Abstract
Complicated grief, or persistent complex bereavement disorder, is a condition that affects approximately 10% of bereaved individuals and is marked by intense longing and yearning for the deceased. Little is known about the neurocognitive mechanisms contributing to this syndrome, but previous research suggests that reward pathways in the brain may play a role. Twenty-five older adults were categorized based on grief severity into one of three groups: complicated grief (CG), non-complicated grief (NCG) and non-bereaved married controls (NB). Neural activation was examined using fMRI while participants viewed a countdown on the screen (anticipation) followed by a photo of their (living or deceased) spouse. There was no significantly differential activation between the three groups for the spouse v. stranger photo contrast, nor for anticipation period v. spouse photo. Post-hoc analyses were conducted using self-reported yearning scores as a regressor across all bereaved participants, which revealed that greater symptoms of yearning predicted greater activation in the subgenual anterior cingulate cortex (sgACC). Given the small sample size, the results should be considered preliminary and in need of replication, but may suggest a more nuanced, transdiagnostic role of the sgACC. This region of the brain has been previously linked to depression and suggests that symptoms of yearning may present an opportune place to intervene to improve outcomes in CG.
Keyword: Neuroscience
1. Introduction
Grief is a common human experience, yet is one of life's greatest and most painful stressors. Despite the difficulty of loss, most widow(er)s are resilient and eventually cope in response to loss. However, some bereaved people do not seem to recover, and their grief, which is especially prolonged and intense, develops into a chronic debilitating condition known as complicated grief (CG) (Shear et al., 2011). CG is distinct from major depressive disorder and post-traumatic stress disorder, although they can be comorbid (Simon et al., 2011). Previous research has found evidence that neural mechanisms may differentiate those who adjust well from those who develop CG (O'Connor et al., 2008). In response to a photo of the deceased (compared to a stranger), those with CG had increased activity in the nucleus accumbens, a brain region implicated in the liking and wanting aspects of reward compared to bereaved participants that didn't meet CG criteria (or non-complicated grief: Non-CG). Moreover, subjective reports of yearning during an interview correlated with activation in this region.
Yearning, or an intense longing to be reunited with the deceased, is a hallmark symptom of CG. The nucleus accumbens is a small structure in the ventral region of the basal ganglia, which is associated with reward (Knutson et al., 2001). One possible interpretation of the aforementioned finding is that the activity in the nucleus accumbens is an instantiation of the yearning for the deceased experienced by those with CG. Subjectively, the bereaved individual desires to be reunited with their loved one in response to memories and cues associated with the deceased. Consequently, cues related to their loved one are perceived as rewarding and therefore reinforce the longing, preventing extinction of that reward response that would occur in resilient adaptation during bereavement.
The present study attempted to replicate the earlier work by O'Connor and colleagues while also manipulating a few key differences to test generalizability. First, the present study used a sample of older adults (instead of middle aged women), who had experienced the death of a spouse (instead of a sister or mother). Second, the task was modified to include five photos of the deceased (instead of a single photograph, embedded with death-related words in the prior study). The task was also modified in an attempt to capture the anticipation of seeing the photo, with a countdown of the numbers 4 through 1 preceding each photo. In addition, comparison to a non-bereaved control group was added. We hypothesized that the nucleus accumbens would discriminate those with CG from the non-bereaved and those with Non-CG.
2. Methods
2.1. Participants
As part of a larger study conducted at the University of California, Los Angeles (Arizmendi et al., 2016), twenty-nine older adults were recruited from the community. The UCLA Institutional Review Board approved all procedures and all participants provided informed consent prior to participation. After removing four participants with excessive head motion or structural abnormalities, the final sample consisted of twenty-five older adults. Participants had either experienced the death of a spouse in the past three years or were non-bereaved, married controls. Among the 16 bereaved participants, 9 met the criteria for CG. The non-bereaved group consisted of 9 married individuals that had not experienced the loss of any first-degree relative in the past three years.
2.2. Procedure
All bereaved participants completed a 19-item Inventory of Complicated Grief, a well-validated measure for determining grief severity (ICG; Prigerson et al., 1995). Each participant provided five photos of their deceased or living spouse, which were matched with five photos of a stranger on age, sex, race, and indoor/outdoor setting. Photos were presented in an event-related design, in a randomized order for a total of 60 trials (sequence optimized using Optseq2, http://surfer.nmr.mgh.harvard.edu/optseq). Each photo was preceded by a countdown of the numbers 4 through 1, and the countdown lasted two seconds. Photos remained on the screen for three seconds.
Participants completed the photo-viewing task in the fMRI scanner. They were instructed to focus on the thoughts, feeling and memories elicited by viewing the photographs, without trying to alter them. As a manipulation check, participants completed a post-scan questionnaire in which they were asked to write about the experience of viewing the photos of their (living or deceased) spouse.
Scanning took place in a Siemens Trio 3T scanner at UCLA Ahmanson-Lovelace Brain Mapping Center. A high-resolution structural T1 weighted image (MPRAGE, TR = 2200 ms; TE = 3.4 ms, TI = 900 ms, flip angle = 10°, FOV = 256 mm, 176 continuous 1 mm slices, 1.0 × 1.0 × 1.0 mm) was collected for each participant for anatomical reference. T2 weighted functional scans (TR – 2500 ms; TE = 25 ms, flip angle = 90°, FOV = 200 mm, 36 3 mm slices, 3.1 × 3.1 × 3.0 mm) were collected during the task.
Image processing and statistical analyses were completed using Statistical Parametric Mapping (SPM12, Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm). Raw functional images were manually reoriented with the origin set to the anterior commissure. Functional images were then realigned, unwarped, and co-registered to the participant's anatomical image using the default algorithms in SPM12. Images were normalized to the MNI template and smoothed with an 8 mm Gaussian kernel, and were resliced to 2 × 2 × 2 mm voxels. Artifact Detection Tools (ART) was used to identify outliers with global intensity >3 standard deviations and scan-to-scan motion >1 mm. These outliers were included as nuisance regressor in the design matrix.
3. Analysis
First level analyses at the single subject level involved contrasts for the different phases of the task (e.g, spouse v. stranger, anticipation v. photo viewing). Second level analyses were at the group level and used F-tests to test for differences between CG, Non-CG and non-bereaved at different phases of the task. Significant F-tests were followed by planned t-tests. All coordinates are reported in MNI format.
4. Results
Participant's average age was 71.4, they were predominantly female (84%), predominantly Caucasian (77%) and average time since the death was 21.19 months. The three groups did not differ on any demographic variables, levels of depression or measures of cognitive function.
To test our primary hypothesis, functional neuroimaging data were compared across groups and conditions (thresholded at p < .001, cluster size <20). Contrary to our hypothesis, the three groups showed no differential activation for spouse photo v. stranger photo, or anticipation countdown v. spouse photo. Additionally, region of interest analysis of the nucleus accumbens did not show activation in these two contrasts.
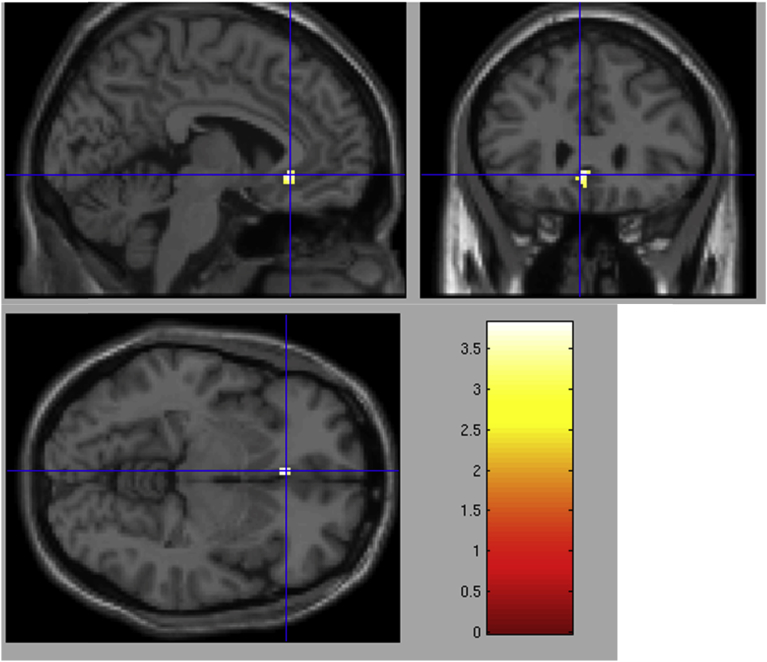
Next, for the bereaved participants (n = 16), we examined the continuous association between yearning and brain activation. Due to the hypothesized link between the CG symptom of yearning and reward processes, and previous positive findings correlated with regional activation (O'Connor et al., 2008), the single yearning item was extracted from the ICG scale. This item assesses yearning for the deceased on a scale from 1 to 4. A multiple regression analysis was performed for the anticipation countdown v. spouse contrast across all bereaved participants, regardless of CG diagnosis. Higher self-reported yearning predicted activation in the subgenual anterior cingulate (sgACC; x = -4, y = 28, z = -6) during the anticipation period compared to viewing a photo of one's spouse (z = 3.11, p <.005, cluster size = 25) (Fig. 1). For discriminative validity, the Beck Depression Inventory (BDI) was also included individually as a regressor, but was not associated with activation in this region. These results should be interpreted with caution, given the risk of Type I error due to the lack of correction for multiple comparisons across the voxelwise analysis and the small sample size in the analysis (n=16).
Fig. 1.
Greater self-reported yearning associated with greater neural activity in the subgenual anterior cingulate cortex (sgACC, x = −4, y = 28, z = −6) across all bereaved participants (Z = 3.11, p < .005, cluster size = 25).
5. Discussion
The present study attempted to replicate and extend the previous neuroimaging findings demonstrating that activation in the nucleus accumbens discriminated bereaved persons with CG and Non-CG. Contrary to our hypothesis, nucleus accumbens activation did not differ among the groups. However, yearning across the bereaved groups was significantly associated with greater activation within the sgACC. Overall, these findings may suggest a more nuanced picture regarding the role of these brain regions in complicated grief and yearning for the deceased. However, given the small sample size in this study and the lack of correction for multiple comparisons across the voxelwise analysis, these findings should be considered highly preliminary and require replication before drawing major conclusions.
First, the lack of a significant finding for our primary hypothesis could be due to a number of factors. Our sample was significantly older than in previous work. The intensity of absolute nucleus accumbens responsiveness may be lower in this age group. Recent studies in older animals have shown changes in nucleus accumbens functioning and behavior (Ruegsegger et al., 2017). However, the small sample size may have limited the statistical power to detect a smaller effect size. There may be distinct regional activation between the groups, but individual differences within the CG group may be greater than the differences between the groups, preventing us from detecting it. Nonetheless, these null results seem worth reporting in the scientific literature.
Second, using the larger bereaved group (n = 16), we found a correlation between yearning and sgACC activity (albeit with a very small cluster size). This region has been reliably associated with depressive symptoms, notably depressive rumination. Cooney et al. (2010) found that participants with depression, relative to controls, exhibited greater sgACC activation during a rumination task compared to distraction. However, we found an association with yearning, and not with scores on the BDI. An alternative possibility is that sgACC activity represents a transdiagnostic construct of maladaptive repetitive thought common to both yearning (Eisma et al., 2015) and depressive rumination (Nolen-Hoeksema et al., 2008). These symptoms share important qualities. For one, both rumination and yearning (in the context of grief), are experienced as uncontrollable, have a negative valence and are self-focused (Kaplan et al., 2018). Activation in these two populations may indicate that the sgACC reflects the degree, or uncontrollability, of the repetitive thought pattern elicited by these psychological events. More research may determine whether the neural correlates of these thought patterns are processed in the sgACC in both psychopathologies.
Declarations
Author contribution statement
Mairead H. McConnell: Analyzed and interpreted the data; Wrote the paper.
William D. S. Killgore: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mary-Frances O'Connor: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Funding statement
The study was supported by a grant from the National Institutes of Aging (K01-AG028404) awarded to Dr. Mary-Frances O'Connor, PhD.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Arizmendi B., Kaszniak A.W., O'Connor M.F. Disrupted prefrontal activity during emotion processing in complicated grief: an fMRI investigation. NeuroImage. 2016;124:968–976. doi: 10.1016/j.neuroimage.2015.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney R.E., Joormann J., Eugène F., Dennis E.L., Gotlib I.H. Neural correlates of rumination in depression. Cognit. Affect Behav. Neurosci. 2010;10(4):470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisma M.C., Schut H.A.W., Stroebe M.S., Boelen P.A., Van Den Bout J., Stroebe W. Adaptive and maladaptive rumination after loss: a three-wave longitudinal study. Br. J. Clin. Psychol. 2015;54(2):163–180. doi: 10.1111/bjc.12067. [DOI] [PubMed] [Google Scholar]
- Kaplan D.M., Palitsky R., Carey A.L., Crane T.E., Havens C.M., Medrano M.R., Reznik S.J., Sbarra D.A., O'Connor M.-F. Maladaptive repetitive thought as a transdiagnostic phenomenon and treatment target: an integrative review. J. Clin. Psychol. 2018:1–11. doi: 10.1002/jclp.22585. 2018. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21(1–5) doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Wisco B.E., Lyubomirsky S. Rethinking rumination. Perspect. Psychol. Sci. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- O'Connor M.-F., Wellisch D.K., Stanton A.L., Eisenberger N.I., Irwin M.R., Lieberman M.D. Craving love? Enduring grief activates the brain's reward center. NeuroImage. 2008;42(2):969–972. doi: 10.1016/j.neuroimage.2008.04.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigerson H.G., Maciejewski P.K., Reynolds C.F., III, Bierhals A.J., Newsom J.T., Fasiczka A., Frank E., Doman J., Miller M. Inventory of complicated grief: a scale to measure maladaptive symptoms of loss. Psychiatr. Res. 1995;59(1–2):65–79. doi: 10.1016/0165-1781(95)02757-2. [DOI] [PubMed] [Google Scholar]
- Ruegsegger G.N., Toedebusch R.G., Childs T.E., Grigsby K.B., Booth F.W. Loss of Cdk5 function in the nucleus accumbens decreases wheel running and may mediate age-related declines in voluntary physical activity. J. Physiol. 2017;595(1):363–384. doi: 10.1113/JP272489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shear M.K., Simon N., Wall M., Zisook S., Neimeyer R., Duan N. Complicated grief and related bereavement issues for DSM-5. Depress. Anxiety. 2011;28(2):103–117. doi: 10.1002/da.20780. (1091–4269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon N., Shear M.K., Thompson E.H., Zalta A.K., Perlman C., Reynolds C.F., III The prevalence and correlates of psychiatric comorbidity in individuals with complicated grief. Compr. Psychiatr. 2011;48(5) doi: 10.1016/j.comppsych.2007.05.002. 395–399, 103–117. [DOI] [PubMed] [Google Scholar]