Abstract
In the past 10 years, with the increase of investment in clinical nano-gene therapy, there are many trials that have been discontinued due to poor efficacy and serious side effects. Therefore, it is particularly important to design a suitable gene delivery system. In this paper, we introduce the application of liposomes, polymers, and inorganics in gene delivery; also, different modifications with some stimuli-responsive systems can effectively improve the efficiency of gene delivery and reduce cytotoxicity and other side effects. Besides, the co-delivery of chemotherapy drugs with a drug tolerance-related gene or oncogene provides a better theoretical basis for clinical cancer gene therapy.
Keywords: gene delivery, nanoparticles, tumor therapy, liposome, polymers, inorganics stimuli response, co-delivery, cellular uptake, nuclear entry
Main Text
Gene therapy is a promising therapeutic strategy aimed at altering or modifying defective and/or missing gene sequences to cure acquired or inherited diseases, including genetic disorders, cancer, cardiovascular diseases, and autoimmune disease, through introducing foreign genetic materials to cells, tissues, or organs.1 RNAi, as a posttranslational gene regulation technology of gene therapy, can specifically inhibit the gene expression of interest triggered by small interfering RNA (siRNA), genome origin microRNA, and double-stranded small hairpin RNA (shRNA).2 In addition, RNA-programmed CRISPR/Cas9 mRNA has proven to be a versatile tool for therapeutic genome editing in mice, as recently reported.3, 4, 5, 6 In just the past 5 years, more than 100 antisense oligonucleotide-based therapies have been tested in phase I clinical trials, and a quarter of them have reached phase II/III. Nusinersen, a modified antisense oligonucleotide to cure spinal muscular atrophy, following Formivisen and Mipomersen treating cytomegalovirus retinitis and high blood cholesterol, respectively, has been approved by the U.S. Food and Drug Administration (FDA).7, 8, 9, 10 The continued improvement of innovative DNA/RNA modifications and delivery carriers, such as nanoparticles (NPs), will aid to solve the challenges and barriers of DNA/RNA-based therapeutics.
It is known that naked DNA/RNA molecules are rapidly degraded by nuclease, with a high clearance rate of kidney, poor efficiency of cell uptake by intravenous injection, and severe unintended side effects when off target.11 Therefore, it is necessary to select a safe and stable transport carrier to deliver it to the target cells and tissues in clinical research. The transport carrier should meet these conditions: (1) it must protect DNA/RNA molecules from degrading and release with control; (2) it must be designed to increase the transfection efficiency, with the ability to penetrate deep into the tumor and reach tumor cells remote from the blood vessels for cellular internalization;12, 13, 14 and (3) it should be stable and safe during blood circulation (no interaction with biomolecules resulting in an immune response) and accumulate in the tumor site, however, it must be able to stick to the cells for cellular uptake.15
Generally, transporters are divided into viral and non-viral vectors. Viral vectors, including retroviruses, adenoviruses, and lentiviruses,16 are more efficient than non-viral vectors in numerous cell lines due to their controlled nucleic acid packing and unpacking in or from the capsid, as well as the ability of the virus to overcome various extracellular and intracellular delivery barriers or defense mechanisms of the targeted cells; however, there are safety concerns about severe off-target immunogenicity, inflammatory response, and toxicity.16, 17, 18, 19 In contrast, non-viral vectors, such as cationic lipid, polymers, and peptides using chemical entities that are able to mimic the main features of viral vectors, are able to compact and deliver nucleic acids in a similar manner. Also, these are much safer compared to viral vectors because the artificial design is usually not recognized (immediately) by the immune system. In addition, the chemical structure is controllable and easier to scale up and synthesize commercially.20, 21, 22 However, there are still some problems with non-viral carriers to confront.
The first is how to improve the efficiency of passive targeting, namely, the enhanced permeability and retention (EPR) effect. The EPR effect is the unique and most crucial phenomenon occurring in tumor tissue, with excessive production of vascular mediators and extravasation of macromolecules from blood vessels into the tumor tissue interstitium.23 Maeda et al.24 summarized the characteristics of the EPR effect of nanomedicines and macromolecular drugs in most, if not all, solid tumors, including biocompatibility, property size (over 40 kDa), weakly negative to near neutral, maintain at least 30 min to achieve tumor site and cleared rapidly in cells, then drug release. The second one is choosing a highly specific molecular target to even a single epitopic antigen, receptor, or kinase, which can modify NPs to target tissue and enhance the therapeutic efficacy,25, 26 in DNA/RNA delivery caused by positively charged materials, the property of electrostatic interaction with the negatively charged DNA/RNA molecules.27
In this review, we introduce the current clinical applications of gene delivery, and we summarize the barriers and challenges to systemic delivery of nucleic acids. In addition, we highlight the exploitation and discovery of different NPs and their modification in DNA/RNA delivery to discover more efficiency and safer NPs in gene delivery.
Clinical Application of NPs-Based RNAi Therapy
In the last decade, several biotechnology companies have invested massive manpower, physical resources, and funds in NPs-based gene therapeutics. The NPs-RNA complex is intended to enhance its circulation, promoting safe delivery to the desired location and silencing of the target mRNAs.28 Most of the NPs-based siRNA/microRNA delivery systems currently are approved for clinical trials in cancer therapy, virus infection, and other diseases (Table 1).
Table 1.
The siRNA/MicroRNA-Based Drugs Targeting Different Diseases in Clinical Trials
| Disease | Target | Vehicle | Drug Name | Sponsor | ClinicalTrials.gov Identifier (Phase) |
|---|---|---|---|---|---|
| Cancer | |||||
| HC, ST, ACC, GNT | siRNA target PLK1 | lipid nanoparticle | TKM 080301 | Tekmira Pharmaceuticals | NCT02191878 (I/II) |
| NCT01262235 (I/II) | |||||
| NCT01437007 (I) | |||||
| ST, MM, NHL | siRNA target MYC | lipid nanoparticle | DCR-MYC | Dicerna Pharmaceuticals | NCT02110563 (I) NCT02314052 (I/II) |
| ST | siRNA target RRM2 | polymer nanoparticle | CALAA-01 | Calando Pharmaceuticals | NCT00689065 (I) |
| ST | siRNA target EphA2 | liposome | siRNA-EphA2-DOPC | M.D.Anderson Cancer Center | NCT01591356 (I) |
| Leukemia | antisense target GRB-2 | neutral liposomes | BP1001 | Bio-Path Holdings | NCT01159028 (I) |
| ASC, PC | siRNA target PKN3 | lipid nanoparticle | Atu027 | Silence Therapeutics | NCT01808638 (I/II) NCT00938574 (I) |
| PDA, PC | siRNA target K-RAS | biodegradable polymer matrix | siG12D LODER | Silenseed | NCT01188785 (I) NCT01676259 (II) |
| Glioblastoma | siRNA target p53 | nanoparticle (NPs) | Temozolomide/SGT-53 | SynerGene Therapeutics | NCT02340156 |
| Lung cancer | siRNA target Fus1 | DOTAP-Chol | Fus1/Erlotinib | Genprex | NCT01455389 (I/II) |
| HC | siRNA target CEBPA | liposomal nanoparticle | MTL-CEBPA | Mina Alpha | NCT02716012 (I) |
| Glioblastoma | siRNA target Bcl2L12 | spherical gold nanoparticle | NU-0129 | Northwestern University | NCT03020017 (early I) |
| IMG | siRNA target UGT1A1*28 | nanoliposomal | CPT-11 | University of California, San Francisco | NCT00734682 (I) |
| Advanced, metastatic cancer, ST | shRNA STMN1 | BIV-lipoplex | pbi-shRNA STMN1 LP | Strike Bio | NCT01505153 (I) |
| Ewing’s sarcoma | shRNA EWS/FLI1 type 1 | BIV-lipoplex | pbi-shRNA EWS/FLI1 Type 1 LPX | Strike Bio | NCT02736565 (I) |
| NR | eIF5AK50R plasmid eIF5A siRNA | polyethylenimine | SNS01-T | Senesco Technologies | NCT01435720 (II) |
| MPM, NSCLC | microRNA -16 mimic target EGFR | EDV | TargomiRs | Asbestos Diseases Research Foundation | NCT02369198 (I) |
| Virus infection | |||||
| EVD | siRNA target VP24, and VP35 regions, EBOV polymerase inhibitor | lipid nanoparticle | Favipiravir | INSERM, France | NCT02329054 (II) |
| Other Disease | |||||
| Hepatic fibrosis | siRNA target HSP47 | lipid nanoparticle | ND-L02 s0201 injection | Bristol-Myers Squibb | NCT02227459 (Ib/II) |
| Hypercholesterolemia | siRNA target APOB | lipid nanoparticle | PRO-040201 | Tekmira Pharmaceuticals | NCT00927459 (I), terminated |
Source: https://clinicaltrials.gov. ACC, adrenocortical carcinoma; ASC, advanced solid cancer; GNT, gastrointestinal neuroendocrine tumors; HC, hepatocellular carcinoma; MM, multiple myeloma; MPM, malignant pleural mesothelioma; NHL, non-Hodgkins lymphoma; NSCLC, non-small-cell lung cancer; PC, pancreatic cancer; PDA, pancreatic ductal adenocarcinoma; ST, solid tumor; ALC, advanced liver cancer; SCLC, squamous cell lung cancer; IMG, intracranial malignant glioma; NR, not recorded; EGFR, epidermal growth factor receptor; GRB-2, Growth Factor Receptor Bound Protein-2; RRM2, Ribonucleotide Reductase Regulatory Subunit M2; PLK1,Polo-Like Kinase 1; HSP47, Heat Shock Protein 47; EphA2, Ephrin type-A receptor 2; eIf5A, Eukaryotic translation initiation factor 5A-1; EDV, EnGeneIC Delivery Vehicle; CEBPA,CCAAT/enhancer-binding protein alpha; BIV-lipoplex, bilamellar invaginated vesicle lipoplex; EVD, Ebola virus disease; PKN3, protein kinase N3; K-Ras oncogene, Kirsten rat sarcoma viral oncogene; APOB, apolipoprotein B; VP24, virus protein 24; VP35, virus protein 3.
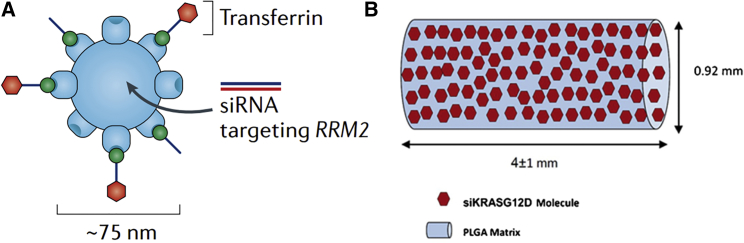
CALAA-01 is a polymer-based NPs siRNA delivery system containing a linear, cationic cyclodextrin-based polymer and a siRNA that targets the M2 subunit of ribonucleotide reductase (RRM2) (Figure 1A). Adamantane polyethylene glycol (PEG) was used as a surface modifier on the NPs to provide steric stabilization and a targeting ligand (human protein transferrin) on its surface as a positive target. The cationic polymer interacts electrostatically with anionic siRNA to assemble into nanocomplexes below approximately 100 nm in diameter that protect the siRNA from nuclease degradation in serum. The siRNA-containing nanocomplexes are targeted to cells that overexpress the transferrin receptor (TfR), and then anti-R2 suppresses RRM2 expression, resulting in cell-cycle arrest and cell death.29The trial has been terminated due to 21% of the patients having an adverse event because of drug instability (specifically the transferrin-targeting ligand).30 Tolerable dose, siRNA plasma concentration, and elevated plasma levels of cytokines have no association with adverse events. In addition, a dose-dependent accumulation of CALAA-01 within tumors, but not adjacent tissue, was found in CALAA-01-specific staining, and the expression of RRM2 in protein and mRNA level were inhibited effectively.31 Thus, how not only to engineer nanomedicines to make them effectively target tumor tissues but also to choose an appropriate delivery system are the keys to developing new-generation nanomedicines of high therapeutic efficacy.
Figure 1.
Schematic Illustrations of the siRNA-Based Therapeutics of CALAA-01 and siG12D-LODER
(A) CALAA-01 is a polymer-based nanoparticle containing a targeting ligand on its surface (the human protein transferrin) and a small interfering RNA (siRNA) that targets the M2 subunit of ribonucleotide reductase (RRM2). Reproduced with permission from Zuckerman and Davis.30 Copyright © 2015 Springer Nature. (B) siG12D-LODER is a polymeric matrix of poly(lactic-co-glycolic) acid (PLGA) in a shape of a small cylindrical rod of 0.8 mm in diameter and 5.5 ± 1 mm in length. Reproduced with permission from Titze-de-Almeida et al.33 Copyright © 2017 Springer Nature.
siG12D LODER is composed of Local Drug EluteR (LODER), a novel biodegradable polymeric matrix that shields drugs against enzymatic degradation and siRNA against G12D-mutated KRAS (siG12D), which is released by LODER slowly (Figure 1B).32, 33 siG12D LODER was implanted regionally as miniature cylindrical pills into pancreatic tumors by endoscope ultrasound (EUS) biopsy for a long term, showing good anticancer effects. It has successfully passed a phase 1/2a clinical trial. Most importantly, LODER is a miniature (millimetric) biodegradable polymeric matrix that is composed of a copolymer of poly (lactic-co-glycolic) acid (PLGA) with good safety, biodegradability, and biocompatibility in this tissue for a long period.34, 35, 36 Furthermore, the knockdown of KRAS activated the alternative pathway of the complement system37 and stimulated immunity under certain conditions.31
TKM-080301, targeting polo-like kinase 1 (PLK1), is a siRNA for intravenous (i.v.) administration formulated in stable nucleic acid lipid particles (SNALPs), and it was evaluated in patients with gastrointestinal neuroendocrine tumors and adrenocortical carcinoma.38 In this delivery system, PLK1 is a protein overexpressed in cancer cells promoting an uncontrolled cell proliferation,39 and researchers used siRNA-silencing PLK1 molecules incorporating into the aqueous inner part with the help of cationic lipids (1,2-dilinoleyloxy-N,N-dimethyl-3-aminopropane,DlinDMA). Helper lipids with DSPC (1,2-distear-oyl-sn-glycero-3-phosphocholine) promote the release of siRNAs from SNALPs in the cell cytoplasm. Polyethylene glycosylated lipids (PEG-C-DMA) offer an external surface that is more hydrophilic and neutral, for stability and crossing barriers. Cholesterol helps to stabilize the lipid particle formulation.40
Nowadays, some of these trials are in phase III and the results will hopefully be approved. Engineering NPs carriers has received wide attention in gene delivery due to their outstanding characteristics, including diverse modifications and high stability biocompatibility to adapt desirable properties in particle forms. Among the nanopaticles used in gene delivery carriers, the cationic polymer and lipid were the most widely used polymer for the delivery of DNA and RNA molecules.
The Challenges and Barriers of Polymer-Mediated Gene Therapy
Cellular Uptake and Endosomal Escape
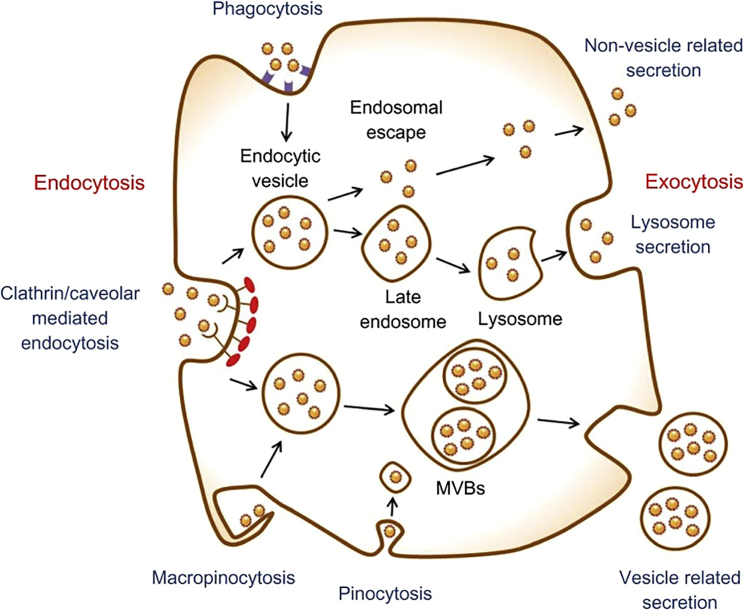
Naked siRNA/microRNA permeating the cell membrane is blocked by its size and negative charge. Thus, using the electropositivity of cationic polymer leads to the formation of complexes containing siRNA/microRNA molecules promoting cell uptake and preventing degradation by RNase. Most complexes interact with the cell membrane electrostatically, because of the anionic cell surface proteoglycans, and enter into cells. For cell uptake, clathrin- and caveolae-mediated endocytosis, which are energy-dependent and controlled by the cell internalized molecules, are the common processes (Figure 2); the other occurs in phagocytes (macrophages, dendritic cells, and neutrophils), and the sizes of engulfing particles are larger than 0.5 μm, driven by actin.41, 42, 43, 44 Additionally, the efficiency of NPs is affected by the physicochemical properties, such as size, shape, and surface chemistry, as well as cell type. Some researchers said that uptake of PLGA copolymer-DNA complexes in Caco-2 cells was size dependent, with the highest uptake seen for particles with a mean diameter of 100 nm;45 but, in COS-7 and HEK293 cell lines, it was higher for particles with mean diameters of 70 and 200 nm, respectively.46 Furthermore, the highest transfection efficiency was seen for polyetherimide (PEI) nanogels with 75- and 87-nm mean diameters, when transfected in complexes with different diameters but with similar surface charge in different cancer cell lines.47 These results suggest that the optimal size for gene transfer of non-targeting cationic vector-DNA complexes is between 70 and 90 nm. As the cellular uptake pathway of receptor targeting vector-DNA complex is different from non-targeting NPs, the effective size needs to be smaller. Several temperature- and pH-sensitive polymers or other smart polymers rapidly used in gene delivery to enhance cell uptake and lysosomal escape are introduced in Different Stimuli-Responsive Gene Delivery Systems.
Figure 2.
Schematic of Endocytosis and Exocytosis Patterns of Nanoparticles
Nanoparticles enter the cell via four types of pathways: clathrin-/caveolar-mediated endocytosis, phagocytosis, macropinocytosis, and pinocytosis. Nanoparticles exit the cell via three types of pathways: lysosome secretion, vesicle-related secretion, and non-vesicle-related secretion. MVBs, multivesicular bodies. Reproduced with permission from Oh and Park.42 Copyright © 2014 Dove Press Ltd.
Nuclear Entry for Plasmid shRNA
For shRNA, the carrier vector must carry it into the nucleus of the host cell, and nuclear localization sequences (NLSs) have been utilized. There are reports of the transfection efficiencies of cationic DNA nanocarriers or polycations coupled with NLS derived from the SV40 large T antigen, which can be recognized and go through the nuclear pore complex (NPC).48, 49, 50 A peptide or protein such as Histon H1 can also improve the efficiency of entering into the nucleus.51 Besides, sugar residue can also transport cargoes into the nucleus, and the pathway is used by neoglycoproteins to enter the nucleus. Lactosylated polylysine (PLL)/cDNA complex induced nuclear localization by binding to a potential lectin-like shuttling protein with galactose/lactose specificity, and this binding interaction was suggested to trigger the nuclear internalization of the complex.52, 53
Some cationic polymers like PEI and T443, a poly-(glycoamidoamine), are capable of inducing nuclear membrane permeability, but they also have the highest level of cytotoxicity.53
Nanotoxicity
Despite the increasing biocompatibility of the material and the decreasing side effects, NPs-mediated toxicity still exists. The physicochemical properties of NPs, such as small size, large surface area, and flexible chemical compositions or structures that facilitate their use in nanomedicine, have also been found to contribute to their enhanced toxicological side effects. Reports have said that smaller sized NPs exhibit higher toxic effects due to the increased surface area,54 and the structure and shape of NPs also contribute to nanotoxicity.55 Besides, the ability of NPs adsorbing with ions and biomolecules influences the cellular responses, resulting in toxicity.56
As Baeg and colleagues57 reviewed, polymeric NPs are safer than inorganic NPs and metallic NPs. Thus, a better understanding of cationic polymer NPs and the mechanisms of cationic polymer-based drug and gene delivery involved in nanotoxicity may help to further develop and utilize NPs in the field of nanomedicine.
The Smart NPs Engineered for Gene Delivery
Now, the researchers are focused on the design of more sophisticated NPs, aimed at addressing multiple challenges at the same time, such as controlled delivery, nucleic acid separation, controlled cell patterning, and gene release. Here we introduce three types of commonly used gene carriers that are lipid based, polymeric, and inorganic. In the subsequent section, different stimuli response modifications of NPs are addressed. Lastly, we discuss how to improve nanomedicine tumor accumulation and penetration (EPR).
Gene Delivery Systems
Lipid-Based Systems
Lipid-based NPs have been developed for systemic delivery of RNA or DNA into tumors, including liposomes, solid lipid NPs (SLNs), and reconstituted high-density lipoprotein (rHDL) NPs. Cationic liposomes have been extensively applied because of their high encapsulation efficiency, effective transfection, and easy-to-surface modification. Zhang et al.58 used a liposome-protamine-IL-22-binding protein mRNA complex to inhibit the growth of C26 tumor cells exhibiting a high mRNA transport and expression efficiency because of the protamine, which can package DNA denser than histones. However, the potential clinical use of cationic liposomes is limited by their instability, rapid systemic clearance, toxicity, and induction of immunostimulatory responses. Therefore, many cationic lipid-containing liposomes are being developed that efficiently capture nucleic acids internally, but there are some NPs with neutral or anionic surface charge, such as lipidic NPs (LNPs).59 Researchers found that 3β-[N-(N',N'-dimethylaminoethane)-carbamoyl]cholesterol/dioleoyl phosphatidylethanolamine (DC-Chol/DOPE) cationic liposomes were easy to aggregate with negatively charged blood components and thus were allowed only by direct injection into local targets, but PEGylated DC-Chol/DOPE-siRNA lipoplexes can effectively reduce the excretion by kidneys and scavenging in liver, prolong the circulation time in vivo, and ultimately increase their preferential tumor accumulation; in addition, the systemic administration of PEGylated lipoplexes won’t lead to any activation of innate immune responses in the immunocompetent mice.60 Zou et al.61 used low-molecular-weight PEI conjugating to lipid and prepared poly(D,L-lactide-co-glycolide) NPs; as a potential effective gene delivery system, it showed low toxicity and higher transduction efficacy when compared to high-molecular-weight PEI in vitro.
There are some other lipid-based NPs systems like cationic SLN62 and rHDL.63, 64 SLNs bounded with p5α-red were developed as a gene vector, and no significant cytotoxicity was observed with high gene-silencing efficiency in vitro.65 However, a main drawback of SLNs is the stability of NPs, which may hinder their implementation for therapeutic purpose. Thus, researchers focus on overcoming this problem and developing effective NPs systems for delivering nucleic acids.
Polymeric Gene Delivery Systems
Despite of lipid, natural polymers like proteins and oligopeptides and synthetic cationic polymers, including cyclodextrin derivatives, PEI, and polyamidoamine (PAMAM), have been explored, owing to their unique physicochemical properties and the ability to form electrostatic complexes with anionic biomolecules, nucleic acids, and proteins used in clinical trials along with their inherent bioactive properties, such as being antimicrobial, antioxidant, stimuli responsive, anti-inflammatory, and antitumor. Also, the delivery efficiency was affected by the molecular weight, charge density, and chemical structure of the polymer.66
Natural Polymers
Cationic chitosan is a deacetylated derivative of chitin, which is the second most abundant natural polymer. The uses of chitosan include with proteins,67 peptides,68 DNA,69 and siRNA delivery.70, 71 Given the cytotoxicity and stability of chitosan in the siRNA delivery process, further developments of chitosan-based delivery systems occurred. Grafting small molecules or polymer chains onto the chitosan backbone or alkalifying the amino groups to modify chitosan were most investigated. Mad2 siRNA-loaded epidermal growth factor receptor (EGFR)-targeted chitosan NPs can effectively inhibit cell growth in a cisplatin-sensitive and -resistant lung cancer model.72 Poly(ethylene glycol)-modified chitosan (PEG-CS) could improve chitosan solubility, by forming stable siRNA-loaded NPs with smaller particle size, and enhance transfection efficiency in cancer cell lines.73, 74 Using a carboxymethyl dextran (CMD)-chitosan NPs platform to encapsulate HMGA2 siRNA and doxorubicin (DOX), it had a high efficiency for siRNA and drug encapsulation (about 78% and 75%, respectively), and it was stable against serum and heparin.75 When CD73-siRNA was encapsulated into chitosan-lactate NPs, it also exhibited low cytotoxicity and high transfection.76 The applications of other natural polymers have been listed in Table 2.
Table 2.
Overview of the Most Widely Used Natural Cationic Polymers in Gene Delivery
 |
Synthetic Polymers
Compared with natural cationic polymers, synthetic cationic polymers are easy to improve the control properties and modify the structure. The bioactive moieties and functional groups can be readily incorporated into synthetic polymer systems to produce therapeutic potential and degradation characteristics of gene delivery. The research most focuses on PEI, PLL, polyacrylic acid (PAA), poly(aliphatic ester) (PAE), and poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA) and some required modification on them (Table 3).
Table 3.
The Overview of the Most Widely Used Synthetic Cationic Polymers in Gene Delivery
 |
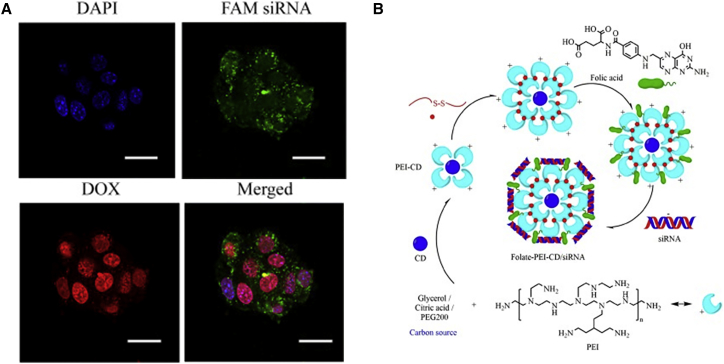
PEI is one of the most prominent and extensively cationic polymers capable of gene transfection. The high charge density of PEI and composition of primary (25%), secondary (50%), and tertiary (25%) amines make it have high gene transfection activity (Figure 3A).77 Primary amines are protonated at physiological pH 7.4, and they enable PEI to bind negatively charged genes effectively via electrostatic interactions. The secondary and tertiary amines are able to sequester protons in the endosomes, namely, the proton-sponge effect.78 Furthermore, the efficacy and toxicity of PEI are strongly correlated with its molecular weight and structure (branched or linear). The higher the molar mass with higher cationic charge densities and better cellular uptake, generally the higher cytotoxicity is.79, 80, 81, 82 Thus, researchers modified PEI structure to improve the transfection efficiency and decrease the cytotoxicity in PEI and gene delivery systems. One strategy is modifying PEI with PEGylation to create a hydrophilic exterior that reduces interactions of the polyplex with plasma proteins and erythrocytes. The other important strategy is modifying PEI with hydrophobic moieties, like lipids, stearic acid, cholesterol, or palmitic acid, to increase cellular uptake and stability with systemic administration.83, 84 For branched PEI (bPEI) (1.8 kDa), conjugation with cholesterol can increase the transfection efficiency and reduce the toxicity.78 In addition, PEI as a functional group to modify carbonaceous quantum dots or gold NPs (AuNPs) can effectively deliver and release a siRNA in the tumor site (Figure 3B).85, 86 Folate-rPEI-CDs with high biocompatibility and gene delivery efficiency can be accumulated in lung cancer cells selectively through receptor-mediated endocytosis, resulting in better gene silencing and anti-cancer effects.
Figure 3.
The Application of PEI for Co-delivery of siRNA and DOX and the Schematic Synthesis of Folate-PEI-CDs/siRNA Nanoparticles
(A) Confocal laser scanning microscope (CLSM) images of B16F10 cells incubated with PEI/siRNA/DOX for 24 hr. Scale bars, 20 μm. Reproduced with permission from Xu et al.77 Copyright © 2017 Elsevier. (B) Schematic diagram of synthesis route of folate-PEI-CDs/siRNA nanoassemblies. Reproduced with permission from He et al.85 Copyright © 2017 Elsevier.
PLL, as one of the most widely studied gene carriers, is a synthetic polypeptide composed of a large number of primary amines, which can interact with negatively charged biomolecules through electrostatic interaction. Due to the proximity group effect in the polymer chain, only a portion of the primary amine groups of the PLL are protonated at physiological pH. Therefore, the PLL exhibits a relatively low buffering capacity (pH 5.7–7.7), whereas PEI does not effectively escape the endosome without the endosomal solubilizing agent and strongly impairs its transfection ability.87, 88, 89 In addition, it has been indicated that high-molecular-weight PLL has shown a tendency to aggregate and precipitate, which may cause cytotoxicity depending on the ionic strength.90 Thus, conjugation to hydrophilic or amphiphilic macromolecules, such as PEG, has been widely investigated.91 However, grafting PEG to the side chains of PLL indeed prolonged its lifetime in blood circulation and tumoral accumulation with loss of the ability to associate with siRNA,92 but it also reduced cell uptake and binding at the target site. Thus, choosing specific targeting moieties, like antibodies, peptides, sugars, and folate, to conjugate to PEG-PLL can improve cellular uptake via receptor-mediated endocytosis and conferred tissue specificity.93 Another method to promote the transfection efficiency is stimuli-responsive polymer, such as pH-sensitive, reduction-sensitive, or enzyme-responsive linkages. We introduce the smart polymer in detail in the next section.
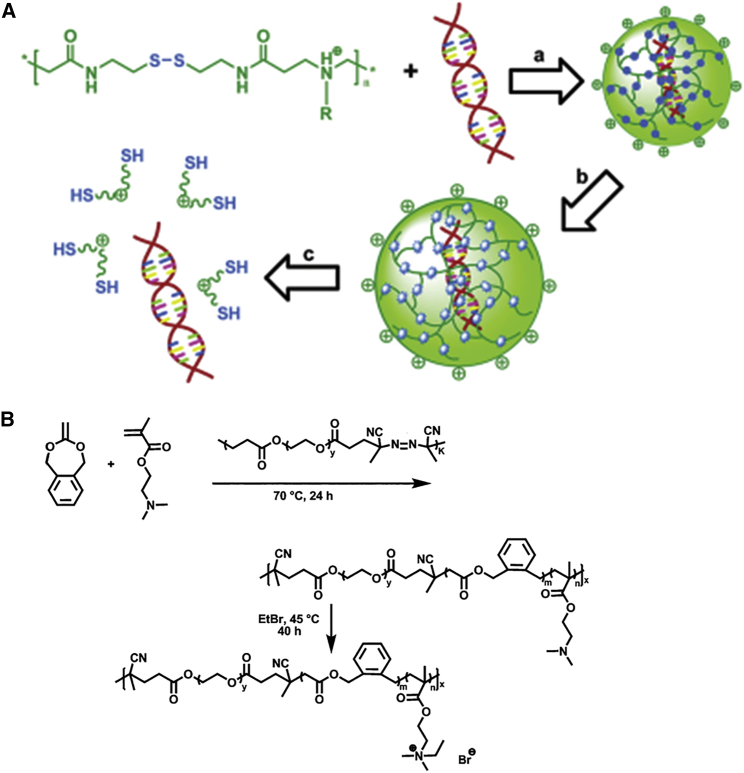
PAA is a bisacrylamide by the addition of a primary or tertiary secondary amine to a Michael-type polyamine used for gene transfer due to their biocompatibility, water solubility, biodegradability, and lower toxicity, including linear and branched PAA. Therein, bioreducible PAAs or poly(disulfide amines) are a unique family of PAAs with lower cytotoxicity and improved gene transfer compared to branched PEI-25 kDa (Figure 4A).94, 95 Synthesized reducible poly(amino ethylenimine)s (SS-PAEIs) exhibited a 2-fold higher efficiency compared with PEI-25 kDa. Moreover, Lin et al.96 found that the branching degree had a significant effect on transfection efficiency. They indicated that low-branched PAA could more effectively compact plasmid DNA (pDNA) into positively charged NPs than both high-branched PAA and linear PAA.
Figure 4.
The Schematic Process of SS-PAA/DNA In Vitro and the Formation of Poly(PEG-co-(BMDO-co-DMAEMA)) with EtBr
(A) The concept of DNA condensation and subsequent intracellular release. (a) Formation of SS-PAAs/DNA polyplexes that are stable in the extracellular environment, (b) intracellular reduction of the disulfide linkages in the polymer of the polyplex, and (c) dissociation of DNA from the degraded polymer are shown. Reproduced with permission from Lin et al.94 Copyright © 2007 American Chemical Society. (B) Synthesis route for the formation of the poly(PEG-co-(BMDO-co-DMAEMA)) and poly(PEG-co-(BMDO-co-DMAEMA))·EtBr. Reproduced with permission Zhang et al.98 Copyright © 2012 American Chemical Society.
PDMAEMA is one of the most important pH-responsive cationic polymers, with combined transfection efficiency and acceptable cytotoxicity.97 There were various modifications that have been investigated in an attempt to improve the transfection of PDMAEMA. PEGylated PDMAEMA not only induced cytokine production by murine macrophages but also it could effectively deliver a DNA vaccine, which can enhance adaptive immune responses by activating innate immunity (Figure 4B).98, 99
Endogenous proteins and synthetic oligopeptides have been used for siRNA or DNA delivery like HAS, transferrin, atelocollagen, poly(Pro-Hyp-Gly), cholestery oligoarginine, and MPG-8, a 21-residue amphipathic peptide. Wang et al.100 developed PEGylated NPs based on the cationic α-helical polypeptide poly(γ-4-((2-(piperidin-1-yl)ethyl)aminomethyl)benzyl-l-glutamate) for the delivery of Cas9 expression plasmid and small guide RNA (sgRNA) to various cell types and gene-editing scenarios. The results showed that the colloidally stable P-HNPs achieved a Cas9 transfection efficiency up to 60% and sgRNA uptake efficiency of 67.4%, representing a versatile gene-editing platform for biological research and therapeutic applications.100
Inorganic Gene Delivery Systems
Inorganic materials are frequently used for drug delivery and imaging, including gold, calcium phosphate, cadinum (quantum dots), and iron oxide. Actually, inorganic substances are biologically inert and afford excellent controls for gene delivery.
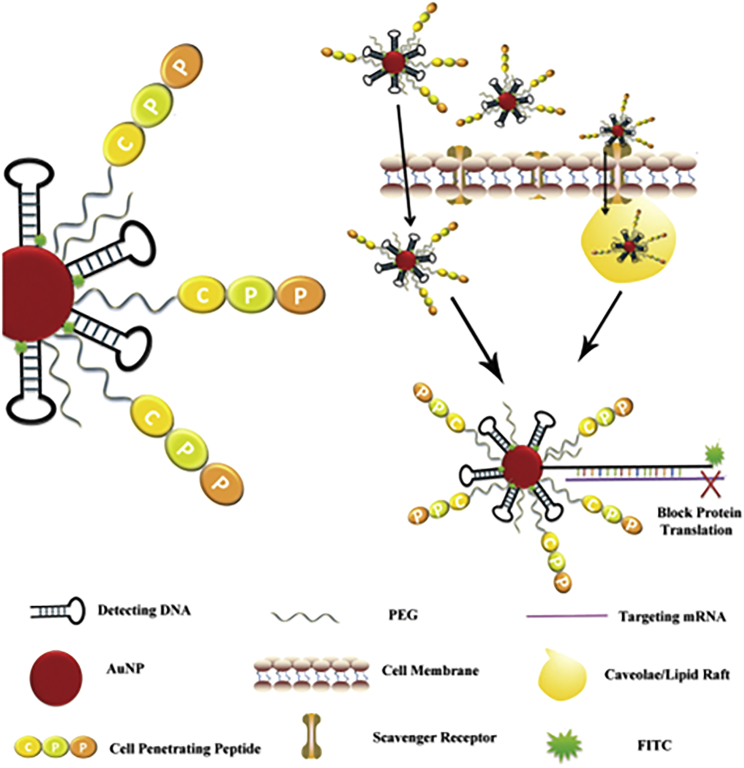
AuNPs can be suitably engineered for applications in gene delivery and as carriers of peptides and proteins. AuNPs can be stabilized by a large variety of stabilizers like polymers, ligands, biomolecules, denrimers, surfactants, etc.;84, 101, 102, 103, 104 the stabilizers affect not only the relative instability and aggregation but also cell uptake and toxicity, which points to the necessity of strategic engineering of NPs surface chemistry.105, 106, 107 Gu and colleagues108 developed a novel AuNP beacon by optimizing the sequence amount, and they modified PEG and cell-penetrating peptide (CPP) on the gold core (Figure 5). When the molecular beacon got into tumor cells through membrane recognition by a scavenger receptor, the detecting DNA identified target mRNA like Akt, mTOR, and HIF-1 mRNA, resulting in the expression of Akt, mTOR, and HIF-1 being blocked and the fluorescence signal amplified.
Figure 5.
Schematic Illustration of the Modification of AuNP Beacon and the Mechanism of Cell Uptake
Reproduced with permission from Li et al.108 Copyright © 2018 John Wiley & Sons, Inc.
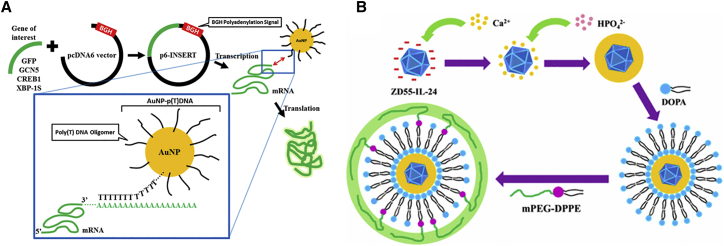
Cationic polymer (chitosan and poly-L-lysis) and cysteine-coated AuNPs also showed good stability and resistance to aggregation in blood circulation, with significant transgene activities in all cell lines.104 Son et al.109 developed an exquisite RNAi-AuNP nanoconstruct with various geometries, which exhibited a precise conjugation and separation of a designated number of therapeutic siRNAs onto AuNP to create various geometries of RNAi-AuNP nanoassemblies, based on the hybridization between complementary nucleic acid base-pairing. Besides, using the property of mesenchymal stem cell (MSC) high affinity to bind arginine-glycine-aspartic (RGD) peptide, modifying RGD to dendrimer-entrapped AuNPs exhibited a highly efficient and specific gene delivery to stem cells. It revealed that the coexistence of RGD and AuNPs allows the design of a dendritic vector with specific stem cell-binding ability to bind to the cell surface through integrin receptors and improve the three-dimensional conformation of dendrimers, which facilitates efficient and specific stem cell gene delivery applications.110 Further, poly(thymine)-functionalized AuNP (AuNP-p(T)-DNA) is also another strategy for gene delivery. The gene of interest was inserted separately into pcDNA6 plasmid vector containing BGH polyadenylation(P(A)) signal; the produced poly(A) tail can hybridize with poly(T) olignucleotide on AuNPs, facilitating the increased production of the respective proteins (Figure 6A).111
Figure 6.
The Schematic Process of AuNP-p(T)DNA and PLC-ZD55-IL-24
(A) Schematic showing the use of AuNP-p(T)DNA in enhancing the translation of different mRNA templates. Genes of interest were inserted separately into pcDNA6 plasmid vector containing BGH polyadenylation (P(A)) signal and transcribed to produce the respective mRNA templates with a poly(A) tail. AuNP-p(T)DNA was added to mRNA templates to allow hybridization between the poly(T) oligonucleotide on AuNP and the poly(A) tail on the mRNA, which facilitated the increased production of the respective proteins. Reproduced with permission from Chan et al.111 Copyright © 2018 American Chemical Society. (B) Synthetic route used to prepare PLC-ZD55-IL-24. Reproduced with permission from Chen et al.121 Copyright © 2016 American Chemical Society.
The application of calcium-based biomaterials in gene delivery causes calcium ion to form ionic complexes with the helical phosphates of DNA, and these complexes have easy transportability across the cell membrane via ion channel-mediated endocytosis, including calcium phosphates, calcium carbonates, calcium silicate, and calcium fluoride. Taking calcium phosphates as an example, there was a large number of -OH groups with Ca2+ cations in their surface, which can effectively adsorb organic molecules with acid groups such as carboxylic(-COO−) and phosphoric groups. In addition, the solubility of calcium phosphates (CaPs) increased with the decrease of the solution pH value and dissolved into ions at a low pH value, revealing themselves to be pH-responsive nanocarriers for gene and drug delivery. A report said that low-to-moderate elevation of Ca2+ concentration (0.2–0.4 μM) triggers apoptosis and higher concentrations of Ca2+ (>1 μM) are associated with necrosis; the normal extracellular Ca2+ concentration is ∼1.2 mM and the cytosolic concentration is ∼0.1 μM.112 However, in the application of CaPs, researchers performed one more layer of CaPs covering DNA-coated CaPs, including PEI,113 liposome,114, 115, 116, 117 hyaluronan (HA),118 or PEG-biophosphonates,119 which can enable the prepared CaP NPs to remain physically stable over a long time and effectively protect siRNAs from enzymatic degradation under physiological conditions, although the elevated Ca2+ concentration was only a transient event and not toxic to the cells.120
Chen et al.121 constructed a PEG/lipid/calcium phosphate-OncoAd (PLC-OncoAd) delivery system for ZD55-IL-24 carried by oncolytic adenovirus (Figure 6B). It exhibited a highly efficient targeted gene delivery to the tumor site without the innate immune response and specific sequestration and toxicity in liver through systemic administration. Besides, using alendronated-hyaluronan graft polymer (AHA) instead of PEG-bp as the outer shell to carry the siRNA exhibited a stronger interaction between calcium ions and negatively charged phosphate, and it enabled CD44-mediated targeting of tumor cells in the study performed by Qiu et al.118 In this delivery system, the negatively charged CaP-AHA/siRNA NPs (−12 mV) could effectively deliver EGFR-targeted siRNA and downregulate EGFR expression through CD44-mediated endocytosis; and, the internalized CaP-AHA/siRNA NPs exhibited a pH-responsive release of siRNA benefitting from CaP.
Nano-sized quantum dots (QDs) exhibit uniquely optical properties that are tunable with different sizes and shapes as attractive vectors for imaging, guided by the properties of emitting narrow symmetric bands under a wide excitation range, having antiphotobleaching stability, and being bio-functionalized on the large surface area. In addition, QDs can potentially promote the efficient delivery of siRNA into target cells and track the distribution of siRNA in cells in vitro or in vivo.85, 86, 122
Different Stimuli-Responsive Gene Delivery Systems
pH-Responsive Systems
The pH gradient is one of the most exploited stimuli to design stimuli-sensitive NPs for DNA/RNA delivery in tumors, because pH exists differently among healthy (pH 7.2–7.4) and tumor (pH 6.5–6.9) sites, cytosol (pH 7.4) and lysosomes (pH 4.5–5) and endosomes (pH 5.5–6).123, 124, 125 These pH-responsive components can be used to design pH-sensitive nanocarriers with the properties of being protonizable, acid labile, and destabilizing.126 At a pH above the acid dissociation constant (pKa), a specific pH that when altered can affect the structure, the side chain amine groups remain non-ionized, allowing the polymer chain to contract while capturing siRNA or DNA. As the pH decreases below the amine pKa, for example, in a lysosome or endosome, the amine group becomes protonated and the polymeric chain swells due to electrostatic repulsion, resulting in the siRNA or DNA being encapsulated by cationic polymer released into the surrounding medium.87
PEI, chitosan, and PAA can be used as pH-responsive carriers because of their primary, secondary, and tertiary amine groups. When poly-histidine and poly-arginine coupled to PEI, the gene transfection abilities were substantially improved with low levels of cytotoxicity.127 In addition, conjugating PEI to biodegradable lipids, such as cholesterol128 or poly-glutamic acid derivates,129 can increase the stability of the siRNA and PEI complexes and reduce the toxicity. Meanwhile, CaP NPs coupled with PEG-grafted carboxymethyl chitosan (CMCS), which have the stabilization of the CaP NPs and the high releasing efficiency of CMCS, showed a highly efficient delivery ability.130 Other calcium-based biomaterials have been introduced in the part of inorganic gene delivery systems. Besides, a carboxybetaine ester functional (CBE) group can also mediate the interaction between DNA and AuNPs via pH.131 The negatively charged phosphate backbone of the DNA interacts with and adsorbs on the positively charged CBE on the self-assembled monolayer (SAM). DNA release can be carried out by hydrolyzing CBE to a zwitterionic carboxybetaine state, and the adsorption of the SAM-modified common gold surface can be controlled by pH control of the negatively charged citrate-terminated AuNPs.
Thermo-responsive Systems
Temperature is among the most often investigated stimuli to control drug release, because the structural properties of some NPs are changed in response to temperature.87 The hyperthermic nature of most inflamed pathological sites and tumors can act as an internal stimulus. Poly(N-isopropylacrylamide) (PNIPAAm)132, 133 or pluronic F-127 as temperature-responsive moieties can incorporate or graft to NPs to achieve the thermo-stimuli; and, PEG attachment can solve the toxicity, immunogenicity, and circulation time concomitant with thermo-sensitive moieties. PNIPAAm-co-2-(dimethylamino)ethyl methacrylate (DMAEMA)-co-butylmethacrylate (BMA) contained 8 mol% DMAEMA and 11 mol% BMA with a lower critical solution temperature (LCST) at 21°C; therefore, the copolymer was insoluble above 21°C and soluble below 21°C. At a temperature above the LCST, the complexes between the polymer and the pCMV-lacZ plasmid encoding for β-galactosidase become more densely bound and better protect the plasmid from enzymatic degradation. When the temperature is lower than the LCST, hydration occurs and the water solubility of PNIPAAm increases, resulting in the complexes being less compact and DNA being released. The transfection efficiency incubated at 37°C for 48 hr was greater than that incubated at 20°C for 3 hr and 37°C for 45 hr.134
Feng et al.135 prepared an efficient nonviral cationic block copolymer gene delivery system containing poly(ethylene glycol)-block-poly{N-[N-(2-aminoethyl)-2-aminoehtyl]aspartamide} [PEG-b-PAsp(DET)] and poly(N-isopropylacrylamide)-blockPAsp(DET) [PNIPAM-b-PAsp(DET)] with high tolerability against nuclease and strong resistance toward protein adsorption. From the results in vitro and in vivo, it showed a higher gene transfection efficiency than that of regular polyplex micelles prepared form solo block copolymer of PEG-b-PAsp (DET)(SPM), with low cytotoxicity and improved colloidal stability.135 In addition, thermo-sensitive PEI-plurinic nanocapsules prepared by an interfacial crosslinking reaction between preactivated Pluronic F-127 and low-molecular-weight PEI conjugated with PEG showed a controlled delivery manner.136 The introduction of isobutyramide groups attached to the hyperbranched PEI side chain also indicated that the nanomedicine has thermal response characteristics.137
Redox-Responsive Systems
The redox-stimuli system is one of the most efficient systems for stimulus-sensitive cancer and gene therapy.138, 139, 140 Glutathione (GSH) is an ubiquitous small molecule involved in important cellular pathways, such as in the maintenance of intracellular redox state because of the different redox conditions between the intracellular (2–10 mM) and extracellular (2–20 μM) compartments.141, 142 Furthermore, tumors can be regarded as a reducing environment because the concentration of GSH in tumor tissues and the cytoplasm of tumor cells is at least four times higher than that in normal tissues.143 The rational design of reduction-sensitive delivery systems containing disulfide linkages has prepared for tumor targeting and intracellular delivery of siRNA.144, 145 The disulfide linkage in the complex can increase siRNA transfection efficiency and decrease toxicity in cells with high levels of GSH, like tumor cells. Hu et al.146 constructed a redox-sensitive, oligopeptide-guided, self-assembling, and efficiency-enhanced (ROSE) system by mixing PEI-hβCD with Ad-SS-PEG and Ad-PEG-SP94 for miR-34a delivery, representing a significant effect improvement over conventional gene delivery strategies. In addition, the semblable strategy of MC11 peptide targeting EGFRs conjugated to PEI-hβCD could efficiently condense pDNA into NPs after mixing with Ad-SS-PEG, and it exhibited high transfection efficiency.147 It is said that, compared to non-reducible polyplexes such as PAA, redox-sensitive polyplexes show higher gene transfection activity in response to intracellular GSH levels.148 Meanwhile, the degradable behaviors of silica nanocomposite with disulfide bonds or embedded drugs revealed the redox-triggered degradable silica NPs for drug and gene delivery.149
Enzyme-Responsive Systems
Different from normal tissue, the levels of certain local enzymes, such as matrix metallo proteinases (MMPs), β-glucuronidase, hyaluronidase, urokinase plasminogen activator (uPA), human leukocyte elastase (HLE), and cancer-associated proteases (CAPs), are out of control, and they have been designed to develop enzyme-sensitive NPs for siRNA/microRNA delivery.150, 151, 152 For example, MMPs involved in cancer metastasis, progression, and invasion are highly expressed in tumor cells, and they have become one of the hotspots in antitumor therapy research.153 Zhu et al.154 used PEG-pp-PEI-PE to co-deliver drug and tumor-targeted siRNA by MMP2-sensitive response, which not only has the ability to co-incorporate different drugs, excellent physical characteristics and passive tumor targeting via the EPR effect, and, most importantly, the ability to release the siRNA preferentially in tumors where upregulated tumor levels of MMP2 triggered the de-shield of PEG and the exposure of the previously hidden PEI.154, 155 An enzyme-responsive system acts as a positive target delivery system that requires two components: the first one is the substrate or a substrate mimic as an enzyme-sensitive moiety, and the second component can lead to macroscopic transitions of nanomaterial and drug and gene release after interacting with the first component.156
Light-Responsive Systems
Light is a clean energy that is easy to apply, is controllable spatially and temporally, and has become another appealing stimulus for gene delivery. There are three regions of light used for triggering drug and gene delivery from appropriately designed nanocarriers: UV (10–400 nm), visible, and near-infrared (NIR) regions (650–900 nm). However, UV irradiation can’t be used in a light-responsive system because it is much more cytotoxic than the other regions of the light spectrum and because of its inability to penetrate deeply into the tissue due to the absorption by endogenous chromophores. For NIR, it is hardly absorbed by water, hemoglobin, and lipid, and it causes less damage to cells than visible light, which gives NIR the characteristics of lower absorption and scattering in tissue; thus, researchers have focused on NIR to induce drug or gene release.157 One strategy is attaching a carrier to the gold surface and then loading the gene onto the carrier, such as PLL. The PLL is attached to the surface of the Au nanoshell as a nucleic acid acceptor via Au-thiol binding and electrostatic binding, and then RNA or DNA was electrostatically attached to the cationic PLL with controllable releasing and no cytotoxicity.158 The other strategy is to covalently attach the gene to the gold surface. Wijaya et al.159 loaded two different DNA onto nanorods through thiol conjugation; the two different DNA were released selectively by the different wavelengths of NIR (λ = 800 or 1,100 nm) irradiation, and they did not exhibit any side effect from NIR or gold nanorods.159, 160
Multi-responsive System
Because of the complex environment of the human body, multi-responsive nanocarriers, as a new development in the field of environmentally responsive gene delivery, are designed to be two or more stimuli responsive, including pH and redox, pH and temperature, pH and light, light and redox, and other stimuli triggers. An et al.161 exploited a pH- and redox-responsive carrier; it not only rapidly released DNA under the presence of 10 mM GSH but also, when the pH reduced to the range of 7.4–6.3, the surface charge markedly increased. It benefited from the disulfide histamine composing the 4-arm PEG-b-poly(disulfide histamine) copolymer.161 Another similar system is a mPEG-SS-PLL15-glutaraldehyde star cationic polymer by Cai et al.162 Yang et al.163 developed polymer NPs with light- and pH-responsive polypeptides (PPPs), namely DSPE-PEG2000-PPP. Under NIR illumination and low pH at the tumor site after intravenous injection, DSPE-PEG2000-PPP could accumulate at tumor sites selectively and internalize into tumor cells.163 Besides, a redox- and light-responsive system like GNR-DSPEI-PEG-RGD could release DNA from the complex at 808-nm irradiation, and it exhibited degradability of disulfide crosslinked short PEI.164 The pH- and temperature-responsive carrier poly(ethylethylene phosphate)-block-poly[2-(dimethylamino)ethyl methacrylate] (PEEP-b-PDMAEMA) can effectively condense DNA because of the property of double-hydrophilic diblock copolymer. From the results of TEM and UV-vis measurement, PEEP-b-PDMAEMA was able to self-assemble into aggregates with different particle sizes and morphologies in aqueous solution in various pH media, and it exhibited a reproducible temperature-responsive behavior with a LCST.165
Design for Tumor Accumulation and Penetration
As an effective penetration system mentioned by Shen and colleagues,14 the NPs must be small, less than 30 nm, which is the opposite of blood circulation and tumor accumulation that favor larger sizes.166 Cationic nanomaterials because of their cationic charges can improve tumor penetration, but a neutral or a slightly negatively charged one can have a long blood circulation.167 They synthesized polycaprolactone (PCL)-block–PEI/amide-folate acid (FA) with high cellular uptake, which can reverse its charge triggered by pH change, because amides with β-carboxylic acids can hydrolyze in acidic conditions to regenerate the amines, giving rise to a negative-to-positive charge reversal.168 Thus, as we design an effective gene delivery system, the NPs should be neutral or negatively charged in blood circulation; once in the tumor’s acidic extracellular medium, they become positively charged for cellular uptake, lysosomal escape, and nuclear localization. Zhang and colleagues169, 170 also synthesized a similar delivery system, poly(l-lysine)-block-poly(l-leucine) with a nuclear target Tat peptide to achieve this purpose169 and FK/p53/PEG-PLLcomplexes composed of FK/p53 with PEG-PLL coated by folate.170 Also a polyion complex coating using micelles was constructed, which was composed of poly (lysine)-b-polycaprolactone (PLys-b-PCL) as the cationic core and poly (glutamic acid)-g-methoxyl poly (ethylene glycol) (PGlu-g-mPEG) as the anionic coating material. The charge reversal profile was manipulated by controlling the polymer chain entanglement and electrostatic interaction in the polyion complex layer through glutaraldehyde-induced shell crosslinking that facilitated the therapeutic effect via tumor pentration.171 In addition, quaternary amines carrying N-propionic 4-acetoxybenzyl ester substituents to make esterase-presponsive polymer were used as a gene carrier because it can undergo a quick intracellular esterase-catalyzed hydrolysis and then triggers the polymer’s charge reversal from cationic to zwitterionic. This esterase-catalyzed hydrolysis can be tracked by monitoring the release of 4-hydroxybenzyl alcohol by high-performance liquid chromatography (HPLC).172
To conjugate with tumor-homing and -penetrating cyclic peptide iRGD, it was shown that the tumor accumulation and penetration enhanced significantly, but also the C-terminally exposed cryptic(R/K)XX(R/K) CendR element can trigger neuropilin-1 (NRP-1) binding, resulting in cellular internalization and malignant tissue penetration.173, 174, 175, 176
Recently, Ji et al. developed peptide-based nanomaterials targeting and depleting cancer-associated fibroblasts (CAFs) to overcome the poor penetration and even low perfusion of molecular drugs177 through remodeling tumor microenvironments to enhance nanomedicine penetration and, thus, efficacy. Like inhibiting platelet-derived growth factor receptor-β expression by imatinib mesylate,178 reducing tumor extracellular matrix by collagenase,179 inhibiting the transforming growth factor β (TGF-β)-signaling pathway by LY364947,180 inhibiting the expression of collagenI and TGF-β by pirfenidone,178 depleting tumor collagen by losartan,181 and disrupting tumor extracellular fibronectins by cyclopamine181 have been shown to significantly improve tumor perfusion, accumulation, and intratumoral distribution.
Future Direction of Cationic Polymer Design in Gene Delivery
As mentioned above, engineering NPs was always designed via conjugation with some functional group to decrease the cytotoxicity and increase the efficiency of gene transfection, especially in preclinical trials with application prospects in both drug and gene delivery. The different modifications have been explored (Tables 2, 3, and 4) in the literature, as detailed above. We have found the advantages of modified NPs in RNAi therapy to be as follows: (1) biologically degradable with minimal cytotoxicity, (2) easy-to-modify physicochemical characteristics and simple purification procedures, (3) functional ligand conjugation with improved targeted delivery of siRNA, (4) most can deliver siRNA and chemotherapeutics for an additive or synergistic effect, and (5) siRNA delivery can be monitored by visualizing themselves or through proper labeling.
Table 4.
Multifunctional Cationic Polymer for Preclinical Gene Delivery Systems
| Functionalization | Advantage |
|---|---|
| PEGylation | stabilization enhanced, circulation time improved, prevention of protein absorption |
| Targeting | gene target efficacy in vivo enhanced |
| Stimulus response | gene target efficacy in vivo enhanced |
| Cell penetrating | cellular uptake enhanced, cross cell membrane |
| Endosome escaping | endosomal escaping enhanced, cross cell membrane |
| Nuclear localization | nuclear localization |
All the modifications are based on maximally decreasing the potential adverse human health effects. This requires that NPs do not impair mitochondrial function, form apoptotic bodies, produce reactive oxygen species under phasilogical conditions in vivo, and maintain their ideal properties. Furthermore, minimizing the problem of off-targeting, which causes an off-target immune response, is the most important consideration. Nevertheless, the different sizes affect the nanomaterials’ chemical block lengths and ratios, as well as stability, mechanical properties, charge, PEG-chain length or density, and even hydrophilic shell thickness. Besides, detailed analysis about tumor accumulation data for various nanomedicines found that only about 0.7% of injected doses actually accumulated in the tumor, because of the tumor’s inherent pathological characteristics.182 The large size of the NPs and tumor’s crosslinked matrix components also hinder the penetration of NPs.
Choosing an appropriate oncogene for gene therapy is also considered in our study. Oncogenes and oncoproteins are involved in the regulation of tumorigenic cell growth,183, 184 and some of them are accepted as tumor markers, such as RAS, WNT, MYC, ERK, and TRK. They include the following: (1) growth factors or mitogens, which can induce cell proliferation like c-sis;185 (2) receptor tyrosine kinases, which transduce signals for cell growth and differentiation, such as EGFR, vascular EGFR (VEGFR), and HER2/neu;186, 187, 188, 189, 190, 191 (3) cytoplasmic tyrosine kinases, which mediate the responses and the activation receptors of cell proliferatioin, migration, differentiation, and survival;84 (4) cytoplasmic serine/threonine kinases and their regulatory subunits involved in organism development; cell survival, differentiation, and apoptosis; cell cycle regulation; and cell proliferation;192, 193, 194 (5) regulatory GTPases such as ras protein, which are involved in signaling pathways of cell proliferation;195, 196 and (6) transcription factors like myc gene, which regulate the transcription of genes that induce cell proliferation.197 Furthermore, DNAzyme/RNAzyme198 and microRNA also are involved in cell growth and gene transcription, especially of oncogenes, and they act as tumor suppressors, such as miR-21, miR-34a, miR-132, and miR-155.199, 200, 201, 202, 203
Another application of NPs is drug delivery; thus, the number of studies on the co-delivery of siRNAs and drugs has been increasing, especially studies for drug-resistant cancer. For multidrug resistance (MDR), siRNA and anti-cancer drugs are delivered into cancer cells simultaneously; the siRNA is chosen to silence the genes related to drug resistance, decreasing the drug efflux pumps and activating apoptosis pathways. Following the release of siRNA, the accumulation of co-delivered anti-cancer drug inside of the cancer cells will increase, resulting in promoted chemotherapeutic effects.204 Co-delivery of siRNAs and anti-cancer drugs has been the most potential therapy to cure cancer.
Author Contributions
Y.X. conducted the writing and literature review of the entire article; K.S. conducted the modification and proofreading of “The smart nanoparticles engineeried for gene delivery”; Y.Q. and B.C. conducted “Clinical application of nanoparticle-based RNAi therapy” and “The challenges and barriers of polymers-mediated gene therapy”; and Z.Q. conducted the conception of the whole review and the final proofreading.
Conflicts of Interest
The authors have no conflicts of interest.
Acknowledgments
This work was financially supported by The National Natural Science Fund for Distinguished Young Scholars (NSFC31525009), the Sichuan Innovative Research Team Program for Young Scientists (2016TD0004), the National Natural Science Foundation of China (NSFC31771096), the National Key R&D Program of China (2016YFC1201700), and the Distinguished Young Scholars of Sichuan University (2011SCU04B18).
References
- 1.Mulligan R.C. The basic science of gene therapy. Science. 1993;260:926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- 2.Sioud M. RNA interference: mechanisms, technical challenges, and therapeutic opportunities. Methods Mol. Biol. 2015;1218:1–15. doi: 10.1007/978-1-4939-1538-5_1. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez A.A., Lim W.A., Qi L.S. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat. Rev. Mol. Cell Biol. 2016;17:5–15. doi: 10.1038/nrm.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu P.D., Lander E.S., Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang F., Zhou K., Ma L., Gressel S., Doudna J.A. STRUCTURAL BIOLOGY. A Cas9-guide RNA complex preorganized for target DNA recognition. Science. 2015;348:1477–1481. doi: 10.1126/science.aab1452. [DOI] [PubMed] [Google Scholar]
- 6.Sternberg S.H., Redding S., Jinek M., Greene E.C., Doudna J.A. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507:62–67. doi: 10.1038/nature13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams B.D., Parsons C., Walker L., Zhang W.C., Slack F.J. Targeting noncoding RNAs in disease. J. Clin. Invest. 2017;127:761–771. doi: 10.1172/JCI84424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corey D.R. Nusinersen, an antisense oligonucleotide drug for spinal muscular atrophy. Nat. Neurosci. 2017;20:497–499. doi: 10.1038/nn.4508. [DOI] [PubMed] [Google Scholar]
- 9.Hoy S.M. Nusinersen: First Global Approval. Drugs. 2017;77:473–479. doi: 10.1007/s40265-017-0711-7. [DOI] [PubMed] [Google Scholar]
- 10.Koo T., Wood M.J. Clinical trials using antisense oligonucleotides in duchenne muscular dystrophy. Hum. Gene Ther. 2013;24:479–488. doi: 10.1089/hum.2012.234. [DOI] [PubMed] [Google Scholar]
- 11.Singh A., Trivedi P., Jain N.K. Advances in siRNA delivery in cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018;46:274–283. doi: 10.1080/21691401.2017.1307210. [DOI] [PubMed] [Google Scholar]
- 12.Sun Q., Sun X., Ma X., Zhou Z., Jin E., Zhang B., Shen Y., Van Kirk E.A., Murdoch W.J., Lott J.R. Integration of nanoassembly functions for an effective delivery cascade for cancer drugs. Adv. Mater. 2014;26:7615–7621. doi: 10.1002/adma.201401554. [DOI] [PubMed] [Google Scholar]
- 13.Sun Q., Radosz M., Shen Y. Challenges in design of translational nanocarriers. J. Control. Release. 2012;164:156–169. doi: 10.1016/j.jconrel.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 14.Sun Q., Zhou Z., Qiu N., Shen Y. Rational Design of Cancer Nanomedicine: Nanoproperty Integration and Synchronization. Adv. Mater. 2017;29:18. doi: 10.1002/adma.201606628. [DOI] [PubMed] [Google Scholar]
- 15.Keles E., Song Y., Du D., Dong W.J., Lin Y. Recent progress in nanomaterials for gene delivery applications. Biomater. Sci. 2016;4:1291–1309. doi: 10.1039/c6bm00441e. [DOI] [PubMed] [Google Scholar]
- 16.Somia N., Verma I.M. Gene therapy: trials and tribulations. Nat. Rev. Genet. 2000;1:91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 17.Dufour B.D., McBride J.L. Intravascular AAV9 Administration for Delivering RNA Silencing Constructs to the CNS and Periphery. Methods Mol. Biol. 2016;1364:261–275. doi: 10.1007/978-1-4939-3112-5_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kootstra N.A., Verma I.M. Gene therapy with viral vectors. Annu. Rev. Pharmacol. Toxicol. 2003;43:413–439. doi: 10.1146/annurev.pharmtox.43.100901.140257. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen J., Szoka F.C. Nucleic acid delivery: the missing pieces of the puzzle? Acc. Chem. Res. 2012;45:1153–1162. doi: 10.1021/ar3000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kullberg M., McCarthy R., Anchordoquy T.J. Systemic tumor-specific gene delivery. J. Control. Release. 2013;172:730–736. doi: 10.1016/j.jconrel.2013.08.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mintzer M.A., Simanek E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009;109:259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 22.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 23.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 24.Maeda H., Tsukigawa K., Fang J. A Retrospective 30 Years After Discovery of the Enhanced Permeability and Retention Effect of Solid Tumors: Next-Generation Chemotherapeutics and Photodynamic Therapy--Problems, Solutions, and Prospects. Microcirculation. 2016;23:173–182. doi: 10.1111/micc.12228. [DOI] [PubMed] [Google Scholar]
- 25.Danhier F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release. 2016;244(Pt A):108–121. doi: 10.1016/j.jconrel.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Li X., Hong L., Song T., Rodríguez-Patón A., Chen C., Zhao H., Shi X. Highly Biocompatible Drug-Delivery Systems Based on DNA Nanotechnology. J. Biomed. Nanotechnol. 2017;13:747–757. [Google Scholar]
- 27.Zhou J., Song X., Han S., Wei T., Wang X., Cao H., Liang X.-J., Liang Z., Cheng Q., Deng L., Dong A. Balancing Biocompatibility, Internalization and Pharmacokinetics of Polycations/siRNA by Structuring the Weak Negative Charged Ternary Complexes with Hyaluronic Acid. J. Biomed. Nanotechnol. 2017;13:1533–1544. doi: 10.1166/jbn.2017.2438. [DOI] [PubMed] [Google Scholar]
- 28.Oliveira C., Ribeiro A.J., Veiga F., Silveira I. Recent Advances in Nucleic Acid-Based Delivery: From Bench to Clinical Trials in Genetic Diseases. J. Biomed. Nanotechnol. 2016;12:841–862. doi: 10.1166/jbn.2016.2245. [DOI] [PubMed] [Google Scholar]
- 29.Heidel J.D., Liu J.Y.C., Yen Y., Zhou B., Heale B.S.E., Rossi J.J., Bartlett D.W., Davis M.E. Potent siRNA inhibitors of ribonucleotide reductase subunit RRM2 reduce cell proliferation in vitro and in vivo. Clin. Cancer Res. 2007;13:2207–2215. doi: 10.1158/1078-0432.CCR-06-2218. [DOI] [PubMed] [Google Scholar]
- 30.Zuckerman J.E., Davis M.E. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat. Rev. Drug Discov. 2015;14:843–856. doi: 10.1038/nrd4685. [DOI] [PubMed] [Google Scholar]
- 31.Zuckerman J.E., Gritli I., Tolcher A., Heidel J.D., Lim D., Morgan R., Chmielowski B., Ribas A., Davis M.E., Yen Y. Correlating animal and human phase Ia/Ib clinical data with CALAA-01, a targeted, polymer-based nanoparticle containing siRNA. Proc. Natl. Acad. Sci. USA. 2014;111:11449–11454. doi: 10.1073/pnas.1411393111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golan T., Khvalevsky E.Z., Hubert A., Gabai R.M., Hen N., Segal A., Domb A., Harari G., David E.B., Raskin S. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6:24560–24570. doi: 10.18632/oncotarget.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Titze-de-Almeida R., David C., Titze-de-Almeida S.S. The Race of 10 Synthetic RNAi-Based Drugs to the Pharmaceutical Market. Pharm. Res. 2017;34:1339–1363. doi: 10.1007/s11095-017-2134-2. [DOI] [PubMed] [Google Scholar]
- 34.Nyska A., Schiffenbauer Y.S., Brami C.T., Maronpot R.R., Ramot Y. Histopathology of biodegradable polymers: challenges in interpretation and the use of a novel compact MRI for biocompatibility evaluation. Polym. Adv. Technol. 2014;25:461–467. [Google Scholar]
- 35.Ramot Y., Nyska A., Markovitz E., Dekel A., Klaiman G., Zada M.H., Domb A.J., Maronpot R.R. Long-term Local and Systemic Safety of Poly(L-lactide-co-epsilon-caprolactone) after Subcutaneous and Intra-articular Implantation in Rats. Toxicol. Pathol. 2015;43:1127–1140. doi: 10.1177/0192623315600275. [DOI] [PubMed] [Google Scholar]
- 36.Ramot Y., Touitou D., Levin G., Ickowicz D.E., Zada M.H., Abbas R., Yankelson L., Domb A.J., Nyska A. Interspecies differences in reaction to a biodegradable subcutaneous tissue filler: severe inflammatory granulomatous reaction in the Sinclair minipig. Toxicol. Pathol. 2015;43:267–271. doi: 10.1177/0192623314534995. [DOI] [PubMed] [Google Scholar]
- 37.Schultheis B., Strumberg D., Santel A., Vank C., Gebhardt F., Keil O., Lange C., Giese K., Kaufmann J., Khan M., Drevs J. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 2014;32:4141–4148. doi: 10.1200/JCO.2013.55.0376. [DOI] [PubMed] [Google Scholar]
- 38.Bulbake U., Doppalapudi S., Kommineni N., Khan W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. 2017;9:E12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X. Targeting Polo-Like Kinases: A Promising Therapeutic Approach for Cancer Treatment. Transl. Oncol. 2015;8:185–195. doi: 10.1016/j.tranon.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanasty R., Dorkin J.R., Vegas A., Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 41.Geiser M. Update on macrophage clearance of inhaled micro- and nanoparticles. J. Aerosol Med. Pulm. Drug Deliv. 2010;23:207–217. doi: 10.1089/jamp.2009.0797. [DOI] [PubMed] [Google Scholar]
- 42.Oh N., Park J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomedicine. 2014;9(Suppl 1):51–63. doi: 10.2147/IJN.S26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee H.Y., Mohammed K.A., Nasreen N. Nanoparticle-based targeted gene therapy for lung cancer. Am. J. Cancer Res. 2016;6:1118–1134. [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang X., Röcker C., Hafner M., Brandholt S., Dörlich R.M., Nienhaus G.U. Endo- and exocytosis of zwitterionic quantum dot nanoparticles by live HeLa cells. ACS Nano. 2010;4:6787–6797. doi: 10.1021/nn101277w. [DOI] [PubMed] [Google Scholar]
- 45.Desai M.P., Labhasetwar V., Walter E., Levy R.J., Amidon G.L. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharm. Res. 1997;14:1568–1573. doi: 10.1023/a:1012126301290. [DOI] [PubMed] [Google Scholar]
- 46.Prabha S., Zhou W.Z., Panyam J., Labhasetwar V. Size-dependency of nanoparticle-mediated gene transfection: studies with fractionated nanoparticles. Int. J. Pharm. 2002;244:105–115. doi: 10.1016/s0378-5173(02)00315-0. [DOI] [PubMed] [Google Scholar]
- 47.Xu D.M., Yao S.D., Liu Y.B., Sheng K.L., Hong J., Gong P.J., Dong L. Size-dependent properties of M-PEIs nanogels for gene delivery in cancer cells. Int. J. Pharm. 2007;338:291–296. doi: 10.1016/j.ijpharm.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 48.Dean D.A., Dean B.S., Muller S., Smith L.C. Sequence requirements for plasmid nuclear import. Exp. Cell Res. 1999;253:713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aronsohn A.I., Hughes J.A. Nuclear localization signal peptides enhance cationic liposome-mediated gene therapy. J. Drug Target. 1998;5:163–169. doi: 10.3109/10611869808995871. [DOI] [PubMed] [Google Scholar]
- 50.Collins E., Birchall J.C., Williams J.L., Gumbleton M. Nuclear localisation and pDNA condensation in non-viral gene delivery. J. Gene Med. 2007;9:265–274. doi: 10.1002/jgm.1015. [DOI] [PubMed] [Google Scholar]
- 51.Zaitsev S., Buchwalow I., Haberland A., Tkachuk S., Zaitseva I., Haller H., Böttger M. Histone H1-mediated transfection: role of calcium in the cellular uptake and intracellular fate of H1-DNA complexes. Acta Histochem. 2002;104:85–92. doi: 10.1078/0065-1281-00633. [DOI] [PubMed] [Google Scholar]
- 52.Klink D.T., Chao S., Glick M.C., Scanlin T.F. Nuclear translocation of lactosylated poly-L-lysine/cDNA complex in cystic fibrosis airway epithelial cells. Mol. Ther. 2001;3:831–841. doi: 10.1006/mthe.2001.0332. [DOI] [PubMed] [Google Scholar]
- 53.Grandinetti G., Reineke T.M. Exploring the mechanism of plasmid DNA nuclear internalization with polymer-based vehicles. Mol. Pharm. 2012;9:2256–2267. doi: 10.1021/mp300142d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grabinski C., Hussain S., Lafdi K., Braydich-Stolle L., Schlager J. Effect of particle dimension on biocompatibility of carbon nanomaterials. Carbon. 2007;45:2828–2835. [Google Scholar]
- 56.Li X., Liu W., Sun L., Aifantis K.E., Yu B., Fan Y., Feng Q., Cui F., Watari F. Effects of physicochemical properties of nanomaterials on their toxicity. J. Biomed. Mater. Res. A. 2015;103:2499–2507. doi: 10.1002/jbm.a.35384. [DOI] [PubMed] [Google Scholar]
- 57.Khanna P., Ong C., Bay B.H., Baeg G.H. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials (Basel) 2015;5:1163–1180. doi: 10.3390/nano5031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang R., Men K., Zhang X., Huang R., Tian Y., Zhou B., Yu C., Wang Y., Ji X., Hu Q., Yang L. Delivery of a Modified mRNA Encoding IL-22 Binding Protein (IL-22BP) for Colon Cancer Gene Therapy. J. Biomed. Nanotechnol. 2018;14:1239–1251. doi: 10.1166/jbn.2018.2577. [DOI] [PubMed] [Google Scholar]
- 59.Sakurai F., Nishioka T., Yamashita F., Takakura Y., Hashida M. Effects of erythrocytes and serum proteins on lung accumulation of lipoplexes containing cholesterol or DOPE as a helper lipid in the single-pass rat lung perfusion system. Eur. J. Pharm. Biopharm. 2001;52:165–172. doi: 10.1016/s0939-6411(01)00165-5. [DOI] [PubMed] [Google Scholar]
- 60.Lee J., Ahn H.J. PEGylated DC-Chol/DOPE cationic liposomes containing KSP siRNA as a systemic siRNA delivery Carrier for ovarian cancer therapy. Biochem. Biophys. Res. Commun. 2018;503:1716–1722. doi: 10.1016/j.bbrc.2018.07.104. [DOI] [PubMed] [Google Scholar]
- 61.Zou L., Lee S.Y., Wu Q., Zhang H., Bastian A., Orji C., Payne G., Galvez A., Thomas T., Zhang Z., Dou H. Facile Gene Delivery Derived from Branched Low Molecular Weight Polyethylenimine by High Efficient Chemistry. J. Biomed. Nanotechnol. 2018;14:1785–1795. doi: 10.1166/jbn.2018.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fàbregas A., Prieto-Sánchez S., Suñé-Pou M., Boyero-Corral S., Ticó J.R., García-Montoya E., Pérez-Lozano P., Miñarro M., Suñé-Negre J.M., Hernández-Munain C., Suñé C. Improved formulation of cationic solid lipid nanoparticles displays cellular uptake and biological activity of nucleic acids. Int. J. Pharm. 2017;516:39–44. doi: 10.1016/j.ijpharm.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 63.Li M., Su Y., Zhang F., Chen K., Xu X., Xu L., Zhou J., Wang W. A dual-targeting reconstituted high density lipoprotein leveraging the synergy of sorafenib and antimiRNA21 for enhanced hepatocellular carcinoma therapy. Acta Biomater. 2018;75:413–426. doi: 10.1016/j.actbio.2018.05.049. [DOI] [PubMed] [Google Scholar]
- 64.Wang R., Zhao Z., Han Y., Hu S., Opoku-Damoah Y., Zhou J., Yin L., Ding Y. Natural Particulates Inspired Specific-Targeted Codelivery of siRNA and Paclitaxel for Collaborative Antitumor Therapy. Mol. Pharm. 2017;14:2999–3012. doi: 10.1021/acs.molpharmaceut.7b00192. [DOI] [PubMed] [Google Scholar]
- 65.Akbaba H., Erel Akbaba G., Kantarcı A.G. Development and evaluation of antisense shRNA-encoding plasmid loaded solid lipid nanoparticles against 5-α reductase activity. J. Drug Deliv. Sci. Technol. 2018;44:270–277. [Google Scholar]
- 66.Howard K.A. Delivery of RNA interference therapeutics using polycation-based nanoparticles. Adv. Drug Deliv. Rev. 2009;61:710–720. doi: 10.1016/j.addr.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 67.Soppimath K.S., Aminabhavi T.M., Kulkarni A.R., Rudzinski W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 68.Ma Z., Lim T.M., Lim L.Y. Pharmacological activity of peroral chitosan-insulin nanoparticles in diabetic rats. Int. J. Pharm. 2005;293:271–280. doi: 10.1016/j.ijpharm.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 69.Mansouri S., Lavigne P., Corsi K., Benderdour M., Beaumont E., Fernandes J.C. Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: strategies to improve transfection efficacy. Eur. J. Pharm. Biopharm. 2004;57:1–8. doi: 10.1016/s0939-6411(03)00155-3. [DOI] [PubMed] [Google Scholar]
- 70.Ballarín-González B., Howard K.A. Polycation-based nanoparticle delivery of RNAi therapeutics: adverse effects and solutions. Adv. Drug Deliv. Rev. 2012;64:1717–1729. doi: 10.1016/j.addr.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Fang J.-K., Chen L., Lu X.-G., Cao D., Guo L.-L., Zhang Y.-S., Li L.B., Zhang L.F., Kuang Y.T., Wang S.L. Optimization of Transforming Growth Factor-β1 siRNA Loaded Chitosan-Tripolyphosphate Nanoparticles for the Treatment of Colorectal Cancer Hepatic Metastasis in a Mouse Model. J. Biomed. Nanotechnol. 2016;12:1489–1500. doi: 10.1166/jbn.2016.2265. [DOI] [PubMed] [Google Scholar]
- 72.Nascimento A.V., Gattacceca F., Singh A., Bousbaa H., Ferreira D., Sarmento B., Amiji M.M. Biodistribution and pharmacokinetics of Mad2 siRNA-loaded EGFR-targeted chitosan nanoparticles in cisplatin sensitive and resistant lung cancer models. Nanomedicine (Lond.) 2016;11:767–781. doi: 10.2217/nnm.16.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun P., Huang W., Jin M., Wang Q., Fan B., Kang L., Gao Z. Chitosan-based nanoparticles for survivin targeted siRNA delivery in breast tumor therapy and preventing its metastasis. Int. J. Nanomedicine. 2016;11:4931–4945. doi: 10.2147/IJN.S105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corbet C., Ragelle H., Pourcelle V., Vanvarenberg K., Marchand-Brynaert J., Préat V., Feron O. Delivery of siRNA targeting tumor metabolism using non-covalent PEGylated chitosan nanoparticles: Identification of an optimal combination of ligand structure, linker and grafting method. J. Control. Release. 2016;223:53–63. doi: 10.1016/j.jconrel.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 75.Siahmansouri H., Somi M.H., Babaloo Z., Baradaran B., Jadidi-Niaragh F., Atyabi F., Mohammadi H., Ahmadi M., Yousefi M. Effects of HMGA2 siRNA and doxorubicin dual delivery by chitosan nanoparticles on cytotoxicity and gene expression of HT-29 colorectal cancer cell line. J. Pharm. Pharmacol. 2016;68:1119–1130. doi: 10.1111/jphp.12593. [DOI] [PubMed] [Google Scholar]
- 76.Jadidi-Niaragh F., Atyabi F., Rastegari A., Mollarazi E., Kiani M., Razavi A., Yousefi M., Kheshtchin N., Hassannia H., Hadjati J., Shokri F. Downregulation of CD73 in 4T1 breast cancer cells through siRNA-loaded chitosan-lactate nanoparticles. Tumour Biol. 2016;37:8403–8412. doi: 10.1007/s13277-015-4732-0. [DOI] [PubMed] [Google Scholar]
- 77.Xu C.-N., Tian H.-Y., Wang Y.-B., Du Y., Chen J., Lin L., Guo Z.-P., Chen X.-S. Anti-tumor effects of combined doxorubicin and siRNA for pulmonary delivery. Chin. Chem. Lett. 2017;28:807–812. [Google Scholar]
- 78.Li L., Wei Y., Gong C. Polymeric Nanocarriers for Non-Viral Gene Delivery. J. Biomed. Nanotechnol. 2015;11:739–770. doi: 10.1166/jbn.2015.2069. [DOI] [PubMed] [Google Scholar]
- 79.Fischer D., Bieber T., Li Y., Elsässer H.P., Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 1999;16:1273–1279. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 80.Kunath K., von Harpe A., Fischer D., Petersen H., Bickel U., Voigt K., Kissel T. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J. Control. Release. 2003;89:113–125. doi: 10.1016/s0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 81.Liechty W.B., Kryscio D.R., Slaughter B.V., Peppas N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010;1:149–173. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petersen H., Fechner P.M., Fischer D., Kissel T. Synthesis, characterization, and biocompatibility of polyethylenimine-graft-poly(ethylene glycol) block copolymers. Macromolecules. 2002;35:6867–6874. [Google Scholar]
- 83.Kichler A. Gene transfer with modified polyethylenimines. J. Gene Med. 2004;6(Suppl 1):S3–S10. doi: 10.1002/jgm.507. [DOI] [PubMed] [Google Scholar]
- 84.Wu J., Liu B., Wu H., Wu Y., Zhang W., Zhao S., Zhang L., Pan X., Gao W., Wang X. A Gold Nanoparticle Platform for the Delivery of Functional TGF-β1 siRNA Into Cancer Cells. J. Biomed. Nanotechnol. 2016;12:800–810. doi: 10.1166/jbn.2016.2217. [DOI] [PubMed] [Google Scholar]
- 85.He Z.-Y., Jin Z.-H., Zhan M., Qin Z., Li Z., Xu T. Advances in quantum dot-mediated siRNA delivery. Chin. Chem. Lett. 2017;28:1851–1856. [Google Scholar]
- 86.Kim J., Park J., Kim H., Singha K., Kim W.J. Transfection and intracellular trafficking properties of carbon dot-gold nanoparticle molecular assembly conjugated with PEI-pDNA. Biomaterials. 2013;34:7168–7180. doi: 10.1016/j.biomaterials.2013.05.072. [DOI] [PubMed] [Google Scholar]
- 87.Samal S.K., Dash M., Van Vlierberghe S., Kaplan D.L., Chiellini E., van Blitterswijk C., Moroni L., Dubruel P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012;41:7147–7194. doi: 10.1039/c2cs35094g. [DOI] [PubMed] [Google Scholar]
- 88.Sato A., Choi S.W., Hirai M., Yamayoshi A., Moriyama R., Yamano T., Takagi M., Kano A., Shimamoto A., Maruyama A. Polymer brush-stabilized polyplex for a siRNA carrier with long circulatory half-life. J. Control. Release. 2007;122:209–216. doi: 10.1016/j.jconrel.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 89.Martin M.E., Rice K.G. Peptide-guided gene delivery. AAPS J. 2007;9:E18–E29. doi: 10.1208/aapsj0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park T.G., Jeong J.H., Kim S.W. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 2006;58:467–486. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 91.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016;99(Pt A):28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kano A., Moriyama K., Yamano T., Nakamura I., Shimada N., Maruyama A. Grafting of poly(ethylene glycol) to poly-lysine augments its lifetime in blood circulation and accumulation in tumors without loss of the ability to associate with siRNA. J. Control. Release. 2011;149:2–7. doi: 10.1016/j.jconrel.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 93.Zhao X., Komatsu D.E., Hadjiargyrou M. Delivery of rhBMP-2 Plasmid DNA Complexes via a PLLA/Collagen Electrospun Scaffold Induces Ectopic Bone Formation. J. Biomed. Nanotechnol. 2016;12:1285–1296. doi: 10.1166/jbn.2016.2250. [DOI] [PubMed] [Google Scholar]
- 94.Lin C., Zhong Z., Lok M.C., Jiang X., Hennink W.E., Feijen J., Engbersen J.F. Novel bioreducible poly(amido amine)s for highly efficient gene delivery. Bioconjug. Chem. 2007;18:138–145. doi: 10.1021/bc060200l. [DOI] [PubMed] [Google Scholar]
- 95.Jeong H., Lee E.-S., Jung G., Park J., Jeong B., Ryu K.H., Hwang N.S., Lee H. Bioreducible-Cationic Poly(amido amine)s for Enhanced Gene Delivery and Osteogenic Differentiation of Tonsil-Derived Mesenchymal Stem Cells. J. Biomed. Nanotechnol. 2016;12:1023–1034. doi: 10.1166/jbn.2016.2223. [DOI] [PubMed] [Google Scholar]
- 96.Lin C., Blaauboer C.-J., Timoneda M.M., Lok M.C., van Steenbergen M., Hennink W.E., Zhong Z., Feijen J., Engbersen J.F. Bioreducible poly(amido amine)s with oligoamine side chains: synthesis, characterization, and structural effects on gene delivery. J. Control. Release. 2008;126:166–174. doi: 10.1016/j.jconrel.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 97.Cherng J.Y., van de Wetering P., Talsma H., Crommelin D.J., Hennink W.E. Effect of size and serum proteins on transfection efficiency of poly ((2-dimethylamino)ethyl methacrylate)-plasmid nanoparticles. Pharm. Res. 1996;13:1038–1042. doi: 10.1023/a:1016054623543. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y., Zheng M., Kissel T., Agarwal S. Design and biophysical characterization of bioresponsive degradable poly(dimethylaminoethyl methacrylate) based polymers for in vitro DNA transfection. Biomacromolecules. 2012;13:313–322. doi: 10.1021/bm2015174. [DOI] [PubMed] [Google Scholar]
- 99.Qiao Y., Huang Y., Qiu C., Yue X., Deng L., Wan Y., Xing J., Zhang C., Yuan S., Dong A., Xu J. The use of PEGylated poly [2-(N,N-dimethylamino) ethyl methacrylate] as a mucosal DNA delivery vector and the activation of innate immunity and improvement of HIV-1-specific immune responses. Biomaterials. 2010;31:115–123. doi: 10.1016/j.biomaterials.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 100.Wang H.X., Song Z., Lao Y.H., Xu X., Gong J., Cheng D., Chakraborty S., Park J.S., Li M., Huang D. Nonviral gene editing via CRISPR/Cas9 delivery by membrane-disruptive and endosomolytic helical polypeptide. Proc. Natl. Acad. Sci. USA. 2018;115:4903–4908. doi: 10.1073/pnas.1712963115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim C., Agasti S.S., Zhu Z., Isaacs L., Rotello V.M. Recognition-mediated activation of therapeutic gold nanoparticles inside living cells. Nat. Chem. 2010;2:962–966. doi: 10.1038/nchem.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim H.S., Son Y.J., Mao W., Leong K.W., Yoo H.S. Atom Transfer Radical Polymerization of Multishelled Cationic Corona for the Systemic Delivery of siRNA. Nano Lett. 2018;18:314–325. doi: 10.1021/acs.nanolett.7b04183. [DOI] [PubMed] [Google Scholar]
- 103.Uz M., Bulmus V., Alsoy Altinkaya S. Effect of PEG Grafting Density and Hydrodynamic Volume on Gold Nanoparticle-Cell Interactions: An Investigation on Cell Cycle, Apoptosis, and DNA Damage. Langmuir. 2016;32:5997–6009. doi: 10.1021/acs.langmuir.6b01289. [DOI] [PubMed] [Google Scholar]
- 104.Lazarus G.G., Singh M. Cationic modified gold nanoparticles show enhanced gene delivery in vitro. Nanotechnol. Rev. 2016;5:425–434. [Google Scholar]
- 105.Chandran P., Riviere J.E., Monteiro-Riviere N.A. Surface chemistry of gold nanoparticles determines the biocorona composition impacting cellular uptake, toxicity and gene expression profiles in human endothelial cells. Nanotoxicology. 2017;11:507–519. doi: 10.1080/17435390.2017.1314036. [DOI] [PubMed] [Google Scholar]
- 106.Boisselier E., Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 107.Poletaeva J., Dovydenko I., Epanchintseva A., Korchagina K., Pyshnyi D., Apartsin E., Ryabchikova E., Pyshnaya I. Non-Covalent Associates of siRNAs and AuNPs Enveloped with Lipid Layer and Doped with Amphiphilic Peptide for Efficient siRNA Delivery. Int. J. Mol. Sci. 2018;19:E2096. doi: 10.3390/ijms19072096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li S., Tian C., Liu Y., Wang Z., Ma Y., Han Z., Du J., Zhang J., Gu Y. Mechanism of Cellular Uptake to Optimized AuNP Beacon for Tracing mRNA Changes in Living Cells. Part. Part. Syst. Charact. 2018;35:1700331. [Google Scholar]
- 109.Son S., Kim N., You D.G., Yoon H.Y., Yhee J.Y., Kim K., Kwon I.C., Kim S.H. Antitumor therapeutic application of self-assembled RNAi-AuNP nanoconstructs: Combination of VEGF-RNAi and photothermal ablation. Theranostics. 2017;7:9–22. doi: 10.7150/thno.16042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kong L., Alves C.S., Hou W., Qiu J., Möhwald H., Tomás H., Shi X. RGD peptide-modified dendrimer-entrapped gold nanoparticles enable highly efficient and specific gene delivery to stem cells. ACS Appl. Mater. Interfaces. 2015;7:4833–4843. doi: 10.1021/am508760w. [DOI] [PubMed] [Google Scholar]
- 111.Chan K.P., Chao S.H., Kah J.C.Y. Universal mRNA Translation Enhancement with Gold Nanoparticles Conjugated to Oligonucleotides with a Poly(T) Sequence. ACS Appl. Mater. Interfaces. 2018;10:5203–5212. doi: 10.1021/acsami.7b16390. [DOI] [PubMed] [Google Scholar]
- 112.Landon E.J., Naukam R.J., Rama Sastry B.V. Effects of calcium channel blocking agents on calcium and centrilobular necrosis in the liver of rats treated with hepatotoxic agents. Biochem. Pharmacol. 1986;35:697–705. doi: 10.1016/0006-2952(86)90369-2. [DOI] [PubMed] [Google Scholar]
- 113.Klesing J., Chernousova S., Epple M. Freeze-dried cationic calcium phosphate nanorods as versatile carriers of nucleic acids (DNA, siRNA) J. Mater. Chem. 2012;22:199–204. [Google Scholar]
- 114.Lecaros R.L., Huang L., Lee T.C., Hsu Y.C. Nanoparticle Delivered VEGF-A siRNA Enhances Photodynamic Therapy for Head and Neck Cancer Treatment. Mol. Ther. 2016;24:106–116. doi: 10.1038/mt.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang J., Howard C.B., Mahler S.M., Thurecht K.J., Huang L., Xu Z.P. Enhanced delivery of siRNA to triple negative breast cancer cells in vitro and in vivo through functionalizing lipid-coated calcium phosphate nanoparticles with dual target ligands. Nanoscale. 2018;10:4258–4266. doi: 10.1039/c7nr08644j. [DOI] [PubMed] [Google Scholar]
- 116.Wu Z., Chen J., Sun Y., Shen M., Huang L., Xu Q., Duan Y., Li Y. Tumor Microenvironment-Response Calcium Phosphate Hybrid Nanoparticles Enhanced siRNAs Targeting Tumors InVivo. J. Biomed. Nanotechnol. 2018;14:1816–1825. doi: 10.1166/jbn.2018.2606. [DOI] [PubMed] [Google Scholar]
- 117.Ru D., Ding C., Liu L., Liu H., Shen M., Duan Y., Li H. pH Sensitive Triptolide-Loaded Liposome Calcium Phosphate Nanoparticles Exhibit Enhanced Anti-Tumor Activities Against Ovarian Cancer Without Damaging the Reproductive System. J. Biomed. Nanotechnol. 2017;13:1413–1424. doi: 10.1166/jbn.2017.2429. [DOI] [PubMed] [Google Scholar]
- 118.Qiu C., Wei W., Sun J., Zhang H.-T., Ding J.-S., Wang J.-C., Zhang Q. Systemic delivery of siRNA by hyaluronan-functionalized calcium phosphate nanoparticles for tumor-targeted therapy. Nanoscale. 2016;8:13033–13044. doi: 10.1039/c6nr04034a. [DOI] [PubMed] [Google Scholar]
- 119.Pittella F., Cabral H., Maeda Y., Mi P., Watanabe S., Takemoto H., Kim H.J., Nishiyama N., Miyata K., Kataoka K. Systemic siRNA delivery to a spontaneous pancreatic tumor model in transgenic mice by PEGylated calcium phosphate hybrid micelles. J. Control. Release. 2014;178:18–24. doi: 10.1016/j.jconrel.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 120.Tseng Y.C., Yang A., Huang L. How does the cell overcome LCP nanoparticle-induced calcium toxicity? Mol. Pharm. 2013;10:4391–4395. doi: 10.1021/mp400028m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen J., Gao P., Yuan S., Li R., Ni A., Chu L., Ding L., Sun Y., Liu X.Y., Duan Y. Oncolytic Adenovirus Complexes Coated with Lipids and Calcium Phosphate for Cancer Gene Therapy. ACS Nano. 2016;10:11548–11560. doi: 10.1021/acsnano.6b06182. [DOI] [PubMed] [Google Scholar]
- 122.Wu Y.F., Wu H.C., Kuan C.H., Lin C.J., Wang L.W., Chang C.W., Wang T.W. Multi-functionalized carbon dots as theranostic nanoagent for gene delivery in lung cancer therapy. Sci. Rep. 2016;6:21170. doi: 10.1038/srep21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dicheva B.M., Koning G.A. Targeted thermosensitive liposomes: an attractive novel approach for increased drug delivery to solid tumors. Expert Opin. Drug Deliv. 2014;11:83–100. doi: 10.1517/17425247.2014.866650. [DOI] [PubMed] [Google Scholar]
- 124.Torchilin V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014;13:813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sawant R.R., Torchilin V.P. Multifunctional nanocarriers and intracellular drug delivery. Curr. Opin. Solid State Mater. Sci. 2012;16:269–275. [Google Scholar]
- 126.Jhaveri A.M., Torchilin V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 2014;5:77. doi: 10.3389/fphar.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parhiz H., Hashemi M., Hatefi A., Shier W.T., Amel Farzad S., Ramezani M. Arginine-rich hydrophobic polyethylenimine: potent agent with simple components for nucleic acid delivery. Int. J. Biol. Macromol. 2013;60:18–27. doi: 10.1016/j.ijbiomac.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 128.Wang D.A., Narang A.S., Kotb M., Gaber A.O., Miller D.D., Kim S.W., Mahato R.I. Novel branched poly(ethylenimine)-cholesterol water-soluble lipopolymers for gene delivery. Biomacromolecules. 2002;3:1197–1207. doi: 10.1021/bm025563c. [DOI] [PubMed] [Google Scholar]
- 129.Chen L., Tian H., Chen J., Chen X., Huang Y., Jing X. Multi-armed poly(L-glutamic acid)-graft-oligoethylenimine copolymers as efficient nonviral gene delivery vectors. J. Gene Med. 2010;12:64–76. doi: 10.1002/jgm.1405. [DOI] [PubMed] [Google Scholar]
- 130.Xie Y., Qiao H., Su Z., Chen M., Ping Q., Sun M. PEGylated carboxymethyl chitosan/calcium phosphate hybrid anionic nanoparticles mediated hTERT siRNA delivery for anticancer therapy. Biomaterials. 2014;35:7978–7991. doi: 10.1016/j.biomaterials.2014.05.068. [DOI] [PubMed] [Google Scholar]
- 131.Filip J., Popelka A., Bertok T., Holazova A., Osicka J., Kollar J., Ilcikova M., Tkac J., Kasak P. pH-Switchable Interaction of a Carboxybetaine Ester-Based SAM with DNA and Gold Nanoparticles. Langmuir. 2017;33:6657–6666. doi: 10.1021/acs.langmuir.7b00568. [DOI] [PubMed] [Google Scholar]
- 132.Stayton P.S., Shimoboji T., Long C., Chilkoti A., Chen G., Harris J.M., Hoffman A.S. Control of protein-ligand recognition using a stimuli-responsive polymer. Nature. 1995;378:472–474. doi: 10.1038/378472a0. [DOI] [PubMed] [Google Scholar]
- 133.Park J.S., Yang H.N., Woo D.G., Jeon S.Y., Park K.-H. Poly(N-isopropylacrylamide-co-acrylic acid) nanogels for tracing and delivering genes to human mesenchymal stem cells. Biomaterials. 2013;34:8819–8834. doi: 10.1016/j.biomaterials.2013.07.082. [DOI] [PubMed] [Google Scholar]
- 134.Kurisawa M., Yokoyama M., Okano T. Gene expression control by temperature with thermo-responsive polymeric gene carriers. J. Control. Release. 2000;69:127–137. doi: 10.1016/s0168-3659(00)00297-2. [DOI] [PubMed] [Google Scholar]
- 135.Feng G., Chen H., Li J., Huang Q., Gupte M.J., Liu H., Song Y., Ge Z. Gene therapy for nucleus pulposus regeneration by heme oxygenase-1 plasmid DNA carried by mixed polyplex micelles with thermo-responsive heterogeneous coronas. Biomaterials. 2015;52:1–13. doi: 10.1016/j.biomaterials.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 136.Lee S.H., Choi S.H., Kim S.H., Park T.G. Thermally sensitive cationic polymer nanocapsules for specific cytosolic delivery and efficient gene silencing of siRNA: swelling induced physical disruption of endosome by cold shock. J. Control. Release. 2008;125:25–32. doi: 10.1016/j.jconrel.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 137.Wu Y., Li G., Bai L., Li W., Wang S., Ba X., Zhou G., Zhao H. Synthesis backbone-dual-responsive of hyperbranched poly(bis(N,N-ethyl acrylamide))s by RAFT. Macromol. Res. 2014;22:1196–1202. [Google Scholar]
- 138.Thamphiwatana S., Fu V., Zhu J., Lu D., Gao W., Zhang L. Nanoparticle-stabilized liposomes for pH-responsive gastric drug delivery. Langmuir. 2013;29:12228–12233. doi: 10.1021/la402695c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rajpoot P., Bali V., Pathak K. Anticancer efficacy, tissue distribution and blood pharmacokinetics of surface modified nanocarrier containing melphalan. Int. J. Pharm. 2012;426:219–230. doi: 10.1016/j.ijpharm.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 140.Chen G., Ma B., Wang Y., Gong S. A Universal GSH-Responsive Nanoplatform for the Delivery of DNA, mRNA, and Cas9/sgRNA Ribonucleoprotein. ACS Appl. Mater. Interfaces. 2018;10:18515–18523. doi: 10.1021/acsami.8b03496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cheng R., Feng F., Meng F., Deng C., Feijen J., Zhong Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release. 2011;152:2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 142.Yu F., Huang J., Yu Y., Lu Y., Chen Y., Zhang H., Zhou G., Sun Z., Liu J., Sun D. Glutathione-Responsive Multilayer Coated Gold Nanoparticles for Targeted Gene Delivery. J. Biomed. Nanotechnol. 2016;12:503–515. doi: 10.1166/jbn.2016.2177. [DOI] [PubMed] [Google Scholar]
- 143.Torchilin V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur. J. Pharm. Biopharm. 2009;71:431–444. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim S.H., Jeong J.H., Kim T.I., Kim S.W., Bull D.A. VEGF siRNA delivery system using arginine-grafted bioreducible poly(disulfide amine) Mol. Pharm. 2009;6:718–726. doi: 10.1021/mp800161e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vader P., van der Aa L.J., Engbersen J.F.J., Storm G., Schiffelers R.M. Disulfide-based poly(amido amine)s for siRNA delivery: effects of structure on siRNA complexation, cellular uptake, gene silencing and toxicity. Pharm. Res. 2011;28:1013–1022. doi: 10.1007/s11095-010-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu Q., Wang K., Sun X., Li Y., Fu Q., Liang T., Tang G. A redox-sensitive, oligopeptide-guided, self-assembling, and efficiency-enhanced (ROSE) system for functional delivery of microRNA therapeutics for treatment of hepatocellular carcinoma. Biomaterials. 2016;104:192–200. doi: 10.1016/j.biomaterials.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 147.Ping Y., Hu Q., Tang G., Li J. FGFR-targeted gene delivery mediated by supramolecular assembly between β-cyclodextrin-crosslinked PEI and redox-sensitive PEG. Biomaterials. 2013;34:6482–6494. doi: 10.1016/j.biomaterials.2013.03.071. [DOI] [PubMed] [Google Scholar]
- 148.Manickam D.S., Li J., Putt D.A., Zhou Q.-H., Wu C., Lash L.H., Oupický D. Effect of innate glutathione levels on activity of redox-responsive gene delivery vectors. J. Control. Release. 2010;141:77–84. doi: 10.1016/j.jconrel.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang Q., Shen C., Zhao N., Xu F.-J. Redox-Responsive and Drug-Embedded Silica Nanoparticles with Unique Self-Destruction Features for Efficient Gene/Drug Codelivery. Adv. Funct. Mater. 2017;27:1606229. [Google Scholar]
- 150.Kaur S., Prasad C., Balakrishnan B., Banerjee R. Trigger responsive polymeric nanocarriers for cancer therapy. Biomater. Sci. 2015;3:955–987. doi: 10.1039/c5bm00002e. [DOI] [PubMed] [Google Scholar]
- 151.Molla M.R., Prasad P., Thayumanavan S. Protein-induced supramolecular disassembly of amphiphilic polypeptide nanoassemblies. J. Am. Chem. Soc. 2015;137:7286–7289. doi: 10.1021/jacs.5b04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rozema D.B., Blokhin A.V., Wakefield D.H., Benson J.D., Carlson J.C., Klein J.J., Almeida L.J., Nicholas A.L., Hamilton H.L., Chu Q. Protease-triggered siRNA delivery vehicles. J. Control. Release. 2015;209:57–66. doi: 10.1016/j.jconrel.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 153.Huang S., Shao K., Kuang Y., Liu Y., Li J., An S., Guo Y., Ma H., He X., Jiang C. Tumor targeting and microenvironment-responsive nanoparticles for gene delivery. Biomaterials. 2013;34:5294–5302. doi: 10.1016/j.biomaterials.2013.03.043. [DOI] [PubMed] [Google Scholar]
- 154.Zhu L., Perche F., Wang T., Torchilin V.P. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials. 2014;35:4213–4222. doi: 10.1016/j.biomaterials.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Veiman K.-L., Künnapuu K., Lehto T., Kiisholts K., Pärn K., Langel Ü., Kurrikoff K. PEG shielded MMP sensitive CPPs for efficient and tumor specific gene delivery in vivo. J. Control. Release. 2015;209:238–247. doi: 10.1016/j.jconrel.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 156.Karimi M., Ghasemi A., Sahandi Zangabad P., Rahighi R., Moosavi Basri S.M., Mirshekari H., Amiri M., Shafaei Pishabad Z., Aslani A., Bozorgomid M. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016;45:1457–1501. doi: 10.1039/c5cs00798d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yi Q., Sukhorukov G.B. UV light stimulated encapsulation and release by polyelectrolyte microcapsules. Adv. Colloid Interface Sci. 2014;207:280–289. doi: 10.1016/j.cis.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 158.Huschka R., Barhoumi A., Liu Q., Roth J.A., Ji L., Halas N.J. Gene silencing by gold nanoshell-mediated delivery and laser-triggered release of antisense oligonucleotide and siRNA. ACS Nano. 2012;6:7681–7691. doi: 10.1021/nn301135w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wijaya A., Schaffer S.B., Pallares I.G., Hamad-Schifferli K. Selective release of multiple DNA oligonucleotides from gold nanorods. ACS Nano. 2009;3:80–86. doi: 10.1021/nn800702n. [DOI] [PubMed] [Google Scholar]
- 160.Yang C., Gao S., Song P., Dagnæs-Hansen F., Jakobsen M., Kjems J. Theranostic Niosomes for Efficient siRNA/MicroRNA Delivery and Activatable Near-Infrared Fluorescent Tracking of Stem Cells. ACS Appl. Mater. Interfaces. 2018;10:19494–19503. doi: 10.1021/acsami.8b05513. [DOI] [PubMed] [Google Scholar]
- 161.An K., Zhao P., Lin C., Liu H. A pH and redox dual responsive 4-arm poly(ethylene glycol)-block-poly(disulfide histamine) copolymer for non-viral gene transfection in vitro and in vivo. Int. J. Mol. Sci. 2014;15:9067–9081. doi: 10.3390/ijms15059067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cai X., Dong C., Dong H., Wang G., Pauletti G.M., Pan X., Wen H., Mehl I., Li Y., Shi D. Effective gene delivery using stimulus-responsive catiomer designed with redox-sensitive disulfide and acid-labile imine linkers. Biomacromolecules. 2012;13:1024–1034. doi: 10.1021/bm2017355. [DOI] [PubMed] [Google Scholar]
- 163.Yang Y., Xie X., Yang Y., Li Z., Yu F., Gong W., Li Y., Zhang H., Wang Z., Mei X. Polymer Nanoparticles Modified with Photo- and pH-Dual-Responsive Polypeptides for Enhanced and Targeted Cancer Therapy. Mol. Pharm. 2016;13:1508–1519. doi: 10.1021/acs.molpharmaceut.5b00977. [DOI] [PubMed] [Google Scholar]
- 164.Wang F., Shen Y., Zhang W., Li M., Wang Y., Zhou D., Guo S. Efficient, dual-stimuli responsive cytosolic gene delivery using a RGD modified disulfide-linked polyethylenimine functionalized gold nanorod. J. Control. Release. 2014;196:37–51. doi: 10.1016/j.jconrel.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 165.Liu X., Ni P., He J., Zhang M. Synthesis and Micellization of pH/Temperature-Responsive Double-Hydrophilic Diblock Copolymers Polyphosphoester-block-poly 2-(dimethylamino)ethyl methacrylate Prepared via ROP and ATRP. Macromolecules. 2010;43:4771–4781. [Google Scholar]
- 166.Cabral H., Matsumoto Y., Mizuno K., Chen Q., Murakami M., Kimura M., Terada Y., Kano M.R., Miyazono K., Uesaka M. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 167.Yim H., Park S.J., Bae Y.H., Na K. Biodegradable cationic nanoparticles loaded with an anticancer drug for deep penetration of heterogeneous tumours. Biomaterials. 2013;34:7674–7682. doi: 10.1016/j.biomaterials.2013.06.058. [DOI] [PubMed] [Google Scholar]
- 168.Xu P., Van Kirk E.A., Zhan Y., Murdoch W.J., Radosz M., Shen Y. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angew. Chem. Int. Ed. Engl. 2007;46:4999–5002. doi: 10.1002/anie.200605254. [DOI] [PubMed] [Google Scholar]
- 169.Han S.-S., Li Z.-Y., Zhu J.-Y., Han K., Zeng Z.-Y., Hong W., Li W.X., Jia H.Z., Liu Y., Zhuo R.X., Zhang X.Z. Dual-pH Sensitive Charge-Reversal Polypeptide Micelles for Tumor-Triggered Targeting Uptake and Nuclear Drug Delivery. Small. 2015;11:2543–2554. doi: 10.1002/smll.201402865. [DOI] [PubMed] [Google Scholar]
- 170.Chen S., Rong L., Lei Q., Cao P.-X., Qin S.-Y., Zheng D.-W., Jia H.Z., Zhu J.Y., Cheng S.X., Zhuo R.X., Zhang X.Z. A surface charge-switchable and folate modified system for co-delivery of proapoptosis peptide and p53 plasmid in cancer therapy. Biomaterials. 2016;77:149–163. doi: 10.1016/j.biomaterials.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 171.Gou J., Liang Y., Miao L., Guo W., Chao Y., He H., Zhang Y., Yang J., Wu C., Yin T. Improved tumor tissue penetration and tumor cell uptake achieved by delayed charge reversal nanoparticles. Acta Biomater. 2017;62:157–166. doi: 10.1016/j.actbio.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 172.Qiu N., Liu X., Zhong Y., Zhou Z., Piao Y., Miao L., Zhang Q., Tang J., Huang L., Shen Y. Esterase-Activated Charge-Reversal Polymer for Fibroblast-Exempt Cancer Gene Therapy. Adv. Mater. 2016;28:10613–10622. doi: 10.1002/adma.201603095. [DOI] [PubMed] [Google Scholar]
- 173.Sun H., Su J., Meng Q., Yin Q., Chen L., Gu W., Zhang P., Zhang Z., Yu H., Wang S., Li Y. Cancer-Cell-Biomimetic Nanoparticles for Targeted Therapy of Homotypic Tumors. Adv. Mater. 2016;28:9581–9588. doi: 10.1002/adma.201602173. [DOI] [PubMed] [Google Scholar]
- 174.Peng Z.-H., Kopeček J. Enhancing Accumulation and Penetration of HPMA Copolymer-Doxorubicin Conjugates in 2D and 3D Prostate Cancer Cells via iRGD Conjugation with an MMP-2 Cleavable Spacer. J. Am. Chem. Soc. 2015;137:6726–6729. doi: 10.1021/jacs.5b00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liang D.S., Su H.T., Liu Y.J., Wang A.T., Qi X.R. Tumor-specific penetrating peptides-functionalized hyaluronic acid-d-α-tocopheryl succinate based nanoparticles for multi-task delivery to invasive cancers. Biomaterials. 2015;71:11–23. doi: 10.1016/j.biomaterials.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 176.Cai D., Gao W., He B., Dai W., Zhang H., Wang X., Wang J., Zhang X., Zhang Q. Hydrophobic penetrating peptide PFVYLI-modified stealth liposomes for doxorubicin delivery in breast cancer therapy. Biomaterials. 2014;35:2283–2294. doi: 10.1016/j.biomaterials.2013.11.088. [DOI] [PubMed] [Google Scholar]
- 177.Ji T., Ding Y., Zhao Y., Wang J., Qin H., Liu X., Lang J., Zhao R., Zhang Y., Shi J. Peptide assembly integration of fibroblast-targeting and cell-penetration features for enhanced antitumor drug delivery. Adv. Mater. 2015;27:1865–1873. doi: 10.1002/adma.201404715. [DOI] [PubMed] [Google Scholar]
- 178.Ji T., Li S., Zhang Y., Lang J., Ding Y., Zhao X., Zhao R., Li Y., Shi J., Hao J. An MMP-2 Responsive Liposome Integrating Antifibrosis and Chemotherapeutic Drugs for Enhanced Drug Perfusion and Efficacy in Pancreatic Cancer. ACS Appl. Mater. Interfaces. 2016;8:3438–3445. doi: 10.1021/acsami.5b11619. [DOI] [PubMed] [Google Scholar]
- 179.Villegas M.R., Baeza A., Vallet-Regí M. Hybrid Collagenase Nanocapsules for Enhanced Nanocarrier Penetration in Tumoral Tissues. ACS Appl. Mater. Interfaces. 2015;7:24075–24081. doi: 10.1021/acsami.5b07116. [DOI] [PubMed] [Google Scholar]
- 180.Zuo Z.-Q., Chen K.-G., Yu X.-Y., Zhao G., Shen S., Cao Z.-T., Luo Y.L., Wang Y.C., Wang J. Promoting tumor penetration of nanoparticles for cancer stem cell therapy by TGF-β signaling pathway inhibition. Biomaterials. 2016;82:48–59. doi: 10.1016/j.biomaterials.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 181.Cun X., Ruan S., Chen J., Zhang L., Li J., He Q., Gao H. A dual strategy to improve the penetration and treatment of breast cancer by combining shrinking nanoparticles with collagen depletion by losartan. Acta Biomater. 2016;31:186–196. doi: 10.1016/j.actbio.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 182.Wilhelm S., Tavares A.J., Chan W.C.W. Reply to “Evaluation of nanomedicines: stick to the basics.”. Nat. Rev. Mater. 2016;1:16074. [Google Scholar]
- 183.Croce C.M., Zhang K., Wei Y.Q. Announcing Signal Transduction and Targeted Therapy. Signal Transduct. Target. Ther. 2016;1:15006. doi: 10.1038/sigtrans.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chen C., Yue D., Lei L., Wang H., Lu J., Zhou Y., Liu S., Ding T., Guo M., Xu L. Promoter-Operating Targeted Expression of Gene Therapy in Cancer: Current Stage and Prospect. Mol. Ther. Nucleic Acids. 2018;11:508–514. doi: 10.1016/j.omtn.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Press R.D., Misra A., Gillaspy G., Samols D., Goldthwait D.A. Control of the expression of c-sis mRNA in human glioblastoma cells by phorbol ester and transforming growth factor beta 1. Cancer Res. 1989;49:2914–2920. [PubMed] [Google Scholar]
- 186.Ali R., Wendt M.K. The paradoxical functions of EGFR during breast cancer progression. Signal Transduct. Target. Ther. 2017;2:16042. doi: 10.1038/sigtrans.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ande S.R., Xu Y.X.Z., Mishra S. Prohibitin: a potential therapeutic target in tyrosine kinase signaling. Signal Transduct. Target. Ther. 2017;2:17059. doi: 10.1038/sigtrans.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gschwind A., Fischer O.M., Ullrich A. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat. Rev. Cancer. 2004;4:361–370. doi: 10.1038/nrc1360. [DOI] [PubMed] [Google Scholar]
- 189.Hossain J., Ystaas L., Latif A., Joseph J., Talasila K., Ninzima S., Riecken K., Fehse B., Bjerkvig R., Miletic H. Recurrent xenograft tumors upregulate EGFR after lentiviral vector mediated suicide gene therapy for glioblastoma, but are resistant to combinatorial treatment with erlotinib. Neuro Oncol. 2017;19:vi88. [Google Scholar]
- 190.Dalal A.R., Homsy S., Balkhi M.Y. Third-Generation Human Epidermal Growth Factor Receptor 2 Chimeric Antigen Receptor Expression on Human T Cells Improves with Two-Signal Activation. Hum. Gene Ther. 2018;29:845–852. doi: 10.1089/hum.2017.244. [DOI] [PubMed] [Google Scholar]
- 191.Zhang H., Wang Y., Wu Y., Jiang X., Tao Y., Yao Y., Peng Y., Chen X., Fu Y., Yu L. Therapeutic potential of an anti-HER2 single chain antibody-DM1 conjugates for the treatment of HER2-positive cancer. Signal Transduct. Target. Ther. 2017;2:17015. doi: 10.1038/sigtrans.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Luedtke D.A., Niu X., Pan Y., Zhao J., Liu S., Edwards H., Chen K., Lin H., Taub J.W., Ge Y. Inhibition of Mcl-1 enhances cell death induced by the Bcl-2-selective inhibitor ABT-199 in acute myeloid leukemia cells. Signal Transduct. Target. Ther. 2017;2:17012. doi: 10.1038/sigtrans.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hiraki M., Maeda T., Mehrotra N., Jin C., Alam M., Bouillez A., Hata T., Tagde A., Keating A., Kharbanda S. Targeting MUC1-C suppresses BCL2A1 in triple-negative breast cancer. Signal Transduct. Target. Ther. 2018;3:13. doi: 10.1038/s41392-018-0013-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dai L., Pan Q., Peng Y., Huang S., Liu J., Chen T., Wang X., Chen D., Wang J., Zhu Y. p53 Plays a Key Role in the Apoptosis of Human Ovarian Cancer Cells Induced by Adenovirus-Mediated CRM197. Hum. Gene Ther. 2018;29:916–926. doi: 10.1089/hum.2017.186. [DOI] [PubMed] [Google Scholar]
- 195.El-Sayed A., Harashima H. Endocytosis of gene delivery vectors: from clathrin-dependent to lipid raft-mediated endocytosis. Mol. Ther. 2013;21:1118–1130. doi: 10.1038/mt.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bucci C., Parton R.G., Mather I.H., Stunnenberg H., Simons K., Hoflack B., Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- 197.Felsher D.W., Bishop J.M. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 198.Somasuntharam I., Yehl K., Carroll S.L., Maxwell J.T., Martinez M.D., Che P.L., Brown M.E., Salaita K., Davis M.E. Knockdown of TNF-α by DNAzyme gold nanoparticles as an anti-inflammatory therapy for myocardial infarction. Biomaterials. 2016;83:12–22. doi: 10.1016/j.biomaterials.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhao Y., Chen J., Wei W., Qi X., Li C., Ren J. The dual-inhibitory effect of miR-338-5p on the multidrug resistance and cell growth of hepatocellular carcinoma. Signal Transduct. Target. Ther. 2018;3:3. doi: 10.1038/s41392-017-0003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kojima K., Fujita Y., Nozawa Y., Deguchi T., Ito M. MiR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate. 2010;70:1501–1512. doi: 10.1002/pros.21185. [DOI] [PubMed] [Google Scholar]
- 201.Wu J., Tan X., Lin J., Yuan L., Chen J., Qiu L., Huang W. Minicircle-oriP-miR-31 as a Novel EBNA1-Specific miRNA Therapy Approach for Nasopharyngeal Carcinoma. Hum. Gene Ther. 2017;28:415–427. doi: 10.1089/hum.2016.136. [DOI] [PubMed] [Google Scholar]
- 202.Clauss J., Obenaus M., Miskey C., Ivics Z., Izsvák Z., Uckert W., Bunse M. Efficient Non-Viral T-Cell Engineering by Sleeping Beauty Minicircles Diminishing DNA Toxicity and miRNAs Silencing the Endogenous T-Cell Receptors. Hum. Gene Ther. 2018;29:569–584. doi: 10.1089/hum.2017.136. [DOI] [PubMed] [Google Scholar]
- 203.Peng Y., Croce C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016;1:15004. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sun H., Yarovoy I., Capeling M., Cheng C. Polymers in the Co-delivery of siRNA and Anticancer Drugs for the Treatment of Drug-resistant Cancers. Top. Curr. Chem. (Cham) 2017;375:24. doi: 10.1007/s41061-017-0113-z. [DOI] [PubMed] [Google Scholar]