Abstract
Objective
To assess the relationship between serum endostatin (ES) and coronary artery calcification (CAC) in type 2 diabetic (T2DM) patients.
Methods
The study included 110 participants with coronary artery disease (CAD); 55 with T2DM, for serum ES levels by enzyme-linked immunosorbent assay and CAC by contrast-enhanced spiral computed tomography (CT).
Results
Mean serum ES value was 66.54 ng/mL [95% confidence interval (CI), 61.77–71.32 ng/mL]. Serum ES levels positively correlated with Agatston score index [ASI; r = 0.701, p < 0.001; high sensitive C-reactive protein (hs-CRP) r = 0.783, p < 0.001]. On multiple regression analysis, the highest three ES quartiles (2, 3, and 4) were related to ASI in diabetic patients, adjusted ES level was an independent predictor of CAD [odds ratio (OR) = 1.065; 95% CI, 1.008–1.126; p = 0.026] and for the number of coronary vessels affected (OR = 1.089; 95% CI, 1.018–1.164; p = 0.013) in T2DM patients. Receiver operating characteristics (ROC) analysis showed serum ES at a cutoff value of 86.5 ng/mL can predict the risk of CAC in T2DM, with a sensitivity of 74.1%, specificity of 71.4%, p < 0.001 and area under curve (AUC) of 0.776.
Conclusion
Measurement of serum ES levels can improve diagnosis of CAC and could be useful as a high sensitive marker for the presence and progression of atherosclerosis in T2DM patients.
Keywords: Coronary calcification, Coronary heart disease, Endostatin, Type 2 DM
Abbreviations
- ES
Endostatin
- CAC
Coronary artery calcification
- T2DM
Type 2 diabetic
- CAD
Coronary artery disease
- ASI
Agatston score index
- NO
Nitric oxide
- VSMCs
Vascular smooth muscle cells
- CT
Cardiac computed tomography
- TTE
Trans-thoracic echocardiography
- 2D
Two-dimensional
- LVEDD
Left ventricular end diastolic diameter
- LVESD
Left ventricular end systolic diameter
- IVS
Interventricular septum
- EF
Ejection fraction
- CACS
Coronary artery calcium score
- CCTA
Coronary computed tomographic angiography
- FBG
Fasting blood glucose
- T.C
Total cholesterol
- LDL
Low-density lipoprotein
- HDL
High-density lipoprotein
- hs-CRP
C-reactive protein
- HOMA-IR
The homeostasis model of insulin resistance
- GFR
Glomerular filtration rate
- ROC
Receiver Operating Characteristics
- CHD
Coronary heart disease
1. Introduction
Diabetes mellitus is an established cardiovascular major risk factor [1], labeled as a coronary artery disease (CAD) “risk equivalent” and its prevalence has been increasing globally [2].
Coronary artery disease (CAD) causes more deaths in patients with T2DM than patients without diabetes [3]. The pathophysiology of vascular disease in T2DM includes reduced production and bioavailability of nitric oxide (NO) with endothelial dysfunction, vascular smooth muscle cells (VSMCs) abnormalities, disturbed platelet function, enhanced inflammation, and subsequent atherosclerosis [4].
Diabetes strongly associates with coronary artery calcification (CAC)—assessed using cardiac computed tomography (CT)—which is a well-established surrogate marker of the total atherosclerotic burden. Progression and the total amount of CAC can be used to improve the prediction of the cardiovascular events risk beyond the standard risk factors [4], [5].
Vascular calcification is a complex pathobiology process, it is of two principal types; intimal (associates atherosclerosis) and medial (arteriosclerosis or known as Mönckeberg’s sclerosis) [3]. These two distinct types should be considered as a continuum [6], [7].
Endostatin is a fragment of the C-terminal domain NC1 of collagen XVIII [8], found in vessel walls (elastic fibers) and basement membranes with a strong angiogenesis modulating effect [9]. It inhibits endothelial cell migration, induces endothelial cell apoptosis [10], plays an important role in endothelial cell adhesion [11], and impairs blood vessel maturation in wound healing [12].
Diabetes [13], physical activity [14], inflammation, and obesity can affect the circulating serum levels of ES. Disturbed ES serum level is associated with cerebrovascular diseases [15], organ damage in hypertension [16], and can predict cardiovascular mortality in elderly people [17].
This study investigates the relationship between circulating endostatin level and the degree of CAC in T2DM with CAD.
2. Materials and methods
This case-control study included 110 patients who previously verified with coronary artery disease of different severity after invasive coronary angiography or cardiac computed tomography. CAD was defined as nonobstructive coronary artery stenosis <50% or obstructive lesion (≥50%) stenosis [18], [19]. Patients were recruited from the outpatient Clinics of Cardiology and Internal Medicine at Zagazig University Hospital in Egypt based on the following inclusion criteria: patients were aged between 50 and 70 years at enrollment, symptomatic or previously recognized coronary artery disease for all the study population, and type 2 diabetic patients with the onset of type 2 diabetes occurred at age 30 years or older with no history of ketoacidosis for Group II participants. Exclusion criteria: left ventricular ejection fraction (LVEF) ≤40%; chronic heart failure, unstable angina, myocardial infarction, arrhythmia, valvular heart disease, coronary artery bypass surgery, history of stent placement, liver diseases, malignancy, glomerular filtration rate index <35 mL/min/m2, serum creatinine level >4.5 mg/dL, body mass index >30 kg/m2 or <15 kg/m2, pulmonary edema, stroke, acute infections, severe trauma, recent surgery, inflammatory conditions, thyrotoxicosis, pregnancy, and known allergic reactions.
Patients were divided into two groups based on presence or absence of T2DM; Group I (55 CAD patients without T2DM) and Group II (55 CAD patients with T2DM).
Review and approval of the study were obtained from the Ethical Committee of the Faculty of Medicine, Zagazig University, Zagazig, Egypt.
After giving a written informed consent, all participants were subjected to the following: (1) full history taking and thorough clinical examination; (2) electrocardiographic examination; and (3) transthoracic echocardiography (TTE). TTE was performed for every patient using GE Vivid 9 set (part no GA 091568, Norway 2010 – Chicago, Illinois, United States by GE Healtcare) using 5 MHz transducer. Images were taken while the patient was supine or in left lateral position, utilizing two-dimensional (2D), M-mode and Doppler echocardiographic techniques for evaluation of: left ventricular end diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD), interventricular septum (IVS), and ejection fraction (EF); and (4) cardiac CT angiography. Computed tomography data acquisition and post processing.
One hour before the scan, 25–50 mg of metoprolol was given orally to the patients with heart rates >60 beats/minute and had no contraindications to beta-blockers. Sublingual nitroglycerin was given to all patients before CT examination. A 64-slice CT scanner (Sensation 64, Siemens Medical System, Henkestr, Germany) was used. Scanning started from near the lower margin of the main pulmonary artery bifurcation with the individual holding breath during inspiration for 35–40 seconds and proceeded caudally.
Coronary artery calcium score (CACS) evaluation was done initially through a nonenhanced prospective electrocardiogram (ECG)-gated scan with a section collimation (0.6 mm), rotation time (330 ms), tube voltage (100–120 kV), slice width (3.0 mm), and tube current (50 mA). Coronary computed tomographic angiography (CCTA) was performed using retrospective ECG gating with a section collimation (64 × 0.6 mm), rotation time (330 ms), tube voltage (100–120 kV), and tube current (400–800 mA). ECG-based tube current modulation was applied to 65% of the R–R interval. Iodixanol (270 mg/mL-as a contrast agent) was given. Contrast timing was tested by an initial bolus-timing scan using 15 mL followed by a 30-mL saline chaser. The scan time was adjusted by adding 3 seconds after the peak of time-enhancement curve of the ascending aorta to ensure maximum opacification of the coronary arteries. The contrast-enhanced scan was obtained using a volume of 60–100 mL of contrast individually adapted to the selected table feed and scan range followed by a 50-mL saline chaser. Both contrast and saline were injected at a rate of 6 mL/s by using a dual-syringe injector (Stellant D; Medrad, Indianola, PA, USA). Image reconstruction was performed by Vitrea Workstation (Vitrea software, version 6.5.1, Vital Images, Minnesota, USA). Reconstruction of axial images was done retrospectively at 65% of the R–R interval for each cardiac cycle. The reconstructed image data sets were sent to an offline workstation for image analysis. Maximum intensity projection and multi-planar reconstruction techniques on a short axis and along multiple longitudinal axes were used for examining each identified lesion then classified into three categories according to the luminal stenotic percentage: normal (absence of plaque/no luminal stenosis), nonobstructive lesion (up to 49% stenosis), and obstructive lesion (≥50% stenosis).
Calcified coronary artery lesions were identified when a minimum density of 130 Hounsfield units and a minimum area of three pixels (1.03 mm). Calcium score for each region was assessed and calculated as 1 for 130–199 Hounsfield units, 2 for 200–299 Hounsfield units, 3 for 300–399 Hounsfield units, and 4 for >399 Hounsfield units. A total CAC score in Agatston units was determined by adding up scores for all slices separately for left main, left anterior descending, circumflex, and right coronary arteries by the workstation using a standard algorithm [20].
2.1. Laboratory investigations
Blood samples were collected after an overnight fast. The samples were centrifuged at 3000 g at 4 °C for 15 minutes. The supernatants were blind coded and frozen at −80 °C until assayed. Fasting blood glucose (FBG), serum creatinine, total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and HbA1c were measured using standard methods. High sensitive C-reactive protein (hs-CRP) levels were measured by the nephelometric method. The homeostasis model of insulin resistance (HOMA-IR) index was calculated as (fasting serum glucose × fasting serum insulin/22.5). ES levels were measured using a sandwich enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s instructions endostatin ELISA kit (R&D Systems, Minneapolis, MN, USA).
Patients with acute coronary syndrome, coronary artery bypass, history of stenting, manifest arrhythmia, more than mild valvular heart disease, acute heart failure, liver diseases, malignancy, glomerular filtration rate (GFR) index <35 mL/min/m2, serum creatinine level >4.5 mg/dL, obesity (body mass index >30 kg/m2), stroke, acute infections, severe trauma, recent surgery, inflammatory conditions, thyrotoxicosis, pregnancy, and known allergic reactions were excluded from the study.
2.2. Statistical analysis
Statistical analysis of received data was conducted using the program SPSS version 20 (SPPS Inc., Chicago, IL, USA). Data were tested for normality using the Kolmogorov–Smirnov test. Baseline of study population characteristics were summarized using frequency and percent distribution [n (%)] for categorical variables, whereas mean and standard deviation (mean value ± SD) values were used for scale variables. All association between the different study variables was performed using X2/Fisher exact test for categorical variables and a Mann–Whitney U test/Student t test for continuous variables based on shape of distribution. Pearson correlation was performed between CAC, ES, and other risk factors of CAD. Determination of cutoff values with associated sensitivity and specificity was performed using receiver operating characteristics (ROC) analysis. Multivariate logistic regression analysis was used to detect independent predictor of certain parameter. A calculated difference of p < 0.05 was considered significant.
3. Results
Baseline of study population characteristics: patient groups (I and II) showed no statistically significant difference in terms of age, sex, and comorbidities. Based on %HbA1c, the calculated mean of patients in Group I was 4.73 ± 0.45, and 8.41 ± 0.8 in Group II (p < 0.001). Circulating hs-CRP level, HOMA-IR, and severity of CAC measured by ASI were significantly higher in Group II when compared with Group I, (p < 0.001; Table 1).
Table 1.
Baseline characteristics of Group I (CAD without T2DM) and Group II (CAD with T2DM) patients.
| Variables | Group I (n = 55) | Group II (n = 55) | p* |
|---|---|---|---|
| Age (yr) | 55.92 ± 4.66 | 57.7 ± 5.66 | >0.05 |
| Male | 34 (61.8) | 33 (60) | >0.05 |
| Hypertension | 29 (52.7) | 27 (49.1) | >0.05 |
| Hyperlipidemia | 34 (61.8) | 32 (58.2) | >0.05 |
| Smoking | 26 (47.3) | 25 (45.5) | >0.05 |
| Waist/hip ratio | 0.90 ± 0.089 | 0.94 ± 0.1 | >0.05 |
| BMI (kg/m2) | 24.32 ± 2.82 | 25.3 ± 3.5 | >0.05 |
| HbA1c | 4.73 ± 0.45 | 8.41 ± 0.8 | <0.001 |
| HOMA-IR | 3.74 ± 0.46 | 9.6 ± 0.9 | <0.001 |
| hs-CRP (mg/L) | 4.57 ± 1.11 | 8.33 ± 1.76 | <0.001 |
| Creatinine (mg/dL) | 0.96 ± 0.17 | 1.02 ± 0.2 | >0.05 |
| TC (mg/dL) | 201.69 ± 21.23 | 208.1 ± 15.64 | >0.05 |
| Mean systolic BP, mmHg | 126.9 ± 11.52 | 130.27 ± 12.56 | >0.05 |
| Mean diastolic BP (mmHg) | 81 ± 8.84 | 83.09 ± 9.3 | >0.05 |
| LVEF (%) | 49.87 ± 5.7 | 48.2 ± 4.2 | >0.05 |
| Coronary artery obstructive disease: | |||
| 1 vessel | 6 (10.9) | 8 (14.5) | >0.05 |
| 2 vessels | 3 (5.5) | 7 (12.7) | |
| 3 vessels | 8 (14.5) | 11 (20) | |
| ACEI/ARBs | 49 (89.1) | 52 (94.5) | >0.05 |
| Aspirin | 48 (87.3) | 53 (96.4) | >0.05 |
| Statins | 38 (69.1) | 43 (78.2) | >0.05 |
Data are presented as n (%) or mean ± SD.
ACEI = angiotensin converting enzyme inhibitor; ARBs = angiotensin-2 receptor blockers; BMI = body mass index; HbA1c = glycated hemoglobin; HOMA-IR = homeostatic model assessment of insulin resistance; hs-CRP = high sensitive C-reactive protein; HU = Hounsfield units; LVEF = left ventricular ejection fraction; TC = total cholesterol.
p < 0.05 is considered statistically significant.
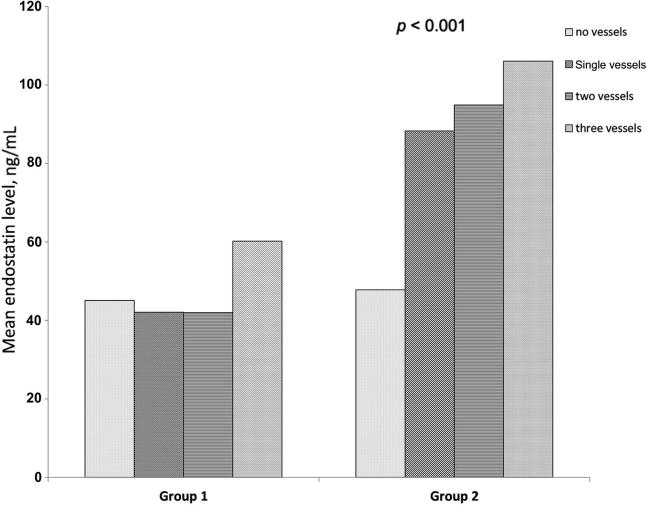
A significantly higher overall ES level among diabetic Group II (85.43 ± 18.9) than nondiabetic Group I patients (47.67 ± 14.27) (p < 0.001). The number of affected vessels was associated with significantly higher mean ES serum level in Group II than in Group I, p < 0.001 (Table 2 and Fig. 1).
Table 2.
Comparison of main serum endostatin levels in Group 1 (CAD without T2DM) and Group 2 (CAD with T2DM) according to the number of coronary vessels affected.
| Number of coronary vessel(s) affected | Group 1 (mean ± SD) | Group 2 (mean ± SD) | p |
|---|---|---|---|
| No vessels | 45.12 ± 13.50 | 74.83 ± 16.55 | p < 0.001 |
| Single vessels | 42.11 ± 12.60 | 88.25 ± 19.52 | |
| 2 vessels | 41.98 ± 12.53 | 94.85 ± 20.98 | |
| 3 vessels | 60.21 ± 18.53 | 106.12 ± 23.47 |
CAD = coronary artery disease; SD = standard deviation; T2DM = type 2 diabetic.
Figure 1.
Bar graph shows comparison of mean serum ES levels in Group I and Group II according to the number of coronary vessels affected. ES = endostatin.
Data analysis showed that ES mean value was 66.4 ng/mL [95% confidence interval (CI) = 61.77–71.32 ng/mL] in all study participants. Depending on ASI, the distribution of serum ES levels were: 51.93 ng/mL (95% CI = 42.17–61.69 ng/mL) for one, 51.64 ng/mL (95% CI = 46.38–56.91 ng/mL) for two, 59.49 ng/mL (95% CI = 51.95–67.04 ng/mL) for three, and 80.59 ng/mL (95% CI = 73.34–87.83 ng/mL) for four.
The overall ASI was significantly higher in Group II (499.5 ± 212.82) than Group I (311.94 ± 120.53) with p < 0.001. The distribution of serum ES for both groups of patients according to ASI is shown in Table 3. There were significant differences between serum ES levels in both groups of patients with all ASI levels.
Table 3.
Serum endostatin distribution for Group I (CAD without T2DM) and Group II (CAD with T2DM) according to Agatston score index.
| Agatston score index (ASI) | Group I (n = 55) |
Group II (n = 55) |
p* | ||
|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | ||
| 1 (130–199 HU) | 37.62 | 33.07– 42.17 | 54.6 | 45.64–63.56 | <0.001 |
| 2 (200–299 HU) | 45.73 | 41.41–50.04 | 69.1 | 53.63–84.57 | <0.001 |
| 3 (300–399 HU) | 47.96 | 42.37–53.54 | 76.81 | 73.71–79.83 | <0.001 |
| 4 (400 + HU) | 54.6 | 45.63–63.56 | 97.36 | 93.13–101.59 | <0.001 |
CI = confidence interval; HU = Hounsfield units.
A p value <0.05 is considered statistically significant.
ES serum levels had significantly direct correlations to ASI (r = 0.701, p < 0.001), hs-CRP (r = 0.783, p < 0.001), total cholesterol (TC; r = 0.366, p < 0.001), age (r = 0.305, p = 0.001), sex (r = 0.263, p = 0.006 for male), smoking (r = 0.217, p = 0.023), and a negative correlation to LVEF (r = −0.306, p = 0.001).
Multiple linear regression analysis adjusted for age, sex, smoking, TC, hs-CRP, and LVEF are shown in Table 4. In multiple regression analyses CAC assessed using ASI was a dependent variable and serum ES quartiles were independent variables in both groups of patients. The predictive strength of serum ES for detection of CAC was tested before and after adjustment for potential confounders including age, sex, smoking, TC, hs-CRP, and LVEF. In Group I, serum ES quartile 4 was an independent predictor of CAC assessed using ASI. In Group II, adjusted ES level within all the quartiles was a predictive marker for CAC except for the first quartiles where adjustment attenuated the predictive strength of ES for CAC detection.
Table 4.
Serum endostatin levels predict the presence of coronary artery calcification in multivariate logistic regression analysis model.
| ES quartiles | ES concentration, ng/mL | Unadjusted |
Adjusted |
||
|---|---|---|---|---|---|
| Mean ± SD | β | p | β | p | |
| Group 1 | |||||
| Q1 | 33.59 ± 2.55 | 0.399 | 0.157 | 0.239 | 0.537 |
| Q2 | 41.31 ± 1.84 | 0.299 | 0.299 | 0.282 | 0.434 |
| Q3 | 48.1 ± 2.72 | 0.462 | 0.131 | −0.758 | 0.218 |
| Q4 | 68.22 ± 12.5 | 0.772 | 0.002* | 0.751 | 0.024* |
| Group 2 | |||||
| Q1 | 60.5 ± 11.81 | 0.711 | 0.004* | 0.272 | 0.602 |
| Q2 | 81.17 ± 3.98 | 0.799 | 0.001* | 0.805 | 0.017* |
| Q3 | 93.6 ± 3.52 | 0.807 | <0.001* | 0.645 | 0.042* |
| Q4 | 108.06 ± 6.84 | 0.765 | 0.002* | 1.296 | 0.029* |
Q1 = first quartile, adjusted for age, sex, smoking, total cholesterol, hs-CRP, and LVEF.
A p value <0.05 is considered statistically significant.
ROC-analysis was used to determine the serum ES concentration at a cutoff value of 86.5 ng/mL could be used as a predictor of severity of coronary calcification assessed using ASI in Group II diabetic patients; with a sensitivity of 74.1%; a specificity of 71.4%, area under curve (AUC) = 0.776, p < 0.001. Without adjustment for other risk factors, serum ES was predictive for obstructive CAD in diabetic patients (OR = 1.064; 95% CI 1.022–1.107; p = 0.003) but not for nondiabetic individuals (OR = 1.02; 95% CI, 0.982–1.062; p = 0.297). Multivariable analysis with the use of logistic regression models to obtain adjusted odds ratios for coronary artery obstruction in Group II diabetic patients showed that ES was an independent predictor for obstructive coronary artery disease, after adjustment for age, sex, smoking, CRP, TC, and HOMA-IR (OR = 1.065; 95% CI, 1.008–1.126; p = 0.026). The results showed that ES serum levels were directly related to the number of affected coronary arteries in Group II (r = 0.547, p < 0.001) but no significant results obtained in Group I (r = 0.265, p = 0.051). In Group II participants, ES was an independent factor associated with the number of coronary vessels affected (OR = 1.093; 95% CI, 1.036–1.153; p = 0.001) and this association remained significant after adjustment for conventional risk factors (OR = 1.089; 95% CI, 1.018–1.164; p = 0.013).
4. Discussion
Blood vessel calcification is a dynamic process and a pathological feature of atherosclerosis. Environmental and therapeutic factors had a wide effect on such process [21]. In asymptomatic patients with extensive CAC, mild and moderate CAC score increased the risk of first acute coronary events [22]. CAC score >40 was considered as a predictor for atherosclerotic events in T2DM patients with low to intermediate Framingham risk Score [23]. Diabetes causes an impairment of vasa vasorum, neovascularization, and increases the risk of atherosclerosis [24]. In diabetic patients, calcification of blood vessels includes both the intima and media [25]. Atherosclerosis is mainly related to calcification of the intimal plaque [6], [7].
This study showed that serum ES significantly correlates with CAC, obstruction, and the number of diseased coronary vessels in T2DM population with known CAD.
As known, age, sex, dyslipidemia, and smoking are conventional risk factors for atherosclerosis. But according to some studies, T2DM patients have a higher CAC score [26], [27] than can be attributed to known risk factors [26], which could be more predictive as an independent risk factor than traditional risk factors [28], with high prevalence of CAC in asymptomatic T2DM patients [29]. Therefore, CAC score can be considered as an important cardiovascular risk index in diabetics. T2DM is coronary heart disease (CHD) equivalent and leads to accelerated atherosclerotic process [2].
The multivariate logistic regression analysis in this study — either with or without adjustments for the conventional cardiovascular risk factors — has shown that serum ES had a stronger association with CAC than other known risk factors, and could be considered as an independent risk factor for CAC in CAD patients with T2DM. Several studies have proven that high serum ES levels can predict cardiovascular mortality independently and were associated with acute myocardial infarction [30].
In comparison with other expected markers for CAC, Jenny et al [31] examined cross-sectional associations of C-reactive protein (CRP), interleukin-6 (IL-6), and fibrinogen with CAC presence (Agatston score >0 by computed tomography) in 6783 Multi-Ethnic Study of Atherosclerosis (MESA) participants. They concluded that inflammatory markers were weakly associated with CAC presence and burden in MESA.
It has been found that a higher serum ES level was a significant predictor of CAC progression, as it was associated with the severity of vessel obstruction and the number of coronary vessels affected. This association was seen in T2DM than nondiabetic patients. Although all ES quartiles in diabetics and quartile four in nondiabetic patients were associated with calcification of coronary arteries, it still has a greater predictive value of CAC in T2DM patients than nondiabetic patients. This may be related partially to chronic hyperglycemia. Medial arterial calcification in T2DM patients were strongly related to chronic hyperglycemia and carried a higher risk for incident CVD [32]. Also, no independent correlation between either adjusted or unadjusted ES quartiles indexed as 1, 2, and 3 in Group I participants and unadjusted first ES quartile in Group II diabetic patients and CAC in multiple linear regression analysis; thus, serum ES may be related to CAC above a certain cutoff point which was 86.5 ng/mL in Group II diabetic patients.
Although the mechanism of inducing coronary calcification by ES is not that clear, higher serum ES level associated with valvular calcification was detected in CAD patients. ES was involved in the active process of valvular calcification which is significantly similar to pathogenesis of atherosclerosis [33]. The direct mechanisms related to the serum ES level and calcified atherosclerotic changes may be related to cleavage of ES from collagen XVIII by proteinases [34] such as MMP-9 which has a role in initiation and advancement of atherosclerosis [35], [36]. However, increased levels of metalloproteinases (MMPs), circulating Cathepsin D as well as PMN (neutrophil) elastase, and other proteases are closely linked with the presence of T2DM [37], [38], [39].
To the best of our knowledge, this study is the first to demonstrate a link between elevated ES and CAC progression in T2DM patients with coronary heart disease as it included both T2DM and non-DM CAD participants. Even though the small size of the study sample may affect the data analytic power, there was a sufficient power to find significant associations between ES level and CAC, extent of coronary artery obstruction and number of affected vessels in T2DM.
5. Conclusion
This study demonstrated that elevated serum ES levels were independently associated with increased risk, severity, and progression of CAC independent of traditional cardiovascular disease risk factors in T2DM patients with known CAD. Measurement of serum ES levels can improve diagnosis of coronary artery calcification and can be useful as a high sensitive marker for the presence and progression of atherosclerosis in T2DM patients.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Kannel W.B., McGee D.L. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 2.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421. [PubMed]
- 3.Elkeles R.S. Coronary artery calcium and cardiovascular risk in diabetes. Atherosclerosis. 2010;210:331–336. doi: 10.1016/j.atherosclerosis.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Bertoni A.G., Kramer H., Watson K., Post W.S. Diabetes: Insights from the multi-ethnic study of atherosclerosis. Glob Heart. 2016;11:337–342. doi: 10.1016/j.gheart.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Detrano R., Guerci A.D., Carr J.J., Bild D.E., Burke G., Folsom A.R. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 6.Kovacic J.C., Moreno P., Nabel E.G., Hachinski V., Fuster V. Cellular senescence, vascular disease, and aging: Part 2 of a 2-part review: Clinical vascular disease in the elderly. Circulation. 2011;123:1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- 7.McCullough P.A., Agrawal V., Danielewicz E., Abela G.S. Accelerated atherosclerotic calcification and Monckeberg’s sclerosis: s continuum of advanced vascular pathology in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1585–1598. doi: 10.2215/CJN.01930408. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki T., Larsson H., Tisi D., Claesson-Welsh L., Hohenester E., Timpl R. Endostatins derived from collagens XV and XVIII differ in structural and binding properties, tissue distribution, and antiangiogenic activity. J Mol Biol. 2000;301:1179–1190. doi: 10.1006/jmbi.2000.3996. [DOI] [PubMed] [Google Scholar]
- 9.Zatterstrom U.K., Felbor U., Fukai N., Olsen B.R. Collagen XVIII/endostatin structure and functional role in angiogenesis. Cell Struct Funct. 2000;25:97–101. doi: 10.1247/csf.25.97. [DOI] [PubMed] [Google Scholar]
- 10.O'Reilly M.S., Boehm T., Shing Y., Fukai N., Vasios G., Lane W.S. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 11.Dixelius J., Cross M., Matsumoto T., Sasaki T., Timpl R., Claesson-Welsh L. Endostatin regulates endothelial cell adhesion and cytoskeletal organization. Cancer Res. 2002;62:1944–1947. [PubMed] [Google Scholar]
- 12.Bloch W., Huggel K., Sasaki T., Grose R., Bugnon P., Addicks K. The angiogenesis inhibitor endostatin impairs blood vessel maturation during wound healing. FASEB J. 2000;14:2373–2376. doi: 10.1096/fj.00-0490fje. [DOI] [PubMed] [Google Scholar]
- 13.Sponder M., Dangl D., Kampf S., Fritzer-Szekeres M., Strametz-Juranek J. Exercise increases serum endostatin levels in female and male patients with diabetes and controls. Cardiovasc Diabetol. 2014;13:6. doi: 10.1186/1475-2840-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sponder M., Sepiol K., Lankisch S., Priglinger M., Kampf S., Litschauer B. Endostatin and physical exercise in young female and male athletes and controls. Int J Sports Med. 2014;35:1138–1142. doi: 10.1055/s-0034-1375692. [DOI] [PubMed] [Google Scholar]
- 15.Arenillas J.F., Alvarez-Sabin J., Montaner J., Rosell A., Molina C.A., Rovira A. Angiogenesis in symptomatic intracranial atherosclerosis: predominance of the inhibitor endostatin is related to a greater extent and risk of recurrence. Stroke. 2005;36:92–97. doi: 10.1161/01.STR.0000149617.65372.5d. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson A.C., Ruge T., Sundström J., Ingelsson E., Larsson A., Lind L. Association between circulating endostatin, hypertension duration, and hypertensive target-organ damage novelty and significance. Hypertension. 2013;62:1146–1151. doi: 10.1161/HYPERTENSIONAHA.113.02250. [DOI] [PubMed] [Google Scholar]
- 17.Ärnlöv J., Ruge T., Ingelsson E., Larsson A., Sundström J., Lind L. Serum endostatin and risk of mortality in the elderly significance. Arterioscler Thromb Vasc Biol. 2013;33:2689–2695. doi: 10.1161/ATVBAHA.113.301704. [DOI] [PubMed] [Google Scholar]
- 18.Pizzi C., Xhyheri B., Costa G.M., Faustino M., Flacco M.E., Gualano M.R. Nonobstructive versus obstructive coronary artery disease in acute coronary syndrome: a meta-analysis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maddox T.M., Stanislawski M.A., Grunwald G.K., Bradley S.M., Ho P.M., Tsai T.T. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–1763. doi: 10.1001/jama.2014.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopp A.F., Ohnesorge B., Becker C., Schroder S., Heuschmid M., Kuttner A. Reproducibility and accuracy of coronary calcium measurements with multi-detector row versus electron-beam CT. Radiology. 2002;225:113–119. doi: 10.1148/radiol.2251010173. [DOI] [PubMed] [Google Scholar]
- 21.Li G., Lu W.-h., Ai R., Yang J.-h., Chen F., Tang Z.-z. The relationship between serum hypoxia-inducible factor 1α and coronary artery calcification in asymptomatic type 2 diabetic patients. Cardiovasc Diabetol. 2014;13:;52 doi: 10.1186/1475-2840-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shemesh J., Tenenbaum A., Fisman E.Z., Koren-Morag N., Grossman E. Coronary calcium in patients with and without diabetes: first manifestation of acute or chronic coronary events is characterized by different calcification patterns. Cardiovasc Diabetol. 2013;12:161. doi: 10.1186/1475-2840-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lau K.K., Wong Y.K., Chan Y.H., Yiu K.H., Teo K.C., Li L.S. Prognostic implications of surrogate markers of atherosclerosis in low to intermediate risk patients with type 2 diabetes. Cardiovasc Diabetol. 2012;11:101. doi: 10.1186/1475-2840-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veerman K., Venegas-Pino D., Shi Y., Khan M., Gerstein H., Werstuck G. Hyperglycaemia is associated with impaired vasa vasorum neovascularization and accelerated atherosclerosis in apolipoprotein-E deficient mice. Atherosclerosis. 2013;227:250–258. doi: 10.1016/j.atherosclerosis.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Doherty T.M., Fitzpatrick L.A., Inoue D., Qiao J.H., Fishbein M.C., Detrano R.C. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–672. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 26.Schurgin S., Rich S., Mazzone T. Increased prevalence of significant coronary artery calcification in patients with diabetes. Diabetes Care. 2001;24:335–338. doi: 10.2337/diacare.24.2.335. [DOI] [PubMed] [Google Scholar]
- 27.Meigs J.B., Larson M.G., D'Agostino R.B., Levy D., Clouse M.E., Nathan D.M. Coronary artery calcification in type 2 diabetes and insulin resistance: The Framingham offspring study. Diabetes Care. 2002;25:1313–1319. doi: 10.2337/diacare.25.8.1313. [DOI] [PubMed] [Google Scholar]
- 28.Greenland P., LaBree L., Azen S.P., Doherty T.M., Detrano R.C. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 29.Anand D.V., Lim E., Hopkins D., Corder R., Shaw L.J., Sharp P. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J. 2006;27:713–721. doi: 10.1093/eurheartj/ehi808. [DOI] [PubMed] [Google Scholar]
- 30.Seko Y., Fukuda S., Nagai R. Serum levels of endostatin, vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in patients with acute myocardial infarction undergoing early reperfusion therapy. Clin Sci. 2004;106:439–442. doi: 10.1042/CS20030365. [DOI] [PubMed] [Google Scholar]
- 31.Jenny N.S., Brown E.R., Detrano R., Folsom A.R., Saad M.F., Shea S. Associations of inflammatory markers with coronary artery calcification: results from the multi-ethnic study of atherosclerosis. Atherosclerosis. 2010;209:226–229. doi: 10.1016/j.atherosclerosis.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehto S., Niskanen L., Suhonen M., Ronnemaa T., Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in noninsulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 33.Sponder M., Fritzer-Szekeres M., Litschauer B., Binder T., Strametz-Juranek J. Endostatin and osteopontin are elevated in patients with both coronary artery disease and aortic valve calcification. IJC Metab Endocr. 2015;9:5–9. [Google Scholar]
- 34.Heljasvaara R., Nyberg P., Luostarinen J., Parikka M., Heikkila P., Rehn M. Generation of biologically active endostatin fragments from human collagen XVIII by distinct matrix metalloproteases. Exp Cell Res. 2005;307:292–304. doi: 10.1016/j.yexcr.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Ma Y., Yabluchanskiy A., Hall M.E., Lindsey M.L. Using plasma matrix metalloproteinase-9 and monocyte chemoattractant protein-1 to predict future cardiovascular events in subjects with carotid atherosclerosis. Atherosclerosis. 2014;232:231–233. doi: 10.1016/j.atherosclerosis.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng X., Chen J., Miller Y.I., Javaherian K., Moulton K.S. Endostatin binds biglycan and LDL and interferes with LDL retention to the subendothelial matrix during atherosclerosis. J. Lipid Res. 2005;46:1849–1859. doi: 10.1194/jlr.M500241-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Liu L., Chen B., Zhang X., Tan L., Wang D.W. Increased cathepsin D correlates with clinical parameters in newly diagnosed type 2 diabetes. Dis Markers. 2017;2017:1–6. doi: 10.1155/2017/5286408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woo M., Patterson E.K., Cepinskas G., Clarson C., Omatsu T., Fraser D.D. Dynamic regulation of plasma matrix metalloproteinases in human diabetic ketoacidosis. Pediatr Res. 2015;79:295–300. doi: 10.1038/pr.2015.215. [DOI] [PubMed] [Google Scholar]
- 39.Derosa G., D'Angelo A., Tinelli C., Devangelio E., Consoli A., Miccoli R. Evaluation of metalloproteinase 2 and 9 levels and their inhibitors in diabetic and healthy subjects. Diabetes Metab. 2007;33:129–134. doi: 10.1016/j.diabet.2006.11.008. [DOI] [PubMed] [Google Scholar]