Abstract
Background
Liver injury is a known feature of severe malaria, but is only incidentally investigated in uncomplicated disease. In such cases, drug-induced hepatotoxicity is often thought to be the primary cause of the observed liver injury, and this can be a major concern in antimalaria drug development. We investigated liver function test (LFT) abnormalities in patients with imported uncomplicated malaria, and in Controlled Human Malaria Infection (CHMI) studies.
Methods
Clinical and laboratory data from 484 imported malaria cases and 254 CHMI participants were obtained from the Rotterdam Malaria Cohort database, and the Radboud University Medical Center database (between 2001 and 2017), respectively. Routine clinical LFTs, clinical profiles, parasite densities, hematological, and inflammation parameters were assessed in 217 patients with imported falciparum malaria upon admission, and from longitudinal data of 187 CHMI participants.
Findings
Upon admission, the proportion of patients with imported uncomplicated malaria and elevated liver enzymes was 128/186 (69%). In CHMI, 97/187 (52%) participants showed LFT abnormalities, including mild (64%, >1.0 ≤ 2.5× upper limit of normal (ULN)), moderate (20%, >2.5 ≤ 5.0xULN) or severe (16%, >5.0xULN). LFT abnormalities were primarily ALT/AST elevations and to a lesser extent γGT and ALP. LFT abnormalities peaked shortly after initiation of treatment, regardless of drug regimen, and returned to normal within three to six weeks. Positive associations were found with parasite burden and inflammatory parameters, including cumulative inflammatory cytokine responses and oxidative stress markers (r = 0·65, p = 0·008, and r = −0·63, p = 0·001, respectively).
Interpretation
This study shows that reversible liver injury is a common feature of uncomplicated falciparum malaria, most likely caused by an enduring pro-inflammatory response post treatment. The recognition of this phenomenon is of clinical relevance for individual patient care as well as clinical development of (new) antimalarial drugs.
Fund
PATH Malaria Vaccine Initiative (MVI)
Research in context.
Evidence before this study
We conducted an online literature search on liver function test (LFT) abnormalities in uncomplicated malaria using PubMed for articles published up to May 1st, 2018. Articles were searched by title and abstract using the terms “hepatic dysfunction” or “liver injury” or “liver function test” or “liver enzymes” or “transaminases”, with either “malaria” or “uncomplicated malaria” or “non-severe malaria” without language restrictions. Some articles cited in the screened publications were also included. We screened 58 articles, 26 of which described liver injury in malaria focusing on severe disease. Only four contained information on LFT abnormalities in uncomplicated disease specifically. Most other studies described hepatic adverse reactions in the context of antimalarial drugs. The most descriptive study was a recent retrospective study by Woodford et al… that provides data on LFT dynamics. Ramirez et al described the proportion of LFT abnormalities on admission day in a case control study. In these studies, no clear associations were found between clinical and demographic factors, and LFT abnormalities in uncomplicated malaria. The pathophysiological basis of liver injury has only been described in animals to date.
Added value of this study
To our knowledge, this is the first study using longitudinal and prospective data from CHMI studies to characterize liver injury specifically in uncomplicated falciparum malaria. This study adds novel insights into clinical relevance, dynamics and pathophysiological basis of liver injury. We provide evidence that the underlying pathogenesis of liver injury in uncomplicated malaria is unlikely to be related to hepatotoxicity of antimalarials, and conceivably differs from that during severe malaria. Induced inflammation per se may be a major driver of liver injury since parasite load in CHMI is extremely low but sufficient to effect systemic inflammation and oxidative stress.
Implications of all the available evidence
Recognizing and understanding this relatively common phenomenon is of significant importance for both drug-related clinical decision making and development of antimalarial drugs. Incorrect attribution of liver injury to an antimalarial drug can lead to unnecessary discontinuation or shifts in drug regimens; instead, a more expectative approach might be warranted. Clinical trials testing the safety and efficacy of novel antimalarials should carefully (re)consider unnecessary early abrogation of promising drugs with supposed hepatotoxic effects. Supportive therapeutics with hepatotoxic potential during a malaria infection should, however, be limited or given with appropriate monitoring.
Alt-text: Unlabelled Box
1. Introduction
Malaria continues to play a critical role in the global infectious disease burden with significant morbidity and mortality, especially in sub-Saharan Africa. International travelers are also at risk in >90 countries worldwide, mainly in Africa, Asia, and the Americas [1]. Clinical malaria is characterized by a systemic inflammatory response induced by asexual Plasmodium parasites. Plasmodium falciparum is responsible for most malaria-related deaths globally [1]. Severity of disease is determined by multiple factors, including parasite species and timing of antimalarial treatment [2]. The majority of severe falciparum malaria manifestations are most likely related to cytoadherence of parasitized red blood cells (RBCs) to the vascular endothelium, to each other (platelet-mediated agglutination) [3], or to uninfected erythrocytes (rosetting) [4,5]. The subsequent sequestration in the microvasculature results in decreased local oxygen supply and a disturbed metabolism, eventually leading to vital organ failure.
Hepatic dysfunction and jaundice are common features of severe malaria [[6], [7], [8], [9]]. Histopathological changes in the liver range from hepatocyte necrosis, granulomatous lesions, Kupffer cell hyperplasia, malarial pigmentation, cholestasis, monocyte infiltrations to malarial nodules [7,10,11]. These complications can contribute significantly to liver failure and other systemic complications [9,12,13]. The underlying pathogenesis of liver damage is largely unknown. Furthermore, liver involvement has only been incidentally investigated in uncomplicated malaria [[14], [15], [16]].
Previously, we regularly observed pronounced elevations in liver function tests (LFTs) often shortly upon initiation of curative treatment in patients with imported uncomplicated malaria. We hypothesized that liver injury might arise through a mechanism other than drug-induced hepatotoxicity, or the substantial sequestration observed in severe disease [4,17]. Understanding this clinical feature of uncomplicated malaria could support clinicians in drug-related decision making, and in particular drug development, where frequency of adverse hepatic reactions is a leading concern. Here, we performed a retrospective analysis to determine the frequency of LFT abnormalities in patients with imported uncomplicated malaria, and used longitudinal prospective data of controlled human malaria infection (CHMI) studies to further investigate the significance, dynamics and pathophysiological basis of liver injury.
2. Methods
2.1. Study design and population
Clinical and laboratory data from 484 imported malaria cases and 254 individuals participating in 17 CHMI studies were obtained from the Rotterdam Malaria Cohort database, and the Radboud University Medical Center database, respectively. The Rotterdam Malaria Cohort consists of patients diagnosed with imported malaria who presented at the Rotterdam Harbour Hospital or the Erasmus University Medical Center between 2002 and 2017. For this study, clinical and laboratory data from patients with imported P. falciparum malaria were selected (S1 figure). Patients with a known history of liver disease, or concomitant infections at admission were excluded (e.g. hepatitis B, HIV, schistosomiasis, urinary tract infections, and respiratory tract infections). Furthermore, patients that received antimalarial treatment prior admission were also excluded. Data on concomitant medication use other than antimalarial treatment, antipyretic therapy (e.g. acetaminophen) or information on (chronic) alcohol intake were not available. Because of the retrospective nature of the analysis and use of anonymized data, ethical approval or informed consent was not necessary, as stated in the Medical Research involving Human Subject Act (WMO).
Healthy malaria-naïve male and female participants aged 18–35 years were recruited for the CHMI studies between 2001 and 2016. Extended screening included hematology and biochemistry parameters, serology for asexual stages of P. falciparum, and hepatitis B, C and HIV, as previously described [16,18]. All study participants provided written informed consent at screening visit. All clinical trials were approved by the Radboudumc Committee on Research Involving Human Subjects (CMO), the Central Committee on Research Involving Human Subjects (CCMO) of the Netherlands, or the Western Institutional Review Board (WIRB). The studies were conducted according to the principles outlined in the Declaration of Helsinki and Good Clinical Practice standards, and registered at ClinicalTrials.gov. Study EHMI-3, conducted in 2001, was not registered in an online database such as ClinicalTrials.gov.
2.2. Controlled human malaria infection
CHMI participants were all challenged by bites of malaria-infected mosquitoes or direct venous inoculation of sporozoites/blood stage parasites. The challenges were conducted with different parasite clones: NF54 (airport malaria) [19], 3D7 (clone of NF54) [20], NF135 (Cambodia) [21], and NF166 (Guinea, West Africa) [22]. Daily blood sampling included parasitological assessment (thick blood smears and qPCR) and/or safety laboratory parameters.
2.3. Laboratory investigations
All clinical laboratory data were assessed by standard hematological and biochemical tests on peripheral blood specimens. Biochemical liver tests included: aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (γGT), alkaline phosphatase (ALP), lactate dehydrogenase (LD), and total bilirubin. Reference laboratory values for AST were < 30 or < 35 U/L (female or male); ALT <35 or < 45 U/L (female or male); γGT <40 or < 55 U/L (female or male); ALP <100 or < 115 U/L (female or male); total bilirubin <17 μmol/L; LD <250 U/L (S2 table).
2.4. Inflammatory cytokines and oxidative stress analysis
Inflammatory cytokines (IL-1β, IFN-α, IFN-γ, TNF-α, MCP-1, IL-6, IL-8, IL-10, IL-12p70, IL-17A, IL-18, IL-23, and IL-33) were determined in study CHMI-trans1 using flow-based multiplex (Legendplex). For the oxidative stress analysis, TaqMan gene expression Assays (Applied Biosystem) were used to measure antioxidant gene expression levels for Superoxide dismutases (SOD1 and SOD2), Nitric oxide synthase (NOS3), Catalase (CAT), Peroxiredoxin (PRDX1) and Glutathione S-transferase Kappa (GSTK1) from whole blood by using qPCR (S1 Material). The cumulative anti-oxidant and cytokine responses were determined through transformation of data into a Z-score (each value subtracted by the mean of the group and divided by the SD of the group).
2.5. Definitions
Liver function test (LFT) abnormalities were graded using an adaptation of the World Health Organization (WHO) Adverse Event Grading System 2003: mild LFT elevations (>1·0 ≤ 2·5× upper limit of normal (ULN)), moderate (>2·5 ≤ 5·0xULN), and severe (>5·0XULN) elevations. Imported malaria patients were classified as having uncomplicated malaria by absence of WHO criteria 2014 [23] for severe P. falciparum malaria on admission or during hospitalization. Abnormal bilirubin levels were not included in the incidence or grading of LFT abnormalities, since the bilirubin increases found in the imported malaria cases most likely reflect haemolysis and could possibly bias the elevated liver enzyme prevalence in imported malaria. Similarly, lactate dehydrogenase (LD) was not included in the incidence or grading of LFT abnormalities, due to its strong relation to haemolysis and low liver specificity. Patients with imported malaria were considered partially-, semi-, or non-immune. In short, patients born in a malaria-endemic country living in the Netherlands were considered partially immune. Patients that were born and still residing in a malaria-endemic area were presumed semi-immune. All other patients were considered non-immune [24]. Data on previous episodes of malaria were not available.
The parasite load was estimated by measuring the area under the curve of parasitaemia over time, and computed by GraphPad Prism 5 (2009, San Diego California USA).
2.6. Statistical analysis
All data were analyzed with GraphPad Prism 5 or SPSS software version 24 (IBM Inc., Chicago, IL, USA). Descriptive analyses are presented as percentages, mean with standard deviation, or median with inter-quartile range. Mann Whitney U test was used for comparison of non-normal continuous data or independent groups/samples. Chi square test or Fisher's exact test was used for comparison of qualitative variables and for determining association between various variables. Correlation between different parameters was assessed by Spearman's rho. A P-value of <0·05 was considered as statistically significant.
3. Results
The general characteristics on admission of the 217 included patients with imported P. falciparum malaria are shown in Table 1 and S1 table. On admission day, LFTs were increased in 128/186 (69%) of patients with uncomplicated falciparum malaria (Table 1) and 27/31 (87%) in severe disease (S1 table). In uncomplicated disease, abnormalities appeared to be mild in 93/186 (50%), moderate in 26/186 (14%), and severe in 9/186 (4·8%) of the cases. The median ALT of all uncomplicated malaria patients with LFT abnormalities was 48 U/L (range 15–242 U/L, IQR 34–68 U/L), and the median AST was 41 U/L (range 9–326 U/L, IQR 31–63 U/L). The median γGT was 71 U/L (range 20–447 U/L, IQR 49–113 U/L), the median ALP was 83 U/L (range 36–265 U/L, IQR 64–101 U/L), the median total bilirubin was 22 μmol/L(3–115 μmol/L, IQR 17–43 μmol/L), and the LD was 286 μmol/L (range 52–656 μmol/L, IQR 113–346 μmol/L). No statistically significant differences were found between ages, gender, or immune status in patients with- or without LFT abnormalities. Furthermore, LFT abnormalities appeared in all treatment groups (Table 1). In a sub-group analysis of the malaria cohort, increases in LFT abnormalities were also found in 23/50 (46%) of the patients with a Plasmodium vivax infection (S1 table). No significant differences in other parameters than in LFTs between cases with and without LFT abnormalities were observed (S1 table).
Table 1.
General characteristics of patients with imported uncomplicated falciparum malaria on admission day.
| No LFT# |
LFT# |
p-value |
|
|---|---|---|---|
| (n = 58) | (n = 128) | ||
| Age | 37 (29–50) | 39 (30–47) | 0·960 |
| Gender | 0·390 | ||
| Female | 18 (31%) | 32 (25%) | |
| Male | 40 (69%) | 96 (75%) | |
| Immunity | 0·270 | ||
| Unknown | 3 (5%) | 1 (1%) | |
| Non-immune | 27 (47%) | 57 (45%) | |
| Partially | 26 (45%) | 65 (51%) | |
| Semi | 2 (3%) | 5 (4%) | |
| Parasitaemia/μL | 7·611 (1·582–44·550) | 5·466 (1·146–47·100 | 0·811 |
| AST (U/L) | 21 (17–26) | 41 (31–63) | <0·001 |
| ALT (U/L) | 25 (19–30) | 48 (34–68) | <0·001 |
| γGT (U/L) | 30 (20–37) | 71 (49–113) | <0·001 |
| Alkaline phosphatase (U/L) | 65 (57–79) | 83 (64–101) | <0·001 |
| Bilirubin (total) (μmol/L) | 21 (10–28) | 22 (15–31) | 0·039 |
| LD (U/L) | 275 (196–304) | 286 (113–346) | 0·181 |
| Haemoglobin (mmol/L) | 8·1 (7·4–8·9) | 8·7 (7·7–9·4) | 0·006 |
| Leukocytes (x109/L) | 4·9 (4·0–5·8) | 4·9 (3·6–6·4) | 0·863 |
| Lymphocytes (x109/L) | 0·9 (0·6–1·4) | 0·9 (0·6–1·4) | 0·998 |
| Thrombocytes (x109/L) | 125 (69–174) | 87 (56–131) | 0·008 |
| CRP (mg/L) | 64 (30–116) | 106 (56–158) | 0·001 |
| Creatinine (μmol/L) | 95 (81–108) | 95 (81–108) | 0·400 |
| Urea (mmol/L) | 4·9 (3·9–6·2) | 5·2 (4·0–6·4) | 0·498 |
| Sodium (mmol/L) | 136 (133–138) | 135 (133–138) | 0·578 |
| Potassium (mmol/L) | 3·8 (3·5–4·2) | 3·7 (3·5–4·0) | 0·299 |
| Lactate (mmol/L) | 1·3 (1·0–1·5) | 1·4 (1·1–1·9) | 0·017 |
| Temperature (°C) | 37·6 (36·9–38·8) | 38·9 (37·5–39·5) | 0·001 |
| Treatment | 0·532 | ||
| atovaquone/proguanil | 50 (86%) | 108 (84%) | |
| quinine | 4 (7%) | 4 (3%) | |
| artemether/lumefantrine | 4 (7%) | 2 (2%) | |
| artesunate | 13 (10%) | ||
| chloroquine | 1 (1%) | ||
| Severity LFT# | n/a | ||
| Mild LFT# | n/a | 93 (73%) | |
| Moderate LFT# | n/a | 26 (20%) | |
| Severe LFT# | n/a | 9 (7%) |
All data are presented as median (IQR) or N (%). LFT# = liver function test abnormalities. Statistical differences are tested using Mann-Whitney or Chi square. Clinical laboratory reference ranges can be found in S2 table.
To better define the dynamics and basis of liver injury, we used data of CHMI studies, which entails extensive screening to minimise confounding effects. Longitudinal data of 17 CHMI studies with a total of 254 participants with patent parasitaemia, showed that LFTs were elevated in 97/187 (52%) of the participants with LFTs measured during infection (Table 2). In most cases standard LFT measurement time-points were obtained at baseline, 2 days post-treatment, and at the end of study. Elevations were primarily limited to ALT/AST elevations, and to a lesser extent, to γGT and ALP. Increased transaminases were found in 83/129 (64%) of the CHMI participants when treated at positive thick smear (treatment threshold of ~ > 5 parasites/μL), including mild (50/83 (60%), >1·0 ≤ 2.5xULN), moderate (18/83 (22%), >2·5 ≤ 5·0xULN) and severe (15/83 (18%), >5·0xULN) elevations (Table 2). The incidence of LFT elevations was 14/58 (24%) in volunteers treated at positive qPCR (treatment threshold of >0·1 parasites/μL), including mild (12/14 (86%)), moderate (1/14 (7%)), and severe (1/14 (7%)) elevations (Table 2;). Among all CHMI participants the median peak ALT was 69 U/L (range 13–870 U/L, IQR 46–98 U/L), and median peak AST was 52 U/L (range 22–723 U/L, IQR 43–85 U/L) from all sample collection time-points during infection. Median peak γGT was 40 U/L (range 11–184 U/L, IQR 26–68 U/L), median peak ALP was 79 U/L (range 17–218 U/L, IQR 67–94 U/L), median peak total bilirubin was 11 μmol/L (range 3–31 μmol/L, IQR 9–15 μmol/L), and median peak LD was 422 μmol/L (range 170–981 μmol/L, IQR 198–396 μmol/L). Only 9/187 (5%) showed mild elevations (>1·0 ≤ 2·5xULN) of total bilirubin levels.
Table 2.
Overview of CHMI studies.
| Study | Year | Challenge | Treatment drug | Subjects with parasitaemia (n) | Mean peak parasiteamia (Pf/ μL) | Subjects with LFT#a (n(%)) | Severity |
Treatment criterion | LFT timepoints | Registered | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mild | moderate | severe | ||||||||||
| EHMI-3 | 2001 | 3D7- mosquito bite | chloroquine | 5 | 21.6 | 4 (80) | 2 | 2 | 0 | 48 h after positive TSb | multiple timepointse | – |
| EHMI-8A | 2007 | NF54- mosquito bite | artemether-lumefantrine | 5 | 15.1 | LFTs not measured during infectiond | positive TSb | – | NCT00442377 | |||
| LSA-3 | 2008 | NF54- mosquito bite | artemether-lumefantrine | 18 | 26.7 | 18 (100) | 10 | 4 | 4 | positive TSb | multiple timepointse | NCT00509158 |
| EHMI-8B | 2009 | NF54- mosquito bite | atovaquone-proguanil | 7 | 3.7 | 4 (57) | 4 | 0 | 0 | positive TSb | Standardc | NCT00757887 |
| TIP1 | 2010 | NF54-NF135 mosquito bite | atovaquone-proguanil | 10 | 17.6 | LFTs not measured during infectiond | positive TSb | – | NCT01002833 | |||
| TIP2 | 2010/2011 | NF54- PfSPZ I.M.⁎ | atovaquone-proguanil | 15 | 43.6 | 5 (33) | 4 | 0 | 1 | positive TSb | Standardc | NCT01086917 |
| ZonMw1 | 2011 | NF54- mosquito bite | atovaquone-proguanil | 12 | 28.5 | LFTs not measured during infectiond | positive TSb | – | NCT01218893 | |||
| EHMI-9 | 2011 | 3D7- mosquito bite or BS challenge⁎⁎ | atovaquone-proguanil | 19 | 9.5 | 11 (58) | 9 | 1 | 1 | positive TSb | standardc | NCT01236612 |
| ZonMw2 | 2012 | NF54- mosquito bite | atovaquone-proguanil | 9 | 27.8 | 6 (67) | 6 | 0 | 0 | positive TSb | standardc | NCT01422954 |
| TIP4 | 2012 | NF135- mosquito bite | atovaquone-proguanil | 19 | 81.1 | 8 (42) | 6 | 2 | 0 | positive TSb | standardc | NCT01660854 |
| TIP3 | 2012 | NF54-NF135-NF166 mosquito bite | atovaquone-proguanil | 15 | 37.4 | LFTs not measured during infectiond | positive TSb | – | NCT01627951 | |||
| TIP5 | 2012/2013 | NF54- mosquito bite | atovaquone-proguanil | 21 | 15.5 | 11 (52) | 4 | 6 | 1 | positive TSb | standardc | NCT01728701 |
| BMGF2a | 2014 | NF54-NF135-NF166 mosquito bite | atovaquone-proguanil | 20 | 20.1 | LFTs not measured during infectiond | 2 pos. qPCR (≥500Pf/mL) | – | NCT02149550 | |||
| BMGF1 | 2014 | NF54- mosquito bite | atovaquone-proguanil | 8 | 0.1 | 0 (0) | 0 | 0 | 0 | pos. qPCR (>100Pf/mL) | standardc | NCT02080026 |
| BMGF2b | 2015 | NF54-NF135-NF166 mosquito bite | atovaquone-proguanil | 31 | 1.5 | 7 (23) | 6 | 0 | 1 | pos. qPCR (>100Pf/mL) | standardc | NCT02098590 |
| BCG-EHMI | 2016 | NF54- mosquito bite | atovaquone-proguanil | 19 | 1.0 | 7 (37) | 6 | 1 | 0 | pos. qPCR (>100Pf/mL) | standardc | NCT02692963 |
| CHMI-trans1 | 2016 | 3D7- mosquito bite | sulfadoxine-pyrimethamine/ piperaquine | 16 | 20.0 | 16 (100) | 5 | 3 | 8 | positive TSb | multiple timepointse | NCT02836002 |
| Total subjects with LFTs measured | 187 | 97 (52) | 62 (33) | 19 (10) | 16 (9) | |||||||
| Total subjects with LFTs measured (thick smear studies) | 129 | 83 (64) | 50 (39) | 18 (14) | 15 (12) | |||||||
| Total subjects with LFTs measured (qPCR studies) | 58 | 14 (24) | 12 (21) | 1 (2) | 1 (2) | |||||||
liver function test abnormalities.
TS = thick smear.
standard LFT measurements at 3 time-points: baseline, 2 days after treatment, and at the end of study.
LFTs are only measured on baseline and at the end of study.
>3 LFT measurement time-points (range of 4–13).
intra-muscular injection.
blood stage challenge.
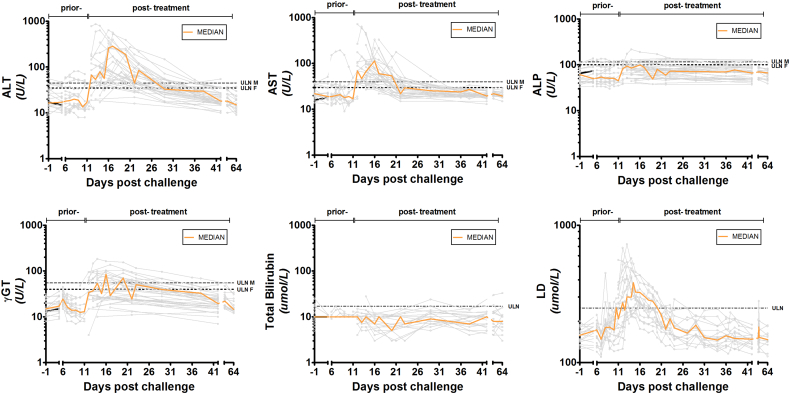
When more frequently assessed over the course of the study (2 CHMI studies, n = 34), mild LFT elevations became apparent just prior to initiation of treatment (Fig. 1). The majority of LFT abnormalities exceeded the upper limit of normal on the day of treatment, and peaked around 2–6 days after initiation of antimalarials. This corroborates with data from EHMI-3 (n = 5) where treatment is initiated 48 h after a positive thick smear. The observed abnormalities were transient, normalizing at study end (around day 35–42 after challenge infection). Furthermore, a distinct pattern of timing of peak of transaminase elevations is found between individuals. Some participants show peak elevations early after treatment and others more delayed. These specific patterns, as well as the severity of the observed abnormalities, seem regardless of different drug regimens used.
Fig. 1.
Course of liver function test (LFT) levels during CHMI. Multiple LFT measurements of the LSA-3 and CHMI-trans1 study (n = 34). LFT sampling time-points differ between studies and individual participants. The line interruption indicates sampling time-points prior- and post-treatment. ALT = alanine aminotransferase, AST = aspartate aminotransferase, γGT = gamma glutamyl transferase, ALP = alkaline phosphatase. Orange curves are the median of all grey individual participants curves per LFT.
To compare the dynamics of LFT abnormalities between CHMI and imported cases, data of 51 patients with imported uncomplicated malaria with multiple LFTs measurements were also obtained to analyze the course of LFT elevations (S2 figure). A similar LFT pattern was found in both naturally-acquired infections and CHMI participants, where LFT elevations peaked after treatment and normalized during follow-up. In contrast to CHMI-participants, total bilirubin levels in patients with imported P. falciparum malaria were increased on admission day, suggesting a concomitant haemolysis most likely due to 2–3 log higher parasite densities.
3.1. Association between parasite load & liver function tests
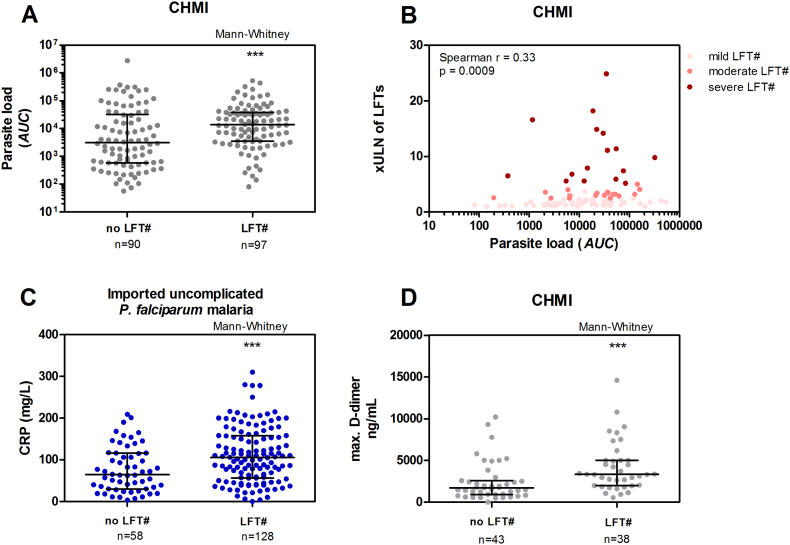
CHMI participants with LFT abnormalities presented with a higher parasite load than those without LFT abnormalities (p < 0.001), although the range was relatively large (Fig. 2A). There was a positive association between LFT values and parasite load in the CHMI studies (r = 0·33, p < 0.001) (Fig. 2B). No significant differences were found between the different groups of LFT severity grades. In addition, in clinical cases with imported uncomplicated P. falciparum infections, a positive association was found between parasite density on admission day, and LFT abnormalities (r = 0·25, p = 0.005).
Fig. 2.
Parasite load, inflammation markers, and liver function test (LFT) abnormalities.
A) Parasite load in CHMI without- (no LFT#), and with LFT abnormalities (LFT#). Error bars represent median with inter-quartile range. Parasite load as the area under the curve (AUC) represents the total parasite exposure over time. B) Association of parasite load and severity of liver function abnormalities in x the upper limit of normal of LFTs (xULN of LFTs) in CHMI subjects. C) C-reactive protein (CRP) levels of patients with imported uncomplicated P. falciparum malaria on admission day. D) Maximum d-dimer levels measured in participants with LFT abnormalities (LFT#) in CHMI. D-dimer data are from 6 CHMI studies (n = 81) with multiple samples measured during challenge infection. Error bars represent the median with inter-quartile range. ***p < 0.001.
4. Clinical laboratory parameters and liver function tests
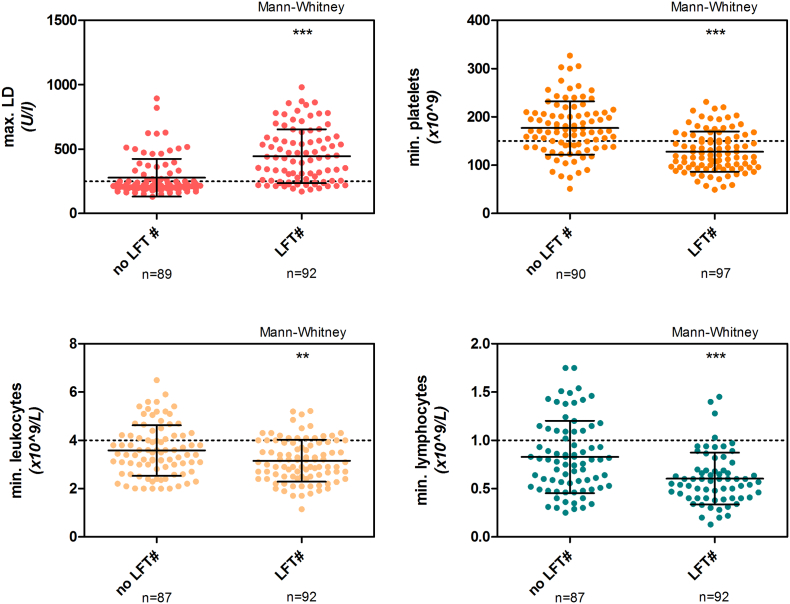
Analysis of clinical laboratory parameters in CHMI participants showed that lowest platelet, leukocyte, and lymphocyte counts, as well as maximum LD levels, independently associated with LFT abnormalities (p < 0.0001, p = 0.01, p < 0.0001, p < 0.0001, respectively) (Fig. 3). However, prior to treatment these parameters poorly predicted LFT abnormalities.
Fig. 3.
Clinical laboratory parameters and liver function test (LFT) abnormalities in CHMI.
Maximum lactate dehydrogenase (LD), and minimum platelets, leukocytes, and lymphocytes measured in CHMI participants without-, and with LFT abnormalities (LFT#). Error bars represent the mean with SD. **P = 0·004; ***p < 0·001.
We hypothesized that inflammation, well known to be involved in malaria pathogenesis, might play a role in these abnormalities. In the imported uncomplicated malaria cases, C-reactive protein (CRP) was increased on the day of admission in patients with LFT abnormalities (p < 0.001) (Fig. 2C). A statistically significant difference was found in maximum d-dimer levels in CHMI participants with or without LFT abnormalities (p < 0.001) (Fig. 2D).
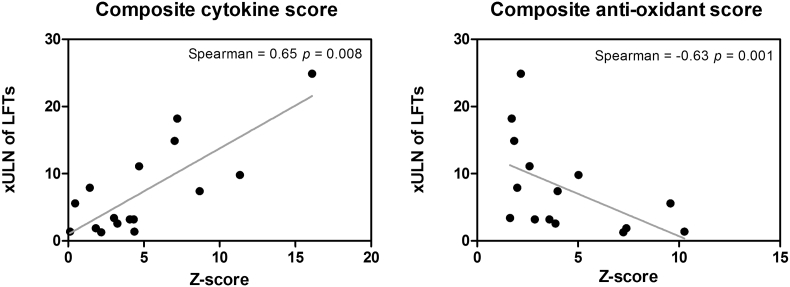
A more extended pro-inflammatory cytokine panel was measured in one CHMI study (CHMI-trans1, n = 16) one day after treatment, showing a positive correlation between IFNγ, IL-6, and IL-8, and peak of the LFT abnormalities, p = 0.039, p = 0.049, and p = 0.015, respectively (S3 table). A strong positive association was also found between the cumulative inflammatory responses (IFNγ, MCP-1, IL-6, IL-8, IL-10, IL12p70, IL-17a, IL-18) and LFT abnormalities (r = 0.65, p = 0.008) (Fig. 4). In a second study (EHMI-8B study, n = 7), a similar positive trend was found for d-dimer, CRP, IFNγ, and IL-6 values (S3 figure).
Fig. 4.
Association between liver function test abnormalities, and cumulative cytokine levels and cumulative anti-oxidant gene expression. The cumulative cytokine score comprises IFNγ, MCP-1, IL-6, IL-8, IL-10, IL12p70, IL-17a, IL-18 and the cumulative anti-oxidant gene expression comprises SOD1, SOD2, NOS3, CAT, GTSK1, and PRDX1measured on 1 day after treatment (DT + 1) in CHMI-trans1. Values are standardized as a Z-score. xULN of LFTs = x upper limit of normal range of liver function tests (LFTs). The xULN of LFTs values are the peak LFT abnormalities measured from day 2 after treatment and the samples available onwards.
Finally, a number of anti-oxidant markers were measured to further define the nature of the induced inflammatory response in relation to LFT abnormalities. A significant reduction in the average of anti-oxidant gene expression SOD1 (p = 0.03), SOD2 (p = 0.001), NOS3 (p < 0.0001), CAT (p < 0.0001), and GTSK1 (p < 0.0001) and an increase in expression of PRDX1 (p = 0.007) was observed during the malaria infection (one day after treatment) (S4 figure). The cumulative anti-oxidant response of these markers through transformation into a Z-score, showed a negative association between the anti-oxidant response and the LFT abnormalities (r = −0.63, p = 0.001) (Fig. 4). All markers normalized by the end of the study.
5. Discussion
This study shows that abnormalities in liver function test (LFT) are a relatively frequent finding in uncomplicated falciparum malaria, and occur regardless of the choice of drug regimen. These abnormalities are transient in nature, resolving within 3–6 weeks. The typical pattern of LFT elevations is primarily limited to ALT/AST elevations, and to a lesser extent, to γGT and ALP, and peaks in particular two to six days after initiation of treatment. Furthermore, the study shows a significant association between LFT elevations and parasite load, inflammatory markers and reduced expression of oxidative stress markers. Early treatment based on qPCR thresholds can decrease the occurrence and severity of LFT abnormalities in CHMI.
Hepatic dysfunction is a well-known feature of severe malaria, contributing to clinically significant complications such as hypoglycaemia, metabolic acidosis, impaired drug metabolism, and finally organ failure [8,12]. Malaria-associated liver damage in uncomplicated malaria, however, has rarely been investigated so far [25]. Observational studies are often restricted in number and limited to admission samples only [15], therefore, underestimating incidence and severity. Other studies do not differentiate non-severe from severe disease in their analysis [9,14,26]. This study specifically advocates the relevance in relation to potential clinical implications in uncomplicated disease, which constitutes the vast majority of clinical malaria cases. This study highlights that LFT abnormalities can be severe (up to >20 xULN), but are apparently fully reversible upon parasitological cure, corroborating previous reports [[25], [26], [27]].
The observed liver injury seems to have a hepatocellular basis, as indicated by the disproportionate elevation in serum transaminases relative to alkaline phosphatase. Peak bilirubin levels precede antimalarial treatment in imported malaria cases but not CHMI. This suggests that these elevations are likely due to haemolysis leading from higher parasitaemia in these patients compared to CHMI volunteers. Similarly, Woodford et al. show that bilirubin elevations in clinical cases peak on admission day, resolving early after treatment in contrast to the later occurring peak of transaminases [14]. The absence of clearly elevated bilirubin levels in CHMI adduces that these events may not result in a functional liver impairment, since conjugation of bilirubin occurred normally. Serum albumin or clotting factors were not tested in the current study, and therefore, it cannot be formally excluded that biosynthetic capacity of the liver may be compromised. The absolute increase in serum transaminases is of little prognostic value since the liver can recover from most forms of acute injury, due to its large regenerative capacity. In absence of symptoms such as haemorrhage or altered consciousness, significant hepatic dysfunction seems highly unlikely in patients without history of liver disease.
The potential contribution of infection transformation, from the liver stage to blood stage infection, to LFT abnormalities remains uncertain. However, similar LFT abnormalities are found CHMI studies with a blood stage challenge, where the liver stage of the parasite is circumvented [14]. This, together with the observation that no substantial LFT abnormalities are found on day 6–7 (day of liver schizont rupture), makes it less likely that the liver stage can cause significant LFT abnormalities on its own. It is unknown if the liver stage could influence the severity of the observed abnormalities.
This study provides evidence that the underlying pathogenesis of liver injury in uncomplicated malaria conceivably differs from severe malaria. In severe falciparum malaria, tissue damage is thought to be at least partly related to decreased oxygen supply and disturbed metabolisms due to parasite sequestration in the microvascular capillary system [7,17]. However, in CHMI only very low parasite densities are present and sequestration likely limited. Systemic inflammation with leukocytopaenia, thrombocytopaenia, increased d-dimer, CRP, and IFN-y concentrations is a typical hallmark of malaria even at the very low density of parasitaemias as observed after CHMI [28,29]. This study shows a clear relationship between these parameters and LFT abnormalities in falciparum malaria. Together with the timing of LFT abnormalities post treatment, and the presence of low-density parasitaemia, this suggests an inflammation-based mechanism of liver injury. Similarly, lung injury with impaired alveolar-capillary function is described to deteriorate after antimalarial treatment, reflecting a prolonged inflammatory response to parasite killing [30]. Histopathology of lung tissue in vivax malaria supports this hypothesis with an increase in alveolar-capillary monocytes and pulmonary phagocytic cell activity after initiation of treatment [31]. An adjacent hypothesis describes platelet sequestration as a downstream promoter of liver injury by exacerbating local inflammation and leukocyte accumulation [32], which correlates with the observed thrombocytopaenia and leukocytopaenia. Interestingly, although inflammation-induced liver injury is common in other infectious diseases (e.g. extrahepatic bacterial infection and sepsis), it seems to predominantly have a cholestatic nature in contrast to uncomplicated malaria [33].
Systemic inflammation is the net result of an interplay between pro- and anti-inflammatory responses, and a shift towards a pro-inflammatory status can cause malfunctioning of the host's mitochondria in malaria, leading to organ damage [34]. The resulting oxidative stress as observed in uncomplicated malaria is known to play a role in pathogenesis [35]. For instance, lipid peroxidation and antioxidant enzyme activity associate with cholestatic jaundice in P. vivax patients [36], and oxidative stress causes hepatocyte apoptosis in murine malaria models [37,38]. In this study, we show that anti-oxidant gene expression is down-regulated for SOD1, SOD2, NOS3, CAT, and GSTK1 during infection, and up-regulated for PRDX1. This corroborates with data from rodent studies [39,40] and a non-human primate study with P. knowlesi [41], where hepatic superoxide scavenging systems, glutathione S-transferase, superoxide dismutase and catalase, decrease during the course of a malaria infection. This net result may be reduced clearance of radicals and subsequent failure to protect cellular constituents from oxidative damage.
Our findings reflect a need to understand and reassess LFT abnormalities in malaria. This will have significant impact on clinical decision-making and in drug development, where they are often interpreted as drug-induced liver injury, particularly in malaria drug studies [27,42]. For example, drug-related serious adverse events in a recent multicentre trial comparing pyronaridine–artesunate or dihydroartemisinin–piperaquine versus current first-line therapies for uncomplicated malaria were associated mainly with increased liver enzymes [43]. Incorrect attribution of liver injury to an antimalarial drug can lead to unnecessary discontinuation or shifts in drug regimens, or impede drug development. Given the diversity of drug regimens used, it is unlikely that the observed LFT abnormalities in this study are caused by antimalarials. Moreover, no marked deterioration of LFTs was observed after a second, higher drug dose [16]. Our data does not support distinct differences between drugs and severity or occurrence of LFT abnormalities. In line with findings from Woodford et al., our data does not show a clear relation between the severity of abnormalities found, the pattern of early- or delayed transaminase elevations, and the different drug regimens used in CHMI. Furthermore, the timing, varied and often limited use of supportive medication (such as paracetamol, known for dose-dependent hepatotoxicity) in CHMI does not support a clear relationship with LFT abnormalities. It seems similarly improbable that the observed injury is parasite strain-specific. Geographically distinct P. falciparum strains were used in CHMI, and the imported falciparum malaria cases were presumably caused by a wide diversity of strains. In addition, non-falciparum malaria (e.g. Plasmodium vivax) was also shown to increase LFT abnormalities.
The limitations of this study are related to the retrospective observational nature of some of the data. However, the longitudinal CHMI data from healthy participants excludes the majority of confounding factors, and provides a much more thorough description of LFT dynamics. Although, we observed differences between CHMI and naturally-acquired malaria cases, patients with sequential sampling data from the latter seem to have similar LFT dynamics, and affirm previous observations [14].
This study shows that liver function test abnormalities are an under-recognized but common feature of uncomplicated falciparum malaria. The pathophysiological basis is most likely a pro-inflammatory response associated with oxidative stress resulting in transient liver tissue injury. Given the rapid and spontaneous resolution, and the absence of clear functional liver impairment in uncomplicated malaria, these events are unlikely to result in permanent subclinical liver damage. Treatment should remain focused on fast parasite clearance, and a more expectative approach might be warranted to avert unnecessary discontinuation or shifts in antimalarials due to supposed drug-induced liver injury. Nevertheless, supportive drugs with hepatotoxic potential should be limited or given only with appropriate monitoring, and adjunctive therapy that mediates systemic inflammation and oxidative stress may have a potential role in helping to constrain malaria progress into severe disease. Clinical trials testing the safety and efficacy of novel antimalarials should consider the temporal relationship between LFT abnormalities and infection. Importantly, unnecessary early abrogation of further clinical development of promising drugs related to potential or supposed hepatotoxic effects should be carefully (re)considered.
Contributors
IJR, PJJG, and RWS designed the study, which was performed by IJR, LAS, QM, AJV, TB, RWS. IJR, GMJ, JW, LAS, and RK collected the data, and IJR, GMJ, JJH, PJJG, and RWS analyzed and interpreted the clinical data. IJR, XZY, and MA performed, analyzed, and interpreted the cytokine and oxidative stress markers assays, and supervised by AF and RWS. IJR wrote the first manuscript, which was critically reviewed and approved by all authors.
Funding sources
IJR and RWS are supported by funds from PATH Malaria Vaccine Initiative. MA is supported by Swedish Society for Medical Research. TB is supported by a fellowship from the European Research Council (ERC-2014-StG 639,776). PATH Malaria Vaccine Initiative was involved in the study design of 1 CHMI study (CHMI-trans1). The funding sources had no other involvement in the current study. IJR and RWS had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
All authors declare no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
The authors sincerely thank the CHMI study participants for their participation with repeated study visits and blood donations, and the study staff for their dedication to the studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.09.018.
Contributor Information
Isaie J. Reuling, Email: isaie.reuling@radboudumc.nl.
Robert W. Sauerwein, Email: robert.sauerwein@radboudumc.nl.
Appendix A. Supplementary data
Supplementary material
References
- 1.World Health Organization . 2017. World Malaria Report. [Google Scholar]
- 2.Gachot B., Ringwald P. Severe malaria. Rev Prat. 1998;48(3):273–278. [PubMed] [Google Scholar]
- 3.Pain A., Ferguson D.J., Kai O. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc Natl Acad Sci U S A. 2001;98(4):1805–1810. doi: 10.1073/pnas.98.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White N.J., Pukrittayakamee S., Hien T.T., Faiz M.A., Mokuolu O.A., Dondorp A.M. Malaria. Lancet. 2014;383(9918):723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 5.Ho M., Davis T.M., Silamut K., Bunnag D., White N.J. Rosette formation of Plasmodium falciparum-infected erythrocytes from patients with acute malaria. Infect Immun. 1991;59(6):2135–2139. doi: 10.1128/iai.59.6.2135-2139.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirsky I.A., Brecht R.V., Williams L.D. Hepatic Dysfunction in Malaria. Science. 1944;99(2558):20–21. doi: 10.1126/science.99.2558.20. [DOI] [PubMed] [Google Scholar]
- 7.Anand A.C., Puri P. Jaundice in malaria. J Gastroenterol Hepatol. 2005;20(9):1322–1332. doi: 10.1111/j.1440-1746.2005.03884.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaeley N., Ahmad S., Shirazi N. Malarial hepatopathy: a 6-year retrospective observational study from Uttarakhand, North India. Trans R Soc Trop Med Hyg. 2017;111(5):220–225. doi: 10.1093/trstmh/trx042. [DOI] [PubMed] [Google Scholar]
- 9.Jain A., Kaushik R., Kaushik R.M. Malarial hepatopathy: Clinical profile and association with other malarial complications. Acta Trop. 2016;159:95–105. doi: 10.1016/j.actatropica.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 10.Murthy G.L., Sahay R.K., Sreenivas D.V., Sundaram C., Shantaram V. Hepatitis in falciparum malaria. Trop. Gastroenterol. 1998;19(4):152–154. [PubMed] [Google Scholar]
- 11.Srivastava A., Khanduri A., Lakhtakia S., Pandey R., Choudhuri G. Falciparum malaria with acute liver failure. Trop. Gastroenterol. 1996;17(3):172–174. [PubMed] [Google Scholar]
- 12.Joshi Y.K., Tandon B.N., Acharya S.K., Babu S., Tandon M. Acute hepatic failure due to Plasmodium falciparum liver injury. Liver. 1986;6(6):357–360. doi: 10.1111/j.1600-0676.1986.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 13.Kochar D.K., Agarwal P., Kochar S.K. Hepatocyte dysfunction and hepatic encephalopathy in Plasmodium falciparum malaria. QJM. 2003;96(7):505–512. doi: 10.1093/qjmed/hcg091. [DOI] [PubMed] [Google Scholar]
- 14.Woodford J., Shanks G.D., Griffin P., Chalon S., McCarthy J. The dynamics of liver function test abnormalities after malaria infection: A retrospective observational study. Am J Trop Med Hyg. 2018;98(4):1113–1119. doi: 10.4269/ajtmh.17-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramirez J.F., Porras B., Borrero E., Martinez S.P. Factors associated with the severity and complication of patients with malaria hospitalized between 2009 and 2013 in three municipalities of Colombia, case control study. Malar J. 2016;15(1):514. doi: 10.1186/s12936-016-1554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuling I.J., van de Schans L.A., Coffeng L.E. A randomized feasibility trial comparing four antimalarial drug regimens to induce Plasmodium falciparum gametocytemia in the controlled human malaria infection model. eLife. 2018:7. doi: 10.7554/eLife.31549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molyneux M.E., Looareesuwan S., Menzies I.S. Reduced hepatic blood flow and intestinal malabsorption in severe falciparum malaria. Am J Trop Med Hyg. 1989;40(5):470–476. doi: 10.4269/ajtmh.1989.40.470. [DOI] [PubMed] [Google Scholar]
- 18.Walk J., Reuling I.J., Behet M.C. Modest heterologous protection after Plasmodium falciparum sporozoite immunization: a double-blind randomized controlled clinical trial. BMC Med. 2017;15(1):168. doi: 10.1186/s12916-017-0923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delemarre B.J., Van der Kaay H.J. Tropical malaria contracted the natural way in the Netherlands. Ned Tijdschr Geneeskd. 1997;123(46):1981–1982. [PubMed] [Google Scholar]
- 20.Bijker E.M., Bastiaens G.J., Teirlinck A.C. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A. 2013;110(19):7862–7867. doi: 10.1073/pnas.1220360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teirlinck A.C., Roestenberg M., van de Vegte-Bolmer M. NF135.C10: a new Plasmodium falciparum clone for controlled human malaria infections. J Infect Dis. 2013;207(4):656–660. doi: 10.1093/infdis/jis725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCall M.B.B., Wammes L.J., Langenberg M.C.C. Infectivity of Plasmodium falciparum sporozoites determines emerging parasitemia in infected volunteers. Sci Transl Med. 2017;9(395) doi: 10.1126/scitranslmed.aag2490. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization . Vol. 19. 2014. Tropical Medicine and International Health - Severe Malaria; pp. 7–131. suppl. 1. [DOI] [PubMed] [Google Scholar]
- 24.Koopmans L.C., van Wolfswinkel M.E., Hesselink D.A. Acute kidney injury in imported Plasmodium falciparum malaria. Malar J. 2015;14:523. doi: 10.1186/s12936-015-1057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grobusch M.P., Kremsner P.G. Uncomplicated malaria. Curr Top Microbiol Immunol. 2005;295:83–104. [PubMed] [Google Scholar]
- 26.Ghoda M.K. Falciparum hepatopathy: a reversible and transient involvement of liver in falciparum malaria. Trop. Gastroenterol. 2002;23(2):70–71. [PubMed] [Google Scholar]
- 27.Silva-Pinto A., Ruas R., Almeida F. Artemether-lumefantrine and liver enzyme abnormalities in non-severe Plasmodium falciparum malaria in returned travellers: a retrospective comparative study with quinine-doxycycline in a Portuguese centre. Malar J. 2017;16(1):43. doi: 10.1186/s12936-017-1698-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermsen C.C., Konijnenberg Y., Mulder L. Circulating concentrations of soluble granzyme A and B increase during natural and experimental Plasmodium falciparum infections. Clin Exp Immunol. 2003;132(3):467–472. doi: 10.1046/j.1365-2249.2003.02160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladhani S., Lowe B., Cole A.O., Kowuondo K., Newton C.R. Changes in white blood cells and platelets in children with falciparum malaria: relationship to disease outcome. Br J Haematol. 2002;119(3):839–847. doi: 10.1046/j.1365-2141.2002.03904.x. [DOI] [PubMed] [Google Scholar]
- 30.Anstey N.M., Handojo T., Pain M.C. Lung injury in vivax malaria: pathophysiological evidence for pulmonary vascular sequestration and posttreatment alveolar-capillary inflammation. J Infect Dis. 2007;195(4):589–596. doi: 10.1086/510756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anstey N.M., Jacups S.P., Cain T. Pulmonary manifestations of uncomplicated falciparum and vivax malaria: cough, small airways obstruction, impaired gas transfer, and increased pulmonary phagocytic activity. J Infect Dis. 2002;185(9):1326–1334. doi: 10.1086/339885. [DOI] [PubMed] [Google Scholar]
- 32.Nowatari T., Murata S., Fukunaga K., Ohkohchi N. Role of platelets in chronic liver disease and acute liver injury. Hepatol. Res. 2014;44(2):165–172. doi: 10.1111/hepr.12205. [DOI] [PubMed] [Google Scholar]
- 33.Geier A., Fickert P., Trauner M. Mechanisms of disease: mechanisms and clinical implications of cholestasis in sepsis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(10):574–585. doi: 10.1038/ncpgasthep0602. [DOI] [PubMed] [Google Scholar]
- 34.Clark I.A., Budd A.C., Alleva L.M., Cowden W.B. Human malarial disease: a consequence of inflammatory cytokine release. Malar J. 2006;5:85. doi: 10.1186/1475-2875-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plewes K., Kingston H.W.F., Ghose A. Cell-free hemoglobin mediated oxidative stress is associated with acute kidney injury and renal replacement therapy in severe falciparum malaria: an observational study. BMC Infect Dis. 2017;17(1):313. doi: 10.1186/s12879-017-2373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fabbri C., de Cassia Mascarenhas-Netto R., Lalwani P. Lipid peroxidation and antioxidant enzymes activity in Plasmodium vivax malaria patients evolving with cholestatic jaundice. Malar J. 2013;12:315. doi: 10.1186/1475-2875-12-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guha M., Kumar S., Choubey V., Maity P., Bandyopadhyay U. Apoptosis in liver during malaria: role of oxidative stress and implication of mitochondrial pathway. FASEB J. 2006;20(8):1224–1226. doi: 10.1096/fj.05-5338fje. [DOI] [PubMed] [Google Scholar]
- 38.Dey S., Guha M., Alam A. Malarial infection develops mitochondrial pathology and mitochondrial oxidative stress to promote hepatocyte apoptosis. Free Radic Biol Med. 2009;46(2):271–281. doi: 10.1016/j.freeradbiomed.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 39.Srivastava P., Arif A.J., Pandey V.C. Status of hepatic glutathione-S-transferase(s) during Plasmodium berghei infection and chloroquine treatment in Mastomys natalensis. Int J Parasitol. 1995;25(2):203–205. doi: 10.1016/0020-7519(94)00089-7. [DOI] [PubMed] [Google Scholar]
- 40.Siddiqi N.J., Pandey V.C. Studies on hepatic oxidative stress and antioxidant defence systems during arteether treatment of Plasmodium yoelii nigeriensis infected mice. Mol Cell Biochem. 1999;196(1–2):169–173. [PubMed] [Google Scholar]
- 41.Srivastava P., Puri S.K., Dutta G.P., Pandey V.C. Status of oxidative stress and antioxidant defences during Plasmodium knowlesi infection and chloroquine treatment in Macaca mulatta. Int J Parasitol. 1992;22(2):243–245. doi: 10.1016/0020-7519(92)90109-x. [DOI] [PubMed] [Google Scholar]
- 42.Duparc S., Borghini-Fuhrer I., Craft C.J. Safety and efficacy of pyronaridine-artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials. Malar J. 2013;12:70. doi: 10.1186/1475-2875-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West African Network for Clinical Trials of Antimalarial D Pyronaridine-artesunate or dihydroartemisinin-piperaquine versus current first-line therapies for repeated treatment of uncomplicated malaria: a randomised, multicentre, open-label, longitudinal, controlled, phase 3b/4 trial. Lancet. 2018;391(10128):1378–1390. doi: 10.1016/S0140-6736(18)30291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.