Abstract
Radiation-induced pulmonary fibrosis (RIPF) is a common complication in patients with lung cancer and breast cancer after receiving thoracic radiotherapy. The average incidence of RIPF is 16%-28% after radiotherapy. RIPF includes a heterogeneous group of lung disorders characterized by progressive and irreversible destruction of lung architecture and disruption of gas exchange. The clinical signs of RIPF include increasing dyspnea, deteriorating lung function, and accumulation of interstitial fluid, eventually leading to respiratory failure. No medical therapy for RIPF has been approved for routine clinical use despite the apparent need for an effective treatment. Numerous signaling pathways are involved in the initiation and progression of RIPF. Also, various approaches for RIPF treatments have focused on several aspects of the current understanding of the molecular pathology of RIPF. This review used the mechanistic categories of associated cell signaling pathways, epithelial cell dysfunction and senescence, abnormal lung remodeling, and aberrant innate and adaptive immunity to review the published literature on RIPF to date and then to identify potential areas for the effective treatment of RIPF.
Introduction
Lung cancer is the leading cause of cancer death all over the world. Its overall 5-year survival rate is only approximately 15%. Although the best outcomes are achieved with surgery, more than 64.3%±4.7% of patients with non–small cell lung cancer require radiation therapy at least once because of medical comorbidities or extensiveness of the disease [1]. Irrespective of the extensive use of stereotactic radiotherapy, which limits the exposure of normal lung tissue to irradiation, as many as 35% of patients with lung cancer and breast cancer receiving thoracic radiotherapy develop radiation pneumonitis and are at strong risk of developing radiation-induced pulmonary fibrosis (RIPF) months and years after initial radiotherapy [2], [3], [4]. According to old radiation therapy oncology group data, the average incidence of RIPF was 28% after two-dimensional radiotherapy. Cella et al. reported an overall RIPF incidence of 16% (8% symptomatic and 8% asymptomatic) in 115 patients with Hodgkin's lymphoma who received three-dimensional radiotherapy [5]. RIPF includes a heterogeneous group of lung disorders characterized by progressive and irreversible destruction of lung architecture and disruption of gas exchange [6]. The clinical signs of RIPF include increasing dyspnea, deteriorating lung function, and accumulation of interstitial fluid, eventually leading to respiratory failure. While steroids and other forms of anti-inflammatory therapy have been established to control acute pulmonary inflammation, no medical therapy for RIPF has been approved for routine clinical use despite the apparent need for an effective treatment [7].
Prevailing hypotheses suggest that RIPF is an epithelial-fibroblastic disorder. Ionizing radiation injures pulmonary epithelial and endothelial cells and causes the release of proinflammatory cytokines that recruit macrophages and lymphocytes to the sites of injury [6]. Activated myofibroblasts play a central role during the pathogenesis of pulmonary fibrosis by synthesizing and depositing extracellular matrix (ECM) proteins. Myofibroblasts are likely derived from various cells, including 1) resident stromal fibroblasts, 2) bone marrow–derived “fibrocytes,” and 3) alveolar type II epithelial cells, a subset of which undergoes epithelial-to-mesenchymal transition (EMT) [8]. During EMT, epithelial cells lose apical-basal polarity, basement membrane attachment, and cell-cell contact. They gain mesenchymal characteristics associated with increased migratory behavior, cytoskeletal rearrangements, and their migration into the lung interstitium where they produce excess ECM. During the normal healing process, alveolar-capillary permeability is restored and inflammation is resolved. Radiation to the thorax causes pulmonary fibrosis as lung injury, inflammation, and remodeling persist [9]. Numerous signaling pathways are involved in the initiation and progression of RIPF. Also, various approaches for RIPF treatments have focused on several aspects of the current understanding of the molecular pathology of RIPF.
This study used the mechanistic categories of associated cell signaling pathway, epithelial cell dysfunction and senescence, abnormal lung remodeling, and aberrant innate and adaptive immunity to review the published literature in RIPF to date and then to identify potential areas for the effective treatment of RIPF.
Associated Cell Signaling Pathway
Latency-associated peptide (LAP) interacts with proteins of the latent transforming growth factor-β (TGF-β) binding protein family, which anchor latent TGF-β to the extracellular matrix [10]. LAP and TGF-β are secreted as a complex in which TGF-β is latent. The release of TGF-β from LAP is a highly regulated step in TGF-β signaling [11]. TGF-β is a multifunctional regulator of cell growth, EMT, and differentiation in response to injuries [12], [13], [14]. The expression of TGF-β induced by radiation has been reported as part of RIPF [15], [16], [17]. Once the ligand/receptor complex of TGF-β is formed, intracellular effector molecules are phosphorylated by the receptor to induce numerous intracellular pathways [18]. Hypoxia-inducible factor -1α (HIF-1α) can affect the irradiation-induced endothelial-to-mesenchymal transition (EndMT) via the TGFβ-R1/Smad3 signaling pathway [19]. HIF-1α siRNA inhibits radiation-induced EndMT accompanied by a decrease in TGFβ-R1/Smad3 signaling [20], [21], [22]. Serine palmitoyltransferase (SPT) catalyzes the first step in the chain of reactions leading to the formation of complex sphingolipids, determines the rate of de novo sphingolipid biosynthesis [23], and provides increased sphingoid base supply affecting dihydrosphingosine-dihydroceramide levels. Targeting SPT decreases sphingosine-kinase-1 (SphK1) activity in the lung, reduces the levels of sphingosine-1-phosphate (S1P) and dihydro-sphingosine-1-phosphate (DHS1P) in the lung and circulation, and delays the onset of RIPF [24].
The platelet-derived growth factor (PDGF)-PDGFR system is involved in idiopathic pulmonary fibrosis (IPF) and asbestos, bleomycin, and RIPF [25], besides fibrosis in other organs such as the kidneys, liver, skin, and heart [26], [27]. During fibrosis, the PDGF-PDGFR system may be a promising target for treating fibrotic disease [28]. The role of TGF-β and PDGF signaling in the development of pulmonary fibrosis has been well established [29], [30]. Also, the upregulation of components of the TGF-β and PDGF signaling cascades was reported in lung tissues after thoracic RT [15], [31]. The development of radiation-induced fibrosis was attenuated by blocking either TGF-β or PDGF effects in animal models [31], [32].
Cannabinoid receptor-1 (CB1) is known to be expressed in the lung tissue, bronchial epithelial cells, and alveolar type II cells. Activation of CB1 may exert proinflammatory or pro-oxidant effects, further leading to RIPF [33], [34].
Ecto-5'-nucleotidase (CD73) acts in concert with ectonucleoside triphosphate diphosphohydrolase 1 (CD39) to generate adenosine from extracellularly released ATP/ADP. Extracellular adenosine acts through four different G protein–coupled adenosine receptors that are widely expressed and have various biological functions aimed at maintaining or restoring tissue homeostasis. Both CD73 and adenosine are critical in balancing tissue inflammation and repair processes in pulmonary fibrosis; regulating leukocyte extravasation and function; and modulating epithelial cell behavior, vascular function, and cell death [35].
Epithelial Cell Dysfunction and Senescence
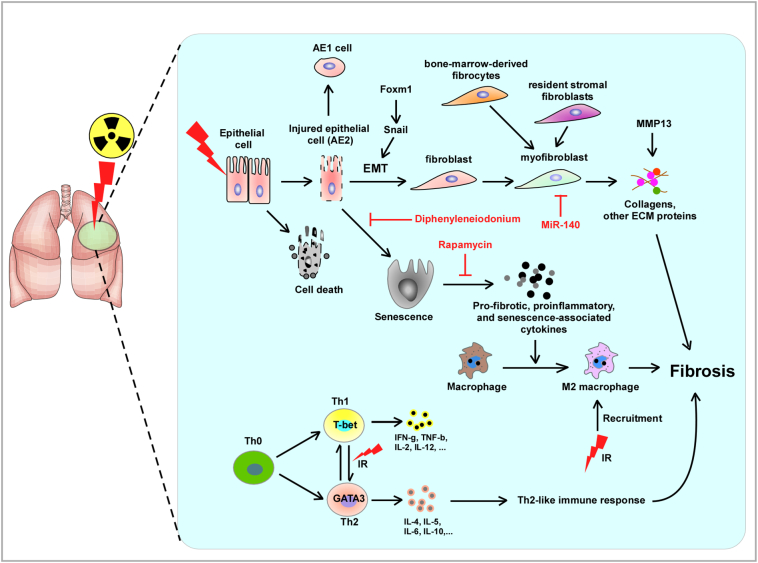
Airway epithelial cell II (AEC II) can function as the alveolar stem cell, proliferating in response to injury and differentiating into both AEC I and AEC II [36]. AEC II depletion is hypothesized to be a major contributor to ineffective alveolar repair [37], [38], leading to epithelial stress and fibroproliferation [39]. Extensive AEC II loss stimulates macrophage influx and proinflammatory cytokine elaboration, resulting in fibrosis [38], [40]. The severity of AEC II depletion correlates with the degree of senescence [41]. Senescent cells secrete proinflammatory cytokines, such as interleukin 6, TGF-β, and interleukin 1-α, which are implicated in RIPF [42], [43]. A time- and dose-dependent increase of AEC II senescence and pneumocyte depletion after exposure to fibrosis-inducing doses of irradiation was reported [41]. AEC II senescence is involved in RIPF and may serve as a novel target for intervention (Figure 2).
Figure 2.
Epithelial cell injury, abnormal lung remodeling, and related potential therapeutic areas. Diphenyleneiodonium (DPI), an inhibitor of NADPH oxidase (NOX), prevents AECII senescence and markedly reduces RIPF. Rapamycin inhibits radiation-induced signaling downstream of mTOR; reduces expression of profibrotic, proinflammatory, and senescence-associated cytokines in irradiated lungs; and prevents RIPF. Forkhead box M1 (Foxm1) can decrease the expression of Snail1 mRNA and protein and attenuate RIPF by repressing EMT. MiR-140 inhibits myofibroblast differentiation and inflammation and prevents RIPF. MMP13 is relevant with ECM deposition and remodeling of lung architecture. MMP13 knockouts dramatically reduce lung density and shrinkage of lung volume and attenuate RIPF. GATA-3 develops type 2 phenotype and produces type 2 cytokines while inhibiting Th1 cells. Inhibition of GATA-3 attenuates RIPF.
Superoxide dismutase (SOD) belongs to a family of metalloprotein enzymes important in protection from oxidative damage. Several SOD gene therapy studies in animals have suggested the protective effect of SOD against radiation toxicity in the lung [44]. Extracellular SOD (EC-SOD) is the predominant antioxidant enzyme; in the lung, it is primarily located to type II pneumocytes and macrophages. The overexpression of EC-SOD in transgenic mice confers protection against RIPF, with a corresponding decrease in oxidative stress [45].
Abnormal Lung Remodeling
The numbers of cells undergoing EMT during pulmonary fibrosis were estimated to be between 5% and 20% depending on the mouse model or human samples [46], [47], [48]. EMT is controlled by a network of signaling and transcriptional events mediated in part by TGF-β signaling. Snail and Twist family members of transcription factors are also important regulators of EMT, repressing E-cadherin and activating the mesenchymal transcriptomes [49]. Snail1 can induce EMT and the expression of EMT-associated genes, and may act as a switch to promote EMT program in epithelial cells [50]. EMT is also controlled by the Wnt/β-catenin pathway. Further, aberrant activation of β-catenin has been demonstrated in idiopathic pulmonary fibrosis [50], [51], [52], [53]. Regulatory T cells (Tregs) contribute to RIPF though promotion of EMT and β-catenin accumulation in the alveolar epithelium. They also accelerate EMT partly through β-catenin [54].
Myofibroblasts are likely derived from bone marrow (BM)–derived fibroblast progenitor cells that are crucial in the fibrotic process [55] (Figure 2). The BM-derived fibrocytes express a chemokine receptor CXCR4. Activation of the CXCR4 by its ligand stromal cell–derived factor 1 (SDF-1/CXCL12) may be important in the development of RIPF. Therefore, the CXCR4/CXCL12 axis is critical in recruiting BM-derived precursors that differentiate into the fibroblasts that cause RIPF.
Matrix metalloproteinases (MMPs) constitute a family of extracellular zinc- and calcium-dependent proteases that degrade ECM and other extracellular proteins, the extensive remodeling of which can result in fibrogenesis [56] (Figure 2). MMP13 is a highly specific interstitial collagenase in rodents capable of degrading insolubale fibrillar collagens [56]. MMP13 is also a key activator of a whole cascade of proinflammatory reactions in the lungs. The presence of MMP13 may cause an increase of ECM in mouse lungs [57].
Intercellular adhesion molecule 1 (ICAM-1) and E-selection are two cell adhesion proteins located on endothelial cells of the microvasculature. Leukocytes subsequently adhere to the endothelial cells via these adhesion molecules. ICAM-1−/− mice showed no inflammatory cell infiltration of their lungs after thoracic irradiation [58]. Also, irradiated ICAM-1−/− mice had lower levels of hydroxyproline in their lungs and improved pulmonary compliance than did irradiated ICAM+/+ mice. The improvement in pulmonary compliance accounted for the attenuation of RIPF.
Matricellular protein connective tissue growth factor (CTGF) not only is an essential mediator for the fibrotic activity of TGF-β but also can act independently of TGF-β. It can modulate the formation of myofibroblasts by regulating the transdifferentiation of fibroblasts or epithelial cells or by enabling edema leading to the deposition of provisional matrix on which the epithelial cells undergo EMT. CTGF stimulates myofibroblasts to express chemokines and cytokines that recruit leukocytes and regulate their activity and to deposit and remodel ECM, leading to changes in organ structure and function [59].
Aberrant Innate and Adaptive Immunity
Thorax irradiation triggered the recruitment of various immune cells into the lung. Preclinical and clinical studies have shown that activated T lymphocytes constitute a significant part of immune cells infiltrating the lung tissue on thorax irradiation; they are also an important cellular component involved in the control of RIPF. Naive CD4+ T-helper (Th0) cells can be differentiated into at least two functional subsets during the immune response: Th1 cells (type 1), which secrete Th1 cytokines such as INF-γ, TNF-β, IL-2, and IL-12, and Th2 cells (type 2), which secrete Th2 cytokines such as IL-4, IL-5, IL-6, IL-10, and IL-13 [60]. The differentiation of Th0 cells into Th1 or Th2 cells is regulated by the following transcription factors: T-box expressed in T cells (T-bet) and GATA-binding protein-3 (GATA-3). The expression of Th2-related cytokines increased and that of Th1-related cytokines decreased after radiation exposure. Then, alveolar macrophage accumulation increased in the irradiated tissue, and TGF-β was expressed at a higher level. In TGF-β-Smad–dependent pathways, the expression of TGF-β1, TβR, and phosphor-Smad 2/3 was upregulated and the expression of Smad 7 was downregulated, leading to the development of RIPF [61]. Han et al. reported that Th2-like immune response was involved in RIPF, and GATA-3 had an important role in promoting RIPF [62]. Irradiation can induce lung fibrosis in tumor-bearing animals more easily than in healthy mice. This may be because tumors deliver type 2 cytokines or other profibrogenic signals into normal lung tissue via the circulation, eventually leading to RIPF [63]. The signaling of Toll-like receptors (TLRs) can influence innate and adaptive immune responses [64], [65]. The activation of TLR-9 in part regulates the type 1/type 2 immune environment in irradiated lung tissue by enhancing type 1 immunity and alleviates RIPF [66]. The activation of TLR-5 has also been reported to be quite effective in protecting cells, mice, and nonhuman primates against radiation-induced lethality [67], [68].
The bronchoalveolar lavage of patients after radiotherapy contained increased numbers of mast cells and neutrophils [69]. Animal models of RIPF also showed an increase in the levels of tissue mast cells and lavage neutrophils [70]. Inflammatory monocytes, which are derived from the bone marrow and express CCR2, were shown to migrate into the lung after radiation exposure. CCR2+ infiltrating monocyte-derived macrophages played a critical role in the development of RIPF [71].
Potential Areas for Effective Treatment of RIPF
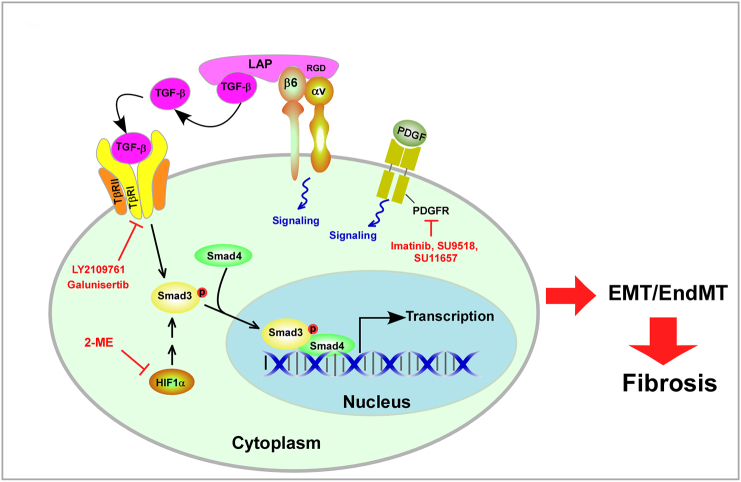
Integrin ɑvβ6 is a major TGF-β activator in the lung, which interacts with the amino acid sequence arginine-glycine-aspartic acid (RGD) located near the C terminus of LAP, a peptide derived from the N-terminal region of the TGF-β gene product that is part of the TGF-β-LAP complex [72] (Figure 1). The ɑvβ6-mediated TGF-β activation was a major contributor to fibrosis [73]. Also, low-level doses of ɑvβ6 inhibition prevented fibrosis without altering bronchoalveolar lavage (BAL) cell counts or the levels of selected inflammatory mediators. Direct inhibition of ɑvβ6 and prevention of its upregulation are potentially useful strategies to treat or prevent RIPF. LY2109761 is a novel small-molecule TGF-β receptor I serine/threonine kinase inhibitor (Figure 1). It not only attenuated profibrotic signaling directly but also balanced the complex TGF-β/bone morphogenetic proteins (TGF-β/BMP) signaling [32]. Moreover, it reduced the radiation-induced expression of downstream signaling genes of TGF-β, including Smads 6, 7, and 9 and MDM2. Galunisertib is a highly selective inhibitor of TGFβR1 that has been demonstrated to completely inhibit the phosphorylation and activation of several downstream targets of the receptor [74] (Figure 1). 2-Methoxyestradiol (2-ME), a metabolite of 17-beta-estradiol, has been suggested to effectively inhibit the action of HIF-1α (Figure 1). 2-ME inhibited the radiation-induced increase in the expression of HIF-1α in vivo, leading to a decrease in EndMT and concomitant deposition of vascular collagen during the development of RIPF. 2-ME also reduced EMT and the fibrotic phase [19]. Hence, 2-ME may be used to prevent RIPF. Myriocin is a specific inhibitor of SPT. It can block the de novo biosynthesis of sphingolipids. Targeting SPT with myriocin can counteract TGF-β–induced signaling, defer radiation-induced SphK1 activation and RIPF progression, and serve as a novel therapeutic target for managing RIPF. Syndecan-2 attenuated pulmonary fibrosis in mice exposed to radiation and inhibited TGF-β1–induced fibroblast-myofibroblast differentiation, migration, and proliferation by downregulating P13K/Akt/ROCK signaling and blocking the binding of serum response factor to α-SMA promoter via CD148. It can be used as an antifibrotic therapy in RIPF [75]. PDGF receptor tyrosine kinase inhibitors (Imatinib/Gleevec, SU9518, and SU11657) markedly attenuated the development of fibroblast foci, the hallmark of pulmonary fibrosis, and the subsequent remodeling of the lung architecture (Figure 1). It is unlikely that single pathway inhibition can completely prevent lung fibrosis in humans, considering the intricate genetic networking [76], [77]. However, the PDGF receptor tyrosine kinase inhibitors are undergoing clinical trials and may have appropriate potency, selectivity, and safety profiles for treating RIPF. SU9518 is a highly selective inhibitor of PDGF receptors α and β. It has been shown to block PDGFR kinase activity and PDGFR-induced cellular proliferation [78], [79]. SU14816 has activity against c-Kit and PDGF receptors α and β (Figure 1). Animal studies demonstrated that the dual-pathway blockade showed additional benefits regarding the development of pulmonary fibrosis and mouse survival on using Galunisertib, SU9518, and SU14816 after thoracic irradiation compared with the inhibition of each pathway alone [80]. AM6545, a pharmacological inhibition of CB1, not only significantly attenuated RIPF but also prolonged the survival of animals. AM6545 can selectively affect CB1-mediated signaling in peripheral organs without affecting neurotransmission in the central nervous system because of its lower brain/plasma ratio. Pegylated adenosine deaminase (PEG-ADA) can be used to decrease tissue adenosine levels. PEG-ADA and targeting CD73 with monoclonal antibody TY/23 significantly reduced RIPF. Modulating adenosine may be effective in limiting RIPF [35]. More recently, molecular advances on radiation-induced fibrogenesis showed an essential role in epigenetic regulation. The bromodomain and extra-terminal inhibitor JQ1 attenuated radiological and histological presentations of RIPF [81].
Figure 1.
Associated cell signaling pathway and related potential therapeutic areas. Integrin avβ6 interacts with the amino acid sequence RGD, which is located near the C-terminus of LAP. TGF-β can be activated after release from LAP. The inhibition of avβ6-mediated TGF-β activation prevents RIPF. Both LY2109761, a small molecule TGF-β receptor 1 serine/threonine kinase inhibitor, and galunisertib, a highly selective inhibitor of TGFβR1, attenuate RIPF by inhibiting TGF-β–associated downstream targets. Further, 2-methoxyestradiol (2-ME) effectively inhibits the action of HIF-1α, reducing EndMT, EMT, and concomitant deposition of vascular collagen and eventually attenuating the development of RIPF. PDGF receptor tyrosine kinase inhibitors (imatinib, SU9518, and SU11657) markedly attenuated the development of fibroblast foci and subsequent remodeling of lung architecture.
NADPH oxidase (NOX) is a critical mediator of radiation-induced AEC II senescence and pulmonary fibrosis [41]. Diphenyleneiodonium (DPI), an inhibitor of NOX, was sufficient to prevent AEC II senescence and markedly reduced RIPF (Figure 2). The mTOR signaling pathway is known to play an essential role in aging and senescence. The signaling of mTORC 1 is rapamycin sensitive and thought to mediate the effects of the pathways on aging and senescence through p70S6 kinase (p70S6K) or S6 kinase 1 (S6K1) [82], [83]. Treatment with rapamycin resulted in inhibition of radiation-induced signaling downstream of mTOR; reduced the expression of profibrotic, proinflammatory, and senescence-associated cytokines in irradiated lungs; exhibited a marked reduction in macrophage accumulation, collagen content, and AEC II senescence; reduced RIPF; and significantly prolonged survival after lethal thoracic irradiation in C57BL/6NCr mice [84] (Figure 2). SOD protected normal lung tissue from radiation by decreasing oxidative stress, thereby attenuating RIPF with the same dose of radiation therapy. Amifostine, a synthetic sulfhydryl compound, plummeted the accumulation of reactive oxygen species (ROS) and macrophages and decreased the expression of TGF-β1 in irradiated lung tissues of rats. It is the only drug approved by the US Food and Drug Administration as a radioprotector [85]. Cytochrome P450 2E1 (CYP2E1) regulated the levels of endoplasmic reticulum stress and ROS in alveolar epithelial type II (AE2) cells and lung fibroblasts. Inhibition of CYP2E1 significantly attenuated EMT transition, apoptosis of AE2 cells, and myofibroblast formation [86].
Forkhead box M1 (FOXM1) is a member of the Forkhead family of transcription factors that share homology in the Winged Helix/Forkhead box DNA-binding domain. Conditional deletion of FOXM1 from alveolar type II cells and siRNA-mediated depletion of FOXM1 from cultured A459 cells can decrease the expression of Snail1 mRNA and protein and then attenuate RIPF by repressing EMT (Figure 2). As a subset of CD4+ T lymphocytes, Tregs express the surface marker CD25 and the transcription factor Foxhead box protein 3 (Foxp3). The anti-CD25–directed antibody is always used specifically to deplete Tregs [87]. The monoclonal anti-CD25 antibody treatment was found to partially attenuate RIPF probably through inhibiting the involvement of Tregs in immune response [54]. MSX-122 is a novel small molecule and partial CXCR4 antagonist. It was discovered as one of the most potent CXCR4 inhibitors with reasonable bioavailability and has been considered as a clinical drug candidate. It displays high-affinity binding to CXCR4 and inhibition of receptor function in the sub-nM range without metal-chelating capability [88]. MSX-122 can alleviate RIPF in a mouse model [89]. The possible use of MSX-122 for blocking RIPF among patients undergoing thoracic irradiation warrants further investigations. MMP13 knockouts dramatically reduced lung density and shrinkage of the lung volume, directly reducing lung fibrosis. MMP13 is more relevant in the later fibrogenesis phase with ECM deposition, remodeling of the architecture, and lung fibrosis than in the acute pneumonitis phase [57]. MMP13 can be a potential drug target to attenuate RIPF. Mice with a homozygous null mutation in the ICAM-1 gene had less pulmonary fibrosis and reduced thickening of the alveolar septum. Blocking ICAM-1 function or expression should be explored for its effects on the prevention of RIPF. FG-3019, a human monoclonal antibody to CTGF, reprogrammed fibrogenesis via normalization of the radiation-induced expression of genes involved in inflammation associated with M2 macrophage influx, EMT, myofibroblast activation, remodeling, and ECM deposition. FG-3019 reduced fibrotic disease activity by normalizing radiation-induced SMA and the expression of osteopontin protein and mRNA. Transient administration of FG-3019 to irradiated mice prevented and reversed lung remodeling, preserved lung function, and provided a survival benefit [59]. The loss of miR-140 is a marker of fibrotic lung tissue in vivo. MiR-140 knockout primary lung fibroblasts have a higher percentage of myofibroblasts, and miR-140 deficiency promotes the accumulation of M2 macrophages in irradiated lung tissues. MiR-140 serves a key protective molecule against RIPF through inhibiting myofibroblast differentiation and inflammation [90].
CpG-oligodexynucleotide (ODN), a nonmethylated, short, single-stranded synthetic DNA molecule, is a TLR ligand [91]. CpG-ODN treatment following irradiation significantly increased the expression of type 1 inflammatory cytokines (IL-12 and INF-γ) and decreased type 2 inflammatory cytokines (IL-13 and IL-5) and TGF-β. It also attenuated RIPF by reversing the type 2 immunosuppressive microenvironment in fibrotic renal tissue [92]. Meanwhile, CpG-ODN reduced the injury caused by ROS in mice after irradiation, thereby alleviating RIPF [93].
TLR activation requires a host of intracellular adaptor proteins proximal to TLR, with the most prominent being myeloid differentiation primary response factor 88 (MyD 88) [94]. MyD 88 contains two prominent domains, the Toll-like receptor/interleukin 1 receptor domain and a death domain [95]. MyD 88 is a key control element for innate immune signaling in response to pathogenic stimuli and mechanical and environmental stresses [96], [97], [98], [99]. MyD 88 supported survival from radiation-induced injury through regulating inflammatory factors that aided in recovery from irradiation [100]. The absence of MyD 88 resulted in unresolved pulmonary infiltrates, enhanced collagen deposition, and elevated levels of Th2 cytokines in long-term survivors of irradiation.
Natural resources may be a better choice for attenuating RIPF due to their multitargeted activity. Podophyllotoxin possesses the properties of cell-cycle arrest in the G2/M phase, regulation of DNA repair pathway, and cellular proliferation in both in vitro and in vivo model systems. Rutin demonstrates strong proton-donating and free radical–stabilizing properties. A formulation (G-003M) prepared by combining two molecules podophyllotoxin and rutin was reported to modulate immunity and inhibit RIPF by attenuating oxidative/nitrosative stress and downregulating the expression of inflammatory/fibrogenic cytokines [101].
Conclusions
No treatment method for RIPF has been approved for routine clinical use to date despite an urgent need to improve the treatment of RIPF. Many signal pathways and potential targets are involved in RIPF. They apparently attenuate fibrosis levels in RIPF models. Based on the findings, it would be plausible to presume that RIPF will be cured in the future.
However, parallel to the drug development, it would be also important to identify predictive biomarker that helps in selecting susceptible patients for administering a lower radiation dose and avoiding the occurrence of RIPF. Efforts should be made in the future to gain more knowledge on novel RIPF inhibitors so as to improve the treatment of patients with RIPF.
The authors declare no potential conflicts of interest.
Acknowledgements
This work was supported by grants from Tianjin Research Program of Application Foundation and Advantage Technology (15JCYBJC29100).
Contributor Information
Zhongjie Chen, Email: zchen01@tmu.edu.cn.
Wen Ning, Email: ningwen108@nankai.edu.cn.
References
- 1.Tyldesley S, Boyd C, Schulze K, Walker H, Mackillop WJ. Estimating the need for radiotherapy for lung cancer: an evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys. 2001;49:973–985. doi: 10.1016/s0360-3016(00)01401-2. [DOI] [PubMed] [Google Scholar]
- 2.Abid SH, Malhotra V, Perry MC. Radiation-induced and chemotherapy-induced pulmonary injury. Curr Opin Oncol. 2001;13:242–248. doi: 10.1097/00001622-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 3.TK Yu, Whitman GJ, Thames HD, Buzdar AU, Strom EA, Perkins GH, Schechter NR, McNeese MD, Kau SW, Thomas ES. Clinically relevant pneumonitis after sequential paclitaxel-based chemotherapy and radiotherapy in breast cancer patients. J Natl Cancer Inst. 2004;96:1676–1681. doi: 10.1093/jnci/djh315. [DOI] [PubMed] [Google Scholar]
- 4.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 5.Cella L, Liuzzi R, D'Avino V, Conson M, Di Biase A, Picardi M, Pugliese N, Solla R, Salvatore M, Pacelli R. Pulmonary damage in Hodgkin's lymphoma patients treated with sequential chemo-radiotherapy: Predictors of radiation-induced lung injury. Acta Oncol. 2014;53:613–619. doi: 10.3109/0284186X.2013.850739. [DOI] [PubMed] [Google Scholar]
- 6.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams JP, Johnston CJ, Finkelstein JN. Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction? Curr Drug Targets. 2010;11:1386–1394. doi: 10.2174/1389450111009011386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson-Sjoland A, Nihlberg K, Eriksson L, Bjermer L, Westergren-Thorsson G. Fibrocytes and the tissue niche in lung repair. Respir Res. 2011;12:76. doi: 10.1186/1465-9921-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardie WD, Hagood JS, Dave V, Perl AK, Whitsett JA, Korfhagen TR, Glasser S. Signaling pathways in the epithelial origins of pulmonary fibrosis. Cell Cycle. 2010;9:2769–2776. doi: 10.4161/cc.9.14.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyytiainen M, Penttinen C, Keski-Oja J. Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci. 2004;41:233–264. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- 11.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 12.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991;88:6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 14.Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 15.Rube CE, Uthe D, Schmid KW, Richter KD, Wessel J, Schuck A, Willich N, Rube C. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000;47:1033–1042. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- 16.Vujaskovic Z, Marks LB, Anscher MS. The physical parameters and molecular events associated with radiation-induced lung toxicity. Semin Radiat Oncol. 2000;10:296–307. doi: 10.1053/srao.2000.9424. [DOI] [PubMed] [Google Scholar]
- 17.Tsoutsou PG, Gourgoulianis KI, Petinaki E, Mpaka M, Efremidou S, Maniatis A, Molyvdas PA. ICAM-1, ICAM-2 and ICAM-3 in the sera of patients with idiopathic pulmonary fibrosis. Inflammation. 2004;28:359–364. doi: 10.1007/s10753-004-6647-6. [DOI] [PubMed] [Google Scholar]
- 18.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 19.Choi SH, Hong ZY, Nam JK, Lee HJ, Jang J, Yoo RJ, Lee YJ, Lee CY, Kim KH, Park S. A Hypoxia-Induced Vascular Endothelial-to-Mesenchymal Transition in Development of Radiation-Induced Pulmonary Fibrosis. Clin Cancer Res. 2015;21:3716–3726. doi: 10.1158/1078-0432.CCR-14-3193. [DOI] [PubMed] [Google Scholar]
- 20.Sanders KA, Hoidal JR. The NOX on pulmonary hypertension. Circ Res. 2007;101:224–226. doi: 10.1161/CIRCRESAHA.107.158246. [DOI] [PubMed] [Google Scholar]
- 21.Basu RK, Hubchak S, Hayashida T, Runyan CE, Schumacker PT, Schnaper HW. Interdependence of HIF-1alpha and TGF-beta/Smad3 signaling in normoxic and hypoxic renal epithelial cell collagen expression. Am J Physiol Renal Physiol. 2011;300:F898–F905. doi: 10.1152/ajprenal.00335.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ueno M, Maeno T, Nomura M, Aoyagi-Ikeda K, Matsui H, Hara K, Tanaka T, Iso T, Suga T, Kurabayashi M. Hypoxia-inducible factor-1alpha mediates TGF-beta-induced PAI-1 production in alveolar macrophages in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;300:L740–L752. doi: 10.1152/ajplung.00146.2010. [DOI] [PubMed] [Google Scholar]
- 23.Linn SC, Kim HS, Keane EM, Andras LM, Wang E, Merrill AJ. Regulation of de novo sphingolipid biosynthesis and the toxic consequences of its disruption. Biochem Soc Trans. 2001;29:831–835. doi: 10.1042/0300-5127:0290831. [DOI] [PubMed] [Google Scholar]
- 24.Gorshkova I, Zhou T, Mathew B, Jacobson JR, Takekoshi D, Bhattacharya P, Smith B, Aydogan B, Weichselbaum RR, Natarajan V. Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase-1 expression. J Lipid Res. 2012;53:1553–1568. doi: 10.1194/jlr.M026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tada H, Ogushi F, Tani K, Nishioka Y, Miyata JY, Sato K, Asano T, Sone S. Increased binding and chemotactic capacities of PDGF-BB on fibroblasts in radiation pneumonitis. Radiat Res. 2003;159:805–811. doi: 10.1667/0033-7587(2003)159[0805:ibacco]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Beljaars L, Weert B, Geerts A, Meijer DK, Poelstra K. The preferential homing of a platelet derived growth factor receptor-recognizing macromolecule to fibroblast-like cells in fibrotic tissue. Biochem Pharmacol. 2003;66:1307–1317. doi: 10.1016/s0006-2952(03)00445-3. [DOI] [PubMed] [Google Scholar]
- 27.Ponten A, Li X, Thoren P, Aase K, Sjoblom T, Ostman A, Eriksson U. Transgenic overexpression of platelet-derived growth factor-C in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy. Am J Pathol. 2003;163:673–682. doi: 10.1016/S0002-9440(10)63694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Ingram JL, Bonner JC. EGF and PDGF receptor tyrosine kinases as therapeutic targets for chronic lung diseases. Curr Mol Med. 2006;6:409–421. doi: 10.2174/156652406777435426. [DOI] [PubMed] [Google Scholar]
- 30.Koli K, Myllarniemi M, Keski-Oja J, Kinnula VL. Transforming growth factor-beta activation in the lung: focus on fibrosis and reactive oxygen species. Antioxid Redox Signal. 2008;10:333–342. doi: 10.1089/ars.2007.1914. [DOI] [PubMed] [Google Scholar]
- 31.Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, Lee LB, McMahon G, Grone HJ, Lipson KE. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201:925–935. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flechsig P, Dadrich M, Bickelhaupt S, Jenne J, Hauser K, Timke C, Peschke P, Hahn EW, Grone HJ, Yingling J. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res. 2012;18:3616–3627. doi: 10.1158/1078-0432.CCR-11-2855. [DOI] [PubMed] [Google Scholar]
- 33.Rajesh M, Batkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horvath B, Holovac E, Cinar R, Liaudet L, Mackie K. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes. 2012;61:716–727. doi: 10.2337/db11-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jourdan T, Godlewski G, Cinar R, Bertola A, Szanda G, Liu J, Tam J, Han T, Mukhopadhyay B, Skarulis MC. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19:1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wirsdorfer F, de Leve S, Cappuccini F, Eldh T, Meyer AV, Gau E, Thompson LF, Chen NY, Karmouty-Quintana H, Fischer U. Extracellular Adenosine Production by ecto-5'-Nucleotidase (CD73) Enhances Radiation-Induced Lung Fibrosis. Cancer Res. 2016;76:3045–3056. doi: 10.1158/0008-5472.CAN-15-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbas-Filho JV, Ferreira MA, Sesso A, Kairalla RA, Carvalho CR, Capelozzi VL. Evidence of type II pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary fibrosis (IFP)/usual interstitial pneumonia (UIP) J Clin Pathol. 2001;54:132–138. doi: 10.1136/jcp.54.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sisson TH, Mendez M, Choi K, Subbotina N, Courey A, Cunningham A, Dave A, Engelhardt JF, Liu X, White ES. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 40.Osterholzer JJ, Olszewski MA, Murdock BJ, Chen GH, Erb-Downward JR, Subbotina N, Browning K, Lin Y, Morey RE, Dayrit JK. Implicating exudate macrophages and Ly-6C(high) monocytes in CCR2-dependent lung fibrosis following gene-targeted alveolar injury. J Immunol. 2013;190:3447–3457. doi: 10.4049/jimmunol.1200604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Citrin DE, Shankavaram U, Horton JA, Shield WR, Zhao S, Asano H, White A, Sowers A, Thetford A, Chung EJ. Role of type II pneumocyte senescence in radiation-induced lung fibrosis. J Natl Cancer Inst. 2013;105:1474–1484. doi: 10.1093/jnci/djt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 43.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Epperly MW, Defilippi S, Sikora C, Gretton J, Kalend A, Greenberger JS. Intratracheal injection of manganese superoxide dismutase (MnSOD) plasmid/liposomes protects normal lung but not orthotopic tumors from irradiation. Gene Ther. 2000;7:1011–1018. doi: 10.1038/sj.gt.3301207. [DOI] [PubMed] [Google Scholar]
- 45.Kang SK, Rabbani ZN, Folz RJ, Golson ML, Huang H, D Yu, Samulski TS, Dewhirst MW, Anscher MS, Vujaskovic Z. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2003;57:1056–1066. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 46.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanjore H, XC Xu, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180:657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marmai C, Sutherland RE, Kim KK, Dolganov GM, Fang X, Kim SS, Jiang S, Golden JA, Hoopes CW, Matthay MA. Alveolar epithelial cells express mesenchymal proteins in patients with idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;301:L71–L78. doi: 10.1152/ajplung.00212.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 51.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim TH, Kim SH, Seo JY, Chung H, Kwak HJ, Lee SK, Yoon HJ, Shin DH, Park SS, Sohn JW. Blockade of the Wnt/beta-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J Exp Med. 2011;223:45–54. doi: 10.1620/tjem.223.45. [DOI] [PubMed] [Google Scholar]
- 53.Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014;69:760–765. doi: 10.1136/thoraxjnl-2013-204608. [DOI] [PubMed] [Google Scholar]
- 54.Xiong S, Pan X, L Xu, Yang Z, Guo R, Y Gu, Li R, Wang Q, Xiao F, L Du. Regulatory T Cells Promote beta-Catenin--Mediated Epithelium-to-Mesenchyme Transition During Radiation-Induced Pulmonary Fibrosis. Int J Radiat Oncol Biol Phys. 2015;93:425–435. doi: 10.1016/j.ijrobp.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uchinami H, Seki E, Brenner DA, D'Armiento J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006;44:420–429. doi: 10.1002/hep.21268. [DOI] [PubMed] [Google Scholar]
- 57.Flechsig P, Hartenstein B, Teurich S, Dadrich M, Hauser K, Abdollahi A, Grone HJ, Angel P, Huber PE. Loss of matrix metalloproteinase-13 attenuates murine radiation-induced pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 2010;77:582–590. doi: 10.1016/j.ijrobp.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 58.Hallahan DE, Geng L, Shyr Y. Effects of intercellular adhesion molecule 1 (ICAM-1) null mutation on radiation-induced pulmonary fibrosis and respiratory insufficiency in mice. J Natl Cancer Inst. 2002;94:733–741. doi: 10.1093/jnci/94.10.733. [DOI] [PubMed] [Google Scholar]
- 59.Bickelhaupt S, Erbel C, Timke C, Wirkner U, Dadrich M, Flechsig P, Tietz A, Pfohler J, Gross W, Peschke P. Effects of CTGF Blockade on Attenuation and Reversal of Radiation-Induced Pulmonary Fibrosis. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djw339. [DOI] [PubMed] [Google Scholar]
- 60.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 61.Zhang C, Zhao H, Li BL, Fu-Gao, Liu H, Cai JM, Zheng M. CpG-oligodeoxynucleotides may be effective for preventing ionizing radiation induced pulmonary fibrosis. Toxicol Lett. 2018;292:181–189. doi: 10.1016/j.toxlet.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 62.Han G, Zhang H, Xie CH, Zhou YF. Th2-like immune response in radiation-induced lung fibrosis. Oncol Rep. 2011;26:383–388. doi: 10.3892/or.2011.1300. [DOI] [PubMed] [Google Scholar]
- 63.Chen J, Wang Y, Mei Z, Zhang S, Yang J, Li X, Yao Y, Xie C. Radiation-induced lung fibrosis in a tumor-bearing mouse model is associated with enhanced Type-2 immunity. J Radiat Res. 2016;57:133–141. doi: 10.1093/jrr/rrv077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Netea MG, Van der Meer JW, Sutmuller RP, Adema GJ, Kullberg BJ. From the Th1/Th2 paradigm towards a Toll-like receptor/T-helper bias. Antimicrob Agents Chemother. 2005;49:3991–3996. doi: 10.1128/AAC.49.10.3991-3996.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amati L, Pepe M, Passeri ME, Mastronardi ML, Jirillo E, Covelli V. Toll-like receptor signaling mechanisms involved in dendritic cell activation: potential therapeutic control of T cell polarization. Curr Pharm Des. 2006;12:4247–4254. doi: 10.2174/138161206778743583. [DOI] [PubMed] [Google Scholar]
- 66.Chen J, Tian X, Mei Z, Wang Y, Yao Y, Zhang S, Li X, Wang H, Zhang J, Xie C. The effect of the TLR9 ligand CpG-oligodeoxynucleotide on the protective immune response to radiation-induced lung fibrosis in mice. Mol Immunol. 2016;80:33–40. doi: 10.1016/j.molimm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vijay-Kumar M, Aitken JD, Sanders CJ, Frias A, Sloane VM, Xu J, Neish AS, Rojas M, Gewirtz AT. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J Immunol. 2008;180:8280–8285. [Google Scholar]
- 69.Majori M, Poletti V, Curti A, Corradi M, Falcone F, Pesci A. Bronchoalveolar lavage in bronchiolitis obliterans organizing pneumonia primed by radiation therapy to the breast. J Allergy Clin Immunol. 2000;105:239–244. doi: 10.1016/s0091-6749(00)90071-x. [DOI] [PubMed] [Google Scholar]
- 70.Haston CK, Begin M, Dorion G, Cory SM. Distinct loci influence radiation-induced alveolitis from fibrosing alveolitis in the mouse. Cancer Res. 2007;67:10796–10803. doi: 10.1158/0008-5472.CAN-07-2733. [DOI] [PubMed] [Google Scholar]
- 71.Groves AM, Johnston CJ, Williams JP, Finkelstein JN. Role of Infiltrating Monocytes in the Development of Radiation-Induced Pulmonary Fibrosis. Radiat Res. 2018;189:300–311. doi: 10.1667/RR14874.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Annes JP, Rifkin DB, Munger JS. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett. 2002;511:65–68. doi: 10.1016/s0014-5793(01)03280-x. [DOI] [PubMed] [Google Scholar]
- 73.Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, Devitt ML, Horan GS, Weinreb PH, Lukashev ME. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177:82–90. doi: 10.1164/rccm.200706-806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bueno L, de Alwis DP, Pitou C, Yingling J, Lahn M, Glatt S, Troconiz IF. Semi-mechanistic modelling of the tumour growth inhibitory effects of LY2157299, a new type I receptor TGF-beta kinase antagonist, in mice. Eur J Cancer. 2008;44:142–150. doi: 10.1016/j.ejca.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 75.Tsoyi K, Chu SG, Patino-Jaramillo NG, Wilder J, Villalba J, Doyle-Eisele M, McDonald J, Liu X, EI-Chemaly S, Perrella MA. Syndecan-2 Attenuates Radiation-induced Pulmonary Fibrosis and Inhibits Fibroblast Activation by Regulating PI3K/Akt/ROCK Pathway via CD148. Am J Respir Cell Mol Biol. 2018;58:208–215. doi: 10.1165/rcmb.2017-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdollahi A, Hahnfeldt P, Maercker C, Grone HJ, Debus J, Ansorge W, Folkman J, Hlatky L, Huber PE. Endostatin's antiangiogenic signaling network. Mol Cell. 2004;13:649–663. doi: 10.1016/s1097-2765(04)00102-9. [DOI] [PubMed] [Google Scholar]
- 77.Huber PE, Hauser K, Abdollahi A. Genome wide expression profiling of angiogenic signaling and the Heisenberg uncertainty principle. Cell Cycle. 2004;3:1348–1351. doi: 10.4161/cc.3.11.1209. [DOI] [PubMed] [Google Scholar]
- 78.Yamasaki Y, Miyoshi K, Oda N, Watanabe M, Miyake H, Chan J, Wang X, Sun L, Tang C, McMahon G. Weekly dosing with the platelet-derived growth factor receptor tyrosine kinase inhibitor SU9518 significantly inhibits arterial stenosis. Circ Res. 2001;88:630–636. doi: 10.1161/01.res.88.6.630. [DOI] [PubMed] [Google Scholar]
- 79.Li M, Ping G, Plathow C, Trinh T, Lipson KE, Hauser K, Krempien R, Debus J, Abdollahi A, Huber PE. Small molecule receptor tyrosine kinase inhibitor of platelet-derived growth factor signaling (SU9518) modifies radiation response in fibroblasts and endothelial cells. BMC Cancer. 2006;6:79. doi: 10.1186/1471-2407-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dadrich M, Nicolay NH, Flechsig P, Bickelhaupt S, Hoeltgen L, Roeder F, Hauser K, Tietz A, Jenne J, Lopez R. Combined inhibition of TGFbeta and PDGF signaling attenuates radiation-induced pulmonary fibrosis. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2015.1123366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J, Zhou F, Li Z, Mei H, Wang Y, Ma H, Shi L, Huang A, Zhang T, Lin Z. Pharmacological targeting of BET proteins attenuates radiation-induced lung fibrosis. Sci Rep. 2018;8:998. doi: 10.1038/s41598-018-19343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature. 2010;468:1100–1104. doi: 10.1038/nature09584. [DOI] [PubMed] [Google Scholar]
- 83.Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012;4:159–165. doi: 10.18632/aging.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chung EJ, Sowers A, Thetford A, McKay-Corkum G, Chung SI, Mitchell JB, Citrin DE. Mammalian Target of Rapamycin Inhibition With Rapamycin Mitigates Radiation-Induced Pulmonary Fibrosis in a Murine Model. Int J Radiat Oncol Biol Phys. 2016;96:857–866. doi: 10.1016/j.ijrobp.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist. 2007;12:738–747. doi: 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- 86.Son B, Kwon T, Lee S, Han I, Kim W, Youn H, Youn B. CYP2E1 regulates the development of radiation-induced pulmonary fibrosis via ER stress- and ROS-dependent mechanisms. Am J Physiol Lung Cell Mol Physiol. 2017;313:L916–L929. doi: 10.1152/ajplung.00144.2017. [DOI] [PubMed] [Google Scholar]
- 87.Meyer ZHG, Cordes S, Mausberg AK, Zozulya AL, Wessig C, Sparwasser T, Mathys C, Wiendl H, Hartung HP, Kieseier BC. FoxP3+ regulatory T cells determine disease severity in rodent models of inflammatory neuropathies. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Z Liang W, Zhan A, Zhu Y, Yoon S, Lin M, Sasaki JM, Klapproth H, Yang HE, Grossniklaus J Xu. Development of a unique small molecule modulator of CXCR4. PLoS One. 2012;7:e34038. doi: 10.1371/journal.pone.0034038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shu HK, Yoon Y, Hong S, Xu K, Gao H, Hao C, Torres-Gonzalez E, Nayra C, Rojas M, Shim H. Inhibition of the CXCL12/CXCR4-axis as preventive therapy for radiation-induced pulmonary fibrosis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duru N, Zhang Y, Gernapudi R, Wolfson B, Lo PK, Yao Y, Zhou Q. Loss of miR-140 is a key risk factor for radiation-induced lung fibrosis through reprogramming fibroblasts and macrophages. Sci Rep. 2016;6:39572. doi: 10.1038/srep39572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 92.Xin BM, Wang XX, Jin W, Yan HM, Cui B, Zhang XW, Hua F, Yang HZ, Hu ZW. Activation of Toll-like receptor 9 attenuates unilateral ureteral obstruction-induced renal fibrosis. Acta Pharmacol Sin. 2010;31:1583–1592. doi: 10.1038/aps.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li X, Xu G, Qiao T, Yuan S, Zhuang X, Zhang J, Sun HB. Effects of CpG Oligodeoxynucleotide 1826 on transforming growth factor-beta 1 and radiation-induced pulmonary fibrosis in mice. J Inflamm (Lond) 2016;13:16. doi: 10.1186/s12950-016-0125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hacker H, Vabulas RM, Takeuchi O, Hoshino K, Akira S, Wagner H. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J Exp Med. 2000;192:595–600. doi: 10.1084/jem.192.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Y Xu, Tao X, Shen B, Horng T, Medzhitov R, Manley JL, Tong L. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 2000;408:111–115. doi: 10.1038/35040600. [DOI] [PubMed] [Google Scholar]
- 96.Rudd BD, Schaller MA, Smit JJ, Kunkel SL, Neupane R, Kelley L, Berlin AA, Lukacs NW. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J Immunol. 2007;178:5820–5827. doi: 10.4049/jimmunol.178.9.5820. [DOI] [PubMed] [Google Scholar]
- 97.Rahman AH, Cui W, Larosa DF, Taylor DK, Zhang J, Goldstein DR, Wherry EJ, Kaech SM, Turka LA. MyD88 plays a critical T cell-intrinsic role in supporting CD8 T cell expansion during acute lymphocytic choriomeningitis virus infection. J Immunol. 2008;181:3804–3810. doi: 10.4049/jimmunol.181.6.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Doz E, Noulin N, Boichot E, Guenon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180:1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 99.Babcock AA, Toft-Hansen H, Owens T. Signaling through MyD88 regulates leukocyte recruitment after brain injury. J Immunol. 2008;181:6481–6490. doi: 10.4049/jimmunol.181.9.6481. [DOI] [PubMed] [Google Scholar]
- 100.Brickey WJ, Neuringer IP, Walton W, Hua X, Wang EY, Jha S, Sempowski GD, Yang X, Kirby SL, Tilley SL. MyD88 provides a protective role in long-term radiation-induced lung injury. Int J Radiat Biol. 2012;88:335–347. doi: 10.3109/09553002.2012.652723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Verma S, Kalita B, Bajaj S, Prakash H, Singh AK, Gupta ML. A Combination of Podophyllotoxin and Rutin Alleviates Radiation-Induced Pneumonitis and Fibrosis through Modulation of Lung Inflammation in Mice. Front Immunol. 2017;8:658. doi: 10.3389/fimmu.2017.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]