Summary
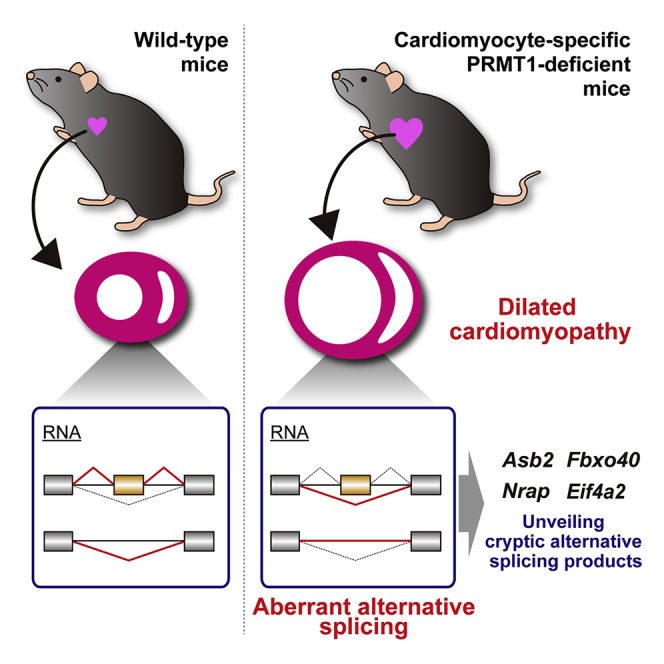
Protein arginine methyltransferase 1 (PRMT1) catalyzes the asymmetric dimethylation of arginine residues in proteins and methylation of various RNA-binding proteins and is associated with alternative splicing in vitro. Although PRMT1 has essential in vivo roles in embryonic development, CNS development, and skeletal muscle regeneration, the functional importance of PRMT1 in the heart remains to be elucidated. Here, we report that juvenile cardiomyocyte-specific PRMT1-deficient mice develop severe dilated cardiomyopathy and exhibit aberrant cardiac alternative splicing. Furthermore, we identified previously undefined cardiac alternative splicing isoforms of four genes (Asb2, Fbxo40, Nrap, and Eif4a2) in PRMT1-cKO mice and revealed that eIF4A2 protein isoforms translated from alternatively spliced mRNA were differentially ubiquitinated and degraded by the ubiquitin-proteasome system. These findings highlight the essential roles of PRMT1 in cardiac homeostasis and alternative splicing regulation.
Subject Areas: Genetics, Molecular Biology, Cell Biology, Developmental Biology
Graphical Abstract
Highlights
-
•
PRMT1 deficiency in cardiomyocytes causes dilated cardiomyopathy in juvenile mice
-
•
PRMT1-deficient heart shows abnormal alternative splicing patterns
-
•
Previously undefined cardiac splicing events are revealed by transcriptome analysis
-
•
eIF4A2 isoforms are differentially ubiquitinated and degraded
Genetics; Molecular Biology; Cell Biology; Developmental Biology
Introduction
Arginine methylation, a post-translational modification, is found in a broad range of proteins, including histones, transcription factors, membrane proteins, and RNA-binding proteins (RBPs) (Yang and Bedford, 2013). In mammalian cells, 10 protein arginine methyltransferases (PRMTs) are responsible for arginine methylation and have been classified into three groups according to their catalytic machinery (Yang and Bedford, 2013, Hatanaka et al., 2017). PRMT1, PRMT2, PRMT3, PRMT4 (also called CARM1), PRMT6, PRMT8, and METTL23 belong to type I PRMTs, which catalyze the formation of mono-methylarginine and asymmetric dimethylarginine. PRMT5 and PRMT9 are known as type II PRMTs, which are capable of producing mono-methylarginine and symmetric dimethylarginine (Hadjikyriacou et al., 2015, Yang et al., 2015). PRMT7 has been characterized as a type III enzyme synthesizing only mono-methylarginine (Zurita-Lopez et al., 2012).
PRMT1, a ubiquitously expressed type I enzyme, was the first identified PRMT, and it regulates a wide variety of cellular processes, such as transcription, DNA damage response, and signal transduction (Wang et al., 2001, Yamagata et al., 2008, Yang and Bedford, 2013). The in vivo functions of PRMT1 were not identified in mammals for a long time, because conventional PRMT1-deficient mice showed early embryonic lethality (Pawlak et al., 2000). In recent years, two independent reports using heterozygous Prmt1-deficient mice revealed that PRMT1 controls hepatic gluconeogenesis and neuronal excitability in the CNS (Choi et al., 2012, Kim et al., 2016). Another group reported that lack of PRMT1 in murine muscle stem cells impairs muscle regeneration (Blanc et al., 2017). On the other hand, we demonstrated that CNS-specific PRMT1-deficient mice exhibited hypomyelination, indicating a vital role of PRMT1 in oligodendrocyte development (Hashimoto et al., 2016). Furthermore, we also showed that loss of PRMT1 in vascular endothelial cells results in angiodysplasia in mouse embryo (Ishimaru et al., 2017). Thus, an increasing number of studies indicate that PRMT1 has crucial roles in multiple tissue functions and development.
Several reports have investigated the protein expression levels of PRMTs in the rodent heart and show that the cardiac tissue expresses PRMT1, PRMT3, PRMT4, PRMT5, and PRMT7 (Bulau et al., 2007). In addition, PRMT5 directly methylates GATA4 transcription factor, and inhibits phenylephrine-induced cardiomyocyte hypertrophy in neonatal rat ventricular myocytes (Chen et al., 2014). PRMT4 has been shown to regulate aging-related autophagy in mouse heart (Li et al., 2017). Interestingly, cardiac PRMT1 expression is increased in patients with coronary artery disease and in rats with isoproterenol-induced heart failure (Chen et al., 2006, Li et al., 2012), suggesting the importance of PRMT1 in the heart. However, the physiological function of PRMT1 in the heart remains to be elucidated.
A number of reports have demonstrated that genetic deletion of RBP causes abnormal alternative splicing, resulting in lethal heart failure (Xu et al., 2005, Guo et al., 2012, Ye et al., 2015, Wei et al., 2015). SRSF1, a serine/arginine-rich family of splicing factors, is essential for the splicing switch of the Ca2+/calmodulin-dependent kinase II δ (Camk2d) gene, and its deficiency in mouse cardiomyocytes leads to dilated cardiomyopathy (DCM) (Xu et al., 2005). Moreover, mutations in RNA-binding motif protein 20 (RBM20) gene, a regulator of titin (Ttn) mRNA splicing, has been shown to cause human DCM (Guo et al., 2012, Wells et al., 2013, Beqqali et al., 2016). Other reports have shown that hnRNP U and RBFOX2 are necessary for alternative splicing of some genes and maintenance of cardiac function in mouse models (Ye et al., 2015, Wei et al., 2015). Interestingly, using a proteomic approach, it has been shown that many types of RBPs, such as SRSFs, RBMs, hnRNPs, and RBFOXs, are methylated in the arginine residues (Guo et al., 2014). Although it has been reported that PRMT1 methylates various RBPs, including hnRNP A2, hnRNP U, and RBM15 (Nichols et al., 2000, Herrmann et al., 2004, Zhang et al., 2015), there is no in vivo evidence that PRMT1 regulates alternative splicing.
In this study, we aimed to assess whether the deletion of PRMT1 affects cardiac functions and alternative splicing events in the heart. To this end, we generated cardiomyocyte-specific PRMT1-deficient mice and found that they display features of heart failure, including contractile dysfunction and cardiac chamber dilation. Furthermore, we also found that the deletion of PRMT1 impairs alternative splicing of mRNA in the heart and detected uncharacterized alternative splicing isoforms in some genes. Finally, we demonstrated that protein products translated from alternatively spliced mRNA variants exhibit distinct ubiquitination status in C2C12 myoblast cells. Our results clarify the essential role of PRMT1 in cardiac homeostasis and alternative splicing.
Results
PRMT1 Is Expressed in Both Cardiomyocytes and Non-myocytes
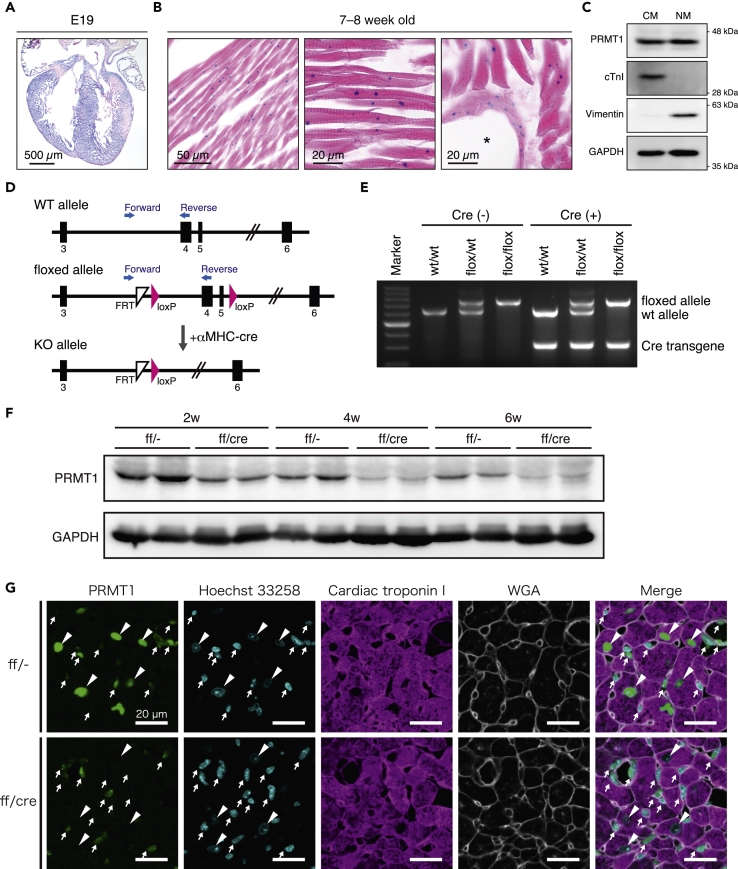
To elucidate the role of PRMT1 in the heart, we first examined the type of cells expressing PRMT1 using Prmt1tm1a (EUCOMM) Wtsi knockin (PRMT1KI) mice (Skarnes et al., 2011). Prmt1tm1a (EUCOMM) Wtsi allele contains FRT-flanked lacZ/neomycin sequence within intron 3 in Prmt1 gene. As PRMT1KI mice express β-galactosidase (β-gal) driven by endogenous Prmt1 promoter, we performed X-gal staining using hearts from embryos and adult mice. At embryonic day 19, β-gal activity was ubiquitously detected in the heart section, including atrial and ventricular walls (Figure 1A). In the adult mice, β-gal activity was found in the center of cardiomyocytes and in the vascular wall cells (Figure 1B). To further investigate PRMT1-expressing cell types, we examined the protein expression levels of PRMT1 in isolated primary cardiomyocytes and non-myocytes from cardiac tissues of C57BL/6 mice. Consistent with the results of X-gal staining, PRMT1 was expressed in cardiomyocytes and non-myocytes in the mouse heart (Figure 1C).
Figure 1.
Generation of Cardiomyocyte-Specific PRMT1-Deficient Mice
(A and B) X-gal staining of (A) fetal and (B) adult hearts in PRMT1KI mice. The asterisk indicates cardiac artery.
(C) Western blotting of PRMT1 in neonatal mouse cardiomyocytes (CM) and non-myocytes (NM). Cardiac troponin I (cTnI) and vimentin were used as specific markers for CM and NM, respectively. GAPDH was used as loading control.
(D) Schematic diagram of Prmt1 gene targeting. Black rectangles indicate exons of Prmt1, and blue allows show primer set for genotyping.
(E) Representative images of genotyping in PRMT1-cKO mice.
(F) Western blotting analysis of PRMT1 in the hearts of control (ff/-) and PRMT1-cKO (ff/cre) mice. The hearts were harvested from 2-, 4-, and 6-week-old (shown as 2w, 4w, and 6w in the figure) mice.
(G) Representative images of cardiac sections stained with anti-PRMT1 antibody (green), Hoechst 33258 (blue), anti-cardiac troponin I antibody (magenta), and WGA (gray). Arrowheads indicate nuclei of cardiomyocytes. Arrows indicate non-cardiomyocytes.
Cardiomyocyte-Specific PRMT1 Deletion Causes Dilated Cardiomyopathy in Juvenile Mice
To examine the function of PRMT1 in cardiomyocytes, we generated cardiomyocyte-specific PRMT1-deficient (hereafter referred to as PRMT1-cKO) mice. These mice were obtained by crossing Prmt1flox/flox mice with α myosin heavy chain (αMHC)-Cre transgenic mice (Figures 1D and 1E). PRMT1 protein levels were decreased in the heart of PRMT1-cKO mice compared with Prmt1flox/flox (control) mice (Figure 1F). Immunohistochemical analysis revealed that PRMT1 is mainly expressed in the nuclei of cardiomyocytes and non-myocytes in control mice (Figure 1G, upper panels). Furthermore, in both control and cKO mice, non-myocytes showed various levels of green fluorescence, suggesting that PRMT1 expression levels are highly heterogeneous among non-myocytes depending on the cell types (Figure 1G, arrows). We found that the signals of PRMT1 were dramatically reduced in the cardiomyocytes of PRMT1-cKO mice, whereas non-myocytes normally expressed PRMT1 (Figure 1G, lower panels). These data clearly show that PRMT1 is specifically deleted in the cardiomyocytes of PRMT1-cKO mice.
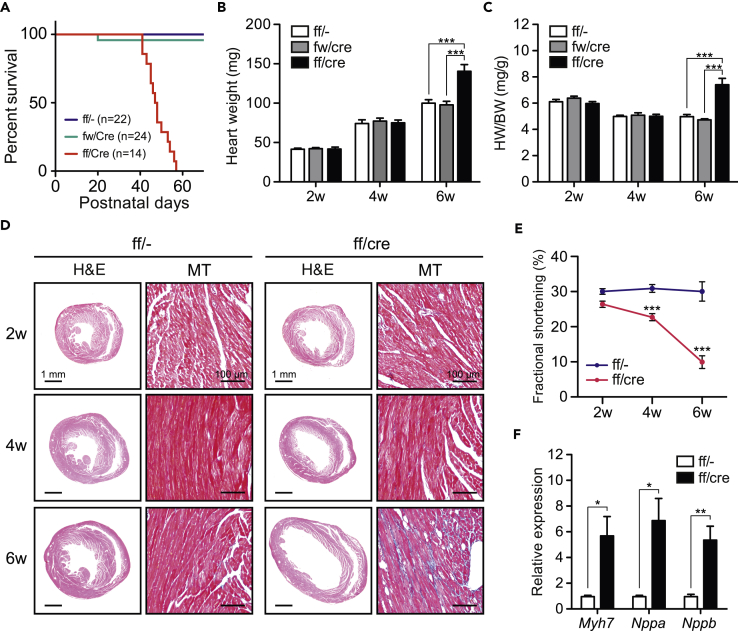
To determine the influence of PRMT1 deletion on the heart, we analyzed the cardiac morphology and function in PRMT1-cKO mice. Although PRMT1-cKO mice were born in a normal Mendelian ratio (data not shown), they died within 60 days of birth (Figure 2A). Heart weight and heart/body weight ratio were significantly increased in 6-week-old PRMT1-cKO mice compared with age-matched control and Prmt1 hetero-deficient mice (Figures 2B and 2C). PRMT1-cKO mice exhibited marked cardiac chamber dilation and fibrosis at 6 weeks of age (Figure 2D, lower panels). Cardiac systolic function of PRMT1-cKO mice began to reduce within 4 weeks after birth, and further reduction was observed in 6-week-old mice (Figure 2E). Furthermore, expression levels of βMHC (Myh7), ANP (Nppa), and BNP (Nppb) genes, which are well-characterized markers of heart failure, were significantly elevated in the heart of PRMT1-cKO mice at 4 weeks of age (Figure 2F).
Figure 2.
Dilated Cardiomyopathy of PRMT1-cKO Mice
(A) Survival rate of control, heterozygous (fw/cre), and PRMT1-cKO mice.
(B and C) (B) Heart weight and (C) heart weight/body weight ratio (HW/BW) of 2-, 4-, and 6-week-old mice (n = 4–6).
(D) H&E and Masson's trichrome (MT) staining of the hearts at different ages.
(E) Cardiac contractility of control and PRMT1-cKO mice at different ages.
(F) Gene expression analysis for marker genes of cardiac dysfunction in the heart of 4-week-old control and PRMT1-cKO mice (n = 4).
All data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared with age-matched control mice.
We further conducted a comprehensive analysis of gene expression changes in the heart of 6-week-old PRMT1-cKO mice using RNA sequencing (RNA-seq). Gene expression analysis identified 2,037 differentially expressed genes between control and PRMT1-cKO mice: 1,044 genes were upregulated and 993 genes were downregulated (Table S1, Figures S1A and S1B). Consistent with the results of histological analysis, fibrosis-related genes, such as Tgfb2, Ctgf, and collagen family, were induced in the heart of PRMT1-cKO mice (Table S1). On the other hand, Kyoto Encyclopedia of Genes and Genomics pathway analysis of decreased genes showed an enrichment in pathways related to energy metabolism, mitochondrial function, and DCM (Figure S1C). This transcriptional profile of the PRMT1-cKO mice coincides with that of a DCM mouse model carrying a missense mutation in the phospholamban gene (Burke et al., 2016). These observations clearly demonstrated that juvenile PRMT1-cKO mice develop lethal DCM and that PRMT1 plays critical roles in maintaining cardiac homeostasis.
Cardiomyocyte-Specific Loss of PRMT1 Causes Aberrant Alternative Splicing of mRNA in the Heart
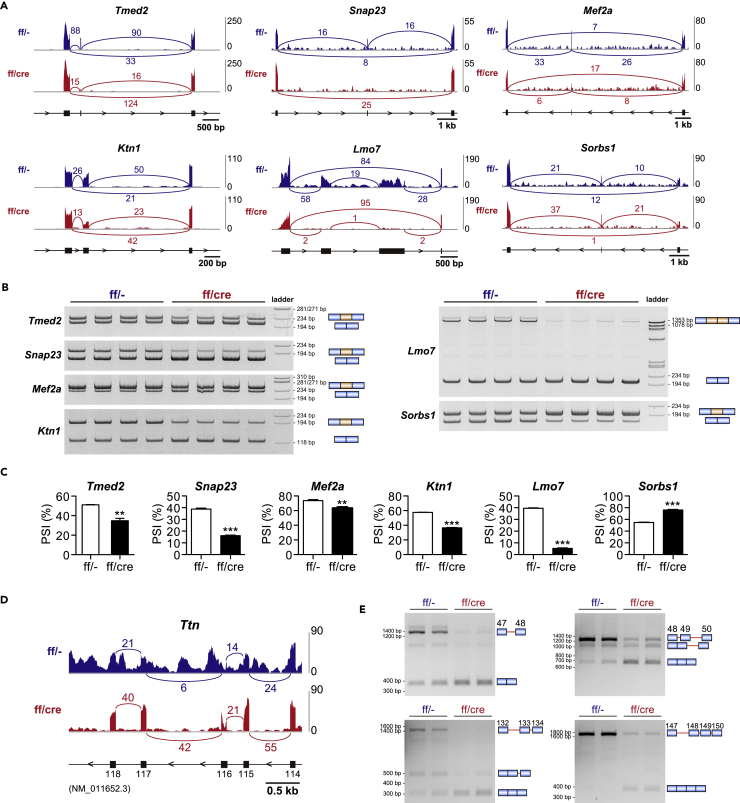
To examine the effect of PRMT1 deletion on cardiac alternative splicing, we focused on the known alternative splicing events that are observed in the loss of function or the gain of function in animal models for RBPs in the heart (Table S2), and analyzed RNA-seq data. Sashimi plots revealed differences in exon usage of several genes between control and PRMT1-cKO mice at 6 weeks of age (Figure 3A). These results were confirmed by RT-PCR using the heart of 4-week-old mice, indicating that alternative splicing abnormality occurs in the early stage of cardiac pathogenesis in PRMT1-cKO mice (Figures 3B and 3C).
Figure 3.
Aberrant Alternative Splicing in the Heart of PRMT1-cKO Mice
(A) Representative images of Sashimi plot depicting alternative splicing pattern in the heart of 6-week-old mice. Read counts for each sample are indicated on the y axis.
(B and C) (B) Gel images of RT-PCR products and (C) percent spliced in (PSI) value calculated from the band intensities for assessing changes in alternative splicing in the heart of 4-week-old mice (n = 4).
(D) Representative images of Sashimi plot depicting alternative splicing of Titin (Ttn) gene in the heart of 6-week-old mice. Read counts for each sample are indicated on the y axis.
(E) Gel images of RT-PCR products for Titin gene. Blue rectangles and red lines indicate exons and introns of Titin gene, respectively.
All data are presented as mean ± SEM. **p < 0.01, ***p < 0.001 compared with control mice.
Alternative splicing defects or mutations of Ttn gene, which encodes a giant sarcomeric protein, are closely associated with DCM (Guo et al., 2012, Herman et al., 2012, Schafer et al., 2017). Consistent with a previous work using rat model (Li et al., 2013), intron retention was observed within the regions encoding middle Ig domains and PEVK regions in the heart of control mice, whereas it was clearly reduced in those of PRMT1-cKO mice aged 6 weeks (Figures 3D and 3E). Similar changes in alternative splicing of Ttn gene have been found in RBM20 knockout rats and cardiomyocyte-specific hnRNP U-deficient mice (Guo et al., 2012, Li et al., 2013, Ye et al., 2015). These results indicate that PRMT1 modulates cardiac alternative splicing events, regulated by a wide-range of RBPs.
The Heart of PRMT1-cKO Mouse Provides Valuable Tissues for Exploration of Unidentified Alternative Splicing Events
To further investigate the effects of PRMT1 deletion on alternative splicing in the heart, we searched for the genes that display abnormal splicing pattern in PRMT1-cKO mice. From RNA-seq data, we extracted genes that have differences in expression levels of splicing isoform between control and PRMT1-cKO mice and found 74 candidate genes (Table S3). A gene ontology analysis of the 74 candidates showed enrichment of genes implicated in muscle development, such as muscle cell development, myofibril assembly, and actin cytoskeleton organization (Figure S2). This hallmark was similar to that of some RBP-deficient animals (Guo et al., 2012, Wei et al., 2015, Dixon et al., 2015). Among the 74 genes, the alternative splicing of Ttn, Lmo7, Tnnt2, Rtn4, and Obscn has been shown to be directly regulated by RBM20 (Maatz et al., 2014). The alterations in Pdlim5 and Ldb3 splicing have been observed in RBM20-, RBFOX2-, and MBNL1-deficient animals (Guo et al., 2012, Wei et al., 2015, Dixon et al., 2015). In addition, alternative splicing events of Pkm, Sptan1, Ppfibp1, and Popdc2 genes in the heart have already been reported (Gao and Cooper, 2013, Cianci et al., 1999, Kalsotra et al., 2008). Thus, these results led us to believe that our screening protocol was sensitive enough to pick up other unknown splicing events that occur in the pathologic hearts. Next, we validated those new alternative splicing events that have not been characterized in mouse hearts to date.
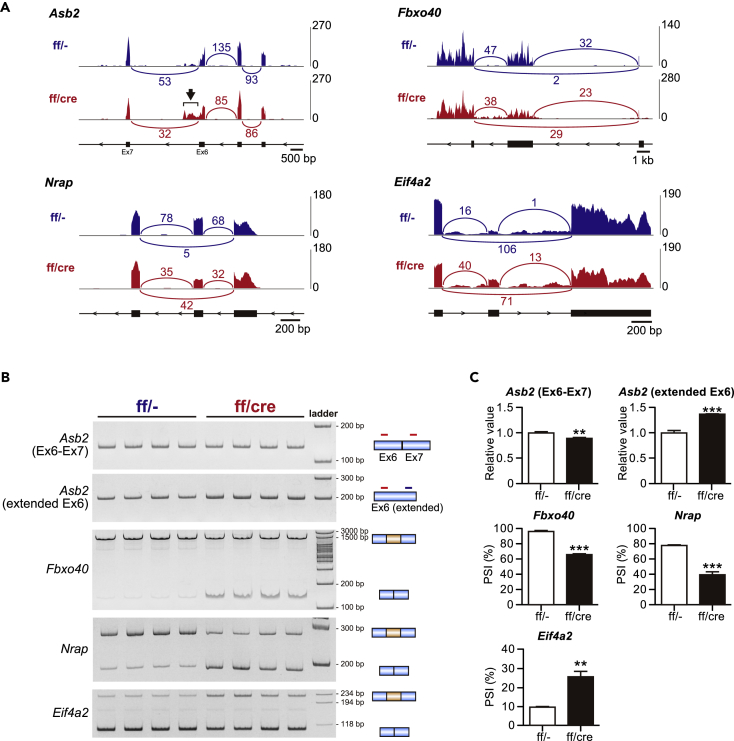
ASB2, a component of ECS-type (ElonginBC-Cullin-SOCS box) E3 ligase complex, has been shown to be essential for the cardiomyocyte maturation in zebrafish model (Fukuda et al., 2017). In the heart of PRMT1-cKO mice, a large number of reads were detected following Asb2 exon 6 compared with control mice, indicating the existence of the extended form of exon 6 (Figure 4A). This extended exon 6 has not been registered in the NCBI, Ensembl, or UCSC database. To verify the presence of the novel isoform of exon 6, we designed a primer set for the detection of the extended exon 6 and performed RT-PCR analysis. Although the expression level of the known transcript that includes exons 6 and 7 was slightly but significantly decreased in PRMT1-cKO mice, the extended exon 6 expression was increased in PRMT1-cKO hearts (Figures 4B and 4C). Because the extended exon 6 contains a stop codon and a putative poly(A) signal, this exon may serve as a composite terminal exon (Figure S3A; Tian and Manley, 2013). This suggests that this novel transcript encodes truncated ASB2 protein lacking SOCS box, which is required for the formation of E3 ligase complex (Figure S3A; Okumura et al., 2012).
Figure 4.
Novel Alternative Splicing Events in the Heart
(A) Representative Sashimi plots depicting alternative splicing pattern in the heart of 6-week-old mice. Read counts for each sample are indicated on the y axis.
(B and C) (B) Gel images of RT-PCR products and (C) relative expression (for Asb2) or PSI value (for Fbxo40, Nrap, and Eif4a2) calculated from the band intensities in the heart of 4-week-old mice (n = 4).
All data are presented as mean ± SEM. **p < 0.01, ***p < 0.001 compared with control mice.
FBXO40 is a component of SCF (Skp, Cullin, and F box) E3 ligase complex, modulating insulin-like growth factor 1 (IGF1) receptor signaling pathway via ubiquitination of IRS1 in skeletal muscle (Shi et al., 2011). A large part of FBXO40 coding sequence is encoded by exon 3 (Ensembl Transcript ID: ENSMUST00000075869.12). Although most of the Fbxo40 mRNA was contained in exon 3 in the control hearts, exon 3 skipping was significantly increased in the PRMT1-cKO hearts (Figures 4A–4C).
Nrap (nebulin-related anchoring protein) gene encodes a cytoskeletal protein (Bang and Chen, 2015). It has been reported that the expression of NRAP is upregulated in mouse models of DCM (Ehler et al., 2001). Furthermore, several studies investigated the alternative splicing of Nrap exon 12 included in skeletal muscle isoform, but not in cardiac isoform (Mohiddin et al., 2003, Berger et al., 2011). In addition to exon 12, the NCBI database suggests that Nrap exon 2 is alternatively spliced (NM_001286552.1); however, this splicing event has not been characterized in the heart. Although alternative splicing defect of Nrap has been found in the heart of Rbfox2-KO mice, a model of DCM, which exons are affected by Rbfox2 deficiency in the heart has not been addressed (Wei et al., 2015). We found that exon skipping of Nrap exon 2, encoding an LIM domain of NRAP protein, was significantly increased in PRMT1-cKO mice (Figures 4A–4C).
Eif4a2 gene encodes eIF4A2 DEAD-box RNA helicase associated with translation initiation, microRNA-mediated gene repression, and stress granule formation (Meijer et al., 2013, Jongjitwimol et al., 2016). A previous report demonstrated that knockdown of splicing factor U2AF65 or U2AF35 induces the inclusion of a 107-nucleotide (nt) exon of EIF4A2 in HeLa cells (Shao et al., 2014). Importantly, the exon-intron structure of eIF4A2 gene is highly conserved between human and mouse (Figure S3B, a–c). In the PRMT1-cKO mice, the 107-nt exon inclusion of Eif4a2 was significantly increased compared with control mice (Figures 4A–4C).
Taken together, we discovered previously unrecognized cardiac alternative splicing events through the use of PRMT1-cKO mice.
eIF4A2 Isoforms Are Differentially Ubiquitinated and Degraded in C2C12 Cells
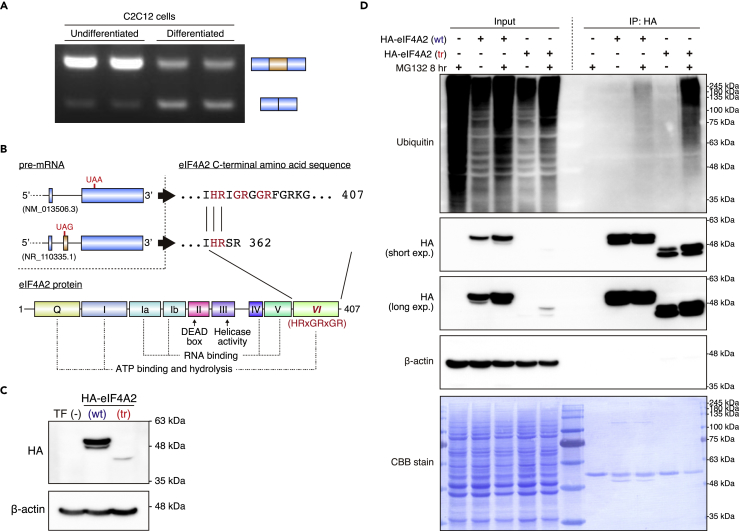
Finally, we explored the significance of alternative splicing of Eif4a2 under more physiological conditions. It has been reported that Eif4a2 mRNA expression is increased in differentiating C2C12 mouse myoblast cells, and that double knockdown of Eif4a1 and Eif4a2 suppresses myoblast differentiation into myotubes (Galicia-Vazquez et al., 2014). The differentiation process of C2C12 cells is intimately involved in alternative splicing (Bland et al., 2010, Singh et al., 2014), whereas the association between alternative splicing of Eif4a2 and C2C12 differentiation is undefined. Therefore, we investigated whether C2C12 differentiation is linked to alternative splicing change of Eif4a2. We found that the undifferentiated C2C12 cells predominantly expressed long Eif4a2 transcripts (Figure 5A). Furthermore, the long Eif4a2 isoform expression was reduced in differentiating C2C12 cells, whereas that of the short Eif4a2 isoform was markedly increased (Figure 5A). Although changes in alternative splicing of Eif4a2 were found in PRMT1-deficient hearts (Figure 4) and U2AF knockdown cells (Shao et al., 2014), data suggested that these splicing changes were also induced by physiological stimuli, such as myoblast differentiation.
Figure 5.
Differential Ubiquitination Status of eIF4A2 Isoforms
(A) RT-PCR analysis for detecting Eif4a2 alternative splicing in undifferentiated and differentiated C2C12 cells.
(B) Schematic diagrams of Eif4a2 pre-mRNA 3′ end, C-terminal sequences of eIF4A2, and eIF4A2 protein domains.
(C) Western blot analysis of HA-eIF4A2 wild-type (wt) and truncated (tr) isoforms in C2C12 cells 24 hr after transfection. β-Actin was used as a loading control.
(D) Immunoprecipitation assay for detecting ubiquitination of HA-eIF4A2 isoforms in C2C12 cells. At 16 hr after transfection, cells were treated with MG132 (10 μM) for 8 hr.
The NCBI database predicts that the long Eif4a2 transcript (NR_110335.1), upregulated in the heart of PRMT1-cKO mice (Figure 4), is a candidate for nonsense-mediated mRNA decay (NMD). Indeed, the 107-nt exon of long Eif4a2 transcript contains a premature termination codon (PTC) positioned more than 50–55 nt upstream of the last exon-exon junction (Figure 5B). However, several reports have demonstrated that the PTC-containing transcript escapes NMD, and is translated into a truncated protein that plays an important physiological role (Holbrook et al., 2004, Bamber et al., 1999, Donnadieu et al., 2003). Furthermore, our data showed that PTC-containing long Eif4a2 transcript is expressed at substantial levels in the heart of PRMT1-cKO mice and in undifferentiated C2C12 cells (Figures 4B and 5A), suggesting that long Eif4a2 transcript is translated into truncated eIF4A2 protein (Figure 5B). Although eIF4A2 possesses motif VI, which probably functions as a trigger of ATP hydrolysis, at its C terminus (Pause and Sonenberg, 1992, Schutz et al., 2008), truncated eIF4A2 isoform partially lacks motif VI (Figure 5B).
To investigate the functional difference of eIF4A2 isoforms, expressed differentially in the heart of control and PRMT1-cKO mice, we generated hemagglutinin (HA)-tagged eIF4A2 (HA-eIF4A2-wt) and HA-tagged truncated-eIF4A2 (HA-eIF4A2-tr) expression vectors. Although equivalent amount of vectors was transfected into C2C12 cells, unexpectedly, the protein level of HA-eIF4A2-tr was remarkably lower than that of HA-eIF4A2-wt (Figure 5C). A previous study showed that eIF4A2 protein is sumoylated on K226 in mammalian cells (Jongjitwimol et al., 2016), whereas some proteomic analyses indicated that eIF4A2 protein contains several ubiquitination sites (Danielsen et al., 2011, Kim et al., 2011; curated information is available on PhosphoSitePlus, a database of post-translational modifications). Therefore, we hypothesized that HA-eIF4A2-tr protein is ubiquitinated and degraded by the ubiquitin-proteasome system. To examine this hypothesis, we treated C2C12 cells with MG132 after transfection and assessed the ubiquitination levels of HA-eIF4A2 isoforms. MG132 treatment increased both HA-eIF4A2-wt and HA-eIF4A2-tr protein levels (Figure 5D, input). Furthermore, poly-ubiquitination of HA-eIF4A2-wt and HA-eIF4A2-tr was detected in MG132-treated C2C12 cells (Figure 5D, immunoprecipitation [IP]: HA). It is noteworthy that HA-eIF4A2-tr protein was highly ubiquitinated compared with HA-eIF4A2-wt (Figure 5D, IP: HA), indicating that the 47 amino acid residues in the C terminus of eIF4A2-wt protect against ubiquitination and subsequent degradation by the ubiquitin-proteasome system.
Discussion
In the present study, we generated cardiomyocyte-specific PRMT1-deficient mice and demonstrated the vital role of PRMT1 in maintaining cardiac function. Moreover, cardiac alternative splicing was disturbed in the heart of PRMT1-cKO mice, indicating the critical role of PRMT1 in the regulation of alternative splicing in vivo. In addition, using this animal model, we successfully detected novel alternative splicing products in the heart.
PRMT1 Is Essential for Cardiac Development in Mice
Accumulating evidence demonstrates that PRMT1, a dominant type I PRMT in mammalian cells, controls tissue development, regeneration, and homeostasis in vivo (Hashimoto et al., 2016, Blanc et al., 2017). In the present study, we showed that cardiomyocyte-specific deletion of PRMT1 provoked DCM in juvenile mice. Although PRMT1 has been suggested to be involved in the pathogenesis of heart diseases via overproduction of free asymmetric dimethylarginine followed by its nitric oxide synthase (NOS)-inhibiting effect (Haghikia et al., 2011, Li et al., 2012), our data clearly demonstrated that PRMT1 has essential roles in cardiac development and homeostasis under physiological conditions. Despite the parallel expression of PRMT1 in cardiomyocytes and non-myocytes, PRMT1 deficiency in cardiomyocytes led to DCM in juvenile mice, demonstrating an indispensable role of arginine methylation catalyzed by PRMT1 in cardiomyocytes.
Association between PRMT1 and Cardiac Splicing Regulation
In the postnatal heart, many physiological and structural changes occur, becoming fully functional by adulthood. Although these processes are transcriptionally regulated, alternative splicing also participates in postnatal cardiac development (Baralle and Giudice, 2017). Interestingly, it has been demonstrated that the majority of alternative splicing transitions in cardiomyocytes occur during the first 4 weeks after birth in mice (Giudice et al., 2014). In the present study, we found that PRMT1-cKO mice showed cardiac alternative splicing defects and reduction of cardiac contractility at 4 weeks after birth. We therefore postulated that alternative splicing transition fails in PRMT1-cKO mice. Indeed, we showed that exon skipping of Tmed2 and Snap23 was significantly increased in 4-week-old PRMT1-cKO mice (Figures 3B and 3C); these splicing patterns resemble the feature in neonatal hearts (Giudice et al., 2014). These observations suggest that PRMT1 regulates alternative splicing transition in the postnatal hearts.
In this study, we found and validated the four novel splicing events in the heart (Figure 4). Among these, we discovered a new Asb2 isoform that is not registered in the NCBI, Ensemble, or UCSC database. This novel Asb2 transcript, which has the extended exon 6 containing a stop codon and a putative poly(A) signal, seems to encode truncated ASB2 protein that lacks SOCS box, suggesting the failure to form E3 ligase complex. On the other hand, ankyrin repeat, a putative substrate recognition motif (Li et al., 2006, Andresen et al., 2014), partially remained in truncated ASB2 protein. Therefore, this truncated ASB2 might function as a dominant-negative isoform to trap substrates. To detect the truncated ASB2 protein, we used commercially available anti-ASB2 antibody that is raised against the N-terminal of ASB2. We were unable to confirm the expression of truncated ASB2 protein in cardiac lysate, whereas weak signal from the full-length ASB2 protein was seen (data not shown), probably because of a lower expression level of truncated ASB2 than full-length protein. Since the expression level of novel Asb2 isoform was increased in the PRMT1-deficient hearts, PRMT1 may be involved in determining alternative polyadenylation sites in the cardiomyocytes.
Although splicing events of three other genes (Fbxo40, Nrap, and Eif4a2) are recorded in at least one database, there is no evidence demonstrating that these splicing events actually occur in the hearts. It has been reported that FBXO40, a component of SCF E3 ligase complex, regulates IGF1 signaling in the skeletal muscle (Shi et al., 2011). FBXO40 is also expressed in human and rodent hearts (Ye et al., 2007), but its function remains unclear. We found that skipping of the exon encoding a large part of FBXO40 protein was significantly increased in the PRMT1-cKO heart (Figures 4B and 4C). The Ensembl database predicts that this transcript does not contain an open reading frame, suggesting that this short form of Fbxo40 transcript exists as long non-coding RNA (lncRNA). Interestingly, a recent study revealed that lncRNA, which is produced by alternative splicing of protein-coding gene, plays an important role in tumor progression (Grelet et al., 2017). This raises the possibility that lnc-Fbxo40 has pathophysiological roles in the heart, and PRMT1 acts as a molecular switch to regulate Fbxo40 gene function. However, further studies are needed to confirm whether the short form of Fbxo40 transcript functions as an lncRNA.
We showed that the usage of Nrap exon 2 was significantly decreased in the heart of PRMT1-cKO mice (Figures 4B and 4C). Nrap transcript that lacks exon 2 encodes an NRAP protein deficient for the LIM domain. NRAP is dominantly expressed in cardiac and skeletal muscles, and is upregulated in the heart of DCM mouse models (Bang and Chen, 2015). NRAP is localized in Z-disk precursors during myofibrillogenesis, whereas specific localization of NRAP at the intercalated disks is found in the adult heart (Bang and Chen, 2015). A previous report revealed that the LIM domain of NRAP strongly binds to talin: talin binds the cytoplasmic domain of β1D integrin subunit, whereas NRAP super-repeats bind to actin, suggesting that NRAP is a component of the protein complex that anchors actin filaments to the plasma membrane (Luo et al., 1999). Although integrin and talin are mainly localized in the costamere, they are also found at the intercalated disks in the heart (Israeli-Rosenberg et al., 2014, Manso et al., 2013). Interestingly, a recent study demonstrated that talin1/talin2 double knockout mice exhibit DCM (Manso et al., 2017). These observations suggest that increase in NRAP lacking the LIM domain may affect the mechanotransduction system through the absence of binding to integrin and talin in the intercalated disks and may contribute to cardiac pathogenesis. Because the role of integrin system in the intercalated disks is largely unknown, it is interesting to investigate the implication in Nrap isoform shift and the function of NRAP lacking LIM domain in the heart.
It has been shown that knockdown of splicing factor U2AF increases the 107-nt exon inclusion of EIF4A2 in HeLa cells (Shao et al., 2014). In the present study, we found that this 107-nt exon of mouse Eif4a2 is alternatively spliced in the heart and C2C12 cells, suggesting that alternative splicing of Eif4a2 occurs under physiological conditions in the cardiac and skeletal muscle cells. We assumed that long Eif4a2 transcript encodes truncated eIF4A2 protein and examined its protein function. Unexpectedly, we found that the eIF4A2 protein is constantly ubiquitinated, and that the truncated isoform is more efficiently ubiquitinated and degraded than the eIF4A2-wt protein, indicating that the splicing switch of Eif4a2 may regulate its protein levels utilizing the ubiquitin-proteasome system. It has been reported that eIF4A is overexpressed in lung and cervical cancer, and inhibitors of eIF4A have antitumor activity (Bhat et al., 2015). Therefore, an understanding of the molecular basis for alternative splicing and ubiquitination of eIF4A2 may provide new insight into the pathogenesis of heart failure, muscle differentiation, and cancer therapy.
PRMT1 Is a Possible Upstream Regulator of RBPs
In the past decade, a number of studies have revealed the critical roles of the RBPs in the cardiac alternative splicing regulation by analyzing genetically engineered mice. On the other hand, we showed that deletion of PRMT1 impairs the cardiac alternative splicing of a broad spectrum of genes, including already reported genes that are affected by the deletion or mutation of RBPs. These observations raise the possibility that PRMT1 participates in alternative splicing regulation through the methylation of RBPs in the cardiomyocytes. It has been reported that cardiomyocyte-specific deletion of hnRNP U (also known as SAF-A) results in splicing defects and severe DCM (Ye et al., 2015). Importantly, a previous report demonstrated that PRMT1 methylates hnRNP U, whereas arginine methylation of hnRNP U did not affect its nuclear localization (Herrmann et al., 2004). SRSF1 (as known as SF2/ASF) deficiency in cardiomyocytes also contributes to aberrant splicing and DCM (Xu et al., 2005). Although which PRMTs are involved is unknown, arginine methylation controls SRSF1 nuclear localization (Sinha et al., 2010). Furthermore, proteomic analyses suggested that other RBPs that are related to the cardiac alternative splicing, such as RBFOX1, RBFOX2, MBNL1, and SRSF10, have putative arginine methylation sites (Guo et al., 2014, Larsen et al., 2016, Geoghegan et al., 2015). Our results suggest that PRMT1 may serve as a key regulator of RBPs functions, such as nuclear localization, RNA-binding properties, and binding of other splicing factors, in the cardiomyocytes.
Taken together, our findings identify an essential role of PRMT1 in the cardiac alternative splicing in vivo, and the heart of PRMT1-cKO mouse is an effective tool for understanding the relationship between alternative splicing regulation and heart failure.
Limitations of the Study
In the present study, we showed that PRMT1 deficiency in cardiomyocytes causes aberrant alternative splicing in the heart. As it is currently not known whether the changes in alternative splicing are due to direct effects of PRMT1 deficiency or secondary consequences (e.g., an influence of DCM), we examined the effect of PRMT1 knockdown on alternative splicing of Eif4a2 in C2C12 cells. However, we were unable to accurately assess the contribution of PRMT1 to Eif4a2 splicing in undifferentiated or differentiated C2C12 cells (data not shown). This may be because knockdown of PRMT1 was inefficient for changing Eif4a2 alternative splicing, or PRMT1 regulates alternative splicing in a cell-type-dependent manner. Therefore, other experimental models are needed to investigate whether the changes in alternative splicing are due to the lack of PRMT1.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dr. Tsuyoshi Waku for technical advice. We also thank members of the Fukamizu Laboratory for helpful discussions and encouragement. This work was supported by the Uehara Memorial Foundation (to A.F.), Grant-in-Aid for Scientific Research (A) (to A.F., Grant No. 25252062), for Scientific Research on Innovative Areas (23116004 to A.F.), and for Scientific Research (C) (to J.-D.K., Grant No. 18K054298) from the Japan Society for the Promotion of Science (JSPS).
Author Contributions
Conceptualization, K.M. and A.F.; Methodology, K.M., J.I., and A.F.; Investigation, K.M., W.L., M.H., N.O., and K.N.; Formal Analysis, K.M., M.M., and J.-D.K.; Writing – Original Draft, K.M.; Writing – Review & Editing, K.M., M.H., J.I., and A.F.; Supervision, S.E. and A.F.; Funding Acquisition, A.F.
Declaration of Interests
The authors declare no competing interests.
Published: October 26, 2018
Footnotes
Supplemental Information includes Transparent Methods, three figures, and three tables and can be found with this article online at https://doi.org/10.1016/j.isci.2018.09.023.
Data and Software Availability
The accession number for the data reported in this study is GEO: GSE112938.
Supplemental Information
References
- Andresen C.A., Smedegaard S., Sylvestersen K.B., Svensson C., Iglesias-Gato D., Cazzamali G., Nielsen T.K., Nielsen M.L., Flores-Morales A. Protein interaction screening for the ankyrin repeats and suppressor of cytokine signaling (SOCS) box (ASB) family identify Asb11 as a novel endoplasmic reticulum resident ubiquitin ligase. J. Biol. Chem. 2014;289:2043–2054. doi: 10.1074/jbc.M113.534602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamber B.A., Beg A.A., Twyman R.E., Jorgensen E.M. The Caenorhabditis elegans unc-49 locus encodes multiple subunits of a heteromultimeric GABA receptor. J. Neurosci. 1999;19:5348–5359. doi: 10.1523/JNEUROSCI.19-13-05348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang M.L., Chen J. Roles of nebulin family members in the heart. Circ. J. 2015;79:2081–2087. doi: 10.1253/circj.CJ-15-0854. [DOI] [PubMed] [Google Scholar]
- Baralle F.E., Giudice J. Alternative splicing as a regulator of development and tissue identity. Nat. Rev. Mol. Cell Biol. 2017;18:437–451. doi: 10.1038/nrm.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beqqali A., Bollen I.A., Rasmussen T.B., van den Hoogenhof M.M., van Deutekom H.W., Schafer S., Haas J., Meder B., Sorensen K.E., van Oort R.J. A mutation in the glutamate-rich region of RNA-binding motif protein 20 causes dilated cardiomyopathy through missplicing of titin and impaired Frank-Starling mechanism. Cardiovasc. Res. 2016;112:452–463. doi: 10.1093/cvr/cvw192. [DOI] [PubMed] [Google Scholar]
- Berger D.S., Moyer M., Kliment G.M., van Lunteren E., Ladd A.N. Expression of a dominant negative CELF protein in vivo leads to altered muscle organization, fiber size, and subtype. PLoS One. 2011;6:e19274. doi: 10.1371/journal.pone.0019274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M., Robichaud N., Hulea L., Sonenberg N., Pelletier J., Topisirovic I. Targeting the translation machinery in cancer. Nat. Rev. Drug Discov. 2015;14:261–278. doi: 10.1038/nrd4505. [DOI] [PubMed] [Google Scholar]
- Blanc R.S., Vogel G., Li X., Yu Z., Li S., Richard S. Arginine methylation by PRMT1 regulates muscle stem cell fate. Mol. Cell. Biol. 2017;37 doi: 10.1128/MCB.00457-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland C.S., Wang E.T., Vu A., David M.P., Castle J.C., Johnson J.M., Burge C.B., Cooper T.A. Global regulation of alternative splicing during myogenic differentiation. Nucleic Acids Res. 2010;38:7651–7664. doi: 10.1093/nar/gkq614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulau P., Zakrzewicz D., Kitowska K., Leiper J., Gunther A., Grimminger F., Eickelberg O. Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L18–L24. doi: 10.1152/ajplung.00076.2006. [DOI] [PubMed] [Google Scholar]
- Burke M.A., Chang S., Wakimoto H., Gorham J.M., Conner D.A., Christodoulou D.C., Parfenov M.G., DePalma S.R., Eminaga S., Konno T. Molecular profiling of dilated cardiomyopathy that progresses to heart failure. JCI Insight. 2016;1:e86898. doi: 10.1172/jci.insight.86898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Yi B., Sun J. Inhibition of cardiomyocyte hypertrophy by protein arginine methyltransferase 5. J. Biol. Chem. 2014;289:24325–24335. doi: 10.1074/jbc.M114.577494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Niroomand F., Liu Z., Zankl A., Katus H.A., Jahn L., Tiefenbacher C.P. Expression of nitric oxide related enzymes in coronary heart disease. Basic Res. Cardiol. 2006;101:346–353. doi: 10.1007/s00395-006-0592-5. [DOI] [PubMed] [Google Scholar]
- Choi D., Oh K.J., Han H.S., Yoon Y.S., Jung C.Y., Kim S.T., Koo S.H. Protein arginine methyltransferase 1 regulates hepatic glucose production in a FoxO1-dependent manner. Hepatology. 2012;56:1546–1556. doi: 10.1002/hep.25809. [DOI] [PubMed] [Google Scholar]
- Cianci C.D., Zhang Z., Pradhan D., Morrow J.S. Brain and muscle express a unique alternative transcript of alphaII spectrin. Biochemistry. 1999;38:15721–15730. doi: 10.1021/bi991458k. [DOI] [PubMed] [Google Scholar]
- Danielsen J.M., Sylvestersen K.B., Bekker-Jensen S., Szklarczyk D., Poulsen J.W., Horn H., Jensen L.J., Mailand N., Nielsen M.L. Mass spectrometric analysis of lysine ubiquitylation reveals promiscuity at site level. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.003590. M110.003590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D.M., Choi J., El-Ghazali A., Park S.Y., Roos K.P., Jordan M.C., Fishbein M.C., Comai L., Reddy S. Loss of muscleblind-like 1 results in cardiac pathology and persistence of embryonic splice isoforms. Sci. Rep. 2015;5:9042. doi: 10.1038/srep09042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnadieu E., Jouvin M.H., Rana S., Moffatt M.F., Mockford E.H., Cookson W.O., Kinet J.P. Competing functions encoded in the allergy-associated F(c)epsilonRIbeta gene. Immunity. 2003;18:665–674. doi: 10.1016/s1074-7613(03)00115-8. [DOI] [PubMed] [Google Scholar]
- Ehler E., Horowits R., Zuppinger C., Price R.L., Perriard E., Leu M., Caroni P., Sussman M., Eppenberger H.M., Perriard J.C. Alterations at the intercalated disk associated with the absence of muscle LIM protein. Exp. Cell Res. 2001;153:763–772. doi: 10.1083/jcb.153.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R., Gunawan F., Beisaw A., Jimenez-Amilburu V., Maischein H.M., Kostin S., Kawakami K., Stainier D.Y. Proteolysis regulates cardiomyocyte maturation and tissue integration. Nat. Commun. 2017;8:14495. doi: 10.1038/ncomms14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galicia-Vazquez G., Di Marco S., Lian X.J., Ma J.F., Gallouzi I.E., Pelletier J. Regulation of eukaryotic initiation factor 4AII by MyoD during murine myogenic cell differentiation. PLoS One. 2014;9:e87237. doi: 10.1371/journal.pone.0087237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Cooper T.A. Reexpression of pyruvate kinase M2 in type 1 myofibers correlates with altered glucose metabolism in myotonic dystrophy. Proc. Natl. Acad. Sci. U S A. 2013;110:13570–13575. doi: 10.1073/pnas.1308806110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan V., Guo A., Trudgian D., Thomas B., Acuto O. Comprehensive identification of arginine methylation in primary T cells reveals regulatory roles in cell signalling. Nat. Commun. 2015;6:6758. doi: 10.1038/ncomms7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice J., Xia Z., Wang E.T., Scavuzzo M.A., Ward A.J., Kalsotra A., Wang W., Wehrens X.H., Burge C.B., Li W. Alternative splicing regulates vesicular trafficking genes in cardiomyocytes during postnatal heart development. Nat. Commun. 2014;5:3603. doi: 10.1038/ncomms4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelet S., Link L.A., Howley B., Obellianne C., Palanisamy V., Gangaraju V.K., Diehl J.A., Howe P.H. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat. Cell Biol. 2017;19:1105–1115. doi: 10.1038/ncb3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A., Gu H., Zhou J., Mulhern D., Wang Y., Lee K.A., Yang V., Aguiar M., Kornhauser J., Jia X. Immunoaffinity enrichment and mass spectrometry analysis of protein methylation. Mol. Cell. Proteomics. 2014;13:372–387. doi: 10.1074/mcp.O113.027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Schafer S., Greaser M.L., Radke M.H., Liss M., Govindarajan T., Maatz H., Schulz H., Li S., Parrish A.M. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat. Med. 2012;18:766–773. doi: 10.1038/nm.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikyriacou A., Yang Y., Espejo A., Bedford M.T., Clarke S.G. Unique features of human protein arginine Methyltransferase 9 (PRMT9) and its substrate RNA splicing factor SF3B2. J. Biol. Chem. 2015;290:16723–16743. doi: 10.1074/jbc.M115.659433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghikia A., Missol-Kolka E., Tsikas D., Venturini L., Brundiers S., Castoldi M., Muckenthaler M.U., Eder M., Stapel B., Thum T. Signal transducer and activator of transcription 3-mediated regulation of miR-199a-5p links cardiomyocyte and endothelial cell function in the heart: a key role for ubiquitin-conjugating enzymes. Eur. Heart J. 2011;32:1287–1297. doi: 10.1093/eurheartj/ehq369. [DOI] [PubMed] [Google Scholar]
- Hashimoto M., Murata K., Ishida J., Kanou A., Kasuya Y., Fukamizu A. Severe hypomyelination and developmental defects are caused in mice lacking protein arginine Methyltransferase 1 (PRMT1) in the central nervous system. J. Biol. Chem. 2016;291:2237–2245. doi: 10.1074/jbc.M115.684514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka Y., Tsusaka T., Shimizu N., Morita K., Suzuki T., Machida S., Satoh M., Honda A., Hirose M., Kamimura S. Histone H3 methylated at arginine 17 is essential for reprogramming the paternal genome in zygotes. Cell Rep. 2017;20:2756–2765. doi: 10.1016/j.celrep.2017.08.088. [DOI] [PubMed] [Google Scholar]
- Herman D.S., Lam L., Taylor M.R., Wang L., Teekakirikul P., Christodoulou D., Conner L., DePalma S.R., McDonough B., Sparks E. Truncations of titin causing dilated cardiomyopathy. N. Engl. J. Med. 2012;366:619–628. doi: 10.1056/NEJMoa1110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann F., Bossert M., Schwander A., Akgun E., Fackelmayer F.O. Arginine methylation of scaffold attachment factor A by heterogeneous nuclear ribonucleoprotein particle-associated PRMT1. J. Biol. Chem. 2004;279:48774–48779. doi: 10.1074/jbc.M407332200. [DOI] [PubMed] [Google Scholar]
- Holbrook J.A., Neu-Yilik G., Hentze M.W., Kulozik A.E. Nonsense-mediated decay approaches the clinic. Nat. Genet. 2004;36:801–808. doi: 10.1038/ng1403. [DOI] [PubMed] [Google Scholar]
- Ishimaru T., Ishida J., Kim J.D., Mizukami H., Hara K., Hashimoto M., Yagami K.I., Sugiyama F., Fukamizu A. Angiodysplasia in embryo lacking protein arginine methyltransferase 1 in vascular endothelial cells. J. Biochem. 2017;161:255–258. doi: 10.1093/jb/mvw095. [DOI] [PubMed] [Google Scholar]
- Israeli-Rosenberg S., Manso A.M., Okada H., Ross R.S. Integrins and integrin-associated proteins in the cardiac myocyte. Circ. Res. 2014;114:572–586. doi: 10.1161/CIRCRESAHA.114.301275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongjitwimol J., Baldock R.A., Morley S.J., Watts F.Z. Sumoylation of eIF4A2 affects stress granule formation. J. Cell Sci. 2016;129:2407–2415. doi: 10.1242/jcs.184614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsotra A., Xiao X., Ward A.J., Castle J.C., Johnson J.M., Burge C.B., Cooper T.A. A postnatal switch of CELF and MBNL proteins reprograms alternative splicing in the developing heart. Proc. Natl. Acad. Sci. U S A. 2008;105:20333–20338. doi: 10.1073/pnas.0809045105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Jeong M.H., Kim K.R., Jung C.Y., Lee S.Y., Kim H., Koh J., Vuong T.A., Jung S., Yang H. Protein arginine methylation facilitates KCNQ channel-PIP2 interaction leading to seizure suppression. Elife. 2016;5:e17159. doi: 10.7554/eLife.17159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Bennett E.J., Huttlin E.L., Guo A., Li J., Possemato A., Sowa M.E., Rad R., Rush J., Comb M.J. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S.C., Sylvestersen K.B., Mund A., Lyon D., Mullari M., Madsen M.V., Daniel J.A., Jensen L.J., Nielsen M.L. Proteome-wide analysis of arginine monomethylation reveals widespread occurrence in human cells. Sci. Signal. 2016;9:rs9. doi: 10.1126/scisignal.aaf7329. [DOI] [PubMed] [Google Scholar]
- Li C., Yu L., Xue H., Yang Z., Yin Y., Zhang B., Chen M., Ma H. Nuclear AMPK regulated CARM1 stabilization impacts autophagy in aged heart. Biochem. Biophys. Res. Commun. 2017;486:398–405. doi: 10.1016/j.bbrc.2017.03.053. [DOI] [PubMed] [Google Scholar]
- Li J., Mahajan A., Tsai M.D. Ankyrin repeat: a unique motif mediating protein-protein interactions. Biochemistry. 2006;45:15168–15178. doi: 10.1021/bi062188q. [DOI] [PubMed] [Google Scholar]
- Li S., Guo W., Dewey C.N., Greaser M.L. Rbm20 regulates titin alternative splicing as a splicing repressor. Nucleic Acids Res. 2013;41:2659–2672. doi: 10.1093/nar/gks1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang X., Guo Y., Deng N., Zheng P., Xu Q., Wu Y., Dai G. Regulation of endothelial nitric oxide synthase and asymmetric dimethylarginine by matrine attenuates isoproterenol-induced acute myocardial injury in rats. J. Pharm. Pharmacol. 2012;64:1107–1118. doi: 10.1111/j.2042-7158.2012.01502.x. [DOI] [PubMed] [Google Scholar]
- Luo G., Herrera A.H., Horowits R. Molecular interactions of N-RAP, a nebulin-related protein of striated muscle myotendon junctions and intercalated disks. Biochemistry. 1999;38:6135–6143. doi: 10.1021/bi982395t. [DOI] [PubMed] [Google Scholar]
- Maatz H., Jens M., Liss M., Schafer S., Heinig M., Kirchner M., Adami E., Rintisch C., Dauksaite V., Radke M.H. RNA-binding protein RBM20 represses splicing to orchestrate cardiac pre-mRNA processing. J. Clin. Invest. 2014;124:3419–3430. doi: 10.1172/JCI74523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manso A.M., Li R., Monkley S.J., Cruz N.M., Ong S., Lao D.H., Koshman Y.E., Gu Y., Peterson K.L., Chen J. Talin1 has unique expression versus talin 2 in the heart and modifies the hypertrophic response to pressure overload. J. Biol. Chem. 2013;288:4252–4264. doi: 10.1074/jbc.M112.427484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manso A.M., Okada H., Sakamoto F.M., Moreno E., Monkley S.J., Li R., Critchley D.R., Ross R.S. Loss of mouse cardiomyocyte talin-1 and talin-2 leads to beta-1 integrin reduction, costameric instability, and dilated cardiomyopathy. Proc. Natl. Acad. Sci. U S A. 2017;114:E6250–E6259. doi: 10.1073/pnas.1701416114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer H.A., Kong Y.W., Lu W.T., Wilczynska A., Spriggs R.V., Robinson S.W., Godfrey J.D., Willis A.E., Bushell M. Translational repression and eIF4A2 activity are critical for microRNA-mediated gene regulation. Science. 2013;340:82–85. doi: 10.1126/science.1231197. [DOI] [PubMed] [Google Scholar]
- Mohiddin S.A., Lu S., Cardoso J.P., Carroll S., Jha S., Horowits R., Fananapazir L. Genomic organization, alternative splicing, and expression of human and mouse N-RAP, a nebulin-related LIM protein of striated muscle. Cell Motil. Cytoskeleton. 2003;55:200–212. doi: 10.1002/cm.10123. [DOI] [PubMed] [Google Scholar]
- Nichols R.C., Wang X.W., Tang J., Hamilton B.J., High F.A., Herschman H.R., Rigby W.F. The RGG domain in hnRNP A2 affects subcellular localization. Exp. Cell Res. 2000;256:522–532. doi: 10.1006/excr.2000.4827. [DOI] [PubMed] [Google Scholar]
- Okumura F., Matsuzaki M., Nakatsukasa K., Kamura T. The role of elongin BC-containing ubiquitin ligases. Front. Oncol. 2012;2:10. doi: 10.3389/fonc.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause A., Sonenberg N. Mutational analysis of a DEAD box RNA helicase: the mammalian translation initiation factor eIF-4A. EMBO J. 1992;11:2643–2654. doi: 10.1002/j.1460-2075.1992.tb05330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak M.R., Scherer C.A., Chen J., Roshon M.J., Ruley H.E. Arginine N-Methyltransferase 1 is required for early postimplantation mouse development, but cells deficient in the enzyme are viable. Mol. Cell. Biol. 2000;20:4859–4869. doi: 10.1128/mcb.20.13.4859-4869.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer S., de Marvao A., Adami E., Fiedler L.R., Ng B., Khin E., Rackham O.J., van Heesch S., Pua C.J., Kui M. Titin-truncating variants affect heart function in disease cohorts and the general population. Nat. Genet. 2017;49:46–53. doi: 10.1038/ng.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutz P., Bumann M., Oberholzer A.E., Bieniossek C., Trachsel H., Altmann M., Baumann U. Crystal structure of the yeast eIF4A-eIF4G complex: an RNA-helicase controlled by protein-protein interactions. Proc. Natl. Acad. Sci. U S A. 2008;105:9564–9569. doi: 10.1073/pnas.0800418105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C., Yang B., Wu T., Huang J., Tang P., Zhou Y., Zhou J., Qiu J., Jiang L., Li H. Mechanisms for U2AF to define 3' splice sites and regulate alternative splicing in the human genome. Nat. Struct. Mol. Biol. 2014;21:997–1005. doi: 10.1038/nsmb.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Luo L., Eash J., Ibebunjo C., Glass D.J. The SCF-Fbxo40 complex induces IRS1 ubiquitination in skeletal muscle, limiting IGF1 signaling. Dev. Cell. 2011;21:835–847. doi: 10.1016/j.devcel.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Singh R.K., Xia Z., Bland C.S., Kalsotra A., Scavuzzo M.A., Curk T., Ule J., Li W., Cooper T.A. Rbfox2-coordinated alternative splicing of Mef2d and Rock2 controls myoblast fusion during myogenesis. Mol. Cell. 2014;55:592–603. doi: 10.1016/j.molcel.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R., Allemand E., Zhang Z., Karni R., Myers M.P., Krainer A.R. Arginine methylation controls the subcellular localization and functions of the oncoprotein splicing factor SF2/ASF. Mol. Cell. Biol. 2010;30:2762–2774. doi: 10.1128/MCB.01270-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes W.C., Rosen B., West A.P., Koutsourakis M., Bushell W., Iyer V., Mujica A.O., Thomas M., Harrow J., Cox T. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Manley J.L. Alternative cleavage and polyadenylation: the long and short of it. Trends Biochem. Sci. 2013;38:312–320. doi: 10.1016/j.tibs.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Huang Z.Q., Xia L., Feng Q., Erdjument-Bromage H., Strahl B.D., Briggs S.D., Allis C.D., Wong J., Tempst P. Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science. 2001;293:853–857. doi: 10.1126/science.1060781. [DOI] [PubMed] [Google Scholar]
- Wei C., Qiu J., Zhou Y., Xue Y., Hu J., Ouyang K., Banerjee I., Zhang C., Chen B., Li H. Repression of the central splicing regulator RBFox2 is functionally linked to pressure overload-induced heart failure. Cell Rep. 2015;10:1521–1533. doi: 10.1016/j.celrep.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells Q.S., Becker J.R., Su Y.R., Mosley J.D., Weeke P., D'Aoust L., Ausborn N.L., Ramirez A.H., Pfotenhauer J.P., Naftilan A.J. Whole exome sequencing identifies a causal RBM20 mutation in a large pedigree with familial dilated cardiomyopathy. Circ. Cardiovasc. Genet. 2013;6:317–326. doi: 10.1161/CIRCGENETICS.113.000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Yang D., Ding J.H., Wang W., Chu P.H., Dalton N.D., Wang H.Y., Bermingham J.R., Jr., Ye Z., Liu F. ASF/SF2-regulated CaMKIIdelta alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell. 2005;120:59–72. doi: 10.1016/j.cell.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Yamagata K., Daitoku H., Takahashi Y., Namiki K., Hisatake K., Kako K., Mukai H., Kasuya Y., Fukamizu A. Arginine methylation of FOXO transcription factors inhibits their phosphorylation by Akt. Mol. Cell. 2008;32:221–231. doi: 10.1016/j.molcel.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Yang Y., Bedford M.T. Protein arginine methyltransferases and cancer. Nat. Rev. Cancer. 2013;13:37–50. doi: 10.1038/nrc3409. [DOI] [PubMed] [Google Scholar]
- Yang Y., Hadjikyriacou A., Xia Z., Gayatri S., Kim D., Zurita-Lopez C., Kelly R., Guo A., Li W., Clarke S.G. PRMT9 is a type II methyltransferase that methylates the splicing factor SAP145. Nat. Commun. 2015;6:6428. doi: 10.1038/ncomms7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Beetz N., O'Keeffe S., Tapia J.C., Macpherson L., Chen W.V., Bassel-Duby R., Olson E.N., Maniatis T. hnRNP U protein is required for normal pre-mRNA splicing and postnatal heart development and function. Proc. Natl. Acad. Sci. U S A. 2015;112:E3020–E3029. doi: 10.1073/pnas.1508461112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Zhang Y., Xu J., Zhang Q., Zhu D. FBXO40, a gene encoding a novel muscle-specific F-box protein, is upregulated in denervation-related muscle atrophy. Gene. 2007;404:53–60. doi: 10.1016/j.gene.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Zhang L., Tran N.T., Su H., Wang R., Lu Y., Tang H., Aoyagi S., Guo A., Khodadadi-Jamayran A., Zhou D. Cross-talk between PRMT1-mediated methylation and ubiquitylation on RBM15 controls RNA splicing. Elife. 2015;4:e07938. doi: 10.7554/eLife.07938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita-Lopez C.I., Sandberg T., Kelly R., Clarke S.G. Human protein arginine methyltransferase 7 (PRMT7) is a type III enzyme forming omega-NG-monomethylated arginine residues. J. Biol. Chem. 2012;287:7859–7870. doi: 10.1074/jbc.M111.336271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.