Abstract
Introduction
Guideline-recommended surveillance reduces likelihood of colorectal cancer (CRC) recurrence, yet surveillance rates are low in the United States (US). Little is known about CRC surveillance rates among patients without health insurance and their primary care clinicians/oncologists' attitudes towards surveillance care.
Methods
A retrospective study of 205 patients diagnosed with Stage I-III CRC from 2008-2010 was conducted in an integrated system with a network of providers delivering care to patients lacking health insurance coverage. Surveillance patterns were characterized from medical records and logistic regression models examined correlates of guideline-concordant surveillance. 41 Parkland primary care physicians (PCPs) and 24 oncologists completed surveys to assess their attitudes and practices regarding CRC surveillance.
Results
38% of CRC patients received guideline-concordant surveillance; those with early stage cancers were less likely to receive surveillance (OR=0.35; 95 CI: 0.14, 0.87). PCPs and oncologists differed markedly on who is responsible for cancer surveillance care. 77% of oncologists responded that PCPs evaluated patients for cancer recurrence while 76% of PCPs responded that these services were either ordered by oncologists or shared with PCPs. 67% of oncologists said they rarely provide a treatment and surveillance care plan to survivors and over half said that they infrequently communicate with patients' other physicians about who will follow patients for their cancer and other medical issues.
Discussion
Care coordination between PCP and oncologist is needed to improve CRC surveillance. New models of shared care clearly delineating roles for oncologists and PCPs are needed to improve CRC survivorship care.
Keywords: Colorectal cancer, surveillance, primary care physicians, oncologists
Introduction
Colorectal cancer (CRC) is the third most common cancer in both men and women worldwide with over 60% of individuals in the United States (US) surviving at least 5-years.1 During 2016, there were over 1.45 million CRC survivors living in the US 1, a figure that is projected to increase 35% by 2020. Globally, the burden of CRC is expected to increase 60% by 2030.2 Guideline-recommended post-cancer surveillance reduces likelihood of CRC recurrence; yet rates of CRC surveillance in the US are low.3
The transition from active treatment for cancer to surveillance is pivotal to the long-term health of cancer survivors. Many survivors, especially those that are socio-economically vulnerable, report that they are unaware of the cancer treatments received, long-term health risks, and follow-up care needed to manage their disease.4 Studies globally have shown that being a racial/ethnic minority, older, and of lower socioeconomic status is associated with underuse of cancer surveillance care. In the US, this is particularly concerning for racial/ethnic minority population because they have the highest mortality rates.5 Furthermore, studies globally indicate that poor health literacy and multiple comorbidities are associated with poor care coordination.6, 7
Despite clear clinical guidelines on CRC surveillance care processes, there is lack of consensus about who is responsible for providing and coordinating CRC surveillance care.8. Depending on the health care setting, primary care, oncology, or gastroenterology providers may be involved in ordering and following up on CRC surveillance tests with limited, deliberate organization of care and exchange of information between specialties to facilitate appropriate delivery CRC surveillance care 9 Notably, in a nationally representative study conducted in the US, primary care providers (PCPs) and oncologists differed markedly in their knowledge, attitudes, and practices with respect to care of breast and colorectal cancer survivors. 10 Studies suggest that a lack of coordination between specialties, coupled with health disparities that exist among socio-economically vulnerable populations, may result in more pronounced negative effects, such as a higher 5-year mortality rate, increased comorbidities, secondary cancers, and recurrence.11
To date, few studies have investigated rates of and factors associated with guideline-concordant surveillance among CRC survivors with inadequate or no health insurance, referred as under- and uninsured CRC survivors. Further, there is limited knowledge about PCPs and oncologists' perspectives on surveillance for CRC patients receiving care in an integrated health system, where infrastructure should support coordination. Therefore, drawing from both medical records and physician surveys, the purpose of this study is twofold: (1) To characterize CRC surveillance patterns and correlates of receiving guideline-concordant CRC surveillance among under- and uninsured CRC survivors, and (2) to compare PCPs' and oncologists' attitudes and practices regarding care of CRC cancer survivors in an integrated safety-net health system. We hypothesize that PCPs and oncologists practicing within an integrated system would demonstrate clear allocation of responsibilities in the follow-up care of cancer survivors that would derive from practicing in a single institution with uniform structures, policies, and procedures
Methods
Study Design
We conducted a retrospective cohort study including all patients diagnosed with Stage I-III CRC (CRC survivors) from 2008 to 2010 and who received care at Parkland Health & Hospital System (Parkland) and a cross-sectional survey of PCPs and oncologists practicing within the Parkland network using the validated Survey of Physician Attitudes Regarding Care of Cancer Survivors (SPARCCS) survey over a three-month period in 2013. Institutional review board approval was obtained through University of Texas Southwestern Medical Center IRB.
Study Setting
Parkland is an integrated public safety-net system that organizes and delivers health care and other health-related services to those who are under- and uninsured residents of Dallas County, Texas. It is one of the largest public health hospital systems in the US serving more than 1 million patient visits a year and delivering care across 20 community-based clinics. At the time of this study, Parkland coordinated care for all patients through a centralized group of schedulers who assisted patients with clinic appointments and by empaneling patients to specific clinics and providers. However, there were no care coordination services dedicated to cancer survivors.
Data Collection
A research assistant experienced in medical record audits extracted data from patients' medical records following a protocol developed by the principal researcher (BB). We included 205 patients diagnosed with Stage I, II, and III CRC as a single, incident cancer between 2008 and 2010 and receiving care at Parkland. We excluded patients with Stage IV cancer because surveillance guidelines are relevant only for non-metastatic cancers (Stages I, II, and III).
We also administered the validated SPARCCS 12 survey to PCPs and oncologists affiliated with Parkland in the period, March-December 2013. The SPARCCS was developed by the National Cancer Institute using previously developed questionnaires and later revised following a series of cognitive interviews. Separate questionnaires were developed for oncologists and PCPs to include specialty-specific items. However, the questionnaires contain identical items assessing PCP and oncologists' knowledge, practices, and perceived roles and responsibilities regarding post-treatment follow-up care of CRC survivors. 10, 12 Four data collectors distributed surveys to physicians at group forums regularly convened by Parkland for ongoing training and quality improvement. Physicians completed the surveys and handed them back to the data collectors with no identifying information. The length of time to complete the survey ranged between 15-30 minutes. The full survey instruments are available as supplementary material online.
Measures
Guideline-recommended CRC surveillance was assessed using National Comprehensive Cancer Network (NCCN) guidelines13. Up-to-date with cancer surveillance was defined as survivors who received any of the following interventions per NCCN guidelines, with all time points calculated from date of end of active treatment:13 (1) carcinoembryonic antigen levels (CEA, within 6 months), (2) endoscopy (colon or rectum, within 15 months), (3) at least one primary care visit within 1 year, and (4) computed tomography (CT) scan within 6 months or 12 months. Endoscopy was defined as 15 months from end of active treatment because subjects may not have been able to schedule tests at exactly 12 months. The surveillance period began at the end of active CRC treatment, defined as receipt of a definitive surgical procedure or adjuvant chemotherapy and/or radiotherapy as first course of treatment after diagnosis. Surveillance modalities were each dichotomized as “yes [1]” or “no [0]”. In addition, we assessed a composite measure by examining if patients received all three (CEA, endoscopy, and CT scan) surveillance modalities.
SPARCCS survey measures included items to assess three distinct domains; (1) clinicians' perceived skills and knowledge of survivorship care and surveillance, (2) care delivery practices clinicians used for survivors, and (3) care coordination practices clinicians used for survivors.
Covariates included patient sex, age (continuous), race/ethnicity (White Non-Hispanic, Black Non-Hispanic, Hispanic, and Other), and health insurance (Private/Commercial, Medicare, Medicaid, Uninsured, Other), and cancer stage (I, II, III). Number of comorbidities was summed up across twenty-three comorbidity diagnoses provided on the medical records and comorbidity status was categorized as 0, 1, 2, and 3+.
Statistical Analyses
We analyzed medical record data by generating descriptive statistics (tabulations and means) to examine distribution of patient demographics, tumor characteristics, and surveillance modalities among the total sample as well as stratified by age. Because clear disparities in cancer surveillance modalities emerged, we used bivariate and multivariate logistic regression models to evaluate the effects of demographics, cancer stage, comorbidities, and place of diagnosis on survivors receiving a CEA, endoscopy, and CT scan per NCCN surveillance guidelines. The multivariate model controlled for covariates identified a priori as potential confounders (demographics, insurance, number of comorbidities, place of diagnosis, physical exam in the past 6 months, and stage of cancer).
We analyzed physician surveys descriptively by examining response frequency distributions and computing Fisher's exact tests to examine differences between PCP and oncologist responses. When specific survey items were not answered, we excluded those respondents' item from analyses. All analyses were conducted using Stata 14.0 software.
Results
Cancer follow-up care
Of the 205 patient records reviewed, 19% were Stage I, 36% stage and 46% were Stage III with the average age at diagnosis of 56.1 years (Table 1). The majority of CRC survivors were male (54%), non-White (80%), and uninsured (47%). Additionally, 54% of survivors had more than three comorbid conditions, with hypertension being the most prevalent condition. Results from the medical record audits show that only 38% of CRC survivors received all guideline-recommended surveillance tests/procedures (CEA, endoscopy, and CT scan).
Table 1. Sample demographics and logistic regression testing the relationship between demographics, comorbidities, cancer stage, and receiving a CEA, endoscopy, and CT scan within NCCN surveillance guidelines (n=205).
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| N (%) | OR | 95% CI | OR | 95% CI | |
|
|
|||||
| Age (mean, SD) | 56.1 (11) | 0.98 | 0.96, 1.01 | 0.97 | 0.94, 1.00 |
| Gender | |||||
| Female | 94 (46) | - | - | - | - |
| Male | 111 (54) | 0.82 | 0.46, 1.44 | 0.62 | 0.33, 1.19 |
| Race/Ethnicity | |||||
| White Non-Hispanic | 41 (20) | - | - | - | - |
| Black Non-Hispanic | 78 (38) | 2.34* | 1.03, 5.32 | 2.38 | 0.96, 5.87 |
| Hispanic | 77 (38) | 1.74 | 0.76, 3.99 | 2.40 | 0.92, 6.28 |
| Other | 9 (4) | 0.34 | 0.04, 3.05 | 0.51 | 0.05, 5.24 |
| Health Insurance | |||||
| Private/Commercial | 12 (6) | - | - | - | - |
| Medicare | 68 (33) | 0.59 | 0.06, 5.47 | 0.79 | 0.07, 9.20 |
| Medicaid | 5 (2) | 1.85 | 0.97, 3.53 | 2.76* | 1.20, 6.31 |
| Uninsured | 97 (47) | 2.74 | 0.85, 8.84 | 2.17 | 0.60, 7.76 |
| Other | 22 (11) | 2.34 | 0.91, 6.01 | 2.25 | 0.73, 6.92 |
| Number of Comorbidities | |||||
| 0 | 21 (10) | - | - | - | - |
| 1 | 36 (18) | 1.27 | 0.41, 3.93 | 0.99 | 0.29, 3.40 |
| 2 | 38 (19) | 0.71 | 0.22, 2.28 | 0.85 | 0.24, 3.09 |
| 3+ | 110 (54) | 1.49 | 0.56, 3.99 | 1.37 | 0.41, 4.53 |
| Physical exam (6 months) | 21 (10) | 0.35 | 0.11, 1.08 | 1.37 | 0.71, 2.67 |
| Cancer stage | |||||
| I | 38 (19) | 0.43* | 0.19, 0.96 | 0.35* | 0.14, 0.87 |
| II | 72 (36) | 0.43* | 0.22, 0.82 | 0.36** | 0.18, 0.74 |
| III | 92 (46) | - | - | - | - |
| Recommended surveillance modalities | |||||
| Carcinoembryonic antigen levels (CEA)a | 156 (76) | ||||
| Endoscopyc | 104 (51) | ||||
| CEAa + Endoscopyc | 89 (43) | ||||
| CEAa + Endoscopyc + CT scanb | 78 (38) | ||||
| Computed tomography (CT) | 146 (71) | ||||
| scana | |||||
| CT scanb | 164 (80) | ||||
| Physical examb | 36 (18) | ||||
-6 months,
-12 months,
-15 months
p-value < 0.05;
p-value < 0.01
In addition to descriptive characteristics, Table 1 provides bivariate and multivariate associations between stage of cancer, demographics, number of comorbidities, and guideline-recommended surveillance. Multivariate regression models revealed that cancer survivors diagnosed with stage I and stage II cancer had significantly lower odds (OR=0.35; 95 CI: 0.14, 0.87 for stage I; OR=0.36; 95% CI 0.18, 0.74 for stage II) of receiving cancer surveillance as compared to stage III cancer survivors.
PCP and oncologist attitudes and practices towards CRC surveillance
The overall response rate for the SPARCCS survey was 52% with 41 PCPs and 24 oncologists affiliated with Parkland responding to the survey. This response rate is within the range of previous studies using the SPARCCS survey and in diverse populations. 14, 15
Surveillance Care Practices
Results from the SPARCCS illustrated in Table 2 show statistically significant differences between PCPs and oncologists regarding delivery of cancer care including screening for recurrent cancer (p < .01) and new cancers (p < .01), evaluation for cancer recurrence (p < .01) and long-term effects (p < .01), and evaluation for psychological effects of cancer and treatment (p < .01). Most oncologists responded that PCPs were responsible for screening patients for recurrent cancer (77%), evaluating patients for cancer recurrence (80%) and evaluating patients for adverse late or long-term physical effects of cancer and its treatment (77%). Although it is less clear amongst PCPs responsibilities around delivery of cancer care, an overwhelming majority (89%) responded that PCPs should be responsible for screening for other new primary cancers. In regards to evaluating patients for adverse psychological effects of cancer or cancer treatment, only 5% of oncologists responded that they were responsible while half of PCPs responded that psychological evaluation is a shared responsibility.
Table 2. Surveillance care practices.
| PCP n=41 N (%) | Oncologist n=24 N (%) | Fishers exact p-value | |
|---|---|---|---|
|
| |||
| Delivery of care | |||
| Screening for recurrent cancer | <0.001 | ||
| PCP orders this service | 7 (19) | 17 (77) | |
| Oncologist orders this service | 15 (41) | 1 (5) | |
| Oncologists and PCP share responsibility | 13 (35) | 2 (9) | |
| Another specialist orders this service | 0 (0) | 1 (5) | |
| I am not involved in this care | 2 (5) | 1 (5) | |
| Screening for other new primary cancers | <0.001 | ||
| PCP orders this service | 32 (89) | 8 (36) | |
| Oncologist orders this service | 1 (3) | 8 (36) | |
| Oncologists and PCP share responsibility | 3 (8) | 5 (23) | |
| Another specialist orders this service | 0 (0) | 0 (0) | |
| I am not involved in this care | 0 (0) | 1 (5) | |
| Evaluating patients for recurrence of cancer | <0.001 | ||
| PCP orders this service | 6 (17) | 16 (80) | |
| Oncologist orders this service | 16 (44) | 0 (0) | |
| Oncologists and PCP share responsibility | 13 (36) | 3 (15) | |
| Another specialist orders this service | 0 (0) | 1 (5) | |
| I am not involved in this care | 1 (3) | 0 (0) | |
| Evaluating patients for adverse late or long-term physical effects of cancer or its treatment | <0.001 | ||
| PCP orders this service | 3 (9) | 17 (77) | |
| Oncologist orders this service | 14 (44) | 0 (0) | |
| Oncologists and PCP share responsibility | 14 (44) | 5 (23) | |
| Another specialist orders this service | 1 (3) | 0 (0) | |
| I am not involved in this care | 0 (0) | 0 (0) | |
| Evaluating patients for adverse psychological effects of cancer or its treatment | <0.001 | ||
| PCP orders this service | 10 (28) | 11 (50) | |
| Oncologist orders this service | 8 (22) | 1 (5) | |
| Oncologists and PCP share responsibility | 16 (50) | 10 (45) | |
| Another specialist orders this service | 0 (0) | 0 (0) | |
| I am not involved in this care | 2(6) | 0 (0) | |
| Delivery of behavioral health care | |||
| Counseling on diet and physical activity | 0.495 | ||
| PCP orders this service | 21 (57) | 8 (36) | |
| Oncologist orders this service | 2 (5) | 2 (9) | |
| Oncologists and PCP share responsibility | 14 (38) | 12 (55) | |
| Another specialist orders this service | 0 (0) | 0 (0) | |
| I am not involved in this care | 0 (0) | 0 (0) | |
| Counseling on smoking cessation | 0.505 | ||
| PCP orders this service | 21 (58) | 8 (36) | |
| Oncologist orders this service | 1 (3) | 1 (5) | |
| Oncologists and PCP share responsibility | 14 (39) | 12 (55) | |
| Another specialist orders this service | 0 (0) | 1 (5) | |
| I am not involved in this care | 0 (0) | 0 (0) | |
| Delivery of quality of life care | |||
| Treating pain related to cancer treatment | 0.01 | ||
| PCP orders this service | 6 (17) | 12(55) | |
| Oncologist orders this service | 12 (34) | 1 (5) | |
| Oncologists and PCP share responsibility | 13 (37) | 9 (41) | |
| Another specialist orders this service | 1 (3) | 0 (0) | |
| I am not involved in this care | 3 (9) | 0 (0) | |
| Treating depression and/or anxiety | 0.02 | ||
| PCP orders this service | 23 (62) | 6 (27) | |
| Oncologist orders this service | 0 (0) | 5 (19) | |
| Oncologists and PCP share responsibility | 13 (35) | 11 (50) | |
| Another specialist orders this service | 1 (3) | 0 (0) | |
| I am not involved in this care | 0 (0) | 0 (0) | |
| Treating fatigue | 0.99 | ||
| PCP orders this service | 16 (43) | 9 (41) | |
| Oncologist orders this service | 4 (11) | 3 (14) | |
| Oncologists and PCP share responsibility | 17 (46) | 10 (45) | |
| Another specialist orders this service | 0 (0) | 0 (0) | |
| I am not involved in this care | 0 (0) | 0 (0) | |
| Treating sexual dysfunction | 0.02 | ||
| PCP orders this service | 18 (50) | 4 (18) | |
| Oncologist orders this service | 3 (8) | 8 (36) | |
| Oncologists and PCP share responsibility | 12 (33) | 7 (32) | |
| Another specialist orders this service | 1 (3) | 0 (0) | |
| I am not involved in this care | 2 (6) | 3 (14) | |
| Managing adverse late or long-term outcomes of cancer treatment | 0.004 | ||
| PCP orders this service | 7 (19) | 15 (68) | |
| Oncologist orders this service | 8 (22) | 0 (0) | |
| Oncologists and PCP share responsibility | 20 (56) | 7 (32) | |
| Another specialist orders this service | 0 (0) | 0 (0) | |
| I am not involved in this care | 1 (3) | 0 (0) | |
PCP=Primary care physician
Results did not show a statistically significant difference in delivery of behavioral health care including counseling for diet, exercise, and smoking cessation. However, the majority of oncologists indicated that these were shared responsibilities between providers while the majority of PCPs responded that the responsibility was on the PCPs to deliver these services.
Results also show a statistically significant difference between PCPs and oncologists regarding the delivery of quality of life care, specifically treating pain related to cancer treatment (p = .01), depression and/or anxiety (p = .02), sexual dysfunction (p = .02), and managing adverse long-term outcomes of cancer treatment (p < .01). The majority of oncologists indicated that either the PCP is responsible or shares responsibility with the oncologist to treat pain related to cancer treatment (95%) and managed adverse late or long-term outcomes of cancer treatment (86%). Most PCPs, on the other hand, responded that these services were either ordered by oncologists or shared between PCPs and oncologists. Additionally, oncologists and PCPs both indicated that either managing depression/anxiety, fatigue, and sexual dysfunction was ordered by PCPs or that it was a shared responsibility.
Oncologist-Patient and Oncologist-Physician Communication
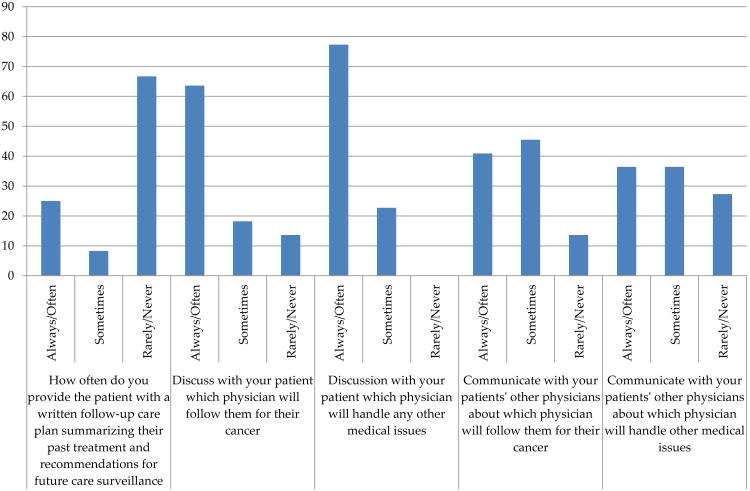
The oncologists' responses to communication practices with PCPs and CRC survivors about cancer surveillance are shown in Figure 1. The majority of oncologists (67%) indicated they rarely or never provide a care plan summarizing cancer treatments and surveillance recommendations to CRC survivors. Yet, most oncologists responded that they notify their patients that a particular physician will continue to provide follow up care for medical issues (78%) and long-term effects of cancer (62%). However, over half of the oncologists indicated that they do not discuss with the patients physicians (59%) about who will follow patients for their cancer and handle other medical issues.
Figure 1. Oncologist-patient and oncologist-physician communications.
Perceived skills and knowledge of PCPs
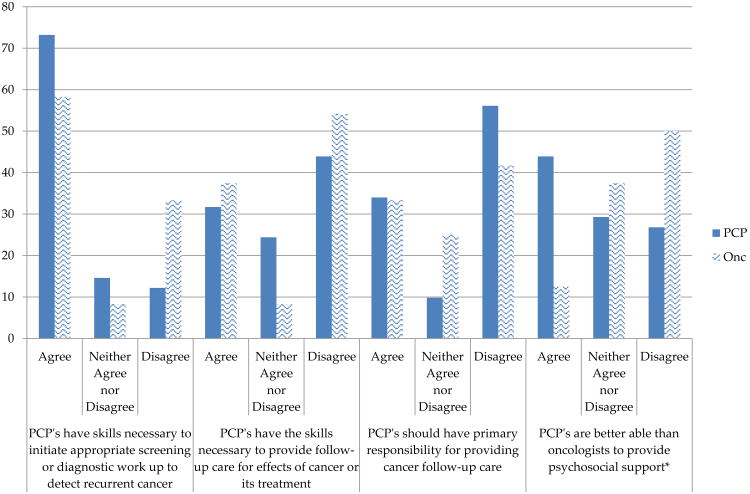
PCP and oncologist's knowledge and skills regarding follow-up care for CRC survivors is illustrated in Figure 2. Although the majority of PCPs and oncologists indicated that PCPS have the skills necessary to initiate appropriate screening or work up to detect recurrent cancers, a lower percentage of PCPs (56%) and oncologists (42%) responded that PCPs should have primary responsibility for providing cancer follow-up care. Furthermore, 44% of PCPs and 54% of oncologists felt that PCPs did not have the skills to provide follow-up care for effects of cancer or its treatment. There was also statistically significant difference between PCPs and oncologists regarding the ability to provide psychosocial support with 44% of PCPs responding that they are better able to provide psychosocial support as compared to only 13% of oncologists (p<0.05).
Figure 2. PCP and oncologists' perceptions about PCPs skills and knowledge.
*p-value<0.05
PCP=primary care physician; Onc= oncologist
Discussion
Our study based at an integrated health system serving socio-economically disadvantaged CRC patients shows that only 38% of CRC survivors received guideline-concordant surveillance. Further, CRC survivors diagnosed with early stage cancer (Stage I and II vs. Stage III) were less likely to receive guideline-concordant surveillance. These results are similar to a cluster analysis of CRC survivors in France describing surveillance patterns across three groups ranging from 29-47% with tumor stage as a predictor of surveillance patterns.16 On surveying PCPs and oncologists using the validated SPARCCS survey instrument, we found statistically significant differences between PCPs and oncologists on whether PCPs should have primary responsibility for providing cancer follow-up care. More notably, there were significant differences between PCPs and oncologists on who was responsible for ordering surveillance care services. Most oncologists reported saying that PCPs screened and evaluated patients for cancer recurrence, while PCPs reported that either oncologists ordered these services or that it was a shared responsibility.
The rate of CRC surveillance observed in our sample of safety-net patients (38%) is consistent with what has been observed in studies utilizing SEER-Medicare data (40-43%) 17, suggesting the problem of suboptimal CRC surveillance after treatment is not limited to vulnerable or safety-net populations. Notably, survivors diagnosed with early stage cancer were less likely to receive recommended surveillance than those diagnosed with Stage III cancer. This relationship remained significant after controlling for a number of covariates, including comorbidities. If preventing cancer recurrence is the objective, then ensuring the highest rates of surveillance among the early stage cancers provides the highest probability of success. In our study, we were unable to ascertain reasons for the lower surveillance among early stage cancers but other studies have identified patient, provider, and system barriers, which may play a role in our sample as well. 18, 19 Thus, it is critically important to implement patient education and quality improvement strategies to increase rates of surveillance in Stage I and II CRC survivors.
Suboptimal CRC surveillance rates may be due to a lack of consensus in the medical care community about who is responsible for surveillance for cancer survivors. While past studies have shown a lack of consensus among physicians within non-integrated systems 10 and across international settings 20, results from this study demonstrate that PCPs and oncologists within an integrated system also have a lack of consensus around the responsible for cancer surveillance care. Specifically, in contrast to national findings, oncologists believed that PCPs were responsible for cancer surveillances while primary care physicians preferred a shared care model. Our study also revealed limited communication and transfer of information between PCPs and oncologists about who will follow patients for their cancer and other medical issues, which further compromises the likelihood of delivering high quality surveillance care. These findings support studies conducted in not only in the US but also from Canada 21, United Kingdom 22, and the Netherlands 23 around poor communication between PCPs and cancer specialists.
Our findings should be considered in light of limitations. The sample size of patients from the medical record audit was small and therefore, we may have decreased power and limited ability to study determinants. Additionally, generalizability to current care is unclear; as indicated, in recent years, Parkland actively undertook systematic initiatives in communication and care coordination across their primary care clinics. We also administered the survey among oncologists from multiple subspecialties and were unable to differentiate oncologists providing care for CRC patients from others to correlate precisely these responses to CRC surveillance results. The response rate was also relatively low and we did not have data to distinguish characteristics between respondents and non-respondents. Finally, we did not collect data from patients about their perspectives and experiences around CRC surveillance. Future studies should include patient perspectives of care coordination.
Notwithstanding these limitations, our study is one of the first to study patterns of CRC surveillance care and physician attitudes, practices, and communication in an integrated safety-net system. This context is significant because many health systems in the US are increasing integration through accountable care organizations emphasizing on value-based care and cost savings. 24 The patient population we studied is also significant as racial/ethnic and socioeconomic disparities in cancer care are increasing and it is not clear which strategies to increase patient adherence to surveillance are best. More research and evidence of gaps in care is needed from minority and under- and uninsured groups of patients.
In conclusion, we observed suboptimal CRC surveillance among socio-economically vulnerable patients and lack of consensus among physicians in an integrated system about who was responsible for follow-up of CRC survivors. The results of our study show that even within an integrated health system there is significant room for improvement. Well-coordinated, patient-centered care calls for a care model reflecting a multi-team system, specifying PCP/oncology paths, creating a shared mental model, engaging physician champions and leadership, and creating closed-loop communication. 25 As care models continue to evolve, future research should focus on creating and evaluating such approaches across diverse health system settings to optimize survival for cancer patients.
Supplementary Material
Acknowledgments
We appreciate clinicians and staff throughout Parkland's Community-oriented Primary Care clinics and oncology clinics who made data collection possible. We also want to thank Joshua Yudkin for his work in preparing and editing the manuscript.
Funding: Support for this study provided by ACS-IRG 02-196 (Balasubramanian) and R01CA203856 (Lee, Balasubramanian), and NIH/NIDDK K23 DK104065 (Bowen). Additional support through the NCI Cancer Center Support Grant (5P30CA142543) and from the UT Southwestern Center for Patient-Centered Outcomes Research (R24 HS022418) for Dr. Lee.
Footnotes
Conflict of interest: All authors declare that they have no conflict of interest
Compliance with Ethical Standards: Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.American Cancer Society. Cancer treatment & survivorship: Facts & Figures 2016-2017. Atlanta, GA: 2016. [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2015;136:E359–E86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Salz T, Weinberger M, Ayanian JZ, et al. Variation in use of surveillance colonoscopy among colorectal cancer survivors in the United States. BMC Health Serv Res. 2010;10:256. doi: 10.1186/1472-6963-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faul LA, Shibata D, Townsend I, Jacobsen PB. Improving survivorship care for patients with colorectal cancer. Cancer control : journal of the Moffitt Cancer Center. 2010;17:35–43. doi: 10.1177/107327481001700105. [DOI] [PubMed] [Google Scholar]
- 5.Ball JK, Elixhauser A. Treatment differences between blacks and whites with colorectal cancer. Medical care. 1996;34:970–84. doi: 10.1097/00005650-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Hawley ST, Janz NK, Lillie SE, et al. Perceptions of care coordination in a population-based sample of diverse breast cancer patients. Patient education and counseling. 2010;81S1:S34–S40. doi: 10.1016/j.pec.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoen C, Osborn R, How SK, Doty MM, Peugh J. In chronic condition: experiences of patients with complex health care needs, in eight countries, 2008. Health affairs (Project Hope) 2009;28:w1–16. doi: 10.1377/hlthaff.28.1.w1. [DOI] [PubMed] [Google Scholar]
- 8.Virgo KS, Lerro CC, Klabunde CN, Earle C, Ganz PA. Barriers to Breast and Colorectal Cancer Survivorship Care: Perceptions of Primary Care Physicians and Medical Oncologists in the United States. Journal of Clinical Oncology. 2013;31:2322–36. doi: 10.1200/JCO.2012.45.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz EM, Pineda N, Lonhart J, Davies SM, McDonald KM. A systematic review of the care coordination measurement landscape. BMC Health Services Research. 2013;13:119. doi: 10.1186/1472-6963-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potosky AL, Han PK, Rowland J, et al. Differences between primary care physicians' and oncologists' knowledge, attitudes and practices regarding the care of cancer survivors. Journal of general internal medicine. 2011;26:1403–10. doi: 10.1007/s11606-011-1808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpentier MY, Vernon SW, Bartholomew LK, Murphy CC, Bluethmann SM. Receipt of Recommended Surveillance among Colorectal Cancer Survivors: A Systematic Review. Journal of cancer survivorship : research and practice. 2013;7:464–83. doi: 10.1007/s11764-013-0290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Cancer Institute. Survey of Physician Attitudes Regarding the Care of Cancer Survivors (SPARCCS) Washington, DC: National Cancer Institute, Division of Cancer Control and Population Sciences; 2015. [Google Scholar]
- 13.Engstrom PF, Arnoletti JP, Benson AB, et al. Colon Cancer: Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network. 2009;7:778–831. doi: 10.6004/jnccn.2009.0056. [DOI] [PubMed] [Google Scholar]
- 14.Giudice MED, Grunfeld E, Harvey BJ, Piliotis E, Verma S. Primary Care Physicians' Views of Routine Follow-Up Care of Cancer Survivors. Journal of Clinical Oncology. 2009;27:3338–45. doi: 10.1200/JCO.2008.20.4883. [DOI] [PubMed] [Google Scholar]
- 15.Ayanian JZ, Zaslavsky AM, Arora NK, et al. Patients' Experiences With Care for Lung Cancer and Colorectal Cancer: Findings From the Cancer Care Outcomes Research and Surveillance Consortium. Journal of Clinical Oncology. 2010;28:4154–61. doi: 10.1200/JCO.2009.27.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boulin M, Lejeune C, Le Teuff G, et al. Patterns of surveillance practices after curative surgery for colorectal cancer in a French population. Diseases of the colon and rectum. 2005;48:1890–9. doi: 10.1007/s10350-005-0096-7. [DOI] [PubMed] [Google Scholar]
- 17.Brawarsky P, Neville BA, Fitzmaurice GM, Earle C, Haas JS. Surveillance after resection for colorectal cancer. Cancer. 2013;119:1235–42. doi: 10.1002/cncr.27852. [DOI] [PubMed] [Google Scholar]
- 18.Underwood JM, Townsend JS, Stewart SL, et al. Surveillance of demographic characteristics and health behaviors among adult cancer survivors: Behavioral Risk Factor Surveillance System, 2009. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002) 2012;61:1–23. [PubMed] [Google Scholar]
- 19.Salz T, Weinberger M, Ayanian JZ, et al. Variation in use of surveillance colonoscopy among colorectal cancer survivors in the United States. BMC Health Serv Res. 2010;10 doi: 10.1186/1472-6963-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunfeld E, Moineddin R, Gunraj N, et al. Cancer screening practices of cancer survivors: Population-based, longitudinal study. Canadian Family Physician. 2012;58:980–6. [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien MA, Grunfeld E, Sussman J, Porter G, Mobilio MH. Views of family physicians about survivorship care plans to provide breast cancer follow-up care: exploration of results from a randomized controlled trial. Current oncology (Toronto, Ont) 2015;22:252–9. doi: 10.3747/co.22.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson EK, Sugden EM, Rose PW. Views of primary care physicians and oncologists on cancer follow-up initiatives in primary care: an online survey. J Cancer Surviv. 2010;4:159–66. doi: 10.1007/s11764-010-0117-y. [DOI] [PubMed] [Google Scholar]
- 23.Roorda C, Berendsen AJ, Haverkamp M, van der Meer K, de Bock GH. Discharge of breast cancer patients to primary care at the end of hospital follow-up: a cross-sectional survey. European journal of cancer (Oxford, England : 1990) 2013;49:1836–44. doi: 10.1016/j.ejca.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Zapka J, Sterba KR, LaPelle N, Armeson K, Burshell DR, Ford ME. Physician perspectives on colorectal cancer surveillance care in a changing environment. Qualitative health research. 2015;25:831–44. doi: 10.1177/1049732315580557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craddock Lee SJ, Clark MA, Cox JV, Needles BM, Seigel C, Balasubramanian BA. Achieving Coordinated Care for Patients With Complex Cases of Cancer: A Multiteam System Approach. Journal of Oncology Practice. 2016;12:1029–38. doi: 10.1200/JOP.2016.013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.