Abstract
Objective
Intramyocellular lipid (IMCL) storage negatively associates with insulin resistance, albeit not in endurance-trained athletes. We investigated the putative contribution of lipid droplet (LD) morphology and subcellular localization to the so-called athlete's paradox.
Methods
We performed quantitative immunofluorescent confocal imaging of muscle biopsy sections from endurance Trained, Lean sedentary, Obese, and Type 2 diabetes (T2DM) participants (n = 8/group). T2DM patients and Trained individuals were matched for IMCL content. Furthermore we performed this analysis in biopsies of T2DM patients before and after a 12-week exercise program (n = 8).
Results
We found marked differences in lipid storage morphology between trained subjects and T2DM: the latter group mainly store lipid in larger LDs in the subsarcolemmal (SS) region of type II fibers, whereas Trained store lipid in a higher number of LDs in the intramyofibrillar (IMF) region of type I fibers. In addition, a twelve-week combined endurance and strength exercise program resulted in a LD phenotype shift in T2DM patients partly towards an ‘athlete-like’ phenotype, accompanied by improved insulin sensitivity. Proteins involved in LD turnover were also more abundant in Trained than in T2DM and partly changed in an ‘athlete-like’ fashion in T2DM patients upon exercise training.
Conclusions
Our findings provide a physiological explanation for the athlete's paradox and reveal LD morphology and distribution as a major determinant of skeletal muscle insulin sensitivity.
Keywords: Insulin sensitivity, Athlete's paradox, Intramyocellular lipid, Lipid droplets
Highlights
-
•
Distinct lipid droplet morphology and distribution underlies the athletes' paradox.
-
•
Muscle lipid storage occurs in a fiber type specific manner.
-
•
Exercise training shifts LD morphology towards an athletes' phenotype.
1. Introduction
Compromised insulin stimulated glucose uptake in skeletal muscle is the culprit in type 2 diabetes and correlates negatively with excessive storage of intramyocellular lipid (IMCL) [1], [2], [3]. Paradoxically, IMCL levels are also elevated in endurance trained athletes, while being very insulin sensitive [4]. This phenomenon is known as the athlete's paradox and indicates that factors other than total IMCL storage per se must be responsible for compromised insulin sensitivity in the diabetic state.
Total IMCL storage is defined as the product of the number and size of lipids dispersed in lipid droplets (LDs) containing predominantly neutral lipids. LDs are covered by a phospholipid monolayer coated with proteins involved in lipid droplet (LD) synthesis and degradation, rendering LDs into dynamic organelles that can range substantially in size and number depending on tissue distribution [5], subcellular location, and metabolic state [6].
Upon a combined dietary-exercise intervention in obese individuals without diabetes, LD size decreased, and the change in LD size correlated with the change in insulin sensitivity [7]. Muscle fiber types have been shown to differ in LD morphology and localization [8] as well as insulin sensitivity [9]. Importantly, muscle fiber typology is affected by exercise training as well as by aging and inactivity (associated with a higher fraction type II fibers). At the muscle fiber level, storage of lipid in either the intramyofibrillar (IMF) space, where LDs are closely linked to mitochondria [8], [10], as opposed to the subsarcolemmal (SS) region, may influence lipotoxic effects.
Thus, despite similar levels of IMCL in trained individuals and patients with type 2 diabetes, one can hypothesize that differences in LD size, number, and subcellular distribution, along with fiber type specific differences, may contribute to the negative association between IMCL content and insulin sensitivity in insulin resistant subjects and the seemingly paradoxical absence of such an association in trained athletes.
The dynamic nature of LDs involves interplay of a variety of LD coat proteins like PLIN2, PLIN3, and PLIN5 supposedly involved in regulating LD synthesis and degradation [11], [12], [13] and the main TAG lipase ATGL [14] and its coactivator CGI-58 [15]. Compromised dynamic behavior often is paralleled by hampered cell function [5]. Given the putative role of these proteins in LD dynamics and their responsiveness to exercise training [16], [17], [18], levels of LD coat proteins and lipolytic proteins may contribute to the athletes’ paradox.
LD number, size, subcellular distribution, and muscle fiber type dependency have been studied using different approaches in various (patho)physiological states. A comprehensive and systematic analysis of all these LD characteristics against the background of similar IMCL content, however, has never been performed. Thus, we aimed to examine the athlete's paradox in individuals with similar levels of IMCL but over a wide range of insulin sensitivity. We therefore cross-sectionally studied LD characteristics and marker proteins of LD dynamics in highly insulin sensitive, lean endurance-trained individuals (Trained), and insulin resistant obese patients with type 2 diabetes (T2DM) matched to IMCL content, along with normoglycemic lean sedentary individuals (Lean) and normoglycemic obese sedentary individuals (Obese) characterized by intermediate levels of insulin sensitivity. We hypothesized LD characteristics and content of marker proteins of LD dynamics to be different between Trained and T2DM. In addition, we examined in T2DM patients if a training intervention induced a shift of the LD phenotype, resembling the LD phenotype observed in Trained.
2. Research design and methods
2.1. Participant selection
From our database of endurance-trained athletes (Trained; VO2max > 55 ml/kg/min), lean sedentary (Lean; VO2max < 45 ml/kg/min), obese sedentary (Obese), and T2DM participants (from Vosselman et al. [19] and Brouwers et al. [20]), eight participants per group were semi-randomly selected for inclusion (Supplemental Figure 1), targeting at similar IMCL levels for Trained and T2DM, as measured with widefield microscopy via staining with Bodipy 493/503 (D3922, Molecular Probes, Leiden, The Netherlands) and calculating the area fraction covered by LDs (Table 1). Following this, Obese were matched to T2DM for age, BMI and VO2max and lean sedentary to Trained for age and BMI. For studying the effects of a 12-week training program, eight T2DM patients who increased whole body insulin sensitivity (GIR) upon the training program were randomly selected (from the studies of Meex et al. [21] and Brouwers et al. [20]) (Supplemental Figure 1). For both the cross-sectional and the training study, all participants gave written informed consent before participating in the original study. Furthermore, these studies were approved by the Medical Ethical Committee of Maastricht University and performed in agreement with the Declaration of Helsinki.
Table 1.
Subject characteristics cross-sectional study.
| Parameter | Trained | Lean | Obese | T2DM |
|---|---|---|---|---|
| Age (years) | 26.0 ± 1.8 | 23.5 ± 1.2 | 54.1 ± 3.1a,b | 60.6 ± 2.0a,b |
| Body weight (kg) | 72.4 ± 2.6 | 73.1 ± 2.3 | 96.1 ± 3.3a,b | 95.9 ± 2.8a,b |
| % Fat mass | 13.4 ± 0.5 | 18.4 ± 1.5a | 28.9 ± 0.9a,b | 28.3 ± 0.9a,b |
| BMI (kg/m2) | 21.0 ± 0.6 | 22.2 ± 0.6 | 29.4 ± 0.4a,b | 29.6 ± 0.8a,b |
| Fasting glucose (mmol/L) | 5.1 ± 0.1 | 5.2 ± 0.1 | 4.9 ± 0.3 | 7.4 ± 0.5a,b,c |
| Glucose infusion rate (GIR) (μmol/kg LBM/min) | 93.8 ± 6.6 | 70.4 ± 5.7a | 38.0 ± 2.9a,b | 25.7 ± 5.3a,b |
| VO2max (ml O2/kg LBM/min) | 71.0 ± 1.6 | 51.8 ± 1.7a | 39.8 ± 1.3a,b | 36.8 ± 1.5a,b |
| IMCL (%) (widefield) | 3.49 ± 0.69 | 1.31 ± 0.43 | 2.77 ± 0.82 | 2.48 ± 0.29 |
| Fasting insulin (mU/L) | – | – | – | 15.5 ± 3.7 |
| HOMA-IR (%) | – | – | – | 5.5 ± 1.0 |
| Glucose-lowering medication (number of patients) | Metformin (n = 7) | |||
| DPP-4 inhibitor (n = 2) | ||||
| Sulfonylureas (n = 2) |
Mean ± SEM. Statistical significance based on one-way ANOVA if p ≤ 0.05.
Versus Trained.
Versus Lean.
Versus Obese.
2.2. Muscle biopsy and metabolic measurements
After an overnight fast a muscle biopsy was taken from the m. vastus lateralis, directly frozen in melting isopentane and stored at −80 °C until histochemical analyses. Directly after the muscle biopsy, glucose infusion rate (GIR) was determined during a hyperinsulinemic-euglycemic clamp at 40 mU/m2/min of insulin as a measure for whole body insulin sensitivity. Detailed methods can be found in Meex et al. [21] and Brouwers et al. [20]. Body composition was measured with a DXA scan and plasma glucose was determined. In addition, maximal oxidative capacity (VO2max) was determined by a graded maximal cycling test until exhaustion via indirect calorimetry (Omnical, Maastricht, The Netherlands). To account for differences in fat mass, both GIR and VO2max are expressed per kg lean body mass (LBM), as opposed to per kg body weight which was used for inclusion. For the training study, the same measurements were performed again after the 12 weeks of training. Muscle biopsies were obtained 48–72 h after the last exercise session.
2.3. Training program
VO2max and maximal power output (Wmax) were defined during a graded maximal cycling test until exhaustion before and after the training. One-repetition maximum (1RM) was measured to determine maximal strength. The 12-week exercise training protocol was supervised, progressive and combined aerobic and resistance exercise. Details of the training program can be found in Meex et al. [21] and Brouwers et al. [20]. In short, aerobic exercise was performed twice a week for 30 min on a cycling ergometer and resistance exercise once a week, focusing on the large muscle groups.
2.4. Histochemical analyses
To determine IMCL content, LD size and number, 7 μm thick sections were cut and mounted on glass slides. To minimize staining intensity variability between groups or time points, on each slide a section from each group or pre- and post-training from the same participant were mounted on the same glass slide. Cryosections were fixed with 3.7% formaldehyde for 30 min. After blocking with blocking buffer (150 mM NaCl, 20 mM Tris pH 6.8 and 2% BSA) and permeabilization for 5 min with 0.25% TX-100 (648466, Merck, Darmstadt, Germany), sections were incubated with primary antibodies against Laminin (L9393; Sigma) and myosin heavy chain type I (A4.840; Developmental Studies Hybridoma Bank, Iowa City, Iowa, USA) for 60 min. Subsequently, sections were incubated for 1.5 h with the appropriate secondary antibodies conjugated with AlexaFluor 405 and AlexaFluor 555 and Bodipy 493/503 (D3922, Invitrogen-ThermoFisher, The Netherlands) 1:100 at 37 °C. Sections were mounted with Mowiol, covered with #1 coverslips and stored in the dark until imaging.
2.5. Image acquisition and analysis
For the analysis of IMCL content, LD size and number cross-sectional images were taken on Leica TCS SPE and SP8 confocal microscopes using a 63 × 1.3 N.A. oil immersion objective and 1.1 optical zoom using 2048 by 2048 pixels, resulting in a pixel size of 81.95 nm by 81.95 nm. Laminin, myosin heavy chain type I and Bodipy 493/503 were imaged using the 405 nm, 532 nm, and 488 nm laser lines, respectively. Type I fibers were identified based on positive myosin heavy chain type I staining, all other fibers were considered to be type II fibers. The fiber type ratio was determined using a Nikon E800 fluorescence microscope prior to confocal imaging to account for bias by differences in fiber type ratio. For each participant, cross-sections of 20 fibers were imaged with the confocal microscope with the previously determined type I to type II ratio. After image acquisition images were deconvolved using Huygens Essential software (Scientific Volume Imaging B.V., Hilversum, The Netherlands). For each fiber type, lipid area fraction, LD size, and number were analyzed by using ImageJ [22]. LD size distribution and LD-membrane distances were calculated with Matlab R2012a (The Mathworks, Inc., Natick, Massachusetts, USA). Based on these LD-membrane distances, LDs were separated in LDs located in near vicinity of the cell membrane (SS region) or LDs located more towards the core of the myofiber (IMF region). Starting at the cell membrane, the SS region was set to be within 5% of the maximal distance from the cell center to the cell membrane, resulting in an SS surface area of around 8% for all cells. This approach was based upon combined examination of available transmission electron microscopy images and the published conversion of this for application in widefield microscopy that used a fixed distance of 2 μm from the sarcolemma to the core of the fiber [23]. As the SS region contains SS mitochondria (which are more abundant in type I fibers than in type II fibers) and muscle fiber cross sectional area and mitochondrial density is different between trained individuals and T2DM patients, we converted the fixed distance of 2 μm into a value that was relative to cell size, resulting in a value of 5%. This converts into an SS area of ∼8% of total cell area, which is somewhat higher than the ∼4% reported in literature for obese sedentary middle-aged males [24]; this can be explained by the inclusion of lean trained and untrained individuals in the present study.
2.6. Western blots
Equal amounts of protein were loaded onto the gels for western blots. Western blots were incubated with primary antibodies against PLIN2 (GP40, Progen Biotechnik, Heidelberg, Germany), PLIN3 (M6PRBP1, Acris, Herford, Germany), PLIN5 (GP31, Progen Biotechnik, Heidelberg, Germany), ATGL (2138, Cell Signaling Technology, Bioké, Leiden, The Netherlands) and CGI-58 (NB110-41576, Novus Biologicals, Littleton, Colorado, USA). IRDye700-or IRDye800-conjugated secondary antibodies were used for visualization of PLIN2, PLIN3, PLIN5, and ATGL by an Odyssey infrared scanner (LI-COR Biosciences, Westburg, Leusden, The Netherlands). CGI-58 was detected via enhanced chemiluminescence with the use of an HRP-conjugated secondary antibody.
2.7. Statistics
Results are presented as mean ± SEM. Statistical analyses were performed using SPSS version 21.0 (SPSS, Chicago, IL, USA). For the cross-sectional study, statistical differences between groups and fiber types were tested with mixed model ANOVA with fiber type as within subject factor for LD morphology. If the interaction effect for fiber type was significant One-way ANOVA was used to test for statistical differences between groups for each fiber type. A Bonferroni post-hoc test was performed to specify which groups statistically differed. A paired sample t-test was used to test for each group significant differences between fiber types. For examining statistical differences between subcellular locations (SS and IMF), mixed model ANOVA with location as within subject factor was performed for each fiber type. In case of a significant interaction effect, subsequent statistical testing was done as described above. To test for training effects and fiber type differences in LD morphology, two-way repeated measures ANOVA was performed with fiber type and time point (pre- and post-training) as within subject factors. In case of a significant interaction effect for fiber type and/or time point, a paired sample t-test was used to test for significant differences between fiber types at each time point or between time points for each fiber type, respectively. Paired sample t-test were used to test for differences in LD morphology in all fibers. Pearson's correlation coefficients were used to test for significant linear association between variables. P < 0.05 was considered to be statistically significant.
3. Results
3.1. Participant characteristics
Participant characteristics of the cross-sectional study are shown in Table 1. By design, Obese and T2DM participants were significantly older and had a higher body weight, BMI, and percentage fat mass than Trained and Lean. In addition, Obese and T2DM participants had a lower GIR and oxidative capacity compared to Lean and Trained (p < 0.01). By definition, T2DM patients had higher fasting glucose levels compared to the other groups (p < 0.01). Trained had a higher GIR (p < 0.01) and VO2max (p < 0.01) compared to other groups. Participant characteristics of the training study are shown in Table 2. Upon the exercise training, GIR increased significantly (p < 0.001). In addition, body fat percentage (p < 0.05), fasting glucose (p < 0.05) and VO2max (p < 0.05) improved with exercise training.
Table 2.
Subject characteristics training study.
| Parameter | Pre | Post |
|---|---|---|
| Age (years) | 61.6 ± 1.5 | – |
| Body weight (kg) | 90.7 ± 4.0 | 89.8 ± 4.0 |
| % Fat mass | 31.3 ± 1,8 | 28.5 ± 1,7a |
| BMI (kg/m2) | 28.1 ± 0.7 | 27.9 ± 0.7 |
| Fasting glucose (mmol/L) | 8.8 ± 0.8 | 8.1 ± 0.6a |
| Glucose infusion rate (GIR) (μmol/kg LBM/min) | 22.9 ± 2.7 | 31.4 ± 3.7a |
| VO2max (ml O2/kg LBM/min) | 35.5 ± 2.4 | 39.3 ± 3.2a |
| Fasting insulin (mU/L) | 17.6 ± 3.1 | 13.6 ± 1.6 |
| HOMA-IR | 7.2 ± 1.4 | 5.1 ± 0.8 |
| Glucose-lowering medication (number of patients) | Metformin (n = 6) | – |
| Sulfonylureas (n = 2) |
Mean ± SEM. Statistical significance based on paired sample T-test if p ≤ 0.05.
Versus Pre.
3.2. Differential lipid storage between trained and T2DM patients associates with insulin sensitivity
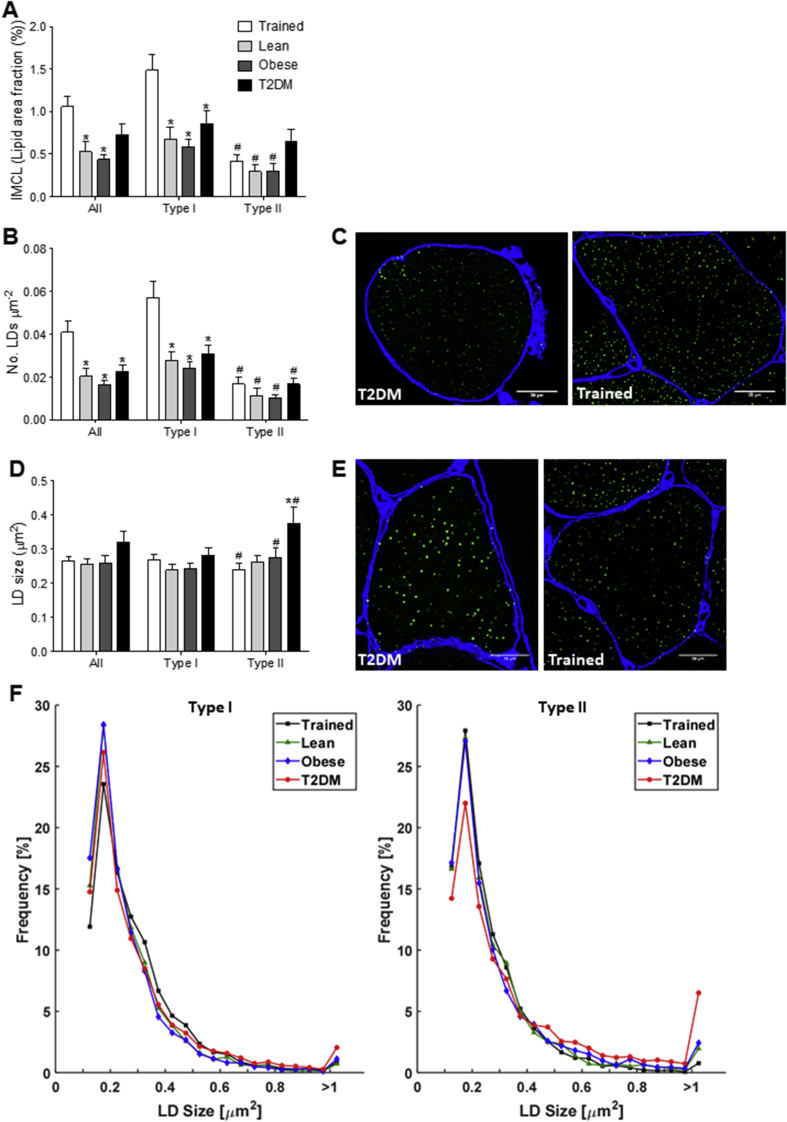
By design, total IMCL content showed no differences between Trained and T2DM patients (p = 0.274, Figure 1A), whereas a significantly lower IMCL content was observed in Lean and Obese compared to Trained (p < 0.05 and p < 0.01 respectively, Figure 1A). In trained, IMCL content in type I fibers was almost 2-fold higher than in T2DM (p < 0.05, Figure 1A). Moreover, in Trained the IMCL content was higher in type I fibers than in type II fibers (p < 0.05), whereas for T2DM patients IMCL content was comparable for both fiber types (p = 0.12, Figure 1A). The higher IMCL content of Trained versus Lean and Obese originates from significantly more LDs (p < 0.01, Figure 1B,C). Conversely, in T2DM, the high IMCL content compared to Obese and Lean was explained by an increase in LD size rather than number, an observation that was accounted for by type II fibers (p < 0.05, Figure 1D,E). Detailed examination of LD size frequency distribution revealed that the higher average LD size in T2DM in type II fibers compared to the other groups originates from a trend towards fewer small LDs (≤0.25 μm2, p = 0.075) and significantly more large LDs compared to other groups (≥1.00 μm2, p < 0.05) (Figure 1F). No such observations were made in type I fibers (Figure 1F). So, in the face of similar IMCL content, Trained predominantly store their lipids in a larger number of small sized LDs in type I fibers, while in T2DM patients the lipid is stored in fewer but larger LDs, predominantly in type II fibers.
Figure 1.
IMCL content and lipid droplet morphology differ between groups of the cross-sectional study. Quantification of IMCL content (lipid area fraction) (A), LD number per fiber area (B), and LD size (D) of all fiber types, type I fibers and type II fibers of Trained (black), Lean (dark grey), Obese (light grey), and T2DM (black) determined with confocal microscopy. Representative confocal images of type I fibers (C) and type II fibers (E) of Trained (right) and T2DM (left) in cross-sectional view with cellular membrane (blue) and LDs (green). Frequency distribution of LD size in type I and type II fibers of Trained (black square), Lean (green triangle), Obese (blue diamond), and T2DM (red circle) (F). *p < 0.05 vs. Trained; #p < 0.05 vs. type I fibers.
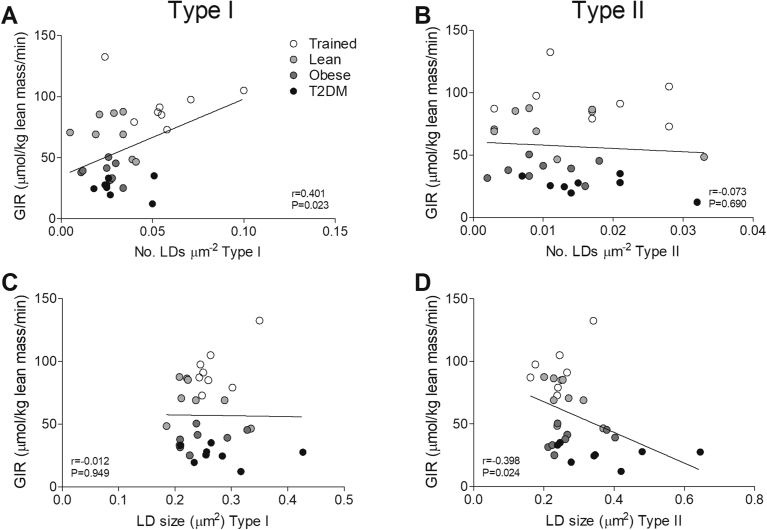
Next, we explored if this differential IMCL storage pattern was associated with the differences in peripheral insulin sensitivity, expressed by GIR. A positive correlation was observed between the number of LDs and insulin sensitivity in type I fibers (r = 0.401, p < 0.05, Figure 2A) but not in type II fibers (r = −0.073, p = 0.690, Figure 2B). Conversely, LD size correlated negatively with insulin sensitivity in type II fibers (r = −0.398, p < 0.05, Figure 2D), but not in type I fibers (r = −0.012, p = 0.949, Figure 2C).
Figure 2.
LD size in type II and LD number in type I fibers correlates with insulin sensitivity. Correlations of GIR with LD number of type I fibers (A) and type II fibers (B) and with LD size of type I fibers (C) and type II fibers (D).
3.3. Subsarcolemmal lipid storage is higher in T2DM patients
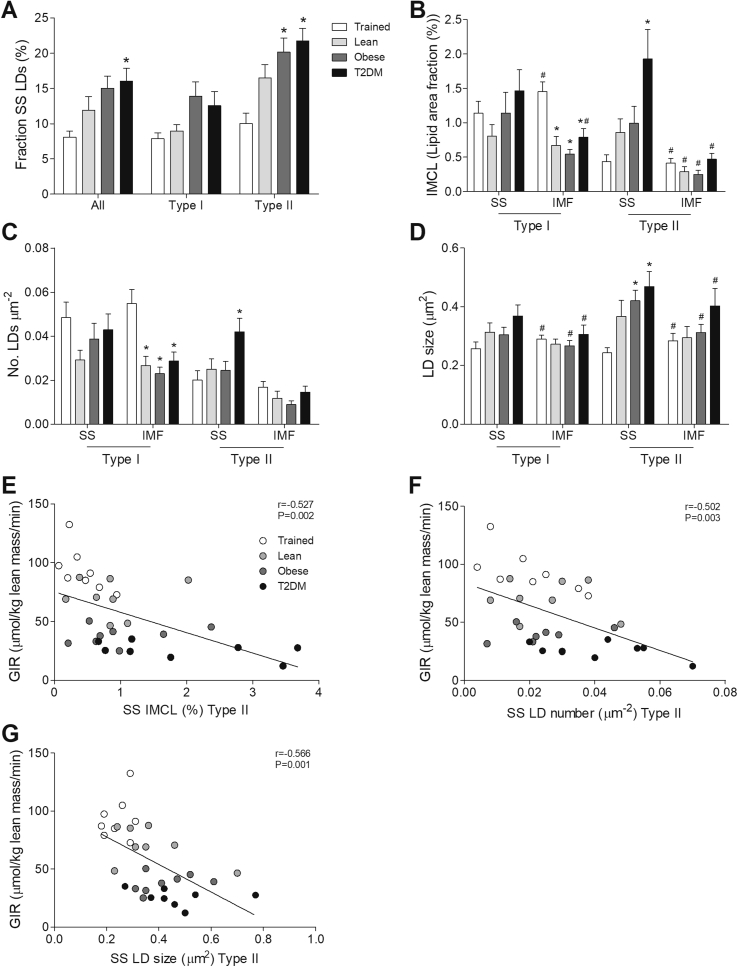
Subsequently, we examined if the differences in LD morphology were specific for the SS or IMF region. Of all LDs measured, Trained only store 8.1% in the SS region, whereas T2DM store 2-fold more LDs (16.1%, p < 0.01, Figure 3A) in the SS region. This difference in the fraction of SS LDs predominantly originates from differences in type II fibers (10.0% vs. 21.8% in Trained and T2DM, respectively; p < 0.001, Figure 3A). Interestingly, also obese normoglycemic individuals store a significantly higher fraction of their LDs in the SS region of type II fibers than Trained (10.0% vs. 20.2% in Trained and Obese, respectively; p < 0.01 Figure 3A). In T2DM, the increased fraction of LDs in type II fibers translated in a strikingly 4.5-fold higher SS IMCL content than in Trained (p < 0.01, Figure 3B). This in contrast to the Obese, in which the fraction of SS LDs in type II fibers was high, whereas IMCL content in the SS region was not elevated (Figure 3B). The high IMCL content in T2DM in the SS region of type II fibers compared to Trained was accounted for by a significantly higher number of LDs (0.042 vs 0.020, p < 0.05, Figure 3C) as well as significantly larger LDs (0.47 vs 0.24, p < 0.01, Figure 3D) The high IMCL content in the IMF region of type I fibers in Trained (Figure 3B) originates from a high number of LDs (Figure 3C) similar in size compared to the other groups (Figure 3D). To summarize, Trained store IMCL predominantly in the IMF region of type I fibers in many normally sized LDs. Conversely, T2DM patients IMCL predominantly in the SS region of type II fibers in more LDs of larger size.
Figure 3.
T2DM patients have increased lipid storage in the SS region. Fraction of LDs located in the SS region of all fiber types, type I fibers and type II fibers of Trained, Lean, Obese, and T2DM (A). Quantification of IMCL content (lipid area fraction) (B), LD number per fiber area (C) and LD size (D) in the SS and IMF region of type I fibers and type II fibers of Trained, Lean, Obese and T2DM. Correlations of GIR with SS IMCL content (lipid area fraction) (E), SS LD number (F) and SS LD size (G) in type II fibers. *p < 0.05 vs. Trained; #p < 0.05 vs. SS.
In addition, we examined if IMCL content, LD number, and size in the SS or IMF region correlated with whole-body insulin sensitivity expressed by GIR. In type II fibers, IMCL content (r = −0.527, p < 0.01, Figure 3E), LD number (r = −0.502, p < 0.01, Figure 3F) and LD size (r = −0.566, p < 0.01, Figure 3G) in the SS region correlated negatively with insulin sensitivity. LD size in the SS region also associated negatively with insulin sensitivity in type I fibers (r = −0.363, p < 0.05, data not shown). Furthermore, in type I fibers in the IMF region a positive correlation with insulin sensitivity was observed for both IMCL content (r = 0.397, p < 0.05, data not shown) and LD number (r = 0.408, p < 0.05, data not shown), but not LD size (r = −0.023, p = 0.091, data not shown).
3.4. Reduced LD size parallels improved insulin sensitivity upon exercise training in T2DM patients
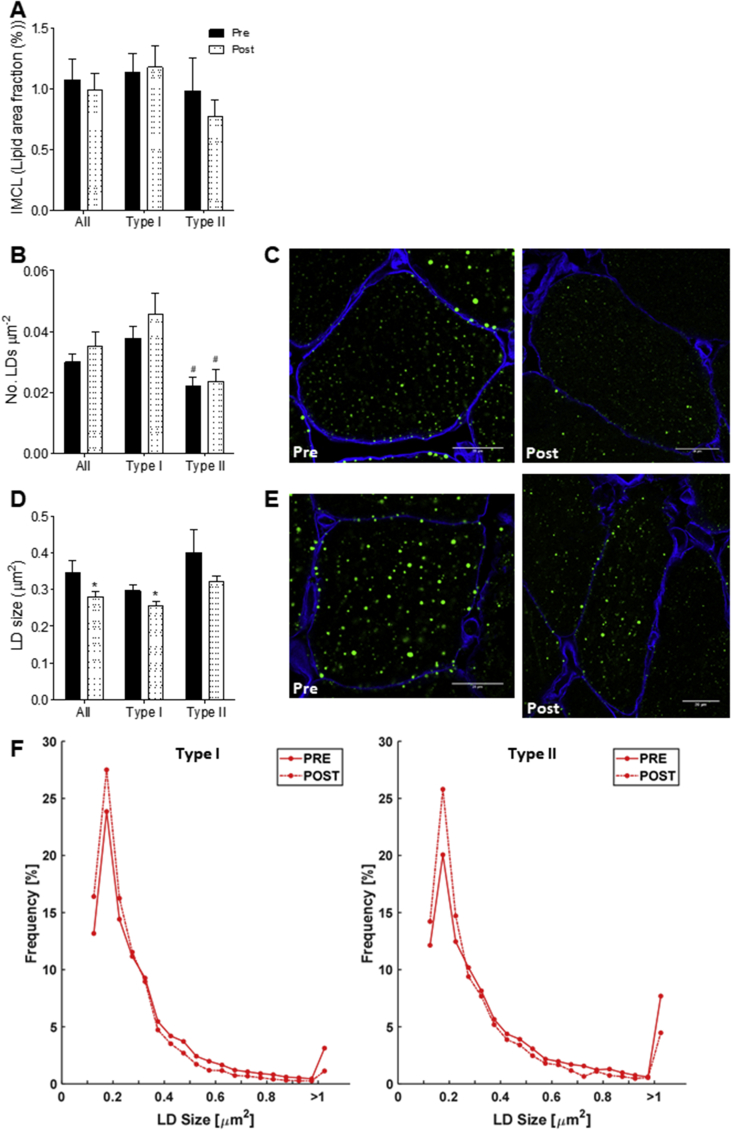
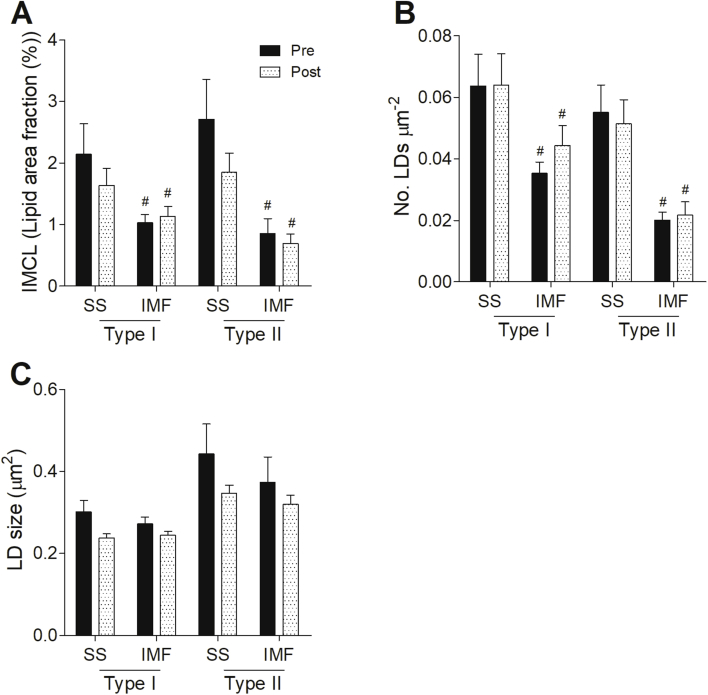
The pronounced differences in the pattern of IMCL storage between Trained and T2DM triggered us to examine if a 12-week exercise training program in T2DM patients would elicit changes in LD morphology and localization resembling the phenotype observed in Trained. Neither total IMCL content nor the number of LDs was significantly affected by 12 weeks of training in T2DM (Figure 4A,B). Upon training, however, LD size was reduced (p < 0.05) in type I and II fibers (Figure 4C,E), a finding that reached statistical significance in type I fibers (p < 0.05, Figure 4D). This change in LD size in type I fibers originates from a training-associated shift towards a higher fraction of smaller LDs (Figure 4F). Although the average size change in type II fibers upon training failed to reach significance (p = 0.146), also in type II fibers, the fraction of small LDs (≤0.25 μm2) increased at the expense of large (≥1.00 μm2) LDs (Figure 4F). The training-associated shift in LD size frequency distribution in both fiber types hence matches our cross-sectional observation of Trained having more LDs of smaller size than T2DM. The 12-week training program did not affect subcellular LD localization; the overall drop in LD size was not specific for either the SS or IMF region (Supplemental Figure 2).
Figure 4.
Training reduced LD size in T2DM patients. Quantification of IMCL content (lipid area fraction) (A), LD number per fiber area (B) and LD size (D) in all fiber types, type I fibers and type II fibers pre (black) and post (white speckled) training. Representative confocal images of type I fibers (C) and type II fibers (E) pre (left) and post (right) training in cross-sectional view with cellular membrane (blue) and LDs (green). Frequency distribution of LD size in type I and type II fibers pre (solid line) and post (dashed line) training of T2DM patients (F). *P < 0.05 vs. pre training, #p < 0.05 vs. type I fibers.
3.5. Differential protein levels of LD coat proteins
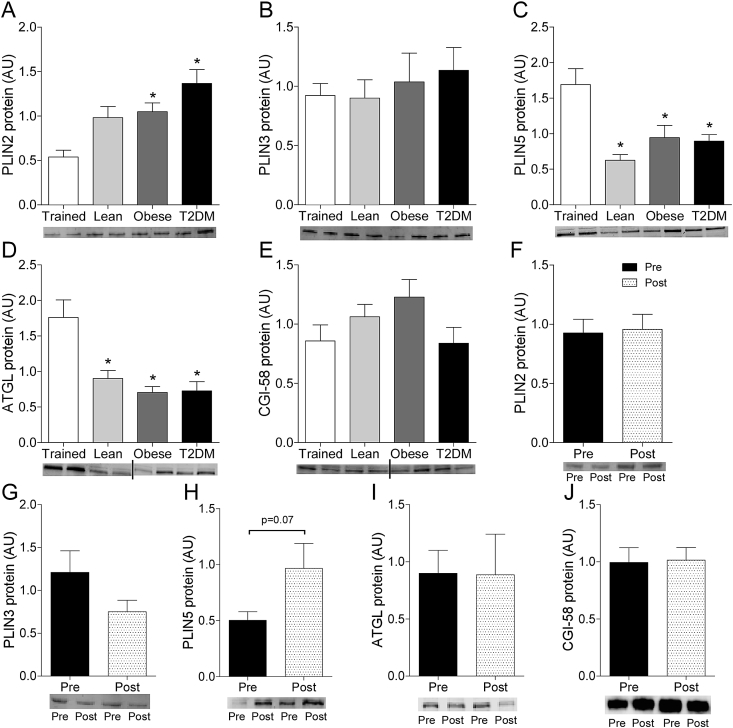
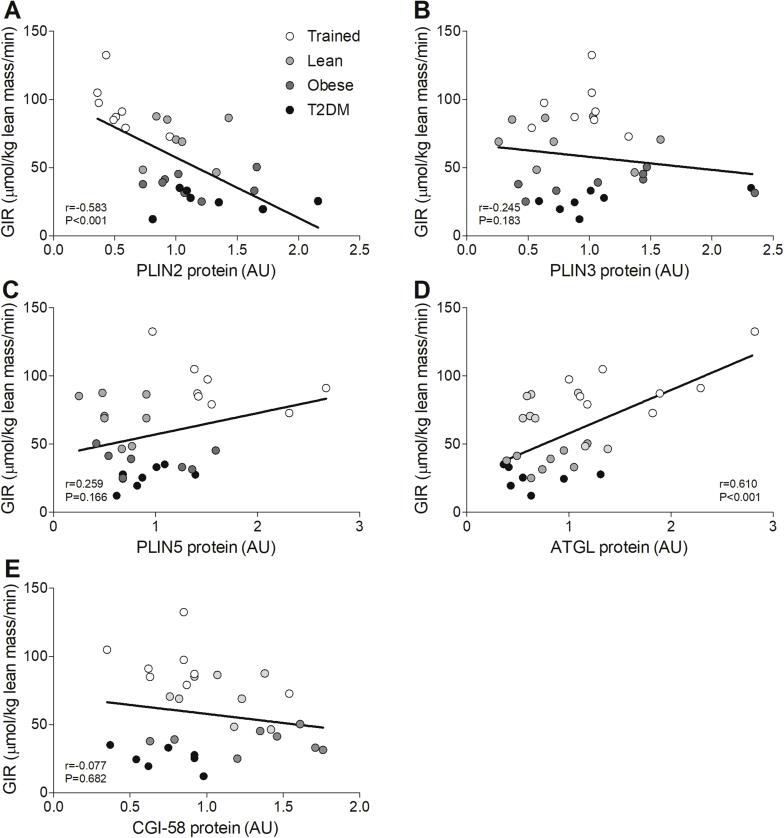
To study the hypothesis that the differential protein coating of LDs may (partly) underlie the cross-sectionally observed differences in the LD storage pattern, we examined protein content of the LD coating proteins PLIN2, PLIN3, PLIN5, ATGL and CGI-58 in Trained, Lean, Obese, and T2DM. PLIN2 protein levels were higher in Obese and T2DM compared to Trained (Figure 5A) while PLIN5 and ATGL protein content was higher in Trained compared to all other groups (Figure 5B,D). No differences between groups were observed for PLIN3 (Figure 5C) and CGI-58 (Figure 5E). PLIN2 correlated negatively with insulin sensitivity (r = −0.583, p < 0.001, Supplemental Figure 3A), while ATGL correlated positively with insulin sensitivity (r = 0.610, p < 0.001, Supplemental Figure 3D). No correlations were observed for PLIN3, PLIN5, and CGI-58 with insulin sensitivity (PLIN3: r = −0.245, p = 0.183, Supplementary Figure 3B; PLIN5: r = 0.259, p = 0.166, Supplementary Figure 3C; CGI-58: r = −0.077, p = 0.682, Supplementary Figure 3E).
Figure 5.
PLIN2, PLIN5 and ATGL protein levels differ between Trained and T2DM. Protein levels of PLIN2, PLIN3, PLIN5, ATGL, and CGI-58 of Trained, Lean, Obese, and T2DM (A–E) and pre and post training (F–J) determined via western blotting with representative blot images. Dividing lines in blot images indicate grouping of images of different parts of blots. *p < 0.05 vs. Trained.
Subsequently, we tested whether these LD coating proteins changed upon training in T2DM. PLIN2 (p = 0.874, Figure 5F), PLIN3 (p = 0.142, Figure 5G), ATGL (p = 0.978, Figure 5I) and CGI-58 protein content (p = 0.877, Figure 5J) remained unaffected by training, whereas PLIN5 tended to increase upon training (p = 0.071, Figure 5H).
4. Discussion
We aimed to examine characteristics of LDs in relation to the athlete's paradox. We show that T2DM patients and Trained, with similar total IMCL content but significantly different in insulin sensitivity, exhibit a vastly different lipid storage pattern. T2DM patients store more IMCL in fewer but larger LDs, located predominantly in type II fibers in the SS area, whereas Trained store IMCL in numerous normally sized LDs in type I fibers, predominantly in the IMF region. Although a high number of LDs in type I fibers has previously been reported in trained vs T2DM [23], our study is the first to report this in the context of similar IMCL levels. In the past, LD size has been linked to insulin sensitivity [7], here we specified this negative association to originate from large LDs in type II fibers.
To explore these fiber type specific differences and its relationship with insulin sensitivity in more detail, we developed a confocal microscopy-based approach to assess LD characteristics in the SS and IMF space in a fiber type specific fashion. Thus, we observed that in T2DM patients, IMCL content was almost 4-fold higher in the SS region of type II fibers compared to Trained. This difference originates from larger LDs in the SS region of type II fibers and extends non-fiber-type specific observations by electron microscopy [25]. In type II fibers, IMCL content, LD size and number in the SS region correlated negatively with insulin sensitivity, suggesting that specifically SS LDs may somehow interfere with insulin sensitivity. This is in line with the negative association of specifically SS LD diameter and insulin sensitivity observed previously in healthy young men [26]. LDs in the SS region are in direct vicinity of cellular membrane, a site where LDs or bioactive LDs released from these droplets, can readily interfere with insulin signaling. LDs in the IMF region are in close proximity of mitochondria [8], [27]. Spatially, this facilitates direct transfer of intrinsically cytotoxic fatty acids to mitochondria upon demand [28]. In that respect, it is interesting to note that it has recently been reported that bio-active insulin desensitizing lipids, i.e. DAGs and sphingolipids, in T2DM originate from the subsarcolemma as opposed to cytosolic LDs [29] and that differences in myocellular lipid synthesis and partitioning between Trained and T2DM exist [30].
To extend our cross-sectional observation that high IMCL content in insulin resistant T2DM originates from large LDs in the SS area of type II muscle, whereas high IMCL content in highly insulin sensitivity trained individuals originates from many small-sized LDs in the IMF area of type I muscle fibers, we analyzed muscle biopsies from insulin resistant individuals undergoing a 12-week exercise training program. We observed that a 12-week combined endurance exercise-resistance training program improved insulin sensitivity without a significant effect on total IMCL. This is somewhat surprising as in untrained lean individuals, with low IMCL levels prior to training, IMCL mostly increases upon training. However, in T2DM patients, training does not necessarily augment IMCL content [31] and sometimes a reduction has been reported [32], [33]. In the present study, LD size decreased significantly in type I fibers and near significantly in type II fibers upon training. A specific decrease in SS IMCL content and SS LD size has previously been observed upon endurance training [24], [25], [26], [34]. Clearly, these exercise-mediated changes push the insulin resistant LD phenotype into a phenotype mimicking the trained insulin sensitive individuals. Thus, differences in LD size along with subcellular and fiber type specific distribution of LDs can be at the basis of the athlete's paradox. Exactly how LDs interfere with insulin sensitivity mechanistically, remains elusive. However, large LDs have a lower surface-to-volume ratio than small LDs, rendering them less accessible for lipolysis or recruitment of proteins activating or inhibiting lipolysis [35], [36]. This is of particular relevance in type II fibers in which the lipolytic machinery is not abundant [37], [38]. Hence, control of regulated lipolysis may be less tight for large LDs, rendering large LDs prone to incomplete lipolysis. Although literature on this is inconsistent, incomplete LD lipolysis may result in release rather than sequestration of bioactive (insulin desensitizing) lipid moieties.
Upon exploring content of proteins involved in LD dynamics, we observed that PLIN2 was higher in T2DM than in trained and correlated negatively with insulin sensitivity. This observation matches the notion that large LD with low lipolytic rates are coated with PLIN2 [11], [39]. However, this cannot directly be reconciled with increasing PLIN2 levels upon training previously observed [18], [40], although we could not confirm these observations in this study. Conversely, ATGL and PLIN5 protein levels were higher in Trained than in T2D with ATGL correlating positively with insulin sensitivity and PLIN5 being induced upon exercise training. These results partly match with previous literature, in which trained athletes reportedly had higher protein levels of PLIN3, PLIN5, ATGL, and CGI-58 compared to lean untrained [16]. Furthermore, exercise training increased muscle perilipins and ATGL protein content in obese subjects [17], [41] and in young, healthy volunteers [18]. We previously reported that unilateral overexpression of PLIN5 in rat skeletal muscle prevented high-fat diet induced insulin resistance in the transfected leg [42] and promoted LD-mitochondrial tethering [42], [43]. This underscores our previous notion of Trained having significantly more PLIN5 coated LDs than in patients with type II diabetes along with the observation that the number of PLIN5 coated LDs significantly contributed to the variance in insulin sensitivity (glucose infusion rate) [44]. For a more detailed understanding of these changes in protein content and its putative effect on LD lipolysis, subcellular distribution analysis is warranted.
Strengths of our study include the combination of both a cross-sectional and intervention component and the comprehensive analysis of LD phenotype across the full spectrum of insulin sensitivity. Furthermore, our study is the first to examine the contribution of LD phenotype and localization to the athlete's paradox in light of similar IMCL levels. The obese and T2DM participants in the present study and the individuals undergoing the training intervention were selected from two previous studies [20], [21]. The inclusion criteria of these studies were similar, albeit not identical, and there were modest differences in the training program with respect to the balance endurance and resistance exercise. Subgroup analysis revealed that these modest differences in inclusion and training did not affect the endpoints of the present study. The difference in age between the Trained and T2DM can be considered a limitation. Although this limitation is partly overcome by adding two control groups (lean young to match the Trained and Obese normoglycemic to match the T2DM), we cannot discriminate between the effects of obesity and age in this study. Furthermore, we measured total levels of LD coating proteins in whole muscle lysates. This does not directly relate to protein levels or distribution on the surface of LDs, which would be interesting to address in future studies.
5. Conclusion
In conclusion, revisiting the athletes’ paradox over a wide range of insulin sensitivity, but in the face of similar muscle fat content, reveals that this paradox can be explained from a physiological perspective rather than being truly paradoxical. Insulin sensitive trained individuals possess high levels of muscle fat that is predominantly dispersed in small lipid droplets in oxidative type I muscle fibers in the intermyofibrillar space, where fatty acids released form the LDs are deemed to fuel oxidation. On the other hand, in the insulin resistant type 2 diabetic state, most of the muscle fat is found in large lipid droplets in the subsarcolemmal space of type II muscle fibers, a region where interference with insulin signaling is likely from a spatial perspective. With high protein levels of ATGL and PLIN5 and low levels of PLIN2 in the trained state, the capacity to promote LD turnover in Trained is most likely high. These cross-sectional observations are strengthened by the observation that upon exercise training insulin sensitivity improved in patients with type 2 diabetes without affecting total muscle fat content while the LD phenotype and the content of proteins involved in LD turnover in patients with type 2 diabetes partly shifted towards the phenotype observed in trained individuals.
Funding
SD is partly supported by Dutch Diabetes Research Foundation (grant DF 2014.00.1756) and by the NUTRIM—School of Nutrition and Translational Research in Metabolism – NWO Graduate Program financially supported from Netherlands Organization for Scientific Research (022.003.011). AG and MKCH acknowledge financial support from the NanoNextNL, a micro and nanotechnology consortium of the Government of the Netherlands and 130 partners. The work of SD, AG, PS and MKCH is partly supported by the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation (CVON2014-02 ENERGISE).
Authors’ contributions
SD and AG performed the experiments. SD, AG, PS, and MKCH designed the study. JH, RCRM, and BB acquired data. PRH, GS, JJ and EK performed a subset of the analysis. SD, AG, MKCH, and PS interpreted the data and wrote the manuscript. PRH, JH, RCRM, GS, and BB revised the manuscript critically.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.08.004.
Conflict of interest
The authors have no conflict of interest to disclose.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Figure 1.
Overview of participant selection.
Supplementary Figure 2.
No changes in SS and IMF lipid droplet distribution upon training in T2DM. Quantification of IMCL content (lipid area fraction) (A), LD number per fiber area (B) and LD size (C) in the SS and IMF region in type I fibers and type II fibers pre and post training. #p < 0.05 vs. SS.2
Supplementary Figure 3.
PLIN2 (negative) and ATGL (positive) correlate with insulin sensitivity. Correlations of GIR with PLIN2 (A), PLIN3 (B), PLIN5 (C), ATGL (D), and CGI-58 (E) protein content.3
References
- 1.Pan D.A., Lillioja S., Kriketos A.D., Milner M.R., Baur L.A., Bogardus C. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46(6):983–988. doi: 10.2337/diab.46.6.983. [DOI] [PubMed] [Google Scholar]
- 2.Krssak M., Falk Petersen K., Dresner A., DiPietro L., Vogel S.M., Rothman D.L. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42(1):113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster B.H., Theriault R., Watkins S.C., Kelley D.E. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49(4):467–472. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 4.Goodpaster B.H., He J., Watkins S., Kelley D.E. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. Journal of Clinical Endocrinology and Metabolism. 2001;86(12):5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 5.Walther T.C., Farese R.V., Jr. Lipid droplets and cellular lipid metabolism. Annual Review of Biochemistry. 2012;81:687–714. doi: 10.1146/annurev-biochem-061009-102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paar M., Jungst C., Steiner N.A., Magnes C., Sinner F., Kolb D. Remodeling of lipid droplets during lipolysis and growth in adipocytes. Journal of Biological Chemistry. 2012;287(14):11164–11173. doi: 10.1074/jbc.M111.316794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J., Goodpaster B.H., Kelley D.E. Effects of weight loss and physical activity on muscle lipid content and droplet size. Obesity Research. 2004;12(5):761–769. doi: 10.1038/oby.2004.92. [DOI] [PubMed] [Google Scholar]
- 8.Shaw C.S., Jones D.A., Wagenmakers A.J. Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochemistry and Cell Biology. 2008;129(1):65–72. doi: 10.1007/s00418-007-0349-8. [DOI] [PubMed] [Google Scholar]
- 9.Albers P.H., Pedersen A.J., Birk J.B., Kristensen D.E., Vind B.F., Baba O. Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes. 2015;64(2):485–497. doi: 10.2337/db14-0590. [DOI] [PubMed] [Google Scholar]
- 10.Tarnopolsky M.A., Rennie C.D., Robertshaw H.A., Fedak-Tarnopolsky S.N., Devries M.C., Hamadeh M.J. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. American Journal of Physiology Regulatory Integrative and Comparative Physiology. 2007;292(3):R1271–R1278. doi: 10.1152/ajpregu.00472.2006. [DOI] [PubMed] [Google Scholar]
- 11.Listenberger L.L., Ostermeyer-Fay A.G., Goldberg E.B., Brown W.J., Brown D.A. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. The Journal of Lipid Research. 2007;48(12):2751–2761. doi: 10.1194/jlr.M700359-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Bulankina A.V., Deggerich A., Wenzel D., Mutenda K., Wittmann J.G., Rudolph M.G. TIP47 functions in the biogenesis of lipid droplets. The Journal of Cell Biology. 2009;185(4):641–655. doi: 10.1083/jcb.200812042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H., Bell M., Sreenivasan U., Hu H., Liu J., Dalen K. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. Journal of Biological Chemistry. 2011;286(18):15707–15715. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmermann R., Strauss J.G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306(5700):1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 15.Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metabolism. 2006;3(5):309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Koves T.R., Sparks L.M., Kovalik J.P., Mosedale M., Arumugam R., DeBalsi K.L. PPARgamma coactivator-1alpha contributes to exercise-induced regulation of intramuscular lipid droplet programming in mice and humans. The Journal of Lipid Research. 2013;54(2):522–534. doi: 10.1194/jlr.P028910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louche K., Badin P.M., Montastier E., Laurens C., Bourlier V., de Glisezinski I. Endurance exercise training up-regulates lipolytic proteins and reduces triglyceride content in skeletal muscle of obese subjects. Journal of Clinical Endocrinology and Metabolism. 2013;98(12):4863–4871. doi: 10.1210/jc.2013-2058. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd S.O., Cocks M., Tipton K.D., Ranasinghe A.M., Barker T.A., Burniston J.G. Sprint interval and traditional endurance training increase net intramuscular triglyceride breakdown and expression of perilipin 2 and 5. Journal of Physiology. 2013;591(3):657–675. doi: 10.1113/jphysiol.2012.240952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vosselman M.J., Hoeks J., Brans B., Pallubinsky H., Nascimento E.B., van der Lans A.A. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. International Journal of Obesity (London) 2015;39(12):1696–1702. doi: 10.1038/ijo.2015.130. [DOI] [PubMed] [Google Scholar]
- 20.Brouwers B., Schrauwen-Hinderling V.B., Jelenik T., Gemmink A., Sparks L.M., Havekes B. Exercise training reduces intrahepatic lipid in people with and people without non-alcoholic fatty liver. American Journal of Physiology. Endocrinology and Metabolism. 2017 doi: 10.1152/ajpendo.00266.2017. ajpendo 00266 2017. [DOI] [PubMed] [Google Scholar]
- 21.Meex R.C., Schrauwen-Hinderling V.B., Moonen-Kornips E., Schaart G., Mensink M., Phielix E. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2010;59(3):572–579. doi: 10.2337/db09-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Loon L.J., Koopman R., Manders R., van der Weegen W., van Kranenburg G.P., Keizer H.A. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. American Journal of Physiology Endocrinology and Metabolism. 2004;287(3):E558–E565. doi: 10.1152/ajpendo.00464.2003. [DOI] [PubMed] [Google Scholar]
- 24.Li Y., Lee S., Langleite T., Norheim F., Pourteymour S., Jensen J. Subsarcolemmal lipid droplet responses to a combined endurance and strength exercise intervention. Physiological Reports. 2014;2(11) doi: 10.14814/phy2.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen J., Mogensen M., Vind B.F., Sahlin K., Hojlund K., Schroder H.D. Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. American Journal of Physiology Endocrinology and Metabolism. 2010;298(3):E706–E713. doi: 10.1152/ajpendo.00692.2009. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen J., Christensen A.E., Nellemann B., Christensen B. Lipid droplet size and location in human skeletal muscle fibers are associated with insulin sensitivity. American Journal of Physiology Endocrinology and Metabolism. 2017;313(6):E721–E730. doi: 10.1152/ajpendo.00062.2017. [DOI] [PubMed] [Google Scholar]
- 27.Vock R., Hoppeler H., Claassen H., Wu D.X., Billeter R., Weber J.M. Design of the oxygen and substrate pathways. VI. structural basis of intracellular substrate supply to mitochondria in muscle cells. Journal of Experimental Biology. 1996;199(Pt 8):1689–1697. doi: 10.1242/jeb.199.8.1689. [DOI] [PubMed] [Google Scholar]
- 28.MacPherson R.E., Peters S.J. Piecing together the puzzle of perilipin proteins and skeletal muscle lipolysis. Applied Physiology Nutrition and Metabolism. 2015;40(7):641–651. doi: 10.1139/apnm-2014-0485. [DOI] [PubMed] [Google Scholar]
- 29.Perreault L., Newsom S.A., Strauss A., Kerege A., Kahn D.E., Harrison K.A. Intracellular localization of diacylglycerols and sphingolipids influences insulin sensitivity and mitochondrial function in human skeletal muscle. JCI Insight. 2018;3(3) doi: 10.1172/jci.insight.96805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergman B.C., Perreault L., Strauss A., Bacon S., Kerege A., Harrison K. Intramuscular triglyceride synthesis: importance in muscle lipid partitioning in humans. American Journal of Physiology Endocrinology and Metabolism. 2018;314(2):E152–E164. doi: 10.1152/ajpendo.00142.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonker J.T., de Mol P., de Vries S.T., Widya R.L., Hammer S., van Schinkel L.D. Exercise and type 2 diabetes mellitus: changes in tissue-specific fat distribution and cardiac function. Radiology. 2013;269(2):434–442. doi: 10.1148/radiol.13121631. [DOI] [PubMed] [Google Scholar]
- 32.Bruce C.R., Kriketos A.D., Cooney G.J., Hawley J.A. Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with Type 2 diabetes. Diabetologia. 2004;47(1):23–30. doi: 10.1007/s00125-003-1265-7. [DOI] [PubMed] [Google Scholar]
- 33.Solomon T.P., Sistrun S.N., Krishnan R.K., Del Aguila L.F., Marchetti C.M., O'Carroll S.M. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. Journal of Applied Physiology (1985) 2008;104(5):1313–1319. doi: 10.1152/japplphysiol.00890.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devries M.C., Samjoo I.A., Hamadeh M.J., McCready C., Raha S., Watt M.J. Endurance training modulates intramyocellular lipid compartmentalization and morphology in skeletal muscle of lean and obese women. Journal of Clinical Endocrinology and Metabolism. 2013;98(12):4852–4862. doi: 10.1210/jc.2013-2044. [DOI] [PubMed] [Google Scholar]
- 35.Thiam A.R., Beller M. The why, when and how of lipid droplet diversity. Journal of Cell Science. 2017;130(2):315–324. doi: 10.1242/jcs.192021. [DOI] [PubMed] [Google Scholar]
- 36.Hesselink M.K., Mensink M., Schrauwen P. Intramyocellular lipids and insulin sensitivity: does size really matter? Obesity Research. 2004;12(5):741–742. doi: 10.1038/oby.2004.88. [DOI] [PubMed] [Google Scholar]
- 37.Minnaard R., Schrauwen P., Schaart G., Jorgensen J.A., Lenaers E., Mensink M. Adipocyte differentiation-related protein and OXPAT in rat and human skeletal muscle: involvement in lipid accumulation and type 2 diabetes mellitus. The Journal of Cinical Endocrinology and Metabolism. 2009;94(10):4077–4085. doi: 10.1210/jc.2009-0352. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd S.O., Cocks M., Tipton K.D., Witard O.C., Ranasinghe A.M., Barker T.A. Resistance training increases skeletal muscle oxidative capacity and net intramuscular triglyceride breakdown in type I and II fibres of sedentary males. Experimental Physiology. 2014;99(6):894–908. doi: 10.1113/expphysiol.2014.078014. [DOI] [PubMed] [Google Scholar]
- 39.McIntosh A.L., Senthivinayagam S., Moon K.C., Gupta S., Lwande J.S., Murphy C.C. Direct interaction of Plin2 with lipids on the surface of lipid droplets: a live cell FRET analysis. American Journal of Physiology - Cell Physiology. 2012;303(7):C728–C742. doi: 10.1152/ajpcell.00448.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw C.S., Shepherd S.O., Wagenmakers A.J., Hansen D., Dendale P., van Loon L.J. Prolonged exercise training increases intramuscular lipid content and perilipin 2 expression in type I muscle fibers of patients with type 2 diabetes. American Journal of Physiology Endocrinology and Metabolism. 2012;303(9):E1158–E1165. doi: 10.1152/ajpendo.00272.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shepherd S.O., Cocks M., Meikle P.J., Mellett N.A., Ranasinghe A.M., Barker T.A. Lipid droplet remodelling and reduced muscle ceramides following sprint interval and moderate-intensity continuous exercise training in obese males. International Journal of Obesity. 2017;41(12):1745–1754. doi: 10.1038/ijo.2017.170. [DOI] [PubMed] [Google Scholar]
- 42.Bosma M., Sparks L.M., Hooiveld G.J., Jorgensen J.A., Houten S.M., Schrauwen P. Overexpression of PLIN5 in skeletal muscle promotes oxidative gene expression and intramyocellular lipid content without compromising insulin sensitivity. Biochimica et Biophysica Acta. 2013;1831(4):844–852. doi: 10.1016/j.bbalip.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Wang H., Sreenivasan U., Hu H., Saladino A., Polster B.M., Lund L.M. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. The Journal of Lipid Research. 2011;52(12):2159–2168. doi: 10.1194/jlr.M017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gemmink A., Daemen S., Brouwers B., Huntjens P.R., Schaart G., Moonen-Kornips E. Dissociation of intramyocellular lipid storage and insulin resistance in trained athletes and type 2 diabetes patients; involvement of perilipin 5? Journal of Physiology. 2017 doi: 10.1113/JP275182. [DOI] [PMC free article] [PubMed] [Google Scholar]