicity using a dielectrophoretic approach. Analyst. 2016;141:6408–6415. doi: 10.1039/c6an01596d. [DOI] [PubMed] [Google Scholar]
- Ho J-N, Byun S-S, Lee S, Oh JJ, Hong SK, Lee SE, Yeon JS. Synergistic antitumor effect of triptolide and cisplatin in cisplatin resistant human bladder cancer cells. The Journal of urology. 2015;193:1016–1022. doi: 10.1016/j.juro.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Köberle B, Tomicic MT, Usanova S, Kaina B. Cisplatin resistance: preclinical findings and clinical implications. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2010;1806:172–182. doi: 10.1016/j.bbcan.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Khoshyomn S, Manske GC, Lew SM, Wald SL, Penar PL. Synergistic action of genistein and cisplatin on growth inhibition and cytotoxicity of human medulloblastoma cells. Pediatric neurosurgery. 2000;33:123–131. doi: 10.1159/000028993. [DOI] [PubMed] [Google Scholar]
- Kim CW, Lu JN, Go S-I, Jung JH, Yi SM, Jeong J-H, Hah Y-S, Han MS, Park JW, Lee WS. p53 restoration can overcome cisplatin resistance through inhibition of Akt as well as induction of Bax. International journal of oncology. 2013;43:1495–1502. doi: 10.3892/ijo.2013.2070. [DOI] [PubMed] [Google Scholar]
- Lee H-H, Ho C-T, Lin J-K. Theaflavin-3, 3′-digallate and penta-O-galloyl-β-d-glucose inhibit rat liver microsomal 5α-reductase activity and the expression of androgen receptor in LNCaP prostate cancer cells. carcinogenesis. 2004;25:1109–1118. doi: 10.1093/carcin/bgh106. [DOI] [PubMed] [Google Scholar]
- Li-Weber M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer letters. 2013;332:304–312. doi: 10.1016/j.canlet.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Li W, Wu J-x, Tu Y-y. Synergistic effects of tea polyphenols and ascorbic acid on human lung adenocarcinoma SPC-A-1 cells. Journal of Zhejiang University-Science B. 2010;11:458–464. doi: 10.1631/jzus.B0900355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limtrakul P, Pitchakarn P, Suzuki S. Kuguacin J, a Triterpenoid from Momordica charantia Linn: A Comprehensive Review of Anticarcinogenic Properties. INTECH Open Access Publisher; 2013. [Google Scholar]
- Lin J-K, Chen Y-W, Lin-Shiau S-Y. Inhibition of breast cancer cell proliferation by theaflavins from black tea through suppressing proteasomal activities. AACR 2006 [Google Scholar]
- Mayr C, Wagner A, Neureiter D, Pichler M, Jakab M, Illig R, Berr F, Kiesslich T. The green tea catechin epigallocatechin gallate induces cell cycle arrest and shows potential synergism with cisplatin in biliary tract cancer cells. BMC complementary and alternative medicine. 2015;15:194. doi: 10.1186/s12906-015-0721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harbor perspectives in biology. 2015;7 doi: 10.1101/cshperspect.a026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto AM, Paddenberg R, Schubert S, Mannherz HG. Cell-cycle arrest, micronucleus formation, and cell death in growth inhibition of MCF-7 breast cancer cells by tamoxifen and cisplatin. Journal of cancer research and clinical oncology. 1996;122:603–612. doi: 10.1007/BF01221192. [DOI] [PubMed] [Google Scholar]
- Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. The EMBO journal. 1992;11:961. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Kaur J, Roy P, Kalra N, Shukla Y. Theaflavins induce G2/M arrest by modulating expression of p21 waf1/cip1, cdc25C and cyclin B in human prostate carcinoma PC-3 cells. Life sciences. 2007;81:1323–1331. doi: 10.1016/j.lfs.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Soucek T, Pusch O, Hengstschläger-Ottnad E, Adams PD, Hengstschläger M. Deregulated expression of E2F-1 induces cyclin A-and E-associated kinase activities independently from cell cycle position. Oncogene. 1997;14 doi: 10.1038/sj.onc.1201061. [DOI] [PubMed] [Google Scholar]
- Sun S, Pan S, Miao A, Ling C, Pang S, Tang J, Chen D, Zhao C. Active extracts of black tea (Camellia Sinensis) induce apoptosis of PC-3 prostate cancer cells via mitochondrial dysfunction. Oncology reports. 2013;30:763–772. doi: 10.3892/or.2013.2504. [DOI] [PubMed] [Google Scholar]
- Tu Y, Kim E, Gao Y, RANkIN GO, Li B, ChEN YC. Theaflavin-3, 3’-digallate induces apoptosis and G2 cell cycle arrest through the Akt/MDM2/p53 pathway in cisplatin-resistant ovarian cancer A2780/CP70 cells. International journal of oncology. 2016;48:2657–2665. doi: 10.3892/ijo.2016.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ui T, Morishima K, Saito S, Sakuma Y, Fujii H, Hosoya Y, Ishikawa S, Aburatani H, Fukayama M, Niki T. The HSP90 inhibitor 17-N-allylamino-17-demethoxy geldanamycin (17-AAG) synergizes with cisplatin and induces apoptosis in cisplatin-resistant esophageal squamous cell carcinoma cell lines via the Akt/XIAP pathway. Oncology reports. 2014;31:619–624. doi: 10.3892/or.2013.2899. [DOI] [PubMed] [Google Scholar]
- Vara JÁF, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer treatment reviews. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Wang K, Liu Z, Huang J, BEKHIT AED, Liu F, Dong X, Gong Y, Fu D. The inhibitory effects of pure black tea theaflavins on the growth of four selected human cancer CELLS. Journal of Food Biochemistry. 2011;35:1561–1567. [Google Scholar]
- Wang X, Jiang P, Wang P, Yang CS, Wang X, Feng Q. EGCG enhances cisplatin sensitivity by regulating expression of the copper and cisplatin influx transporter CTR1 in ovary cancer. PloS one. 2015;10:e0125402. doi: 10.1371/journal.pone.0125402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RS. Apoptosis in cancer: from pathogenesis to treatment. Journal of Experimental & Clinical Cancer Research. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jin Y, Wu Y, Tu Y. Isolation and purification of four individual theaflavins using semi-preparative high performance liquid chromatography. Journal of Liquid Chromatography & Related Technologies. 2010;33:1791–1801. [Google Scholar]
- Zhao H, Zhu W, Xie P, Li H, Zhang X, Sun X, Yu J, Xing L. A phase I study of concurrent chemotherapy and thoracic radiotherapy with oral epigallocatechin-3-gallate protection in patients with locally advanced stage III non-small-cell lung cancer. Radiotherapy and Oncology. 2014;110:132–136. doi: 10.1016/j.radonc.2013.10.014. [DOI] [PubMed] [Google Scholar]


