Abstract
Ever since the ureteral stent design was fitted with a curl on both sides to prevent it from migrating up or down the ureter some 40 years ago, its use has gained tremendous momentum, aiding in the rise and evolution of endourology and has confidently kept its place in modern time urology. Over the past four decades, several designs, coating and biomaterials have been developed, trying to reduce infection, encrustation and other stent related symptoms. As the ideal stent has not yet been discovered, different ways of helping patients with their complaints have been researched. This review will cover these aspects of stent use in urolithiasis.
Keywords: Ureteral stent, Prosthesis, Urolithiasis, Stent-related symptom, Alpha-blocker
1. Introduction
The first description of using a ureteral catheter dates back to over a century ago, when Shoemaker [1] described its first appliance in women. Ever since, ureteral stents have been used for a variety of urological conditions. Although Zimskind et al. [2] described the first use of an indwelling open-ended silicone stent in a ureter for malignant obstruction in 1967, the design was still prone to migration. To overcome this issue, Gibbons et al. [3] designed a new stent with a distal flange to prevent proximal migration and sharply pointed barbs to prevent distal migration, which became commercially available in 1974. Shortly thereafter, Finney [4] and Hepperlen et al. [5], almost simultaneously, reported on a new stent design to prevent both proximal and distal migration with a J-shaped curl on each side of the stent, the still known double pigtail or double-J stent. Most new stent designs are alterations to this model and will be discussed in more detail below.
2. Indications
Ureteral stents are implants used to provide drainage of the upper urinary tract, when obstruction of the ureter is present or anticipated. This obstruction may be due to internal or external issues, such as edema after manipulation of the ureter, ureteral stricture, passage of stone fragments or external compression of the ureter. Stents are also frequently used in reconstructive surgery, where they serve as a scaffold over which the ureteral reconstruction is to heal. This review will be limited to the use of ureteral stents for urolithiasis.
The three main indications for placement of a ureteral stent in urolithiasis are:
-
i.
Drainage of obstructed ureter by stone fragment(s);
-
ii.
Following ureterorenoscopy;
-
iii.
Prophylactic insertion before extracorporeal shock wave lithotripsy (ESWL) or (flexible) ureterorenoscopy.
2.1. Acute drainage of obstructing urolithiasis
Drainage of the upper urinary tract is not mandatory for each obstructing stone. It is however absolutely indicated in cases of bilateral obstruction, obstruction of a solitary kidney, uncontrollable pain or when the obstruction is associated with a urinary tract infection (UTI) or sepsis. Depending on multiple variables, including center and surgeon preference, either a stent or nephrostomy may be preferred to acquire drainage. In the most recent European Association of Urology (EAU) guidelines, ureteral stents and percutaneous nephrostomy tubes are considered equally effective (level of evidence 1b) for decompression of the renal collecting system [6]. To date, only two randomized controlled trials (RCTs) have directly compared effectiveness of retrograde ureteral catheterization and percutaneous nephrostomy in adult cases of infected hydronephrosis associated with ureteral calculi. They found no statistically significant difference in drainage efficiency or recovery time from sepsis [7], [8]. In a large retrospective study by Sammon et al. [9], using the Nationwide Inpatient Sample of the United States between 1999 and 2009, the authors demonstrated that a stent was placed for renal decompression in 87.7% of patients. Patients undergoing percutaneous nephrostomy placement were more likely to have comorbidities, and had a higher rate of sepsis and in-hospital mortality. This probably reflects the fact that sicker patients were more likely to receive a nephrostomy tube rather than demonstrating causality of outcome with a nephrostomy tube [9]. This was corroborated by a smaller retrospective study, indicating indeed that patients receiving a nephrostomy tube were more acutely ill and had larger stones than patients receiving a stent [10].
In the pediatric population, Elsheemy and associates [11] compared acute drainage of bilateral obstructing ureteral calculi with unilateral nephrostomy to bilateral ureteral stenting. As percutaneous nephrostomy tube insertion was associated with more, albeit low grade, complications and as double-J stent placement facilitated subsequent endourological procedures, the authors would advocate stent placement rather than nephrostomy tube insertion in this setting. For stones >2 cm, a percutaneous nephrostomy tube would be preferred as attempts at stent placement resulted more frequently in mucosal complications in this subgroup.
2.2. Insertion of stent after ureterorenoscopy
Both the EAU and American Urological Association (AUA) guidelines state that stent placement after uncomplicated ureteroscopy can be omitted in select cases [6], [12]. In case of a solitary kidney, anatomic abnormalities, ureteral perforation, residual fragments or other risk of complication, ureteral stents are still suggested.
These recommendations are based on the initial report of safely omitting a stent after uncomplicated ureteroscopy by Denstedt et al. [13] and many others that followed afterwards corroborating these results [14]. The most recent meta-analysis by Wang et al. [14] identified 22 RCTs, involving stenting after ureteroscopy or after ESWL including a total of 2552 patients. The authors demonstrated pain, dysuria, hematuria and irritative urinary symptoms to be significantly more common in the stented group (OR and [95%CI] were respectively 2.69 [1.43, 5.06], 3.97 [2.24, 7.01], 3.09 [1.45, 6.60] and 4.40 [2.12, 9.10]). Operating time was on average 5 min longer in the stented group and no significant differences were seen in stone free rate, length of hospital stay and infection rate. The risk of unplanned readmissions was significantly higher in the unstented group (OR=0.54, 95%CI [0.34, 0.87], p < 0.01).
Despite compelling evidence in favour of not stenting a patient after an uncomplicated ureteroscopy, it is still common practice to routinely do so. This is reflected in an analysis of the Clinical Research Office of the Endourological Society (CROES) Ureteroscopy Global Study, a large prospective, observational study conducted in 114 urology departments across 32 countries including 11 885 patients [15]. The authors identified that a stent was placed in 63.2% after ureteral stone treatment and in 79.5% after renal stone treatment. Interestingly, there were great geographical differences in routine stent placement practice ranging from 28.9% in Iran to 96.1% in Japan [15].
Whether or not to place a stent after the use of a ureteral access sheath (UAS) is still a topic of debate. Although expert opinion would indicate that it is recommended, retrospective data nuances that it would be safe to omit a stent in pre-stented patients treated with ureteroscopy with a UAS [16], [17], [18]. To date, only one RCT has been published demonstrating that it is safe and feasible to omit stent placement after the use of a UAS during ureteroscopy [19].
In a climate of increasing society healthcare costs, the question of cost when placing or omitting a stent after uncomplicated ureteroscopy is gaining importance. An Austrian study by Seklehner et al. [20] in 2017 simulated the total cost for both scenarios including costs of stent placement, stent removal and postoperative complication management after uncomplicated semirigid ureteroscopy as well as costs of unplanned hospital visits and readmissions. Based on a decision tree model extracted from 12 RCTs, they calculated a total cost increment of €138.25 in public insured and €599.82 in privately insured patients for placing a stent. Although these data were based on the Austrian health care system and cannot necessarily be extrapolated to other national healthcare systems, they unveiled a previously unreported issue in the debate. The discrepancy may become even more apparent when including cost to society by work incapacity [21].
Additional to the debate of whether or not to place a stent, the ideal indwelling time after ureteroscopy is also controversial. The heterogeneity in dwell-time in the RCTs, ranging from 3 to 28 days, demonstrates the lack of consensus [14]. The only two studies on the subject are retrospective and would indicate that a dwelling time >15 days has an increased risk of adverse events and fever and that a ureteroscopy performed in patients that had a stent indwelling for >30 days have a higher risk of post-ureteroscopic sepsis [22], [23]. To date, there is no prospective randomized data to determine the most ideal stent indwelling time.
Ultimately, it is up to the treating physician to decide whether or not a procedure was uncomplicated and to estimate the risk of post-operative complications, weighing the absence of stent-related symptoms against the risk of a higher readmission rate when omitting stent placement.
2.3. Pre-stenting in ESWL or (flexible) ureterorenoscopy
Prophylactic stenting prior to ESWL has been a common practice in the past to avoid ureteral obstruction by passage of stone fragments or steinstrasse formation after lithotripsy.
A meta-analysis of eight RCTs, including 876 patients, failed to demonstrate a significant difference in stone-free rate, auxiliary treatment rate, UTI, hematuria or pain [24]. The overall results would indicate that stents do prevent steinstrasse, however this result was heavily skewed by the data of Al-Awadi et al. [25], that treated stones of 15–35 mm. In subgroup analyses, there was no significant difference between stented or non-stented patients. The incidence of lower urinary tract symptoms was significantly higher in the stented group, compared with the stentless group (RR=4.10, 95%CI [2.21, 7.61], p < 0.000 01) [24]. Based on these results, stent placement in prevention of steinstrasse when treating very large stones (>15–20 mm) with ESWL can be beneficial.
The jury is still out on whether or not pre-stenting prior to ureteroscopic treatment of urinary stone disease should be routine practice. So far, no prospective randomized data are available demonstrating a significant benefit. The primary failure rate of ureteroscopy, i.e. the incapacity of reaching the calculus to be treated with the ureteroscope, is generally less than 10% and is more common in younger females [26]. Passive dilation by ureteral stenting has been reported to occur after 2 weeks [27].
Retrospective data suggest that stone-free rates of ureteroscopy are higher in pre-stented patients. A subanalysis of the CROES Ureteroscopy Global Study identified 11.9% of patients with ureteric calculi and 36.4% of patients with renal calculi to have been pre-stented [28]. Indications for stent placement were not recorded, but pre-stented patients had more comorbidities and were more likely to have smaller stones. The analysis demonstrated a higher stone free rate for pre-stented renal stones, but no benefit for ureteric stones [28]. These results were confirmed in a recent meta-analysis of nine retrospective studies, including 11 239 patients, identifying a stone-free rate in favour of pre-stented patients (OR [95%CI]: 1.6 [1.19, 2.15]) [29].
Based on these retrospective studies, the EAU guidelines state that it is not necessary to routinely pre-stent all patients before ureteroscopy, but that it may improve the stone-free rate and reduce complications [6].
Additionally, placement of a UAS has been shown to be easier in pre-stented patients [30].
Considering the low level of evidence, heterogeneity of data and contradicting results, together with the fact that stents cause considerable stent-related symptoms in a large part of patients, it may be prudent to reserve pre-stenting for patients in whom primary ureteroscopy has failed or in whom stent placement rather than primary ureteroscopy was indicated at presentation. A second ureteroscopy can be attempted within 2–4 weeks.
3. Stent-related complications
Despite the obvious benefits of ureteral stents, they may also induce adverse events. Ureteral stents are notoriously fraught with relevant patient discomfort, negatively affecting quality of life. The presence of an indwelling foreign body can also lead to biofilm-formation, which may promote development of UTIs or formation of encrustations, complicating subsequent stent removal.
3.1. Stent-related symptoms (SRS) and quality of life
Joshi et al. [31] identified 80% of patients with indwelling ureteral stents to have at least one urinary symptom. These SRS range from bladder/flank pain to storage symptoms to hematuria. Although the underlying pathophysiology behind these complaints are not yet fully elucidated, bladder wall and trigonal irritation by the distal coil as well as vesicoureteral reflux and retrograde pressure transmission have been proposed as underlying mechanisms [32], [33]. Apart from being bothersome for the patient, stent-related symptoms can also lead to socio-economic burden. Leibovici et al. [34] identified that among working patients, 45% lost at least 2 labor days during the first 14 days after stent insertion and 32% were still absent from work by Day 30. For Swiss patients the median cost associated with stent placement, removal, work incapacity and treatment of SRS was calculated by Staubli et al. [21] to be USD455 (113–11 948) per patient for the entire indwelling time and USD15 (4–398) per patient per day. Work incapacity was the biggest cost (78.1%), with younger patients showing a higher tendency to miss at least 1 working day.
Not all patients are equally bothered by stents and patient-reported outcome measures can help in quantifying patient discomfort. The absence of a comprehensive follow-up tool stimulated Joshi et al. [35] to develop a disease-specific questionnaire, the Ureteral Stent Symptom Questionnaire (USSQ), consisting of six sections: Urinary symptoms, body pain, general health, work performance, sexual matters and additional problems. This validated questionnaire has proven to be extremely useful in comparing treatments and stent designs in research for SRS and has been translated and validated in several languages [36], [37], [38], [39], [40], [41], [42].
3.2. Biofilms, UTIs and encrustations
Biofilm formation on the stent surface has been implicated as an important step in the process of stent associated UTI, stent encrustation and SRS. Biofilm formation is a multistep process, resulting in a complex, multilayered organized structure composed of organic molecules, fluid-filled spaces and bacteria that adhere to the stent surface [43]. Within this biofilm, microorganisms are protected from host defenses and antibiotics, which may lead to an accelerated development of antibiotic resistance. The deposition of conditioning film molecules, the first step of biofilm formation, starts almost immediately after stent insertion in the human body [44]. Bacterial colonization was reported in 24% before 4 weeks, 33% after 4–6 weeks and 71% thereafter [43]. Additionally, diabetes mellitus, chronic renal failure and pregnancy were associated with a higher risk of stent related bacteriuria [45]. Routine screening for and treatment of asymptomatic bacteriuria however is not recommended. A continuous low-dose antibiotic treatment during the entire stent-indwelling time showed no reduction in quantity and severity of UTIs and has no effect on SRS, compared to a single peri-interventional antibiotic prophylaxis at stent placement [46].
Urease producing bacteria in the biofilm and lithogenic characteristics of urine in stone formers seem to be the most likely culprits influencing encrustation of the stent surface [47], [48] (Fig. 1). The indwelling time is the most important risk factor for encrustation with encrustations presenting on stents in 9.2%–26.8% before 6 weeks, 47.5%–56.9% after 6 to 12 weeks and 75.9%–76.3% thereafter [49], [50]. These encrustations may block urinary drainage, resulting in patient symptoms or significantly complicate stent removal [51].
Figure 1.
Encrustations on stent surface after 2 weeks indwelling time in chronically infected urinary tract system.
Although several modifications of the stent surface to reduce biofilm formation and bacterial colonization have been investigated, at this moment no available materials or coatings have been proven to prevent or reduce biofilm formation to a clinically relevant extent [43].
3.3. The “forgotten stent”
Every stent that has been placed, will eventually need to be removed or replaced. Despite best efforts however, forgotten stents still emerge, often leading to major complications like encrustation, fragmentation, obstruction of urinary flow, renal failure, and even death [52]. In a series of 22 forgotten stents, Monga et al. [51] found that after a mean indwelling time of 22.7 months, 68% of stents were calcified, 45% were fragmented and 14% were both calcified and fragmented (Fig. 2). Due to these issues, removal of the device may be a challenging endeavor, often requiring multiple procedures combining different endourological or even open or laparoscopic approaches [51], [53]. As such, the removal of a forgotten stent can be up to 7-fold more expensive than the timely removal of a stent [54]. To avoid both legal and surgical consequences of forgotten stents, several approaches have been developed to monitor indwelling ureteral stents. Almost all of these are based on computer programs in which stent placement is registered and an automatic reminder is sent to patient and/or urologist after a preset period of time [55], [56], [57]. As this still requires proper registration of every stent insertion, human error cannot be eliminated completely.
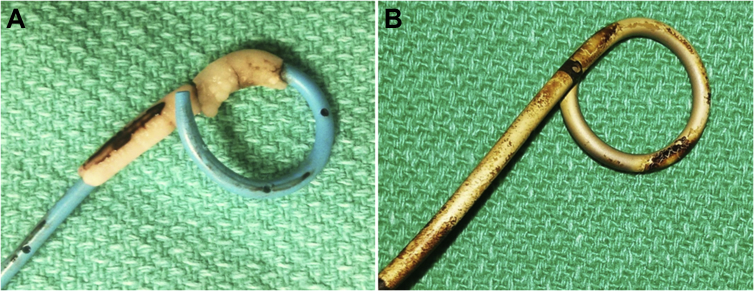
Figure 2.
Not all forgotten stents encrust over time. (A) Forgotten stent after 1 year in a cystinuric patient. (B) Forgotten stent after 2 years in a paraplegic patient.
3.4. Treatment and prevention of stent complications
Since Finney [4] described the first double-J stent in 1978, there has been a continuous search for the “perfect” or “ideal” stent. This stent should provide excellent drainage, resist migration, encrustation and infection, provoke no reaction or symptoms in the patient, be inexpensive and be easy to insert and remove. The main approaches for prevention and treatment of SRS and complications are stent design and drug therapy. Other focuses are stent positioning, patient education and intravesical drug application [58].
4. Developments in improving stent characteristics
4.1. Stent composition
4.1.1. Biomaterials and biocompatibility
A biomaterial is defined as a natural or synthetic substance that interfaces with tissue [59]. With increasing biocompatibility, there is a decreased reaction of the human body on the stent biomaterial. Currently used biomaterials for stent construction are synthetic polymers or (proprietary) copolymers such as silicone, polyethylene, polyurethane, C-Flex® (Cook Medical, IN, USA), Silitek® (Surgitek, WI, USA), Pellethane® (Bard Medical, GA, USA), Vertex® (Applied Medical, CA, USA) and Percuflex™ (Boston Scientific, MA, USA).
Although silicone is still the most biocompatible material currently available, its softness and high friction coefficient can make stent insertion in a tortuous or obstructed ureter very difficult [59], [60]. The stiffer and easier to insert polyethylene is no longer used for stent manufacturing due to biocompatibility issues and fragmentation of stents over time [60]. Polyurethane is an inexpensive polymer combining the flexibility of silicone with the stiffness of polyethylene. Unfortunately, polyurethane stents appear to be poorly biocompatible as they caused more epithelial ulceration and erosion than other stents in an animal study [59], [61]. Multiple proprietary (co)polymer biomaterials have been developed in search of an ideal combination of stiffness to increase ease of handling, flexibility to decrease stent related symptoms and biocompatibility to prevent encrustations and infections. Based on in vitro testing, Mardis and colleagues [59] suggested C-flex® and Percuflex™ to be the most suitable biomaterials. Results from an animal study demonstrated silicone and C-flex® to cause fewer tissue reactions in dog ureters [61]. Additionally, silicone stents were more resistant to encrustations compared to polyurethane of Percuflex™ stents in an in vitro model [62].
4.1.2. Hardness/durometer
Durometer is a measure of the resistance of the material to a calibrated pin gauge under standard test conditions, with a higher durometer indicating increased hardness. Variations are seen as a result of strength of cross-links in biomaterials used, with more cross links resulting in higher durometer [63]. A “softer” biomaterial would intuitively cause less urinary symptoms than a “firmer” ureteral stent. Lennon et al. [64] performed an RCT involving 155 patients comparing polyurethane to Sof-Flex® stents (Cook Medical, IN, US). Besides a significantly higher incidence of dysuria, renal and suprapubic pain in the firm stent group, no other differences in tolerance, encrustation or stent positioning could be identified. Normal activity and return to work was higher in patients with softer stents (67% vs. 45%). A similar RCT by Joshi et al. [63] with placement of a firm (Percuflex™ 6 Fr, Boston Scientific, MA, USA) versus soft (Contour™ 6 Fr, Boston Scientific, MA, USA) stent in 130 patients demonstrated no significant differences in USSQ-score between the two groups at 1 or 4 weeks after stent insertion.
Dual-durometer stents, such as the Sof-Curl™ (ACMI, MA, USA) and the Polaris™ (Microvasive/Boston Scientific, MA, USA), incorporate a smooth transition from a firm biomaterial at the proximal end to a softer biomaterial at the distal end, to minimize bladder discomfort. Two small RCTs however failed to demonstrate a significant benefit of the Polaris™ stent compared to the Percuflex™ (Boston Scientific, MA, USA) or InLay® (Bard Medical, GA, USA) stent [65], [66]. Park et al. [65] were able to identify some advantages in terms of pain, physical activities, work, and antibiotics use in favor of a softer stent tail.
4.2. Stent design
4.2.1. Stent diameter and drainage capacities
The total urinary flow in a stented ureter consists of intraluminal and extraluminal flow. Brewer and colleagues [67] demonstrated in an in vivo porcine model that with increasing stent diameter, as expected, the intraluminal flow increases. Efforts have been made to increase extraluminal flow with several stent designs such as the Towers stent (Cook Medical, IN, USA) and the LithoStent™ (ACMI, MA, USA), which have grooves spiraling down the exterior of the stent, or the Spirastent™ (Urosurge Inc, Coralville, Iowa), which is spiral shaped [68]. The Towers stent however was demonstrated to provide a lower total flow than other stents in an experimental setup [69]. Experimental results for the Spirastent™ were not consistent and a human trial failed to demonstrate its supposed benefits [69], [70], [71].
Side drainage holes may promote drainage, but can also make the stent more prone to breakage or buckling during insertion [72]. The importance of side holes in promoting urine flow seems to increase with narrowing of the ureteral lumen [73].
Interestingly, two RCTs demonstrated that larger diameter stents do not cause more SRS. The authors found no significant differences in terms of pain or irritative symptoms. There was however a tendency to higher migration in the 4.7 Fr stent group [74], [75]. This could persuade the physician to place a larger stent instead of a small caliber stent. On the other hand, a smaller stent would intuitively allow larger stone fragments to pass alongside the stent. The MicroStent™ (Percutaneous Systems, CA, USA) is a 3 Fr stent that utilizes a film anchor that is deployed above the ureteral obstruction as proximal retaining mechanism. In an experimental study by Lange et al. [76] the MicroStent™ showed a drainage capacity equivalent to a 4.7 Fr stent and better than a 3 Fr double-J stent. No results of human trials with this novel stent have been published to date.
4.2.2. Stent length
Most contemporary ureteral stents are manufactured in a variety of sizes. As several groups have reported that a stent crossing the midline of the bladder is a strong predictor of stent symptoms, placing a properly sized stent is of importance [77], [78], [79]. Additionally, a stent that is too short can result in stent migration, with the need for additional manipulations [80]. Although efforts have been made to identify a reliable predictor of the proper stent length for each patient, the gold standard for measuring the required stent length remains insertion of a graduated ureteral catheter, measuring the distance between ureteropelvic junction (UPJ) and ureterovesical junction (UVJ) [81]. Interestingly, post-mortem measurements of ureteric length could not identify a significant correlation with any anthropomorphic measurement [82]. Measurements deducted from pre-operative CT imaging or acquired during the procedure by fluoroscopic measurement appear to provide an adequate estimate of the appropriate stent length [81], [83], [84]. Ideal stent length for children has been formulated by Palmer JS and Palmer LS [85] as “child's age +10 cm”.
In an effort to provide a “one-size-fits-all” solution, reducing stock with stents of different lengths and obviating the process of estimating the appropriate stent length, variable length stents were designed. Although this results in more stent material in the bladder, this material is coiled up, preventing it from crossing the midline of the bladder. Calvert et al. [86] compared the standard Contour™ 6 Fr, 24 cm stent (Boston Scientific, MA, USA)) with a Contour multi-length stent, Contour VL™ 6 Fr, 22–30 cm (Boston Scientific, MA, USA) and found no difference in USSQ score on all domains.
In an observational study, El-Nahas and associates [79] identified calyceal positioning of the upper coil as an independent predictor of SRS. In contrast, Liatsikos et al. [87] demonstrated in a small RCT that upper pole positioning of the proximal coil was associated with fewer SRS. This can probably be attributed to a shorter intravesical stent portion when the stent is positioned higher in the kidney.
4.2.3. Distal stent coil adjustments
As the distal coil of the stent is hypothesized to be in part responsible for SRS, several design alterations have been proposed to reduce SRS. The conventional distal coil has been replaced by a loop, a tail and a simple suture in several trials [88], [89], [90].
Lingeman et al. [88] assessed two new stent designs, in which the distal pigtail end was replaced by a short or long 3 Fr loop. These stents were prospectively evaluated with the USSQ in 236 patients next to two standard stents (Polaris™ and Percuflex Plus™; Boston Scientific, MA, USA). Although the authors suggested an improved stent comfort, they could not demonstrate a significant benefit of the Loop stents over the conventional stents. The Tail stent (Boston Scientific, MA, USA) consists of a 7 Fr proximal pigtail and a 7 Fr shaft which tapers to a lumenless straight 3 Fr tail to decrease stent-related bladder irritability. In a small RCT, a significant 21% decrease in overall lower tract symptoms was identified in comparison to standard stents (Percuflex™) [79]. Olweny et al. [70] demonstrated the stent to provide similar drainage in comparison to conventional stent designs. Despite these advantages, the stent is unfortunately no longer available.
To mimic this design, Vogt et al. [90] designed a self-adjusted ureteral stent by removing the distal coil of a standard polyurethane stent and attaching a 0.3 Fr suture for subsequent removal of the stent. In their prospective study with 79 patients, they identified a significant decline in SRS in 24 patients after a classic stent was replaced by a suture stent. Interestingly, the suture appeared to provide adequate ureteral dilation, allowing subsequent ureteroscopy. The authors observed migration of the suture into the urethra in 13 patients, without causing incontinence and proximal migration in three patients.
Chew and associates [91] recently reported on a new design concept for ureteral stents. They evaluated pain scores in 15 patients that had a Percuflex Helical™ stent inserted and compared them with a historical patient group, stented with a standard Percuflex™ stent (Boston Scientific, MA, USA). The Helical stent is a standard Percuflex™ stent, cut in a spiral fashion in order to provide more flexibility and the ability to conform to the natural anatomy of the ureter. The Helical™ stent-group required significantly less analgesics and had equivalent pain scores. SRS by means of USSQ was unfortunately not inquired.
4.2.4. Anti-reflux mechanism
Another factor implicated with SRS is vesicoureteral reflux (VUR), induced by the stent bypassing the antireflux valve. Yossepowitch and colleagues [92] reported 27% of stented patients to have VUR immediately after insertion, which increased to 76% after an average of 9 weeks. Similarly, Sameh and Eid [33] demonstrated 80% of patients to have pressure transmission from the bladder to the kidney with pressure flow studies in 20 patients that had both a double-J stent and nephrostomy tube. To prevent this phenomenon from occurring, several stents with an antireflux mechanism have been designed. At the distal end of the stent, they consist of a valve mechanism that allows drainage of the kidney but closes with increasing intravesical pressure [94], [95], [96].
Ritter et al. [93] included 29 patients in an RCT comparing an antirefluxive stent to a regular stent. Although the antirefluxive stent appears to cause less SRS, the results were not statistically significant, probably due to a small sample size. In a larger RCT, including 133 patients, Ecke et al. [94] reached a significantly lower complication rate and higher acceptance rate with an antirefluxive stent. Kim and colleagues [95] developed a flexible polymeric flap valve that can be attached to the intravesical portion of a ureteral stent for the prevention of VUR. This flap valve closes off when intravesical pressure rises above 20 cmH2O. In an in vivo porcine model, a significantly lower VUR grade was noted without occurrence of antegrade urinary obstruction.
Although many promising design alterations have been developed, these have not penetrated in routine clinical practice yet. Larger, multi-center, well-designed studies will be needed to confirm these preliminary findings and persuade widespread adoption of stents that cause fewer symptoms.
4.2.5. Coatings
To increase biocompatibility in order to reduce device associated UTI or encrustations or to decrease the surface friction coefficient to facilitate stent manipulation, several different coatings have been applied on stent surfaces. Only a few of these have been investigated in human subjects.
4.2.5.1. Surface friction
A hydrogel is a polymeric biomaterial that results in a hydrophilic layer when hydrated, thus decreasing the surface friction coefficient and facilitating stent insertion [59]. In vitro results have not been able to consistently support the theoretical advantage against bacterial colonization and encrustation [96], [97], [98]. Hydrogel-coated stents impregnated with antibiotics however did show an antibacterial effect in an in vitro environment [97].
4.2.5.2. Resistance to bacterial colonization and encrustation
In an in vivo human trial, phosphorylcholine-coated ureteral stents showed less encrustation and colonization by bacterial biofilm than conventional stents in the same patients after a 12-week period [99]. Poly(vinyl pyrollidone)-coated biomaterials were demonstrated in an in vitro study to be very hydrophilic and more resistant to encrustation and bacterial adherence than uncoated polyurethane or silicone [100].
Heparin, a strong negatively charged glycosaminoglycan, has been demonstrated to resist encrustations in comparison to uncoated polyurethane stents after 1 month indwelling time in a human trial [101]. The authors also reported on two patients in whom stents had remained free of encrustations after 10 and 12 months [101]. Interestingly the heparin coating was not successful in preventing bacterial adhesion in an in vitro study [102].
In an observational study with 10 patients that were previously known with encrusting stents, diamond like carbon, a chemically inert substance, coated onto ureteral stents appeared to decrease encrustations and friction during insertion [103].
4.2.5.3. Drug-eluting stents
After promising animal tests with the Triumph® stent (Boston Scientific, MA, USA), a triclosan-eluting stent, two human trials were performed [104], [105], [106]. In short-term stented patients, the Triumph® stent demonstrated a significant reduction in SRS without reducing biofilm formation or encrustation [107]. In chronically stented patients, fewer symptomatic UTI were identified with a reduction in antibiotics use, while no fewer positive urine cultures were recorded [105]. It should be noted however that in both studies, patients receiving a control stent were given a course of post-operative antibiotics while they did not receive any antibiotics when the Triumph® stent was placed.
A ketoroloac-eluting stent, the Lexington™ stent (Boston Scientific, MA, USA), was designed with the goal of reducing stent-related pain. In a large multi-center RCT, Krambeck et al. [107] could not demonstrate a significant benefit over conventional stents in the entire population. Only in a subgroup of young men, there was a statistically lower need for pain medication.
Based on experience with intravesical instillations with ketorolac for stent symptoms [108], a ketorolac-eluting stent was designed (Lexington™ stent, Boston Scientific, MA, USA). In a multicenter, randomized prospective study with 276 patients this stent appeared safe, but showed no clear advantage in prevention of SRS. Only in a subgroup of young men, there was a statistically lower need for pain medication [107].
In an RCT with 126 patients, El-Nahas et al. [109] could not identify a clinically significant benefit of silver sulfadiazine coated stents over non-coated stents.
Although a large variety of other coatings has been and is being tested in vitro demonstrating promising results, human trials are still lacking to support their commercial use [110].
5. Medication
5.1. Alpha 1-blockers
Alpha1-blockers are often prescribed for outflow obstruction due to prostatic hyperplasia, but based on its working mechanism and the location of alpha1-adrenoreceptors in the ureter and bladder trigone, they can also affect SRS [111], [112]. The first placebo-controlled RCT, published in 2006, demonstrated alfuzosin to confer a significant benefit to stented patients with a relief in SRS [113]. The most recent meta-analysis on the effect of alpha 1-blockers on SRS by He et al. [114], included 16 studies with a total of 1 489 patients. Despite considerable heterogeneity in data, alpha-blockers appear to provide a significant relief in SRS on all USSQ subdomains except on work and additional issues [114]. Although alfuzosin and tamsulosin are the most investigated drugs to this purpose, a recent RCT including 239 patients also demonstrated a significant benefit of doxazosin for SRS [115].
5.2. Antimuscarinics
As SRS may resemble complaints of an overactive bladder (i.e. frequency, urgency, urge-incontinence), the use of antimuscarinics has been advocated and studied. Wang et al. [116] performed a meta-analysis of the available RCTs evaluating solifenacin or its combination with tamsulosin for the treatment of SRS, including ten studies comprising 1786 patients. Solifenacin monotherapy provided a significant reduction in total USSQ and hematuria-score and was not superior to tamsulosin. Although no published literature is available on the use of mirabegron, a beta-agonist, several trials are ongoing for its use in treating SRS (clinicaltrials.gov: NCT02095665, NCT02462837, NCT02744430).
5.3. Combination products
Whereas Wang and associates [116] concluded that the combination of solifenacin and tamsulosin provides no significant benefit over solifenacin monotherapy, Zhang et al. [117], in a meta-analysis also including RCTs on other antimuscarinics in combination with alpha-blockers, reported a significant improvement in International Prostate Symptom Score and quality of life with combination therapy in comparison to either monotherapy. With only three of the included studies reporting USSQ scores, a sub-analysis of these studies then again could not identify a benefit of combination therapy.
5.4. Analgesics
Since pain in the bladder, lumbar, flank, groin and genital region is frequently stent-related complaints, analgesic substances (paracetamol, non-steroidal anti-inflammatory drugs, opioids) are regularly prescribed. There are however, surprisingly, no trials assessing the influence of classical analgesics on SRS [58].
5.5. Intravesical treatment
To minimize side effects of oral medication, medical treatment can also be instilled in the bladder. In a double-blind RCT an intravesical instillation with oxybutynin, alkalinized lidocaine, ketorolac or a control solution was given immediately after stent placement at time of shock wave lithotripsy. The authors found a significant decrease in stent-related discomfort 1 h after instillation with intravesical ketorolac and oxybutynin in comparison to the control group [108]. These effects were however only short-lived (maximum 2 h), which renders it impractical in clinical settings.
Two small studies investigated the effect of injections in the bladder wall. Sur et al. [118] gave five injections of 2 mL 0.5% ropivacaine around the ureteral orifice and compared this with placebo injections. A slightly decreased pain and symptom score 8 h postoperatively until stent removal could be noted, but none of these differences was statistically significant. In an RCT, Gupta et al. [119] injected botulinum toxin in the detrusor muscle in three locations around the ureteral orifice of 30 patients and showed a significant decrease in pain score, but no improvement in USSQ scores. Due to lack of data, no clear conclusions can be made.
6. Patient education
When planning to stent a patient, the patient should be informed of the possible side-effects of this treatment as part of the informed consent. Abt and colleagues [120] aimed to quantify the influence of patient education on the patient's experience. The authors identified a small, but significant correlation (Pearson r = −0.40, 95%CI [−0.58, −0.19], p = 0.02) between USSQ-scores and high-quality patient education, without however demonstrating a reduction in incidence of symptoms. These results however do warrant further research on the influence of patient education on stent related symptoms and complications.
7. Procedures for stent removal
An indwelling stent after stone treatment has to be removed at some point in time, which is generally performed by flexible cystoscopy in an outpatient setting. To avoid possible complications associated with this procedure, several new methods of stent removal have been designed.
7.1. Mechanical (self-)removal with suture
Multiple reports discuss the use of tethered stents that have a string attached to the distal end of the stent. This string is left outside of the urethra, so the stent can be removed by the patient, physician or nurse and obviates cystoscopy. Apart from avoiding the possible complications related to a cystoscopy, this method can also reduce healthcare-related costs produced by a cystoscopy. An Australian group reported a cost-saving of A$864.5 per case when using tethered stents [121]. There are however concerns of potential dislodgement, discomfort due to the string and risk of developing a UTI. In an RCT with 68 patients, there was no significant difference in USSQ scores, pain at removal or UTI rates between conventional stents and tethered stents [122]. Fifteen percent of patients unwittingly removed the stent prematurely without however the need for replacement. Althaus et al. [123] reported a similar dislodgement rate, with a clear predominance of women over men (24.4% vs. 5.3%). Consequently, if longer-term stenting is desired, a non-tethered stent may prevent inadvertent premature removal.
In a retrospective cohort, Fröhlich et al. [124] could not demonstrate a significant risk of UTI for tethered stents (7.9%) in comparison to conventional stents (5.6%). Freifeld and associates [125] on the other hand did identify more UTI in patients with tethered stents in comparison to regular stents or non-stented patients after ureteroscopy (6.7% vs. 3% vs. 2.1% respectively) with an OR of 7.7 (95%CI: [1.01, 58.9], p = 0.049). In subgroup analysis, this effect was only significant in the male population. Both studies however are limited by the retrospective design and significant differences in patient populations.
Loh-Doyle et al. [126] surveyed 571 patients about their experience during stent removal. The majority of stents (44%) was removed by office cystoscopy, 17% by cystoscopy in the operation room and 39% by use of an extraction string (27% by doctor, 12% by patient). Although self-removal of a tethered stent appeared to be less painful than cystoscopic removal, it caused considerably more delayed pain, resulting in a higher rate of emergency department visits (14.71% vs. 4.17%) [126].
These results were corroborated by Kim and associates [127] who nuanced that stent extraction by string is significantly less painful only in the male subgroup. The group also confirmed earlier findings of Barnes et al. [122], reporting no differences in most of the USSQ domains. Sexually active patients in the tethered stent cohort however had considerably more complaints regarding sexual matters [127].
Patient counseling on the subsequent necessity of stent removal after placement including potential side-effects and education about the possibility of self-removal with a string may aid in the adoption of this technique by both physicians and patients.
7.2. Mechanical removal with magnet
In an effort to prevent cystoscopic removal of a ureteral stent, stents with a metal bead attached to the distal tip have been developed to facilitate removal by a magnet-tip urethral catheter [128], [129], [130], [131].
All studies reported high success rates of 86%–97% for removal by catheter [128], [129], [130]. Rassweiler et al. [130] compared magnet-tip stents (Blackstar®, Urotech, Germany) to conventional stents in an RCT and demonstrated an equivalent USSQ-score. The magnetic stent however caused more pain in the groin and bladder region whereas the regular stent caused more flank pain. Removal of the magnet-stent was significantly faster (9.55 min vs. 21.35 min), less painful and less costly than cystoscopic stent removal.
7.3. Biodegradable stents
In an effort to avoid any manipulation for stent removal and to reduce the forgotten stent phenomenon to 0, biodegradable stents are under development. Lingeman et al. [132] reported on the first in human trial of a biodegradable device in 87 patients. The stent was effectively retained in 80.5% of patients for at least 48 h. In 17 patients this failed because of early stent extrusion. Despite urethral discomfort in 68.2% during elimination, the authors noted a high degree of patient satisfaction. In three patients there was retention of stent fragments beyond 3 months, for which additional interventions (ESWL and/or ureteroscopy) were needed to clear the fragments.
Uriprene™, a biodegradable stent made of a variation of biodegradable copolymers similar to those used in absorbable sutures, is engineered to degrade from the distal to the proximal end to avoid ureteral obstruction by fragments. The third generation of this stent was designed to degrade over a shorter period of time and was evaluated in vivo in a porcine model, demonstrating complete degradation by Day 28 in 90% while providing excellent drainage [133].
Barros et al. [134] developed a biodegradable ureteral stent made from natural origin polysaccharides. In an in vitro study, breakdown in urine was seen after 14 to 60 days and the stent showed good resistance to bacterial adhesion. This new technology could also lend itself to incorporating drugs into the biomaterial, acting as a sustained release mechanism during stent degradation [135], [136].
Although these stents may not become commercially available in the next few years, human trials with this very promising technology will demonstrate whether or not it can hold the promise of obviating cystoscopic removal and eliminate the forgotten stent issue.
8. Conclusion
Ureteral stents have proven their worth in the treatment of urolithiasis. Although indications for stent placement have decreased, the presence of a ureteral stent can cause considerable complaints in patients. Despite current evidence supporting several strategies in preventing stent related symptoms or complication, widespread adoption often lags behind. Several knowledge gaps still exist due to the absence of adequately powered, prospective RCTs. Despite the vast amount of research on different biomaterials, stent coatings and novel designs, the ideal stent has not yet been developed. Although results so far are not always consistently in favour of new developments, the future goal of reduced stent symptoms is not unrealistic and is being approached one step at the time.
Author contributions
Both of the authors equally contributed to each part of the paper, from conception to data acquisition, data analysis, drafting the manuscript as well as the critical revision.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Shoemaker G.E., IV An improvement in the technique of catheterization of the ureter in the female. Ann Surg. 1895;22:650–654. doi: 10.1097/00000658-189507000-00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimskind P.D., Fetter T.R., Wilkerson J.L. Clinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopically. J Urol. 1967;97:840–844. doi: 10.1016/S0022-5347(17)63130-6. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons R.P., Mason J.T., Correa R.J. Experience with indwelling silicone rubber ureteral catheters. J Urol. 1974;111:594–599. doi: 10.1016/s0022-5347(17)60023-5. [DOI] [PubMed] [Google Scholar]
- 4.Finney R.P. Experience with new double J ureteral catheter stent. J Urol. 1978;120:678–681. doi: 10.1016/s0022-5347(17)57326-7. [DOI] [PubMed] [Google Scholar]
- 5.Hepperlen T.W., Mardis H.K., Kammandel H. Self-retained internal ureteral stents: a new approach. J Urol. 1978;119:731–734. doi: 10.1016/s0022-5347(17)57613-2. [DOI] [PubMed] [Google Scholar]
- 6.Türk C., Petřík A., Sarica K., Seitz C., Skolarikos A., Straub M. EAU guidelines on interventional treatment for urolithiasis. Eur Urol. 2016;69:475–482. doi: 10.1016/j.eururo.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 7.Pearle M.S., Pierce H.L., Miller G.L., Summa J.A., Mutz J.M., Petty B.A. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J Urol. 1998;160:1260–1264. [PubMed] [Google Scholar]
- 8.Mokhmalji H., Braun P.M., Martinez Portillo F.J., Siegsmund M., Alken P., Köhrmann K.U. Percutaneous nephrostomy versus ureteral stents for diversion of hydronephrosis caused by stones: a prospective, randomized clinical trial. J Urol. 2001;165:1088–1092. [PubMed] [Google Scholar]
- 9.Sammon J.D., Ghani K.R., Karakiewicz P.I., Bhojani N., Ravi P., Sun M. Temporal trends, practice patterns, and treatment outcomes for infected upper urinary tract stones in the United States. Eur Urol. 2013;64:85–92. doi: 10.1016/j.eururo.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 10.Goldsmith Z.G., Oredein-McCoy O., Gerber L., Bañez L.L., Sopko D.R., Miller M.J. Emergent ureteric stent vs percutaneous nephrostomy for obstructive urolithiasis with sepsis: patterns of use and outcomes from a 15-year experience. BJU Int. 2013;112:E122–E128. doi: 10.1111/bju.12161. [DOI] [PubMed] [Google Scholar]
- 11.Elsheemy M.S., Shouman A.M., Shoukry A.I., Elshenoufy A., Aboulela W., Daw K. Ureteric stents vs percutaneous nephrostomy for initial urinary drainage in children with obstructive anuria and acute renal failure due to ureteric calculi: a prospective, randomised study. BJU Int. 2015;115:473–479. doi: 10.1111/bju.12768. [DOI] [PubMed] [Google Scholar]
- 12.Assimos D., Krambeck A., Miller N.L., Monga M., Murad M.H., Nelson C.P. Surgical management of stones: American Urological Association/Endourological Society Guideline, PART II. J Urol. 2016:1–9. doi: 10.1016/j.juro.2016.05.090. [DOI] [PubMed] [Google Scholar]
- 13.Denstedt J.D., Wollin T.A., Sofer M., Nott L., Weir M., D’A Honey R.J. A prospective randomized controlled trial comparing nonstented versus stented ureteroscopic lithotripsy. J Urol. 2001;165:1419–1422. [PubMed] [Google Scholar]
- 14.Wang H., Man L., Li G., Huang G., Liu N., Wang J. Meta-analysis of stenting versus non-stenting for the treatment of ureteral stones. PLoS One. 2017;12:e0167670. doi: 10.1371/journal.pone.0167670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muslumanoglu A.Y., Fuglsig S., Frattini A., Labate G., Nadler R.B., Martov A. Risks and benefits of postoperative double-J stent placement after ureteroscopy: results from the Clinical Research Office of Endourological Society Ureteroscopy Global Study. J Endourol. 2017;31:446–451. doi: 10.1089/end.2016.0827. [DOI] [PubMed] [Google Scholar]
- 16.Giusti G., Proietti S., Villa L., Cloutier J., Rosso M., Gadda G.M. Current standard technique for modern flexible ureteroscopy: tips and tricks. Eur Urol. 2016;70:188–194. doi: 10.1016/j.eururo.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Astroza G., Catalán M., Consigliere L., Selman T., Salvadó J., Rubilar F. Is a ureteral stent required after use of ureteral access sheath in presented patients who undergo flexible ureteroscopy? Cent Eur J Urol. 2017;70:88–92. doi: 10.5173/ceju.2016.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torricelli F.C., De S., Hinck B., Noble M., Monga M. Flexible ureteroscopy with a ureteral access sheath: when to stent? Urology. 2014;83:278–281. doi: 10.1016/j.urology.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Sirithanaphol W., Jitpraphai S., Taweemonkongsap T., Nualyong C., Chotikawanich E. Ureteral stenting after flexible ureterorenoscopy with ureteral access sheath; Is it really needed?: A prospective randomized study. J Med Assoc Thail. 2017;100:S174–S178. [Google Scholar]
- 20.Seklehner S., Sievert K., Lee R., Engelhardt P.F., Riedl C., Kunit T. A cost analysis of stenting in uncomplicated semirigid ureteroscopic stone removal. Int Urol Nephrol. 2017;49:753–761. doi: 10.1007/s11255-017-1538-6. [DOI] [PubMed] [Google Scholar]
- 21.Staubli S.E.L., Mordasini L., Engeler D.S., Sauter R., Schmid H.P., Abt D. Economic aspects of morbidity caused by ureteral stents. Urol Int. 2016;97:91–97. doi: 10.1159/000443379. [DOI] [PubMed] [Google Scholar]
- 22.Shigemura K., Yasufuku T., Yamanaka K., Yamahsita M., Arakawa S., Fujisawa M. How long should double J stent be kept in after ureteroscopic lithotripsy? Urol Res. 2012;40:373–376. doi: 10.1007/s00240-011-0426-2. [DOI] [PubMed] [Google Scholar]
- 23.Nevo A., Mano R., Baniel J., Lifshitz D.A. Ureteric stent dwelling time: a risk factor for post-ureteroscopy sepsis. BJU Int. 2017;120:117–122. doi: 10.1111/bju.13796. [DOI] [PubMed] [Google Scholar]
- 24.Shen P., Jiang M., Yang J., Li X., Li Y., Wei W. Use of ureteral stent in extracorporeal shock wave lithotripsy for upper urinary calculi: a systematic review and meta-analysis. J Urol. 2011;186:1328–1335. doi: 10.1016/j.juro.2011.05.073. [DOI] [PubMed] [Google Scholar]
- 25.Al-Awadi K.A., Abdul Halim H., Kehinde E.O., Al-Tawheed A. Steinstrasse: a comparison of incidence with and without J stenting and the effect of J stenting on subsequent management. BJU Int. 1999;84:618–621. doi: 10.1046/j.1464-410x.1999.00280.x. [DOI] [PubMed] [Google Scholar]
- 26.Fuller T.W., Rycyna K.J., Ayyash O.M., Ferroni M.C., Mitchell C.R., Ohmann E. Defining the rate of primary ureteroscopic failure in unstented patients: a multi-institutional study. J Endourol. 2016;30:970–974. doi: 10.1089/end.2016.0304. [DOI] [PubMed] [Google Scholar]
- 27.Hubert K.C., Palmer J.S. Passive dilation by ureteral stenting before ureteroscopy: eliminating the need for active dilation. J Urol. 2005;174:1079–1080. doi: 10.1097/01.ju.0000169130.80049.9c. [DOI] [PubMed] [Google Scholar]
- 28.Assimos D., Crisci A., Culkin D., Xue W., Roelofs A., Duvdevani M. Preoperative JJ stent placement in ureteric and renal stone treatment: results from the Clinical Research Office of Endourological Society (CROES) Ureteroscopy (URS) Global Study. BJU Int. 2016;117:648–654. doi: 10.1111/bju.13250. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y., Tang Y., Bai Y., Wang X., Feng D., Han P. Preoperative double-J stent placement can improve the stone-free rate for patients undergoing ureteroscopic lithotripsy: a systematic review and meta-analysis. Urolithiasis. 2017:1–7. doi: 10.1007/s00240-017-1012-z. [DOI] [PubMed] [Google Scholar]
- 30.Kawahara T., Ito H., Terao H., Ishigaki H., Ogawa T., Uemura H. Preoperative stenting for ureteroscopic lithotripsy for a large renal stone. Int J Urol. 2012;19:881–885. doi: 10.1111/j.1442-2042.2012.03046.x. [DOI] [PubMed] [Google Scholar]
- 31.Joshi H.B., Okeke A., Newns N., Keeley F.X., Timoney A.G. Characterization of urinary symptoms in patients with ureteral stents. Urology. 2002;59:511–516. doi: 10.1016/s0090-4295(01)01644-2. [DOI] [PubMed] [Google Scholar]
- 32.Koprowski C., Kim C., Modi P.K., Elsamra S.E. Ureteral stent-associated pain: a review. J Endourol. 2016;30:744–753. doi: 10.1089/end.2016.0129. [DOI] [PubMed] [Google Scholar]
- 33.Sameh W.M., Eid A.A. Pressure transmission through ureteric stents: a novel in vivo human study. Urology. 2012;79:766–770. doi: 10.1016/j.urology.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 34.Leibovici D., Cooper A., Lindner A., Ostrowsky R., Kleinmann J., Velikanov S. Ureteral stents: morbidity and impact on quality of life. Isr Med Assoc J. 2005;7:491–494. [PubMed] [Google Scholar]
- 35.Joshi H.B., Newns N., Stainthorpe A., MacDonagh R.P., Keeley F.X., Timoney A.G. Ureteral stent symptom questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060–1064. doi: 10.1097/01.ju.0000049198.53424.1d. [DOI] [PubMed] [Google Scholar]
- 36.Giannarini G., Keeley F.X., Valent F., Milesi C., Mogorovich A., Manassero F. The Italian linguistic validation of the Ureteral Stent Symptoms Questionnaire. J Urol. 2008;180:624–628. doi: 10.1016/j.juro.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 37.Park J., Shin D.W., You C., Chung K.J., Han D.H., Joshi H.B. Cross-cultural application of the Korean version of Ureteral Stent Symptoms Questionnaire. J Endourol. 2012;26:1518–1522. doi: 10.1089/end.2012.0235. [DOI] [PubMed] [Google Scholar]
- 38.Sanguedolce F., Millán-Rodriguez F., Santillana-Altimira J.M., Fantova-Alonso A., Sánchez-Martín F.M., Angerri-Feu O. The Spanish linguistic validation of the Ureteral Stent Symptom Questionnaire. J Endourol. 2014;28:237–242. doi: 10.1089/end.2013.0325. [DOI] [PubMed] [Google Scholar]
- 39.Puichaud A., Larré S., Bruyère F., Auger J., Bret N., Chevreste A. [The French linguistic validation of the Ureteric Stent Symptom Questionnaire (USSQ)] Prog Urol. 2010;20:210–213. doi: 10.1016/j.purol.2009.09.007. [Article in French] [DOI] [PubMed] [Google Scholar]
- 40.Tanidir Y., Mangir N., Sahan A., Sulukaya M. Turkish version of the Ureteral Stent Symptoms Questionnaire: linguistic and psychometric validation. World J Urol. 2017;35:1149–1154. doi: 10.1007/s00345-016-1958-4. [DOI] [PubMed] [Google Scholar]
- 41.El-Nahas A.R., Elsaadany M.M., Tharwat M., Mosbah A., Metwally A.H., Hawary A. Validation of the Arabic linguistic version of the Ureteral Stent Symptoms Questionnaire. Arab J Urol. 2014;12:290–293. doi: 10.1016/j.aju.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee C., Kuskowski M., Premoli J., Skemp N., Monga M. Randomized evaluation of ureteral stents using validated symptom questionnaire. J Endourol. 2005;19:990–993. doi: 10.1089/end.2005.19.990. [DOI] [PubMed] [Google Scholar]
- 43.Zumstein V., Betschart P., Albrich W.C., Buhmann M.T., Ren Q., Schmid H.P. Biofilm formation on ureteral stents - incidence, clinical impact, and prevention. Swiss Med Wkly. 2017;147:w14408. doi: 10.4414/smw.2017.14408. [DOI] [PubMed] [Google Scholar]
- 44.Tieszer C., Reid G., Denstedt J. Conditioning film deposition on ureteral stents after implantation. J Urol. 1998;160:876–881. doi: 10.1016/S0022-5347(01)62825-8. [DOI] [PubMed] [Google Scholar]
- 45.Akay A.F., Aflay U., Gedik A., Sahin H., Bircan M.K. Risk factors for lower urinary tract infection and bacterial stent colonization in patients with a double J ureteral stent. Int Urol Nephrol. 2007;39:95–98. doi: 10.1007/s11255-006-9150-1. [DOI] [PubMed] [Google Scholar]
- 46.Moltzahn F., Haeni K., Birkhäuser F.D., Roth B., Thalmann G.N., Zehnder P. Peri-interventional antibiotic prophylaxis only vs. continuous low-dose antibiotic treatment in patients with JJ stents: a prospective randomised trial analysing the effect on urinary tract infections and stent-related symptoms. BJU Int. 2013;111:289–295. doi: 10.1111/j.1464-410X.2012.11592.x. [DOI] [PubMed] [Google Scholar]
- 47.Sighinolfi M.C., Sighinolfi G.P., Galli E., Micali S., Ferrari N., Mofferdin A. Chemical and mineralogical analysis of ureteral stent encrustation and associated risk factors. Urology. 2015;86:703–706. doi: 10.1016/j.urology.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 48.Broomfield R.J., Morgan S.D., Khan A., Stickler D.J. Crystalline bacterial biofilm formation on urinary catheters by urease-producing urinary tract pathogens: a simple method of control. J Med Microbiol. 2009;58:1367–1375. doi: 10.1099/jmm.0.012419-0. [DOI] [PubMed] [Google Scholar]
- 49.El-Faqih S.R., Shamsuddin A.B., Chakrabarti A., Atassi R., Kardar A.H., Osman M.K. Polyurethane internal ureteral stents in treatment of stone patients: morbidity related to indwelling times. J Urol. 1991;146:1487–1491. doi: 10.1016/s0022-5347(17)38146-6. [DOI] [PubMed] [Google Scholar]
- 50.Kawahara T., Ito H., Terao H., Yoshida M., Matsuzaki J. Ureteral stent encrustation, incrustation, and coloring: morbidity related to indwelling times. J Endourol. 2012;26:178–182. doi: 10.1089/end.2011.0385. [DOI] [PubMed] [Google Scholar]
- 51.Monga M., Klein E., Castañeda-Zúñiga W.R., Thomas R. The forgotten indwelling ureteral stent: a urological dilemma. J Urol. 1995;153:1817–1819. [PubMed] [Google Scholar]
- 52.Singh V., Srinivastava A., Kapoor R., Kumar A. Can the complicated forgotten indwelling ureteric stents be lethal? Int Urol Nephrol. 2005;37:541–546. doi: 10.1007/s11255-004-4704-6. [DOI] [PubMed] [Google Scholar]
- 53.Bultitude M.F., Tiptaft R.C., Glass J.M., Dasgupta P. Management of encrusted ureteral stents impacted in upper tract. Urology. 2003;62:622–626. doi: 10.1016/s0090-4295(03)00506-5. [DOI] [PubMed] [Google Scholar]
- 54.Sancaktutar A.A., Söylemez H., Bozkurt Y., Penbegül N., Atar M. Treatment of forgotten ureteral stents: how much does it really cost? A cost-effectiveness study in 27 patients. Urol Res. 2012;40:317–325. doi: 10.1007/s00240-011-0409-3. [DOI] [PubMed] [Google Scholar]
- 55.Lynch M.F., Ghani K.R., Frost I., Anson K.M. Preventing the forgotten ureteral stent: implementation of a web-based stent registry with automatic recall application. Urology. 2007;70:423–426. doi: 10.1016/j.urology.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 56.Sancaktutar A.A., Tepeler A., Söylemez H., Penbegül N., Atar M., Bozkurt Y. A solution for medical and legal problems arising from forgotten ureteral stents: initial results from a reminder short message service (SMS) Urol Res. 2012;40:253–258. doi: 10.1007/s00240-011-0404-8. [DOI] [PubMed] [Google Scholar]
- 57.Ziemba J.B., Ludwig W.W., Ruiz L., Carvalhal E., Matlaga B.R. Preventing the forgotten ureteral stent by using a mobile point-of-care application. J Endourol. 2017;31:719–724. doi: 10.1089/end.2017.0118. [DOI] [PubMed] [Google Scholar]
- 58.Betschart P., Zumstein V., Piller A., Schmid H.P., Abt D. Prevention and treatment of symptoms associated with indwelling ureteral stents: a systematic review. Int J Urol. 2017;24:250–259. doi: 10.1111/iju.13311. [DOI] [PubMed] [Google Scholar]
- 59.Mardis H.K., Kroeger R.M., Morton J.J., Donovan J.M. Comparative evaluation of materials used for internal ureteral stents. J Endourol. 1993;7:105–115. doi: 10.1089/end.1993.7.105. [DOI] [PubMed] [Google Scholar]
- 60.Denstedt J.D., Wollin T.A., Reid G. Biomaterials used in urology: current issues of biocompatibility, infection, and encrustation. J Endourol. 1998;12:493–500. doi: 10.1089/end.1998.12.493. [DOI] [PubMed] [Google Scholar]
- 61.Marx M., Bettmann M.A., Bridge S., Brodsky G., Boxt L.M., Richie J.P. The effects of various indwelling ureteral catheter materials on the normal canine ureter. J Urol. 1988;139:180–185. doi: 10.1016/s0022-5347(17)42349-4. [DOI] [PubMed] [Google Scholar]
- 62.Gorman S.P., Garvin C.P., Quigley F., Jones D.S. Design and validation of a dynamic flow model simulating encrustation of biomaterials in the urinary tract. J Pharm Pharmacol. 2003;55:461–468. doi: 10.1211/002235702856. [DOI] [PubMed] [Google Scholar]
- 63.Joshi H.B., Chitale S.V., Nagarajan M., Irving S.O., Browning A.J., Biyani C.S. A prospective randomized single-blind comparison of ureteral stents composed of firm and soft polymer. J Urol. 2005;174:2303–2306. doi: 10.1097/01.ju.0000181815.63998.5f. [DOI] [PubMed] [Google Scholar]
- 64.Lennon G.M., Thornhill J.A., Sweeney P.A., Grainger R., McDermott T.E., Butler M.R. “Firm” versus “soft” double pigtail ureteric stents: a randomised blind comparative trial. Eur Urol. 1995;28:1–5. doi: 10.1159/000475010. [DOI] [PubMed] [Google Scholar]
- 65.Park H.K., Paick S.H., Kim H.G., Lho Y.S., Bae S. The impact of ureteral stent type on patient symptoms as determined by the ureteral stent symptom questionnaire: a prospective, randomized, controlled study. J Endourol. 2015;29:367–371. doi: 10.1089/end.2014.0294. [DOI] [PubMed] [Google Scholar]
- 66.Davenport K., Kumar V., Collins J., Melotti R., Timoney A.G., Keeley F.X. New ureteral stent design does not improve patient quality of life: a randomized, controlled trial. J Urol. 2011;185:175–178. doi: 10.1016/j.juro.2010.08.089. [DOI] [PubMed] [Google Scholar]
- 67.Brewer A.V., Elbahnasy A.M., Bercowsky E., Maxwell K.L., Shalhav A.L., Kahn S.A. Mechanism of ureteral stent flow: a comparative in vivo study. J Endourol. 1999;13:269–271. doi: 10.1089/end.1999.13.269. [DOI] [PubMed] [Google Scholar]
- 68.Lam J.S., Gupta M. Update on ureteral stents. Urology. 2004;64:9–15. doi: 10.1016/j.urology.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Stoller M.L., Schwartz B.F., Frigstad J.R., Norris L., Park J.B., Magliochetti M.J. An in vitro assessment of the flow characteristics of spiral-ridged and smooth-walled JJ ureteric stents. BJU Int. 2000;85:628–631. doi: 10.1046/j.1464-410x.2000.00489.x. [DOI] [PubMed] [Google Scholar]
- 70.Olweny E.O., Portis A.J., Afane J.S., Brewer A.V., Shalhav A.L., Luszczynski K. Flow characteristics of 3 unique ureteral stents: investigation of a Poiseuille flow pattern. J Urol. 2000;164:2099–2103. [PubMed] [Google Scholar]
- 71.Gerber R., Nitz C., Studer U.E., Danuser H. Spiral stent versus standard stent in patients with midsize renal stones treated with extracorporeal shock wave lithotripsy: which stent works better? A prospective randomized trial. J Urol. 2004;172:965–966. doi: 10.1097/01.ju.0000134544.78768.12. [DOI] [PubMed] [Google Scholar]
- 72.Chua M.E., Morales M.L., Jr. Spontaneous fracture of indwelling polyurethane ureteral stents: a case series and review of literature. Can Urol Assoc J. 2012;6:386–392. doi: 10.5489/cuaj.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim H.-H., Choi Y.H., Lee S.B., Baba Y., Kim K.W., Suh S.H. Numerical analysis of urine flow through the side holes of a double J stent in a ureteral stenosis. Technol Health Care. 2017;25:63–72. doi: 10.3233/THC-171307. [DOI] [PubMed] [Google Scholar]
- 74.Erturk E., Sessions A., Joseph J.V. Impact of ureteral stent diameter on symptoms and tolerability. J Endourol. 2003;17:59–62. doi: 10.1089/08927790360587342. [DOI] [PubMed] [Google Scholar]
- 75.Damiano R., Autorino R., De Sio M., Cantiello F., Quarto G., Perdonà S. Does the size of ureteral stent impact urinary symptoms and quality of life? A prospective randomized study. Eur Urol. 2005;48:673–678. doi: 10.1016/j.eururo.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 76.Lange D., Hoag N.A., Poh B.K., Chew B.H. Drainage characteristics of the 3F MicroStent using a novel film occlusion anchoring mechanism. J Endourol. 2011;25:1051–1056. doi: 10.1089/end.2010.0722. [DOI] [PubMed] [Google Scholar]
- 77.Al-Kandari A.M., Al-Shaiji T.F., Shaaban H., Ibrahim H.M., Elshebiny Y.H., Shokeir A.A. Effects of proximal and distal ends of double-J ureteral stent position on postprocedural symptoms and quality of life: a randomized clinical trial. J Endourol. 2007;21:698–702. doi: 10.1089/end.2007.9949. [DOI] [PubMed] [Google Scholar]
- 78.Giannarini G., Keeley F.X., Valent F., Manassero F., Mogorovich A., Autorino R. Predictors of morbidity in patients with indwelling ureteric stents: results of a prospective study using the validated Ureteric Stent Symptoms Questionnaire. BJU Int. 2011;107:648–654. doi: 10.1111/j.1464-410X.2010.09482.x. [DOI] [PubMed] [Google Scholar]
- 79.El-Nahas A.R., El-Assmy A.M., Shoma A.M., Eraky I., El-Kenawy M.R., El-Kappany H.A. Self-retaining ureteral stents: analysis of factors responsible for patients' discomfort. J Endourol. 2006;20:33–37. doi: 10.1089/end.2006.20.33. [DOI] [PubMed] [Google Scholar]
- 80.Breau R.H., Norman R.W. Optimal prevention and management of proximal ureteral stent migration and remigration. J Urol. 2001;166:890–893. [PubMed] [Google Scholar]
- 81.Barrett K., Foell K., Lantz A., Ordon M., Lee J.Y., Pace K.T. Best stent length predicted by simple CT measurement rather than patient height. J Endourol. 2016;30:1029–1032. doi: 10.1089/end.2016.0105. [DOI] [PubMed] [Google Scholar]
- 82.Novaes H.F.F., Leite P.C.S., Almeida R.A., Sorte N.C.B., Barroso U. Analysis of ureteral length in adult cadavers. Int Braz J Urol. 2013;39:248–256. doi: 10.1590/S1677-5538.IBJU.2013.01.14. [DOI] [PubMed] [Google Scholar]
- 83.Barrett K., Ghiculete D., Sowerby R.J., Farcas M., Pace K.T., Honey R.J.D. Intraoperative radiographic determination of ureteral length as a method of determining ideal stent length. J Endourol. 2017;31:S101–S105. doi: 10.1089/end.2016.0709. [DOI] [PubMed] [Google Scholar]
- 84.Shrewsberry A.B., Al-Qassab U., Goodman M., Petros J.A., Sullivan J.W., Ritenour C.W.M. A +20% adjustment in the computed tomography measured ureteral length is an accurate predictor of true ureteral length before ureteral stent placement. J Endourol. 2013;27:1041–1045. doi: 10.1089/end.2013.0041. [DOI] [PubMed] [Google Scholar]
- 85.Palmer J.S., Palmer L.S. Determining the proper stent length to use in children: age plus 10. J Urol. 2007;178:1566–1569. doi: 10.1016/j.juro.2007.03.191. [DOI] [PubMed] [Google Scholar]
- 86.Calvert R.C., Wong K.Y., Chitale S.V., Irving S.O., Nagarajan M., Biyani C.S. Multi-length or 24 cm ureteric stent? A multicentre randomised comparison of stent-related symptoms using a validated questionnaire. BJU Int. 2013;111:1099–1104. doi: 10.1111/j.1464-410X.2012.11388.x. [DOI] [PubMed] [Google Scholar]
- 87.Liatsikos E.N., Gershbaum D., Kapoor R., Fogarty J., Dinlenc C.Z., Bernardo N.O. Comparison of symptoms related to positioning of double-pigtail stent in upper pole versus renal pelvis. J Endourol. 2001;15:299–302. doi: 10.1089/089277901750161854. [DOI] [PubMed] [Google Scholar]
- 88.Lingeman J.E., Preminger G.M., Goldfischer E.R., Krambeck A.E. Assessing the impact of ureteral stent design on patient comfort. J Urol. 2009;181:2581–2587. doi: 10.1016/j.juro.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dunn M.D., Portis A.J., Kahn S.A., Yan Y., Shalhav A.L., Elbahnasy A.M. Clinical effectiveness of new stent design: randomized single-blind comparison of tail and double-pigtail stents. J Endourol. 2000;14:195–202. doi: 10.1089/end.2000.14.195. [DOI] [PubMed] [Google Scholar]
- 90.Vogt B., Desgrippes A., Desfemmes F.N. Changing the double-pigtail stent by a new suture stent to improve patient's quality of life: a prospective study. World J Urol. 2015;33:1061–1068. doi: 10.1007/s00345-014-1394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chew B.H., Rebullar K.A., Harriman D., McDougall E., Paterson R.F., Lange D. Percuflex helical ureteral stents significantly reduce patient analgesic requirements compared to control stents. J Endourol. 2017;31:1321–1325. doi: 10.1089/end.2017.0349. [DOI] [PubMed] [Google Scholar]
- 92.Yossepowitch O., Lifshitz D.A., Dekel Y., Ehrlich Y., Gur U., Margel D. Assessment of vesicoureteral reflux in patients with self-retaining ureteral stents: implications for upper urinary tract instillation. J Urol. 2005;173:890–893. doi: 10.1097/01.ju.0000147747.89028.64. [DOI] [PubMed] [Google Scholar]
- 93.Ritter M., Krombach P., Knoll T., Michel M.S., Haecker A. Initial experience with a newly developed antirefluxive ureter stent. Urol Res. 2012;40:349–353. doi: 10.1007/s00240-011-0415-5. [DOI] [PubMed] [Google Scholar]
- 94.Ecke T.H., Bartel P., Hallmann S., Ruttloff J. Evaluation of symptoms and patients' comfort for JJ-ureteral stents with and without antireflux-membrane valve. Urology. 2010;75:212–216. doi: 10.1016/j.urology.2009.07.1258. [DOI] [PubMed] [Google Scholar]
- 95.Kim H.W., Park C.J., Seo S., Park Y., Lee J.Z., Shin D.G. Evaluation of a polymeric flap valve-attached ureteral stent for preventing vesicoureteral reflux in elevated intravesical pressure conditions: a pilot study using a porcine model. J Endourol. 2016;30:428–432. doi: 10.1089/end.2015.0711. [DOI] [PubMed] [Google Scholar]
- 96.Desgrandchamps F., Moulinier F., Daudon M., Teillac P., Le Duc A. An in vitro comparison of urease-induced encrustation of JJ stents in human urine. Br J Urol. 1997;79:24–27. doi: 10.1046/j.1464-410x.1997.02775.x. [DOI] [PubMed] [Google Scholar]
- 97.John T., Rajpurkar A., Smith G., Fairfax M., Triest J. Antibiotic pretreatment of hydrogel ureteral stent. J Endourol. 2007;21:1211–1216. doi: 10.1089/end.2007.9904. [DOI] [PubMed] [Google Scholar]
- 98.Gorman S.P., Tunney M.M., Keane P.F., Van Bladel K., Bley B. Characterization and assessment of a novel poly(ethylene oxide)/polyurethane composite hydrogel (Aquavene) as a ureteral stent biomaterial. J Biomed Mater Res. 1998;39:642–649. doi: 10.1002/(sici)1097-4636(19980315)39:4<642::aid-jbm20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 99.Stickler D.J., Evans A., Morris N., Hughes G. Strategies for the control of catheter encrustation. Int J Antimicrob Agents. 2002;19:499–506. doi: 10.1016/s0924-8579(02)00091-2. [DOI] [PubMed] [Google Scholar]
- 100.Tunney M.M., Gorman S.P. Evaluation of a poly(vinyl pyrollidone)-coated biomaterial for urological use. Biomaterials. 2002;23:4601–4608. doi: 10.1016/s0142-9612(02)00206-5. [DOI] [PubMed] [Google Scholar]
- 101.Cauda F., Cauda V., Fiori C., Onida B., Garrone E. Heparin coating on ureteral Double J stents prevents encrustations: an in vivo case study. J Endourol. 2008;22:465–472. doi: 10.1089/end.2007.0218. [DOI] [PubMed] [Google Scholar]
- 102.Lange D., Elwood C.N., Choi K., Hendlin K., Monga M., Chew B.H. Uropathogen interaction with the surface of urological stents using different surface properties. J Urol. 2009;182:1194–1200. doi: 10.1016/j.juro.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 103.Laube N., Kleinen L., Bradenahl J., Meissner A. Diamond-like carbon coatings on ureteral stents—a new strategy for decreasing the formation of crystalline bacterial biofilms? J Urol. 2007;177:1923–1927. doi: 10.1016/j.juro.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 104.Cadieux P.A., Chew B.H., Knudsen B.E., Dejong K., Rowe E., Reid G. Triclosan loaded ureteral stents decrease proteus mirabilis 296 infection in a rabbit urinary tract infection model. J Urol. 2006;175:2331–2335. doi: 10.1016/S0022-5347(06)00252-7. [DOI] [PubMed] [Google Scholar]
- 105.Cadieux P.A., Chew B.H., Nott L., Seney S., Elwood C.N., Wignall G.R. Use of triclosan-eluting ureteral stents in patients with long-term stents. J Endourol. 2009;23:1187–1194. doi: 10.1089/end.2008.0437. [DOI] [PubMed] [Google Scholar]
- 106.Mendez-Probst C.E., Goneau L.W., MacDonald K.W., Nott L., Seney S., Elwood C.N. The use of triclosan eluting stents effectively reduces ureteral stent symptoms: a prospective randomized trial. BJU Int. 2012;110:749–754. doi: 10.1111/j.1464-410X.2011.10903.x. [DOI] [PubMed] [Google Scholar]
- 107.Krambeck A.E., Walsh R.S., Denstedt J.D., Preminger G.M., Li J., Evans J.C. A novel drug eluting ureteral stent: a prospective, randomized, multicenter clinical trial to evaluate the safety and effectiveness of a ketorolac loaded ureteral stent. J Urol. 2010;183:1037–1042. doi: 10.1016/j.juro.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 108.Beiko D.T., Watterson J.D., Knudsen B.E., Nott L., Pautler S.E., Brock G.B. Double-blind randomized controlled trial assessing the safety and efficacy of intravesical agents for ureteral stent symptoms after extracorporeal shockwave lithotripsy. J Endourol. 2004;18:723–730. doi: 10.1089/end.2004.18.723. [DOI] [PubMed] [Google Scholar]
- 109.El-Nahas A.R., Lachine M., Elsawy E., Mosbah A., El-Kappany H. A randomized controlled trial comparing antimicrobial (silver sulfadiazine)-coated ureteral stents with non-coated stents. Scand J Urol. 2017:1–5. doi: 10.1080/21681805.2017.1376353. [DOI] [PubMed] [Google Scholar]
- 110.Yang L., Whiteside S., Cadieux P.A., Denstedt J.D. Ureteral stent technology: drug-eluting stents and stent coatings. Asian J Urol. 2015;2:194–201. doi: 10.1016/j.ajur.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sigala S., Dellabella M., Milanese G., Fornari S., Faccoli S., Palazzolo F. Evidence for the presence of alpha1 adrenoceptor subtypes in the human ureter. Neurourol Urodyn. 2005;24:142–148. doi: 10.1002/nau.20097. [DOI] [PubMed] [Google Scholar]
- 112.Sigala S., Peroni A., Mirabella G., Fornari S., Palazzolo F., Pezzotti G. Alpha 1 adrenoceptor subtypes in human urinary bladder: sex and regional comparison. Life Sci. 2004;76:417–427. doi: 10.1016/j.lfs.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 113.Deliveliotis C., Chrisofos M., Gougousis E., Papatsoris A., Dellis A., Varkarakis I.M. Is there a role for alpha1-blockers in treating double-J stent-related symptoms? Urology. 2006;67:35–39. doi: 10.1016/j.urology.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 114.He F., Man L.B., Li G.Z., Liu N. Efficacy of α-blocker in improving ureteral stent-related symptoms: a meta-analysis of both direct and indirect comparison. Drug Des Dev Ther. 2016;10:1783–1793. doi: 10.2147/DDDT.S103195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang L., Li J., Pan M., Han W., Liu S., Xiao Y. Doxazosin oral intake therapy to relieve stent-related urinary symptoms and pain: a prospective, randomized, controlled study. Int Braz J Urol. 2016;42:727–733. doi: 10.1590/S1677-5538.IBJU.2015.0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang J., Zhang X., Zhang T., Mu J., Bai B., Lei Y. The role of solifenacin, as monotherapy or combination with tamsulosin in ureteral stent-related symptoms: a systematic review and meta-analysis. World J Urol. 2017;35:1669–1680. doi: 10.1007/s00345-017-2051-3. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Y.M., Chu P., Wang W.J. PRISMA-combined α-blockers and antimuscarinics for ureteral stent-related symptoms: a meta-analysis. Medicine (Baltim) 2017;96:e6098. doi: 10.1097/MD.0000000000006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sur R.L., Haleblian G.E., Cantor D.A., Springhart W.P., Albala D.M., Preminger G.M. Efficacy of intravesical ropivacaine injection on urinary symptoms following ureteral stenting: a randomized, controlled study. J Endourol. 2008;22:473–478. doi: 10.1089/end.2007.9847. [DOI] [PubMed] [Google Scholar]
- 119.Gupta M., Patel T., Xavier K., Maruffo F., Lehman D., Walsh R. Prospective randomized evaluation of periureteral botulinum toxin type A injection for ureteral stent pain reduction. J Urol. 2010;183:598–602. doi: 10.1016/j.juro.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 120.Abt D., Warzinek E., Schmid H.P., Haile S.R., Engeler D.S. Influence of patient education on morbidity caused by ureteral stents. Int J Urol. 2015;22:679–683. doi: 10.1111/iju.12782. [DOI] [PubMed] [Google Scholar]
- 121.Okullo A., Yuminaga Y., Ziaziaris W., Ende D., Lau H., Brooks A. Comparison of cost of care for tethered versus non-tethered ureteric stents in the management of uncomplicated upper urinary tract stones. ANZ J Surg. 2017;87:505–508. doi: 10.1111/ans.13945. [DOI] [PubMed] [Google Scholar]
- 122.Barnes K.T., Bing M.T., Tracy C.R. Do ureteric stent extraction strings affect stent-related quality of life or complications after ureteroscopy for urolithiasis: a prospective randomised control trial. BJU Int. 2014;113:605–609. doi: 10.1111/bju.12541. [DOI] [PubMed] [Google Scholar]
- 123.Althaus A.B., Li K., Pattison E., Eisner B., Pais V., Steinberg P. Rate of dislodgment of ureteral stents when using an extraction string after endoscopic urological surgery. J Urol. 2015;193:2011–2014. doi: 10.1016/j.juro.2014.12.087. [DOI] [PubMed] [Google Scholar]
- 124.Fröhlich M., Fehr J., Sulser T., Eberli D., Mortezavi A. Extraction strings for ureteric stents: is there an increased risk for urinary tract infections? Surg Infect (Larchmt) 2017;18:936–940. doi: 10.1089/sur.2017.165. [DOI] [PubMed] [Google Scholar]
- 125.Freifeld Y., Goldin D., Khalili L., Friedman B., Boyarsky L., Klein I. Does the use of ureteral stents with extraction strings increase urinary infection rates? Int Urol Nephrol. 2017;49:763–767. doi: 10.1007/s11255-017-1533-y. [DOI] [PubMed] [Google Scholar]
- 126.Loh-Doyle J.C., Low R.K., Monga M., Nguyen M.M. Patient experiences and preferences with ureteral stent removal. J Endourol. 2015;29:35–40. doi: 10.1089/end.2014.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim D.J., Son J.H., Jang S.H., Lee J.W., Cho D.S., Lim C.H. Rethinking of ureteral stent removal using an extraction string; what patients feel and what is patients' preference? : a randomized controlled study. BMC Urol. 2015;15:121. doi: 10.1186/s12894-015-0114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Macaluso J.N., Deutsch J.S., Goodman J.R., Appell R.A., Prats L.J., Wahl P. The use of the Magnetip double-J ureteral stent in urological practice. J Urol. 1989;142:701–703. doi: 10.1016/s0022-5347(17)38858-4. [DOI] [PubMed] [Google Scholar]
- 129.Taylor W.N., McDougall I.T. Minimally invasive ureteral stent retrieval. J Urol. 2002;168:2020–2023. doi: 10.1016/S0022-5347(05)64286-3. [DOI] [PubMed] [Google Scholar]
- 130.Rassweiler M.C., Michel M.S., Ritter M., Honeck P. Magnetic ureteral stent removal without cystoscopy: a randomized controlled trial. J Endourol. 2017;31:762–766. doi: 10.1089/end.2017.0051. [DOI] [PubMed] [Google Scholar]
- 131.Wang J., Feng J., Hu W., Song Y., Xu X., Fan M. Preclinical evaluation of a newly designed ureteral stent and magnetic retrieval catheter for minimally invasive stent removal. Urology. 2014;84:960–966. doi: 10.1016/j.urology.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 132.Lingeman J.E., Preminger G.M., Berger Y., Denstedt J.D., Goldstone L., Segura J.W. Use of a temporary ureteral drainage stent after uncomplicated ureteroscopy: results from a phase II clinical trial. J Urol. 2003;169:1682–1688. doi: 10.1097/01.ju.0000055600.18515.a1. [DOI] [PubMed] [Google Scholar]
- 133.Chew B.H., Paterson R.F., Clinkscales K.W., Levine B.S., Shalaby S.W., Lange D. In vivo evaluation of the third generation biodegradable stent: a novel approach to avoiding the forgottenstent syndrome. J Urol. 2013;189:719–725. doi: 10.1016/j.juro.2012.08.202. [DOI] [PubMed] [Google Scholar]
- 134.Barros A.A., Rita A., Duarte C., Pires R.A., Sampaio-Marques B., Ludovico P. Bioresorbable ureteral stents from natural origin polymers. J Biomed Mater Res Part B Appl Biomater. 2015;103:608–617. doi: 10.1002/jbm.b.33237. [DOI] [PubMed] [Google Scholar]
- 135.Barros A.A., Oliveira C., Reis R.L., Lima E., Duarte A.R.C. In vitro and ex vivo permeability studies of paclitaxel and doxorubicin from drug-eluting biodegradable ureteral stents. J Pharmacol Sci. 2017;106:1466–1474. doi: 10.1016/j.xphs.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 136.Barros A.A., Oliveira C., Reis R.L., Lima E., Rita A., Duarte C. Ketoprofen-eluting biodegradable ureteral stents by CO2 impregnation: in vitro study. Int J Pharm. 2015;495:651–659. doi: 10.1016/j.ijpharm.2015.08.040. [DOI] [PubMed] [Google Scholar]