Highlights
-
•
Based on the characteristics of the antimicrobial spectrum of Cefotaxime, low incidence of allergy, and lack of adverse effects, Cefotaxime has been used successfully for prophylaxis of a number of infectious diseases.
-
•
Drug reactions like FDE are very frequently seen by dermatologists in the day to day practice. FDE to Cefotaxime is not usual.
-
•
Healthcare professionals should have a high index of suspicion should aware of the possibility of reactions to Cephalosporin.
Keywords: Cefotaxime, Fixed drug eruption, Antibiotic, Dermatology
Abstract
Fixed drug eruption (FDE) is the most common cutaneous adverse drug reaction. Cefotaxime, a broad-spectrum third-generation cephalosporin, appeared to be a safe and effective therapy in greater than 90% of infections including cellulitis, abscesses and necrotizing ulcers of the skin and subcutaneous tissues but here we report a rare case of 36 years old female patient developed generalized bullous FDE after intravenous administration of Cefotaxime.
1. Introduction
Fixed drug eruption (FDE) is drug-induced dermatoses characterized by an appearance of multiple or single, oval or round lesions which may occur in any part of the skin or mucous membrane. It is commonly seen in lips, genital areas, and perianal areas. It is an erythematous patch which occurs after administration of an offending drug within hours and heals with residual hyperpigmentation and reappears when the same drug was readministered [1,2]. Initially, the lesions are dusky red macules with erythematous accompanied by burning sensation, itching with bulla formation and crusting [3].
Cefotaxime is the first ‘third generation’ semisynthetic cephalosporin antibiotic with bactericidal activity. Cefotaxime inhibits mucopeptide synthesis by binding to and inactivating penicillin-binding proteins thereby interfering with the final transpeptidation step required for cross-linking of peptidoglycan units which are a component of bacterial cell walls. This results in a reduction of cell wall stability and causes cell lysis. It is used to treat obstetric and gynecological infections, lower respiratory tract infections, bacteremia, complicated urinary tract infections, uncomplicated gonorrhea, meningitis, infection of the skin and soft tissue and reduces the incidence of postsurgical bacterial infection [4].
It does not have activity against Pseudomonas aeruginosa but active against multidrug-resistant Enterobacteriaceae. It is active against the infections caused by mixed aerobic/anaerobic organisms in soft tissue infections. Biological half-life is approximately one hour. In most Gynecological infections, cephalosporins are drugs of the first choice because of their broad spectrum, their β-lactamase stability and their lack of toxicity. It is also used as prophylaxis for most of the hospitalized patients [5]. FDE due to Cefotaxime is a rare adverse event. Therefore, here we discuss a case of bullous FDE due to Cefotaxime administration.
2. Case report
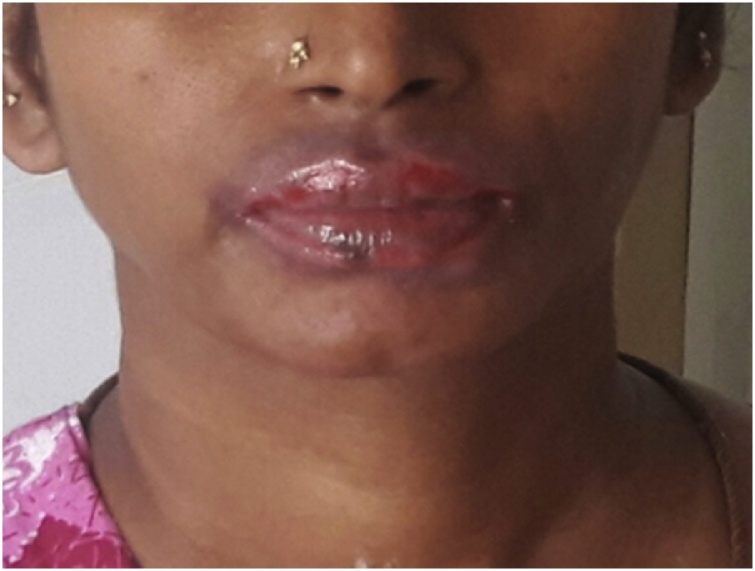
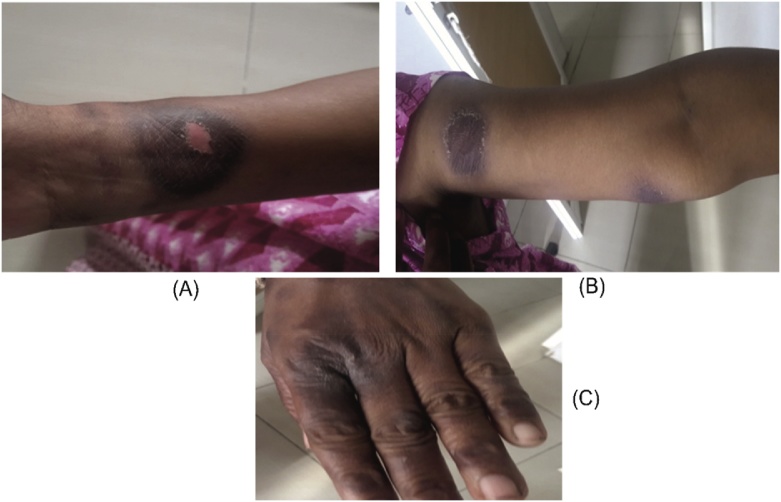
A 36 years old female patient came to the hospital with complaints of dysmenorrhea, heavy flow for the past 1 month and was admitted to the gynecology ward. She was prescribed with Tranexamic acid. The patient had a previous history of allergic to drugs like Pheniramine, Cefixime, and Ranitidine. Her obstetric history was P3L3. Last childbirth was 13 years back. Sterilization was done 13 years back. USG has shown Nabothian cyst in the uterine cervix and was diagnosed to be abnormal uterine bleeding. The patient was advised to do a minor surgical procedure called Dilation and curettage. After the minor surgical procedure patient was given postoperative antibiotic Cefotaxime 1 g intravenously. After two days, the patient developed generalized erythematous skin with blistering oval lesions on the wrist of the right hand, left arm, genital areas, and lips (Figs. 1 & 2 ). Physician stopped the medication and Patient was shifted to the dermatology ward. Physical examination revealed bullae ruptured, edema of lips, erythematous to hyperpigmentation patches, plaques over both hands, hemorrhagic crusting over lips were seen. The lesions cause burning sensation and pruritic. On clinical signs along with past allergic history to Cefixime, the patient was diagnosed with FDE and angioedema on lips. Therefore, Cefotaxime was diagnosed to cause generalized bullous FDE. Nicholsky sign was positive. She was afebrile and all the routine laboratory parameters were within the normal range. However, patch test and oral provocation test was not done as the patient did not consent for the same. The patient was treated with dexamethasone 10 mg/ml once daily, Azithromycin 250 mg twice daily, Levocetirizine 10 mg once daily, liquid paraffin for lips, Saline soaks and Mupirocin ointment for lips and genitals and other supportive measures. After 5 days of treatment, lesions were healed and resolved and so the steroid dose was tapered. patient’s condition was stable and improved. The patient was discharged with medication card template which has the patient’s allergic history of drugs and advised to show the medication card whenever she visits the Physician in future.
Fig. 1.
Edema of Lips.
Fig. 2.
Bullous lesion on right wrist (A), left arm (B) and left-hand fingers (C).
3. Discussion
FDE is one of the most common cutaneous drug reactions which involves the similar lesions and also reoccur in the same site that heals with residual hyperpigmentation which may also use for site recognition and remain for months and years [6]. FDE has multiple variants, including generalized, linear, bullous, urticarial, pigmenting, nonpigmenting, wandering, eczematous, psoriasiform, erythema dyschromicum perstans like vulvitis and oral FDE [7]. Till now pathogenesis of FDE is unknown but cell-mediated immunity, certain serum factors, and antibodies are some of the causative factors. FDE occurs by a CD8+-mediated reaction which is a delayed type hypersensitivity reaction. The offending drug activities CD8+ cells by damaging surrounding keratinocytes and release cytokines such as interferon gamma in localized epidermal and dermal tissue which cause localized tissue damage [8]. Staphylococcus aureus is a common pathogen that leads to skin and systemic infections in hospitalized patients [9]. The other most frequently involved microorganisms are Gram-negative bacilli, coagulase-negative staphylococci, Enterococcus spp. and Escherichia coli [10]. The most commonly used drugs which cause FDE are Paracetamol, metronidazole, tetracycline, Cotrimoxazole, Diclofenac, Tinidazole, Mefenamic acid, Metamizole, Erythromycin, Ibuprofen, Ampicillin, Phenobarbitone, Phenylbutazone, albendazole, clindamycin, indomethacin, belladonna, griseofulvin, allopurinol, diflunisal and acetylsalicylic acid [11]. Apart from the above-listed drugs, studies prove that the 5-day combination of once-daily 80 mg gentamicin with a second-generation cephalosporin is effective in female patients with chorioamnionitis and endometritis [12]. Cephalosporins have many side effects but FDE has been rarely reported. A single case of FDE due to ceftriaxone of cephalosporin class has been reported earlier in Turkish Woman [13]. To the extent of our knowledge, in the literature, there is no published report of Cefotaxime - induced FDE.
In this case, Naranjo's algorithm [14] was used to determine a plausible reaction due to Cefotaxime. The following criteria were considered: There were previous conclusion reports on this reaction (0); the adverse event appeared after Cefotaxime was administered (+2); adverse reaction improved when Cefotaxime was discontinued (+1); adverse event reappeared when Cefotaxime was readministered (0); alternate causes (other than the drug) that could on their own have caused the reaction (+2); the reaction reappeared when placebo was given (0); the drug detected in blood (or other fluids) in concentrations known to be toxic (0); the reaction more severe when the dose was increased or less severe when the dose was decreased (+1); the patient has a similar reaction to the same or similar drugs in any previous exposure (+1); adverse event confirmed any objective evidence (+1). Based on the total score of 8, this FDE was categorized as “probable” reaction to Cefotaxime administration. Severity was assessed by using Modified Hartwig and Siegel scale [15] and found the severity is at level-3.
As most case reports concern suspected adverse drug reactions, pharmacovigilance can be problematic. Also, adverse reactions are rarely specific for the drug without specific diagnostic tests and a rechallenge is rarely ethically justified. Therefore, we have also used WHO-UMC causality assessment system [16]. As per the system, in our case, the association was 'certain' reaction to Cefotaxime.
4. Conclusion
FDE to Cefotaxime is uncommon. To our knowledge, this is the first case reported in the literature which describes FDE elicited by Cefotaxime use. It was concluded that any cephalosporin derivatives can cause FDE. So before prescribing cephalosporins, it is very important for every clinician to take a proper history, clinical examination and history of allergies of the patient. Healthcare professionals should have a high index of suspicion and should aware of the possibility of reactions to Cephalosporin.
Conflict of interest
All the authors declared no competing interests.
Ethical approval
Not Applicable.
Patient consent
Written informed consent was obtained from the patient for publication of photographs in this case report.
Acknowledgment
We would like to thank Prof. K.S. Lakshmi, Dean, SRM College of Pharmacy for her kind support throughout the study.
References
- 1.Ozkaya-Bayazit E. Specific site involvement in fixed drug eruption. J. Am. Acad. Dermatol. 2003;49(6):1003–1007. doi: 10.1016/s0190-9622(03)01588-3. [DOI] [PubMed] [Google Scholar]
- 2.Jain S.P., Jain P.A. Bullous fixed drug eruption to ciprofloxacin: a case report. J. Clin. Diagn. Res. 2013;7(4):744–745. doi: 10.7860/JCDR/2013/4757.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiohara T., Mizukawa Y. Fixed drug eruption: a disease mediated by self-inflicted responses of intraepidermal T cells. Eur. J. Dermatol. 2007;17(3):201–208. doi: 10.1684/ejd.2007.0149. [DOI] [PubMed] [Google Scholar]
- 4.Todd P.A., Brogden R.N. Cefotaxime. An update of its pharmacology and therapeutic use. Drugs. 1990;40(4):608–651. doi: 10.2165/00003495-199040040-00008. [DOI] [PubMed] [Google Scholar]
- 5.Melmon K.L., Morelli H.F., Hoffman B.B., Nierenberg D.W., editors. Melmon and Morrelli’s Clinical Pharmacology. 3rd ed. McGraw-Hill, Inc.; New York: 1992. Pp. 707–708. [Google Scholar]
- 6.Pai V.V., Bhandari P., Kikkeri N.N., Athanikar S.B., Sori T. Fixed drug eruption to fluconazole: a case report and review of literature. Indian J. Pharmacol. 2012;44(5):643–645. doi: 10.4103/0253-7613.100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Özkaya E. Oral mucosal fixed drug eruption: characteristics and differential diagnosis. J. Am. Acad. Dermatol. 2013;69:e51–e58. doi: 10.1016/j.jaad.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Morillas L., Rojas Pérez-Ezquerra P., González Morales M.L., Mayorga C., González-Mendiola R., Laguna Martínez J.J. Fixed drug eruption due to norfloxacin and cross‑reactivity with other quinolones. Allergol. Immunopathol. (Madr.) 2013;41(1):60–61. doi: 10.1016/j.aller.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Ungureanu A., Zlatian O., Mitroi G., Drocaş A., Ţîrcă T., Călina D., Dehelean C., Docea A.O., Izotov B.N., Rakitskii V., Cioboată R., Spandidos D.A., Tsatsakis A.M., Găman A. Staphylococcus aureus colonisation in patients from a primary regional hospital. Mol. Med. Rep. 2017;16:8771–8780. doi: 10.3892/mmr.2017.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calina D., Docea A.O., Roşu L., Zlatian O., Roşu A.F., Anghelina F., Rogoveanu O., Arsene A., Nicolae A.C., Drăgoi C.M., Tsiaoussis J., Tsatsakis A.M., Spandidos D.A., Drakoulis N., Gofiţă E. Antimicrobial resistance development following surgical site infections. Mol. Med. Rep. 2017;15(2):681–688. doi: 10.3892/mmr.2016.6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahboob A., Haroon T.S. Haroon. Drugs causing fixed eruptions: a study of 450 cases. Int. J. Dermatol. 1998;37(11):833–838. doi: 10.1046/j.1365-4362.1998.00451.x. [DOI] [PubMed] [Google Scholar]
- 12.Sifakis S., Angelakis E., Makrigiannakis A., Orfanoudaki I., Christakis-Hampsas M., Katonis P., Tsatsakis A., Koumantakis E. Chemoprophylactic and bactericidal efficacy of 80 mg gentamicin in a single and once-daily dosing. Arch. Gynecol. Obstet. 2005;272(September (3)):201–206. doi: 10.1007/s00404-004-0698-7. [DOI] [PubMed] [Google Scholar]
- 13.Ozkaya E., Mirzoyeva L., Jhaish M.S. Ceftriaxone induced fixed drug eruption: first report. Am. J. Clin. Dermatol. 2008;9(5):345–347. doi: 10.2165/00128071-200809050-00011. [DOI] [PubMed] [Google Scholar]
- 14.Naranjo C.A., Busto U., Sellers E.M., Sandor P., Ruiz I. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 15.Padmavathi S., Manimekalai K., Ambujam S. Causality, severity and preventability assessment of adverse cutaneous drug reaction: a prospective observational study in a tertiary care hospital. J. Clin. Diagn. Res. 2013;7(12):2765–2767. doi: 10.7860/JCDR/2013/7430.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The use of the WHO-UMC system for standardised case causality assessment. Available from: http://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf. (Last Accessed on 01 October 2018).