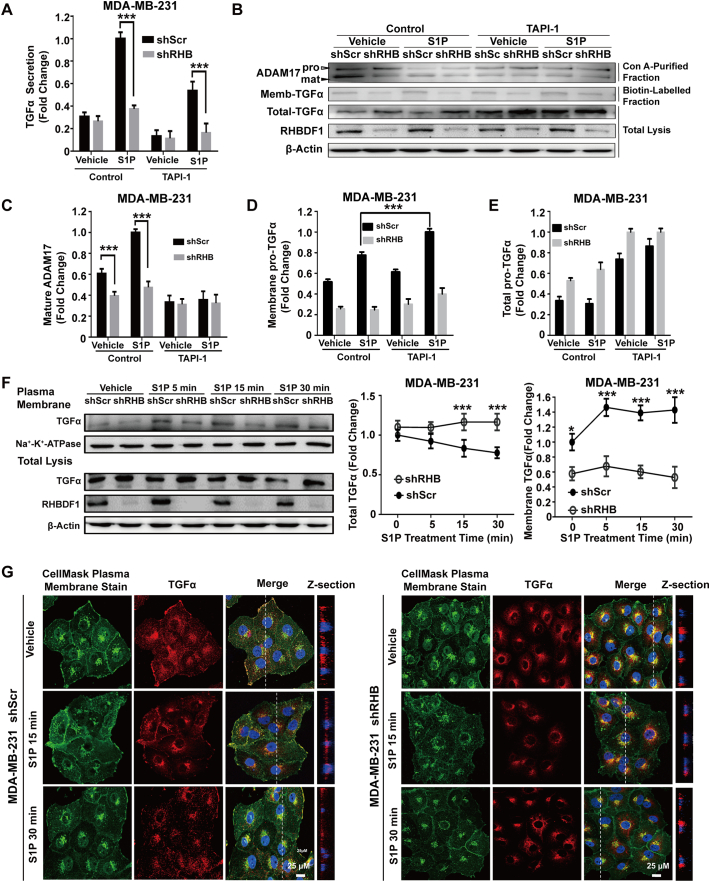
Fig. 2.
RHBDF1 not only modulates ADAM17-dependent shedding but also alters membrane trafficking of pro-TGFα. (A) shScr- or shRHB-treated cells were preincubated in the presence or absence of ADAM17 inhibitors TAPI-1 (20 μM) for 1 h before S1P stimulation for another 20 min; then, the TGFα in supernatant was detected by ELISA. (B) The above lysates were enriched for glycoproteins with concanavalin A (Con A) and immunoblotted for ADAM17 to detect its maturation. The cell membrane was biotin-labelled and enriched to enable detection of the level of TGFα on the cell plasma membrane. The empty arrowhead indicates immature form, whereas the black arrowhead indicates mature forms of ADAM17. (C) The percentage of mature ADAM17 was calculated by normalizing the amount of immature ADAM17. The relative levels of TGFα on the cell membrane (D) and in total lysis (E) was quantified based on densitometry. (F) Plasma membrane-bound or cell-associated total TGFα in shScr- or shRHB-treated cells were detected by western blotting analysis at indicated time intervals following S1P treatment; Na-K-ATPase, control for plasma membrane proteins. (G). Representative images of plasma membrane staining with CellMask Plasma Membrane Stain (green) and immune-fluorescence staining of pro-TGFα (red). Z-section shows the transition of pro-TGFα from locations near the nucleus to the plasma membrane. Data are means ± SEM. Student t-test, *p < 0.05, **p < 0.01 and *** p < 0.001.