Abstract
Context
Most heritable causes of low bone mass in children occur due to mutations affecting type 1 collagen. We describe two related patients with low bone mass and fracture without mutations in the type 1 collagen genes.
Case description
We describe the index case of a 10-year-old girl with low-impact fractures in childhood and her 59-year-old father with traumatic fractures in adulthood, both with low bone mineral density. They were found to have the same heterozygous missense mutation in the WNT1 gene (p.Gly222Arg), occurring in a highly conserved WNT motif in close proximity to the Frizzled binding site.
Conclusions
The WNT-ligand WNT1, signaling through the canonical WNT-βcatenin pathway, plays a critical role in skeletal development, adult skeletal homeostasis, and bone remodeling. Biallelic mutations have been described and are associated with moderate to severe osteogenesis imperfecta, in some cases with extra-skeletal manifestations. Patients with monoallelic mutations, as in our case, seem to present with low bone mineral density and less severe disease. The phenotypic difference between biallelic and monoallelic mutations highlights that the aberrant protein in monoallelic mutations may exert a dominant negative effect on the wild type protein as heterozygous carriers in families with biallelic disease are usually asymptomatic. With better understanding of disorders associated with WNT1 mutations, therapies targeting this signaling pathway may offer therapeutic benefit.
Keywords: Osteopenia, fracture, Monoallelic mutation, Biallelic mutation, WNT1 mutation
1. Introduction
The most frequently encountered heritable disorder of low bone mass in children is osteogenesis imperfecta (OI) (Makitie et al., 2016). OI types I-IV occur due to mutations in the COL1A1 or COL1A2 gene, affecting type 1 collagen and altering matrix structure, resulting in low bone mass and fragility fracture. In this report, we describe the index case of a 10-year-old girl with osteoporosis without mutations in the type 1 collagen genes as well as findings in her 59-year-old father.
2. Case report
A 10-year-old girl presented with a history of multiple fractures over the previous year. Her gestation and delivery were uncomplicated, and she reached developmental milestones normally. At age 9, she sustained a right metatarsal fracture resulting from direct trauma to her foot. Five months later, she sustained a right distal radius fracture after falling on her outstretched hand. Two weeks prior to evaluation, she experienced sudden back spasm while brushing her hair, followed by acute back pain. She was evaluated in an emergency department and found to have marked osteopenia of her vertebral bodies, but no fracture. When seen at Yale, she reported a two-year history of back pain, exacerbated by jumping. Her diet contained approximately 400 mg/day of calcium. At initial evaluation, her height was at the 85th percentile and weight at the 61st percentile. She appeared healthy, was premenarchal, was wearing a back brace, and using a wheelchair. Physical examination revealed normal strength and normal range of motion at all joints. Her sclerae were white and her dentition was normal. The initial laboratory evaluation is summarized in Table 1 and was unremarkable except for an extremely low L-spine BMD.
Table 1.
Laboratory and DXA evaluation.
| Initial Evaluation | Reference Range | Post-Treatment | ||
|---|---|---|---|---|
| Proband | Calcium | 10.2 mg/dL | 8.8–10.2 | 9.3 mg/dL |
| Phosphorus | 4.2 mg/dL | 3.5–5.6 | 4.3 mg/dL | |
| Alkaline Phosphatase | 177 U/L | 50–480 | 219 U/L | |
| Collage Type I C-Telopeptide | 934 pg/mL | 519–2415 | 957 pg/mL | |
| Parathyroid hormone, (mid molecule assay) | 9 nLEq/mL | 10–25 | 26 nL Eq/mL | |
| 25-Hydroxy Vitamin D | 31 ng/mL | 20–50 | 26 ng/mL | |
| L-spine, BMD (by DXA) | 0.349 g/cm2 | 0.489 g/cm2 | ||
| L-spine, Z-score (by DXA) | −4.7 | −2.9 | ||
| Father | L-spine, BMD (by DXA) | 0.853 g/cm2 | ||
| L-spine, T-score (by DXA) | −2.2 |
Laboratory and DXA results of the proband and her father at initial evaluation and post-treatment.
Whole exome sequencing revealed a heterozygous mutation (p.Gly222Arg) in the WNT1 gene. She was treated with zoledronic acid infusions 0.0125 mg/kg every 3 months for two doses, then 0.05 mg/kg every 6 months thereafter. A dietary Ca of 1200 mg/d was recommended. She enrolled in an intensive physical therapy program with marked improvement, progressing to ambulation with walker. After 1 year of zoledronic acid infusions, she was able run and participate in physical education. She gained approximately 5 cm in height during the first year of treatment. She did not reach menarche while on zoledronic acid infusions. Bone mineral density, although low for age, improved considerably over the year (Table 1).
Subsequent evaluation of the patient's 59-year old father revealed the same WNT1 mutation. He had sustained multiple finger fractures as a child and bilateral hand fractures while playing basketball in college. On initial evaluation, he was well-appearing, his vital signs were normal and his height was 6′3″, weight 208 pounds, BMI 26 kg/m2. Physical examination was unremarkable with normal arm span, joints, and virilization. Sclerae were white and his dentition was normal. An extensive evaluation for secondary causes of bone loss was unrevealing. Further evaluation revealed low bone mineral density in the lumbar spine and normal spinal radiographs. Since he had no recent fractures, anti-osteoporotic therapy was not initiated.
3. Discussion
Loss of function mutations in the WNT-ligand, WNT1, have been associated with a spectrum of skeletal dysplasias (Laine et al., 2013). The WNT gene family encodes signaling proteins that act in several crucial developmental and regenerative processes (Baron and Kneissel, 2013). The canonical WNT–βcatenin signaling pathway is required for skeletal development, homeostasis, and remodeling. This pathway is activated by interaction of a WNT ligand with the transmembrane receptor Frizzled, and a co-receptor, LDL-receptor related protein (LRP). Ligand binding leads to accumulation of intracellular β-catenin, which translocates to the nucleus where, in association with transcription factor 4 (TCF4) or lymphoid enhancer binding factor 1 (LEF1), it regulates a wide array of genes in a tissue-specific manner, and in bone, leads to increased formation and reduced resorption (van Amerongen et al., 2008).
Initial reports of OI secondary to WNT1 mutations were associated with an autosomal recessive inheritance pattern (Laine et al., 2013; Keupp et al., 2013; Pyott et al., 2013; Fahiminiya et al., 2013; Aldinger et al., 2016; Faqeih et al., 2013; Won et al., 2017; Stephen et al., 2015). Phenotypically, they manifested with moderate to severe OI (Table 2). Several patients also developed extra-skeletal manifestations, including neurologic and developmental abnormalities. A particularly severe phenotype was described in 2 sisters of a Lao Hmong family with severe osteopenia, fractures in utero, and long bone deformities (Laine et al., 2013).
Table 2.
Summary of WNT1 mutations.
| Reference | Gene mutation | Phenotype | |
|---|---|---|---|
| Monoallelic mutations | Proband and her father | c.666G>A(p.Gly222Arg) |
|
| Keupp et al. (2013) | c.703C > T (p.Arg235Trp) |
|
|
| Laine et al. (2013) | c.652 T > G (p.Cys218Gly) |
|
|
| Compound mutations | Fahiminiya et al. (2013) | c.946_949insAACA (p.Ser317Lysfs) and c.1063G > T (p.Val355Phe) |
|
| Pyott et al. (2013) | c.506dupG (p.Cys170Leufs*6) and c.259C > T (p.Gln87*) |
|
|
| Aldinger Et al (2016) | c.184C > T (p.Gln62*) and c.677C > T (p.Ser226Leu) |
|
|
| Yeon Won et al. (2017) | c.369A > C (p.Glu123Asp) and c.457 T > G (p.Cys153Gly) |
|
|
| Biallelic Mutations | Keupp et al. (2013) | c.859dupC (p.His287Profs*30) |
|
| c.529G > T (p.Gly177Cys) |
|
||
| c.624 + 4A > G |
|
||
| c.565G > T (p.Glu189*) |
|
||
| c.893 T > G (p.Phe298Cys) |
|
||
| Pyott et al. (2013) | c.893 T > G (Phe298Cys) |
|
|
| c.884C > A (p.Ser295*) |
|
||
| c.287_300delAGTTCCGGAATCGC (p.Gln96Profs*54) |
|
||
| Laine et al. (2013) | c.884C > A (p.Ser295*) |
|
|
| Fahiminiya et al. (2013) | c.428G > T (p.Cys143Phe) |
|
|
| c.287_300del (p.Gln96Profs) |
|
||
| Faqeih et al. (2013) | c.990C > A (p.Cys330*) |
|
|
| Stephen et al. (2014) | c.525_536delCTTCGGCCGCCT (p.Phe176_Leu179del) |
|
Gene mutation and phenotypic correlation of reported monoallelic, compound and biallelic WNT1 mutations.
Autosomal dominant mutations have also been described (Laine et al., 2013; Keupp et al., 2013). Affected individuals have presented with fragility fractures and low bone density without extra-skeletal features, as in the family described here. This case report highlights the phenotypic spectrum of a heterozygous missense mutation in the WNT1 gene, in that the daughter had low-impact fractures in early childhood, whereas the father sustained traumatic fractures primarily in young adulthood.
The differences in phenotypic expression of autosomal recessive and autosomal dominant WNT1 mutations points to a potential difference in disease pathogenesis for these two types of mutations. One possibility is that heterozygous missense mutations encode an aberrant protein that interferes with signaling by the wild type protein. Although much more severe phenotypes generally occur with biallelic mutations, there is usually no phenotype associated with heterozygous carriers in families with biallelic disease due to null mutations. This suggests that the product of only one wild type allele is sufficient for normal function, and that the mutation in our case is consistent with a dominant negative effect.
A heterozygous missense mutation in WNT1 previously described in a Finnish family (p.Cys218Gly) is in close proximity to the mutation in our case (Laine et al., 2013). Both mutations occur within the highly conserved WNT1 motif at residues 218–227 of the protein (C-[KR]-C-H-G-[LIVMT]-SG-x-C) (Fig. 1) (Laine et al., 2013). Four of the ten affected members of the Finnish family had lumbar spine z-scores of −2.0 or less. The majority also had multiple vertebral fractures, low-impact peripheral fractures, and no extra-skeletal manifestations. A second family has also been described with dominantly inherited osteoporosis due to a heterozygous mutation in close proximity to the WNT motif (p.Arg235Trp) (Keupp et al., 2013). Affected members of this family had cortical and trabecular bone loss on high-resolution peripheral quantitative CT and history of recurrent fractures.
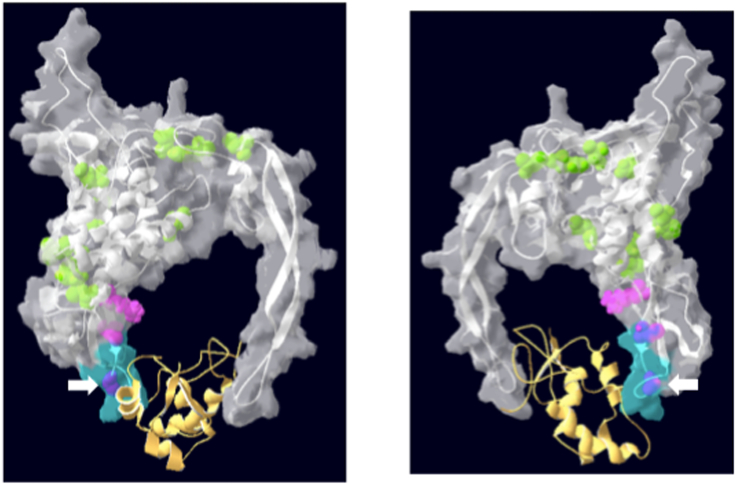
Fig. 1.
Diagram of reported monoallelic and biallelic WNT1 mutations.
Ribbon and space-filling diagram of the WNT1 molecule, depicted as a white ribbon in the grey space-filling model. WNT1 is engaged with Frizzled (yellow ribbon). The two views represent 180° rotations along a vertical axis. The conserved WNT domain (amino acids 218–227) is depicted in blue. The positions of bi-allelic mutations are depicted in green. Pink and purple represent the positions of known mono-allelic mutations (purple designates mutations within the conserved WNT domain, as detailed in Table 2). The white arrow points to the mono-allelic mutation described in the current report. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Given the emerging understanding of bone disorders associated with mutations in WNT1, therapies specifically targeting this signaling pathway offer potential therapeutic benefit. Clinical trials with a humanized anti-sclerostin antibody which activates WNT signaling have shown beneficial effects on bone mineral density as well as significant fracture risk reduction in patients with osteoporosis (Krause et al., 2010; Cosman et al., 2016). Other agents that enhance Wnt signaling, such as an anti-Dkk-1 antibody and SFRP inhibitors, continue to to be evaluated for clinical efficacy (Kim et al., 2013).
In summary, we identified two family members with low bone mass and fractures associated with a heterozygous missense mutation in the WNT1 gene. This specific mutation (p.Gly222Arg) has not been previously described, but occurs in a highly conserved region of the WNT1 molecule and is in close proximity to the Frizzled binding site.
Funding source
R01 GM112182 (Dan Wu, PhD).
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Conflict of interest
The authors have no conflicts of interest to disclose.
Transparency document
Transparency document.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Aldinger K.A., Mendelsohn N.J., Chung B.H. Variable brain phenotype primarily affects the brainstem and cerebellum in patients with osteogenesis imperfecta caused by recessive WNT1 mutations. J. Med. Genet. 2016;53(6):427–430. doi: 10.1136/jmedgenet-2015-103476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R., Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 2013;19(2):179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- Cosman F., Crittenden D.B., Adachi J.D. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016;375(16):1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- Fahiminiya S., Majewski J., Mort J., Moffatt P., Glorieux F.H., Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J. Med. Genet. 2013;50(5):345–348. doi: 10.1136/jmedgenet-2013-101567. [DOI] [PubMed] [Google Scholar]
- Faqeih E., Shaheen R., Alkuraya F.S. WNT1 mutation with recessive osteogenesis imperfecta and profound neurological phenotype. J. Med. Genet. 2013;50(7):491–492. doi: 10.1136/jmedgenet-2013-101750. [DOI] [PubMed] [Google Scholar]
- Keupp K., Beleggia F., Kayserili H. Mutations in WNT1 cause different forms of bone fragility. Am. J. Hum. Genet. 2013;92(4):565–574. doi: 10.1016/j.ajhg.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Liu X., Wang J. Wnt signaling in bone formation and its therapeutic potential for bone diseases. Ther. Adv. Musculoskelet. Dis. 2013;5(1):13–31. doi: 10.1177/1759720X12466608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause C., Korchynskyi O., de Rooij K. Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J. Biol. Chem. 2010;285(53):41614–41626. doi: 10.1074/jbc.M110.153890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine C.M., Joeng K.S., Campeau P.M. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. N. Engl. J. Med. 2013;368(19):1809–1816. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makitie R.E., Haanpaa M., Valta H. Skeletal characteristics of WNT1 osteoporosis in children and young adults. J. Bone Miner. Res. 2016;31(9):1734–1742. doi: 10.1002/jbmr.2841. [DOI] [PubMed] [Google Scholar]
- Pyott S.M., Tran T.T., Leistritz D.F. WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. Am. J. Hum. Genet. 2013;92(4):590–597. doi: 10.1016/j.ajhg.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen J., Girisha K.M., Dalal A. Mutations in patients with osteogenesis imperfecta from consanguineous Indian families. Eur. J. Med. Genet. 2015;58(1):21–27. doi: 10.1016/j.ejmg.2014.10.001. [DOI] [PubMed] [Google Scholar]
- van Amerongen R., Mikels A., Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci. Signal. 2008;1(35):re9. doi: 10.1126/scisignal.135re9. [DOI] [PubMed] [Google Scholar]
- Won J.Y., Jang W.Y., Lee H.R. Novel missense loss-of-function mutations of WNT1 in an autosomal recessive osteogenesis imperfecta patient. Eur. J. Med. Genet. 2017;60(8):411–415. doi: 10.1016/j.ejmg.2017.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.