Highlights
-
•
The specific histamine H4 receptor agonist ST-1006 induces acute itch in mice.
-
•
Histaminergic itch increases neuronal activity in the medial habenula.
-
•
Selective H4R activation in the skin increases neuronal activity in the medial habenula.
Keywords: c-fos, Itch, Brain, Medial habenula, Histamine H4 receptor
Abstract
Background
Strategies to efficiently control itch require a deep understanding of the underlying mechanisms. Several areas in the brain involved in itch and scratching responses have been postulated, but the central mechanisms that drive pruritic responses are still unknown. Histamine is recognized as a major mediator of itch in humans, and has been the most frequently used stimulus as an experimental pruritogen for brain imaging of itch.
Objective
Histaminergic itch via histamine and the selective histamine H4 receptor (H4R) agonist, ST-1006, recruit brain nuclei through c-fos activation and activate specific areas in the brain.
Methods
An acute itch model was established in c-fos-EGFP transgenic mice using ST-1006 and histamine. Coronal brain sections were stained for c-fos immunoreactivity and the forebrain was mapped for density of c-fos + nuclei.
Results
Histamine and ST-1006 significantly increased scratching response in c-fos-EGFP mice compared to vehicle controls. Mapping c-fos immunostained brain sections revealed neuronal activity in the cortex, striatum, hypothalamus, thalamus, amygdala, and the midbrain.
Conclusions
Histaminergic itch and selective H4R activation significantly increased the density of c-fos + nuclei in the medial habenula (MHb). Thus, the MHb may be a new target to investigate and subsequently develop novel mechanism-based strategies to treat itch and possibly provide a locus for pharmacological control of pruritus.
1. Introduction
Itch is the main symptom of allergic skin diseases. Finding strategies to control itch more efficiently requires a better understanding of the underlying itch and scratch mechanisms. Histamine is recognized as a major mediator of itch in humans, and has been the most frequently used stimulus as an experimental pruritogen for brain imaging of itch (Mochizuki et al., 2014a).
Two main neuronal pathways have been described for itch transmission by Davidson and Giesler (2010): one mediated by histamine, and another by non-histaminergic mediated, such as protease activated receptor PAR2. The initial itch signal is transmitted from the periphery, to the dorsal root ganglia (DRG), and into the dorsal horn of the spinal cord.
Ascending signals are transmitted through the lateral spinothalamic tract into the brain, where several itch processing areas have been implicated: thalamic nuclei, cortex (somatosensory S1 and S2, cingulate cortex with ACC and PCC, as well as the dorsolateral prefrontal cortex DLPFC), cerebellum, supplementary motor area (SMA), precuneus, amygdala, nucleus accumbens (NAc), insula and claustrum (Davidson et al., 2009, 2012; Mochizuki and Kakigi, 2015b, a; Leknes et al., 2007; Papoiu et al., 2012). Sensation of itch evokes scratching behaviors, and the pleasant sensations transmitted by scratching may play an important role in the induction of the itch-scratch cycle (Mochizuki and Kakigi, 2015b). Itch can be inhibited through scratching when inhibitory neurons in the spinal cord are activated, as well as through top-down modulation from the supraspinal level (Akiyama et al., 2011; Davidson et al., 2009).
Descending signals include signals from areas that belong to the reward system such as the periaqueductal gray (PAG) (Mochizuki et al., 2003; Papoiu et al., 2013), NAc (Papoiu et al., 2013), and the striatum (Schneider et al., 2008; Ishiuji et al., 2009; Leknes et al., 2007).
In vivo studies in histamine H4 receptor (H4R)-deficient mice have revealed that H4R is involved in itch (Bell et al., 2004; Dunford et al., 2007; Yu et al., 2010), and first clinical studies in humans further support a role for the H4R in mediating pruritus. A selective H4R antagonist inhibited histamine-induced pruritus in healthy volunteers (Kollmeier et al., 2014) and reduced pruritus in patients with atopic dermatitis (Murata et al., 2015). Though H4R plays a part in pruritus, the mechanism remains unknown. Current data suggests that itch stimuli in the skin leads to the activation of H4R in sensory neurons, which then transmit the signal to the central nervous system (Rossbach et al., 2011; Dunford et al., 2007; Desmadryl et al., 2012). Most functional studies on central perception of itch were conducted in primates and humans with the aid of Positron Emission Tomography (PET) and functional Magnetic Resonance Imaging (fMRI) techniques to map brain activity during the itch-scratch cycle. C-fos is widely used as marker for neuronal activation. The strengths of this marker are low expression level under basal conditions, inducibility upon a wide range of stimuli, and induction only minutes after acute challenge with maximal levels of c-fos after 1–3 h (Kovacs, 1998). Transgenic mouse models expressing GFP or EGFP are an elegant research tool and widely used over the last decades (Okabe et al., 1997; Feng et al., 2000).
Here, we report an in vivo mouse model of acute itch with direct immunostaining of c-fos with visualization using 3,3′-diaminobenzidine (DAB) to detect activation on the neuronal level. It is of clinical interest to determine not only the mechanisms that are involved in the histamine (and specifically the H4R)-mediated itch transmission, but the brain areas involved in the itch-scratch cycle.
2. Experimental procedures
2.1. Mice
Transgenic mice B6.Cg-Tg(Fos-tTA,Fos-EGFP*)1Mmay/J (Stock No: 018306, referred to as c-fos-EGFP mice) were originally obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and kindly provided by the Ghashghaei Laboratory, College of Veterinary Medicine, North Carolina State University, North Carolina, USA. Mice were bred and genotyped using PCR primer sequences and methods provided by the Jackson Laboratory. All mice were healthy and housed in groups of four per cage at 22° C (± 2° C) with a 12-h light/dark cycle. Water and a standard diet were available ad libitum. Male c-fos-EGFP mice (10–21 weeks old, 25–30 g) were chosen for the experiments. In vivo studies performed at the College of Veterinary Medicine, North Carolina State University were conducted following the Guide for the Care and Use of Laboratory Animals with the approval of the Institutional Animal Care and Use Committee of the North Carolina State University (IACUC protocol ID #13-058-B). This study is reported in accordance with the ARRIVE guidelines for reporting experiments involving animals.
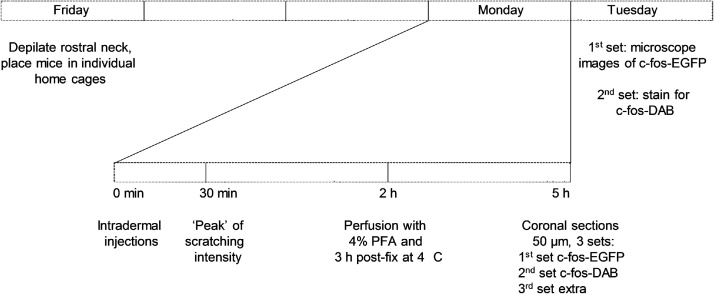
2.2. Timeline for the acute itch model
Experiments were conducted once a week with one animal per group (three animals total). On Friday afternoons, rostral neck hair was removed using NAIR® cream. After sitting for 30 s, the cream was wiped off with soft paper towels soaked in water. Each mouse was then placed in the experimental home cage with ventilated lids in a room with video equipment. These cages matched the housing cages but did not have a water plug-in. The following Monday morning at 7 A M, the mice were injected intradermally with either vehicle (50 μL PBS (phosphate buffered saline, vehicle control), ST-1006 (100 nmol/50 μL) or histamine (0.8 μmol/50 μL, positive control). The mice were then video-monitored for one hour and scratching bouts were counted. A ‘scratching bout’ was defined as lifting a hind leg from the ground and scratching the skin behind the ears and on the back, and then placing the paw back on the ground or grooming it. The videos were also immediately analyzed to ensure successful induction of itch in the treatment group. Exactly two hours following intradermal injection, the mice were anesthetized using 1.25% Avertin solution i.p. (7.5 mg/0.6 mL per mouse) following the guidelines for use of Avertin (University of California, 2018). The use of Avertin was justified, as animals were perfused and not meant to recover from general anesthesia. In addition, the fast induction of deep surgical anesthesia prevents hypoxia of the brain- important in maintaining integrity of the tissue for immunohistochemistry. Cardiac perfusion was performed when the pedal reflex (toe pinch) was absent (1–2 min after injection). For tissue sectioning, 20 mL 4% paraformaldehyde (PFA) solution (room temperature) was used for perfusion and extracted brain was post-fixed in 4% PFA at 4° C for 3 h.
2.3. Tissue sectioning, staining and analysis
Brains were mounted in 1.25% agarose and sliced on the coronal plane at 50 μm in three series using a VT1000S vibratome (Leica Microsystems, Wetzlar, Germany).
For c-fos-EGFP analysis, the first set of slices was directly mounted with Permount mounting media and imaged with confocal microscopy using Nikon AZ100C and NIS-Elements software (AR 4.11.00) within 24 h. A 350x magnification for the area of interest was used and stitching of tile scan images was performed when necessary. Maximum intensity projections were generated for analysis. Imaging was completed within 24 h after sectioning. DAB staining for c-fos was performed as previously described (Ghashghaei and Barbas, 2002). Briefly, floating brain sections were washed with PBS and blocked for one hour in PBS containing 10% rabbit serum and 1% Triton X-100 at room temperature. Sections were then incubated overnight at 4° C in primary goat anti-c-fos polyclonal antibody (Antibody registry: AB_2629503; 1:1000, SC-52-G, Santa Cruz Biotechnology, Dallas, TX, USA), washed three times in PBS, and incubated with secondary biotinylated rabbit anti-goat antibody (Antibody registry: AB_2336126; 1:1000, Vectastain Laboratories, BA-5000, Burlingame, CA, USA) for 1 h at room temperature. After three washes with PBS, the ABC kit (Vectastain Laboratories, Burlingame, CA, USA) in combination with a DAB-Kit (Invitrogen, San Diego, CA, USA) was used following the manufacturer’s recommendations. Sections were mounted with Permount mounting media and the whole slices were imaged with brightfield using the microscope technique as described before. A 200x magnification was used and stitching of tile scan images was performed. Analyzed brain areas were selected following two inclusion criteria: 1. Published literature and 2. The pilot study where a screening at low magnification identified areas with high density of c-fos + neurons. Mapping of the brain was performed based on The Mouse Brain in Stereotactic Coordinates (Paxinos and Franklin, 2001). To confirm EGFP expression in c-fos-EGFP neurons, sections were stained with a chicken anti-GFP polyclonal antibody (1:2000, Abcam, ab13970) overnight, washed three times in PBS and incubated with Cy3 donkey anti-chicken as secondary antibody for two hours (1:4000, Jackson ImmunoResearch Laboratories). Slices were mounted with Permount mounting media and imaged with Nikon TE200 using NIS-Elements software (NIS-Elements V 4.00).
2.4. Statistical evaluation
Results are presented as median with SEM or single values with mean as indicated in the figure. For the acute itch model, a two-way ANOVA was applied to compare scratching bouts over time between the different treatment groups. A Kruskal-Wallis test, followed by Dunn’s post hoc test, was applied to assess differences after the first 30 min of scratching, as well as for analysis of neuronal c-fos density in the brain. GraphPad Prism version 7.0b was used and p-values below 5% probability were considered statistically significant.
2.5. Reagents
The H4R agonist ST-1006 ((N4-(2,6-dichlorobenzyl)-6-(4-methylpiperazin-1-yl)pyrimidine-2,4-diamine, C16H20Cl2N6*2C4H4O4) was synthesized by H. Stark as described previously (Sander et al., 2009). ST-1006 was dissolved in dimethylsulfoxid (DMSO, 10 mg/mL) and further diluted in sterile PBS to the final concentrations as indicated in methods. Histamine dihydrochloride was purchased from Sigma-Aldrich (MO, USA) and directly dissolved in sterile PBS (w/v) to the final concentrations as indicated in methods. Additional reagents were obtained as indicated: PBS, DMSO, Permount, Agarose and Tween 20 (Thermo Fisher Scientific Inc., MA, USA), and PFA 4% in PBS (Alfa Aesar, MA, USA).
3. Results
3.1. Establishing a mouse model for acute itch in male c-fos-EGFP mice
Histamine and the selective H4R agonist ST-1006 significantly increased itch in male c-fos-EGFP mice compared to vehicle treated mice.
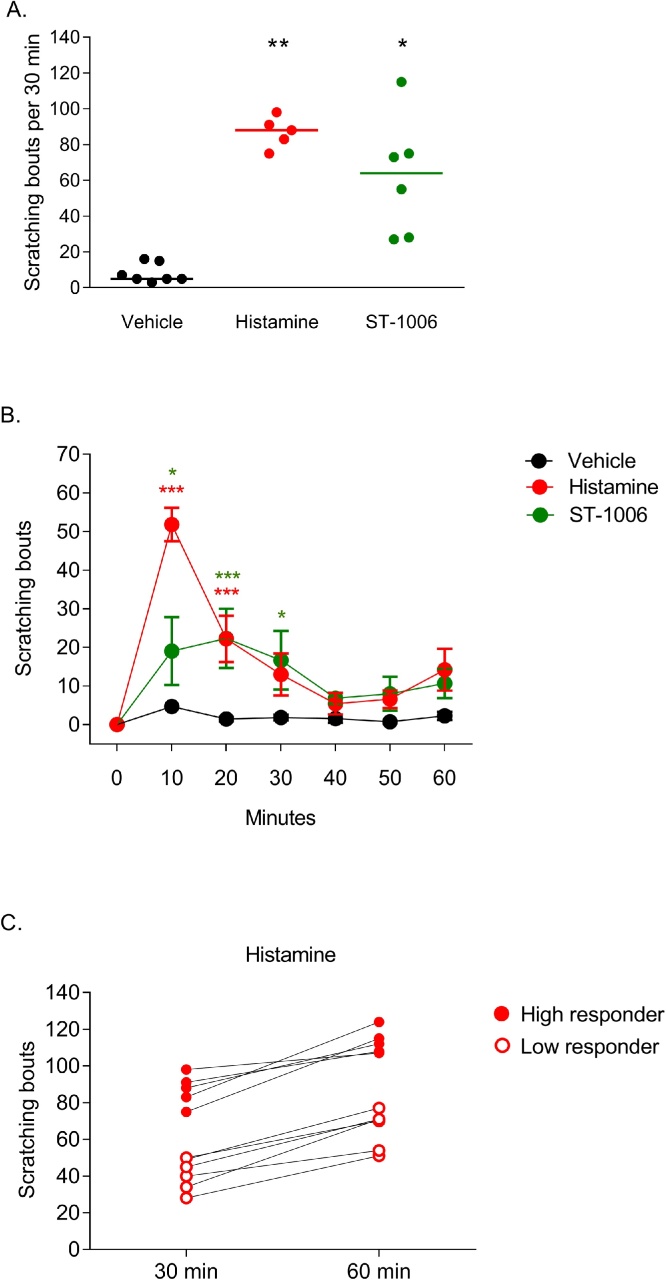
Mice were rid of hair on the rostral back and habituated in the experimental home cage for 3 days. Mice were video monitored for 1 h following injection and perfused 2 h after injection. The brain tissue was harvested, post-fixed for 3 h and prepared for immunostaining (Fig. 1). ST-1006 (62 ± 14) or histamine (87 ± 4) induced significantly more scratching bouts compared to vehicle injected mice (8 ± 2) during the first 30 min of recording trials (Fig. 2A). Itch-inducing concentrations for histamine (0.8 μmol/50 μL) and ST-1006 (100 nmol/50 μL) were determined in pilot experiments (data not shown).
Fig. 1.
Schematic overview of the study design.
The rostral back of the mouse was depilated and each mouse was habituated in its own experimental home cage. Three days later, itch was induced by intradermal injections of histamine 0.8 μmol/50 μL, ST-1006 100 nmol/50 μL or 50 μL vehicle (PBS). Two hours later, the mice were anesthetized, perfused and coronal sections of the brain were taken. Every third section was stained for c-fos-DAB, every second analyzed for c-fos-EGFP.
Fig. 2.
Intradermal injection of the selective H4R agonist ST-1006 and histamine significantly increased scratching bouts in c-fos-EGFP transgenic mice.
A. After 30 min, ST-1006 (62 ± 14 bouts, 100 nmol/ 50 μL) and histamine (87 ± 4 bouts, 0.8 μmol/ 50 μL) induced significantly more scratching bouts in c-fos-EGFP mice compared to vehicle (8 ± 2 bouts) treated mice. Shown are the high responders for histamine. *p < 0.05, **p < 0.01, Kruskal-Wallis test followed by Dunn’s post hoc test, single values with mean, n = 5–7 mice per group. B. Scratching profiles over 1 h show significant differences between the ST-1006 and histamine group compared to the vehicle group during the first 30 min after intradermal injection. Shown are the high responders for histamine. *p < 0.05, **p < 0.01, two-way ANOVA, mean ± SEM. C. Final analysis revealed low and high responders to histamine and brain mapping was performed focused on the high responders (> 100 bouts in 1 h). Single values, n = 5–7 mice per group.
The itch-scratch profiles induced through the selective H4R agonist ST-1006 or histamine indicate significant increase in scratching bouts during the first 30 min compared to vehicle controls (Fig. 2B). Based on this, mice were allowed to scratch for 30 min to establish a constant signal in the brain before they were perfused 90 min later with brain tissue harvested for c-fos analysis.
Scratching results indicate low (n = 6) and high responders (n = 5) to histamine (Fig. 2C) and central c-fos activation was analyzed in the high responders only. This decision was based on Coggeshall (2005), who indicates that the intensity of the stimulus is correlated with the amount of c-fos expression. As histamine injections served as the positive control, we wanted to ensure a strong, detectable c-fos signal to establish the model.
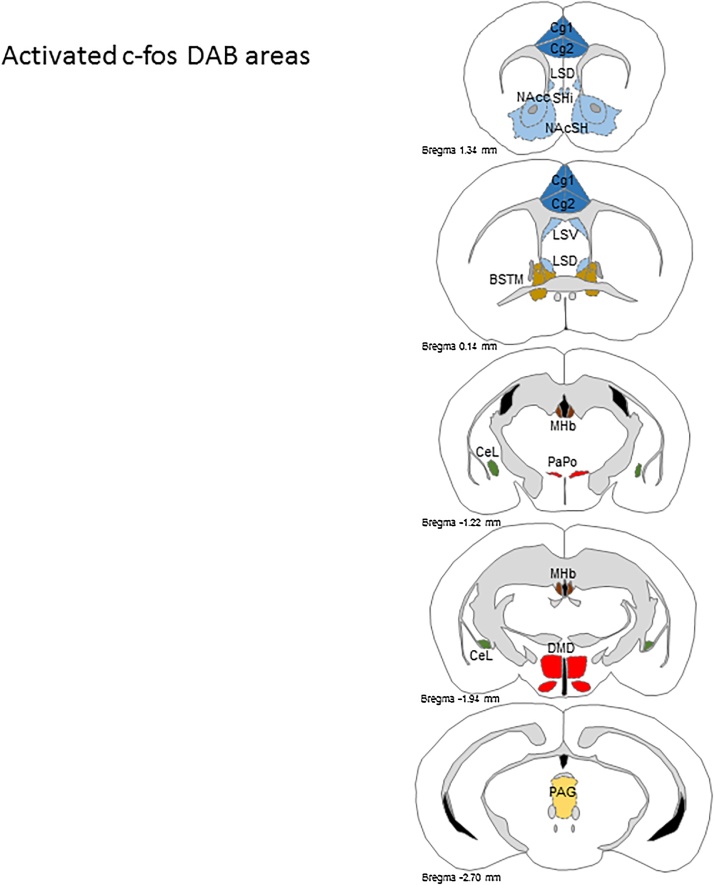
3.2. Overview mapping of nuclei with high c-fos density in the brain
Schematic overview of brain nuclei in coronal sections with high c-fos density as an indicator for neuronal activity in the cortex, striatum, hypothalamus, thalamus, amygdala, and various midbrain nuclei (Fig. 3). Based on pilot experiments (data not shown), analyzed areas include the cingulate cortex (Cg1/2), the striatum with septohippocampal nucleus (Shi), nucleus accumbens core and shell (NAcc, NAcSH), and the ventral and dorsal parts of the hypothalamic lateral septal nucleus (LSV, LSD), as well as the dorsal part of the dorsomedial nucleus (DMD). In the thalamus, the medial habenular (MHb) nucleus, posterior part of the paraventricular hypothalamic nucleus (PaPo) and the lateral part of the central nucleus in the amygdala (CeL). Other regions included the bed nucleus of stria terminalis (BSTMA, BSTMV) in the forebrain and the periaqueductal gray (PAG) in the midbrain. See also Table 1 for c-fos density (positive neurons per mm2) in each analyzed brain nucleus.
Fig. 3.
Schematic overview mapping of the analyzed brain nuclei with high c-fos-DAB density in coronal sections from rostral to caudal.
High c-fos density as indicator for neuronal activity in nuclei in the cortex (dark blue), striatum (light blue), hypothalamus (red), thalamus (dark brown), amygdala (green), and midbrain (yellow). Analyzed areas include cingulate cortex (Cg1/2), septohippocampal nucleus (Shi), nucleus accumbens core and shell (NAcc, NAcSH), ventral and dorsal parts of the hypothalamic lateral septal nucleus (LSV, LSD) as well as the dorsomedial nucleus (DM). In the thalamus, the medial habenular (MHb) nucleus and the lateral part of the central nucleus in the amygdala (CeL). Other regions included the bed nucleus of stria terminalis (BSTMA, BSTMV, light brown), and the periaqueductal gray (PAG). See also Table 1 for c-fos density (positive neurons per mm2) in each analyzed nucleus.
Table 1.
c-fos-DAB+ and c-fos-EGFP+ neuronal density in analyzed brain areas in male c-fos-EGFP mice.
| c-fos-DAB neuronal density (per mm2) |
c-fos-EGFP neuronal density (per mm2) |
|||||
|---|---|---|---|---|---|---|
| Region | Vehicle | Histamine | ST-1006 | Vehicle | Histamine | ST-1006 |
| Cg1 | 3145 (±412) | 3479 (±383) | 3357 (±246) | N/A | N/A | N/A |
| Cg2 | 2710 (±501) | 2867 (±355) | 2994 (±293) | N/A | N/A | N/A |
| SHi | 3211 (±473) | 4200 (±624) | 3550 (±523) | 496 (±170) | 533 (±104) | 875 (±193) |
| LSD | 2137 (±320) | 2033 (±362) | 2826 (±816) | 599 (±80) | 685 (±69) | 1016 (±106) |
| LSV | 3440 (±408) | 2711 (±332) | 3364 (±421) | 746 (±138) | 609 (±97) | 956 (±214) |
| NAcc | 1552 (±524) | 2386 (±356) | 1857 (±441) | N/A | N/A | N/A |
| NAcSH | 1311 (±503) | 1957 (±176) | 1534 (±316) | N/A | N/A | N/A |
| BSTMA/BSTMV | 1488 (±440) | 1033 (±224) | 1285 (±255) | 465 (±88) | 268 (±76) | 730 (±139) |
| MHb | 11 (±2) | 36 (±7)* | 53 (±521)** | 0 (±5) | 101 (±134)** | 201 (±301)* |
| PaPo | 2589 (±464) | 1738 (±486) | 2870 (±276) | 480 (±99) | 467 (±186) | 933 (±281) |
| CeL | 2009 (±447) | 1547 (±270) | 1258 (±357) | 423 (±46) | 1058 (±127) | 552 (±152) |
| DM | 1412 (±219) | 1062 (±140) | 1280 (±259) | 514 (±102) | 988 (±157) | 382 (±96) |
| PAG | 1157 (±330) | 1326 (±236) | 1954 (±295) | 304 (±53) | 421 (±88) | 384 (±140) |
| LHb | N/A | N/A | N/A | 229 (±48) | 409 (±129) | 156 (±53) |
*p < 0.05, **p < 0.01, Kruskal-Wallis test followed by Dunn's multiple comparison test, median ± SEM, c-fos density in neurons/mm2, n = 5–7 mice per group. N/A = not analyzed.
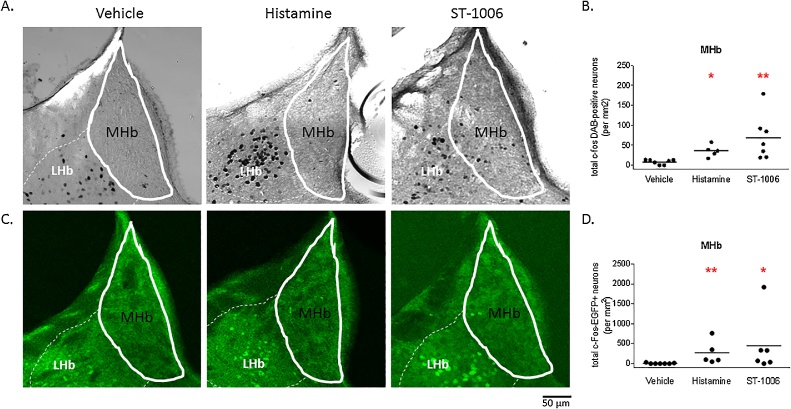
3.3. Histaminergic itch and specific H4R activation increase c-fos density in the medial habenula
Histamine and the specific H4R agonist ST-1006 significantly increased c-fos + neuronal density in the medial habenula.
Although various brain nuclei showed increased cfos density only the medial habenula showed activation in the histamine and ST-1006-treated animals compared to almost no activation in the vehicle group. The medial habenula shows significant increase in median c-fos-DAB density when itch was induced with histamine (36 ± 7), compared to almost no c-fos + neurons in vehicle control animals (11 ± 2). The specific H4R agonist ST-1006 led to a higher density of c-fos + neurons (53 ± 21) than histamine (Fig. 4A/B). The findings in the c-fos-DAB neurons were confirmed in c-fos-EGFP neurons with significant increase in histamine (101 ± 134) and ST-1006 (201 ± 301), compared to vehicle control (7 ± 5) (Fig. 4C/D, Table 1).
Fig. 4.
c-fos + neuronal density in the medial habenula after intradermal injection of ST-1006, histamine or vehicle.
A./C. Representative single layer confocal c-fos-DAB or c-fos-EGFP images of the left medial habenula area (solid white line). Scale bar 50 μm. B./D. Total c-fos-DAB + or c-fos-EGFP + neurons in the medial habenula after intradermal injection of vehicle (50 μL), histamine (0.8 μmol/ 50 μL) or ST-1006 (100 nmol/ 50 μL). *p < 0.05, **p < 0.01, Kruskal-Wallis one-way ANOVA followed by Dunn’s post hoc test, single values with mean, c-fos density neurons/mm2, n = 5–7 mice per group.
4. Discussion
This study was a first attempt to identify activated brain areas during the histamine-induced itch-scratch cycle through direct analysis on a single neuron level. We successfully established a mouse model for acute itch in male c-fos-EGFP mice, where we demonstrate for the first time that selective H4R agonist ST-1006 induces itch when injected intradermally. In this acute model, histaminergic itch led to significant increase in c-fos + neurons in the medial habenula in mice.
Although various brain nuclei showed increased cfos density only the medial habenula showed significant activation in the histamine and ST-1006-treated animals compared to almost no activation in the vehicle group. The habenula is well conserved among vertebrates and divided into a medial and a lateral division. It is not only a key regulator of the dopaminergic and serotonergic system in the midbrain, but also processes stimuli from the periphery (Boulos et al., 2017; Shelton et al., 2012b). The medial habenula receives inhibitory input from GABAergic neurons from the medial septum and the diagonal band (Qin and Luo, 2009). Excitatory afferents (glutamatergic, ATP or ACh positive neurons) are received from the triangular septal nucleus or septofimbrial nucleus (Qin and Luo, 2009; Boulos et al., 2017). The medial habenula circuitry had not been studied for itch. In mice, Boulos et al. (2017) summarized that behavioral experiments identified the habenula processes nociception and pain control, as well as emotional and cognitive aspects of chronic pain. A role in antinociception is also suggested (Ma et al., 1992; Viswanath et al., 2013). Pain and itch share similar processing pathways (reviewed in Liu and Ji, 2013) and it is of interest to look into existing data about pain processing and analgesia in the CNS. In humans, fMRI and PET studies support a role of the habenula in analgesia and pain processing (Shelton et al., 2012a), but further analysis of the medial habenula has not been possible yet due to the lack of resolution in current MRI techniques (Strotmann et al., 2014, 2013). A limitation of our study is that we cannot exclude the activation of the medial habenula as consequence of emotion, physical activity or effects secondary to itch. For future studies, comparison with painful stimuli such as formalin or capsaicin will be needed to distinguish between activation pattern of itch and pain.
The H4R is known to mediate itch in the periphery. In early clinical studies, a selective H4R antagonist inhibited histamine-induced pruritus in healthy volunteers (Kollmeier et al., 2014), and reduced pruritus in patients with atopic dermatitis (Murata et al., 2015). Here, we show that the selective H4R agonist ST-1006 induces itch peripherally and increased c-fos density in the medial habenula in mice.
Summarized in Mochizuki et al. (2014b), several areas in the brain have been associated with the itch-scratch cycle. Areas in the cortex, especially the insular cortex and the claustrum, are linked to itch processing. The ACC, the amygdala and the NAc are linked to the emotional sensation of itch. The cerebellum, the premotor cortex and the SMA showed activation when scratching was not allowed. In our study, animals were allowed to scratch, because hind leg scratching bouts are an established readout to assess itch in mice. Jeong and Kang (2015) used a capsaicin-induced itch model in rats and performed manganese-enhanced MRI while the animals were allowed to scratch. Compared to their results, we also saw high c-fos density in some thalamic nuclei, PAG, cortex, amygdala, midbrain, and hypothalamus, but it was not significantly increased through histaminergic itch. In other studies, during investigation of experimental pain in rats, the lateral habenula was found to have increased c-fos activity, suggesting a role in pain processing (Smith et al., 1997; Lehner et al., 2004). In our pilot experiments, the lateral habenula did not come into focus. Because of overlapping pathways of pain and itch, involvement of the lateral habenula in itch remains to be elucidated.
Literature provides some explanations for differences between DAB and EGFP signal density. Transgenic mouse models expressing GFP or EGFP are an elegant research tool and widely used over the last decades (Okabe et al., 1997; Feng et al. 2015). Nevertheless, Kovacs (1998) concluded, that there may be threshold differences between different brain areas. The molecular mechanism are not known but might be due to the sensitivity of detection methods.
To our knowledge, this is one of the first studies that screened for activated brain nuclei on neuronal level during peripheral histaminergic itch and scratching. It has been suggested that there is no brain region specifically activated by itch stimuli (Mochizuki and Kakigi, 2015a). So far, only few studies investigated itch and scratch on a neuronal level. A recent study by Yu et al. (2017) found contagious itch to activate the suprachiasmatic nucleus in mice (Yu et al., 2017). A study by Song et al. (2012) found c-fos antibody enhanced neuronal activation in the arcuate nucleus neurons through intradermal chloroquine injection. Descalzi et al. (2013) showed neuronal antibody enhanced c-fos activation of the anterior cingulate cortex during histaminergic and non-histaminergic induced scratching. These studies looked at pre-selected, specific brain areas that were already described in literature. Our unbiased approach was to screen the brain for activation patterns during itch and to possibly identify itch-specific areas. However, except for the medial habenula all regions which were analysed did not differ statistically from each other, thus we still conclude, that the medial habenula might be of relevance in the processing of itch-scratch behavior.
Our results are a first step to better understand the basic mechanisms of itch and scratching, and to emphasize the need for mechanistic studies on the central role of itch and scratching.
Although, there is some overlap in activation of brain areas, itch and scratching appear to be processed differently. Studies in primates and humans identified activation of ventral posterior thalamic nuclei in histamine-induced itch using electrophysiology and fMRI Arterial Spin Labeling (Davidson et al., 2012; Papoiu et al., 2012). Therefore, further studies are needed to determine the differences. Non-histaminergic and histaminergic pruritogens induce itch through different pathways. Nevertheless, they seem to activate similar areas in the brain (Davidson et al., 2012; Papoiu et al., 2012; Jeong and Kang, 2015) and it will be of interest to investigate the activation pattern in the brain of strong non-histaminergic pruritogens on a neuronal level. Further controls such as mice which vigorously exercise (motor areas) and mice exposed to painful stimuli (itch inhibitory effect) would be helpful to further characterize itch specific areas in the brain.
In comparison to acute itch, stimulus-specific patterns of activation differ in diseases where chronic itch is involved and structural changes in the brain may occur over time (Mochizuki et al., 2017). To understand these changes during chronic itch, more studies investigating central mechanisms in acute itch are necessary. In summary, our study suggests, that the medial habenula could be a new, interesting target to investigate and subsequently develop novel mechanism-based strategies to treat itch, and possibly provide a locus for pharmacological control of pruritus.
Declarations of interest
None.
Acknowledgements
We greatly acknowledge the help of Jacob M. Sawyer, Ph.D., Advanced Imaging Specialist, for his excellent technical support with the confocal imaging.
Contributor Information
Sarah Ehling, Email: sarah.ehling81@gmail.com.
Ashley Butler, Email: arbutler@ncsu.edu.
Stephanie Thi, Email: sythi@ncsu.edu.
H. Troy Ghashghaei, Email: tghashg@ncsu.edu.
Wolfgang Bäumer, Email: Wolfgang.baeumer@fu-berlin.de.
References
- Akiyama T., Iodi Carstens M., Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J.K., Mcqueen D.S., Rees J.L. Involvement of histamine H4 and H1 receptors in scratching induced by histamine receptor agonists in Balb C mice. Br. J. Pharmacol. 2004;142:374–380. doi: 10.1038/sj.bjp.0705754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulos L.J., Darcq E., Kieffer B.L. Translating the habenula-from rodents to humans. Biol. Psychiatry. 2017;81:296–305. doi: 10.1016/j.biopsych.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall R.E. Fos, nociception and the dorsal horn. Prog. Neurobiol. 2005;77:299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Davidson S., Giesler G.J. The multiple pathways for itch and their interactions with pain. Trends Neurosci. 2010;33:550–558. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S., Zhang X., Khasabov S.G., Moser H.R., Honda C.N., Simone D.A., Giesler J.G.J. Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J. Neurophysiol. 2012;108:1711–1723. doi: 10.1152/jn.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S., Zhang X.J., Khasabov S.G., Simone D.A., Giesler G.J. Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat. Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descalzi G., Chen T., Koga K., Li X.-Y., Yamada K., Zhuo M. Cortical GluK1 kainate receptors modulate scratching in adult mice. J. Neurochem. 2013;126:636–650. doi: 10.1111/jnc.12351. [DOI] [PubMed] [Google Scholar]
- Desmadryl G., Gaboyard-Niay S., Brugeaud A., Travo C., Broussy A., Saleur A., Dyhrfjeld-Johnsen J., Wersinger E., Chabbert C. Histamine H4 receptor antagonists as potent modulators of mammalian vestibular primary neuron excitability. Br. J. Pharmacol. 2012;167:905–916. doi: 10.1111/j.1476-5381.2012.02049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunford P.J., Williams K.N., Desai P.J., Karlsson L., Mcqueen D., Thurmond R.L. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J. Allergy Clin. Immunol. 2007;119:176–183. doi: 10.1016/j.jaci.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Feng G., Mellor R.H., Bernstein M., Keller-Peck C., Nguyen Q.T., Wallace M., Nerbonne J.M., Lichtman J.W., Sanes J.R. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H.T., Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Ishiuji Y., Coghill R.C., Patel T.S., Oshiro Y., Kraft R.A., Yosipovitch G. Distinct patterns of brain activity evoked by histamine-induced itch reveal an association with itch intensity and disease severity in atopic dermatitis. Br. J. Dermatol. 2009;161:1072–1080. doi: 10.1111/j.1365-2133.2009.09308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong K.Y., Kang J.H. Investigation of the pruritus-induced functional activity in the rat brain using manganese-enhanced MRI. J. Magn. Reson. Imaging. 2015;42:709–716. doi: 10.1002/jmri.24832. [DOI] [PubMed] [Google Scholar]
- Kollmeier A., Francke K., Chen B., Dunford P.J., Greenspan A.J., Xia Y., Xu X.L., Zhou B., Thurmond R.L. The histamine H(4) receptor antagonist, JNJ 39758979, is effective in reducing histamine-induced pruritus in a randomized clinical study in healthy subjects. J. Pharmacol. Exp. Ther. 2014;350:181–187. doi: 10.1124/jpet.114.215749. [DOI] [PubMed] [Google Scholar]
- Kovacs K.J. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem. Int. 1998;33:287–297. doi: 10.1016/s0197-0186(98)00023-0. [DOI] [PubMed] [Google Scholar]
- Lehner M., Taracha E., Skorzewska A., Wislowska A., Zienowicz M., Maciejak P., Szyndler J., Bidzinski A., Plaznik A. Sensitivity to pain and c-Fos expression in brain structures in rats. Neurosci. Lett. 2004;370:74–79. doi: 10.1016/j.neulet.2004.07.089. [DOI] [PubMed] [Google Scholar]
- Leknes S.G., Bantick S., Willis C.M., Wilkinson J.D., Wise R.G., Tracey I. Itch and motivation to scratch: an investigation of the central and peripheral correlates of allergen- and histamine-induced itch in humans. J. Neurophysiol. 2007;97:415–422. doi: 10.1152/jn.00070.2006. [DOI] [PubMed] [Google Scholar]
- Liu T., Ji R.R. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013;465:1671–1685. doi: 10.1007/s00424-013-1284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q.P., Shi Y.S., Han J.S. Further studies on interactions between periaqueductal gray, nucleus accumbens and habenula in antinociception. Brain Res. 1992;583:292–295. doi: 10.1016/s0006-8993(10)80036-8. [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Schut C., Nattkemper L.A., Yosipovitch G. Brain mechanism of itch in atopic dermatitis and its possible alteration through non-invasive treatments. Allergol. Int. 2017;66:14–21. doi: 10.1016/j.alit.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Kakigi R. Central mechanisms of itch. Clin. Neurophysiol. 2015;126:1650–1660. doi: 10.1016/j.clinph.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Kakigi R. Itch and brain. J. Dermatol. 2015;42:761–767. doi: 10.1111/1346-8138.12956. [DOI] [PubMed] [Google Scholar]
- Mochizuki H., Papoiu A.D.P., Yosipovitch G. Brain processing of itch and scratching. In: Carstens E., Akiyama T., editors. Itch: Mechanisms and Treatment. CRC Press; Boca Raton, Florida, United States: 2014. [Google Scholar]
- Mochizuki H., Papoiu A.D.P., Yosipovitch G. Brain processing of itch and scratching. In: Carstens E., Akiyama T., editors. Itch: Mechanisms and Treatment. CRC Press; Boca Raton, Florida, United States: 2014. Chapter 23. [Google Scholar]
- Mochizuki H., Tashiro M., Kano M., Sakurada Y., Itoh M., Yanai K. Imaging of central itch modulation in the human brain using positron emission tomography. Pain. 2003;105:339–346. doi: 10.1016/s0304-3959(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Murata Y., Song M., Kikuchi H., Hisamichi K., Xu X.L., Greenspan A., Kato M., Chiou C.F., Kato T., Guzzo C., Thurmond R.L., Ohtsuki M., Furue M. Phase 2a, randomized, double-blind, placebo-controlled, multicenter, parallel-group study of a H4 R-antagonist (JNJ-39758979) in Japanese adults with moderate atopic dermatitis. J. Dermatol. 2015;42:129–139. doi: 10.1111/1346-8138.12726. [DOI] [PubMed] [Google Scholar]
- Okabe M., Ikawa M., Kominami K., Nakanishi T., Nishimune Y. RE:’ Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407(3):313–319. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- Papoiu A.D., Coghill R.C., Kraft R.A., Wang H., Yosipovitch G. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. Neuroimage. 2012;59:3611–3623. doi: 10.1016/j.neuroimage.2011.10.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoiu A.D.P., Nattkemper L.A., Sanders K.M., Kraft R.A., Chan Y.-H., Coghill R.C., Yosipovitch G. Brain’s reward circuits mediate itch relief. A functional MRI study of active scratching. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Franklin L.B.J. Academic Press; Cambridge, Massachusetts, United States: 2001. The Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Qin C., Luo M. Neurochemical phenotypes of the afferent and efferent projections of the mouse medial habenula. Neuroscience. 2009;161:827–837. doi: 10.1016/j.neuroscience.2009.03.085. [DOI] [PubMed] [Google Scholar]
- Rossbach K., Nassenstein C., Gschwandtner M., Schnell D., Sander K., Seifert R., Stark H., Kietzmann M., Baumer W. Histamine H1, H3 and H4 receptors are involved in pruritus. Neuroscience. 2011;190:89–102. doi: 10.1016/j.neuroscience.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Sander K., Kottke T., Tanrikulu Y., Proschak E., Weizel L., Schneider E.H., Seifert R., Schneider G., Stark H. 2,4-Diaminopyrimidines as histamine H4 receptor ligands--Scaffold optimization and pharmacological characterization. Bioorg. Med. Chem. 2009;17:7186–7196. doi: 10.1016/j.bmc.2009.08.059. [DOI] [PubMed] [Google Scholar]
- Schneider G., Stander S., Burgmer M., Driesch G., Heuft G., Weckesser M. Significant differences in central imaging of histamine-induced itch between atopic dermatitis and healthy subjects. Eur. J. Pain. 2008;12:834–841. doi: 10.1016/j.ejpain.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Shelton L., Becerra L., Borsook D. Unmasking the mysteries of the habenula in Pain and Analgesia. Prog. Neurobiol. 2012;96:208–219. doi: 10.1016/j.pneurobio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton L., Pendse G., Maleki N., Moulton E.A., Lebel A., Becerra L., Borsook D. Mapping pain activation and connectivity of the human habenula. J. Neurophysiol. 2012;107:2633–2648. doi: 10.1152/jn.00012.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W.J., Stewart J., Pfaus J.G. Tail pinch induces fos immunoreactivity within several regions of the male rat brain: effects of age. Physiol. Behav. 1997;61:717–723. doi: 10.1016/s0031-9384(96)00524-0. [DOI] [PubMed] [Google Scholar]
- Song Y., Pan X., Liu C., Xiang H. Role of nociceptive arcuate nucleus neurons in chloroquine-induced pruritic behaviors in mice. J. Huazhong Univ. Sci. Technol. 2012;32:919–922. doi: 10.1007/s11596-012-1058-7. [DOI] [PubMed] [Google Scholar]
- Strotmann B., Heidemann R.M., Anwander A., Weiss M., Trampel R., Villringer A., Turner R. High-resolution MRI and diffusion-weighted imaging of the human habenula at 7 tesla. J. Magn. Reson. Imaging. 2014;39:1018–1026. doi: 10.1002/jmri.24252. [DOI] [PubMed] [Google Scholar]
- Strotmann B., Kogler C., Bazin P.L., Weiss M., Villringer A., Turner R. Mapping of the internal structure of human habenula with ex vivo MRI at 7T. Front. Hum. Neurosci. 2013;7:878. doi: 10.3389/fnhum.2013.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of California S.D. California: University of California; San Diego: 2018. Guidelines for Use of Avertin [Online]. La Jolla.https://blink.ucsd.edu/_files/sponsor-tab/iacuc/GuidelinesForUseOfAvertin.pdf Available: [Accessed April 3rd 2018] [Google Scholar]
- Viswanath H., Carter A.Q., Baldwin P.R., Molfese D.L., Salas R. The medial habenula: still neglected. Front. Hum. Neurosci. 2013;7:931. doi: 10.3389/fnhum.2013.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Bonaventure P., Thurmond R.L. The future antihistamines: histamine H3 and H4 receptor ligands. Adv. Exp. Med. Biol. 2010;709:125–140. doi: 10.1007/978-1-4419-8056-4_12. [DOI] [PubMed] [Google Scholar]
- Yu Y.Q., Barry D.M., Hao Y., Liu X.T., Chen Z.F. Molecular and neural basis of contagious itch behavior in mice. Science. 2017;355:1072–1076. doi: 10.1126/science.aak9748. [DOI] [PMC free article] [PubMed] [Google Scholar]