Abstract
Introduction
Disparities in dementia prevalence across racial/ethnic groups in the United States may not be narrowing over time.
Methods
Data from Health and Retirement Study (2000 to 2012) were analyzed. Dementia was ascertained based on cognitive, functional measures. Logistic regression was used to quantify association between dementia and risk factors, including chronic conditions, use of drug treatment for them, separately for whites, blacks, and Hispanics.
Results
Disparities in dementia prevalence declined between blacks and whites and increased between Hispanics and whites. Adjusting for risk factors reduced but did not eliminate disparities. Compared to no hypertension, untreated hypertension was associated with increased risk of dementia for all racial/ethnic groups while treated hypertension was associated with reduced risk for whites. Diabetes treated with oral drugs was not associated with increased dementia risk.
Discussion
Racial disparities in dementia may be reduced by prevention and management of disease and promoting educational attainment among blacks and Hispanics.
Keywords: Dementia, Disparities, Epidemiology, Ethnicity, Race, Treatment
1. Background
Dementia is a syndrome characterized by difficulties with memory and other cognitive skills [1] that affects about 14% of people aged 71 years and older in the United States [2]. The number of Americans with Alzheimer's disease, the most common form of dementia, is projected to increase 157% between 2010 and 2050, rising from 3.6 million to 9.1 million affected persons and the costs of care are projected to increase from $181 billion to $1.1 trillion over this same period [3], [4]. Numerous studies have documented differences in dementia prevalence among racial and ethnic groups in the United States. Blacks and Hispanics are found to have a higher risk of dementia compared to whites across studies despite differences in designs, sampling methods, and definitions of dementia [2], [5], [6], [7], [8], [9].
Racial and ethnic differences in dementia risk may result from biological, behavioral, sociocultural, and environmental factors including socioeconomic determinants such as education, income, occupation, wealth, and access to health care [10], [11], [12]. Quantifying the differences and analyzing the mechanisms can aid in the development of interventions, therapeutics, and public policy to reduce and eliminate racial and ethnic differences in dementia.
Dementia has multiple causes. Risk factors for dementia include older age, and modifiable factors include low physical activity and poor cardiovascular health (including diabetes, obesity, smoking, hypertension, high cholesterol) [13]. A hypothesized pathway from poor cardiovascular health to dementia is through reduced blood flow to the brain. Low education is also associated with increased risk of dementia and is theorized to operate through lower cognitive reserves. However, a causal pathway has yet to be established as low education is also associated with lower economic status, poor health, and lower utilization of medical treatments. Blacks and Hispanics have worse cardiovascular health [14] and lower levels of education than whites and may explain part of the dementia disparities across racial and ethnic groups [15], [16], [17], [18], [19]. In addition, other factors such as management of disease, physical activity, and wealth may also influence disparities [9], [20], [21], [22]. Little is known about the relative contribution of all these factors on dementia risk and if the associations differ for whites, blacks, and Hispanics.
Recent population-based studies have shown declines in dementia incidence or prevalence in high-income countries [16], [23], [24]. Several studies suggested that improved population cardiovascular health had spillover benefits on risk of dementia [15], [16], [23], [25]. Other studies argued that rising levels of education over the last 25 years were associated with reduction of dementia risk [16], [26], [27], [28]. In the United States, there is a variation in changes over time of educational attainment, prevalence and management of chronic diseases, and behaviors associated with poor cardiovascular health among racial and ethnic groups [29], [30]. How racial and ethnic disparities in dementia prevalence are changing over time will depend on both changes in risk factors and variation in their relative impact for whites, blacks, and Hispanics.
2. Methods
2.1. Study population
The study uses seven waves of a nationally representative prospective cohort of US adults, the Health and Retirement Study (HRS) from 2000 to 2012 [31]. A key feature of the HRS study design since 1992 is minority oversamples of African Americans and Hispanics and minority response rates at the baseline and in the longitudinal follow-ups have been equal to or better than those of majority whites [32].
Since 1992, the study has collected data on a wide range of topics including health, cognition, family, employment, and wealth of US adults aged 51 years and older. The HRS follows respondents biennially till death, and new cohorts are enrolled at different times to maintain population representativeness. Participants were compensated about $80, and verbal informed consent was obtained from all respondents. Ethics approval was obtained from Health Sciences and Behavioral Sciences institutional review board at the University of Michigan.
We restricted our sample to white, black, or Hispanic adults aged 65 years and older, living in the community or in nursing homes. As a result, we have 75,796 person-wave of data from 2000 to 2012 consisting of 18,606 unique individuals with an average of four waves of follow-up data and a maximum of seven waves. Overall, 84.9% were white, 8.6% black, and 6.5% Hispanic. There were slightly more than 10,000 respondents in each wave. Hispanic adults were less likely to be living in the nursing home (2.2%) compared to white (3.8%) and black (4.2%) (P < .05). Missing data were replaced by participant's information in the previous wave(s): hypertension (n = 25, 0.2%), diabetes (n = 26, 0.2%), heart disease (n = 14, 0.1%), stroke (n = 15, 0.1%), body mass index (n = 118, 1.1%), vigorous activity (n = 37, 0.4%), education (n = 3, 0.0%). We included missing indicator variables in our multivariable regression. The study identified a proxy respondent (usually a spouse or adult child) to complete the survey when the respondent is unable or unwilling to participate in the survey. The proportion of proxy interview decreased from 12.5% (n = 1321) in 2000 to 8.2% (n = 864) in 2012. HRS had consistently high response rates of 88% in 2000 and 89% in 2012 [33].
2.2. Exposures of interest
We used self-reported race and ethnicity to identify whites, blacks, and Hispanics. Respondents identified as other were excluded from the analyses because of small sample size. All regression models included a continuous biennial trend variable starting from wave 5 (year 2000) to wave 11 (year 2012).
2.3. Outcome
Cognitive functioning of HRS respondents is assessed using an adapted version of the Telephone Interview for Cognitive Status at each wave. Spanish versions had been developed for each of the questionnaires and have been administered by bilingual interviewers to Spanish-speaking respondents. Sample members who are unable to communicate adequately in either English or Spanish, and for whom interviews with proxy informants could not be obtained, are treated as nonrespondents and have been dropped from the study (http://hrsonline.isr.umich.edu/sitedocs/surveydesign.pdf). HRS imputed these measures when missing because they tend to be missing for the more cognitively impaired. The method is described in the study by Fisher et al. (2013) [34]. When a respondent does not do the cognitive assessment, cognitive status is determined using information provided by a proxy respondent, typically a spouse or other family member [35]. We assign cognitive state based on scores from the assessments [4], [16], [36]. We sum score of three cognitive assessments (range 0–27): immediate and delayed word recall (0–20); counting down from 100 by 7's test score (0–5); and counting back from 20 (0–20). For proxy interviews, the cognition scale (range 0–11) sums the following: number of instrumental activities of daily living (0–5); interviewer impairment rating (0 = no cognitive limitations, 1 = some limitations, 2 = cognitive limitations); and proxy informant's rating of the respondent's memory (from 0 [excellent] to 4 [poor]). Cognition scores are as follows: 0–6 = demented, 7–11 = mild impairment, no dementia, and 12–27 = normal. Proxy scores are as follows: 0–2 = normal, 3–5 = mild impairment, no dementia, and 6–11 = demented. Both proxy and nonproxy scores are combined into one variable.
2.4. Independent variables
We analyze the association of nonmodifiable and modifiable risk factors for dementia for whites, blacks, and Hispanics. Nonmodifiable factors include age (65–69, 70–74, 75–79, ≥80 years old) and gender (male, female). Also included are socioeconomic factors including highest level of educational attainment (less than high school, high school, college or more) and household wealth in 2010 $ (≤$53 000, $53 001–$178 000, $178 001–$470 000, $470 001+). Household wealth was the net value of total wealth including second home less all debt. In our analysis, we focus on the modifiable factors that include self-reported disease conditions associated with cardiovascular health (heart disease, diabetes, hypertension, stroke), utilization of drug treatment for the aforementioned conditions, self-reported body mass index, and exercise (vigorous activity performed never/rarely, some).
2.5. Statistical analysis
In the descriptive analysis, we used HRS sampling weights and reported weighted percentages (Fig. 1, Fig. 2, Table 1). Trend in dementia prevalence by racial/ethnic groups was compared in Fig. 1. Weighted chi-square test was performed to test the association between independent variables across waves by racial and ethnic groups (Table 1). We analyzed racial/ethnic differences in risk factors associated with dementia and used confidence intervals to discern statistically significant differences (Figures 2 and 3). In the multivariate analyses, we pooled data across seven waves from 2000 to 2012. We used mixed-effects logistic regression with both intercept and time trend to vary by individuals, using random-effects unstructured covariance to control for repeated observations [37]. The binary outcome was whether the individual has dementia and the reference group included those with normal cognition or cognitive impairment no dementia (CIND). We combined CIND with normal cognition, as 64% of individuals who had CIND never transition into dementia. We also included a linear time trend for years 2000 to 2012. First, we fitted four separate models (model 1: demographic variables only; model 2: added cardiovascular risk factors and use of drug treatments; model 3: added exercise, BMI; model 4: added education and wealth) to study what factors change the association of dementia and race and ethnicity (Fig. 3, Table 2). Second, we used logistic regression analysis separately for whites, blacks, and Hispanics to study the differential association of risk factors across race and ethnicity (Table 2, Table A.2).
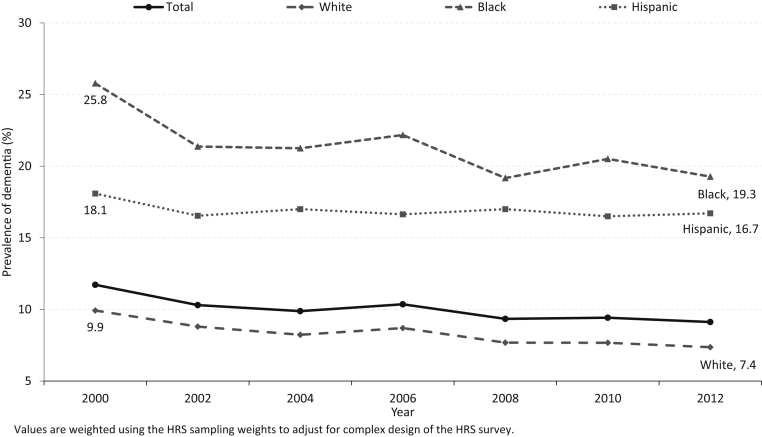
Fig. 1.
Trend in dementia prevalence at the age of 65 years or older by race/ethnicity, 2000–2012. Values are weighted using the HRS sampling weights to adjust for complex design of the HRS survey. Abbreviation: HRS, Health and Retirement Study.
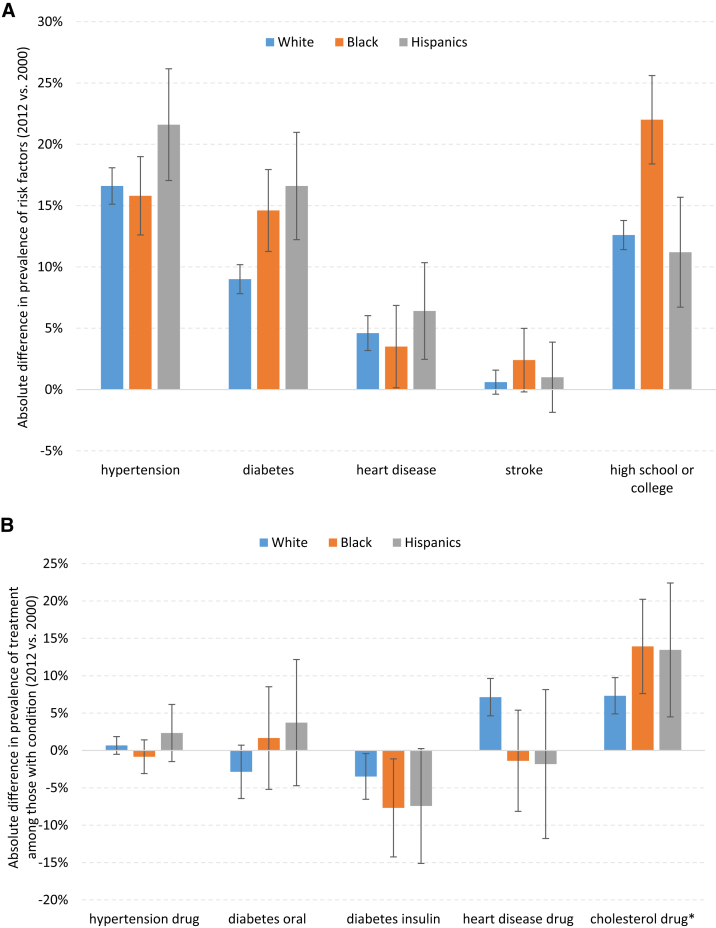
Fig. 2.
(A) Absolute difference in prevalence of risk factors for dementia (2012 vs. 2000). (B) Absolute difference in prevalence of treatment among those with respective medical condition (2012 vs. 2000). *Absolute difference in prevalence of cholesterol treatment among those with heart disease in 2012 versus 2006.
Table 1.
Characteristic by race/ethnicity in years 2000 and 2012
| Characteristic, %∗ | White |
Black |
Hispanics |
|||
|---|---|---|---|---|---|---|
| Y2000 (n = 8474) | Y2012 (n = 8067) | Y2000 (n = 1337) | Y2012 (n = 1478) | Y2000 (n = 738) | Y2012 (n = 975) | |
| Dementia | ||||||
| No | 7661 (90.1) | 7349 (92.6)† | 993 (74.2) | 1173 (80.7)† | 597 (81.9) | 793 (83.3) |
| Yes | 813 (9.9) | 718 (7.4) | 344 (25.8) | 305 (19.3) | 141 (18.1) | 182 (16.7) |
| Age | ||||||
| 65–69 | 2411 (26.9) | 1564 (31.0)† | 485 (31.4) | 330 (33.8) | 252 (31.9) | 208 (34.9) |
| 70–74 | 1985 (24.5) | 2097 (22.9) | 242 (26.9) | 439 (24.1) | 162 (28.6) | 325 (27.3) |
| 75–79 | 1786 (21.8) | 1802 (18.0) | 230 (17.0) | 385 (19.1) | 151 (19.8) | 216 (15.3) |
| ≥80 | 2292 (26.8) | 2604 (28.1) | 380 (24.8) | 324 (23.0) | 173 (19.7) | 226 (22.4) |
| Gender | ||||||
| Male | 3629 (41.6) | 3429 (44.0)† | 507 (38.0) | 548 (39.1) | 310 (42.5) | 408 (41.8) |
| Female | 4845 (58.4) | 4638 (56.0) | 830 (62.0) | 930 (60.9) | 428 (57.6) | 567 (58.2) |
| Hypertension‡,§ | ||||||
| No disease | 4094 (48.0) | 2570 (33.3)† | 412 (31.9) | 232 (17.7)† | 318 (45.9) | 242 (26.8)† |
| With disease, no treatment | 422 (5.0) | 503 (6.2) | 74 (4.8) | 91 (6.6) | 50 (6.1) | 69 (7.0) |
| With disease and treatment | 3784 (45.1) | 4994 (60.5) | 824 (61.7) | 1155 (75.7) | 349 (45.5) | 664 (66.2) |
| Diabetes‡,§ | ||||||
| No disease | 7242 (85.6) | 6172 (76.9)† | 1020 (77.0) | 903 (62.6)† | 552 (75.1) | 566 (59.0)† |
| With disease, no treatment | 201 (2.4) | 432 (5.4) | 37 (2.5) | 96 (6.4) | 17 (2.3) | 52 (5.5) |
| With disease and oral drug | 710 (8.2) | 1046 (12.8) | 161 (11.5) | 306 (19.5) | 113 (15.1) | 268 (26.9) |
| With disease and insulin | 298 (3.5) | 417 (4.9) | 117 (8.8) | 173 (11.5) | 51 (7.0) | 89 (8.7) |
| Heart disease‡,§ | ||||||
| No disease | 5830 (69.1) | 5093 (65.3)† | 951 (71.0) | 1019 (69.1)† | 587 (79.4) | 713 (74.4)† |
| With disease, no treatment | 950 (11.0) | 882 (10.3) | 153 (11.0) | 192 (12.8) | 58 (7.1) | 97 (10.0) |
| With disease and treatment | 1604 (18.7) | 2063 (24.1) | 210 (16.4) | 266 (18.1) | 82 (12.0) | 164 (15.5) |
| Stroke‡ | ||||||
| No | 7505 (88.5) | 6978 (87.9) | 1161 (86.8) | 1246 (84.4) | 671 (90.5) | 869 (89.5) |
| Yes | 969 (11.5) | 1089 (12.1) | 176 (13.2) | 232 (15.6) | 67 (9.5) | 106 (10.5) |
| Body mass index‡ | ||||||
| Underweight (<18.5) | 281 (3.4) | 196 (2.2)† | 50 (4.2) | 37 (2.4)† | 20 (2.2) | 19 (1.5) |
| Normal (18.5–24.9) | 3436 (40.7) | 2645 (31.3) | 384 (28.3) | 360 (25.0) | 244 (33.1) | 276 (29.6) |
| Overweight (25.0–29.9) | 3305 (38.8) | 3051 (38.5) | 525 (37.9) | 535 (34.9) | 294 (40.4) | 372 (38.5) |
| Obese (≥30) | 1452 (17.1) | 2175 (27.9) | 378 (29.6) | 546 (37.7) | 180 (24.3) | 308 (30.5) |
| Vigorous activity‡ | ||||||
| Never or rarely | 5142 (61.0) | 5033 (60.4) | 958 (72.2) | 995 (68.8) | 539 (72.4) | 644 (64.2)† |
| Some activity | 3332 (39.0) | 3034 (39.6) | 379 (27.8) | 483 (31.2) | 199 (27.6) | 331 (35.8) |
| Education‡ | ||||||
| Less than high school | 2168 (25.8) | 1177 (13.1)† | 766 (58.3) | 545 (36.2)† | 535 (71.4) | 594 (60.3)† |
| High school | 3208 (37.8) | 3185 (38.0) | 334 (24.1) | 482 (33.2) | 123 (17.3) | 214 (20.1) |
| College | 3098 (36.5) | 3705 (48.9) | 237 (17.7) | 451 (30.6) | 80 (11.3) | 167 (19.7) |
| Wealth | ||||||
| ≤53,000 | 1537 (19.0) | 1623 (19.6)† | 717 (55.5) | 773 (54.0)† | 427 (58.1) | 521 (54.0)† |
| 53,001–178,000 | 1997 (23.4) | 1758 (21.1) | 421 (30.3) | 393 (25.8) | 186 (24.6) | 235 (21.9) |
| 178,001–470,000 | 2426 (28.5) | 2133 (26.4) | 159 (11.3) | 226 (13.9) | 85 (11.7) | 144 (14.6) |
| 470,001+ | 2514 (29.1) | 2553 (32.9) | 40 (2.9) | 86 (6.4) | 40 (5.6) | 75 (9.5) |
Abbreviation: HRS, Health and Retirement Study.
Weighted percentages were derived using the HRS sampling weights to adjust for the complex design of the HRS survey.
Weighted chi-square test of differences in characteristics across year (2000 and 2012) by race/ethnicity: P < .05.
Missing data were imputed based on participant's information in the most recent previous wave: hypertension (n = 40, 0.19%), diabetes (n = 40, 0.19%), heart disease (n = 28, 0.13%), stroke (n = 21, 0.1%), body mass index (n = 252, 1.17%), vigorous activity (n = 41, 0.19%), education (n = 3, 0.01%).
Percentage does not sum to 100% due to participants who disputed about their conditions: hypertension (white = 2.0%, black = 1.6%, Hispanics = 2.5% in Y2000); diabetes (white = 0.3%, black = 0.2%, Hispanics = 0.5% in Y2000); heart disease (white = 1.5%, black = 1.6%, Hispanics = 1.6% in Y2000 and Y2012).
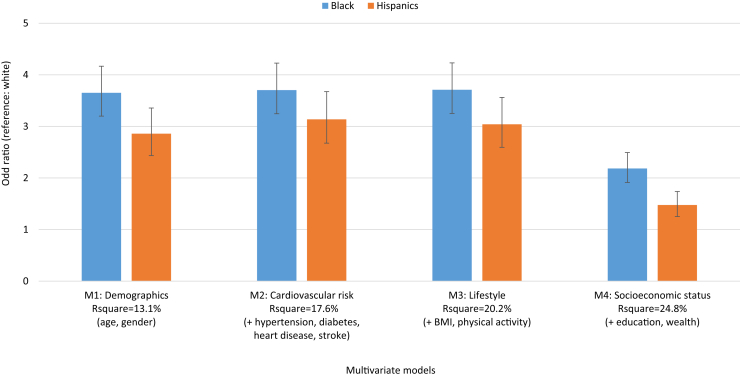
Fig. 3.
Multivariable odds ratio for the presence of dementia in race/ethnic minorities compared to whites, 2000–2012.
Table 2.
Odds ratios for presence of dementia by race/ethnicity, 2000–2012
| Variable | All |
White |
Black |
Hispanics |
|---|---|---|---|---|
| OR [95% CI] | OR [95% CI] | OR [95% CI] | OR [95% CI] | |
| Race | ||||
| White | 1 (ref) | NA | NA | NA |
| Black | 2.18*** [1.91, 2.49] | |||
| Hispanic | 1.47*** [1.25, 1.73] | |||
| Biennial trend | 1.01 [0.99, 1.02] | 1.00 [0.98, 1.02] | 1.01 [0.96, 1.06] | 1.01 [0.95, 1.07] |
| Age | ||||
| 65–69 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 70–74 | 1.46*** [1.34, 1.59] | 1.57*** [1.43, 1.73] | 1.38** [1.11, 1.72] | 1.41* [1.07, 1.85] |
| 75–79 | 2.47*** [2.24, 2.73] | 2.66*** [2.39, 2.97] | 2.38*** [1.83, 3.10] | 2.35*** [1.66, 3.33] |
| ≥80 | 5.72*** [5.15, 6.35] | 6.38*** [5.71, 7.12] | 4.48*** [3.21, 6.24] | 6.01*** [3.96, 9.11] |
| Gender | ||||
| Male | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Female | 0.97 [0.89, 1.05] | 0.95 [0.87, 1.03] | 0.96 [0.75, 1.24] | 1.16 [0.86, 1.57] |
| Stroke | ||||
| No | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 2.83*** [2.48, 3.23] | 2.96*** [2.56, 3.43] | 2.33*** [1.63, 3.35] | 2.89*** [1.64, 5.11] |
| Hypertension | ||||
| No disease | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| With disease, no treatment | 1.85*** [1.58, 2.17] | 1.96*** [1.63, 2.35] | 1.59* [1.04, 2.43] | 1.72* [1.00, 2.94] |
| With disease and treatment | 0.89** [0.81, 0.97] | 0.82*** [0.75, 0.90] | 1.01 [0.76, 1.35] | 1.05 [0.75, 1.47] |
| Diabetes | ||||
| No disease | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| With disease, no treatment | 1.11 [0.92, 1.35] | 1.21 [0.97, 1.51] | 1.07 [0.64, 1.77] | 0.94 [0.52, 1.71] |
| With disease and oral drug | 1.06 [0.95, 1.20] | 1.09 [0.95, 1.25] | 0.95 [0.70, 1.28] | 1.12 [0.79, 1.59] |
| With disease and insulin | 1.42*** [1.19, 1.71] | 1.54*** [1.24, 1.92] | 1.23 [0.83, 1.83] | 1.40 [0.80, 2.44] |
| Heart disease | ||||
| No disease | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| With disease, no treatment | 1.33*** [1.17, 1.51] | 1.37*** [1.19, 1.59] | 1.16 [0.82, 1.64] | 1.44 [0.85, 2.45] |
| With disease and treatment | 1.02 [0.92, 1.13] | 1.00 [0.90, 1.12] | 1.08 [0.78, 1.48] | 1.04 [0.65, 1.66] |
| Body mass index (kg/m2) | ||||
| Underweight (<18.5) | 1.99*** [1.60, 2.49] | 2.00*** [1.55, 2.57] | 1.82 [0.99, 3.35] | 2.08 [0.97, 4.45] |
| Normal (18.5–24.9) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Overweight (25.0–29.9) | 0.70*** [0.64, 0.76] | 0.71*** [0.64, 0.78] | 0.68** [0.53, 0.87] | 0.70* [0.51, 0.96] |
| Obese (≥30) | 0.55*** [0.50, 0.61] | 0.56*** [0.50, 0.63] | 0.47*** [0.34, 0.63] | 0.7 [0.48, 1.01] |
| Vigorous activity | ||||
| Never/rarely | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| Some | 0.42*** [0.39, 0.46] | 0.34*** [0.32, 0.37] | 0.62*** [0.50, 0.77] | 0.55*** [0.42, 0.72] |
| Education | ||||
| Less than high school | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| High school | 0.43*** [0.38, 0.48] | 0.46*** [0.40, 0.52] | 0.37*** [0.27, 0.50] | 0.37*** [0.25, 0.53] |
| College | 0.32*** [0.29, 0.36] | 0.36*** [0.32, 0.41] | 0.22*** [0.16, 0.30] | 0.29*** [0.20, 0.43] |
| Wealth | ||||
| ≤53,000 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| 53,001–178,000 | 0.59*** [0.53, 0.66] | 0.57*** [0.50, 0.65] | 0.60*** [0.48, 0.76] | 0.74* [0.55, 1.00] |
| 178,001–470,000 | 0.47*** [0.42, 0.52] | 0.45*** [0.40, 0.51] | 0.47*** [0.35, 0.64] | 0.63* [0.43, 0.92] |
| 470,001+ | 0.39*** [0.35, 0.44] | 0.37*** [0.32, 0.42] | 0.55** [0.35, 0.86] | 0.48** [0.30, 0.77] |
Abbreviations: CI, confidence interval; NA, not applicable; OR, odds ratio.
NOTE. Exponentiated coefficients; 95% confidence intervals in brackets. Pseudo R2 = 24.8% (overall); 24.1% (white); 21.5% (black); 19.4% (Hispanics). Regression also adjusted for missing dummy variables across person-wave: hypertension (0.3%), diabetes (0.3%), heart attack (5.3%), and vigorous activity (0.15%). *P < .05; **P < .01; ***P < .001.
3. Results
Prevalence of dementia declined from 2000 to 2012 for whites and blacks and declined only between the years 2000 to 2002 among Hispanics (Fig. 1). Both whites and blacks had a 25% reduction in prevalence over the last 12 years (whites: 9.9% in 2000 to 7.4% in 2012; blacks: 25.8% in 2000 to 19.3% in 2012). Dementia prevalence declined by 8.6% between the years 2000 and 2002 for Hispanics (18.1% in 2000 to 16.5% in 2002) and remained unchanged over the next 10 years (16.7% in 2012). Although disparities in dementia prevalence between blacks and whites declined (from 15.9% in 2000 to 11.9% in 2012), the decline was not statistically significant (P = .228). Similarly, while there was a slight increase in dementia prevalence between Hispanics and whites (from 8.2% in 2000 to 9.3% in 2012), this was not statistically significant (P = .292).
Table 1 shows the distribution of characteristics of whites, blacks, and Hispanics in 2000 and 2012. In 2012, compared to blacks and Hispanics, whites were older; were less likely to have hypertension (whites 67%, blacks 82%, Hispanics 73%), diabetes (whites 23%, blacks 37%, Hispanics 41%), and stroke (whites 12%, blacks 16%, Hispanics 11%); were more likely to have heart disease (whites 34%, blacks 31%, Hispanics 26%); were more likely to have normal body mass index (whites 31%, blacks 25%, Hispanics 30%); were more likely to participate in vigorous activity (whites 40%, blacks 31%, Hispanics 36%); were more likely to have attained college education (whites 49%, blacks 31%, Hispanics 20%); and were more likely to have higher household wealth (household wealth greater than US$470 000: whites 33%, blacks 6%, Hispanics 10%).
Comparing across time, there is an increase in prevalence of disease associated with risk of dementia: hypertension, diabetes, and heart disease across all racial and ethnic groups (Table 1 and Fig. 2A). Hispanics had a greater increase in cardiovascular risk factors and lower gains in educational attainment compared to whites and blacks (Fig. 2A). Prevalence of chronic diseases had increased in whites, blacks, and Hispanics with an absolute percentage change in hypertension: 17%, 16%, 22%; diabetes: 9%, 15%, 17%; and heart disease: 5%, 4%, 6%, respectively (Fig. 2A). These factors were statistically different across all racial and ethnic groups (P < .01). On the other hand, there was also an increase in use of drug treatments for whites, blacks, and Hispanics with hypertension, diabetes, and heart diseases (Fig. 2B); thus, the effect on dementia risk of these diseases may have been moderated. The decline in insulin use among those with diabetes is a proxy for declining severity of disease and was higher for blacks than for whites and Hispanics.
Fig. 3 reports the odds ratios for presence of dementia in blacks and Hispanics compared to whites from four different logistic regression models using pooled data from 2000 through 2012. We analyzed how particular risk factors attenuated the estimated level difference between blacks and whites and Hispanics and whites in models with results shown in Table A.1 and illustrated in Fig. 3. The models provide an interpretation of the association of a particular risk factor on dementia prevalence adjusting for differences in race/ethnicity. Adjusting for age and gender only, the likelihood of dementia was 3.7 times for blacks than for whites and 2.9 times for Hispanics than for whites (Fig. 3, Table A.1). The likelihood of dementia was reduced for blacks and Hispanics compared to whites (Table 2) with controls for observable and modifiable risk factors (odds ratio [OR]B = 2.2, CI 1.91–2.49; ORH = 1.5, CI 1.25–1.73) with socioeconomic factors as the main drivers in the reduction in racial and ethnic differences in dementia prevalence (Fig. 3). Declining dementia prevalence over time was explained by the included risk factors (OR = 1.01, CI 0.99–1.02) such as management of chronic diseases with drug treatment and higher levels of educational attainment and wealth over time among successive birth cohorts of whites and blacks (Table A.1).
Table 2 reports the multivariate odds ratios of factors associated with dementia for models stratified by race and ethnicity. Age was associated with dementia across all three racial/ethnic groups with largest effect observed in oldest group (≥80 years) compared 65 to 69 years and with ORs higher for whites (ORw = 6.4, CI 5.71–7.12) and Hispanics (ORH = 6.0, CI 3.96–9.11) compared to blacks (ORB = 4.5, CI 3.21–6.24) but with overlapping confidence intervals.
Ever having a stroke was associated with increased odds of dementia (ORW = 3.0, CI 2.56–3.43; ORB = 2.3, CI 1.63–3.35; ORH = 2.9, CI 1.64–5.11). Untreated hypertension was associated with increased risk of dementia for white, blacks, and Hispanics (ORW = 2.0, CI 1.63–2.35; ORB = 1.6, CI 1.04–2.43; ORH = 1.7, CI 1.00–2.94). Compared to no hypertension, treated hypertension was associated with a reduction in odds of dementia for whites (ORW = 0.82, CI 0.75–0.90) and no difference in odds for blacks and Hispanics (ORB = 1.01, CI 0.76–1.35; ORH = 1.05, CI 0.75–1.47). Older whites, blacks, and Hispanics with diabetes and who report receiving oral drug treatment for the disease did not have a statistically increased odds of dementia compared to those without diabetes (ORW = 1.09, CI 0.95–1.25; ORB = 0.95, CI 0.70–1.28; ORH = 1.12, CI 0.79–1.59). Use of insulin, an indicator of severity of disease, was associated with an increased odds of dementia only for whites (ORW = 1.5, CI 1.24–1.92). Untreated heart disease was associated with increased odds of dementia for whites only (ORW = 1.4, CI 1.19–1.59; ORB = 1.2, CI 0.82–1.64; ORH = 1.4, CI 0.85–2.45).
Among the other modifiable risk factors, being overweight and obese were associated with lower odds of dementia across all racial and ethnic groups (overweight: ORW=ORB=ORH = 0.7). Having some vigorous exercise, relative to none/rare, was associated with lower odds of dementia for whites, blacks, and Hispanics and the association was significantly different and lower for whites compared to blacks and Hispanics (ORW = 0.34, CI 0.32–0.37; ORB = 0.62, CI 0.50–0.77; ORH = 0.55, CI 0.42–0.72). Educational attainment and high household wealth were also significantly associated with lower odds of dementia for whites, blacks, and Hispanics.
3.1. Sensitivity analysis
We performed sensitivity analysis and broke down education into finer categories (0–5 years [reference group], 6–9 years, 10–12 years, 13–15 years, 15 or more years of education). We found similar education gradient, where higher education was associated with lower odds of dementia across all racial/ethnic groups. The protective effect of education on dementia occurred in those with at least 6 years of education in all racial/ethnic groups, and higher education was associated with lower odds of dementia.
In addition, we also ran sensitivity analysis excluding the CIND group from controls (Table A.2) and compared participants with the highest versus lowest cognitive status. The results were qualitatively unchanged while, as expected, the estimated ORs increased. For example, blacks and Hispanics had higher odds of dementia compared to whites [ORBlack = 3.26 (Table A.2) vs. 2.18 (Table 2), ORHispanics = 1.91 (Table A.2) vs. 1.47 (Table 2)].
We also perform sensitivity analysis testing the differences in risk factors by racial/ethnic groups in a fully interacted model. This model included all two-way interaction of risk factors and racial/ethnic groups (i.e., race × risk factors interactions). Race and disease interaction effects (hypertension, diabetes, heart disease, and stroke) were not statistically significant.
4. Discussion
4.1. Racial/ethnic differences in dementia prevalence
To the authors' knowledge, this is the first study of dementia by racial and ethnic groups using a large nationally representative survey of Americans aged 65 years or older and spanning 12 years. Prior evidence on racial/ethnic disparities relied heavily on comparisons of dementia across studies. There have been systematic reviews of dementia prevalence across multiple subpopulations within the United States showing racial/ethnic disparities, but strong variations exist between and within populations in terms of methods used and assessment [38], [39], [40]. One study used health care claims but, without data on socioeconomic status, could not examine if racial/ethnic disparities exist after adjusting for these factors [41]. We used the same diagnostic classification strategy over 12 years for all racial/ethnic groups. We found that racial/ethnic disparity in dementia prevalence continues to persist over 12 years and only narrowing for blacks. After adjustment for modifiable and nonmodifiable risk factors, blacks and Hispanics had 2.0 and 1.5 times the odds of dementia, respectively, compared to whites. This was consistent with other studies [7], [8] that support the robustness of this dementia measure for racial/ethnic minorities.
4.2. Racial/ethnic differences in dementia risk factors
This study focused on disease risk factors and the use of drug treatment for these risk factors and examined differential association with dementia for whites, blacks, and Hispanics. Prevalence of cardiovascular diseases such as hypertension, diabetes, heart disease, and stroke in all racial/ethnic groups increased over time, but the rate of increase was highest among Hispanics. There was also an increase in drug treatment uptake over time and a reduction in insulin use for diabetes suggesting improved management of cardiovascular diseases among all racial/ethnic groups in the United States.
While increased prevalence of cardiovascular diseases was associated with increased odds of dementia, we found dementia risk among people using drug therapy for disease was not different compared to those without these diseases. This study illuminated that these associations were not statistically different for whites, blacks, and Hispanics; however, further study on heterogeneous effects across racial/ethnic groups is needed with larger samples sizes of racial/ethnic minorities to improve the precision of the estimates. Several point estimates, for example, on hypertension, were suggestive of differential effects across racial and ethnic groups.
In additional analyses, we estimated the effects of use of cholesterol-lowering drugs (data only available beginning in 2006). We found that the use of a cholesterol-lowering drug was associated with reduction in odds of dementia by 23% across all groups with higher reductions in whites compared to blacks and Hispanics (ORw = 0.73, CI 0.64–0.83; ORB = 0.84, CI 0.57–1.25; ORH = 0.85, CI 0.50–1.47). This supports research findings using Medicare claims data on the protective association of statins and Alzheimer's disease [25].
With the limited number of novel treatments in development, identifying therapeutics for other diseases that have potential to reduce dementia risk may be a promising approach for reducing risk and addressing racial disparities in care and prevention. Multiple therapies for treatment of high-prevalence conditions such as diabetes, hypertension, and hyperlipidemia have been associated with a reduction in dementia risk. Blacks and Hispanics have higher rates of all three conditions, and identifying drugs for which conditions influence risk, and for whom, may be an effective near-term strategy for reducing dementia development and onset.
We found that education level and wealth were positively associated with reduced dementia risk. Education attainment increased over 12 years, particularly among older blacks and whites. Prior studies also found that higher education was associated with better cognitive function consistent with the cognitive reserve hypothesis that links education with better tolerance of age-related brain changes and thus function [42], [43]. The pathways through which environmental factors, including socioeconomic determinants, function and interact and lead to health differences across populations are complex, shaped in part by geographical and political factors that reduce access to high-quality schooling, constrain employment choices, and diminish financial resources and reduce access to quality health care [10]. Other sociocultural factors, such as poor or negative interactions with the health care system, may reduce health care over the lifecycle, resulting in onset and poor treatment of diseases that increase risk of dementia.
4.3. Racial/ethnic differences in dementia trends over 12 years
Using the same data as in a recent study, we confirmed the decreasing trend in dementia prevalence from 2000 to 2012 [16] but added to our understanding how trends differed for whites, blacks, and Hispanics. Between 2000 and 2012, whites and blacks experienced a 25% reduction in dementia prevalence, whereas the reduction among Hispanics was 8% and was driven by the decline from 2000 and 2002—thereafter, dementia prevalence remained constant among Hispanics. Disparities in dementia prevalence between whites and blacks declined, driven by the larger decline in prevalence among blacks. Disparities between whites and Hispanics increased over this period.
4.4. Limitations
Our study has its limitations. We found that disparities in dementia prevalence were not driven by differences in the relative contributions of the risk factors: the magnitude of association between a given risk factor and dementia risk was not statistically different for whites, blacks, and Hispanics. However, sample size of our populations of blacks and Hispanics is small, and thus, confidence intervals around some estimates of risk factors are large.
Results revealed no increase in risk of dementia associated with chronic diseases among those using drug treatments for the disease relative to not having the disease. For example, we found treated hypertension reduced dementia prevalence relative to those without the disease for whites and there was no difference for blacks and Hispanics. The use of drug treatment may be associated with unobservable factors that are also correlated with dementia and may differ across racial/ethnic groups. The data do not provide sufficient detail to understand the mechanism of the effect or variation by race/ethnicity. For example, there may be differences across race/ethnicity in class of hypertensive drug used. Recent studies reported that blacks had higher rates of poor cardiovascular outcome when using angiotensin-converting enzyme inhibitors compared to whites [44], [45]. In addition, while the HRS has self-reported chronic diseases and treatment, we do not have a full history of patients' diagnosis and how long they had been treated for.
Measurement of dementia is based on cognitive tests and thus subject to measurement error. Measurement error may vary across racial and ethnic groups and may be more severe for blacks and Hispanics if correlated with factors such as educational attainment. Our models, however, control for many factors that may be associated with this type of measurement error. While validation studies based on a clinical evaluation show high concordance with our dementia measure, as described in the study by Langa et al. 2017, validation was not assessed by race/ethnicity.
Differential time trends in dementia across racial and ethnic groups may be affected by the declining proportion of the sample represented by a proxy over time that varied across racial and ethnic groups. We estimated models by race that also controlled for proxy response and the results were robust. The increased risk of dementia for blacks and Hispanics relative to whites is reduced but not eliminated after adjusting for a rich set of observable risk factors associated with dementia that vary across racial and ethnic groups. Future studies are needed to examine other differences across racial and ethnic groups affecting dementia risk such as the presence of APOE ε4 alleles, sleep duration, diet, and type of therapeutic drugs being used to treat chronic conditions.
5. Conclusion
Racial disparities in dementia are significant and have declined over time for blacks but not for Hispanics. While disparities may be reduced by increasing levels of cognitive reserve and prevention and management of disease among blacks and Hispanics, there also remains a complex combination of socioeconomic and cultural factors associated with these disparities. Health disparities often are seen through the lens of access to care or resources, though a lack of diversity in clinical therapeutic development means that surmounting access barriers will not reduce disparities if therapeutics target only a small fraction of the diverse population. Public health prevention programs aimed at reducing cardiovascular risks such as hypertension, diabetes, and stroke or at improving the management of these diseases may both improve the quality of life of the elderly and lower dementia risk. These programs should also consider the complex combination of socioeconomic and cultural factors associated with racial/ethnic disparities in dementia risk, while research must identify treatment options for racial and ethnic minorities by recruiting diverse participants into clinical trials of new therapeutics.
Research in Context.
-
1.
Systematic review: In a PubMed literature review, recent population-based studies have shown declines in dementia incidence or prevalence in high-income countries. Studies on racial and ethnic disparities in dementia prevalence over time are lacking. Disparities over time will depend on both differences in risk factors and variation in their relative impact for whites, blacks, and Hispanics.
-
2.
Interpretation: We found that racial/ethnic disparities in prevalence exist. Both whites and blacks had a 25% reduction in prevalence from 2000 to 2012 and disparities between them declined. Hispanics trend remained unchanged from 2002 to 2012. Better management of chronic diseases and higher levels of educational attainment and wealth explained declining dementia prevalence over time.
-
3.
Future direction: Future research is needed to examine other differences across racial and ethnic groups affecting dementia risk such as the presence of APOE ε4 alleles, sleep, diet, and type of therapeutic drugs used to treat chronic conditions.
Acknowledgments
This research was supported by the National Institutes on Aging (1R03AG054120-01, P30AG043073-01, and 5P30AG024968). Dr. Chen is supported through the Overseas Postdoctoral Fellowship funded by the National University of Singapore. The Health and Retirement Study is performed at the Institute for Social Research, University of Michigan, Ann Arbor. The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Authors' contributions: Drs Chen and Zissimopoulos had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design were performed by Chen and Zissimopoulos. Acquisition, analysis, or interpretation of data were performed by Chen and Zissimopoulos. Drafting of the manuscript was performed by Chen and Zissimopoulos. Critical revision of the manuscript for important intellectual content was performed by Chen and Zissimopoulos. Statistical analysis was conducted by Chen and Zissimopoulos. Study supervision was performed by Zissimopoulos. The contents and views in this article are those of the authors and not the views of the National Institutes of Health or any of the sponsoring organizations and agencies. The authors would like to thank Patricia St. Clair, Yolanda Zhu, and Kenwin Maung for their help in producing and computerizing the data.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.trci.2018.08.009.
Supplementary data
References
- 1.Assoc A. Alzheimer's Association Report 2015 Alzheimer's disease facts and figures. Alzheimers Demen. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurd M.D., Martorell P., Langa K.M. Monetary costs of dementia in the United States. N Engl J Med. 2013;369:489–490. doi: 10.1056/NEJMc1305541. [DOI] [PubMed] [Google Scholar]
- 4.Zissimopoulos J., Crimmins E., St Clair P. The Value of Delaying Alzheimer's Disease Onset. Forum Health Econ Policy. 2014;18:25–39. doi: 10.1515/fhep-2014-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans D.A., Bennett D.A., Wilson R.S., Bienias J.L., Morris M.C., Scherr P.A. Incidence of Alzheimer disease in a biracial urban community - Relation to apolipoprotein E allele status. Arch Neurol. 2003;60:185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 6.Lines L.M., Sherif N.A., Wiener J.M. RTI Press; Research Triangle Park (NC): 2014. Racial and ethnic disparities among individuals with Alzheimer's disease in the United States: A literature review (RTI Press publication No. RR-0024-1412) Available from: https://pdfs.semanticscholar.org/7ed5/b9ed14cd3e002df546134e76766d01c5c4aa.pdf. [Google Scholar]
- 7.Shadlen M.F., Siscovick D., Fitzpatrick A.L., Dulberg C., Kuller L.H., Jackson S. Education, cognitive test scores, and black-white differences in dementia risk. J Am Geriatr Soc. 2006;54:898–905. doi: 10.1111/j.1532-5415.2006.00747.x. [DOI] [PubMed] [Google Scholar]
- 8.Tang M.X., Cross P., Andrews H., Jacobs D.M., Small S., Bell K. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56:49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Yaffe K., Falvey C., Harris T.B., Newman A., Satterfield S., Koster A. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. Bmj-British Med J. 2013:347. doi: 10.1136/bmj.f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill C.V., Pérez-Stable E.J., Anderson N.A., Bernard M.A. The National Institute on Aging Health Disparities Research Framework. Ethn Dis. 2015;25:245–254. doi: 10.18865/ed.25.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilkinson R., Marmot M., editors. Social Determinants of Health: The Solid Facts. Second Edition. World Health Organization, Regional Office for Europe. WHO Library Cataloging in Publication Data; 2003. Available from: http://www.euro.who.int/__data/assets/pdf_file/0005/98438/e81384.pdf. [Google Scholar]
- 12.Stringhini S., Dugravot A., Shipley M., Goldberg M., Zins M., Kivimaki M. Health behaviours, socioeconomic status, and mortality: further analyses of the British Whitehall II and the French GAZEL prospective cohorts. Plos Med. 2011;8:e1000419. doi: 10.1371/journal.pmed.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scriven A. Cognitive Aging: Progress in Understanding and Opportunities for Action. Perspect Public Health. 2016;136:108. 108. [Google Scholar]
- 14.Mensah G.A., Mokdad A.H., Ford E.S., Greenlund K.J., Croft J.B. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 15.Larson E.B., Yaffe K., Langa K.M. New Insights into the Dementia Epidemic. New Engl J Med. 2013;369:2275–2277. doi: 10.1056/NEJMp1311405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langa K.M., Larson E.B., Crimmins E.M., Faul J.D., Levine D.A., Kabeto M.U. A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012. Jama Intern Med. 2017;177:51–58. doi: 10.1001/jamainternmed.2016.6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assoc A. 2012 Alzheimer's disease facts and figures. Alzheimers Demen. 2012;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Sachs-Ericsson N., Blazer D.G. Racial differences in cognitive decline in a sample of community-dwelling older adults - The mediating role of education and literacy. Am J Geriatr Psychiatry. 2005;13:968–975. doi: 10.1176/appi.ajgp.13.11.968. [DOI] [PubMed] [Google Scholar]
- 19.Chin A.L., Negash S., Hamilton R. Diversity and Disparity in Dementia: The Impact of Ethnoracial Differences in Alzheimer Disease. Alzheimer Dis Associated Disord. 2011;25:187–195. doi: 10.1097/WAD.0b013e318211c6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrd D.A., Sanchez D., Manly J.J. Neuropsychological test performance among Caribbean-born and US-born African American elderly: The role of age, education and reading level. J Clin Exp Neuropsychol. 2005;27:1056–1069. doi: 10.1080/13803390490919353. [DOI] [PubMed] [Google Scholar]
- 21.Meng X.F., D'arcy C. Education and Dementia in the Context of the Cognitive Reserve Hypothesis: A Systematic Review with Meta-Analyses and Qualitative Analyses. Plos One. 2012;7 doi: 10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glymour M.M., Manly J.J. Lifecourse Social Conditions and Racial and Ethnic Patterns of Cognitive Aging. Neuropsychol Rev. 2008;18:223–254. doi: 10.1007/s11065-008-9064-z. [DOI] [PubMed] [Google Scholar]
- 23.Satizabal C.L., Beiser A.S., Chouraki V., Chene G., Dufouil C., Seshadri S. Incidence of Dementia over Three Decades in the Framingham Heart Study. New Engl J Med. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews F.E., Arthur A., Barnes L.E., Bond J., Jagger C., Robinson L. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382:1405–1412. doi: 10.1016/S0140-6736(13)61570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zissimopoulos J.M., Barthold D., Brinton R.D., Joyce G. Sex and Race Differences in the Association Between Statin Use and the Incidence of Alzheimer Disease. Jama Neurol. 2017;74:225–232. doi: 10.1001/jamaneurol.2016.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langa K.M., Larson E.B., Karlawish J.H., Cutler D.M., Kabeto M.U., Kim S.Y. Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimers Demen. 2008;4:134–144. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vemuri P., Lesnick T.G., Przybelski S.A., Machulda M., Knopman D.S., Mielke M.M. Association of Lifetime Intellectual Enrichment With Cognitive Decline in the Older Population. Jama Neurol. 2014;71:1017–1024. doi: 10.1001/jamaneurol.2014.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stern Y., Albert S., Tang M.X., Tsai W.Y. Rate of memory decline in AD is related to education and occupation - Cognitive reserve? Neurology. 1999;53:1942–1947. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- 29.Ryan C.L. U.S. Census Bureau, U.S. Department of Commerce; 2016. Educational Attainment in the United States: 2015. Available from: https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. [Google Scholar]
- 30.Crimmins E.M.H.M., Seeman T.E. Race/Ethnicity, Socioeconomic Status, and Health. In: Anderson N.B., Cohen B., editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. National Academies Press (US); Washington (DC): 2004. [PubMed] [Google Scholar]
- 31.Sonnega A., Faul J.D., Ofstedal M.B., Langa K.M., Phillips J.W.R., Weir D.R. Cohort Profile: the Health and Retirement Study (HRS) Int J Epidemiol. 2014;43:576–585. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ofstedal M.B., Weir D.R. Recruitment and retention of minority participants in the health and retirement study. Gerontologist. 2011;51:S8–S20. doi: 10.1093/geront/gnq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Health and Retirement Study (HRS) 2011. Sample Sizes and Response Rates. [Google Scholar]
- 34.Fisher G., Hassan H., Rodgers W., Weir D. 2013. Health and Retirement Study Imputation of Cognitive Functioning Measures 1992–2010.http://hrsonline.isr.umich.edu/ Available from: Accessed June 13, 2017. [Google Scholar]
- 35.Ofstedal M.B., Fisher G., Herzog A.R. 2005. Documentation of Cognitive Functioning Measures in the Health and Retirement Study.http://hrsonline.isr.umich.edu/ Available from: Accessed June 8, 2018. [Google Scholar]
- 36.Crimmins E.M., Kim J.K., Langa K.M., Weir D.R. Assessment of Cognition Using Surveys and Neuropsychological Assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66:162–171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibbons R.D., Hedeker D., DuToit S. Advances in analysis of longitudinal data. Annu Rev Clin Psychol. 2010;6:79–107. doi: 10.1146/annurev.clinpsy.032408.153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta K.M., Yeo G.W. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Demen. 2017;13:72–83. doi: 10.1016/j.jalz.2016.06.2360. [DOI] [PubMed] [Google Scholar]
- 39.Erkinjuntti T., Ostbye T., Steenhuis R., Hachinski V. The effect of different diagnostic criteria on the prevalence of dementia. New Engl J Med. 1997;337:1667–1674. doi: 10.1056/NEJM199712043372306. [DOI] [PubMed] [Google Scholar]
- 40.Glymour M.M., Kosheleva A., Wadley V.G., Weiss C., Manly J.J. Geographic Distribution of Dementia Mortality Elevated Mortality Rates for Black and White Americans by Place of Birth. Alzheimer Dis Associated Disord. 2011;25:196–202. doi: 10.1097/WAD.0b013e31820905e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayeda E.R., Glymour M.M., Quesenberry C.P., Whitmer R.A. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Demen. 2016;12:216–224. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bennett D.A., Wilson R.S., Schneider J.A., Evans D.A., de Leon C.F.M., Arnold S.E. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60:1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 44.Gupta A.K. Racial differences in response to antihypertensive therapy: does one size fits all? Int J Prev Med. 2010;1:217–219. [PMC free article] [PubMed] [Google Scholar]
- 45.Ogedegbe G., Shah N.R., Phillips C., Goldfeld K., Roy J., Guo Y. Comparative Effectiveness of Angiotensin-Converting Enzyme Inhibitor-Based Treatment on Cardiovascular Outcomes in Hypertensive Blacks Versus Whites. J Am Coll Cardiol. 2015;66:1224–1233. doi: 10.1016/j.jacc.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.