Abstract
Background
There is paucity of neurobiological knowledge about major depressive disorder with psychotic features (“psychotic depression”). This study addresses this knowledge gap by using resting state functional magnetic resonance imaging (R-fMRI) to compare functional connectivity in patients with psychotic depression and healthy controls.
Methods
We scanned patients who participated in a randomized controlled trial as well as healthy controls. All patients achieved remission from depressive and psychotic symptoms with sertraline and olanzapine. We employed Independent Component Analysis in independent samples to isolate the default mode network (DMN) and compared patients and controls.
Findings
The Toronto sample included 28 patients (mean [SD], age 56·2 [13·7]) and 39 controls (age 55·1 [13·5]). The Replication sample included 29 patients (age 56·1 [17·7]) and 36 controls (age 48·3 [17·9]). Patients in the Toronto sample demonstrated decreased between-network functional connectivity between the DMN and bilateral insular, somatosensory/motor, and auditory cortices with peak activity in the right planum polare (t = 4·831; p = 0·001, Family Wise Error (FWE) corrected). A similar pattern of between-network functional connectivity was present in our Replication sample with peak activity in the right precentral gyrus (t = 4·144; p = 0·003, FWE corrected).
Interpretation
Remission from psychotic depression is consistently associated with an absence of increased DMN-related functional connectivity and presence of decreased between-network functional connectivity. Future research will evaluate this abnormal DMN-related functional connectivity as a potential biomarker for treatment trajectories.
Funding
National Institute of Mental Health.
Keywords: Default mode network, Functional connectivity, Biomarkers, Remission, Psychosis, Major depressive disorder
Research in context.
Evidence before this study
Major depressive disorder (MDD) with psychotic features (“psychotic depression”) is a severe psychiatric disorder with global impact. Despite its severity, or perhaps because of it, psychotic depression is a psychiatric disorder that is rarely studied and little is known about its neurobiology. Resting state functional magnetic resonance imaging (R-fMRI) is a neurobiological probe that has identified patterns of functional connectivity implicated in depression and psychosis, including functional connectivity abnormalities related to the default mode network (DMN). To date, a single R-fMRI study has focussed on the hypothalamus specifically in unremitted patients with psychotic depression. Another R-fMRI study has examined the DMN in an aggregated sample of unremitted patients with non-psychotic and psychotic depression.
Added value of this study
To our knowledge, this is the first R-fMRI study to specifically examine the DMN in psychotic depression while patients are remitted from their depressive and psychotic symptoms. The present study compared patients with psychotic depression, who were treated to remission with sertraline and olanzapine and subsequently scanned in the remitted state, to healthy controls. An absence of increased DMN-related functional connectivity (in patients relative to controls) and presence of decreased DMN functional connectivity with the bilateral insular, somatosensory/motor, and auditory cortices was found and replicated.
Implications of all the available evidence
A consistent pattern of abnormal functional connectivity has been demonstrated between the subgenual cortex and DMN in non-psychotic depression, as well as in an aggregated sample that included patients with unremitted psychotic depression. The absence of such functional connectivity in remitted psychotic depression suggests that subgenual-DMN functional connectivity may be related to the state of psychotic depression; that remission may normalize such abnormal functional connectivity; and that the patterns of abnormal functional connectivity in this study may be related to the diagnosis of psychotic depression or to the process of remission. Importantly, these replicated patterns of abnormal DMN-related functional connectivity may serve as a biomarker for treatment trajectories.
Alt-text: Unlabelled Box
1. Introduction
Unipolar major depressive disorder (MDD) with psychotic features (“psychotic depression”) is a severe psychiatric disorder with global impact [1]. While depressive and psychotic symptoms can co-occur in other diagnoses such as schizophrenia, schizoaffective disorder, and bipolar I disorder, the hallmark of psychotic depression is the emergence of psychosis during a major depressive episode and the recession of psychosis as the major depressive episode remits. This contrasts with diagnoses of schizophrenia and schizoaffective disorder in which psychotic symptoms remain even after a major depressive episode remits, or bipolar I disorder, which is characterized by manic and depressive episodes with psychosis having the potential to emerge in either mood state [2]. Psychotic features have been found in 20% of patients with MDD [3,4] and 45% of elderly inpatients with MDD [5]. Compared to MDD without psychotic features, MDD with psychotic features is associated with poorer outcomes, including longer recovery, greater disability, and increased mortality [3,4,6]. Despite its severity, or perhaps because of it, psychotic depression is a psychiatric disorder that is rarely studied and little is known about its neurobiology.
Two Studies of the Pharmacotherapy of Psychotic Depression (STOP-PD I and II) have been designed to improve the evidence-based treatment of psychotic depression. STOP-PD I, a 12-week randomized, double-blind placebo-controlled trial, demonstrated the efficacy of combined sertraline and olanzapine in the acute treatment of psychotic depression [7]. The primary goal of STOP-PD II is to compare the efficacy and tolerability of sertraline plus olanzapine versus sertraline plus placebo in preventing relapse following remission from an episode of psychotic depression initially treated with a combination of sertraline and olanzapine. We used resting-state functional magnetic resonance imaging (R-fMRI) in STOP-PD II participants who had been treated to remission to identify biomarkers of psychotic depression since R-fMRI has been used to identify neural circuitry implicated in psychosis that changes with treatment response and may be the target of interventions [8]. We studied participants in remission to avoid confounds related to the state of depression or psychosis present in unremitted patients.
Studies of non-psychotic depression have demonstrated abnormalities within the default mode network (DMN) [9]. This network has been considered a “default” since the same regions show greater activity when no task is being performed. Yet there is evidence that the DMN is active during a range of self-referential functions, including planning for the future and remembering the past [10,11]. There are many resting state networks and the literature has examined how different resting state networks (as a whole or in parts and within or between networks) may be functionally connected in health or demonstrate functional connectivity abnormalities in disease [[12], [13], [14]]. With reference to psychotic depression, functional connectivity abnormalities within the DMN have been identified in unremitted MDD [15]. The sole R-fMRI study on psychotic depression revealed abnormal functional connectivity between the hypothalamus and subgenual cortex, as well as other brain regions [16].
There is mounting evidence for DMN dysfunction in psychiatric disorders and changes in DMN functional connectivity in response to treatment have been demonstrated in non-psychotic depression [17]. Thus, identification of abnormal functional connectivity within the DMN or between the DMN and other brain regions in remitted psychotic depression could serve as a biomarker of this disorder. The goal of the present study was to differentiate remitted psychotic depression from healthy controls. We hypothesized that remitted patients would have patterns of DMN-related functional connectivity that differ from healthy controls.
2. Materials and methods
2.1. Participants
The primary analysis was based on a sample of patients enrolled in STOP-PD II in Toronto. Twenty-eight patients with psychotic depression, recruited from the University Health Network (UHN) and the Centre for Addiction and Mental Health (CAMH), and thirty-nine healthy controls were scanned on a 3 T GE Discovery MR750 at CAMH. Data collected on Siemens 3 T scanners at Cornell University/Nathan Kline Institute (NKI) (patients n = 16; controls n = 16) and the University of Pittsburgh Medical Centre (UPMC) (patients n = 13; controls n = 20) were analyzed to assess replicability of the findings with a sample size comparable to the Toronto sample.
The design of STOP-PD II, including eligibility criteria, has been described in detail [18]. Briefly, STOP-PD II participants were aged 18–85 years and met diagnostic criteria for non-bipolar MDD with psychotic features based on the Structured Clinical Interview for DSM-IV-TR Axis I Disorders administered by a trained research associate. STOP-PD II is divided into three consecutive phases: given the severity of psychotic depression, an acute phase of open-label treatment was pursued with higher doses of sertraline (target dose: 150 − 200 mg/day) and olanzapine (15–20 mg/day) lasting from four to twelve weeks to attain remission, an eight week stabilization phase to ensure that remission is sustained, and a 36 week randomized controlled trial (RCT) comparing the efficacy of sertraline plus olanzapine and sertraline plus placebo in preventing relapse of psychotic depression. At the end of the second phase, to be eligible for randomization into the RCT, participants must be in remission, defined as: [1] having been free of delusions and hallucinations for eight weeks; and [2] having a score of ≤10 on the 17-item Hamilton Depression Rating Scale (HAM-D) for two consecutive weeks or a HAM-D score of 11–15 with ≥50% reduction of the acute phase baseline HAM-D score and a rating of “very much improved” or “much improved” on the Clinical Global Impression (CGI) Scale.
In line with the goal of the present study to differentiate remitted psychotic depression from healthy controls, all participants included in the analysis were scanned at the end of the second phase (i.e., before randomization, when they were in remission and taking sertraline and olanzapine). No data were available on patients receiving sertraline and olanzapine who fail to respond. Moreover, this study occurs prior to randomization and therefore there is no placebo group. Global cognitive impairment and comorbid physical illness burden were assessed in both patients and controls using the Mini-Mental State Examination (MMSE) and Clinical Illness Rating Scale-Geriatrics (CIRS-G), respectively. Patients with MMSE scores <24 were excluded. Moreover, patients with evidence of cognitive decline prior to the index episode were excluded.
Using procedures approved by the local institutional review boards, written informed consent was obtained from all participants or their legal representative prior to the initiation of any research assessment or treatment.
2.2. MRI data acquisition
For 2D axial R-fMRI, participants in the Toronto sample were instructed to “let your mind wander” while keeping their eyes closed and head still. Functional scans were acquired using a spiral in/out gradient echo (GRE) pulse sequence with the following parameters: TR = 2000 msec, TE = 30 msec, flip angle = 60°, matrix = 64 × 64, slice thickness = 5 mm, number of slices = 31. Resting state scan time was seven minutes. At Cornell/NKI, R-fMRI was acquired using an echo planar imaging (EPI) pulse sequence with the following parameters: TR = 2000 msec, TE = 30 msec, flip angle = 80°, matrix = 96 × 96, slice thickness = 2·8 mm, number of slices = 34, with a scan time of seven minutes. At UPMC, R-fMRI scans were acquired using an EPI pulse sequence with the following parameters: TR = 2000 msec, TE = 34 msec, flip angle = 90°, matrix = 128 × 128, slice thickness = 4 mm, number of slices = 28, with a scan time of seven minutes and eight seconds. Acquisition parameters for T1-weighted scans (for registration) are in the appendix.
2.3. R-fMRI data analysis
The Functional MRI of the Brain (FMRIB) Software Library (FSL 5·0·9) was used to preprocess and analyze R-fMRI data [19]. Standard individual preprocessing consisted of removal of the first three volumes to allow for steady-state signal equilibration, removal of temporal spikes using the Analysis of Functional NeuroImages (AFNI) [20] 3ddespike, slice timing correction using AFNI 3dtshift (only for EPI acquisitions at Cornell/NKI and UPMC), head motion correction with AFNI 3dvolreg, brain extraction with the Brain Extraction Tool (BET), and a linear detrend. Timecourses were variance-normalized and single-session Independent Component Analysis (ICA) was performed on an automatically estimated number of ICs using Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) version 3·14 [21]. These single session ICs were labelled as “signal” and “noise”. Noise ICs were regressed from the data using FMRIB's ICA-based Xnoiseifier (FIX) [22]. Given acquisition resolution differences between sites, AFNI 3dBlurToFWHM was then employed to iteratively smooth each participant's R-fMRI image to a final smoothness of 10 mm Full-Width-Half-Maximum. Data were realigned to the MNI template after cleaning with FIX. R-fMRI volumes were registered to individual structural scans using a normal linear search and subsequently spatially normalized to Montreal Neurological Institute (MNI) standard space (MNI152) using a nonlinear transform.
2.4. Correction for motion artefact and other sources of noise
Rigorous detection and correction of motion artefacts is important for mitigating site effects [23]. Mean frame displacement was included in primary statistical analyses, however to further address motion artefact and physiological sources of noise (such as respiration and cardiac effects which were not measured independently) we employed FIX. For the purpose of data cleaning with FIX, individual signal and noise classifiers were trained for each scanning site using a pseudorandom subset of participants (Toronto: twelve patients, twelve controls; Cornell/NKI, ten patients, ten controls; UPMC, nine patients, nine controls). To facilitate this process, ICs from the training set were first labelled as signal (i.e., a resting state network) or noise (i.e., motion or scanner artefacts, non-neuronal physiology, and other nuisance signals) by FIX using the standard training set available with the FIX package. These labels were manually inspected and readjusted with the aid of an in-house html qc interface (https://github.com/edickie/icarus). Readjusted labels were then submitted to FIX for classifier training. Site-specific training data was then used to classify all ICs from all participants at each site. Noise components were regressed out of the original data.
2.5. Group ICA and dual regression
For the Toronto sample collected on a 3 T GE scanner, cleaned data for each participant were temporally concatenated across participants, creating a single 4D dataset for group-wise ICA. The number of ICs was automatically determined to be 42 using standard FSL techniques [24]. The DMN IC included peak activation in the posterior cingulate, bilateral parietal, and medial prefrontal cortices and did not mask out non-default mode regions. This IC was identified among the 42 ICs by visual inspection and spatial correlation against a previously well-defined default mode map [25]. The IC most correlated with this canonical DMN map (r = 0·65) had substantially higher spatial correlation than the next best fitting IC (r = 0·35). The same procedure was followed for the Cornell/NKI and UPMC data. Since these data were collected on 3 T Siemens scanners, data were combined to achieve power comparable to the Toronto sample. Using standard FSL techniques [24], the DMN was identified among 14 ICs that were automatically isolated and the IC most correlated with the canonical DMN map (r = 0·81) had substantially higher spatial correlation than the next best fitting IC (r = 0·10).
Dual regression was then employed for voxel-wise comparisons of functional connectivity between patients and controls [26]. This approach proceeded in three stages. First, for each participant, the group-average DMN were spatially regressed into the participant's 4D space-time dataset. This resulted in a set of participant-specific timeseries representing the DMN timeseries. Second, these participant-specific DMN timeseries were temporally regressed into the same 4D dataset, resulting in a set of participant-specific DMN functional connectivity maps based on the synchronicity between the timeseries and any given voxel. Third, group differences were then tested using FSL's randomise permutation-testing tool (10,000 permutations). Group comparisons employed threshold-free cluster enhancement (TFCE) and were family-wise error (FWE) corrected for multiple comparisons across the brain at p < 0·05 [27].
2.6. Post-hoc analyses
We selected regions of interest (ROIs) informed by the DMN and dual regression results for post-hoc analyses to explore brain regions with the greatest amount of overlap between the Toronto and Replication samples and to probe for medication effects. Using a data driven approach, the conjunction of the Toronto and Replication sample maps of the DMN (thresholded at z < 3) was obtained to form an unbiased search space. Similarly, the conjunction of the Toronto and Replication sample dual regression result maps (thresholded at p < 0·01) was obtained to form an unbiased dual regression result search space. Spherical ROIs (diameter = 10 mm) from the Power atlas were then applied within the search space and ROIs that completely overlapped with the search space were selected [13]. Timeseries data for each ROI was extracted and ROI-ROI correlations were z-transformed. ROIs were noted to be mainly related to the auditory, somatomotor (including the sensory/somatomotor hand and mouth systems), and default mode networks. The mean functional connectivity within the auditory, somatomotor, and default mode networks was then calculated for each participant and included in a linear model for the Toronto and Replication samples with age, sex, years of education, and mean frame displacement as covariates. Results were Bonferroni corrected.
Medication dose at the time of the stabilization scan was available for all but one patient in the Replication sample. Medication effects were examined and given skew as well as dose targets, doses were binned into maximum and less than maximum for sertraline (200 mg and < 200 mg, respectively) and olanzapine (20 mg and < 20 mg). Exploratory models were then pursued to examine the combined as well as separate effects of sertraline and olanzapine on mean functional connectivity within and between the auditory, somatomotor, and default mode networks. Results were Bonferroni corrected.
2.7. Data statement
As per our data sharing plan, our neuroimaging data fits within the ‘phenotypes’ definition of the NIH-funded Genotypes and Phenotypes (dbGaP) database and we plan to submit neuroimaging data to dbGaP within one year of study completion.
3. Results
Participant characteristics are shown in Table 1. In terms of motion, there were no significant differences between patients and controls in mean frame displacement for the Toronto (Patients M = 0·168, SD = 0·089; Controls M = 0·158, SD = 0·110; t = 0·422; p = 0·67) and Replication (Patients M = 0·288, SD = 0·231; Controls M = 0·247, SD = 0·160; t = 0·851; p = 0·40) samples, although taken together, patients and controls in the Toronto sample (M = 0·162, SD = 0·100) demonstrated less mean frame displacement than patients and controls in the Replication sample (M = 0·265, SD = 0·190; t = −3·876; p < 0·001). Given the heterogeneity of participants' age, sex, years of education, and mean frame displacement, these four variables were entered into dual regression as covariates. Additionally, the Replication sample covaried for site. Small but statistically significant differences between patients and controls were observed in total MMSE scores in the Toronto and Replication samples. There was no significant difference between patients and controls in the Toronto sample with respect to cumulative burden of illness (CIRS-G scores) and a significant difference was observed in the Replication sample. There were no significant differences between patients in the Toronto and Replication samples for sertraline (Toronto M = 160·7 mg, SD = 34·3 mg; Replication M = 167·9 mg, SD = 40·6 mg; t = −0·720; p = 0·47) or olanzapine (Toronto M = 14·6 mg, SD = 5·1 mg; Replication M = 14·3 mg, SD = 4·8 mg; t = 0·273; p = 0·79) dose.
Table 1.
Characteristics of patients and healthy controls in the Toronto and Replication Samples. Mean (SD) unless indicated otherwise. Significance is reported for two-sample, two-tailed t-tests, assuming equal variance for baseline measures in the (a) Toronto and (c) Replication samples. Comparison of key baseline and stabilization variables (at time of scanning) are given for the (b) Toronto and (d) Replication samples. Significance for baseline versus stabilization comparisons is reported for paired, one-tailed t-tests, assuming equal variance. F=Female; M = Male. Total MMSE: Mini-Mental State Examination; Total CIRS-G: Total Cumulative Illness Rating Scale-Geriatrics; HAM-D: 17 Item Hamilton Depression Rating Scale; CGI: Clinical Global Impression; BMI: Body Mass Index.
| (a) | ||||
|---|---|---|---|---|
| Patients (n = 28) | Controls (n = 39) | t value (df = 65) | p value | |
| Sex (n) | 16F, 12 M | 22F, 17 M | – | – |
| Age (years) | 56·2 (13·7) | 55·1 (13·5) | 0·322 | 0·748 |
| Education (years) | 13·0 (3·4) | 14·7 (2·2) | -2·472 | 0·016 |
| Total MMSE | 28·3 (2·1) | 29·4 (0·7) | −3·127 | 0·003 |
| Total CIRS-G | 3·0 (2·8) | 2·5 (2·3) | 0·927 | 0·357 |
| (b) | ||||
|---|---|---|---|---|
| Baseline | Stabilization | t value (df = 27) | p value | |
| HAM-D | 28·5 (4·6) | 3·8 (3·0) | 23·951 | <0·001 |
| CGI illness severity score | 5·1 (0·9) | 1·5 (0·8) | 15·363 | <0·001 |
| Weight (kg) | 74·0 (16·0) | 83·1 (17·7) | −8·027 | <0·001 |
| BMI (kg/m2) | 26·3 (4·7) | 29·5 (5·1) | −8·359 | <0·001 |
| (c) | ||||
|---|---|---|---|---|
| Patients (n = 29) | Controls (n = 36) | t value (df = 63) | p value | |
| Sex (n) | 14F, 15 M | 22F, 14 M | – | – |
| Age (years) | 56·1 (17·7) | 48·3 (17·9) | 1·748 | 0·085 |
| Education (years) | 14·4 (3·2) | 15·8 (2·4) | −2·028 | 0·047 |
| Total MMSE | 27·6 (2·2) | 29·1 (1·1) | −3·811 | <0·001 |
| Total CIRS-G | 4·8 (4·9) | 2·3 (2·0) | 2·862 | 0·006 |
| (d) | ||||
|---|---|---|---|---|
| Baseline | Stabilization | t value (df = 28) | p value | |
| HAM-D | 27·8 (4·4) | 7·0 (3·6) | 20·102 | <0·001 |
| CGI illness severity score | 5·1 (0·9) | 1·2 (0·4) | 22·630 | <0·001 |
| Weight (kg) | 70·3 (13·0) | 78·9 (13·6) | −7·027 | <0·001 |
| BMI (kg/m2) | 24·8 (3·5) | 27·8 (3·6) | −7·265 | <0·001 |
Table 1 also presents the clinical characteristics of patients at baseline (i.e., initiation of open label treatment with olanzapine and sertraline) and following successful acute treatment followed by eight weeks of stabilization (i.e., at the time of scanning).
3.1. Toronto sample: group ICA and dual regression
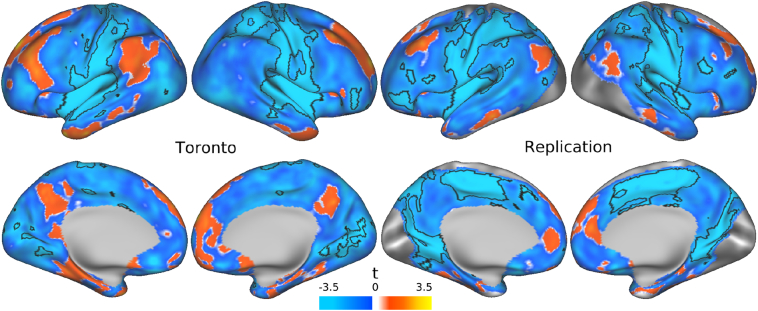
Supplementary Fig. 1 (appendix) illustrates the IC capturing the DMN in the Toronto and Replication samples. Relative to healthy controls, there were no significant increases of within-DMN or between-network functional connectivity observed in patients with psychotic depression (FWE corrected, p < 0·05). In contrast, patients had significant decreased between-network functional connectivity with the DMN when compared with controls (Fig. 1). The most extensive peak of decreased functional connectivity was located within the right planum polare (x = 62 y = −4 z = 2) in a large cluster that extended into the bilateral insula and pre/postcentral gyri (t=4·831, p=0·001; appendix).
Fig. 1.
Main effect of group on default mode network (DMN) related functional connectivity in the Toronto and Replication samples. Dual regression was employed and DMN-related functional connectivity was examined in patients relative to healthy controls in both the Toronto and Replication samples. Relative to controls, there were no brain regions in which patients had significantly increased functional connectivity in either sample. However, patients had significantly decreased functional connectivity between the DMN and regions outside the DMN in the Toronto and Replication samples (Family Wise Error (FWE) corrected, p < 0·05).
3.2. Replication sample: group ICA and dual regression
Relative to healthy controls, there was an absence of significantly increased within-DMN or between-network functional connectivity in the Replication sample (FWE corrected, p < 0·05). There was also a similar pattern of significant decreased between-network functional connectivity with the DMN in a large cluster that extended into the bilateral insula and pre/postcentral gyri, with the most extensive peak in the right precentral gyrus (x = 2 y = −14 z = 58) (Fig. 1; t=4·144, p=0·003; appendix).
3.3. Post-hoc analyses
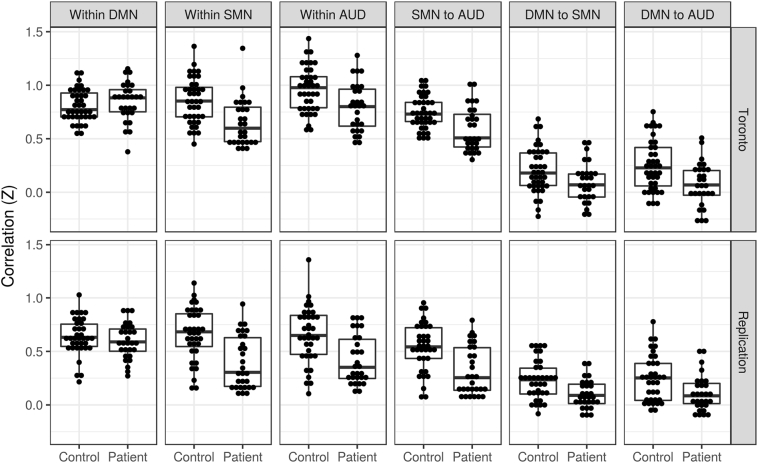
Supplementary Fig. 2 (appendix) illustrates the covariance matrix derived from the Power atlas ROIs. Power ROIs were predominantly related to the default mode (DMN; n = 15), auditory (AUD; n = 11), and somatomotor (SMN; n = 9) networks. To enhance interpretability, ROIs within these networks were averaged together for each participant. With reference to Fig. 2 and considering within-network functional connectivity, there was no significant difference between patients and controls within the DMN for the Toronto (t = 0·873, puncorr = 0·39, pcorr = 1·00) or Replication (t = −0·500, puncorr = 0·62, pcorr = 1·00) samples. However, both samples demonstrated decreased within-network functional connectivity in patients for the SMN (Toronto: t = −3·393, puncorr = 0·001, pcorr = 0·007; Replication: t = −4·172, puncorr = 0·0001, pcorr = 0·0006) and AUD (Toronto: t = −2·927, puncorr = 0·005, pcorr = 0·03; Replication: t = −3·699, puncorr = 0·0005, pcorr = 0·003) networks. In terms of between-network functional connectivity, the SMN to AUD (Toronto: t = −3·322, puncorr = 0·002, pcorr = 0·009; Replication: t = −3·877, puncorr = 0·0003, pcorr = 0·002), DMN to SMN (Toronto: t = −2·427, puncorr = 0·02, pcorr = 0·11; Replication: t = −3·240, puncorr = 0·002, pcorr = 0·01), and DMN to AUD (Toronto: t = −3·404, puncorr = 0·001, pcorr = 0·007; Replication: t = −2·732, puncorr = 0·008, pcorr = 0·05) between-network functional connectivity was decreased in patients in both the Toronto and Replication samples.
Fig. 2.
Post-hoc analyses of brain regions that overlapped in the Toronto and Replication samples. Regions of interest (ROIs) representing brain regions with the greatest amount of overlap between the Toronto and Replication samples were examined. ROIs were noted to be mainly related to the auditory (AUD), somatomotor (SMN), and default mode (DMN) networks. The mean functional connectivity within the auditory, somatomotor, and default mode networks was then calculated for each participant and included in a linear model for the Toronto and Replication samples with age, sex, years of education, and mean frame displacement as covariates. There was no significant (p < 0·05) difference between patients and controls within the DMN for the Toronto or Replication samples. However, both samples demonstrated significantly decreased within-network functional connectivity in patients for the SMN and AUD networks. In terms of between-network functional connectivity, the SMN to AUD, DMN to SMN, and DMN to AUD between-network functional connectivity was significantly decreased in patients in both the Toronto and Replication samples.
An exploratory medication analysis was pursued. As can be seen in Supplementary Figs. 3 and 4 (appendix), sertraline and olanzapine doses were skewed towards high doses. As a result, medication effects were explored by binning patient participants based on maximum or less than maximum doses for sertraline (200 mg (Toronto: n = 9, Replication n = 16) and < 200 mg (Toronto: n = 19; Replication: n = 12)) and olanzapine (20 mg (Toronto: n = 10, Replication: n = 8) and < 20 mg (Toronto: n = 18, Replication: n = 20)). There was no significant relationship between functional connectivity and sertraline and olanzapine when in a combined model in either the Toronto or Replication sample (Supplementary Table 4). When examining the relationship between functional connectivity and sertraline and olanzapine in separate models, only the Replication sample revealed a significant effect of sertraline (t = −2·961, puncorr = 0·006, pcorr = 0·04) and olanzapine (t = −2·423, puncorr = 0·02, pcorr = 0·14) dose on within-DMN functional connectivity. There were no other significant effects of sertraline or olanzapine on any other within or between-network functional connectivity (Supplementary Table 4).
4. Discussion
The main finding of this study is that patients with remitted psychotic depression differ from healthy controls in terms of decreased DMN-related between-network functional connectivity implicating interoceptive and exteroceptive brain regions. A similar pattern of decreased DMN-related functional connectivity was observed in a Replication sample. ROI analyses corroborated these results. Exploratory analyses in the Replication sample suggested an effect of sertraline and olanzapine dose on within-DMN functional connectivity when modeled separately. However, this effect was not significant in the Toronto sample nor in either sample when both sertraline and olanzapine were modeled.
Our findings suggest a pattern of abnormal functional connectivity between the DMN and other key brain regions that may be relevant to remission in psychotic depression. This functional connectivity may be a biomarker of psychotic depression that endures into remission or representative of active neural processes of remission itself. Perhaps the most well known example of between-network relationships relates to the activation and deactivation of different networks during cognition [28]. Such between-network relationships have been examined in depression [29,30]. Many disorders have demonstrated DMN-related functional connectivity abnormalities, yet it has been noted that there may exist unique abnormalities for specific disorders [12]. It should also be noted that the interoceptive and exteroceptive regions found in this study have been implicated in other neural circuits. Previous studies have examined the role of the bilateral insula and somatosensory/motor cortices and how they may support the mental experience of bodily states that accompany emotions, i.e., “feelings” [31]. Furthermore, lesion studies provide evidence that the insula and somatosensory cortices are necessary but not sufficient for the emergence of feelings [32,33]. Our results extend this work and suggest that the neural circuits that have been implicated in feelings have functional connectivity with the DMN and that a disorder such as psychotic depression impacts this functional connectivity. Another possibility relates to a recent quantitative MRI study that suggests insular and somatosensory cortices are responsible for processing trait behaviours associated with empathy [34]. Our study does not permit the exploration of whether the observed functional connectivity patterns specifically relate to processing feelings or empathy. However, the observation remains that DMN-related functional connectivity with interoceptive and exteroceptive brain regions are abnormal in psychotic depression and that this observation replicates in an independent sample.
To our knowledge, these findings are the first functional MRI-based neural correlates of remitted psychotic depression. This is also the first study to specifically examine the DMN in psychotic depression. Within and between-network abnormalities in patients with non-psychotic depression have been reviewed and abnormal connectivity between the subgenual cortex and DMN has been the most consistent pattern of functional connectivity [35]. It is possible that remission was associated with normalization of functional connectivity between the subgenual cortex and the DMN in our patient participants. DMN-related functional connectivity has been previously examined in healthy controls compared to an unremitted sample that aggregated MDD patients with (n = 11) and without (n = 17) psychotic features and revealed increased functional connectivity with the subgenual cingulate, medial prefrontal/orbitofrontal cortex, and precuneus [15]. Our findings contrast with this unremitted sample and are notable for the absence of increased/decreased within-DMN functional connectivity. Our findings are consistent with earlier research showing between-network abnormalities in patients with unremitted psychotic depression, reflected in those investigations by decreased functional connectivity between the hypothalamus and subgenual cingulate [16] and decreased regional cerebral blood flow in the subgenual cingulate, inferior frontal cortex, and insula [36]. Nonetheless, the pattern of between-network abnormalities in these unremitted patients differs from our patients with remitted psychotic depression.
There are several limitations of this study. To begin with study design and medication related limiations, the inclusion of a non-psychotic depression group would have added information related to the specificity of our findings to psychotic depression and would have potentially permitted a direct comparison of effects of antidepressant medication on functional connectivity to that of the combination of antidepressant and antipsychotic medication on functional connectivity. We note, however, that the STOP-PD II RCT did not collect such data and highlight that it remains a unique sample in which all patients remitted on the same antidepressant and antipsychotic medications. Another limitation of this study relates to the general effects of medication use on functional connectivity. While the present cross-sectional study compares medicated patients and healthy controls and does not permit an in-depth exploration of such effects, it is noteworthy that our exploratory medication analysis that combined sertraline and olanzapine did not result in any significant effect of sertraline or olanzapine dose on functional connectivity. Future longitudinal analyses will explore this issue in greater detail. An additional limitation of our cross-sectional study is that we cannot assess whether the DMN-related functional connectivity we observed in patients with psychotic depression predict longer-term remission or relapse. This limitation will be addressed when follow-up scans are obtained, offering the unique opportunity to compare functional connectivity in patients who were randomized to receive sertraline and placebo to those randomized to receive sertraline and olanzapine. It will further offer the opportunity to compare functional connectivity in patients who maintain remission to those who relapse.
Ongoing debate remains on whether to use an eyes closed versus eyes open versus fixated condition when collecting R-fMRI data [[37], [38], [39], [40]]. For example, variable reliability and higher functional connectivity in the auditory network has been described with an eyes closed condition [40]. Although these effects were statistically significant, it should be noted that this study also found the effect size of differences in reliability and consistency of the eyes open, eyes closed, and fixation conditions to be small. Our post-hoc analyses suggest, however, that patients with remitted psychotic depression (relative to healthy controls) have replicable patterns of within and between-network functional connectivity (implicating the auditory network and others) that are evident despite the possibility of the eyes closed condition influencing functional connectivity.
Our study is also potentially limited by its sample size. However, a single R-fMRI [16] study and two task-based fMRI [41,42] studies had a comparable or smaller number of patients with psychotic depression. Nevertheless, the small sample size may have contributed to some inconsistent functional connectivity findings. Despite small sample sizes and varying acquisition parameters, we replicated our findings related to abnormal insular/somatosensory/motor/auditory functional connectivity.
In summary, in a multi-centre study of patients with remitted psychotic depression, we identified abnormal DMN-related functional connectivity, particularly between interoceptive and exteroceptive brain regions and the DMN. When compared to healthy controls, remission from psychotic depression was consistently associated with significantly decreased functional connectivity with these brain regions. Future research will evaluate this abnormal DMN-related functional connectivity as a potential biomarker for treatment trajectories.
Declaration of interests
Dr. Neufeld has nothing to disclose during the conduct of the study. Outside the submitted work, Dr. Neufeld reports grants through a University of Toronto Department of Psychiatry Clinician Scientist Program Norris Scholar Award and grants from the Physicians' Services Incorporated Foundation. Dr. Mulsant reports grants from the National Institute of Mental Health, non-financial support from Pfizer, and non-financial support from Eli Lilly during the conduct of the study. Outside the submitted work, Dr. Mulsant reports grants from Brain Canada, grants from the Canadian Institutes of Health Research, grants from the Centre for Addiction and Mental Health Foundation, grants from the Patient-Centred Outcomes Research Institute, grants and non-financial support the National Institutes of Health, non-financial support from Capital Solution Design LLC, non-financial support from HAPPYneuron, and he directly owns shares of General Electric (less than $5,000). Dr. Dickie has nothing to disclose. Dr. Meyers reports grants from the National Institute of Mental Health, non-financial support from Pfizer, and non-financial support from Eli Lilly during the conduct of the study. Dr. Alexopoulos has nothing to disclose during the conduct of the study. Outside the submitted work, Dr. Alexopoulos reports personal fees from Allergan, personal fees from Otsuka, personal fees from Sunovian, and personal fees from Takeda-Lundbeck. Dr. Rothschild reports grants from the National Institute of Mental Health, non-finanical support from Pfizer, and non-financial support from Eli Lilly during the conduct of the study. Outside the submitted work, Dr. Rothschild reports grants from Allergan, grants from Assure Rx, grants from Jannsen, grants from Takeda, personal fees from Alkermes, personal fees from GlaxoSmithKline, personal fees from Myriad Genetics, personal fees from Sage Therapeutics, personal fees from American Psychiatric Press Inc, personal fees from the University of Massachusetts Medical School, and personal fees from Up-to-Date. Dr. Whyte reports grants from the National Institute of Mental Health, non-financial support from Pfizer, and non-financial support from Eli Lilly during the conduct of the study. Dr. Hoptman reports grants from the University of Toronto/National Institute of Mental Health during the conduct of the study. Outside the submitted work, Dr. Hoptman reports other funding from the New York State Office of Mental Health and the Kessler Research Foundation. Dr. Nazeri has nothing to disclose. Dr. Downar has nothing to disclose during the conduct of the study. Outside the submitted work, Dr. Downar reports personal fees from TMS Health Solutions, personal fees from Restorative Brain Clinics, other support from BrainCheck, and non-financial support from MagVenture. Dr. Flint reports grants from the National Institute of Mental Health, non-financial support from Pfizer, and non-financial support from Eli Lilly during the conduct of the study. Outside the submitted work, Dr. Flint reports grants from Lundbeck. Dr. Voineskos reports grants from the National Institute of Mental Health during the conduct of the study. Outside the submitted work, Dr. Voineskos reports grants from the National Institute of Mental Health, grants from the Canadian Institutes of Health Research, grants from the Canadian Foundation for Innovation, grants from the Ontario Mental Health Foundation, grants from the Brain and Behavior Research Foundation, and grants from the Centre for Addiction and Mental Health Foundation.
Author contributions
NHN and EWD completed the analyses. NHN and ANV drafted the manuscript. All authors were involved in the design of the study, participated in discussing the analyses and interpretation of findings, were involved in the drafting of the manuscript and revision for intellectual content, approved the final version before publication, and agree to be held accountable for the work. ANV is the guarantor for the data and the analysis.
Acknowledgements
Grant support was provided by the National Institute of Mental Health under the following grant numbers: B.S.M (5U01MH062518), A.J.R. (5U01MH062624), E.M.W (5U01MH062565), A.J.F (5U01MH062446), and A.N.V (5R01MH099167). Non-financial support (medication) was provided by Pfizer for sertraline and Eli Lilly for olanzapine and matched placebo pills. The funding source of the study had no role in the study design, data collection, data analysis, data interpretation, writing of this report, or decision to submit for publication. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Footnotes
The supplementary appendix for this article can be found online at https://doi.org/10.1016/j.ebiom.2018.09.025.
Appendix A. Supplementary appendix
Supplementary material
References
- 1.Koyanagi A., Oh H., Stubbs B., Haro J.M., DeVylder J.E. Epidemiology of depression with psychotic experiences and its association with chronic physical conditions in 47 low- and middle-income countries. Psychol Med. 2017;47:531–542. doi: 10.1017/S0033291716002750. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association . American Psychiatric Pub; 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) [Google Scholar]
- 3.Ohayon M.M., Schatzberg A.F. Prevalence of depressive episodes with psychotic features in the general population. Am J Psychiatry. 2002;159:1855–1861. doi: 10.1176/appi.ajp.159.11.1855. [DOI] [PubMed] [Google Scholar]
- 4.Maj M., Pirozzi R., Magliano L., Fiorillo A., Bartoli L. Phenomenology and prognostic significance of delusions in major depressive disorder: a 10-year prospective follow-up study. J Clin Psychiatry. 2007;68:1411–1417. doi: 10.4088/jcp.v68n0913. [DOI] [PubMed] [Google Scholar]
- 5.Meyers B.S., Greenberg R. Late-life delusional depression. J Affect Disord. 1986;11:133–137. doi: 10.1016/0165-0327(86)90019-4. [DOI] [PubMed] [Google Scholar]
- 6.Coryell W., Leon A., Winokur G. Importance of psychotic features to long-term course in major depressive disorder. Am J Psychiatry. 1996;153:483–489. doi: 10.1176/ajp.153.4.483. [DOI] [PubMed] [Google Scholar]
- 7.Meyers B.S., Flint A.J., Rothschild A.J. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD) Arch Gen Psychiatry. 2009;66:838–847. doi: 10.1001/archgenpsychiatry.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarpal D.K., Robinson D.G., Lencz T. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiat. 2015;72:5–13. doi: 10.1001/jamapsychiatry.2014.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu D., Yuan Y., Bai F., You J., Li L., Zhang Z. Abnormal functional connectivity of the default mode network in remitted late-onset depression. J Affect Disord. 2013;147:277–287. doi: 10.1016/j.jad.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Spreng R.N., Mar R.A., Kim A.S.N. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- 11.Schacter D.L., Addis D.R., Buckner R.L. Episodic simulation of future events: concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- 12.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Power J.D., Cohen A.L., Nelson S.M. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uddin L.Q., Kelly A.M., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greicius M.D., Flores B.H., Menon V. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudheimer K., Keller J., Gomez R. Decreased hypothalamic functional connectivity with subgenual cortex in psychotic major depression. Neuropsychopharmacology. 2015;40:849–860. doi: 10.1038/npp.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dichter G.S., Gibbs D., Smoski M.J. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J Affect Disord. 2015;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flint A.J., Meyers B.S., Rothschild A.J. Sustaining remission of psychotic depression: rationale, design and methodology of STOP-PD II. BMC Psychiatry. 2013;13:38. doi: 10.1186/1471-244X-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 21.Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salimi-Khorshidi G., Douaud G., Beckmann C.F., Glasser M.F., Griffanti L., Smith S.M. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawco C., Viviano J.D., Chavez S. A longitudinal human phantom reliability study of multi-center T1-weighted, DTI, and resting state fMRI data. Psychiatry Res Neuroimaging. 2018; published online June 9 doi: 10.1016/j.pscychresns.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 25.Smith S.M., Fox P.T., Miller K.L. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippini N., MacIntosh B.J., Hough M.G. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc Natl Acad Sci. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 28.Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posner J., Cha J., Wang Z. Increased default mode network connectivity in individuals at high familial risk for depression. Neuropsychopharmacology. 2016;41:1759–1767. doi: 10.1038/npp.2015.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damasio A., Carvalho G.B. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci. 2013;14:143–152. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- 32.Mori E., Yamadori A. Rejection behaviour: a human homologue of the abnormal behaviour of Denny-Brown and Chambers' monkey with bilateral parietal ablation. J Neurol Neurosurg Psychiatry. 1989;52:1260–1266. doi: 10.1136/jnnp.52.11.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damasio A., Damasio H., Tranel D. Persistence of feelings and sentience after bilateral damage of the insula. Cereb Cortex. 2013;23:833–846. doi: 10.1093/cercor/bhs077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen M., Frank D., Glen J.C., Fardo F., Callaghan M.F., Rees G. Insula and somatosensory cortical myelination and iron markers underlie individual differences in empathy. Sci Rep. 2017;7 doi: 10.1038/srep43316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dutta A., McKie S., Deakin J.F.W. Resting state networks in major depressive disorder. Psychiatry Res. 2014;224:139–151. doi: 10.1016/j.pscychresns.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Skaf C.R., Yamada A., Garrido G.E.J. Psychotic symptoms in major depressive disorder are associated with reduced regional cerebral blood flow in the subgenual anterior cingulate cortex: a voxel-based single photon emission computed tomography (SPECT) study. J Affect Disord. 2002;68:295–305. doi: 10.1016/s0165-0327(00)00365-7. [DOI] [PubMed] [Google Scholar]
- 37.Van Dijk K.R.A., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan C., Liu D., He Y. Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAvoy M., Larson-Prior L., Nolan T.S., Vaishnavi S.N., Raichle M.E., d’Avossa G. Resting states affect spontaneous BOLD oscillations in sensory and paralimbic cortex. J Neurophysiol. 2008;100:922–931. doi: 10.1152/jn.90426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patriat R., Molloy E.K., Meier T.B. The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. Neuroimage. 2013;78:463–473. doi: 10.1016/j.neuroimage.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrett A., Kelly R., Gomez R., Keller J., Schatzberg A.F., Reiss A.L. Aberrant brain activation during a working memory task in psychotic major depression. Am J Psychiatry. 2011;168:173–182. doi: 10.1176/appi.ajp.2010.09121718. [DOI] [PubMed] [Google Scholar]
- 42.Kelley R., Garrett A., Cohen J. Altered brain function underlying verbal memory encoding and retrieval in psychotic major depression. Psychiatry Res. 2013;211:119–126. doi: 10.1016/j.pscychresns.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material