Abstract
Echinococcosis, also known as Hydatid disease, is caused by the larvae of the tapeworm Echinococcus. It is globally prevalent and is a major clinical health concern. It is endemic in most underdeveloped regions including Asia, the Mediterranean, South America and Africa. There are four species within the genus Echinococcus, with E. granulosus and E. multilocularis being the most common, causing Cystic Echinococcosis (CE) and Alveolar Echinococcosis (AE). The clinical presentation of the disease is non-specific. It commonly involves the liver, lungs, brain and adrenal glands. Pulmonary disease is significant for its propensity to affect children and young adults. This young population accounts for ∼50% of pulmonary hydatid cysts [1]. Cysts are known to grow extensively in size. Many patients are asymptomatic and have only a solitary cyst. Symptoms arise from enlargement of the cyst and from eroding and pressure applied by the cyst to blood vessels and organs. If rupture of the cyst occurs it can lead to immunologic reactions such as asthma and anaphylaxis. Echinococcus in the lung can pose diagnostic dilemmas, as their homogeneous density and tendency to occur alone may cause them to be confused with squamous cell carcinoma, adenocarcinoma, solitary metastasis, and abscess [2]. Our case is of such a patient who was found to have a 6 cm mass in the right middle lobe (RML) found on a chest X-ray during evaluation of back pain. Echinococcus should always be included in a differential diagnosis of any mass lesions especially in immigrant populations from endemic countries.
1. Introduction
We report a case of pulmonary echinococcosis in a patient who was found to have a 6 cm mass in the right middle lobe (RML) of the lung found on a chest X-ray during evaluation of back pain. This case is unique in its perplexing history, surprising diagnosis and favorable outcome. That it is a rare case is proven by the fact that McCorkell and Al Karawi have reported a total of four such cases of pulmonary hydatid cyst aspiration. Furthermore, its complication free resolution lends credence to a newer approach which entails pre-op adjunctive treatment when cystectomy is planned with oral albendazole or mebendazole. This approach has been shown to decrease the risk of recurrence [26] (see Table 1).
Table 1.
Lab Studies.
| Investigations | Findings |
|---|---|
| Pleural fluid cytopathology of original aspiration biopsy | No WBCs, malignant cells, fungus or organisms. |
| Echinococcal Ab EIA | <0.80 (negative) |
| Pleural fluid for Echinococcus | Sample was erroneously not sent to Quest Diagnostics for analysis by the WMC laboratory |
| Pleural fluid AFB stain | negative |
| Pleural fluid analysis | Pale red, blood tinged, 5500 WBC's, 2280 RBC's, 6% PMN, 52% lymphocytes, 12% monocytes, 18% eosinophils, 12% macrophages |
| Pleural fluid chemical analysis | glucose 40 mg/dL, LDH 255 U/L, cholesterol 68 mg/dL, total protein 4.4 g/dL, triglyceride 16/dL, lactic acid 7.5 mmol/L; pH not performed. |
2. Case Report
2.1. Patient Profile
AD is a 26 year old female, born and raised in urban Huancayo, capital of the Junin region of the Peruvian Highlands, having a history of intimate contact with dogs that had exposure to meat products from the local market and were witnessed to be consuming animal entrails.
2.2. Two Months Prior to Presentation
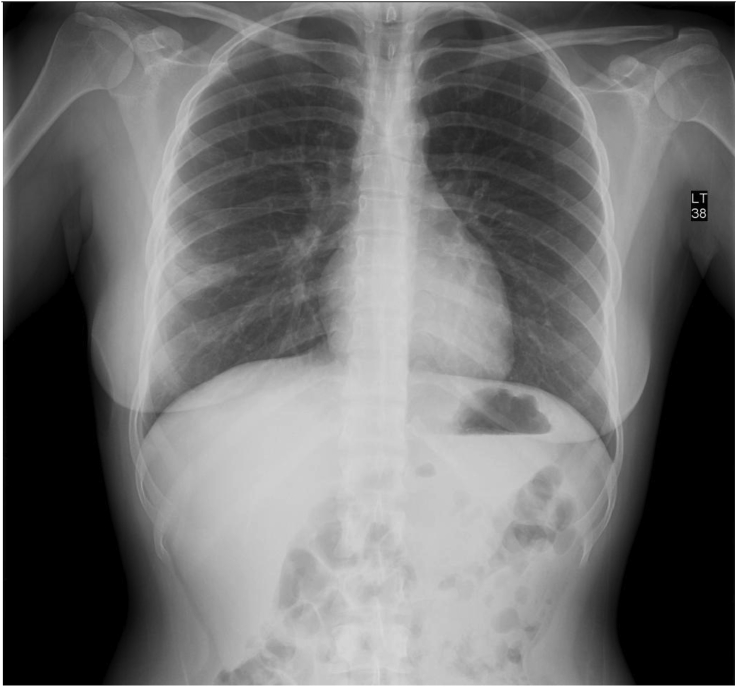
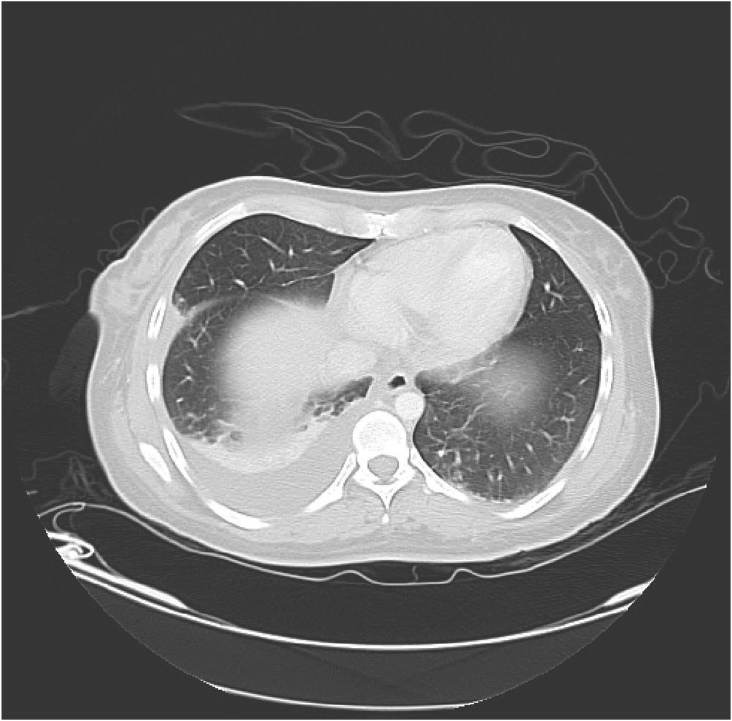
AD began experiencing recurring episodes of spontaneously remitting thoracic spine pain which was 8 or 9 out of 10 in severity, lasting intermittently for 1.5 hours. Routine X-ray demonstrated an RML lesion and a follow-up CAT showed a 4.5 cm × 3.7 cm lobulated mass of low attenuation, pleural-based at the right lateral lung field; referral to outpatient clinic for CAT-guided percutaneous aspiration needle biopsy of the RML lesion previously seen on Chest X-ray (CXR) (see Fig. 1). 20 mL of clear fluid was aspirated and sent for study. Post-procedure, a minimal pleural effusion was noted in the right lung field and there was no evidence of pneumothorax.
Fig. 1.
Chest roentgenogram, prior to biopsy.
3. Emergency Room (ER) and Hospital Course
AD presented to the ER 5 hours after CT-guided needle aspiration biopsy was performed. AD complained of a 30 minute history of drowsiness, weakness, near syncope, and pleuritic pain at the biopsy site. AD took 2 tablets of Advil, without relief. She experienced an occipito-frontal headache with dizziness and subsequently fell. AD denied having any constitutional symptoms as well as any past history of pneumonia or lung infection and had no chest imaging done prior to her current illness. AD acknowledged an unintentional weight loss of 5 pounds over the past 3 months along with a decreased appetite. AD's PPD and HIV status was negative as of February 2007. On physical exam, temperature was 98.8, pulse was 76, respiration was 18, orthostatic blood pressure was 90/51 increased to 106/51, and pulse ox on room air was 97%. AD was alert, awake and in mildly painful distress. Her skin was warm and dry. On chest exam, breath sounds were decreased over the right base, with dullness to percussion, egophony at the right base and no evidence of wheezes, rales, or rhonchi. Tenderness to palpation was present over biopsy site with no evidence of hematoma. Cardiovascular exam revealed no evidence of irregularity, tachycardia, or gallop. A grade III/VI systolic ejection murmur was present, best heard at the pulmonic area. AD's labs revealed leukocytosis of 11.5. Post-biopsy chest x-ray revealed an ill-defined RML opacity with a small amount of fluid in the minor fissure. Repeat chest CT post-biopsy revealed a 3.5 cm × 3.2 cm septated, air-filled juxta-pleural RML lesion previously filled with fluid. Fluid density suggested the substance was unlikely to be blood. A new small, right non-loculated pleural effusion was noted compared to the previous CT, with no evidence of pneumothorax.
3.1. Management
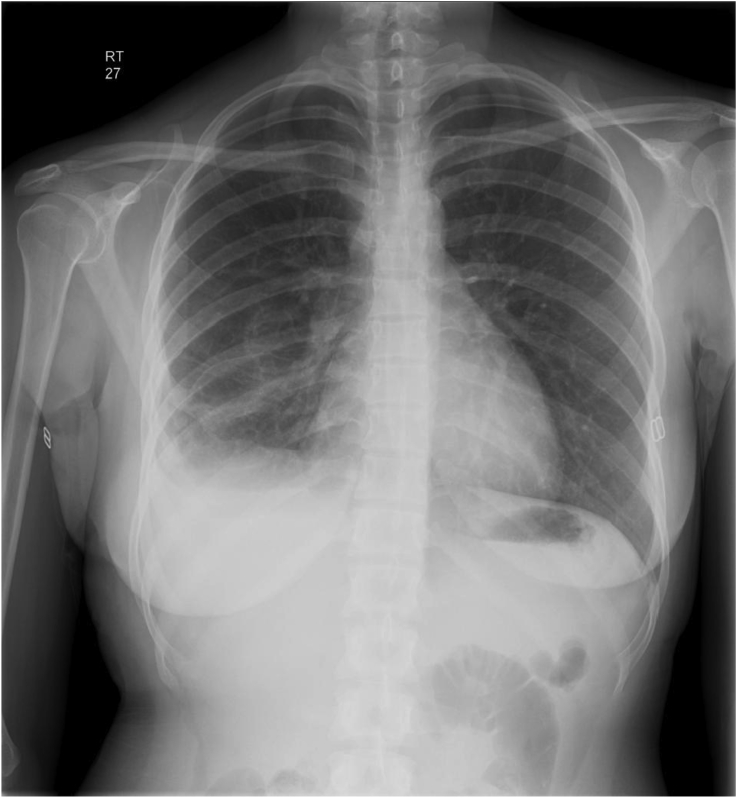
Given the patient's epidemiological characteristics, the clinical setting was felt to be consistent with echinococcal granulosis with a solitary pulmonary lesion, which secondary to needle drainage had now ruptured into the pleural space with resultant signs and symptoms of pleuritis and effusion. Pulmonary and Infectious Disease specialists were consulted; cardiothoracic evaluation was also sought for definitive removal of the tissue in question and long-term albendazole treatment was started as a supportive measure to prevent secondary lesions. Liver function tests, which were obtained to detect liver involvement, were within normal limits and she was ruled out for Tuberculosis with negative PPD and pleural fluid stains. During the course of her hospitalization, she remained afebrile with no aberrations of vital signs to suggest an anaphylactic reaction. She continued to experience pain at the biopsy site that was non-radiating, pleuritic in nature and improved over time with no associated dyspnea. She continued to complain of weakness but denied further episodes of syncope or lightheadedness. Three days after admission, she underwent a right mini-thoracotomy and resection of the RML cyst. 2 chest tubes were also placed for drainage of the pleural effusion. During the procedure she developed cardiovascular collapse most likely from anaphylaxis caused by the cystic fluid. She was treated with IV fluids and methylprednisolone; hypotension subsequently reversed. CXR performed after the procedure revealed a mild right lung atelectasis and effusion and small apical pneumothorax, which were followed to resolution (see Fig. 2, Fig. 5). Both chest tubes were removed by postoperative day (POD) #5. Aside from weakness and slight pain around the chest tube sites, she denied any chest pain, palpitation, dyspnea, fever, nausea or vomiting. With improved pain and respiration and response to appropriate medical treatment, she was discharged on POD #8. She was placed on Albendazole 400 mg twice daily, instructed to continue with incentive spirometry and ambulation. She was instructed to follow-up in the Medicine, Pulmonary and Infectious Disease outpatient clinics (see Fig. 3, Fig. 4, Fig. 6).
Fig. 2.
Chest roentgenogram, following biopsy.
Fig. 5.
Follow up CAT scan of chest at 3 months, showing resolution of pleural effusion and no new masses.
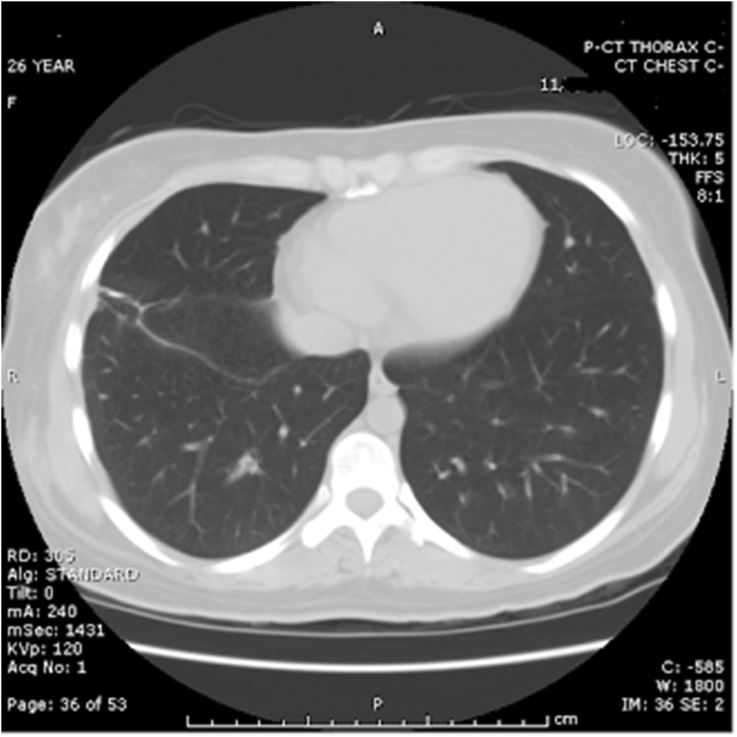
Fig. 3.
CT of chest before biopsy, showing the presence of a cystic structure, as well as pleural effusion.
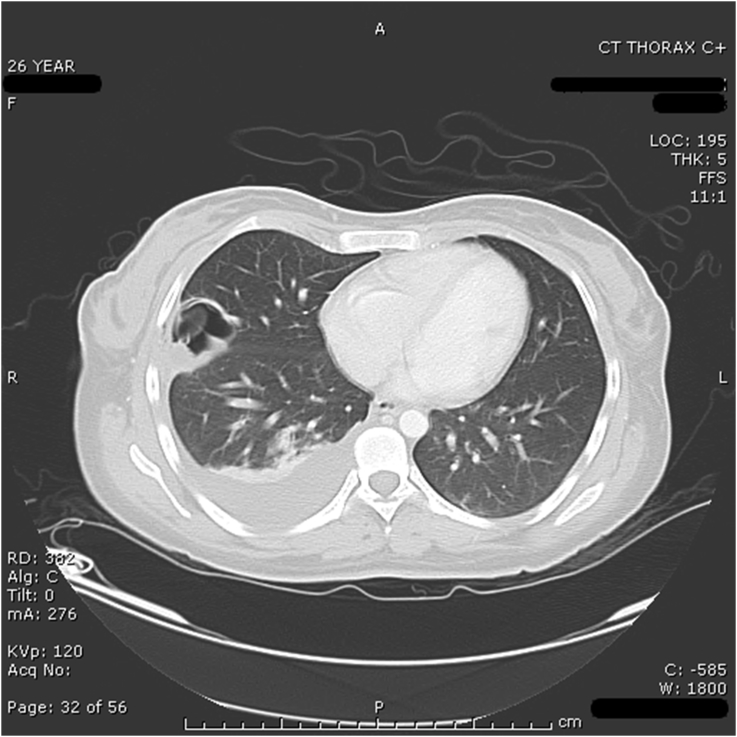
Fig. 4.
CT of chest after biopsy - large effusion and cystic structure no longer present.
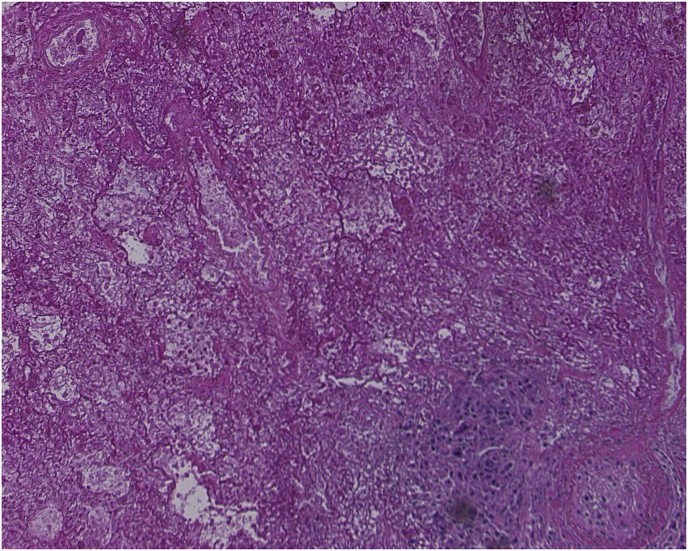
Fig. 6.
Pathology Slide: Microscopic examination of the right lung wedge resection revealed lack of an epithelial lining of the cyst. The cyst wall was laminated and pink, highly suggestive of echinococcal cyst. No scolices or hooklets were seen. Fibrinous exudates, granulation tissue, and lymphoid aggregates were seen adjacent to the cyst. In the surrounding lung parenchyma, edema, hemorrhage and interstitial fibrosis were observed. Grossly, the cyst measured 2.5 × 2.5 × 2 cm, with a smooth, pearly white, glistening inner and outer surfaces and wall thickness less than 0.1 cm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Outpatient Follow Up
Repeat serologic markers became positive for Echinococcus as she continued on Albendazole for an additional 3 months. Follow up CAT scan of chest (see Fig. 5) and Abdominal Ultrasound showed resolution of pleural effusion and no new masses.
4. Lab Studies Conclusion
Pleural fluid (PF) study showed muco-purulent fluid that was exudative in nature. No malignant cells, fungi or other organisms were identified, ruling out neoplasmic, fungal or other causes of disease. PF was seronegative for Echinococcal Antibody EIA; however, false negative results may be seen in up to 35% of cases of pulmonary echinococcosis and so this diagnosis cannot be ruled out based on seronegativity alone [21,22]. Weight loss is concerning for Tuberculosis (TB); however, absence of other classic symptoms (cough, fever, night sweats, hemoptysis, fatigue), as well as negative Acid Fast Bacilli staining of PF eliminate Pulmonary TB from the list of differential diagnoses.
5. Discussion
Echinococcosis, also known as Hydatid disease, is caused by the larvae of the tapeworm Echinococcus. There are 4 species of Echinococcus, which is a major clinical health concern in many underdeveloped parts of the world including Asia, the Mediterranean region, South America, and Africa. Particularly in Peru (our patient's country of origin), studies have shown a high prevalence of cystic Echinococcosis in the central and southern highlands [3,4]. In Hydatid disease, humans play the role of intermediate hosts as they become infected by ingesting tapeworm eggs from an infected source. Common sources of infection include household dogs and cats. Inadvertent ingestion of food or water contaminated with fecal material containing the tapeworm eggs leads to the development of the disease. There are many reports of Echinococcosis found in dogs throughout the world. Our patient also owned dogs as pets in Peru.
The clinical presentation of the disease is non specific. It involves the liver in 95% of cases followed by lungs, brain, and adrenal glands and can infect any organ system [5]. The lungs and liver by virtue of their capillary beds, which are functional sieves, filter out the majority of Echinococcus Granulosis larvae and are consequently the most common sites of disease manifestation [1]. Most patients with hydatid disease are found to be asymptomatic and so several months to years may pass without any apparent symptoms. In addition, many cases have been reported during autopsy. Cysts are known to grow extensively in size depending on their anatomic location and are reported to be as large as a basketball in larger primates. The infection becomes symptomatic if there is a mass effect by the cyst or if there is an acute rupture of the cyst. Most patients are asymptomatic as cysts are found to be well loculated, but occasional rupture of cyst can be the primary manifestation of the disease. A ruptured cyst can lead to symptoms of fever and an acute hypersensitivity reaction. The hypersensitivity reaction is due to immunologic phenomenon caused by antigenic material present in the cyst and when direct communication between the cystic fluid and the circulation occurs, it leads to anaphylaxis [6].
Pulmonary hydatid disease is highly specific to the younger patient population. Pulmonary cysts tend to be solitary, though they have been found to be multiple in 30% of cases [1]. The right lung is affected in 50% of cases, the left lung in 40% of cases and involvement is bilateral in the remaining 10% of cases. The posterior segments and lower lobes are most often affected and occur in 60% of cases [1]. The mechanism of entry of the larvae is via the lymphatic system, hematogenous spread, or by means of a transdiaphragmatic route. The larvae in route pass through the liver where the host's defense mechanisms may kill and suppress cyst formation. Lodging of cysts in the liver or other organs such as the lungs is atypical [1,7,8]. Cysts range in size from 1cm to 20 cm in diameter with a variety of clinical manifestations [1]. The early phase after primary infection is always asymptomatic and can remain latent for many months or even years. Although variable, cyst growth has been reported at a rate of 1 cm per month [9]. The common presenting signs and symptoms of pulmonary hydatid disease include cough with and without expectoration, dyspnea, chest pain, hemoptysis, biliptysis, pneumothorax, pleuritis, lung abscess, eosinophilic pneumonitis, and parasitic lung embolism [[10], [11], [12]]. Other symptoms can occur from compression of nearby organs. Anaphylaxis is a rare complication that occurs with rupture of cystic structure brought on by blunt trauma or due to surgical intervention during therapy, as was the case in our patient [13,14].
The presumptive diagnosis of echinococcal cyst of the lung was not readily apparent from the original complaint of thoracic spinal pain or from the initial imaging studies. Hydatid disease of the lung is difficult to distinguish from lesions caused by tumor of the lungs, abscesses, or other metastatic lesions [2,15,16]. The fluid-filled, cystic nature of the RML lesion was not fully realized until the percutaneous biopsy attempt of the lesion led to aspiration of 20 cc of clear fluid. In our patient's case, a social history that was positive for risk factors (patient from a highly endemic area with environmental exposures) suggested an etiology that, while plausible, is uncommonly found within the United States. In the literature, several instances of accidental aspiration of hydatid cysts have been documented; McCorkell and Al Karawi reported a cumulative of 4 cases of pulmonary hydatid cyst aspiration, which did not result in the most feared complication of anaphylaxis which is reported to occur at an incidence of <10% [6,11,17,18].
Preliminary diagnoses of Hydatid disease require imaging studies such as chest roentgenogram, ultrasound and CAT scan. Further assessment with serologic and pathologic evaluation is required. Ultrasound is considered the gold standard and has a sensitivity and specificity of greater than 88–98%, but it is not useful for evaluation of the lungs [10]. CAT scan is the best method to evaluate for hydatid disease. CAT scan accurately identifies smaller cysts, measures size, determines cyst density and evaluates for any vessel or structural involvement [12]. In one study, CAT scanning diagnosed 61% of patients with liver, lung, kidney, and other organ involvement. It was also useful in determining involvement of vascular structures and with inclusion of serologies the sensitivity increased to 94% [19,20].
Serologic studies identify more than 95% liver and 65% of lung cysts but they are known to have many false positive and false negative tests. However a negative test does not rule out Echinococcosis [21,22]. Cystic lesions can develop as active or inactive lesions depending on whether they have protoscolices. Percutaneous aspiration or biopsy is required for diagnosis when serologic testing is negative. Active cysts will reveal clear fluid and scolices while inactive cyst will reveal cloudy fluid without scolices upon biopsy. During biopsy there is a rare complication of anaphylaxis and dissemination of infected material to other sites in the lung and the pleural cavity. Another option for evaluation is FDG-PET scanning to distinguish the lesions from malignant disease [23].
Surgery is considered the primary modality of treatment in patients with pulmonary Hydatid disease, especially with a solitary cyst. Multiple studies have shown that there is a benefit to surgical removal of the entire cyst with preservation of the lung. There are two methods employed in the removal of intact cysts: removal of cyst after aspiration and enucleation of the cyst without aspiration. Enucleation is preferred over aspiration as there is a smaller chance of spillage of cystic contents into the lung parenchyma and the pleural cavity. Management of ruptured cyst aspiration is followed by removal of the cystic membranes. This is followed by closure of the remaining pulmonary cavity with sutures [24]. Surgery has been known to have a considerable complication rate and morbidity. Complications of anaphylaxis and dissemination are known to occur during operation. During the operation, every attempt is made to avoid spillage of cystic content as there is a risk of anaphylaxis and dissemination of the disease. In our case, the patient developed a severe anaphylactic reaction during surgical intervention which was treated with fluid resuscitation, diphenhydramine and methylprednisolone.
In cases of multiple cysts or inoperable cysts, another procedure called puncture-aspiration-injection-reaspiration (PAIR) is described in the literature. In the PAIR procedure, aspiration of 10–15 ml of cystic fluid is followed by administration of ethanol or hypertonic saline and then reaspiration of the remaining fluid. This has been shown to have higher cure rates but there is a greater risk of rupture and anaphylaxis. PAIR is often performed with concomitant oral medication such as albendazole, which offers the advantage of reducing risk of recurrence and seeding.
While most studies have shown surgical intervention as the primary treatment modality, subjects in one study experienced complete resolution or decrease in size of cysts upon treatment with oral albendazole alone for 3 cycles of 6 weeks [25]. This finding was duplicated in other studies using oral albendazole. A newer approach entails pre-op adjunctive treatment when cystectomy is planned with oral albendazole or mebendazole. This has been shown to decrease the risk of recurrence; dosing is started four days prior to surgery given for four week periods followed by a 2 week period without medication for 3 months [26].
There are no official guidelines after surgical intervention for follow up but serum antibody titers are repeated to assess for relapse of infection [27]. In case of relapse, treatment is continued for an additional 3 months with oral albendazole.
Several features make this case noteworthy with regards to pulmonary hydatid disease. Disease affecting the liver or other organs may manifest more readily due to mass effect, while lung disease remains largely asymptomatic unless cyst rupture or leakage occurs. It is likely that the patient was initially infected between the ages of 16 and 24 with a latent period of 2 years. Such a variable latency for the disease is documented, including a latency of over 50 years in duration. Occasionally it is discovered for the first time during autopsy [28,29].
Though there are no effective drugs or vaccines to protect humans against Echinococcosis, studies have shown the benefit of screening patients in endemic regions [30]. Prevention with prophylactic treatment of dogs in endemic areas is recommended with praziquantel on a monthly basis.
6. Conclusion
Although Echinococcosis has a benign presentation, it is a serious disease and should always be included in the differential diagnosis of any mass lesions occurring in the lungs, especially in immigrant populations from endemic countries. The treatment of choice is surgery. Asymptomatic patients can be monitored by serial imaging or undergo further evaluation with serologic studies. Less invasive medical treatment such as oral albendazole should be offered prior to any radical surgical intervention.
Declarations of Interest
None.
Conflicts of Interest Statement
There are no conflicts of interest.
Sources of Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Contributor Information
Majid Dudha, Email: majid_dudha@yahoo.com.
Zakir Shaikh, Email: zakir.ishaikh@yahoo.com.
Mohammed Bhaiyat, Email: mohammedbhaiyat@yahoo.com.
Ishaq J. Wadiwala, Email: ishaqjwadiwala@gmail.com.
Zainab-Tasneem Bhaiyat, Email: zt.bhaiyat@gmail.com.
References
- 1.Gottstein B., Reichen J. Hydatid lung disease. Clin. Chest Med. 2002;23 doi: 10.1016/s0272-5231(02)00007-2. 397-08. [DOI] [PubMed] [Google Scholar]
- 2.Fraser R.G. W.B. Saunders; Philadelphia, PA: 1978. Diagnosis of Diseases of the Chest; pp. 867–873. [Google Scholar]
- 3.Moro P.L., Bonifacio N., Gilman R.H. Field diagnosis of Echinococcus granulosis infection amount intermediate and definitive hosts in an endemic focus of human cystic Echinococcosis. Trans. R. Soc. Trop. Med. Hyg. 1999;93:611–615. doi: 10.1016/s0035-9203(99)90068-8. [DOI] [PubMed] [Google Scholar]
- 4.Moro P.L., McDonald J., Gilman R.H. Epidemiology of Echinococcus granulosis infection in the central peruvian andes. Bull. World Health Organ. 1997;75:553–561. [PMC free article] [PubMed] [Google Scholar]
- 5.Tuzun M., Hekimoglu B. Various locations of cystic and alveolar hydatid disease: CT appearances. J. Comput. Assist. Tomogr. 2001;25:81–87. doi: 10.1097/00004728-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Al Karawi M., Mohamed A.R., El Tayeb B. Unintentional percutaneous aspiration of a pleural hydatid cyst. Thorax. 1991;46:859–860. doi: 10.1136/thx.46.11.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schantz P. WB Saunders; Philadelphia, PA: 1999. Echinococcosis. Tropical Infectious Diseases: Principles, Pathogens and Practice; pp. 1005–1025. [Google Scholar]
- 8.Gottstein B., Reichen J. Hydatid lung disease. Clin. Chest Med. 2002;23:397–408. doi: 10.1016/s0272-5231(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 9.Eviliyaoglu C., Yuksel M., Gul B. Growth rate of multiple intracranial hydatid cysts assessed by CT from the time of embolisation. Neuroradiology. 1996;40:387–389. doi: 10.1007/s002340050607. [DOI] [PubMed] [Google Scholar]
- 10.Macpherson C.N., Milner R. Performance characteristics and quality control of community based ultrasound surveys for cystic and alveolar Echinococcosis. Acta Trop. 2003;85:203–209. doi: 10.1016/s0001-706x(02)00224-3. [DOI] [PubMed] [Google Scholar]
- 11.McCorkell Sj. Unintended percutaneous aspiration of pulmonary echinococcal cysts. AJR Am. J. Roentgenol. 1984;143:123–126. doi: 10.2214/ajr.143.1.123. [DOI] [PubMed] [Google Scholar]
- 12.Ammann R.W., Eckert J. Cestodes. Echinococcus. Gastroenterol. Clin. North Am. 1996;25:655–689. doi: 10.1016/s0889-8553(05)70268-5. [DOI] [PubMed] [Google Scholar]
- 13.Topuzlar M., Eken C., Ozkurt B. Possible anaphylactic reaction due to pulmonary hydatid cyst rupture following blunt chest trauma: a case report and review of the literature. Wilderness Environ. Med. 2008;19:119–123. doi: 10.1580/07-WEME-CR-1561.1. [DOI] [PubMed] [Google Scholar]
- 14.Gulalp B., Koseoglu Z., Toprak N. Ruptured hydatid cyst following minimal trauma and few signs on presentation. Neth. J. Med. 2007;65:117–118. [PubMed] [Google Scholar]
- 15.Thumler J., Munoz A. Pulmonary and hepatic Echinococcosis in children. Pediatr. Radiol. 1978;7:164–171. doi: 10.1007/BF00975441. [DOI] [PubMed] [Google Scholar]
- 16.Xanthakis D., Efthimiadis M., Papdakis G. Hydatid disease of the chest. Report of 91 patients surgically treated. Thorax. 1972;27:517–528. [Google Scholar]
- 17.Cakmakci M., Sayek I. Prophylactic effect of albendazole in experimental peritoneal hydatidosis. Hepato-Gastroenterology. 1992;39:424–426. [PubMed] [Google Scholar]
- 18.Akhan O., Ozmen M.N., Dincer A. Percutaneous treatment of pulmonary hydatid cysts. Cardiovasc. Intervent. Roadiol. 1994;17:271–275. doi: 10.1007/BF00192450. [DOI] [PubMed] [Google Scholar]
- 19.Di Palma A., Ettorre G.C., Scapati C. The role of computerized tomography in the diagnosis of hydatid disease. Radiol. Med. 1991;82:430–436. [PubMed] [Google Scholar]
- 20.Morar R., Feldman C. Pulmonary echinococcosis. Eur. Respir. J. 2003;21:1069–1077. doi: 10.1183/09031936.03.00108403. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia G. Echinococcus. Semin. Respir. Infect. 1997;12:171. [PubMed] [Google Scholar]
- 22.Zarzosa M.P., Orduna D., Gutierrez P. Evaluation of six serological tests in diagnosis and postoperative control of pulmonary hydatid disease patients. Diagn. Microbiol. Infect. Dis. 1999;35:255–262. doi: 10.1016/s0732-8893(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 23.Yavuz K., Iker S., Iker F. Pulmonary Echinococcosis mimicking multiple lung metastasis of breast cancer: the role of fluoro-deoxy-glucose positron emission tomography. World J. Surg. Oncol. 2008;6:7. doi: 10.1186/1477-7819-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halezeroglu S. Resection of intrathoracic and subdiaphragmatic hydatid cysts. MMCTS. 2005:307. doi: 10.1510/mmcts.2004.000307. [DOI] [PubMed] [Google Scholar]
- 25.Keshmiri M., Baharvahdat H., Fattahi S.H. A placebo controlled study of albendazole in the treatment of pulmonary Echinococcosis. Eur. Respir. J. 1999;14:503–507. doi: 10.1034/j.1399-3003.1999.14c05.x. [DOI] [PubMed] [Google Scholar]
- 26.Mawhorter S., Temeck B., Chang R. Nonsurgical therapy for pulmonary hydatid cyst disease. Chest. 1997;112:1432–1436. doi: 10.1378/chest.112.5.1432. [DOI] [PubMed] [Google Scholar]
- 27.Zarzosa M.P., Orduna Domingo A. Evaluation of six serological tests in diagnosis and postoperative control of pulmonary hydatid disease patients. Diagn. Microbiol. Infect. Dis. 1999;35:255–262. doi: 10.1016/s0732-8893(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 28.Guntz M., Coppo B., Lorimier G. Hydatid cysts of the liver appearing late (10-22 years) after surgical treatment of pulmonary hydatidosis. 1990;127:375–381. [PubMed] [Google Scholar]
- 29.Spruance S.L. Latent period of 53 years in a case of hydatid cyst disease. Arch. Intern. Med. 1974;134:741–742. [PubMed] [Google Scholar]
- 30.Larrieu E., Frider B. Human cystic Echinococcosis: contributions to the natural history of the disease. Ann. Trop. Med. Parasitol. 2001;95:679–687. doi: 10.1080/00034980120094730. [DOI] [PubMed] [Google Scholar]