Abstract
Objective
Chronic ad libitum low protein-high carbohydrate diet (LPHC) increases health- and life-span in mice. A periodized (p) LPHC regimen would be a more practical long-term human lifestyle intervention, but the metabolic benefits of pLPHC are not known. Also, the interactions between LPHC diet and exercise training have not been investigated. Presently, we aimed to provide proof-of-concept data in mice of the efficacy of pLPHC and to explore the potential interactions with concurrent exercise training.
Methods
A detailed phenotypic and molecular characterization of mice undergoing different durations of 14 d LPHC (5 E% protein)/14 d control diet cycles for up to 4 months with or without concurrent access to activity wheels allowing voluntary exercise training.
Results
pLPHC conferred metabolic benefits similar to chronic LPHC, including increased FGF21 and adaptive thermogenesis, obesity-protection despite increased total energy intake and improved insulin sensitivity. The improved insulin sensitivity showed large fluctuations between diet periods and was lost within 14 days of switching back to control diet. Parallel exercise training improved weight maintenance but impaired the FGF21 response to pLPHC whereas repeated pLPHC cycles progressively augmented this response. Both the FGF21 suppression by exercise and potentiation by repeated cycles correlated tightly with Nupr1 mRNA in liver, suggesting dependence on liver integrated stress response.
Conclusion
These results suggest that pLPHC may be a viable strategy to promote human health but also highlight the transient nature of the benefits and that the interaction with other lifestyle-interventions such as exercise training warrants consideration.
Keywords: Dietary restriction, FGF21, Periodized diet, Glucose metabolism, Obesity, Exercise, Integrated stress response
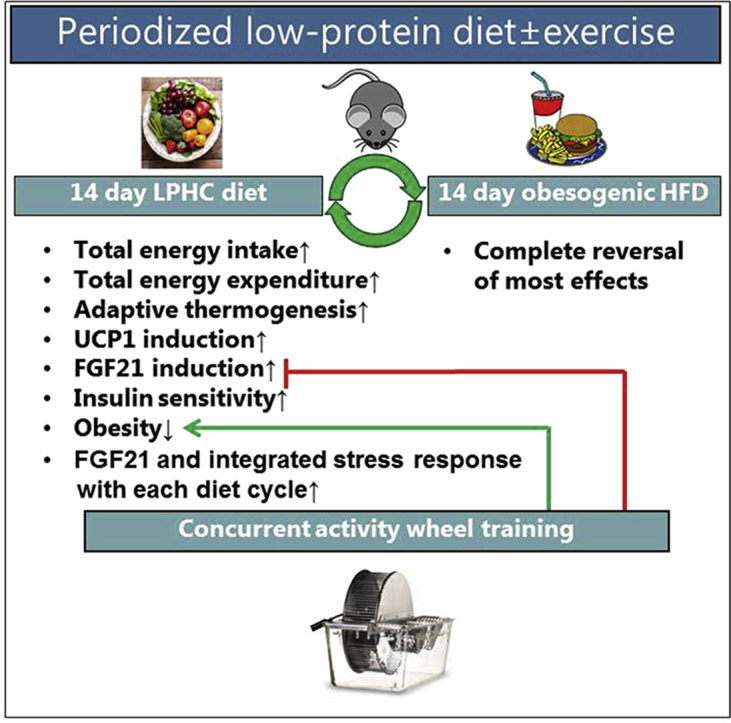
Graphical abstract
Highlights
-
•
Periodized (p) low-protein-high-carbohydrate (LPHC) diet confers metabolic benefits.
-
•
The metabolic improvements by pLPHC diet are highly transient.
-
•
The liver FGF21 and integrated stress response to pLPHC is lowered by exercise and increased by repeated pLPHC cycles.
1. Introduction
Chronic caloric restriction (CR), i.e. reduced total intake of calories of 30–50% without malnutrition, extends health- and life-span in experimental models ranging from drosophila flies to adult rhesus monkeys [1] and likely also in humans [2]. However, long-term adherence to this ascetic regimen is difficult and chronic CR is not feasible to implement broadly as a lifestyle intervention in human [1].
Two promising practical alternatives to chronic CR are intermittent CR and protein restrictive diets. Thus, periodized (p) CR regimens, ranging from alternate day fasting to 5 consecutive days/month, have been shown to increase health-span in mice and indirect measures thereof in humans [3], [4], [5], [6]. Similarly, a low protein-high carbohydrate diet (LPHC) elicited positive effects on mouse health- and life-span similar to calorie-restriction, despite increased ad libitum total energy intake [7], [8], [9], [10], [11]. A human cross-sectional study of >6000 participants showed an association between protein intake and all-cause mortality and cancer, which decreased progressively with age up to ∼65 years and was reversed beyond this age [12]. Furthermore, a LPHC diet acutely improved glucose homeostasis in mice and healthy humans [10], [13]. Recent studies suggest that protein restriction rather than calorie-restriction per se might underlie the anti-aging effects of CR diets [8], [14].
The release of the stress-hormone FGF21 from the liver is necessary in mice to elicit many of the reported benefits of LPHC diets [9], [10], [15], [16], [17], including increased thermogenesis and improved insulin sensitivity. Importantly, protein-diluted diets have been shown to increase circulating FGF21 in humans too [9], [10], [13]. The LPHC diet also suppresses the pro-aging IGF1-Akt-mTOR signaling axis [8], [10], [12], [18], [19], which is associated with health-beneficial outcomes such as decreased tumorigenesis [12] and increased cellular renovation by macroautophagy [20], [21], [22]. Mechanistically, LPHC is proposed to stimulate FGF21 transcription and secretion by the liver via an integrated stress response (ISR) pathway, involving increased activation of a GCN2/eIF2a/ATF4 signaling axis [9], [10], [16] but also requiring independent input from PPARα [9].
Like protein restrictive diets, exercise training is also associated with increased health-span in humans [23]. Single bout and chronic exercise regimens have been reported to increase FGF21 both in mice and humans [24], [25], [26], [27], likely via glucagon [25], [27], and FGF21 may be required for the beneficial effect of glucagon and voluntary exercise training on obesity-associated glucose intolerance [28]. However, exercise training may also signal independently of FGF21. For instance, exercising skeletal muscle releases myokines, such as Irisin [29] and Meteorin-like [30], which may stimulate adipose tissue browning. Furthermore, each bout of exercise stimulates macroautophagy in mouse skeletal muscle [21], [31] and likely in human skeletal muscle [32], [33], [34]. Thus, exercise training might act synergistically with a LPHC diet on health-related outcomes by co-stimulating processes such as thermogenesis and macroautophagy.
In the current study, we hypothesized that periodized protein restriction, similar to chronic protein restriction, would promote thermogenesis and metabolic improvements. Furthermore, we tested whether exercise training and pLPHC diet would have an additive effect on weight gain protection and improvements in glucose metabolism. In support of our hypothesis, we found that mice on pLPHC diet for 14 days on/off progressively increased plasma FGF21 with each diet cycle and increased energy expenditure and decreased feed efficiency (i.e. weight gain/food intake) despite increasing total energy intake, and improved glucose metabolism similar to earlier studies using chronic LPHC regimens. Interestingly, most effects of the pLPHC diet were completely reversed within 14 days after returning to the control diet showing the short-lasting nature of the pLPHC benefits. Exercise training had a strong suppressive effect on LPHC diet-induced Fgf21 mRNA expression in liver, suggesting that combining exercise training and LPHC might impair some of the LPHC-associated health-benefits.
2. Results
2.1. A 2 week LPHC period robustly increased FGF21 in young adult and middle-aged mice
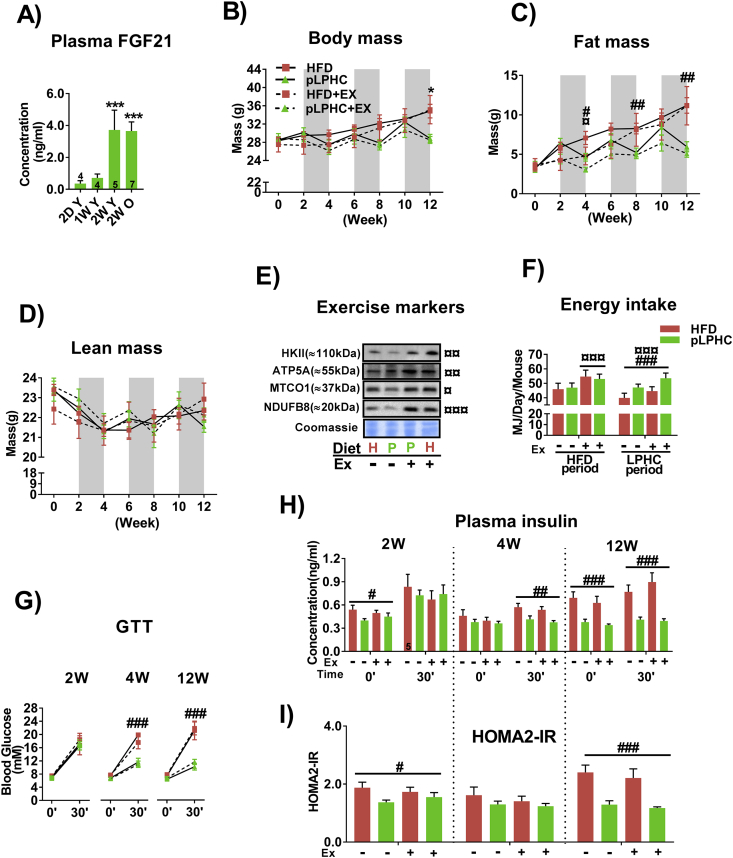
An overview of all experimental interventions is provided in Figure 1. Placing mice on a chronic LPHC diet was previously reported to improve metabolic outcomes in mice whereas high protein-low carbohydrate diet (HPLC) worsened these outcomes [7], [8]. Presently, we utilized a previously published LPHC diet containing 75/5/21 E% carbohydrate/protein/fat [7], [35], using an obesogenic 20/20/60 E% carbohydrate/protein/fat high fat diet (HFD) as reference. Given that FGF21 is necessary for many of the benefits of the LPHC diet, we first sought to verify that our pLPHC regimen elicited a substantial increase in FGF21 reminiscent of the response to chronic LPHC diet. We therefore measured the plasma FGF21 response to 2 days, 1 week, and 2 weeks of LPHC. We found that 2 weeks of LPHC period was required to robustly increase FGF21 in either young adult ∼3 months and middle-aged ∼9 months old female mice to plasma concentrations approaching ∼4 ng/ml (Figure 2A).
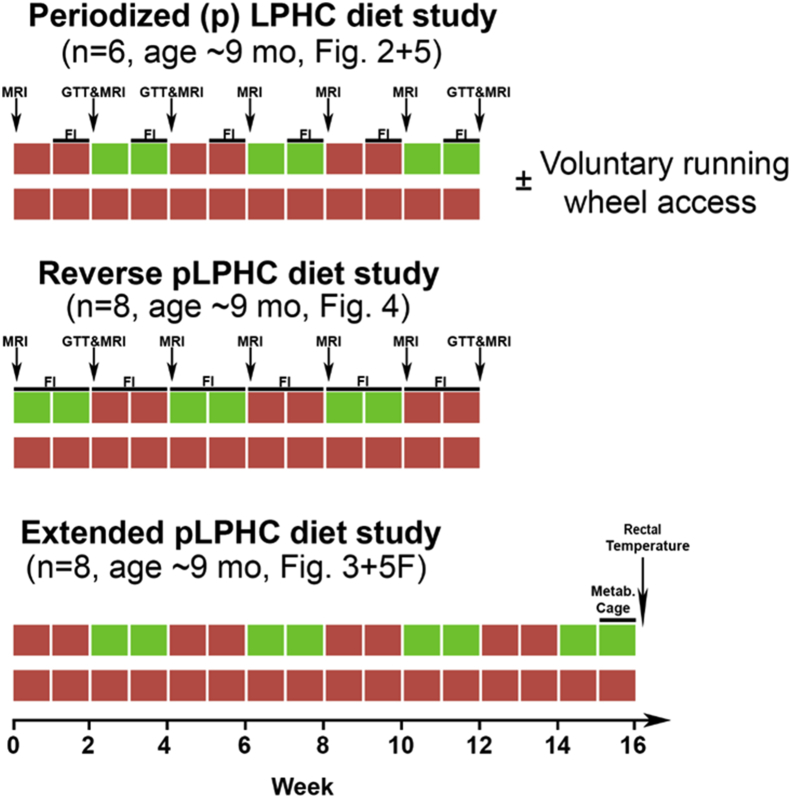
Figure 1.
Experimental overview. Not included is the 2 week low protein-high carbohydrate (LPHC) diet time course experiment in ∼3 month and ∼9 month old mice (Figure 2A) nor the 2 week LPHC diet ± exercise study (Figure 5A). MRI = Magnetic resonance imaging, GTT = modified glucose tolerance test, FI = Food Intake, Metab. Cage = indirect calorimetry cage, Mo = month, Fig. = Figure.
Figure 2.
Periodized low protein-high carbohydrate (pLPHC) diet protected against weight gain and improved glucose tolerance. A) Plasma FGF21 in ∼3 month old (young, Y) or ∼9 month old (old, O) mice subjected LPHC diet for the indicated duration. D, day. W, week. B-D) Body mass, fat mass and lean mass were measured at the indicated time points. The shaded areas indicate LPHC diet periods. The legend for panel B is shared with C, D & G. E) Protein expression of exercise training markers in quadriceps muscle. H = HFD (red). P = pLPHC (green). Ex, exercise. ¤ symbols beside the blots denote statistical significance as explained below. F) Average total energy intake measured during the 2nd week of HFD and LPHC diet periods ± exercise. The legend for panel F is shared with H & I.G) A modified 30 min glucose tolerance test (GTT) was performed at week 2, 4, and 12 during which blood samples were collected for H) insulin concentration and I) HOMA2-IR calculation. For panel A, ***p < 0.001 vs. 2D Y using Tukey's post hoc test. For C-I), ¤/¤¤/¤¤¤ p < 0.05/0.01/0.001, exercise main effect at indicated time-point. #/##/###p < 0.05/0.01/0.001, diet main effect at indicated time point. *p < 0.05 (HFD vs. pLPHC + Ex at week 12 using the Kruskal–Wallis and Dunn's multiple comparison non-parametric test. Data are expressed as mean ± SEM. N = 6, unless indicated otherwise on a given bar.
2.2. Prolonged pLPHC diet protected against weight gain and improved glucose tolerance
We next implemented the 14 days pLPHC diet regimen chronically in ∼9 months old middle-aged female mice. This age was chosen to represent mature adult life prior to onset of age-related functional decline and diseases, a life stage where the LPHC diet is suggested to be more efficacious [12]. In these experiments, the diet was switched between pLPHC and an obesogenic HFD deriving 60 E% from fat every 14 days for 3 months (3 cycles). Since both diet and exercise are considered as cornerstones in health recommendations, we provided additional groups of mice with access to activity wheels to allow concurrent voluntary exercise training to examine the interaction.
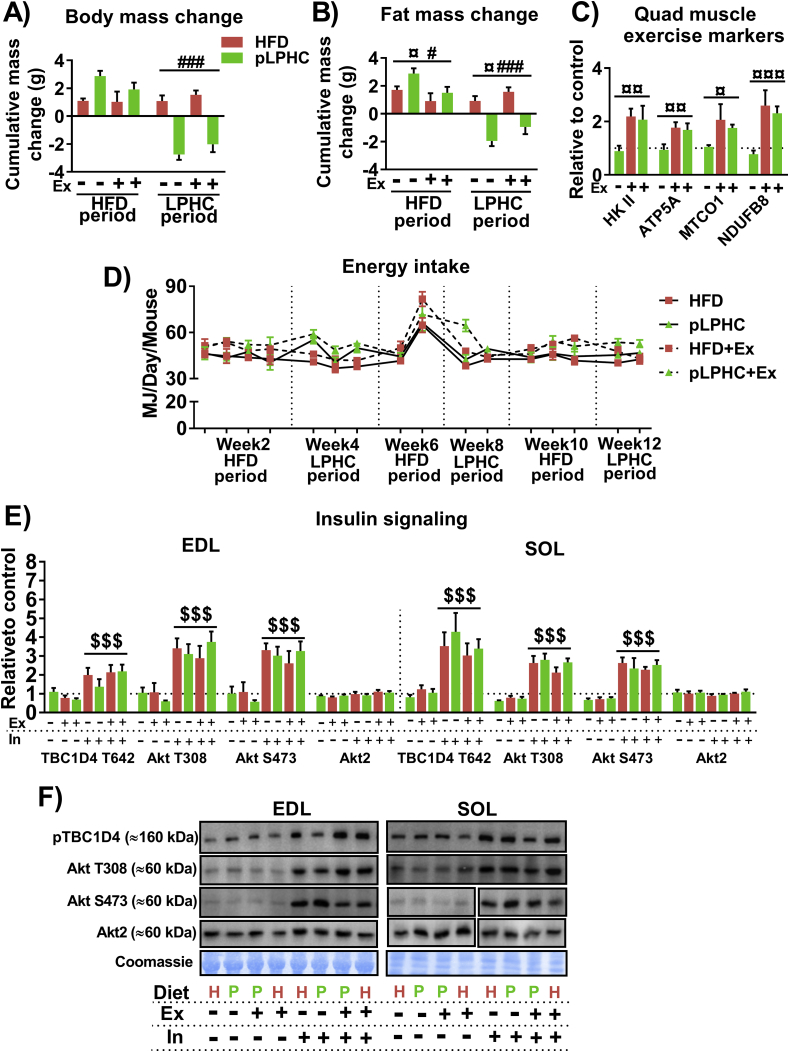
Middle-aged mice on chronic HFD progressively increased their body mass (Figure 2B) and fat mass (Figure 2C). Interestingly, the pLPHC groups significantly lost fat mass with each pLPHC period followed by regain in the following HFD periods (Figure 2B + C). Lean body mass showed similar fluctuations but was not significantly affected by any intervention (Figure 2D). Exercise training tended to attenuate both the regain in body and fat mass in the HFD periods and the body and fat mass loss on pLPHC diet (delta-values in Figure S1A + S1B). Concurrent exercise training caused expected changes in skeletal muscle exercise training markers, including Hexokinase II and mitochondrial complex proteins ATP5A, NDUF8B, and MTCO1 (representative blots in Figure 2E, quantifications in Figure S1C). After 3 cycles of pLPHC diet at 12 weeks, the pLPHC groups had not gained significant body or fat mass compared to their initial weight. Remarkably, the protection against body and fat mass gain in the pLPHC groups occurred despite a similar total energy intake during the HFD periods (when all groups received HFD) and a higher total energy intake in the pLPHC groups vs. chronic HFD groups during the pLPHC periods (Figure 2F, individual timepoints shown in Figure S1D).
To evaluate the effect of pLPHC diet on glucose tolerance and insulin sensitivity, we measured the blood glucose and insulin-response before and 30 min after receiving a 2 g/kg body weight intraperitoneal glucose injection. Whole-body glucose handling, insulin-response, and HOMA2-IR index all indicated improved insulin sensitivity in response to pLPHC diet, with no effect of concurrent exercise training (Figure 2G–I). The LPHC diet effect was evident even after a single cycle at 4 weeks on glucose tolerance and 30 min insulin measurements (Figure 2G,H), but the group separation was clearer after 3 cycles at 12 weeks and HOMA2-IR only significant at this time-point (Figure 2I). Skeletal muscle is the primary site of insulin-stimulated glucose disposal and suggested to be insulin sensitized by both FGF21 [36] and AMPK activation by exercise [37]. We therefore evaluated the sensitivity of insulin-stimulated Akt and TBC1D4 phosphorylation in isolated, ex vivo incubated extensor digitorum longus (EDL) and soleus (SOL) muscles in response to a fixed submaximal 1.8 nM dose of insulin, but found no discernible effect on skeletal muscle insulin signaling (Fig. S1E + S1F). Overall, pLPHC diet decreased feed efficiency (i.e. weight gain/food intake), lowered fat mass and improved whole-body insulin sensitivity independently of skeletal muscle.
2.3. pLPHC diet increased thermogenesis and Ucp1 in brown adipose tissue (BAT)
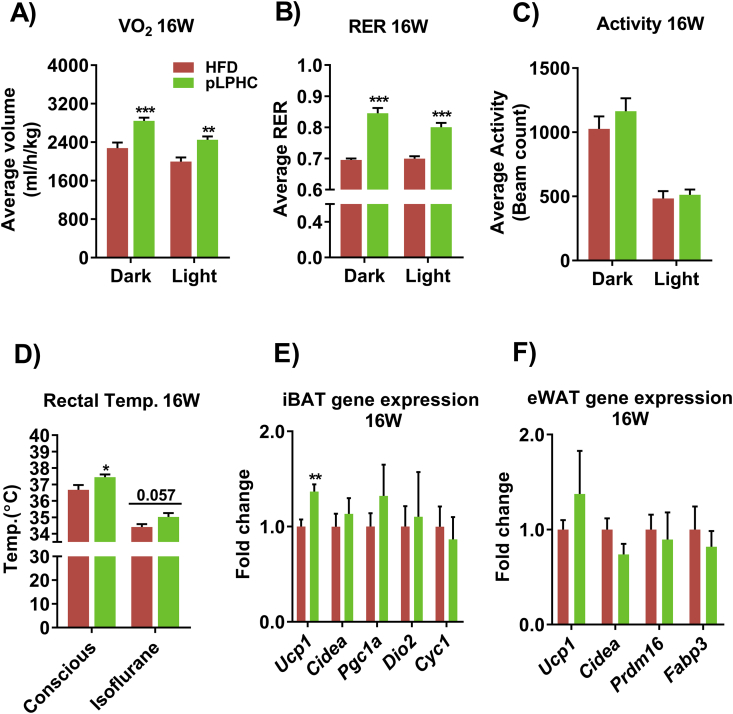
Based on the literature (see introduction), we suspected that the protective effects of pLPHC against weight and fat gain could be explained by increased resting energy expenditure due to adaptive thermogenesis. Indeed, oxygen consumption was higher in pLPHC diet mice vs. controls when measured at 16 weeks after 4 diet cycles (Figure 3A). Respiratory exchange rate was, as expected from the high carbohydrate content, markedly higher on pLPHC diet (Figure 3B). No difference in habitual activity was observed in the mice on pLPHC diet compared to controls (Figure 3C). pLPHC diet cycling for 16 weeks significantly increased rectal temperature in conscious mice and tended to do so in isofluorane anaesthetized mice (Figure 3D), directly demonstrating that pLPHC diet increased thermogenesis. Ucp1 mRNA expression was increased by pLPHC diet in brown adipose tissue (Figure 3E) whereas no significant effects were observed in white adipose tissue browning markers (Figure 3F). These data suggest that the decreased feed efficiency with pLPHC is caused at least in part by increased BAT adaptive thermogenesis.
Figure 3.
Periodized, low protein-high carbohydrate (pLPHC) diet increased thermogenesis and brown adipose tissue Ucp1 mRNA expression. At the end of week 16, A) oxygen consumption (VO2), B) respiratory exchange rate (RER), and C) habitual activity were measured. The legend in panel A is shared with panels B–F. After the metabolic measurements, rectal temperature (D) was recorded before the mice were sacrificed to determine gene expression in E) intrascapular brown adipose tissue (iBAT) and F) epididymal white adipose tissue (eWAT). For A, B, D, E), */**/**p < 0.05/0.01/0.001 vs. HFD using Student's t-test for sub-group comparisons. Data are expressed as mean ± SEM. N = 8.
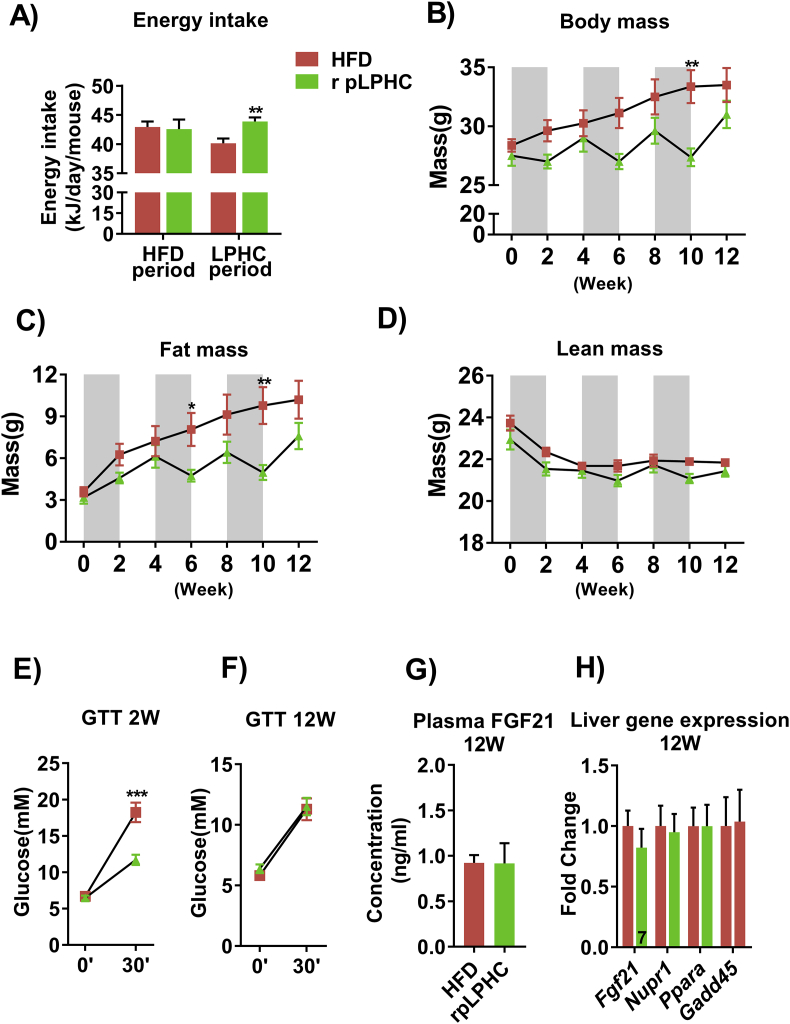
2.4. Reversal of the pLPHC diet order abolished the improved glucose tolerance
Given that our initial studies ended on a LPHC diet period, we next asked if the benefits would persist if the diet order were reversed, i.e. ending on a 14 d HFD period. Similar to our initial pLPHC diet cohort, middle-aged pLPHC mice on the reverse diet order increased their total energy intake during the pLPHC periods (Figure 4A) and exhibited similar fluctuations in body mass (Figure 4B) and fat mass (Figure 4C) but not lean body mass (Figure 4D). Interestingly, improved glucose tolerance was observable after the initial 2 weeks on pLPHC diet (Figure 4E), but this effect was completely absent after 12 weeks on pLPHC diet ending with HFD (Figure 4F). As expected from previous studies showing a rapid reversal of FGF21 levels after switching from acute LPHC diet to control diet in mice and humans [10], plasma FGF21 was comparable to control levels after 3 cycles of reversed pLPHC diet ending on HFD. Liver gene expression of Fgf21 and associated genes such as Pppara, Nupr1, and Gadd45a were not different after 3 cycles of reversed pLPHC diet ending on HFD (Figure 4H). Thus, the beneficial effects of the pLPHC diet dissipate within 14 days when switching back to a reference diet.
Figure 4.
Reversed, periodized, low protein-high carbohydrate (r pLPHC) diet order abolished the improved glucose tolerance. A) Average total energy intake in the HFD and LPHC feeding periods. The legend in panel A is shared with panels B–H. B) Body mass, C) fat mass, and D) lean mass determined by magnetic resonance imaging at the indicated time point. The shaded areas indicate LPHC diet periods. At E) week 2 and F) week 12, a modified glucose tolerance test (GTT) was performed to assess whole body glucose metabolism. After the dietary regimen, the mice were sacrificed and plasma and liver tissue collected to determine G) Plasma FGF21 and H) liver gene expression. For A), **p < 0.01 vs. HFD during the LPHC diet feeding periods. For B & C), */**p < 0.05/0.01, Sidak's post hoc test effect of diet. For E), ***p < 0.001 vs. HFD at 30′ using Student's t-test. Data are expressed as mean ± SEM. N = 8, unless indicated otherwise on a given bar.
2.5. FGF21 was suppressed by concurrent exercise training and increased by repeated pLPHC cycles
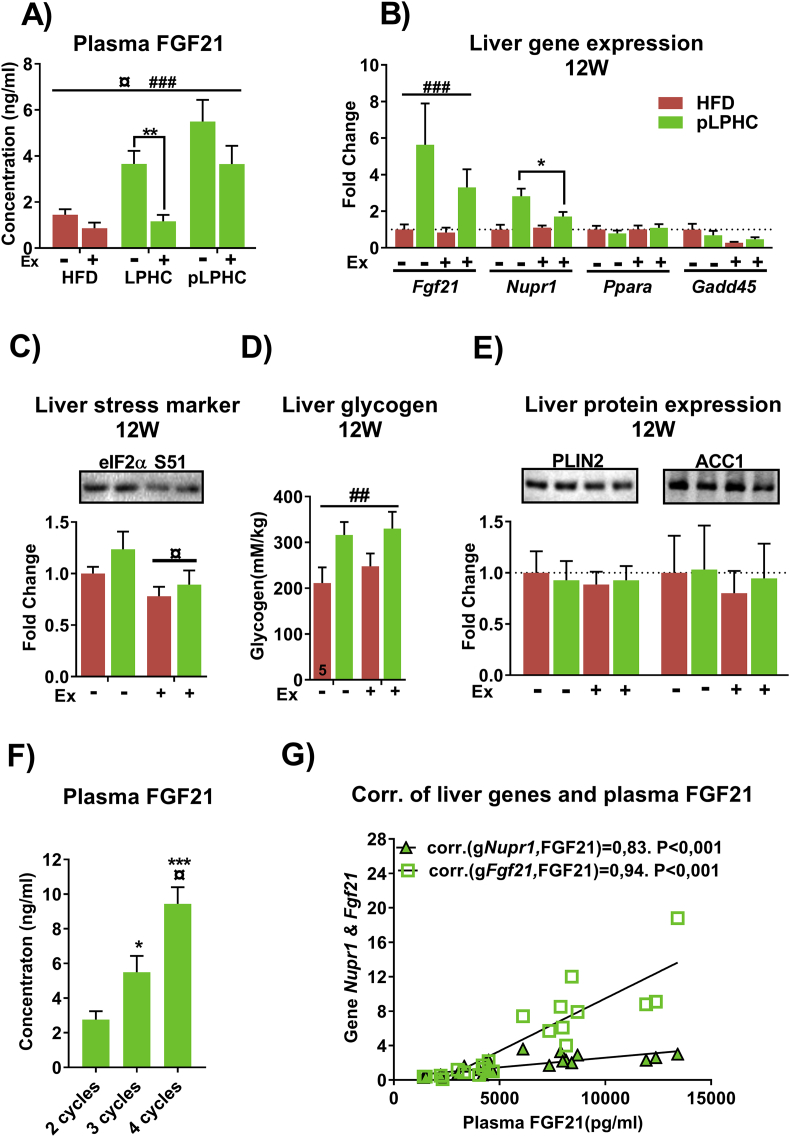
To test the effect of concurrent diet and exercise on FGF21 levels, we measured plasma FGF21 after 1 and 3 full cycles of the pLPHC diet ± activity access. Interestingly, the increase in plasma FGF21 with pLPHC diet was strongly suppressed by concurrent exercise training at both time-points, albeit the exercise-associated suppression was less pronounced after 3 pLPHC diet cycles (Figure 5A). The decrease in plasma FGF21 was associated with suppressed Fgf21 and Nupr1 mRNA expression (Figure 5B) and eIF2α Ser51 phosphorylation (Figure 5C) in the liver, indicating that concurrent exercise lowered the pLPHC-induced stimulation of the ISR pathway. Liver glycogen content was increased by pLPHC, but not affected by exercise (Figure 5D), suggesting that repartitioning of glucose away from the liver was not part of the exercise-induced protection against liver stress. Perilipin (PLIN) 2 and Acetyl CoA carboxylase (ACC) 1 protein expression in liver, biomarkers of hepatic steatosis and de novo lipogenesis, respectively, were not affected by pLPHC or exercise training (Figure 5E). Together with the absent mRNA induction of the fatty acid-activated transcription factor Ppara (Figure 5B), a reported requirement for basal and LPHC diet-induced FGF21 induction independent of eIF2α [9], these data suggest that exercise lowers the liver ISR to pLPHC diet independent of accumulation of liver glycogen or fatty acids.
Figure 5.
FGF21 was suppressed by concurrent exercise training and increased by repeated periodized low protein-high carbohydrate (pLPHC) cycles. A) Plasma FGF21 in ∼9 month old mice fed with LPHC ± activity wheel access for 2 weeks (middle panel) or HFD and pLPHC ± running wheel for 12 weeks (left and right panels). B) Liver gene expression in mice fed with HFD or pLPHC ± running wheel for 12 weeks. The legend in panel B is shared with remaining panels, C) eIF2α Ser51 phosphorylation, D) liver glycogen content and E) liver Perilipin 2 (PLIN2) and acetyl CoA carboxylase (ACC)1 protein expression. F) Plasma FGF21 from mice fed with pLPHC for 8 weeks (2 cycles), 12 weeks of pLPHC (3 cycles) or 16 weeks of pLPHC (4 cycles). G) Spearman correlation analyses of plasma FGF21 and liver Nupr1 or Fgf21 mRNA. For A), ¤ p < 0.05, main effect of exercise. ###p < 0.001, main effect of pLPHC, **p < 0.01 exercise effect using Student's t-test. For B), ###p < 0.001, main effect of pLPHC. *p < 0.05, exercise effect within pLPHC group using Student's t-test. For C & D), ¤/##p < 0.05/0.01, main effect of exercise/pLPHC. For F), */***p < 0.05/0.001 vs 2 cycles, ¤ p < 0.05 vs 3 cycles using Tukey's post hoc test. Data are expressed as mean ± SEM. N = 6–8, unless indicated otherwise on a given bar.
We also tested if multiple cycles of pLPHC diet would affect the plasma FGF21 response. Strikingly, pLPHC cycling progressively increased plasma FGF21 (Figure 5F) despite FGF21 returning to baseline during the HFD diet periods (Figure 4G). The increase in plasma FGF21 strongly correlated with either liver Fgf21 or Nupr1 mRNA (Figure 5G), suggesting that repeated pLPHC diet cycling augments plasma FGF21 via a liver ISR pathway-dependent increase in Fgf21 mRNA expression.
3. Discussion
This study tested whether ad libitum LPHC diet provided in 14 day on/14 day off cycles would confer health-beneficial adaptations in mice, such as lowering of feed efficiency and improvement of glucose metabolism, similar to the effects reported with continuous chronic LPHC diet. Indeed, our data showed that pLPHC diet cycling protected against weight gain, increased thermogenesis and improved glucose tolerance, implying that pLPHC diet may be a viable strategy to promote health in humans. However, our data also add an interesting perspective to LPHC and other diet studies in mice by highlighting the large and rapid fluctuations in body composition and metabolism in mice in response to diet switching and the highly transient nature of the LPHC diet-induced changes.
Dietary restriction and exercise are cornerstones in health-promoting lifestyle interventions and often implemented concurrently. Despite this, diet and exercise are most often studied separately in the laboratory setting. Presently, we found both positive (weight gain-attenuation) and negative synergies (FGF21 release and weight-loss reduction) between LPHC diet and exercise training. Most notably, concurrent exercise prevented much of the regain in weight when the pLPHC mice were switched back to an obesogenic HFD, in agreement with the concept that physical activity protects against weight regain after weight-loss interventions [38], concurrent exercise training clearly suppressed pLPHC diet-induced FGF21 expression and release from liver. Since FGF21 is a major driver of the health beneficial adaptations to low-protein diet in mice [5], [10], [15], [16], [17], concurrent exercise would thus presumably be counter-productive to attaining the effects of eating a LPHC diet. These findings highlight the need to also consider the diet and exercise interactions, rather than focusing exclusively on one or the other.
Remarkably, despite returning to baseline in each HFD period, plasma FGF21 progressively increased with each pLPHC diet cycle. This increase showed excellent correlations with liver mRNA induction of Ffg21 and the eIF2α-ATF4 ISR pathway gene-target Nupr1, an ISR biomarker partially required for the FGF21 increase by a chronic 5E% protein-diluted diet in mice [10]. Conversely, concurrent exercise training lowered the Nupr1 mRNA response to pLPHC diet. Overall, this suggests a convergence between duration of pLPHC diet and exercise training onto Nupr1 gene expression. As the name implies, the integrated stress-response can react to a diverse number of different cell extrinsic and intrinsic cellular insults [39]. Short-term LPHC diet is able to double liver TG content within a week in mice [40], and liver lipid accumulation is associated with an increased liver ISR response and FGF21 release [41]. We currently tested if repeated pLPHC diet cycling and exercise training affected lipid triacylglycerol metabolism but found no changes in markers of liver TG content (PLIN2), de novo lipogenesis (ACC1), or the free fatty acid-activated transcription factor Ppara. Thus, the exact mechanism underlying the progressively increased Nupr1 and Fgf21 mRNA with repeated pLPHC diet cycles remains to be determined.
Similarly, the mechanism behind the dampening effect of exercise training on liver FGF21 and Nupr1 induction by pLPHC diet is currently unclear. We suspected that glucose repartitioning away from the liver to the insulin-sensitized exercising muscles to lower de novo gluconeogenesis [42] might be involved, but found neither liver glycogen nor muscle insulin sensitivity to be altered by training. Alternatively, exercise might increase the muscle breakdown and release of FGF21-supressive non-essential amino acids [10] to fuel gluconeogenesis [43]. This might explain both the current negative effect of exercise training and the previously reported similar effect of calorie restriction on the liver FGF21 response to LPHC diet [9]. Interestingly, the proteolytic response to prolonged fasting in mouse skeletal muscle depends on AMPK-stimulated autophagy [44]. This makes it tempting to hypothesize that an AMPK-dependent amino acid release from muscle dampened the liver ISR pathway in both cases.
The metabolic benefits of ingesting a pLPHC diet were remarkably short-lasting in the current study. This is an important observation for at least two reasons. Firstly, although the faster metabolism and accelerated metabolic response to fasting in mouse vs. man is well-known, our study provides a good example of the fact that mice are highly plastic in their physiological response to any intervention, being able to change their body weight by 10–15% within a 14 days pLPHC diet period. Chronic intervention studies sometimes observe changes in tissue morphology/function, which might be expected to provide durable protective structural changes against obesity and metabolic disease, e.g. the expansion of subcutaneous white adipose tissue by FGF21 treatment [45], but such protective effects might be rapidly reversed without the stimulus. Secondly, from an applied perspective, these data suggest that this dietary regimen, if effective in expanding health- and life-span as chronic diet LPHC diet studies would suggest [8], needs to be applied throughout adult life to be efficacious. Indeed, this is a feature of most if not all remedies against aging- and lifestyle-related diseases.
In conclusion, pLPHC diet applied in 14 d on/14 d off cycles protected against weight gain despite increased ad libitum food intake and improved glucose tolerance in mice. These effects were likely mediated by increased plasma FGF21, the concentration of which was lowered by concurrent exercise and progressively increased with each pLPHC cycle. Furthermore, delineating whether the tight correlation between gene expression of Fgf21 and the ISR marker Nupr1 is a causal relationship explaining mechanism behind the inhibitory effect of exercise training and the stimulatory effect of repeated diet cycles may provide important insight into FGF21 regulation of potential relevance to detection, prevention, and treatment of metabolic disease. The translatability of our findings into humans warrants further investigation.
Author contributions
Conceptualization, TEJ. Methodology, ZL and TEJ. Investigation, ZL, MR, JL, CHO, JK, ABM, EQ, MK, TEJ. Formal analysis, ZL. Visualization, ZL. Writing – Original draft, TEJ. Writing – Review and Editing, ZL with input from all authors. Funding Acquisition, TEJ. Supervision, TEJ.
Acknowledgments
We thank Irene B. Nielsen and Emilija Malogajski for excellent technical assistance. ZL and JL were supported by China Scholarship Council (CSC) PhD stipends. CHO was supported by a CONICYT PhD travel grant. JRK and ABM were supported by Danish Diabetes Academy PhD stipends. MK was supported by a Danish Research Council fellowship grant (DFF – 503-00155). TEJ was supported by a Novo Nordisk Foundation Excellence project grant (#15182).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.molmet.2018.08.008.
Experimental procedures
Animal experiments
LPHC and HFD diets were purchased from Specialty Feeds (Cat.: SF09-048) and Research Diet Inc. (Cat.: D12492) respectively. Following acclimation for 1 week, 8–9 months old C57BL/6JRj female retired breeder mice (Janvier labs, France) were randomized to HFD (n = 12) and pLPHC (n = 12). Half of the mice received access to a running wheel and were single housed at 22–24 °C on a 12-hour light/12-hour dark-cycle with ad libitum access to water and their respective diet regimens for 12 weeks (see Figure 1, periodized LPHC diet study). The HFD group was maintained on the same diet for the entire period whereas the pLPHC group completed 3 cycles of 2 weeks on HFD and 2 weeks on LPHC. Magnetic resonance imaging (MRI) was performed every fortnight to determine body composition. Food intake was measured in the 2nd week after diet switching. A modified GTT was performed at week 2, 4, and 12 to assess whole body glucose metabolism.
For the data shown in Figure 3 and Figure 5F, another cohort of mice were group-housed with ad libitum access to either HFD (n = 8) or 4 cycles of 2 weeks on HFD and 2 weeks on LPHC (n = 8) for 16 weeks (see Figure 1, extended LPHC diet study). Metabolic measurement was performed during week 16 to assess O2 consumption, CO2 production, and habitual activity. 2 days after the metabolic measurement, rectal temperature of these mice was recorded at room temperature at 8 and 9 PM (see below).
For the data in Figure 4, 16 mice were single housed and randomized into HFD group or reverse pLPHC group for 12 weeks. The reverse pLPHC regimen was composed of 3 cycles of 2 weeks on LPHC and 2 weeks on HFD. Food intake was measured every week for 12 weeks, MRI was performed every 2nd week. A modified GTT was conducted at the end of week 2 and 12 (see Figure 1, reverse pLPHC diet study).
The pilot-experiment in Figure 2A to ascertain the duration of pLPHC diet required to substantially increase plasma FGF21 was conducted in ∼3 month old female C57BL/6JRj mice (Janvier labs, France) and the 14 day response then verified in ∼9 month old retired breeder mice. The 2 week LPHC diet ± activity wheel access measuring plasma FGF21 was performed iñ9 month old retired breeder mice.
The individual procedures and analyses are detailed below.
Indirect calorimetry and habitual activity
1 week before the cage measurements, mice were acclimated to single housing in metabolic cages (PhenoMaster, TSE, Germany) with free access to diet and water gel. After acclimation, habitual activity, measured as laser beam break counts, inhaled O2 and expired CO2 were monitored in week 16 across the entire week. The data from the last 24 h were used in the analysis.
Body composition (BC) determination
BC of individual conscious fed mice was determined in an EchoMRI™ 4-in-1-500 Body Composition Analyzer between 9 and 12 A.M. according to the manufacturer's instructions.
Energy intake
Food intake was measured 2–3 times per week between 9 and 11 A.M. The food pellets were weighed before and after a period on the cage to calculate food intake, making sure to collect food pellets in the cage too. The food intake was converted to energy intake by multiplying with the net metabolizable energy of a given diet.
GTT and plasma collection
On the experimental day, the mice were weighed, single housed in clean cages and deprived of food from 7 to 8 A.M. for 6 h. Glucose solution in isotonic saline (1 g/5 ml) was prepared 1 h prior to the experiments and injected at a dose of 10 μg/g body weight. Blood glucose was monitored using a standard glucometer (Contour XT, Bayer's) pre and 30 min after the intraperitoneal glucose injection. Meanwhile, ∼50 μl of blood was collected and centrifuged to recover plasma for insulin measurements. Immediately after the experiment, the mice were allowed access to their respective diets again.
Rectal temperature
Rectal temperature measurements were performed in an unlit room at room temperature at 8 P.M. using a torch light when mouse handling was required. The measurement was first conducted on mice acutely anesthetized by 2–3% Isoflurane, then after 1 h the measurement was repeated on conscious mice. Briefly, once stable anesthesia was reached, a lubricated temperature probe (Model: BIO-TK9882, BIOSEB) was gently inserted in the rectum 1.5–2 cm into the mice. The rectal temperature was recorded approximately 15 s after probe insertion when the measurement was stable. Afterwards, the mice were allowed to gain consciousness and recover for ∼1 h before another measurement was performed on mice restrained by scruffing.
Tissue harvest and muscle incubation
Mice were fasted for 2–3 h from 9 A.M. Quadriceps (Quad) was harvested from mice anesthetized with pentobarbital 6 mg and 0.2 mg lidocaine/100 g body weight. After that, extensor digitorum longus (EDL) and soleus (SOL) muscles were carefully dissected from the mice and then were pinned vertically at resting length onto a custom-made silicone/plastic holder and placed in glass tubes containing Krebs-Ringer-Henseleit (KRH) buffer supplemented with 2 mM pyruvate and 8 mM mannitol at 30 °C with continuous gassing with 95% O2 and 5% CO2. After 30 min, the plastic holders with EDL or SOL muscles were swiftly transferred into other glass tubes containing the same KRH ±1.8 mM insulin. 20 min later, the EDL and SOL muscle were carefully remove from the plastic holders, rinsed in ice-cold KRH, dried on paper tissue and snap frozen in liquid nitrogen (N2).
After EDL and SOL were dissected out, a piece of the most ventral lobe of the liver was collected and snap frozen in liquid N2. Blood was then collected from the punctured heart and thoracic cavity and centrifuged to obtain plasma. The entire tissue collection procedure took approx. 2–3 min/mouse.
Protein extraction and western blotting
∼15 mg of liver or Quad muscle, or the entire EDL and SOL muscles, trimmed free of visible fat and tendons, were homogenized in 300 μl of cold lysis buffer (0.05 M Tris Base pH 7.4, 0.15 M NaCl, 1 mM EDTA and EGTA, 0.05 M sodium fluoride, 5 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 1 mM benzamidine, 0.5% protease inhibitor cocktail (P8340, Sigma Aldrich), and 1% NP-40) using a bead mill homogenizer (TissueLyser II, Retsch) operating at 30 Hz for 1 min. The homogenates were then rotated end-over-end at 4 °C for 30 min followed by centrifugation at 18.320 g at 4 °C for 20 min to recover tissue lysates for western blotting.
Equivalent amounts of protein from liver, Quad, EDL or SOL were separated by 5–15% SDS-PAGE and semi-dry transferred to PVDF membranes. After that, the membranes were blocked in 3–5% BSA or skim milk for 1 h and incubated overnight with primary antibodies. The following day, the membranes were washed in TBS-T and incubated with relevant horseradish peroxidase-conjugated secondary antibodies for 1 h followed by TBS-T washing. The signal of bound antibody was visualized using enhanced chemiluminescence (ECL+; Amersham Biosciences, Little Chalfont, UK) and a ChemiDoc MP Imaging System (Bio-Rad, Hercules, CA, USA). Afterwards, the membranes were washed with TBS-T again and stained with Coomassie Brilliant Blue to verify even transfer and similar total protein loading.
The primary antibodies used were phospho-eIF2α Ser51 (Cell Signaling Technology (CST), 9721), phospho-Akt Thr308 (CST, 13038), phospho-Akt S473 (CST, 4060), phospho-TBC1D4 Thr642 (CST, 8881), Perilipin2 (Novus Biologicals, NB110-40877), Akt2 (CST, 2964), OXPHOS cocktail (ab110413, Abcam), and Hexokinase II (HKII) (CST, 2867). Anti-acetyl CoA carboxylase (ACC)1 was gift from Grahame Hardie, University of Dundee.
Plasma FGF21 and insulin measurements
ELISA kits from BioVendor (Cat. RD291108200R) and ALPCO (Cat. 80-INSMSU-E10) were used to measure plasma FGF21 and insulin concentration, respectively, following the manufacturer's instruction.
Liver glycogen
10–30 μg of frozen liver tissue and 200 μl of 1 N HCL were added to a 1.5 ml tube, then the tubes were tightly capped and heated at 98 °C. 2 h later, the samples were cooled to room temperature. Glycogen content was measured as glycosyl units after acid hydrolysis, and glucose was determined fluorometrically from the neutralized perchloric acid extracts [46].
Preparation of RNA and gene expression analysis
Total RNA from tissues was prepared using RNeasy Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. cDNA synthesis was performed with a QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany). Gene expression was examined with SYBR Green based quantitative PCR (qPCR), using the 7900HT Fast Real-Time PCR System (Thermo Fisher Scientific, Erlangen, Germany). The relative expression levels of each gene were normalized to hypoxanthine phosphoribosyltransferase (Hprt), which served as a housekeeping gene, using the ddCt method. The following forward (for) and reverse (rev) primers were used: Ucp1: for GGCCTCTACGACTCAGTCCA, rev TAAGCCGGCTGAGATCTTGT; Cidea: for AATGGACACCGGGTAGTAAGT, rev CAGCCTGTATAGGTCGAAGGT; Dio2: for TGCCACCTTCTTGACTTTGC, rev GGTTCCGGTGCTTCTTAACC; Pgc1a: for AGCCGTGACCACTGACAACGAG, rev GCTGCATGGTTCTGAGTGCTAAG; Cyc1: for CAGCTTCCATTGCGGACAC, rev GGCACTCACGGCAGAATGAA; Prdm16: for CCGCTGTGATGAGTGTGATG, rev GGACGATCATGTGTTGCTCC; Fabp3: for ACCTGGAAGCTAGTGGACAG, rev TGATGGTAGTAGGCTTGGTCAT; Fgf21: for CTGCTGGGGGTCTACCAAG, rev CTGCGCCTACCACTGTTCC, Nupr1: for ACCCTTCCCAGCAACCTCTAA, rev TCTTGGTCCGACCTTTCCGA; Ppara: for TACTGCCGTTTTCACAAGTGC, rev AGGTCGTGTTCACAGGTAAGA; Gadd45: for AGACCGAAAGGATGGACACG, rev GTACACGCCGACCGTAATG. Primer efficiency was tested.
Statistical analyses of data
Results are expressed as mean ± SEM. Statistical tests were performed using Spearman correlation test, student's T test, ANOVA, or repeated measurements ANOVA followed by Tukey's (independent comparisons) or Sidak's (repeated measures) post hoc testing as indicated in the legends using SPSS 22 and GraphPad Prism 7. The data in Figure 2B failed Levene's equal variance test even after transformation, and the Kruskal–Wallis with Dunn's multiple comparison non-parametric test was instead applied. The significance level was set at p < 0.05. A number written above bars in graphs highlights a tendency, defined as p < 0.1.
Conflicts of interest
The authors declare no competing interests.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Fig. S1.
Net changes in A) body mass and B) fat mass in response to high fat diet (HFD) or periodized low protein high carbohydrate (pLPHC) diet. The legend in panel A is shared with the remaining panels. C) Quantification of exercise markers in quadriceps muscle. D) Energy intake measured in weeks 2, 4, 6, 8, 10, & 12. E) Insulin signaling in extensor digitorum longus (EDL) and soleus (SOL) muscles ex vivo incubated ± 1.8 nM insulin for 20 min after 12 weeks of pLPHC diet vs. HFD. F), representative blots for data in panel E. To fit the panel lay-out, the representative blot consists of rearranged parts of the same membrane. ¤/¤¤/¤¤¤/#/##/###p < 0.05/0/01/0.001 main effect of exercise/pLPHC diet. $$$ p < 0.001, main effect of insulin. Data are expressed as mean ± SEM. N = 4–6. D, diet. H = HFD (red), P = pLPHC diet (green). Ex, exercise. In, insulin.
References
- 1.Fontana L., Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015 Mar 26;161(1):106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longo V.D., Mattson M.P. Fasting: molecular mechanisms and clinical applications. Cell Metabolism. 2014 Feb 4;19(2):181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harvie M.N., Pegington M., Mattson M.P., Frystyk J., Dillon B., Evans G. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. International Journal of Obesity. 2011 May;35(5):714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandhorst S., Choi I.Y., Wei M., Cheng C.W., Sedrakyan S., Navarrete G. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metabolism. 2015 Jul 7;22(1):86–99. doi: 10.1016/j.cmet.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raffaghello L., Lee C., Safdie F.M., Wei M., Madia F., Bianchi G. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2008 Jun 17;105(24):8215–8220. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J., Duan W., Mattson M.P. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. Journal of Neurochemistry. 2002 Sep;82(6):1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 7.Solon-Biet S.M., Mitchell S.J., Coogan S.C., Cogger V.C., Gokarn R., McMahon A.C. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Reports. 2015 Jun 16;11(10):1529–1534. doi: 10.1016/j.celrep.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solon-Biet S.M., McMahon A.C., Ballard J.W., Ruohonen K., Wu L.E., Cogger V.C. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metabolism. 2014 Mar 4;19(3):418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laeger T., Henagan T.M., Albarado D.C., Redman L.M., Bray G.A., Noland R.C. FGF21 is an endocrine signal of protein restriction. Journal of Clinical Investigation. 2014 Sep;124(9):3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maida A., Zota A., Sjoberg K.A., Schumacher J., Sijmonsma T.P., Pfenninger A. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. Journal of Clinical Investigation. 2016 Sep 1;126(9):3263–3278. doi: 10.1172/JCI85946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings N.E., Williams E.M., Kasza I., Konon E.N., Schaid M.D., Schmidt B.A. Restoration of metabolic health by decreased consumption of branched-chain amino acids. The Journal of Physiology. 2018 Feb 15;596(4):623–645. doi: 10.1113/JP275075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine M.E., Suarez J.A., Brandhorst S., Balasubramanian P., Cheng C.W., Madia F. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metabolism. 2014 Mar 4;19(3):407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontana L., Cummings N.E., Arriola Apelo S.I., Neuman J.C., Kasza I., Schmidt B.A. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Reports. 2016 Jul 12;16(2):520–530. doi: 10.1016/j.celrep.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee K.P., Simpson S.J., Clissold F.J., Brooks R., Ballard J.W., Taylor P.W. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proceedings of the National Academy of Sciences of the United States of America. 2008 Feb 19;105(7):2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez-Marti A., Garcia-Guasch M., Tresserra-Rimbau A., Carrilho-Do-Rosario A., Estruch R., Salas-Salvado J. A low-protein diet induces body weight loss and browning of subcutaneous white adipose tissue through enhanced expression of hepatic fibroblast growth factor 21 (FGF21) Molecular Nutrition & Food Research. 2017 Aug;61(8) doi: 10.1002/mnfr.201600725. [DOI] [PubMed] [Google Scholar]
- 16.Laeger T., Albarado D.C., Burke S.J., Trosclair L., Hedgepeth J.W., Berthoud H.R. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Reports. 2016 Jul 19;16(3):707–716. doi: 10.1016/j.celrep.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill C.M., Laeger T., Albarado D.C., McDougal D.H., Berthoud H.R., Munzberg H. Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Scientific Reports. 2017 Aug 15;7(1):8209. doi: 10.1038/s41598-017-07498-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maida A., Chan J.S.K., Sjoberg K.A., Zota A., Schmoll D., Kiens B. Repletion of branched chain amino acids reverses mTORC1 signaling but not improved metabolism during dietary protein dilution. Molecular Metabolism. 2017 Aug;6(8):873–881. doi: 10.1016/j.molmet.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamming D.W., Cummings N.E., Rastelli A.L., Gao F., Cava E., Bertozzi B. Restriction of dietary protein decreases mTORC1 in tumors and somatic tissues of a tumor-bearing mouse xenograft model. Oncotarget. 2015 Oct 13;6(31):31233–31240. doi: 10.18632/oncotarget.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henagan T.M., Laeger T., Navard A.M., Albarado D., Noland R.C., Stadler K. Hepatic autophagy contributes to the metabolic response to dietary protein restriction. Metabolism. 2016 Jun;65(6):805–815. doi: 10.1016/j.metabol.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grumati P., Coletto L., Schiavinato A., Castagnaro S., Bertaggia E., Sandri M. Physical exercise stimulates autophagy in normal skeletal muscles but is detrimental for collagen VI-deficient muscles. Autophagy. 2011 Dec;7(12):1415–1423. doi: 10.4161/auto.7.12.17877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castagnaro S., Pellegrini C., Pellegrini M., Chrisam M., Sabatelli P., Toni S. Autophagy activation in COL6 myopathic patients by a low-protein-diet pilot trial. Autophagy. 2016 Dec;12(12):2484–2495. doi: 10.1080/15548627.2016.1231279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dangardt F.J., McKenna W.J., Luscher T.F., Deanfield J.E. Exercise: friend or foe? Nature Reviews Cardiology. 2013 Sep;10(9):495–507. doi: 10.1038/nrcardio.2013.90. [DOI] [PubMed] [Google Scholar]
- 24.Cuevas-Ramos D., Almeda-Valdes P., Meza-Arana C.E., Brito-Cordova G., Gomez-Perez F.J., Mehta R. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0038022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansen J.S., Pedersen B.K., Xu G., Lehmann R., Weigert C., Plomgaard P. Exercise-induced secretion of FGF21 and follistatin are blocked by pancreatic clamp and impaired in type 2 Diabetes. Journal of Clinical Endocrinology & Metabolism. 2016 Jul;101(7):2816–2825. doi: 10.1210/jc.2016-1681. [DOI] [PubMed] [Google Scholar]
- 26.Kim K.H., Kim S.H., Min Y.K., Yang H.M., Lee J.B., Lee M.S. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berglund E.D., Kang L., Lee-Young R.S., Hasenour C.M., Lustig D.G., Lynes S.E. Glucagon and lipid interactions in the regulation of hepatic AMPK signaling and expression of PPARalpha and FGF21 transcripts in vivo. American Journal of Physiology. Endocrinology and Metabolism. 2010 Oct;299(4):E607–E614. doi: 10.1152/ajpendo.00263.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Loyd C., Magrisso I.J., Haas M., Balusu S., Krishna R., Itoh N. Fibroblast growth factor 21 is required for beneficial effects of exercise during chronic high-fat feeding. Journal of Applied Physiology. 2016 Sep 1;121(3):687–698. doi: 10.1152/japplphysiol.00456.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jedrychowski M.P., Wrann C.D., Paulo J.A., Gerber K.K., Szpyt J., Robinson M.M. Detection and quantitation of circulating human Irisin by tandem mass spectrometry. Cell Metabolism. 2015 Oct 6;22(4):734–740. doi: 10.1016/j.cmet.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao R.R., Long J.Z., White J.P., Svensson K.J., Lou J., Lokurkar I. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014 Jun 5;157(6):1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He C., Bassik M.C., Moresi V., Sun K., Wei Y., Zou Z. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012 Jan 18;481(7382):511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fritzen A.M., Madsen A.B., Kleinert M., Treebak J.T., Lundsgaard A.M., Jensen T.E. Regulation of autophagy in human skeletal muscle: effects of exercise, exercise training and insulin stimulation. The Journal of Physiology. 2016 Feb 1;594(3):745–761. doi: 10.1113/JP271405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moller A.B., Vendelbo M.H., Christensen B., Clasen B.F., Bak A.M., Jorgensen J.O. Physical exercise increases autophagic signaling through ULK1 in human skeletal muscle. Journal of Applied Physiology. 2015 Apr 15;118(8):971–979. doi: 10.1152/japplphysiol.01116.2014. [DOI] [PubMed] [Google Scholar]
- 34.Schwalm C., Jamart C., Benoit N., Naslain D., Premont C., Prevet J. Activation of autophagy in human skeletal muscle is dependent on exercise intensity and AMPK activation. The FASEB Journal. 2015 Aug;29(8):3515–3526. doi: 10.1096/fj.14-267187. [DOI] [PubMed] [Google Scholar]
- 35.Li Z., Rasmussen M.L., Li J., Olguin C.H., Knudsen J.R., Sogaard O. Low- and high-protein diets do not alter ex vivo insulin action in skeletal muscle. Physics Reports. 2018 Jul;6(13) doi: 10.14814/phy2.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Z., Tian H., Lam K.S., Lin S., Hoo R.C., Konishi M. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metabolism. 2013 May 7;17(5):779–789. doi: 10.1016/j.cmet.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Kjobsted R., Munk-Hansen N., Birk J.B., Foretz M., Viollet B., Bjornholm M. Enhanced muscle insulin sensitivity after contraction/exercise is mediated by AMPK. Diabetes. 2016 Oct 26 doi: 10.2337/db16-0530. [DOI] [PubMed] [Google Scholar]
- 38.Foright R.M., Presby D.M., Sherk V.D., Kahn D., Checkley L.A., Giles E.D. Is regular exercise an effective strategy for weight loss maintenance? Physiology & Behavior. 2018 Jan 31;188:86–93. doi: 10.1016/j.physbeh.2018.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pakos-Zebrucka K., Koryga I., Mnich K., Ljujic M., Samali A., Gorman A.M. The integrated stress response. EMBO Reports. 2016 Oct;17(10):1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokota S., Ando M., Aoyama S., Nakamura K., Shibata S. Leucine restores murine hepatic triglyceride accumulation induced by a low-protein diet by suppressing autophagy and excessive endoplasmic reticulum stress. Amino Acids. 2016 Apr;48(4):1013–1021. doi: 10.1007/s00726-015-2149-0. [DOI] [PubMed] [Google Scholar]
- 41.Xu X., Krumm C., So J.S., Bare C.J., Holman C., Gromada J. Preemptive activation of the integrated stress response protects mice from diet-induced obesity and insulin resistance via fibroblast growth factor 21 induction. Hepatology. 2018 Apr 26 doi: 10.1002/hep.30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabol R., Petersen K.F., Dufour S., Flannery C., Shulman G.I. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proceedings of the National Academy of Sciences of the United States of America. 2011 Aug 16;108(33):13705–13709. doi: 10.1073/pnas.1110105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henriksson J. Effect of exercise on amino acid concentrations in skeletal muscle and plasma. Journal of Experimental Biology. 1991 Oct;160:149–165. doi: 10.1242/jeb.160.1.149. [DOI] [PubMed] [Google Scholar]
- 44.Bujak A.L., Crane J.D., Lally J.S., Ford R.J., Kang S.J., Rebalka I.A. AMPK activation of muscle autophagy prevents fasting-induced hypoglycemia and myopathy during aging. Cell Metabolism. 2015 Jun 2;21(6):883–890. doi: 10.1016/j.cmet.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H., Wu G., Fang Q., Zhang M., Hui X., Sheng B. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nature Communications. 2018 Jan 18;9(1):272. doi: 10.1038/s41467-017-02677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lowry O.H., Passonneau J.V. Academic; New York: 1972. A flexible system of enzymatic analysis. [Google Scholar]