Abstract
Aims
To investigate the effect of a free radical scavenger (tempol) after relief of partial bladder outlet obstruction (pBOO) on bladder function in a rat model.
Methods
pBOO was induced in 50 eight-week-old female Sprague-Dawley rats and relieved 3 weeks later. The rats were divided randomly into 5 groups: sham-operated, tempol-treated for 1 week (Treat-1w) or 3 weeks (Treat-3w), and no treatment for 1 week (nonTreat-1w) or 3 weeks (nonTreat-3w). Awaken cystometrograms were obtained 1 or 3 weeks after relief according to the grouping. The bladders were isolated and weighed. H&E, Masson’s trichrome and TUNEL staining were used to analyze histological changes. The oxidative stress assessed using malondialdehyde. The expression of beta-3 adrenoreceptor was examined by Western blotting.
Results
The tempol-treated groups exhibited a significant decrease in the number of non-voiding contractions per voiding cycle (nonTreat-1w vs. Treat-1w, 1.18±0.82 vs. 0.36±0.40, P = 0.010; nonTreat-3w vs. Treat-3w, 1.51±0.69 vs. 0.23±0.25, P = 0.002). The thickness and collagen fiber deposition of the detrusor muscle layer was significantly decreased in the treated groups. Apoptosis detected was mainly observed in the urothelial cell layer, although the rate of apoptosis was significantly decreased in the treated groups (48.9±3.36% vs. 32.7±11.10%, P = 0.024; 25.8±4.67% vs. 15.7±9.83%, P = 0.314). The tempol-treated groups showed significant decreases in the MDA concentrations at both 1 and 3 weeks after relief. The expression of the beta-3 adrenoreceptor was increased in the tempol-treated rats.
Conclusions
Ischemic reperfusion injury after relief of pBOO caused histological and functional changes in the bladder. Free radical scavenger treatment prevented this oxidative stress.
Introduction
Bladder outlet obstruction (BOO) occurs due to a variety of causes, including the posterior urothelial valve in children, urethral stricture in adults, and benign prostatic hyperplasia (BPH) in the elderly [1]. Although the most common cause in the clinic is BPH in older men, BOO may also occur in women with several anatomical and/or functional etiologies, including pelvic organ prolapse, Skene’s gland cyst, primary bladder neck obstruction, and detrusor external sphincter dyssynergia [2]. BOO is one of the most important clinical problems and can cause overactive bladder, urinary incontinence, urinary tract infection, vesicoureteral reflux, hydronephrosis, and renal insufficiency through chronic urinary retention [3].
In patients with severe degenerative bladder due to BOO, surgical treatment may temporarily exacerbate storage bladder dysfunction related to detrusor overactivity (DO) and even cause transient urgency and urgency urinary incontinence [4]. Newly developed urgency urinary incontinence after a HoLEP procedure has been reported in 7.1–44.0% of patients [5]. This de novo urgency urinary incontinence causes significant stress and anxiety not only for the patients but also for the surgeons, because it differs from post-operative complicated stress urinary incontinence. In a recent prospective study of persistent storage symptoms after successful relief of BOO, urodynamic DO was persistent in approximately 40% of the patients [6].
Ischemia reperfusion injury (I/R injury) has been suggested to be a cause of post-operative bladder dysfunction in BPH patients [7]. In patients with chronic BOO due to BPH, a chronic ischemic status is induced in the inner bladder wall due to persistent high intravesical pressure [8]. In this condition, relief of chronic obstruction causes reperfusion and reoxygenation, resulting in the generation of reactive oxygen species (ROS), which cause more severe oxidative damage and cell apoptosis in the bladder wall [9].
Efforts to prevent I/R injuries have been made in various medical fields. Several antioxidants have been used to reduce I/R injury in patients with ischemic diseases, such as coronary artery disease or stroke, and in the transplantation field. For instance, L-alanyl-glutamine attenuates I/R injury in liver transplantation patients [10]. Some antioxidants, such as CoQ10, beta carotene, lycopene, quercetin, resveratrol, vitamin C and vitamin E, have shown preventive and therapeutic benefits for different forms of CVD [11]. However, no studies have investigated methods to prevent I/R injury after BPH surgery. The effects of I/R injury after BPH surgery have only recently drawn researchers’ attention.
In this study, we investigated the effects of oxidative stress caused by I/R injury after relief of partial BOO (pBOO) on bladder functions using a rat model and evaluated the preventive effect of free radical scavenger, tempol, on bladder functions after relief of pBOO.
Materials and methods
Animals and study design
Seven-week-old female Sprague-Dawley rats weighing between 220 and 250 g were used in this study. The rats were housed in a vivarium with free access to food and water in a light-controlled room with a diurnal cycle for 1 week prior to surgery. After surgery, the animals were caged individually and maintained under the same conditions. All animal handling and treatment procedures carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Seoul National University Hospital Institutional Animal Care and Use Committee (Protocol Number: 13-0300-C1A1), which is an AAALAC-accredited facility.
Fifty rats were randomly divided into five groups (n = 10 rats per group). The first group consisted of control sham-operated rats (control group). The other four groups underwent urethral constriction to procedurally induce pBOO, followed by a reversal operation after 3 weeks. The second group underwent a cystometrogram (CMG) without treatment for one week after the reversal operation (unTreat-1w group). The third group underwent a CMG without treatment for 3 weeks after the reversal (unTreat-3w group). The fourth group underwent a CMG with antioxidant treatment for 1 week after the reversal (Treat-1w group). The fifth group underwent a CMG with antioxidant treatment for 3 weeks after the reversal (Treat-3w group). We used tempol as an antioxidant, that has the advantage of being soluble in water and is widely used in rat experiments. The rats in the treated group received tempol (Sigma-Aldrich, St. Louis, MO, USA) by gavage at a dose of 1.5 mmol/kg/day dissolved in water three times per day [12].
Induction of pBOO
Anesthesia was induced by ketamine/xylazine (15 mg/kg and 5 mg/kg; intramuscular injection) and maintained with isoflurane (1–3%). After shaving the skin, asepsis was attained with a 10% povidone-iodine solution while the rat was in a dorsal recumbent position. The bladder was approached through a lower midline abdominal incision. After exposing the proximal urethra, a steel rod 0.9-mm in diameter was placed around the urethra. The bladder neck was ligated using a 3–0 silk, and the steel rod was removed. The bladder was repositioned, and the abdominal wall was closed. In the sham operation group, the bladder neck was very loosely ligated to avoid inducing any obstruction. Three weeks after inducing pBOO, each rat underwent a reversal operation that removed the ligation in the same manner. We measured the body weight of each rat before each procedure.
Procedures for intravesical and intra-abdominal catheter implantation
The catheter implantation procedures were performed 2 days before the functional evaluation. Polyethylene catheters (PE-50; Clay-Adams, Parsippany, NJ, USA) with a cuff were inserted into the dome of the bladder through a lower abdominal incision with a purse-string suture and simultaneously placed on the posterior side of the bladder. The balloon and catheter were filled with distilled water, and the distal end of the catheter was sealed. Both catheters were tunneled subcutaneously to the skin of the back and anchored with a silk ligature. The free end of the catheter was sealed. Each rat was housed individually after the procedures and maintained in the manner described above.
Functional evaluation
CMGs were performed on awakened, unanesthetized, and unrestrained rats in metabolic cages after a minimum of 2 days of recovery from catheterization. The bladder catheter was connected via a 3-way stopcock to a pressure transducer (Research Grade Blood Pressure Transducer; Harvard Apparatus, Holliston, MA, USA) and a microinjection pump (PHD22/2000 pump; Harvard Apparatus). Another pressure transducer was connected to an intra-abdominal balloon catheter to independently record the intra-abdominal pressure (IAP). The micturition volumes were recorded with a fluid collector connected to a force displacement transducer (Research Grade Isometric Transducer; Harvard Apparatus). Room-temperature saline was infused into the bladders at a rate of 0.4 mL/min. Pressures and micturition volumes were recorded continuously with a computerized system (PowerLab, ADInstruments, Colorado Springs, CO, USA) at a sampling rate of 50 Hz. Non-voiding contractions (NVCs) during the filling phase were defined as an increment of intravesical pressure that exceeded 2 cmH2O from the baseline without simultaneous changes in IAP and without fluid expulsion from the bladder [13].
When the bladder contractions became stable, at least five micturition cycles were recorded for each rat. The following CMG parameters were measured, and the mean value of each variable was calculated for the analysis: basal pressure (the lowest pressure during filling), threshold pressure (the pressure immediately before the initiation of micturition), peak micturition pressure (the maximum pressure during micturition), micturition interval (the interval between micturition contractions), micturition volume, micturition duration (the time from initiation to finish of one micturition cycle), post-voided residual volume (the remaining urine after voiding), bladder capacity (the infused volume immediately before the initiation of micturition), bladder compliance (calculated by dividing the micturition volume by the difference between resting and threshold pressures), and the frequency of NVCs (per micturition cycle).
Histology and immunohistochemistry (IHC)
The rats were euthanized after completion of the functional study. The whole bladder was extirpated and weighed. A portion of the bladder divided sagittally was fixed in a 4% formaldehyde solution for the histological evaluation, and the other half was snap frozen immediately in liquid nitrogen and was stored at -80°C for the measurement of malondialdehyde (MDA) and the IHC evaluation.
After obtaining 4-μm serial sections of the paraffin-embedded material, the bladder tissue sections were stained with hematoxylin and eosin or Masson’s trichrome stain. The thickness of the detrusor muscle layer was evaluated in 10 randomly selected hematoxylin and eosin-stained sections. Collagen deposition was measured in 10 high-power (×400) fields from randomly selected Masson’s trichrome-stained sections. Photomicrographs were obtained using a digitalized miscroscopic image system (Nikon Eclipse 80i microscope and Nikon Digital Slight DS-U3; Nikon, Tokyo, Japan). The images were analyzed using the Adobe and ImageJ software (http://rsb.info.nih.gov/ij/).
To detect apoptosis, the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) method was used. In each slide, 10 high-power (×400) fields were randomly selected, and the apoptotic index was expressed as the percentage of apoptotic cells relative to the number of total cells in a given area (nonapoptotic nuclei plus apoptotic cells).
Measurement of malondialdehyde in the bladder
The MDA level in the bladder tissue, which is an index of oxidative stress, was determined using a commercially available kit according to the manufacturer’s instructions. The MDA concentrations were normalized using the protein content (NWLSSTM Malondialdehyde Assay, Northwest Life Science Specialties, LLC, Vancouver, WA, USA).
Western blotting analysis of beta-receptor expression
The membranes were blocked with 5% skim milk for 1 hr at room temperature and incubated overnight at 4°C with primary antibodies against the β3 adrenergic receptors (1:1000, ab59685, Abcam), followed by incubation with appropriate horseradish peroxidase-linked secondary antibodies (1:4000) for 2 hr at room temperature. The bands on the blots were visualized using an enhanced chemiluminescence system (Amersham Bioscience, Buchinghamshire, UK), and densitometric analysis of the Western blots was conducted using VisionWorks LS, version 6.7.1.
Statistical analysis
All data are expressed as the mean and standard error of the mean (SEM). The collected data were analyzed using Student’s t-test or the Mann-Whitney U test depending on whether the data followed a Gaussian distribution. A two-tailed P<0.05 was considered significant. The statistical analyses were performed using IBM SPSS for Windows, Version 24.0 (IBM Inc., Armonk, NY, USA).
Results
Body and bladder weights
No significant differences in body weight were found between the sham-operated and the pBOO-induced groups 1 week after relief of pBOO. However, the urinary bladder weight was significantly increased in the pBOO-induced groups compared with the weights in the sham group 1 week after the reversal operation (28.2±8.3 g vs 12.5±0.7 g, P<0.001). The ratio of bladder weight to total body weight was significantly higher in the pBOO-induced groups than in the sham group. No differences were found in body weight, bladder weight, and the ratio of bladder weight to body weight between the treated and untreated groups.
Comparison of cystometric parameters
Significant differences were found in almost all cystometric parameters, including the threshold pressure, micturition interval, voided volume, NVC, and bladder capacity, between the sham and the pBOO-induced groups. A significant difference was found in the NVC incidence between the tempol-treated and the untreated groups. The number of NVCs per voiding cycle was significantly decreased in the tempol-treated groups compared to the numbers in both the 1- and 3-week untreated groups (unTreat-1w vs. Treat-1w, 1.18±0.82 vs. 0.36±0.40, P = 0.010; unTreat-3w vs. Treat-3w, 1.51±0.69 vs. 0.23±0.25, P = 0.002). However, no other differences in the cystometric parameters were associated with tempol treatment (Table 1).
Table 1. Comparison of urodynamic parameters between conscious sham-operated and obstructed rats.
| sham | 1wk | 3wks | |||
|---|---|---|---|---|---|
| untreated | treated | untreated | treated | ||
| Basal Pressure (mmH2O) | 18.6±3.4 | 20.0±10.9 | 20.9±8.6 | 20.2±10.2 | 19.8±7.7 |
| Threshold Pressure (mmH2O) | 24.8±2.4 | 39.3±13.3* | 37.8±10.3* | 34.1±20.9 | 25.7±12.6 |
| Peak micturition Pressure (mmH2O) | 58.8±15.5 | 52.9±18.9 | 58.2±18.4 | 56.0±36.6 | 48.3±15.9 |
| Micturition interval (sec) | 378.0±115.5 | 210.2±118.3* | 205.6±56.1* | 279.6±59.4 | 238.3±72.8* |
| Micturition duration (sec) | 14.0±3.5 | 15.6±3.9 | 19.1±7.8 | 20.0±15.1 | 19.7±11.2 |
| Voided volume (mL) | 2.14±0.54 | 1.31±0.79* | 1.30±0.32* | 1.81±0.35 | 1.52±0.46* |
| Non-voiding contraction | 0 | 1.18±0.82* | 0.36±0.40*† | 1.51±0.69* | 0.23±0.25† |
| Bladder capacity (mL) | 1.05±0.32 | 1.40±0.79 | 1.37±0.37 | 1.86±0.39* | 1.59±0.49* |
| Residual volume (mL) | 0.15±0.21 | 0.09±0.11 | 0.07±0.09 | 0.05±0.06 | 0.07±0.07 |
*P<0.05 versus sham group
†P<0.05 versus untreated group in the same period (Mann-Whitney U test).
Histological findings
Histologically, a thickened bladder wall was observed in the pBOO-induced bladder specimens. This finding was mainly due to hypertrophy of the detrusor muscle layer. In the untreated group, the thickness of the detrusor muscle layer decreased significantly after 3 weeks compared to 1 week of relief. However, in the tempol-treated group, no significant difference in the thickness of the detrusor muscle layer was observed between 1 and 3 weeks after relief.
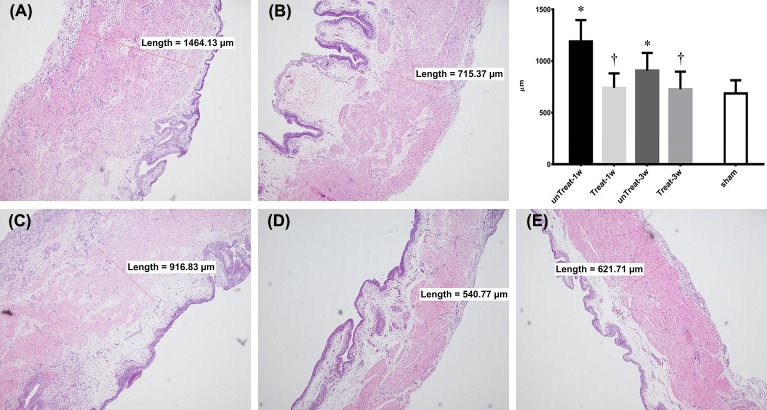
In the comparison with the untreated group, a significant decrease was observed in the detrusor muscle thickness in the treated group after both 1 and 3 weeks of treatment (unTreat-1w vs. Treat-1w, 1164.17±190.58 vs. 776.45±140.78, P<0.001; unTreat-3w vs. Treat-3w, 905.82±161.16 vs. 726.26±162.76, P = 0.043) (Fig 1).
Fig 1. Changes in the detrusor muscle thickness after pBOO and its relief for 1 wk and 3 wks based on tempol treatment.
Representative microscophs of a thin section of the bladder wall (magnification 100×). Bar graphs show the quantitative image analysis. Results are expressed as mean ± standard error of the mean. (A) untreated for 1 week; (B) tempol-treated for 1 week; (C) untreated for 3 weeks; (D) tempol-treated for 3 weeks; (E) sham; *P<0.05 versus sham; †P<0.05 versus untreated group in the same period (Mann-Whitney U test).
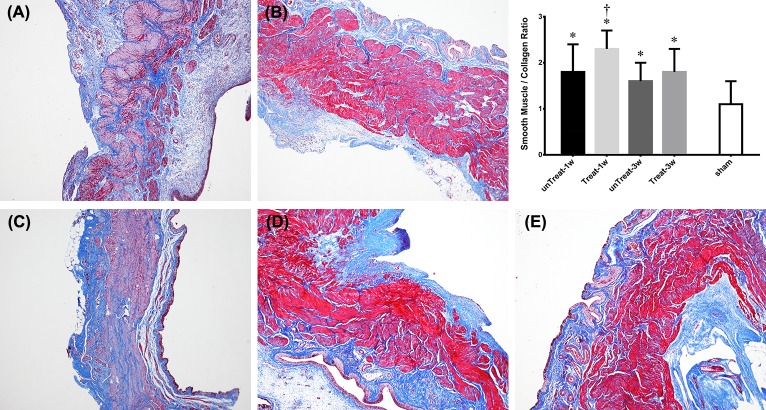
In the Masson’s trichrome-stained sections, tempol treatment reduced the deposition of collagen fibers in the lamina propria and detrusor muscle layer compared with the analysis in the untreated rats. The ratio of collagen to smooth muscle was significantly decreased in the treated rats at 1 week after relief, but not statistically at 3 weeks (Fig 2).
Fig 2. Masson’s trichrome staining.
Representative microscophs show collagen with blue staining and the muscle with purple staining (magnification 20×). Bar graphs show the quantitative image analysis. Results are expressed as mean ± standard error of the mean. (A) untreated for 1 week; (B) tempol-treated for 1 week; (C) untreated for 3 weeks; (D) tempol-treated for 3 wks; (E) sham; *P<0.05 versus sham; †P<0.05 versus untreated group in the same period (Mann-Whitney U test).
TUNEL findings
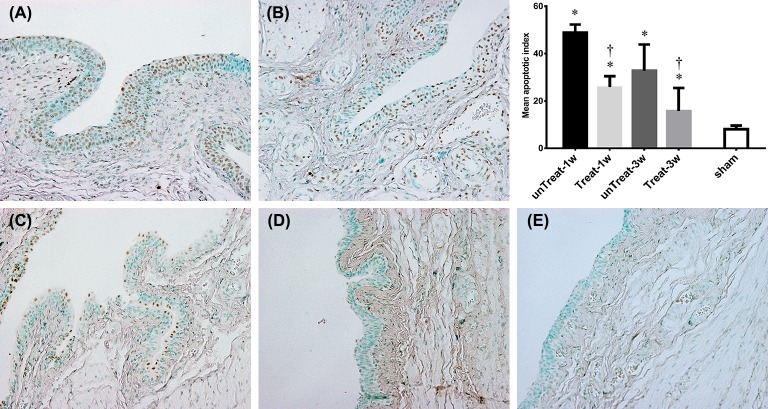
TUNEL-positive cells were observed mainly in the urothelium. The numbers of TUNEL-positive cells were significantly decreased in the tempol-treated groups. This inhibitory effect was observed at both 1 and 3 weeks after relief (unTreat-1w vs. Treat-1w, 48.9±3.36% vs. 32.7±11.10%, P = 0.024; unTreat-3w vs. Treat-3w, 25.8±4.67% vs. 15.7±9.83%, P = 0.314) (Fig 3).
Fig 3. Detection of apoptosis.
Representative microscophs show TUNEL-positive cells as black-brown cells mainly localized in the bladder urothelium (magnification 400×). Bar graphs show the quantitative image analysis. The apoptotic index represents the percentage of apoptotic cells within the total number of cells in a given area. Results are expressed as mean ± standard error of the mean. (A) untreated for 1 week; (B) tempol-treated for 1 week; (C) untreated for 3 weeks; (D) tempol-treated for 3 weeks; (E) sham; *P<0.05 versus sham; †P<0.05 versus untreated group in the same period (Mann-Whitney U test).
MDA
The tempol-treated groups showed significant decreases in the MDA concentrations at both 1 and 3 weeks after relief compared to the concentrations in the untreated groups (unTreat-1w vs. Treat-1w, 0.76±0.08 vs. 0.66±0.07, P = 0.021; unTreat-3w vs. Treat-3w, 0.58±0.10 vs. 0.48±0.06, P = 0.030).
Western blotting
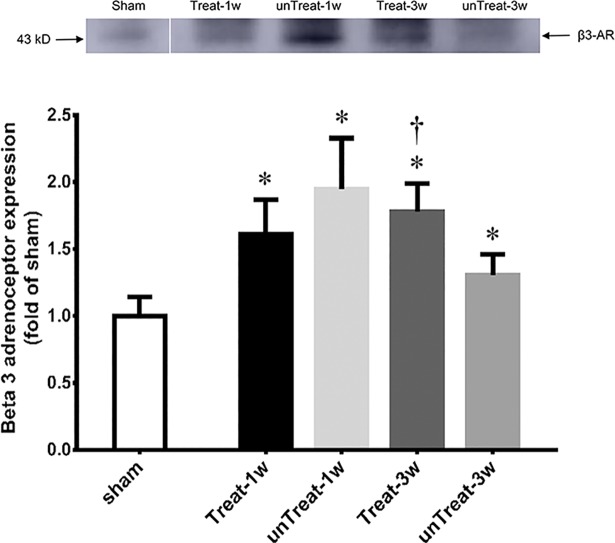
The abundance of the beta-3 adrenoreceptor was increased in the tempol-treated rats 3 week after relief compared to the expression levels in the untreated rats. However, no significant difference in the abundance of the beta-3 adrenoceptor was observed 1 weeks after relief between the treated and untreated rats (Fig 4).
Fig 4. Western blot analysis.
Representative Western blot shows expression of beta-3 receptor protein in the bladder, respectively. Bar graphs show the quantitative analysis for beta-3 receptor protein expression. Data are expressed as folds of corresponding expression in sham-operated rats. Results are expressed as mean ± standard error of the mean. *P<0.05 versus sham; †P<0.05 versus untreated group in the same period (Mann-Whitney U test).
Discussion
In this study, we demonstrated in an animal model that antioxidant agent, such as tempol, prevented bladder I/R injury after relief of pBOO. The preventive effects demonstrated at 1 and 3 weeks. The preventive effect of antioxidants on I/R injury was shown to reduce apoptosis mainly in the mucosal layer and to restore muscle hypertrophy more rapidly in the smooth muscle layer. This effect resulted in a decrease in NVCs in cystometry that was presumably related to beta-3 adrenoreceptor expression.
pBOO causes a chronic ischemic status in the bladder. High pressure due to sustained overdistention can induce a reduction of blood flow to the bladder wall [14]. Additionally, pBOO increases the thickness of the bladder wall through hypertrophy of the detrusor smooth muscle and deposition of collagen tissues, resulting in a reduction of microvascular blood perfusion [15]. In addition, rapid reperfusion due to relief of pBOO in chronically ischemic bladders leads to the generation of free radicals and rapid oxidative stress. Relief of pBOO resolves the intravesical high pressure, although resolving the bladder wall thickness takes at least 3 weeks, and the ongoing reduction of microvascular blood perfusion lasts for a considerable period. The increased metabolic demand on the hypertrophied detrusor can produce more severe damage in the bladder when combined with a reduction in microvascular blood perfusion [16]. The decompensated bladder also causes substantial residual urine, and overdistension of the bladder persists for a considerable period of time, resulting in sustained I/R injury after relief. In our study, this I/R injury persisted for up to 3 weeks after relief.
Chronic I/R injury induced NVCs in the cystometrogram in our study. Oxidative stress results in the generation of free radicals and oxidative damage [17]. Chronic oxidative stress may damage intrinsic nerves, resulting in partial denervation of smooth muscle. Denervation of the detrusor muscle leads to sensitization of the afferent pathway through postjunctional supersensitivity with increased neurotransmitters and upregulation of neurokinin receptors [18]. Denervation and nerve degeneration in sensory pathways during chronic I/R injury also cause an increase in the nerve growth factor level that may induce bladder hypersensitivity [19]. Additionally, chronic oxidative stress leads to the accumulation of calcium in the intracellular medium and the formation of metabolic end products that damage the detrusor musculature [17]. The resulting neurogenic and myogenic damage may cause detrusor overactivity but impaired contractility, resulting in NVCs in the cystometrogram.
Oral administration of antioxidants reduced I/R injury of the bladder after relief. Several studies have proven that antioxidants reduce oxidative stress. Antioxidants have shown preventive and therapeutic benefits in several cardiovascular diseases [11]. Free radical scavengers decrease blood pressure and improve vascular function via biochemical mechanisms, such as normalizing the increased renal sympathetic nerve activity, plasma norepinephrine levels, and angiotensin type I receptor expression and enhancing carotid body chemoreceptor sensitivity to hypoxia [20]. The use of antioxidants after liver transplantation attenuates the effects of I/R-related oxidative stress and reduces lipid peroxidation [10]. However, few reports have shown that antioxidants can reduce oxidative stress in the bladder. I/R injury after BPH surgery has drawn increasing attention with the popularity of the HoLEP procedure, which can completely resect the adenoma. Determining whether the delivery of stable antioxidants to the target organ is possible is important at the time of systemic administration of antioxidants. We confirmed that delivery of effective antioxidant to the bladder was possible in our study. However, studies on bladder-specific delivery may be necessary in the near future.
Apoptosis due to I/R injury was mainly observed in the mucosal layer rather than in the muscle layer. Sensory neurons in the lamina propria, which are susceptible to I/R injury, may be irreversibly damaged by I/R injury [21]. Degeneration of sensory neurons in the mucosal layer may contribute to detrusor instability. In our study, more NVCs occurred in the untreated rats, which were not given antioxidants and sustained more I/R injuries. This finding could be inferred from the influence of apoptosis in the mucosal layer. This finding of our study suggested that the pathophysiology of an overactive bladder may be due to the neuroplasticity of peripheral sensory neurons rather than the myogenic hypothesis.
A difference in beta-3 adrenoceptor expression was observed depending on the degree of I/R injury. In our study, the administration of antioxidants for 3 week prevented the reduction of beta-3 adrenoceptor protein expression compared to the expression level in the untreated group. There are recent studies that the expression of beta-3 adrenoceptor mRNA may be dependent on the degree of obstruction. However, the results are still controversial. In the severe BOO group, the expression level of beta-3 adrenoceptor mRNA in the mucosa of prostatic urethra was significantly lower than that in the mild BOO group [22]. In contrast, pBOO has been reported to increase beta-3 adrenoceptor mRNA expression in bladder of rat models [23]. Activation of beta-3 adrenoceptor in the urothelium induces relaxation of the bladder detrusor muscle [24]. Clinically, some patients exhibit no effect of beta-3 agonists. The difference in the effect of beta-3 agonists may be explained by the differences in beta-3 adrenoceptor expression in the urothelium of the bladder.
Conclusions
Treatment with a free radical scavenger after relief of pBOO shows to prevent histological and functional changes in the bladder. Apoptosis in the urothelium and the deposition of collagen fibers in the detrusor muscle layer were significantly decreased. The incidence of NVCs was also decreased. Additionally, the beta-3 adrenoceptor was associated with bladder overactivity, caused by I/R injury presumably. These preventive effects demonstrated at 1 and 3 weeks.
Supporting information
(CSV)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grant no. 04-2013-0490 from the Seoul National University Hospital research fund.
References
- 1.Keihani S, Kajbafzadeh AM. Concomitant Anterior and Posterior Urethral Valves: A Comprehensive Review of Literature. Urology. 2015;86(1):151–7. Epub 2015/04/13. 10.1016/j.urology.2015.02.019 . [DOI] [PubMed] [Google Scholar]
- 2.Lin CD, Kuo HC, Yang SS. Diagnosis and Management of Bladder Outlet Obstruction in Women. Lower urinary tract symptoms. 2016;8(1):30–7. Epub 2016/01/21. 10.1111/luts.12094 . [DOI] [PubMed] [Google Scholar]
- 3.Solomon E, Yasmin H, Duffy M, Rashid T, Akinluyi E, Greenwell TJ. Developing and validating a new nomogram for diagnosing bladder outlet obstruction in women. Neurourology and urodynamics. 2018;37(1):368–78. Epub 2017/07/01. 10.1002/nau.23307 . [DOI] [PubMed] [Google Scholar]
- 4.Dybowski BA, d'Ancona FC, Langenhuijsen JF, Heesakkers JP. Detrusor overactivity does not predict bothersome storage symptoms after photoselective vaporization of the prostate with lithium triborate laser. Urology. 2014;84(4):898–903. Epub 2014/08/26. 10.1016/j.urology.2014.06.027 . [DOI] [PubMed] [Google Scholar]
- 5.Cho MC, Park JH, Jeong MS, Yi JS, Ku JH, Oh SJ, et al. Predictor of de novo urinary incontinence following holmium laser enucleation of the prostate. Neurourology and urodynamics. 2011;30(7):1343–9. Epub 2011/05/04. 10.1002/nau.21050 . [DOI] [PubMed] [Google Scholar]
- 6.Antunes AA, Iscaife A, Reis ST, Albertini A, Nunes MA, Lucon AM, et al. Can we predict which patients will experience resolution of detrusor overactivity after transurethral resection of the prostate? The Journal of urology. 2015;193(6):2028–32. Epub 2015/01/15. 10.1016/j.juro.2014.12.095 . [DOI] [PubMed] [Google Scholar]
- 7.Chuang YC, Tyagi P, Wang HJ, Huang CC, Lin CC, Chancellor MB. Urodynamic and molecular characteristics of detrusor underactivity in a rat cryoinjury model and effects of low energy shock wave therapy. Neurourology and urodynamics. 2017. Epub 2017/08/03. 10.1002/nau.23381 . [DOI] [PubMed] [Google Scholar]
- 8.Gotoh D, Torimoto K, Tatsumi Y, Hori S, Yamada A, Miyake M, et al. Tadalafil, a phosphodiesterase type 5 inhibitor, improves bladder blood supply and restores the initial phase of lower urinary tract dysfunction in diabetic rats. Neurourology and urodynamics. 2017. Epub 2017/08/07. 10.1002/nau.23372 . [DOI] [PubMed] [Google Scholar]
- 9.Li WJ, Shin MK, Oh SJ. Time dependent bladder apoptosis induced by acute bladder outlet obstruction and subsequent emptying is associated with decreased MnSOD expression and Bcl-2/Bax ratio. Journal of Korean medical science. 2010;25(11):1652–6. Epub 2010/11/10. 10.3346/jkms.2010.25.11.1652 ; PubMed Central PMCID: PMCPmc2967004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barros MA, Vasconcelos PR, Souza CM, Andrade GM, Moraes MO, Costa PE, et al. L-Alanyl-Glutamine Attenuates Oxidative Stress in Liver Transplantation Patients. Transplantation proceedings. 2015;47(8):2478–82. Epub 2015/11/01. 10.1016/j.transproceed.2015.08.001 . [DOI] [PubMed] [Google Scholar]
- 11.Jain AK, Mehra NK, Swarnakar NK. Role of Antioxidants for the Treatment of Cardiovascular Diseases: Challenges and Opportunities. Current pharmaceutical design. 2015;21(30):4441–55. Epub 2015/08/04. . [DOI] [PubMed] [Google Scholar]
- 12.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacological reviews. 2008;60(4):418–69. Epub 2008/12/30. 10.1124/pr.108.000240 ; PubMed Central PMCID: PMCPmc2739999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang YJ, Jin LH, Park CS, Shin HY, Yoon SM, Lee T. Early sequential changes in bladder function after partial bladder outlet obstruction in awake sprague-dawley rats: focus on the decompensated bladder. Korean journal of urology. 2011;52(12):835–41. Epub 2012/01/05. 10.4111/kju.2011.52.12.835 ; PubMed Central PMCID: PMCPMC3246516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chai TC, Gemalmaz H, Andersson KE, Tuttle JB, Steers WD. Persistently increased voiding frequency despite relief of bladder outlet obstruction. The Journal of urology. 1999;161(5):1689–93. Epub 1999/04/21. . [PubMed] [Google Scholar]
- 15.Tong-Long Lin A, Chen KK, Yang CH, Chang LS. Recovery of microvascular blood perfusion and energy metabolism of the obstructed rabbit urinary bladder after relieving outlet obstruction. Eur Urol. 1998;34(5):448–53. Epub 1998/11/06. . [DOI] [PubMed] [Google Scholar]
- 16.Levin RM, Haugaard N, Hypolite JA, Wein AJ, Buttyan R. Metabolic factors influencing lower urinary tract function. Experimental physiology. 1999;84(1):171–94. Epub 1999/03/19. . [DOI] [PubMed] [Google Scholar]
- 17.Bisogni S, Ferreira FT, Amstalden Neto A, Chiarelli LO, Ortiz V. Influence of oxidative stress on inducing micturition dysfunction following chronic infravesical obstruction and the protective role of an antioxidant diet—association of in vivo and in vitro studies in rats. International braz j urol: official journal of the Brazilian Society of Urology. 2012;38(4):552–60. Epub 2012/09/07. . [DOI] [PubMed] [Google Scholar]
- 18.Nomiya M, Andersson KE, Yamaguchi O. Chronic bladder ischemia and oxidative stress: new pharmacotherapeutic targets for lower urinary tract symptoms. International journal of urology: official journal of the Japanese Urological Association. 2015;22(1):40–6. Epub 2014/10/24. 10.1111/iju.12652 . [DOI] [PubMed] [Google Scholar]
- 19.Azadzoi KM, Yalla SV, Siroky MB. Oxidative stress and neurodegeneration in the ischemic overactive bladder. The Journal of urology. 2007;178(2):710–5. Epub 2007/06/19. 10.1016/j.juro.2007.03.096 . [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaugh EG, Savalia KK, Manickam DS, Zimmerman MC. Antioxidant-based therapies for angiotensin II-associated cardiovascular diseases. American journal of physiology Regulatory, integrative and comparative physiology. 2013;304(11):R917–28. Epub 2013/04/05. 10.1152/ajpregu.00395.2012 ; PubMed Central PMCID: PMCPMC3680755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steers WD. Pathophysiology of Overactive Bladder and Urge Urinary Incontinence. Reviews in Urology. 2002;4(Suppl 4):S7–S18. PubMed PMID: PMC1476015. [PMC free article] [PubMed] [Google Scholar]
- 22.Kurizaki Y, Ishizuka O, Imamura T, Ishikawa M, Ichino M, Ogawa T, et al. Relationship between expression of beta3-adrenoceptor mRNA in bladder mucosa and urodynamic findings in men with lower urinary tract symptoms. Neurourology and urodynamics. 2013;32(1):88–91. Epub 2012/06/14. 10.1002/nau.22278 . [DOI] [PubMed] [Google Scholar]
- 23.Park MG, Park HS, Lee JG, Kim HJ. Changes in Awake Cystometry and Expression of Bladder beta-adrenoceptors after Partial Bladder Outlet Obstruction in Male Rats. Int Neurourol J. 2010;14(3):157–63. Epub 2010/12/24. 10.5213/inj.2010.14.3.157 ; PubMed Central PMCID: PMCPMC2998402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi O, Chapple CR. Beta3-adrenoceptors in urinary bladder. Neurourology and urodynamics. 2007;26(6):752–6. Epub 2007/06/30. 10.1002/nau.20420 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.