Abstract
There are currently one million heart failure (HF) patients in France and the rate is progressively increases due to population aging. Acute decompensation of HF is the leading cause of hospitalization in people over 65 years of age with a 25% re-hospitalization rate in the first month. Expenses related to the management of HF in France in 2013 amounted to more than one billion euros, of which 65% were for hospitalizations alone. The management of acute decompensation is a challenge, due to the complexity of clinical and laboratory evaluation leading to therapeutic errors, which in turn leads to longer hospitalization, high early re-hospitalization and complications. Therapeutic adjustment, especially diuretic, in the acute phase (during hospitalization) affects early re-hospitalization rates (within 30 days). These adjustments can be based on clinical estimation and laboratory parameters, but echocardiography has been shown to be superior in estimating filling pressures (FP) compared to clinical and laboratory parameters.
We hypothesize that a simple daily bedside echocardiographic assessment could provide a reproducible estimation of FP with an evaluation of mitral inflow and the inferior vena cava (IVC). This could allow a more reliable estimate of the true blood volume of the patient and thus lead to a more suitable therapeutic adjustment. This in turn should lead to a decrease in early re-admission rate (primary endpoint) and potentially decrease six-month mortality and rate of complications.
Keywords: Heart failure, Doppler echocardiography, Diuretics, Filling pressure, Cava venous, Trans-mitral doppler, Left ventricular ejection fraction
Abbreviations
- DE
Doppler echocardiography
- HF
Heart Failure
- GWTG-HF
Get with the guidelines heart failure
- LVEDP
Left ventricular end diastolic pressure
- IVC
Inferior vena cava
- Nt-ProBNP
N-terminal pro b-type natriuretic peptide
- FP
Filling pressures
- RAP
Right atrial pressure
- RV
Right ventricular
- LV
Left ventricular
- BESPIM
Department of biostatistics, epidemiology, public health and innovation in methodology
- NUH
Nîmes university hospital
- CPP
Commitey for the protection of people
1. Introduction
Heart failure (HF) results in functional limitations, reduced quality of life and increased mortality [1]. Furthermore, acute decompensation HF is the leading cause of hospitalization in people over 65 years and has a 2% re-hospitalization rate in the early months [2]. It poses a high economic burden; up to 2–3% of health-care system expenses in high-income countries are incurred by patients with HF [3]. The prevalence in France alone is estimated at 1–2 million cases, with HF management-related [2] expenses amounting to 1.6 billion euros as of 2013, of which 65% accounts for hospitalizations alone [3,4].
HF management remains a challenge due to the complexity of clinical and laboratory evaluation. Most readmissions within 30 days post-discharge for HF events are due to HF itself, suggesting either incomplete treatment before discharge or lack of adequate discharge planning [5,6]. Readmission consequences include increased economic burden and increased morbidity or mortality [7]. Therefore, it is crucial to prevent readmissions.
Most clinical trials performed in the past decade focusing on interventions in patients with acute decompensated HF have failed to demonstrate an impact on outcomes. Parameters measured in these past trials include dyspnea or functional capacity improvement, although these aspects are difficult to demonstrate [8]. Furthermore, clinical congestion at discharge is associated with worse early post-discharge outcomes. Post hoc analysis of trials such as the DOSE-AHF and CARRESS-HF have shown that a simple clinically determined score (orthodema score, which includes the severity of orthopnea and peripheral edema) identifies patients with high risk of 60-day mortality, re-hospitalization, or unscheduled visits [9].
In order to prevent readmission, prehospitalization measures evaluated included patient education and adherence to therapy or the evaluation of signs of congestion. Congestion can be evaluated by lung impedance or left ventricular end diastolic pressure (LVEDP) in order to detect HF decompensation before onset of clinical symptoms [[10], [11], [12]]. During hospitalization, physical exam signs, plasma electrolyte/biomarker values such as N-terminal pro b-type natriuretic peptide (proNT-BNP), and radiographic studies [13,14] were used to measure treatment efficacy. Clinical evaluation of the intravascular volume status is often difficult in acute settings, especially in patients with obesity, chronic venous stasis, renal dysfunction, and intrinsic pulmonary diseases [15]. The clinical assessment is neither specific nor sensitive. Above all, renal failure affects nearly 40% of patients with heart failure, but renal failure could also be due to heart failure and inversely, leading to the cardiorenal syndrome. Hence, in case of a cardio-renal syndrome, it would be often justified to increase the diuretic treatment, which is counter-intuitive. On the other hand, intrinsic renal impairment could require a therapeutic adjustment (such as discontinuation of diuretic and nephrotoxic therapy, and, in some cases, hydration of the patient) [16,17].
Diuretics are the cornerstone of treating symptoms of patients with decompensated HF. When stabilized, the optimization of medical treatment with beta blockers, angiotensin converting enzyme inhibitor or angiotensin receptor blockers, mineralocorticoids inhibitors, ivabradine and Sacubitril-valsartan has proved to reduce mortality and morbidity for patients with reduced ejection fraction [18] with the lowest possible dose of diuretics.
Until recently, assessing hemodynamic status in acutely and critically ill patients in order to guide fluid/diuretic/inotropic therapies was performed by pulmonary artery catheter, but possible complication and concerns about effectiveness have limited its use [19]. Instead, quantitative Doppler echography (DE) measurements for hemodynamic parameter have been shown to be accurate and comparable to those obtained by pulmonary artery catheter [20,21].
Echocardiography has been shown to be superior to clinical or biological evaluation alone in estimating filling pressures (FP) [22,23]. DE quantifies intracardiac/intrapulmonary pressures mainly based on inferior vena cava (IVC) diameter and percentage of collapse during respiration, valvular regurgitant jet peak velocities, mitral inflow and pulmonary arterial/venous flow patterns, and interventricular/interatrial shunt flow velocities. Right atrial pressure (RAP), right ventricular (RV) systolic and diastolic FP, systolic and mean pulmonary arterial pressures, left atrial pressure, left ventricular (LV) systolic and diastolic FP can then be estimated from these measurements [22]. This assessment is an easily available, easily performed, portable, bedside tool to instantaneously monitor changing hemodynamic status, and therapeutic response during HF management [[23], [24], [25], [26]].
We hypothesize that a daily, simple and rapid echocardiographic evaluation at the patient's bedside in order to measure FP through mitral flow and the IVC evaluation (by DE) would allow a more reliable estimation of pressure volume and thus lead to a personalized tailored treatment, resulting in a decreased 30-day re-hospitalization rate and potential morbidities and mortality.
2. Methods
2.1. Design
2.1.1. Aim of the study
We aim to assess whether a daily, rapid echocardiographic evaluation, performed with DE according to a standard protocol, at the patient's bedside would prevent early readmissions.
2.1.2. Study design and setting
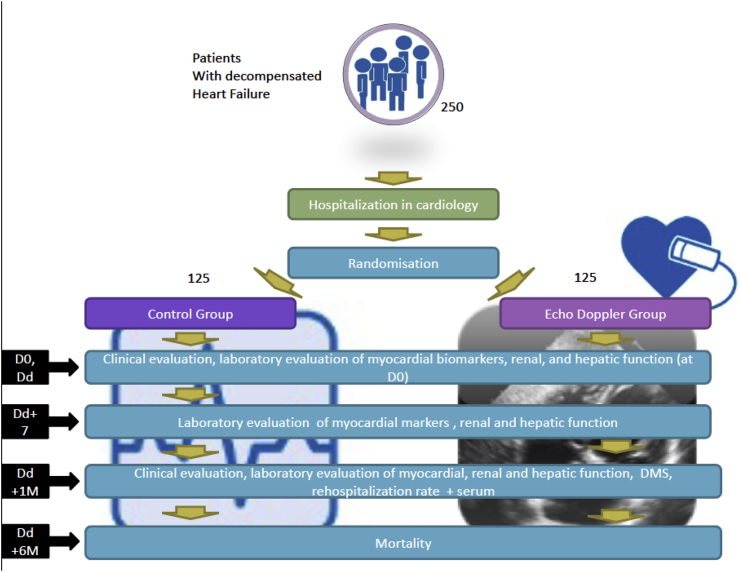
Protocol version 2.0, date 17 July 2017. JECICA will be a bicentric (conducted in Montpellier and Nîmes University Hospitals) prospective study evaluating a randomized open-label treatment strategy with two parallel groups: a group with a DE follow-up during the entire period of hospitalization versus a group without DE follow-up. Patients will be randomized to either study arm in a 1:1 ratio. Randomization lists consisting of centralized randomly-sized blocks will be established per centre. These lists are the responsibility of an independent methodologist at the Department of Biostatistics, Epidemiology, Public Health and Innovation in Methodology (BESPIM). A specifically designed SAS program (Cary, NC, USA) will be used to carry out randomization. The number of subjects per block will be known only to the methodologist.
Groups will be matched by age and severity of comorbidities in order to minimize potential bias. Patients will be cared for in the participating cardiology departments and benefit from a complete clinical and laboratory assessment. An ultrasound will be performed in accordance with standard care in order to confirm the diagnosis, measure baseline LVEF, and elevation of FP, and assess for patients presenting with possible exclusion criteria will be performed. Completion of this first ultrasound examination will condition each patient's entrance into the study (Fig. 1).
Fig. 1.
Study design of the JECICA Trial.
2.2. Characteristics of participants
Adult patients (>18 year of age) hospitalized for acute HF in the cardiology departments of Nîmes and Montpellier who received at least 40 mg IV furosemide, had an LVEF functioning of <50% and an NT-ProBNP value of >1200 pg/ml were eligible for inclusion in the study. Participants are required to be an affiliate or beneficiary of a health insurance plan and available for six-month follow-up and give (or have representative give) free and informed consent with signed consent forms being on file; those lacking these criteria will be excluded from the study. Exclusion criteria include patients participating in another study, pregnant or breastfeeding, those with a history of mechanical or biological mitral prosthesis, mitral stenosis, severe valvulopathy with surgical expiration within one mouth (<30 day), chronic renal failure requiring dialysis, high degree AV block (2nd degree AVB and 3rd AVB), hypertrophic cardiomyopathy, cardiogenic shock, contraindication to furosemide, or those for whom echocardiography is unable to be performed.
3. Intervention
Following application of inclusion and exclusion criteria, the patients are randomized into one of two groups (intervention group vs. control group). Groups will be matched for age and comorbidities.
3.1. Intervention group
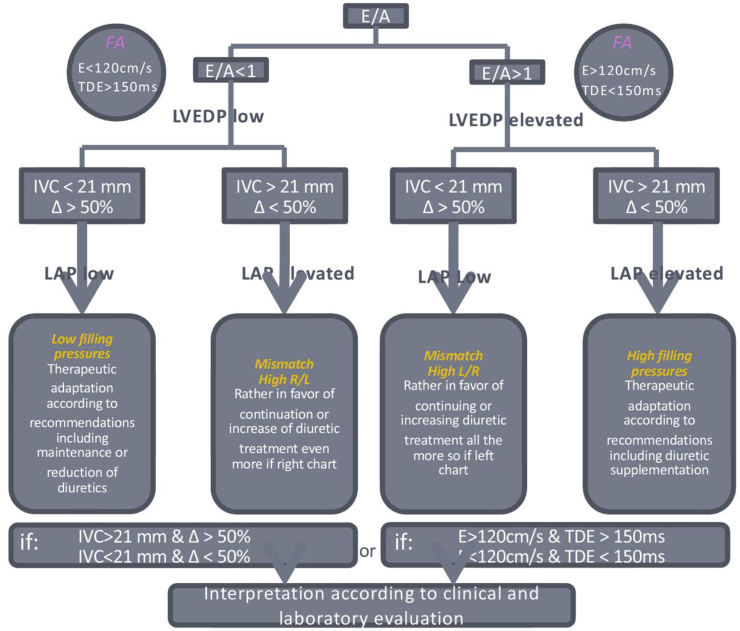
Standard clinical and laboratory evaluations will be performed, in addition to a daily bedside echocardiographic evaluation, by the attending physician, during the patient's daily examination for the duration of the hospitalization. This additional echocardiographic evaluation will assess the transmitral flow and IVC flow and variations. The therapeutic adaptation, including precise dose adjustment, for each patient will be done according to the logical algorithm shown in Fig. 2 following evaluation of each echocardiographic evaluation. These echocardiographic evaluations will allow tailoring of doses to optimize patient care. The objective goal (regardless of group variability) is to use DE to guide treatment in order to reach normo-volemia state for each patient.
Fig. 2.
Algorithm of JECICA trail treatment in the DE arm.
3.2. Control group
Clinical and laboratory evaluation as per standard protocols will be performed and diuretic treatment will be administered as in accordance with the ESC recommendations normo-volemia state. Daily echocardiographic evaluation will not be performed; ultrasound will be performed only upon initiation into the study and during hospitalization when warranted by clinical condition; instances where this is done will be indicated in reports.
4. Follow up
The following visits will be made:
-
-
Inclusion visit (V0)
-
-
Follow-up visit at the end of hospitalization (Dd)
-
-
laboratory evaluation at Dd+7 days.
-
-
Follow-up visit to Dd+30 days.
-
-
The end-of-study telephone call at six months.
Table 1 summarizes the planned interventions throughout the follow-up period.
Table 1.
The planned visits, their chronology, and the acts, examinations, associated samples.
| Visit | Inclusion Visit | Follow up | End of study | ||
|---|---|---|---|---|---|
| Date | D0 | Day of discharge (Dd) | Dd+7 | Dd+30 | Dd+6Mo |
| Physical exam | X | X | X | ||
| Consent form | X | ||||
| Randomization | X | ||||
| Discharge from the hospital | X | ||||
| Blood cells count | X | X | |||
| Nt-ProBNP and troponin levels | X | X | X | X | |
| Renal Function | X | X | X | X | |
| Hospital readmission | X | ||||
| Mortality collection | X | X | |||
| Biological samples collection | X | X | |||
| Adverse events | X | X | X | ||
5. Outcomes
Clinical outcomes will be measured through evaluation of the rate of re-hospitalization at 30 days post-discharge. Failure of the intervention will be declared in case of re-hospitalization in the first 30 days following discharge for one of the following causes:
-
a.
HF decompensation
-
b.
Syncope, malaise, orthostatic hypotension
-
c.
Rhythm disorder (atrial fibrillation flutter, ventricular tachycardia, torsade de pointe) or conduction disorder;
-
d.
Acute coronary syndrome (ACS)
-
e.
Acute renal failure, dehydration or severe electrolyte disorders (hyper/hyponatremia, hyper/hypokalemia).
Evaluation of the failure of the initial therapeutic adjustment in cases of non-rehospitalization between discharge and 30 days post-discharge will be reported by comparison of laboratory values at Dd, Dd+7 and Dd+30 days. For hypervolemic patients, a NT-ProBNP assay will be performed to quantify patient status. For hypovolemic patients, creatinine, urea, sodium and serum potassium will be assessed to quantify status. In the case of outpatient management of therapeutic adjustment, the date of treatment adjustment will indicate the time of initial treatment failure.
Other factors evaluated and reported include six-month mortality, exacerbation of cardiac insufficiency during hospitalization (visual scale of dyspnea from 0 to 10, implementation of oxygen therapy, diuretic treatment or use of nitrates after the first 24 h of hospitalization), duration of hospital stay, evaluation of myocardial (troponin), renal function (GFR, creatinine, urea), liver (AST, ALT, bilirubin) biomarkers during hospitalization at Dd, Dd+7, Dd+30 and laboratory values.
6. Sample size
The 30-day re-hospitalization rate after acute HF management is estimated to be about 25% [3,4]. Furthermore, no comparable study in the literature to estimate the decrease in the number of hospitalizations by a rapid echocardiography technique daily to the bedside (“DE group”) exists, making it difficult to estimate the expected risk education due to this intervention. However, a significant risk reduction is expected due to this study being a strategy study with multiple therapeutic consequences.
We hypothesize a 15% decrease in 30-day re-hospitalization rate in the DE group.
With an alpha risk fixed at 5% a power fixed at 85% in bilateral situation and a drop-out rate at 10% we estimated the sample size at 250 subjects (125 per group).
7. Data management
Data management and statistical analysis is provided by the BESPIM. Data management will be performed in line with ICH requirements. The related documents will be stored on the BESPIM server.
eCRF fields will be formatted so as to enforce homogenous value types and require confirmation especially for out-of-expected-range values. All modifications will be fully traceable (who, when, why?) to allow a complete audit trail.
8. Data and safety monitoring board
Due to the low level of risk added by this research and the lack of interim analyses, a DMC will not be formed.
9. Harms
Serious adverse event reporting will be carried out by the vigilance department of the Nîmes University Hospital (NUH). In accordance with articles L.1123-10, R.1123-42 and R.1123-48 of the French Public Health Code, any suspicion of an unexpected serious adverse event et/or new fact will be transmitted by the NUH to the competent authority, the appropriate commitey for the protection of people (CPP) and if applicable to the other investigators within the legal deadlines.
10. Statistical analysis
An initial analysis of the data will allow the description of the study population. The normality of the distribution of quantitative variables will be explored using the Shapiro-Wilks normality test, as well as Kurtosis and Skewness coefficients.
Results will be presented as means ± standard deviations for quantitative variables with Gaussian distribution, means and 95% confidence intervals (CI) for anti-trans formants (variables whose distribution is Gaussian after transformation), and medians and interquartile range (IQR) for variables with skewed distributions. Qualitative variables will be compared by a Chi-2 test or Fisher's exact test. Gaussian variables will be analyzed by student t-test or analysis of variance. Non-Gaussian variables will be analyzed by Wilcoxon-Mann-Whitney test.
Primary endpoint analysis will include both static and dynamic analyses. The static analysis includes each group's 30-day re-hospitalization rate and will be compared by percentage comparison tests. The dynamic analysis will be performed by Kaplan Meier test within 30 days. These rates will be compared by percentage comparison test, a comparison of the curves constituted by a log rank test.
Each secondary endpoint will be evaluated independently. For the evaluation of the failure of the initial therapeutic adjustments in case of non-re-hospitalization between Dd and Dd+30 days, an analysis identical to that of the main endpoint will be carried out for the intermediate laboratory criteria and for the slope taken into account. An intergroup comparison at each measurement time will be performed using a mean comparison test. Six-month mortality rates in each group will be calculated and rates will be compared by a percentage comparison test.
For the DMS and visual dyspnea scale an intergroup comparison will be performed using a mean comparison test.
For patients lost to follow-up, for whom complete data sets are unattainable, the data existing while still participating in the study will be used. For these patients, secondary endpoints will be evaluated. Regarding cost data, if the patient is lost to follow-up, we will use a regression model for censored data so as not to lose the information at the beginning of the trajectory.
A difference will be considered statistically significant when the significance of the test is less than or equal to 0.05.
The decision to modify the statistical methods provided for in this protocol is made by the methodologist as described above. A change in the statistical method planned for the analysis of the main criterion must be subject to amendment of the protocol. Any changes in the statistical methods planned for the analysis of the secondary criteria will be reported in the study report.
11. Funding
All of the expenses related to the study will be covered by the 2015 Internal Call for Tenders by GCS MERRI Montpellier – Nîmes (Dr RICCI).
12. Ethical consideration
The protocol of this trial was approved by the French institutional review board CPP SUD MEDITERRANEE III on July 11, 2016. The trial protocol was registered at clinicaltrials. gov (NCT02892227) on September 8, 2016. All patients enrolled in the study will have signed informed consent.
The authors do not declare any conflict of interest in relation with this work.
12.1. Roles and responsibilities
The trial sponsor is the CHU Nimes.
The sponsor is not involved in the conception and the management of the study.
The protocol is public (registered online).
The results will be submitted for publication in a scientific journal.
13. Discussion
Most management plans intending to reduce the acute HF related re-hospitalization rates focus on post-hospitalization monitoring and education. However, these approaches are not efficient enough. The main aim of the JECICA trial is to evaluate if a daily, protocolized, simple and rapid echocardiographic evaluation could allow better understanding of the hemodynamic and congestion state. This will allow for a more complete therapeutic management of patients during their hospital stay thereby leading to a reduction in re-hospitalization rates. To our knowledge, this is the first randomized trial to evaluate this treatment plan and its effects in HF patients.
The deployment of portable echocardiographs, the training and development of the ultrasound activity to many other specialties demonstrate the growing interest of doctors for this tool, a real extension of the clinical examination [[24], [25], [26]]. If the JECICA trial is positive, its usefulness outside of the cardiology ward in non-specialized wards would be of great interest, providing a complementary tool for the evaluation of patients with HF, to improve management and outcomes.
14. Limitations
The trial is bicentric and there could be some minor differences in the management of the patients. The physicians are urged to follow the ECS recommendations for treatment. To limit this, a flowchart was provided to ensure optimal treatment.
15. Trial status
Recruiting since November 15, 2016.
Acknowledgments
Acknowledgments to Chloe LOUCHE, Elodie DELELO and Sonia SOLTANI for their excellent data monitoring and to Sarah KABANI for editorial assistance.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.conctc.2018.07.006.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Farmakis D., Parissis J., Lekakis J. Acute heart failure: Epidemiology, risk factors, and prevention. Rev. Esp. Cardiol. 2015 Mar;68(3):245–248. doi: 10.1016/j.rec.2014.11.004. PubMed PMID: 25659507; eng. [DOI] [PubMed] [Google Scholar]
- 2.Tuppin P., Cuerq A., de Peretti C. Two-year outcome of patients after a first hospitalization for heart failure: a national observational study. Arch Cardiovasc. Dis. 2014 Mar;107(3):158–168. doi: 10.1016/j.acvd.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Cook C., Cole G., Asaria P. The annual global economic burden of heart failure. Int. J. Cardiol. 2014 Feb 15;171(3):368–376. doi: 10.1016/j.ijcard.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Ross J.S., Chen J., Lin Z. Recent national trends in readmission rates after heart failure hospitalization. Circ. Heart Fail. 2010 Jan;3(1):97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. PubMed Central PMCID: PMCPMC2830811. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feltner C., Jones C.D., Cene C.W. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann. Intern. Med. 2014 Jun 3;160(11):774–784. doi: 10.7326/M14-0083. [DOI] [PubMed] [Google Scholar]
- 6.Maggioni A.P. Epidemiology of heart failure in Europe. Heart Fail. Clin. 2015 Oct;11(4):625–635. doi: 10.1016/j.hfc.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Bello N.A., Claggett B., Desai A.S. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ. Heart Fail. 2014 Jul;7(4):590–595. doi: 10.1161/CIRCHEARTFAILURE.113.001281. PubMed PMID: 24874200; PubMed Central PMCID: PMCPMC4102617. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavine K.J., Mann D.L. Rethinking phase II clinical trial design in heart failure. Clin. Invest. 2013 Jan 1;3(1):57–68. doi: 10.4155/cli.12.133. PubMed Central PMCID: PMCPMC4204480. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lala A., McNulty S.E., Mentz R.J. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from diuretic optimization strategy evaluation in acute decompensated heart failure (DOSE-AHF) and cardiorenal rescue study in acute decompensated heart failure (CARESS-HF) Circ. Heart Fail. 2015 Jul;8(4):741–748. doi: 10.1161/CIRCHEARTFAILURE.114.001957. PubMed Central PMCID: PMCPMC4512849. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidenreich P.A., Hernandez A.F., Yancy C.W. Get with the Guidelines program participation, process of care, and outcome for Medicare patients hospitalized with heart failure. Circ. Cardiovasc. Qual. Outcomes. 2012 Jan;5(1):37–43. doi: 10.1161/CIRCOUTCOMES.110.959122. PubMed PMID: 22235067; eng. [DOI] [PubMed] [Google Scholar]
- 11.Gorthi J., Hunter C.B., Mooss A.N. Reducing heart failure hospital readmissions: a systematic review of disease management programs. Cardiol. Res. 2014 Oct;5(5):126–138. doi: 10.14740/cr362w. PubMed Central PMCID: PMCPMC5358117. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamson P.B., Abraham W.T., Stevenson L.W. Pulmonary artery pressure-guided heart failure management reduces 30-day readmissions. Circ. Heart Fail. 2016 Jun;9(6) doi: 10.1161/CIRCHEARTFAILURE.115.002600. [DOI] [PubMed] [Google Scholar]
- 13.Weintraub N.L., Collins S.P., Pang P.S. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010 Nov 9;122(19):1975–1996. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 14.Parrinello G., Torres D., Paterna S. The challenge of the volume status assessment in heart failure. Am. Heart J. 2009;157:e19–20. doi: 10.1016/j.ahj.2008.12.001. United States. author reply e21. [DOI] [PubMed] [Google Scholar]
- 15.Remes J., Miettinen H., Reunanen A. Validity of clinical diagnosis of heart failure in primary health care. Eur. Heart J. 1991 Mar;12(3):315–321. doi: 10.1093/oxfordjournals.eurheartj.a059896. eng. [DOI] [PubMed] [Google Scholar]
- 16.Ronco C., Cicoira M., McCullough P.A. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J. Am. Coll. Cardiol. 2012 Sep 18;60(12):1031–1042. doi: 10.1016/j.jacc.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 17.Metra M., Cotter G., Gheorghiade M. The role of the kidney in heart failure. Eur. Heart J. 2012 Sep;33(17):2135–2142. doi: 10.1093/eurheartj/ehs205. [DOI] [PubMed] [Google Scholar]
- 18.Ponikowski P., Voors A.A., Anker S.D. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. 2016 Jul 14. [DOI] [PubMed] [Google Scholar]
- 19.Binanay C., Califf R.M., Hasselblad V. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. Jama. 2005 Oct 5;294(13):1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh S.F., Bhatt R., Vivo R.P. Echocardiographic evaluation of hemodynamics in patients with decompensated systolic heart failure. Circ. Cardiovasc. Imag. 2011 May;4(3):220–227. doi: 10.1161/CIRCIMAGING.111.963496. [DOI] [PubMed] [Google Scholar]
- 21.Galderisi M., Lancellotti P., Donal E. European multicentre validation study of the accuracy of E/e' ratio in estimating invasive left ventricular filling pressure: EURO-FILLING study. Eur. Heart J. Cardiovasc. Imag. 2014 Jul;15(7):810–816. doi: 10.1093/ehjci/jeu022. [DOI] [PubMed] [Google Scholar]
- 22.Vitarelli A., Gheorghiade M. Transthoracic and transesophageal echocardiography in the hemodynamic assessment of patients with congestive heart failure. Am. J. Cardiol. 2000 Aug 17;86(4a):36g–40g. doi: 10.1016/s0002-9149(00)00990-5. (eng) [DOI] [PubMed] [Google Scholar]
- 23.Lei J., Dhamoon A.S., Wang J. Walking the tightrope: using quantitative Doppler echocardiography to optimize ventricular filling pressures in patients hospitalized for acute heart failure. Eur. Heart J. Acute Cardiovasc. Care. 2016 Apr;5(2):130–140. doi: 10.1177/2048872615573517. [DOI] [PubMed] [Google Scholar]
- 24.Goonewardena S.N., Spencer K.T. Handcarried echocardiography to assess hemodynamics in acute decompensated heart failure. Curr. Heart Fail. Rep. 2010 Dec;7(4):219–227. doi: 10.1007/s11897-010-0030-8. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen V.T., Ho J.E., Ho C.Y. Handheld echocardiography offers rapid assessment of clinical volume status. Am. Heart J. 2008 Sep;156(3):537–542. doi: 10.1016/j.ahj.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Wiley B., Mohanty B. Handheld ultrasound and diagnosis of cardiovascular disease at the bedside. J. Am. Coll. Cardiol. 2014 Jul 15;64(2):229–230. doi: 10.1016/j.jacc.2014.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.