Highlights
-
•
Depending on the concentration, dimethyl sulfoxide (DMSO) can be toxic to cells.
-
•
3T3-L1 adipocytes are a well-established model to study anti-obesity properties.
-
•
DMSO doses ≥1% reduced cell viability and promoted cell damage in 3T3-L1 adipocytes.
Abbreviations: DCFH-DA, 2′, 7′-dichlorofluorescein diacetate; DMEM, Dulbecco’s Modified Eagle’s Medium; DMSO, dimethyl sulfoxide; DPBS, Dulbecco’s Phosphate Buffered Saline; FBS, fetal bovine serum; HBSS, Hank’s Balanced Salt Solution; IBMX, 3-isobutyl-1-methylxanthine; JC-1, 5,5′,6,6′-tetrachloro-1,1′,3,3-tetraethylbenzimidazolyl-carbocyanineiodide; MTT, 3-(4,5-Dimethylthiazol-2-yl)-2,5-DiphenyltetrazoliumBromide); ORO, oil red O; ROS, reactive oxygen species
Chemical compounds studied in this article: Dimethyl sulfoxide (PubChem CID: 679)
Keywords: Dimethyl sulfoxide, Cell viability, Oxidative stress, Apoptosis, 3T3-L1 adipocytes
Abstract
Dimethyl sulfoxide (DMSO) is an effective solvent and cytoprotectant agent that can induce diverse actions in experimental settings, ranging from metabolic stress to cytotoxic effects depending on the concentration used. Therefore, for the quality of experiments and reproducibility of results it is essential to establish a precise and non-toxic dose of DMSO within a specific cell system. 3T3-L1 adipocytes, represent a well-established in vitro cell model used to assess the anti-obesity potential of extracts and compounds. Although DMSO is commonly used as a solvent for these experiments, there is limited data available on the compounding effects of using DMSO. The purpose of this study was to assess a concentration-dependent effect of DMSO on lipid content, cell viability and oxidative damage in 3T3-L1 adipocytes. Results showed that DMSO at doses ≥ 0.1% increased mitochondrial membrane potential as measured by JC-1 fluorescent staining, while doses ≥ 10% reduced the lipid content in matured adipocytes. Consistently, higher doses significantly reduced cell viability, elevated reactive oxygen species levels, depleted intracellular glutathione levels, and accelerated apoptosis and cell necrosis. An interesting finding was that a DMSO dose of 0.01% improved glutathione content of 3T3-L1 adipocytes and had minimal effects on cell viability, apoptosis or and necrosis, supporting its antioxidant effect. Therefore, this study provides compelling evidence that precaution should be taken when assessing compounds dissolved in DMSO, particularly doses ≥1% that were shown to induce oxidative stress in 3T3-L1 adipocytes.
1. Introduction
Dimethyl sulfoxide (DMSO, (CH3)2SO) is an organic amphiphilic molecule that is widely used in cell biology for various applications [1]. DMSO exhibits a number of capabilities, including vasodilatory, diuretic, anti-inflammatory and bacteriostatic properties [1,2]. In vitro, DMSO is routinely used for cryopreservation of cells and for many researchers DMSO has been the preferred solvent for the dissolution of small hydrophobic drug molecules [1]. DMSO is known to interact strongly with phospholipids, which makes it efficient at facilitating movement of molecules, especially drugs across biological membranes [3]. In addition, DMSO is also a free radical scavenger demonstrating antioxidant activities at low concentrations but becoming pro-oxidant at higher concentrations [4]. Despite the potential for physiological interference and cytotoxicity, DMSO remains a solvent of choice in biomedical research. Thus, it remains important to understand the possible interactions and establish an optimal and non-toxic concentration for DMSO in a particular cell model and specific experimental settings to ensure accuracy and reproducibility of results [1,[5], [6], [7]].
An in vitro model of 3T3-L1 adipocytes represents an accomplished system to investigate adipocytic lipid metabolism and adiposity. During progression of obesity, adipose tissue metabolism is dysregulated, accompanied by reduced metabolic activity, generation of oxidative stress and subsequent cell damage [8]. Consequently, there has been a drastic increase in the use of 3T3-L1 adipocytes as a model to screen pharmacological and natural compounds [[9], [10], [11], [12]], including those dissolved in DMSO for their therapeutic potential against obesity and its associated complications [13]. While it has been reported that DMSO can inhibit differentiation of 3T3 T mesenchymal stem cells or promote glucose transporter 4 translocation in insulin-stimulated 3T3-L1 adipocytes [14,15], less is known about the dose-dependent effect of DMSO on these cells. This is the first study to report on the concentration-dependent (0.01–100%) effect of DMSO on lipid content and cell viability in 3T3-L1 adipocytes. Furthermore, as contradicting evidence has emerged on the role of DMSO in the modulation of oxidative stress in different cell types, this study investigated the effect of DMSO on the oxidative stress response in 3T3-L1 adipocytes.
2. Materials and methods
2.1. Chemicals
3T3-L1 mouse embryonic fibroblasts were from the American Type Culture Collection (Manassas, USA); Newborn Calf Serum (NBCS) and fetal bovine serum (FBS) were from Biochrom (Berlin, Germany); Dulbecco’s Phosphate Buffered Saline (DPBS), Dulbecco’s Modified Eagle’s Medium (DMEM) and Hank’s Balanced Salt Solution (HBSS) were from Lonza (Basel, Switzerland); 7-amino-4chloromethylcoumarin (CellTracker Blue CMAC) was from Invitrogen Corp. (Carlsbad, USA); annexin V conjugate was from ThermoFisher Scientific, Inc. (Waltham, USA); and 2′, 7′-dichlorfluorescein diacetate (DCFH-DA) fluorescent dye was from Cell Biolabs Inc. (San Diego, USA). All other chemicals, including 3-isobutyl-1-methylxanthine (IBMX), insulin human solution, dexamethasone, 5,5′,6,6′-tetrachloro-1,1′,3,3-tetraethylbenzimidazolyl-carbocyanine iodide (JC-1), oil red O (ORO) and 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) (MTT) stains were obtained from Sigma-Aldrich (St. Louis, USA).
2.2. Culture, differentiation and treatment of 3T3-L1 cells
The 3T3-L1 fibroblasts were grown in pre-adipocyte growth media containing DMEM supplemented with 10% NBCS in T75 flasks until they reached 70–80% confluency. All flasks and tissue culture plates containing cells were incubated under standard culture conditions (37 °C in a water-saturated atmosphere of 5% CO2) whilst pre-adipocyte growth media was refreshed every second day. For assay purposes, pre-adipocytes were seeded in 96 multi-well plates at 4 × 103 cells/well. Upon 100% confluency (day 0), pre-adipocytes were differentiated to mature adipocytes by modifying a previously described method [10]. Briefly, pre-adipocyte growth media was replaced with DMEM supplemented with 10% FBS, 0.5 mM IBMX, 10 μg/mL insulin and 1 μM dexamethasone, and cultured for 72 h. At day 3 of differentiation, media was replaced with DMEM supplemented with 10% FBS and 10 μg/mL insulin, and cultured for further 48 h. Thereafter, cells were further differentiated with DMEM supplemented with 10% FBS until matured adipocytes were obtained on day 8 of differentiation, whilst the media was refreshed daily. The presence of mature adipocytes on day 8 of differentiation was confirmed by microscopy. Differentiated cells were then exposed to various concentrations of DMSO prepared in DPBS ranging from 0.01 to 100 % (v/v) for one hour before subsequent experiments were performed. Time of exposure was based on a previous study and was expected to be enough to induce stress [7]. Cells exposed to DPBS without DMSO were used as an experimental control.
2.3. Determination of lipid content in 3T3-L1 adipocytes
Oil red O is a widely used lysochrome (fat-soluble dye) diazo dye for staining of neutral lipids. Briefly, treated adipocytes in 96 multi-well plates were fixed in 10% (v/v) neutral buffered formalin for 15 min before staining with 0.7% ORO working solution made up in distilled water (v/v) for 30 min at room temperature. Lipid content was quantified by eluting the dye contained in cells with 100% isopropanol and measuring optical density (OD) at 490 nm using a BioTek ELx800 plate reader and Gen 5 software for data acquisition (BioTek Instruments Inc., Winooski, USA). Crystal violet staining, measured at OD570, was used to normalize for cell density as previously described [12]. Photomicrographs of at least 5 fields per well of ORO stained cells were taken with a 20× objective using a Nikon Eclipse Ti inverted microscope and NIS-Elements imaging software (Nikon, Japan).
2.4. Measurement of metabolic activity in 3T3-L1 adipocytes
Colorimetric measurement of the reduction of tetrazolium dye (MTT) to its insoluble formazan is a widely-used method to measure metabolic activity of viable cells [10]. Briefly, after exposure to various doses of DMSO, adipocytes in 96 multi-well plates were stained with a 2 mg/mL MTT solution prepared in DPBS and incubated for 30 min at 37 °C. Thereafter, DMSO and Sorenson's glycine buffer (0.1 M glycine and 0.1 M NaCl, adjusted to pH 10.5 with 0.1 mM NaOH) were added to solubilise the dye before absorbance was read at 570 nm using a BioTek ELx800 plate reader and Gen 5 software for data acquisition.
2.5. Determination of mitochondrial membrane depolarisation in 3T3-L1 adipocytes
Changes in mitochondrial membrane potential (ΔΨm) were assessed by using the JC-1 fluorescent stain, according to a previously described method [16]. Briefly, after exposure to various doses of DMSO, adipocytes in 96 multi-well plates were incubated with 2 μM of JC-1 solution in DMEM without phenol red for 30 min at 37 °C. Thereafter, cells were rinsed once in DPBS before fluorescence was measured at a single excitation of 485 ± 20 nm and dual emission of 530 ± 25 nm and 590 ± 35 nm using BioTek FLx800 plate reader and Gen 5 software for data acquisition. Fluorescent photomicrographs of at least 5 fields per well were taken with a 20× objective using a Nikon Eclipse Ti inverted microscope and NIS-Elements imaging software.
2.6. Assessment of oxidative stress in 3T3-L1 adipocytes
Generation of oxidative stress in cells was measured by quantifying levels of reactive oxygen species (ROS) and glutathione content using DCFH-DA and CellTracker Blue CMAC fluorescent dyes, respectively, as previously described [17]. Briefly, after exposure to various concentrations of DMSO, adipocytes in 96 multi-well plates were incubated for 30 min at 37 °C with either a 1 μM DCFH-DA solution for the detection of ROS or 2.5 μM CellTracker solution for the detection of glutathione content. For detection of ROS, cells were rinsed once with HBSS before DCFH‑DA fluorescence was measured at an excitation/emission spectra of 485 ± 20/ 528 ± 20 nm. For the detection of glutathione content, cells were rinsed once with DPBS before CellTracker Blue CMAC fluorescence was measured at an excitation/ emission spectra of 360 ± 20/ 460 ± 40 nm. Fluorescent intensity was measured using BioTek FLx800 plate reader and Gen 5 software for data acquisition. Fluorescent photomicrographs of at least 5 fields per well were taken with a 20× objective using a Nikon Eclipse Ti inverted microscope and NIS-Elements imaging software.
2.7. Detection of apoptosis in 3T3-L1 adipocytes
Apoptotic and necrotic cells were detected using annexin V conjugate and propidium iodide fluorescent stain, respectively, according to previously described methods [18,19]. Briefly, after exposure to various concentrations of DMSO, adipocytes in 96 multi-well plates were labelled with a combination of 10% annexin V and 1 μg/mL propidium iodide solution dissolved in DPBS for 30 min at 37 °C. Thereafter, cells were rinsed once in DPBS before fluorescence was measured at an excitation/ emission spectra of 485 ± 20/ 528 ± 20 nm for Annexin V and 530 ± 25/ 590 ± 35 nm for propidium iodide, using BioTek FLx800 plate reader and Gen 5 software for data acquisition.
2.8. Statistical analysis
Statistical analysis was performed using GraphPad Prism software version 5.0 (GraphPad Software, Inc. La Jolla, USA). Results are represented as the mean ± standard error of mean (SEM) of three independent biological experiments, with each experiment containing at least six replicates. Comparisons between treatment groups were performed using one-way analysis of variance (ANOVA), followed by a Tukey post hoc test. A p value of <0.05 was considered as statistically significant.
3. Results
3.1. The effect of DMSO on lipid content
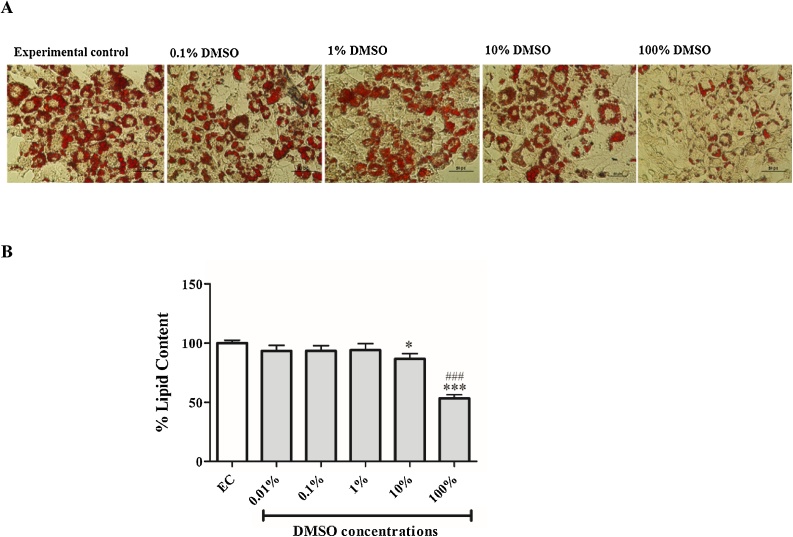
ORO staining confirmed that 3T3-L1 adipocytes presented with enhanced lipid content in our model (Fig. 1A). Thereafter, various doses of DMSO were tested for their effect on lipid content in 3T3-L1 adipocytes after one hour exposure. In comparison to an experimental control (DPBS exposed only cells), DMSO doses of 0.01–1% did not have any effect on lipid content, while doses of 10 and 100% significantly reduced lipid content by 14% (p < 0.05) and 47% (p < 0.001), respectively (Fig. 1A, B). Moreover, the higher dose of DMSO (100%) showed significant reduction of lipid content when compared to the lowest dose tested (0.01%).
Fig. 1.
The effect of dimethyl sulfoxide (DMSO) on lipid content as measured by oil red O assay. (A) Representative images of 3T3-L1 adipocytes exposed to various doses (0.1–100%) of DMSO for 1 h: increased red staining is equivalent to enhanced lipid content. (B) Quantitative analysis of oil red O staining. Results are the mean ± SEM of three independent biological experiments relative to the experimental control (EC). *P < 0.05, and ***P < 0.001 versus EC; ###p < 0.001 versus 0.01% DMSO.
3.2. The effect of DMSO on the metabolic activity
Exposing adipocytes to 0.1–100% DMSO resulted in a concentration-dependent suppression of metabolic activity when compared to the experimental control (Fig. 2). In comparison to an experimental control (DPBS exposed only cells), a DMSO dose of 0.01% did not have any effect on metabolic activity, while doses of 0.1, 1, 10 and 100% significantly reduced metabolic activity by 11% (p < 0.001), 14% (p < 0.001), 40% (p < 0.001) and 90% (p < 0.001), respectively (Fig. 2). Furthermore, in comparison to the lowest dose tested (0.01%), higher DMSO doses (1, 10 and 100%) significantly reduced metabolic activity of cells.
Fig. 2.
The effect of dimethyl sulfoxide (DMSO) on the metabolic activity of 3T3-L1 adipocytes as measured by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) (MTT) assay. Results are the mean ± SEM of three independent biological experiments relative to the experimental control (EC). ***P < 0.001 versus EC; ##p < 0.01, and ###p < 0.001 versus 0.01% DMSO.
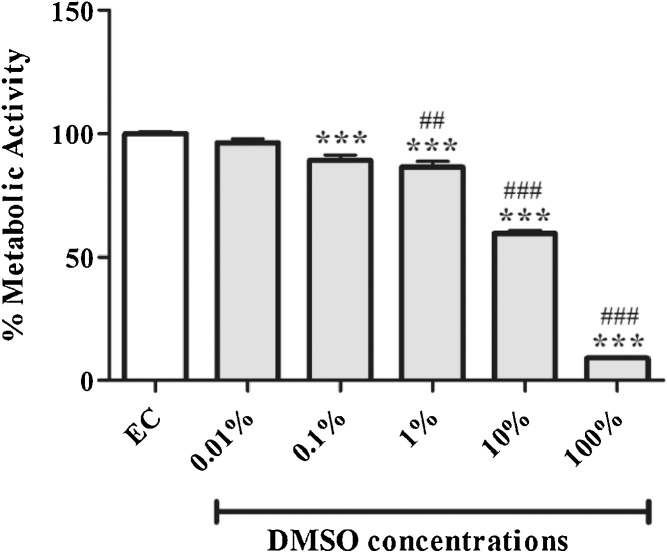
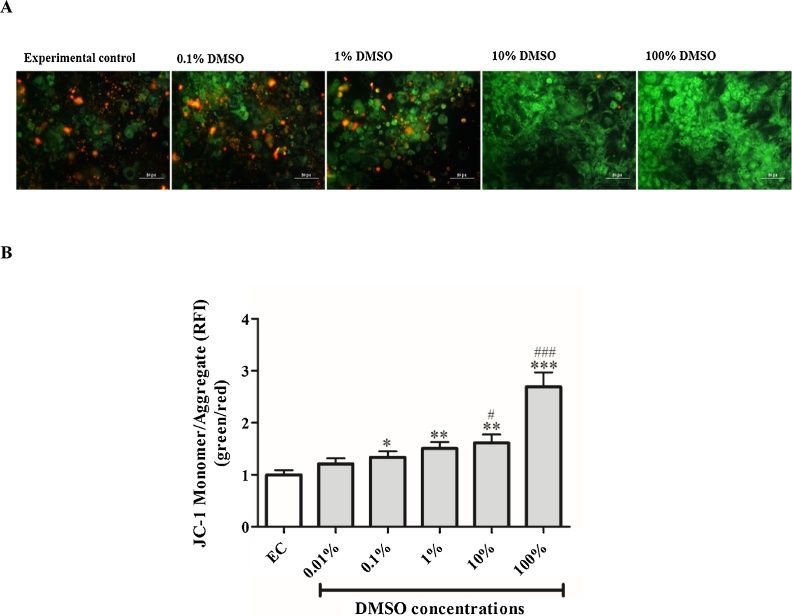
3.3. The effect of DMSO on mitochondrial transmembrane potential (ΔΨm)
Although no significant effect was observed with 0.01% DMSO when compared to the experimental control, doses of 0.1, 1, 10 and 100% markedly increased mitochondrial membrane potential by 0.3 RFI units (p < 0.5), 0.5 RFI units (p < 0.01), 0.6 RFI units (p < 0.01), 1.7 RFI units (p < 0.01), respectively (Fig. 3A, B). The increase in mitochondrial membrane potential was evident by enhanced green fluorescent staining of depolarized cells compared to the experimental control (Fig. 3A). DMSO doses of 10 and 100% further showed aberrant increase in of mitochondrial membrane potential when compared to the lowest dose (0.01%) of DMSO tested.
Fig. 3.
The effect of dimethyl sulfoxide (DMSO) on mitochondrial transmembrane potential (ΔΨm) as measured by JC-1 assay. (A) Representative images of 3T3-L1 adipocytes exposed to various concentrations (0.1–100%) of DMSO for 1 h: orange and green fluorescence are an indication of depolarised and non-depolarised cells, respectively. (B) Quantitative analysis of JC-1 fluorescence staining. Results are the mean ± SEM of three independent biological experiments relative to the experimental control (EC). *P < 0.05, **P < 0.01, and ***P < 0.001 versus EC; #p < 0.05, and ###p < 0.001 versus 0.01% DMSO.
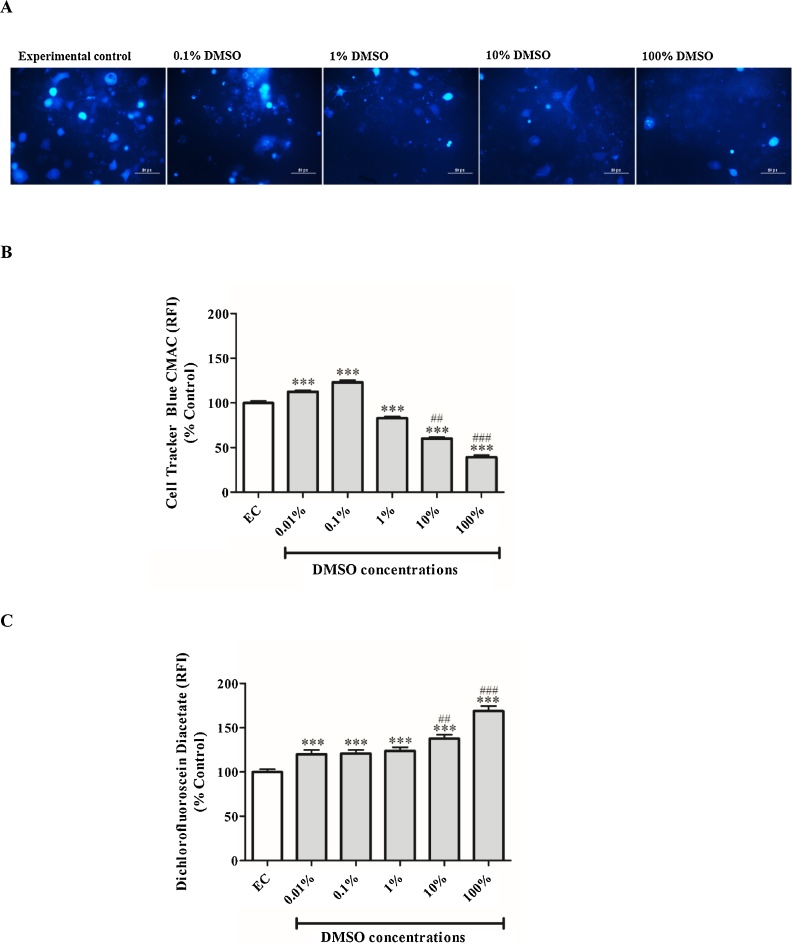
3.4. The effect of DMSO on glutathione content and generation of reactive oxygen species
The lower doses of DMSO (0.01 and 0.1%) increased GSH content of cells by 12% (p < 0.001) and 23% (p < 0.001), respectively, when compared to the experimental control (Fig. 4A, B). While doses of 1, 10 and 100% significantly reduced GSH levels in cells by 17% (p < 0.001), 40% (p < 0.001) and 61% (p < 0.001), respectively (Fig. 4A, B). On the other hand, ROS levels as measured by DCFH-DA stain were dose dependently increased (p < 0.001) when compared to the experimental control (Fig. 4C). Doses of 10 and 100% DMSO further showed significant difference in comparison to the lowest DMSO dose tested (0.01%) for both GSH content and ROS production (Fig. 4B, C).
Fig. 4.
The effect of dimethyl sulfoxide (DMSO) on glutathione content and generation of reactive oxygen species by using CellTracker Blue CMAC and dichlorofluorescein diacetate fluorescent stains, respectively. (A) Representative images of 3T3-L1 adipocytes exposed to various concentrations (0.1–100%) of DMSO for 1 h: an increased blue staining is equivalent to enhanced glutathione content. (B) Quantitative analysis of CellTracker Blue CMAC fluorescence staining. (C) Quantitative analysis of dichlorofluorescein diacetate fluorescence staining. Results are the mean ± SEM of three independent biological experiments relative to the experimental control (EC). ***P < 0.001 versus EC; ##p < 0.01, and ###p < 0.001 versus 0.01% DMSO.
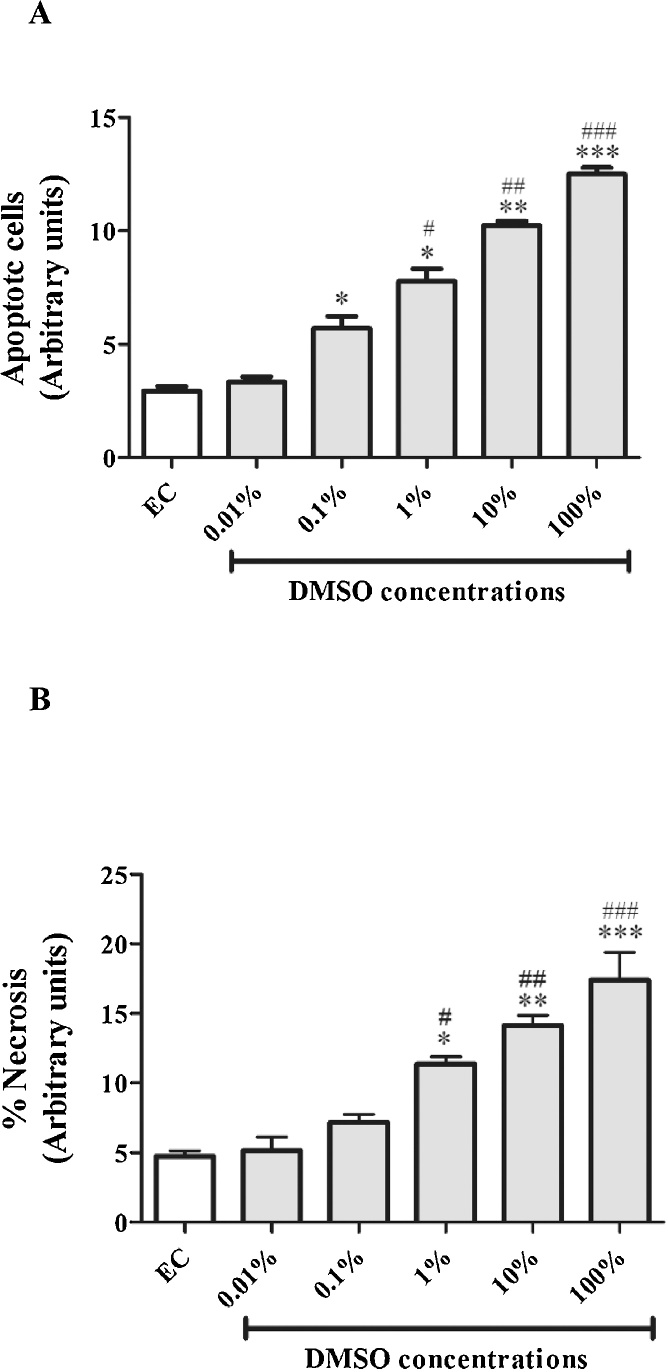
3.5. The effect of DMSO on apoptosis and necrosis markers
Except for the lowest dose of DMSO tested (0.01%), all doses induced a dose-dependent increase in apoptosis and necrosis when compared to the experimental control (Fig. 5A, B). Furthermore, doses of 0.1–100% for apoptosis, and doses of 1–100% for necrosis showed a marked increase when compared to the lowest dose of DMSO tested (0.01%) (Fig. 5A, B).
Fig. 5.
The effect of dimethyl sulfoxide (DMSO) on apoptosis and necrosis markers as measured using annexin V conjugate and propidium fluorescent stains, respectively. (A) Quantitative analysis of annexin V fluorescence staining. (B) Quantitative analysis of propidium iodide staining. Results are the mean ± SEM of three independent biological experiments relative to the experimental control (EC). *P < 0.05, **P < 0.01, and ***P < 0.001 versus EC; #p < 0.05, ##P < 0.01, and ###p < 0.001 versus 0.01% DMSO.
4. Discussion
The use of the 3T3-L1 adipocyte cell model has emerged as a crucial tool to study lipid metabolism and adiposity. This has resulted in an increasing number of studies screening pharmacological or natural compounds, as well as plant extracts for their anti-obesity potential using this model [[10], [11], [12]]. However, a number of these compounds or extracts are non-polar in nature with limited solubility in water [20]. Thus, the use of organic solvents such as DMSO as a vehicle for both polar and non-polar compounds, or extracts both in vitro and in vivo has become the norm over the years. Ideally, a vehicle agent should not have any therapeutic or biological effect, however, as mentioned previously, DMSO is known to have anti-inflammatory, neuroprotective and ROS scavenging effects, amongst others [1,[5], [6], [7],14]. Moreover, studies have shown that the effect of DMSO is concentration-dependent in various cell types [5,6,21], suggesting that while a concentration of 0.01% might not affect some cells, it could be toxic in another cell line. In this study, the effect of DMSO on 3T3-L1 adipocyte function and oxidative stress levels were determined to establish an acceptable concentration to use as a vehicle for dissolving compounds or extracts.
Matured adipocytes have been shown to display a drastic increase in triglyceride storage or lipid content [12]. Correspondingly, ORO staining confirmed that 3T3-L1 adipocytes presented with enhanced lipid content in our model. In comparison to an experimental control (DPBS exposed only cells), only the two highest DMSO doses tested (10 and 100%) significantly reduced lipid content in cells. Currently, there is limited data on the effect of DMSO on lipid content in 3T3-L1 adipocytes. However, DMSO has been linked with inhibition of adipocyte differentiation in 3T3 T mesenchymal stem cells, a related 3T3-L1 cell line [15]. These observations provide an opportunity for further assessment on the various doses of DMSO, especially a dose of 10% on their role in the regulation of adipogenesis in 3T3-L1 pre-adipocytes. In our experiments, it was further shown that exposing adipocytes to DMSO resulted in a concentration-dependent suppression of metabolic activity when compared to the experimental control, with doses of 1–100% showing highest toxicity. These results suggest that these cells are sensitive to DMSO doses ≥1%. While limited studies have reported on the effect of DMSO at concentrations lower than 0.01%, Yuan and colleagues demonstrated that a DMSO concentration of 1% is enough to suppress cell viability in astrocytes as measured by the MTT assay [22]. Similar results are also presented by Laskar and co-workers, showing that DMSO concentrations ≥ 1% reduced cell viability, while increasing apoptosis and necrosis of U937 cells [6]. Although experiments were done on different cell lines [6,22], data presented by these studies is in agreement with our findings in 3T3-L1 adipocytes demonstrating that DMSO doses of 1% and above may induce toxicity.
DMSO-induced toxicity can cause several pathophysiologic consequences that contribute to adipocyte dysfunction. Such disturbances include altered mitochondrial membrane potential, with excess production of ROS and reduced antioxidant capacity leading to cell damage [5,7,22]. Although no significant effect was observed with 0.01% DMSO, consistently, doses of ≥1% were responsible for altered mitochondrial membrane potential, increased oxidative stress-associated damage and apoptosis. However, at concentrations of ≤0.1%, DMSO enhanced GSH content in 3T3-L1 adipocytes was observed. This effect could be explained by the antioxidant potential of DMSO, instigating an enhancement in the endogenous antioxidant potential of cells, however the increase in DMSO concentration may cause it to be pro-oxidant and cytotoxic. This is in line with previous reports showing that lower DMSO concentrations can protect cells by activating autophagic process or at least by enhancing intracellular antioxidant effect [6,21]. Consequently, these beneficial effects should be considered when assessing other antioxidants in combination with a DMSO solvent vehicle. In such cases the interaction on mitochondrial energetics, oxidative stress and other antioxidant enzymes should be considered. These findings also add to the current debate on the danger associated with exposure of individuals to complex mixtures of chemicals via food, water and commercial products consumption, as previously discussed [[23], [24], [25], [26]]. Although DMSO offers various benefits, especially for its use as a solvent, caution should be taken when working with higher concentrations as they can induce cellular stress and cell death. Furthermore, higher concentrations of DMSO could potentially influence the results of other toxicological studies when used in mixture with other chemicals [[23], [24], [25], [26]], however such proposals need to be further investigated.
5. Conclusions
Consistent with previous findings on other cell types, DMSO at concentrations ≥ 1% reduced cell viability and accelerated cellular damage by altering mitochondrial membrane potential and increasing ROS in our model. Interestingly, the exposure of 3T3-L1 adipocytes to concentrations ≤ 0.1% DMSO improved glutathione content in these cells. Overall, we recommend precautions be taken when assessing compounds dissolved in DMSO, especially those higher than 0.1% and when assessing antioxidants as it may interfere with their therapeutic effect, giving false results. Therefore, concentrations lower than 0.1% should be considered when using DMSO as a vehicle in 3T3-L1 adipocytes. Furthermore, we suggest a dose of above 1% DMSO can be effectively used to induce an oxidative stress response of 3T3-L1 adipocytes.
Disclosure statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Transparency document
Acknowledgements
This work was supported by the Biomedical Research and Innovation Platform of the South African Medical Research Council (SAMRC) baseline funding. Ms. Amsha Viraragavan is funded by the South African National Research Foundation (NRF). PV Dludla was partially supported as a Post-Doctoral Fellow by funding from the SAMRC. The grant holders acknowledge that opinions, findings, and conclusions or recommendations expressed in any publication generated by the SAMRC or NRF supported research are those of the authors, and that the SAMRC and NRF accepts no liability whatsoever in this regard.
Contributor Information
Phiwayinkosi V. Dludla, Email: pdludla@mrc.ac.za.
Babalwa Jack, Email: Babalwa.Jack@mrc.ac.za.
References
- 1.Capriotti K., Capriotti A.J.A. Dimethyl sulfoxide: history, chemistry, and clinical utility in dermatology. J. Clin. Aesthet. Dermatol. 2012;5:24–26. [PMC free article] [PubMed] [Google Scholar]
- 2.Rivers-Auty J., Ashton J.C. Vehicles for lipophilic drugs: implications for experimental design, neuroprotection, and drug discovery. Curr. Neurovasc. Res. 2013;10:356–360. doi: 10.2174/15672026113109990021. [DOI] [PubMed] [Google Scholar]
- 3.Notman R., den Otter W.K., Noro M.G., Briels W.J., Anwar J. The permeability enhancing mechanism of DMSO in ceramide bilayers simulated by molecular dynamics. Biophys. J. 2007;93:2056–2068. doi: 10.1529/biophysj.107.104703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanmartin-Suarez C., Soto-Otero R., Sanchez-Sellero I., Mendez-Alvarez E. Antioxidant properties of dimethyl sulfoxide and its viability as a solvent in the evaluation of neuroprotective antioxidants. J. Pharmacol. Toxicol. Methods. 2011;63:209–215. doi: 10.1016/j.vascn.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 5.da Silva Duarte I., Gragnani A., Ferreira L.M. Dimethyl sulfoxide and oxidative stress on cultures of human keratinocytes. Can. J. Plast. Surg. 2004;12:13–16. [PMC free article] [PubMed] [Google Scholar]
- 6.Laskar A., Yuan X.M., Li W. Dimethyl sulfoxide prevents 7β-hydroxycholesterol-induced apoptosis by preserving lysosomes and mitochondria. J. Cardiovasc. Pharmacol. 2010;56:263–267. doi: 10.1097/FJC.0b013e3181eb3063. [DOI] [PubMed] [Google Scholar]
- 7.Sadowska-Bartosz I., Paczka A., Molon M., Bartosz G. Dimethyl sulfoxide induces oxidative stress in the yeast Saccharomyces Cerevisiae. FEMS Yeast Res. 2013;13:820–830. doi: 10.1111/1567-1364.12091. [DOI] [PubMed] [Google Scholar]
- 8.Manna P., Jain S.K. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab. Syndr. Relat. Disord. 2015;13:423–444. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilherme A., Virbasius J.V., Puri V., Czech M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack B.U., Malherbe C.J., Huisamen B., Gabuza K., Mazibuko-Mbeje S., Schulze A.E., Joubert E., Muller C.J.F., Louw J., Pheiffer C. A polyphenol-enriched fraction of Cyclopia intermedia decreases lipid content in 3T3-L1 adipocytes and reduces body weight gain of obese db/db mice. S. Afr. J. Bot. 2017;110:216–229. [Google Scholar]
- 11.Rong J.X., Klein J.L., Qiu Y., Xie M., Johnson J.H., Waters K.M., Zhang V., Kashatus J.A., Remlinger K.S., Bing N., Crosby R., Jackson T.K., Witherspoon S.M., Moore J.T., Ryan T.E., Neill S.D., Strum J.C. Rosiglitazone induces mitochondrial biogenesis in differentiated murine 3T3-L1 and C3H/10T1/2 adipocytes. PPAR Res. 2011;2011 doi: 10.1155/2011/179454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanderson M., Mazibuko S.E., Joubert E., de Beer D., Johnson R., Pheiffer C., Louw J., Muller C.J. Effects of fermented rooibos (Aspalathus linearis) on adipocyte differentiation. Phytomedicine. 2014;21:109–117. doi: 10.1016/j.phymed.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Mazibuko S.E., Muller C.J., Joubert E., de Beer D., Johnson R., Opoku A.R., Louw J. Amelioration of palmitate-induced insulin resistance in C2C12 muscle cells by rooibos (Aspalathus linearis) Phytomedicine. 2013;20:813–819. doi: 10.1016/j.phymed.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Berenguer M., Zhang J., Bruce M.C., Martinez L., Gonzalez T., Gurtovenko A.A., Xu T., Le Marchand-Brustel Y., Govers R. Dimethyl sulfoxide enhances Glut4 translocation through a reduction in Glut4 endocytosis in insulin-stimulated 3T3-L1 adipocytes. Biochimie. 2011;93:697–709. doi: 10.1016/j.biochi.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Wang H., Scott R.E. Inhibition of distinct steps in the adipocyte differentiation pathway in 3T3 T mesenchymal stem cells by dimethyl sulphoxide (DMSO) Cell Prolif. 1993;26:55–66. doi: 10.1111/j.1365-2184.1993.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 16.Sun X., Chen R.C., Yang Z.H., Sun G.B., Wang M., Ma X.J., Yang L.J., Sun X.B. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food Chem. Toxicol. 2014;63:221–232. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 17.Johnson R., Dludla P., Joubert E., February F., Mazibuko S., Ghoor S., Muller C., Louw J. Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis. Mol. Nutr. Food Res. 2016;60:922–934. doi: 10.1002/mnfr.201500656. [DOI] [PubMed] [Google Scholar]
- 18.Dludla P.V., Muller C.J., Joubert E., Louw J., Gabuza K.B., Huisamen B., Essop M.F., Johnson R. Phenylpyruvic acid-2-O-beta-D-glucoside attenuates high glucose-induced apoptosis in H9c2 cardiomyocytes. Planta Med. 2016;82:1468–1474. doi: 10.1055/s-0042-110856. [DOI] [PubMed] [Google Scholar]
- 19.Chellan N. Division of Medical Physiology. Stellenbosch University; South Africa: 2014. The effect of Cyclopia maculata extract on β-cell function, protection against oxidative stress and cell survival.http://scholar.sun.ac.za/handle/10019.1/95861 PhD Research Thesis. Avaiable at. [Accessed 01 August 2017] [Google Scholar]
- 20.Savjani K.T., Gajjar A.K., Savjani J.K. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012 doi: 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song Y.M., Song S.O., Jung Y.K., Kang E.S., Cha B.S., Lee H.C., Lee B.W. Dimethyl sulfoxide reduces hepatocellular lipid accumulation through autophagy induction. Autophagy. 2012;8:1085–1097. doi: 10.4161/auto.20260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan C., Gao J., Guo J., Bai L., Marshall C., Cai Z., Wang L., Xiao M. Dimethyl sulfoxide damages mitochondrial integrity and membrane potential in cultured astrocytes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernández A.F., Tsatsakis A.M. Human exposure to chemical mixtures: challenges for the integration of toxicology with epidemiology data in risk assessment. Food Chem. Toxicol. 2017;103:188–193. doi: 10.1016/j.fct.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Kakutani H., Yuzuriha T., Akiyama E., Nakao T., Ohta S. Complex toxicity as disruption of adipocyte or osteoblast differentiation in human mesenchymal stem cells under the mixed condition of TBBPA and TCDD. Toxicol. Rep. 2018;5:737–743. doi: 10.1016/j.toxrep.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsatsakis A.M., Kouretas D., Tzatzarakis M.N., Stivaktakis P., Tsarouhas K., Golokhvast K.S., Rakitskii V.N., Tutelyan V.A., Hernandez A.F., Rezaee R., Chung G., Fenga C., Engin A.B., Neagu M., Arsene A.L., Docea A.O., Gofita E., Calina D., Taitzoglou I., Liesivuori J., Hayes A.W., Gutnikov S., Tsitsimpikou C. Simulating real-life exposures to uncover possible risks to human health: a proposed consensus for a novel methodological approach. Hum. Exp. Toxicol. 2017;6:554–564. doi: 10.1177/0960327116681652. [DOI] [PubMed] [Google Scholar]
- 26.Tsatsakis A.M., Docea A.O., Tsitsimpikou C. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals’ low dose exposure. Food Chem. Toxicol. 2016;96:174–176. doi: 10.1016/j.fct.2016.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.