Abstract
Background
Although dysfunction of amygdala-related circuits is centrally implicated in major depressive disorder (MDD), little is known about how this dysfunction differs between adult and adolescent MDD patients.
Methods
Voxel-wise meta-analyses of abnormal amygdala resting-state functional connectivity (rsFC) were conducted in adult and adolescent groups separately, followed by a quantitative meta-analytic comparison of the two groups.
Findings
Nineteen studies that included 665 MDD patients (392 adults and 273 adolescents) and 546 controls (341 adults and 205 adolescents) were identified in the current study. Adult-specific abnormal amygdala rsFC in MDD patients compared to that in controls was located mainly within the affective network, including increased connectivity with the right hippocampus/parahippocampus and bilateral ventromedial orbitofrontal cortex and decreased connectivity with the bilateral insula and the left caudate. Adolescent MDD patients specifically demonstrated decreased amygdala rsFC within the cognitive control network encompassing the left dorsolateral prefrontal cortex and imbalanced amygdala rsFC within the default mode network, which was manifested as hyperconnectivity in the right precuneus and hypoconnectivity in the right inferior temporal gyrus. Additionally, direct comparison between the two groups showed that adult patients had strengthened amygdala rsFC with the right hippocampus/parahippocampus as well as the right inferior temporal gyrus and weakened amygdala rsFC with the bilateral insula compared to that in adolescent patients.
Interpretation
Distinct impairments of amygdala-centered rsFC in adult and adolescent patients were related to different network dysfunctions in MDD. Adult-specific amygdala rsFC dysfunction within the affective network presumably reflects emotional dysregulation in MDD, whereas adolescent-specific amygdala rsFC abnormalities in networks involved in cognitive control might reflect the neural basis of affective cognition deficiency that is characteristic of adolescent MDD.
Fund
This study was supported by a grant from the National Natural Science Foundation of China (81671669) and by a Sichuan Provincial Youth Grant (2017JQ0001).
Keywords: Major depressive disorder, Amygdala, Functional connectivity, Adults, Adolescents, Meta-analysis
Research in context.
Evidence before this study
Major depressive disorder (MDD) is primarily a disorder of emotion, and the amygdala is a critical brain region for both bottom-up and top-down processes of emotion generation and regulation. Dysfunctions of amygdala-related networks are associated with MDD, as revealed by resting-state functional connectivity (rsFC) studies in both adult and adolescent MDD patients. An important question is whether the amygdala-based network differs substantially between the two patient groups; however, this matter has not yet been resolved. Thus, the PubMed, Web of Science and EMBASE databases were searched for articles published before March 1st, 2018, to conduct a systematic and comprehensive meta-analysis, which may help to answer this question.
Added value of this study
For the first time, we showed the specific patterns of amygdala-based network abnormalities in adults and adolescents with MDD: adult-specific amygdala rsFC abnormalities compared with healthy controls (HC) were mainly located within the affective network (AN). Adolescent-specific rsFC abnormalities relative to HC were mainly located within the cognitive control network (CCN) and the default mode network (DMN). In addition, direct comparison between the two groups showed that adult patients, compared to adolescent patients, have strengthened amygdala rsFC with the right hippocampus/parahippocampus as well as the right inferior temporal gyrus and weakened amygdala rsFC with the bilateral insula.
Implications of all the available evidence
Our findings may provide clinical treatment insights into the two groups. Alterations in adult patients are localized within the AN, which is also the target of standard antidepressants, presumably reflecting emotional dysregulation. Alterations in adolescent patients are especially prominent in networks involved in cognitive control, which provides a neural basis for the effect of cognitive behavioral therapy in this particular population.
Alt-text: Unlabelled Box
1. Introduction
Major depressive disorder (MDD) is primarily a disorder of emotion and is one of the most common psychiatric illnesses. This condition has affected >350 million people worldwide and accounts for the largest proportion of the global burden of disease, according to WHO [1]. Even so, the pathophysiology of MDD is largely unknown.
As a pivotal component of the affective network (AN), the amygdala has been highlighted in the pathology of MDD [2,3]. This structure is a hub in a wide range of emotion processing, including emotional perception, memory and regulation [4]. Dysfunctions of several amygdala-related circuits are associated with MDD, as revealed by resting-state functional magnetic resonance imaging (rs-fMRI), especially seed-based resting-state functional connectivity (rsFC, an excellent analysis to probe function of neural circuits [5,6]) in recent years. For example, abnormal rsFC was reported, although with increased connectivity in some cases and decreased connectivity in others, within the amygdala-prefrontal circuit in adults with MDD [7,8]. In adolescent MDD, amygdala rsFC was found to be increased within the occipital-parietal and postcingulate cortex (PCC)/precuneus areas and decreased in the hippocampal/parahippocampal region [[9], [10], [11]].
This inconsistency between adult and adolescent MDD patients might come from their inherent differences. Evidence has suggested that adolescent MDD may have different pathology from adult MDD. For instance, from a behavioral perspective, differences in the domains of cognitive control and affective cognition were reported between adolescent and adult patients with MDD [12]. In addition, a meta- and mega-analysis that integrated data from 20 worldwide cohorts reported distinct patterns of structural brain abnormalities between MDD adults and MDD adolescents [13]. Regarding functional brain abnormalities, studies have reported that adults with MDD demonstrated reduced activation in the striatum under affective processing task [14] and reduced activation in the dorsal medial prefrontal cortex during executive tasks [15]. Neural function studies in youth with MDD, however, have yielded findings that diverge from those in adults. A qualitative meta-analysis of this literature has noted hyperactivation in both the thalamus and the parahippocampus during affective processing tasks and hypoactivation in the cuneus and dorsal anterior insula during executive function tasks [16]. Thus, the question of whether amygdala-related circuits also differ substantially between adult and adolescent patients with MDD is a matter that is worth exploring.
Variability due to the different ages of the samples may obscure reliable amygdala rsFC abnormalities in MDD. Apart from age, existing studies that detected amygdala-based network dysfunction in MDD have inherent limitations, including small sample sizes (approximately 30 patients or fewer in the literature), which limits their generalizability and statistical power. In addition, the variable use of the left and right amygdala as a seed might also bring heterogeneity. For instance, one study showed a lateralized pattern in which hypoconnectivity of the left amygdala is associated with more regions, such as the ventral-lateral PFC, precuneus, and temporal areas, than that of the right amygdala in MDD [17]. Another study showed that the right amygdala had greater aberrant connectivity with other brain regions than the left amygdala had [18].
Neuroimaging meta-analysis is a powerful method to summarize findings across studies and can effectively address all the problems mentioned above. This method is also capable of distinguishing spurious results from replicable findings as well as synthesizing and integrating the vast amount of data from studies [19,20]. Moreover, advances in neuroimaging meta-analytic methodology have made it possible to correlate imaging results with clinical characteristics [21] or directly and quantitatively compare different groups [22].
In the current study, we first conducted quantitative meta-analyses of adult and adolescent MDD groups separately to examine their specific amygdala connectivity abnormalities. Second, we performed a meta-analytic comparison to distinguish amygdala rsFC abnormalities in the two groups. Third, we performed a subgroup analysis using the left vs. right amygdala separately as seed regions to explore potential functional lateralization.
2. Materials and methods
2.1. Literature search
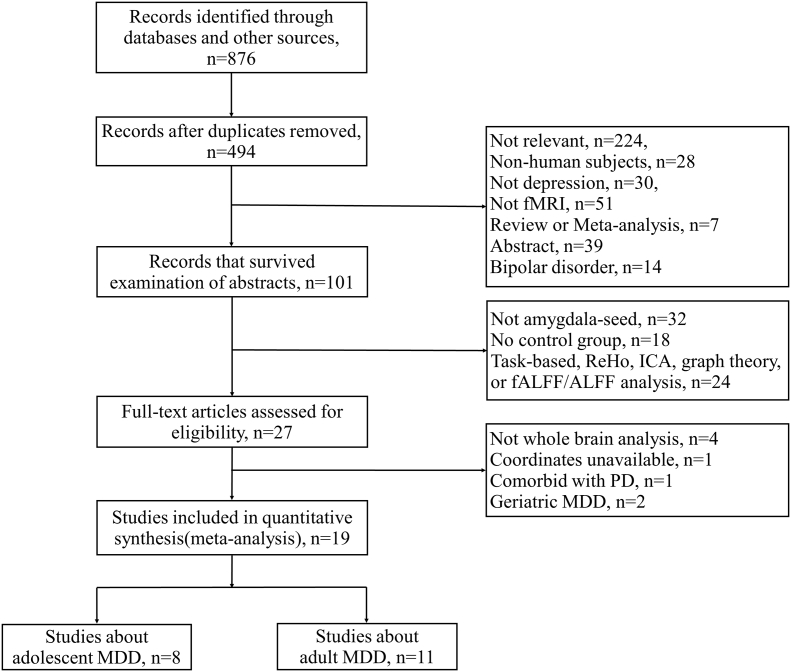
A comprehensive computerized search using the key search terms “depress* AND rest* AND connect* AND amygdal*” (* = truncated) was conducted in the databases PubMed, Web of Science and EMBASE, covering the period before March 1st, 2018. Manual searches were also conducted within the reference lists of identified and review articles to obtain additional reports. Original articles employing rs-fMRI and using whole-brain, seed (amygdala)-based rsFC to compare MDD individuals with a healthy control (HC) group were eligible for inclusion. The exclusion criteria were as follows: (1) no depression group or no HC group; (2) participants diagnosed with bipolar disorder or subthreshold depression; (3) not amygdala-based whole-brain analysis (such as other rsFC methods: independent components analysis (ICA)); (4) comorbidity with other neurological diseases such as Parkinson's disease; (5) overlapping samples; (6) coordinates of brain regions with differences between MDD patients and HC were not available even after contact with the author. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [23], and the study selection procedures are summarized in Fig. 1.
Fig. 1.
Flowchart of the identification of articles. Abbreviations: ReHo, regional homogeneity; ALFF, amplitude of low-frequency fluctuation; ICA, independent components analysis; fALFF, fractional ALFF; PD, Parkinson's disease.
The literature was searched and examined by two investigators independently.
2.2. Meta-analysis
The meta-analysis was performed using the anisotropic effect size version of the signed differential mapping (AES-SDM) software package (http://www.sdmproject.com/software), a powerful statistical technique using peak coordinates for meta-analyzing studies on differences in brain activity or structure. First, we organized the studies into two groups (adults, aged >18 years, vs. adolescents, aged 13–18 years [24]) to identify the abnormal amygdala rsFC in adult MDD and adolescent MDD (relative to HC) separately. Second, selecting the reported peak coordinates ensures that only regions statistically significant at the whole-brain level are considered for inclusion in the meta-analysis. Third, both positive and negative coordinates are reconstructed on the same map, which is important for preventing a particular voxel from erroneously appearing to be significant in exact opposite directions [25]. The SDM approach assigns effect size (standardized mean for one-sample designs or standardized mean difference for two-sample designs) to each voxel, referred to as Hedge's d (or g) at the sample level. In this meta-analysis, a standard Montreal Neurological Institute (MNI) map of the rsFC difference (including positive and negative) was recreated for each included study separately using an anisotropic Gaussian kernel on the voxels close to the peak, which is optimized to recreate the effect size maps and maximize robustness [26].
Subsequently, a quantitative comparison of amygdala rsFC abnormalities between adult MDD and adolescent MDD was performed by calculating the difference between each MDD group in each voxel, and then standard randomization tests were used to establish statistical significance [27]. Next, a conjunction/disjunction analysis was conducted to examine brain regions of contrasting amygdala rsFC abnormalities across both adult and adolescent MDD groups by computing the union of the p values for each MDD group within each voxel while accounting for the presence of noise in the estimation of meta-analytic p values [26]. The meta-analysis uses a default threshold of p < 0.005 with peak |Z| > 1, as this setting was found to optimally balance sensitivity and specificity and to be an approximate equivalent to corrected p value = 0.05 (more accurately, 0.025) in SDM [25]. To improve the reliability of the results, we used a cluster extent of k > 100.
Data extraction was conducted by two investigators and was double-checked.
2.3. Sensitivity analyses
A systematic whole-brain voxel-based jackknife sensitivity analysis was conducted to estimate the robustness of the results. This method discards a different study each time, one by one, and then repeats the analyses. If a significant brain region remains significant in all or most of the combinations of studies, this finding is highly replicable [21].
To examine age-dependent effects, we conducted meta-regression analyses with age as the regressor in two groups separately. The probability threshold was decreased to 0.0005, which is required to detect abnormalities both in the slope and in one of the extremes of the regressor, to minimize the detection of spurious relationships and discarded findings not in the main analyses [21]. Furthermore, we performed leave-one-out analysis for the meta-regression to examine the robustness of the results; in other words, one study was left out each time, and the meta-regression analysis was repeated in the remaining studies. We also conducted meta-regression analyses with the percentage of medicated patients and illness duration as regressors.
We further subdivided the two MDD groups into left amygdala seed and right amygdala seed subgroups to probe potential differences through a subgroup meta-analysis.
2.4. Publication bias
For each significant cluster for MDD-HC comparison, Egger's test was used to assess the asymmetry of funnel plots to examine potential publication bias [28].
3. Results
3.1. Included studies and sample characteristics
Our search strategy yielded 21 primary studies that satisfied the inclusion and exclusion criteria described earlier. However, among these studies, two were about late-life depression; thus, they were not included in our meta-analysis. Altogether, 19 studies with 22 datasets (14 from adults and 8 from adolescents) including 665 MDD patients (392 adults and 273 adolescents) and 546 HCs (341 adults and 205 adolescents) were ultimately identified in this study. Detailed sample characteristics are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the studies included in this meta-analysis.
| Study | MDD subjects |
HC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean age (SD), years | Female, N (%) | Mean illness duration (SD), years | Depression state | Depression severity | Medication status (%) | Comorbidity (N) | N | Mean age (SD), years | Female (N, %) | |
| Samples from adolescents | |||||||||||
| Kim et al., 2015 [11] | 22 | 13.9(1.6) | 8(36) | 6(1.6) | Active | CDI: 40.0 | Medication-naïve (100%) | Pure MDD | 20 | 14.5(1.7) | 6(30) |
| Pannekoek et al., 2014 [9] | 26 | 15.4(1.5) | 23(88) | NA | Active | CDI: 18.6 | Medication-naïve (100%) | Anxiety (18) | 26 | 14.7(1.5) | 23(88.5) |
| Peters et al., 2016 [63] | 23 | 15.61(1.9) | 13(57) | NA | Remitted | CDRS-R: 26.91 | Medicated (52%) | Anxiety (8) | 10 | 15.8(1.99) | 7(70) |
| Chattopadhyay et al., 2017 [64] | 82 | 15.69(1.12) | 64(78) | NA | Active | SMFQ: 18.02 | Medication-naïve (100%) | Pure MDD | 34 | 15.73(1.44) | 27(100) |
| Cullen et al., 2014 [10] | 41 | 15.7(2) | 32(78) | 0.83(0.9) | Active | CRDS: 77 | Medication-naïve (73%) | Anxiety (25) | 29 | 16(2) | 22(76) |
| Connolly et al., 2017 [65] | 48 | 16.1(1.3) | 29(60) | NA | Active | CRDS: 70.2 | Medication-naïve (100%) | Anxiety (14), PTSD (5) | 53 | 16.1(1.3) | 33(62) |
| Cullen et al., 2009 [66] | 12 | 16.5(0.95) | 9(75) | 2.2(2.2) | Active | CRDS: 77 | Medicated (84%) | Anxiety (10) | 14 | 16.8(1.5) | 8(57) |
| Straub et al., 2017 [67] | 19 | 16.76(1.39) | 15(79) | NA | Active | CRDS: 55.91 | Drug-free | Phobia (5) | 19 | 16.35(1.47) | 15(79) |
| Samples from adults | |||||||||||
| Zhang X et al., 2014 [7] | 32 | 20.53(1.78) | 18(56) | NA | Active | CES-D: 38.03 | Medication-naïve (100%) | Pure MDD | 35 | 20.97(1.29) | 17(49) |
| Jacobs et al., 2016(a) [68] | 17 | 22.35(1.80) | 11(65) | 3.13 | Active | HAMD: 18.65 | Medicated (36%) | Anxiety (15) | 26 | 21.15(1.49) | 14(54) |
| Jacobs et al., 2016(b) [68] | 34 | 21.06(1.54) | 25(74) | 7.97 | Remitted | HAMD: 2.35 | Medicated (62%) | Anxiety (12) | 26 | 21.15(1.49) | 14(54) |
| Ye J et al., 2017 (YA) [18] | 34 | 24.15(2.84) | 17(50) | NA | Active | HAMD: 23.59 | Medication-naïve (100%) | Pure MDD | 35 | 24.8(2.14) | 18(51) |
| Altinay et al., 2016 [69] | 15 | 27(10) | 9(60) | NA | Active | HAMD: 20 | Drug-free | Anxiety (15), PTSD (1) | 15 | 29(8) | 9(60) |
| Deligiannidis et al., 2013 [70] | 8 | 28.62(5.93) | 8(100) | NA | Active | QIDS: 11.3 | Drug-free | Anxiety (4), PTSD (1) | 9 | 30.67(3.81) | 9(100) |
| Tang Y et al., 2013 [8] | 28 | 29.3(8.7) | 16(57) | 1.13(1.3) | Active | HAMD: 29 | Medication-naïve (100%) | Pure MDD | 30 | 30.1(8.4) | 15(50) |
| Lui S et al., 2011 (NRD) [71] | 32 | 32(10) | 11(34) | 1.83(1.5) | Active | HAMD: 23.0 | Medication-naïve (100%) | Pure MDD | 48 | 35(12) | 17(35) |
| Wang Y et al., 2016 [72] | 25 | 32.11(11.25) | 11(44) | 0.7(0.2) | Active | HAMD: 29.32 | NA | Pure MDD | 35 | 33.28(8.83) | 16(46) |
| Lui S et al., 2011 (RD)[71] | 28 | 33(11) | 10(37.5) | 16.08(10) | Active | HAMD: 23.3 | Medication-naïve (100%) | Pure MDD | 48 | 35(12) | 17(35) |
| Ramasubbu et al., 2014 [17] | 55 | 36.5(10.4) | 33(60) | 4.19(5) | Active | HAMD: 21.41 | Drug-free | Pure MDD | 19 | 32.89(9.97) | 11(58) |
| Ye J et al., 2017 (OA)[18] | 35 | 37.14(4.15) | 26(74) | NA | Active | HAMD: 23.69 | Medication-naïve (100%) | Pure MDD | 46 | 37.22(4.44) | 36(78) |
| Yang J et al., 2017 [73] | 35 | 44.54(11.15) | 35(100) | 2.68(3.8) | Active | HAMD: 28.29 | Drug-free | Pure MDD | 23 | 39.09(14.3) | 23(100) |
| Tahmasian et al., 2013 [36] | 21 | 51(15) | 11(52) | 14.7(11) | Active | HAMD: 23.8 | Medicated (95%) | Anxiety (6),somatization disorder (2),personality disorder (5) | 20 | 49.6(13.9) | 11(55) |
Abbreviations: N, numbers; CDI, Children's Depression Inventory; CDRS-R, Children's Depression Rating Scale-Revised; CDRS, Children's Depression Rating Scale; SMFQ, Short Mood and Feeling Questionnaire; CES-D, Center for Epidemiological Studies Depression Scale; HAMD, Hamilton Rating Scale for Depression; QIDS, Quick Inventory Depressive Symptoms; NA, not available; YA, young adult; NRD, nonrefractory depression; RD, refractory depression; OA, older adults.
3.2. Abnormal amygdala rsFC in adult and adolescent MDD patients (vs. HC)
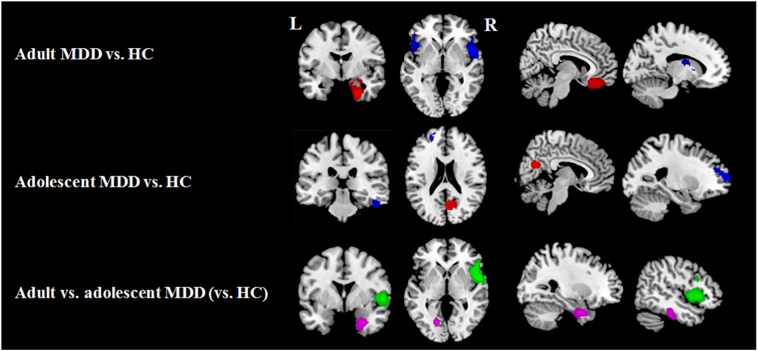
Adult MDD patients, relative to HC, presented significant hyperconnectivity of the amygdala with the right ventromedial orbital frontal cortex (vmOFC) and a large cluster that included the right hippocampus and parahippocampus (Fig. 2 and Table 2). Hypoconnectivity of the amygdala was observed with a large cluster centered at the bilateral insula and extending to the inferior frontal gyrus (IFG) and superior temporal gyrus (STG), the left caudate and a small piece of the left cerebellum (Fig. 2 and Table 2).
Fig. 2.
Results of amygdala rsFC meta-analysis for, from top to bottom, adult patients with major depressive disorder (MDD) relative to healthy controls (HC); adolescent patients with MDD relative to HC (red, MDD patients>HC; blue, MDD patients<HC); and a comparison between MDD adults (vs. HC) and MDD adolescents (vs. HC) (violet, adult>adolescent patients; green, adult<adolescent patients); L, left; R, right.
Table 2.
Meta-analysis results regarding regional differences in amygdala rsFC.
| Local maximum |
Cluster |
Jackknife sensitivityanalysis | ||||
|---|---|---|---|---|---|---|
| Region | MNI coordinates | SDM-Z | p value | No. of voxels | Breakdown (no. of voxels) | |
| Adult MDD vs. HC (No. of datasets: 14) | ||||||
| Hyperconnectivity (MDD > HC) | ||||||
| R-parahippocampal gyrus | 26,0, −32 | 1.639 | <0.0001 | 1206 | R-parahippocampal gyrus (391) | 14 out of 14 |
| R-hippocampus (199) | ||||||
| R-amygdala (215) | ||||||
| R-fusiform gyrus (257) | ||||||
| R-temporal pole (144) | ||||||
| R-vmOFC | 8,32, −20 | 1.367 | <0.001 | 434 | R-vmOFC (325) | 13 out of 14 |
| L-vmOFC (39) | ||||||
| R-SFG (60) | ||||||
| Hypoconnectivity (MDD < HC) | ||||||
| R-insula | 54,4,2 | −2.588 | <0.000005 | 1981 | R-insula (1070) | 14 out of 14 |
| R- IFG, pars opercularis (343) | ||||||
| R-IFG, pars triangularis (441) | ||||||
| R-temporal pole/STG (137) | ||||||
| L-insula | −46,20, −8 | −2.235 | <0.00005 | 1292 | L-insula (498) | 14 out of 14 |
| L-IFG, pars orbitalis (327) | ||||||
| L- temporal pole, STG (293) | ||||||
| L-IFG, pars opercularis (89) | ||||||
| L-IFG, pars triangularis (85) | ||||||
| L-caudate nucleus | −10, −6, 16 | −1.831 | <0.001 | 137 | L-caudate nucleus (100) | 12 out of 14 |
| L-thalamus (37) | ||||||
| L-cerebellum | −32, −66, −48 | −1.551 | <0.005 | 261 | L-cerebellum (261) | 13 out of 14 |
| Adolescent MDD vs. HC (no. of datasets: 8) | ||||||
| Hyperconnectivity (MDD > HC) | ||||||
| R-precuneus | 10, −70, 26 | 1.528 | <0.001 | 388 | R-precuneus (152) | 7 out of 8 |
| R-cuneus cortex (96) | ||||||
| R-calcarine fissure (89) | ||||||
| L-precuneus (18) | ||||||
| L-cuneus cortex (12) | ||||||
| L-calcarine fissure (17) | ||||||
| R-IFG, pars opercularis | 34,12,30 | 1.719 | <0.0005 | 192 | R-IFG, pars opercularis (135) | 7 out of 8 |
| R-IFG, pars triangularis (57) | ||||||
| Hypoconnectivity (MDD < HC) | ||||||
| L-DLPFC | −24,64,12 | −1.34 | <0.0005 | 306 | L-DLPFC (246) | 7 out of 8 |
| L-MFG (60) | ||||||
| R-ITG | 52, −30, −30 | −1.033 | <0.005 | 142 | R-ITG (117) | 7 out of 8 |
| R-fusiform gyrus (25) | ||||||
| Adult MDD (vs. HC) vs. adolescent MDD (vs. HC) | ||||||
| Adult MDD > adolescent MDD | ||||||
| R-parahippocampal gyrus | 26,2, −32 | 1.299 | <0.0005 | 503 | R-parahippocampal gyrus (190) | |
| R-hippocampus (100) | ||||||
| R-amygdala (115) | ||||||
| R-fusiform gyrus (28) | ||||||
| R-temporal pole (70) | ||||||
| R-ITG | 52, −30, −30 | 1.15 | <0.001 | 276 | R-ITG (217) | |
| R-fusiform gyrus (59) | ||||||
| Adult MDD < adolescent MDD | ||||||
| R-insula | 52,4,0 | −2.307 | <0.0001 | 2232 | R-insula (742) | |
| R-rolandic operculum (393) | ||||||
| R-IFG, pars triangularis (491) | ||||||
| R-IFG, pars opercularis(479) | ||||||
| R-temporal pole/STG (137) | ||||||
| L-insula | −40,18, −12 | −1.593 | <0.005 | 122 | L-insula (62) | |
| L-IFG, pars orbitalis (60) | ||||||
Abbreviations: rsFC, resting-state functional connectivity; MDD, major depressive disorder; HC, healthy controls; MNI, Montreal Neurological Institute; SDM, signed differential mapping; L, left; R, right; DLPFC, dorsal lateral prefrontal gyrus; vmOFC, ventromedial orbital frontal cortex; SFG, superior frontal gyrus; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; STG, superior temporal gyrus; ITG, inferior temporal gyrus.
Adolescent MDD patients, relative to HC, presented hyperconnectivity of the amygdala with the bilateral precuneus as well as the right IFG and hypoconnectivity of the amygdala with the left dorsolateral prefrontal cortex (DLPFC) as well as the right inferior temporal gyrus (ITG) (Fig. 2 and Table 2).
3.3. Amygdala rsFC comparison of adult vs. adolescent MDD patients (vs. HC)
Adult MDD patients demonstrated strengthened amygdala rsFC with the right hippocampus/parahippocampus and the right ITG but weakened amygdala rsFC with bilateral insula compared to adolescent patients (Fig. 2 and Table 2). Further analyses demonstrated that contrasting findings were also observed in the right parahippocampus (MNI coordinates: 26, 4, 32) and ITG (MNI coordinates: 46, 24, 24), where adolescent patients showed an increase in amygdala rsFC relative to HC, while adults showed a decrease (Fig. S2 in online supplements).
3.4. Meta-regression analysis
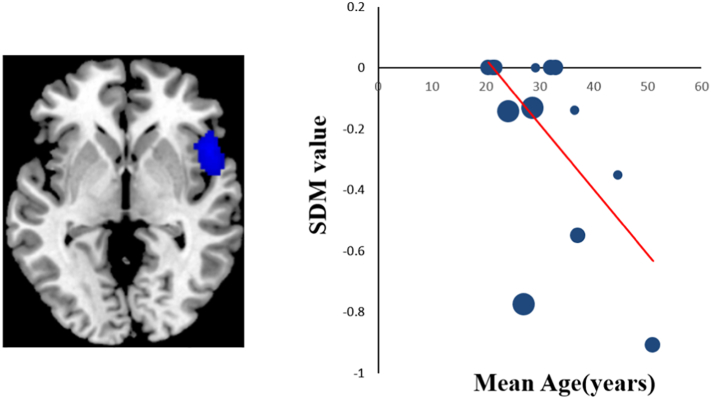
The meta-regression analysis revealed that the age of adult-group MDD patients was negatively and significantly correlated with decreased rsFC between the amygdala and the right insula (peak voxel coordinate: 50, 14, −2; p < 0.0001) (Fig. 3). That is, as age increases in studies including MDD patients, the rsFC between the amygdala and the right insula is predicted to decrease. This result remained significant in the leave-one-out analysis for all 11 combinations of studies preserved. There was no association between amygdala rsFC abnormalities and the ages of adolescent-group MDD patients. There is also no correlation between amygdala FC changes and the percentage of medicated patients or illness duration.
Fig. 3.
Meta-regression results showing that the age of adult MDD patients is negatively correlated with the rsFC in the right insula (peak voxel coordinate: 50, 14, −2, r = 0.604, p < 0.0001). In the graphs, the effect sizes needed to create this plot have been extracted from the peak of the maximum slope significance, and each dataset is represented as a dot, whose size reflects the sample size. Large dots indicate samples with 20–40 patients, and small dots represent samples with <20 patients. The regression line (meta-regression signed differential mapping slope) is shown.
3.5. Subgroup analyses: left vs. right amygdala seed
In adult patients, we found increased left amygdala connectivity with a large cluster centered at the right hippocampus/parahippocampus as well as bilateral vmOFC and decreased left amygdala connectivity with the left insula extending to the IFG as well as bilateral STG. We also found increased right amygdala connectivity with the left lingual gyrus as well as the right temporal gyrus and decreased right amygdala connectivity with the left caudate, the left thalamus and the right IFG (Table S2 and Fig. S1 in online supplements).
In adolescent patients, we found increased left amygdala connectivity with the right IFG, pars opercularis and bilateral precuneus, as well as decreased left amygdala connectivity with the right precentral gyrus. We also found increased right amygdala connectivity with a wide range of the left temporal gyrus and decreased amygdala connectivity with the left DLPFC, bilateral anterior cingulate cortex (ACC), right lingual gyrus and right ITG (Table S2 and Fig. S1 in online supplements).
3.6. Jackknife analyses
In the adult MDD group, the jackknife sensitivity analyses showed that the results in the right hippocampus/parahippocampus and bilateral insula were highly replicable with all 11 combinations of studies preserved; in the adolescent MDD group, bilateral precuneus, the left DLPFC and the right ITG remained significant in all but one combination of studies. The large majority of results in subgroup analysis were robust under jackknife sensitivity analyses. In addition, among all studies, only two studies (one in each group) were performed on subjects in a remitted depressive state; therefore, we discarded those two studies and repeated the analyses. The results remained unchanged. The details are shown in Table S3–8 in online supplements.
3.7. Publication bias
The results of Egger's test were nonsignificant (p > 0.05 for all comparisons except one, Fig. S3–12 in online supplements), suggesting that there was no publication bias.
4. Discussion
By conducting a comprehensive meta-analysis, we show for the first time the specific patterns of amygdala-based network abnormalities in adults and adolescents with MDD, thus providing novel information about within- and between-networks functional deficits beyond the effects reported in a previous meta-analysis [29]. We found that in MDD patients, adult-specific amygdala rsFC abnormalities were mainly located within the AN, including vmOFC, hippocampus/parahippocampus, insula and caudate, compared with HC. In contrast, adolescent-specific rsFC abnormalities were mainly located within the cognitive control network (CCN, i.e., DLPFC) and default mode network (DMN, i.e., precuneus and ITG) relative to HC. In addition, direct comparison between the two groups showed that adult patients have strengthened amygdala rsFC with the right hippocampus/parahippocampus as well as the right ITG and weakened amygdala rsFC with the bilateral insula relative to adolescent patients.
4.1. Adult-specific abnormal amygdala rsFC within the AN
In MDD patients, adults-specific amygdala rsFC abnormalities were mainly within the AN, with increased amygdala rsFC with the vmOFC as well as the hippocampus/parahippocampus and decreased amygdala rsFC with the insula and caudate relative to controls. Dysfunction of the AN has been suggested to underlie the abnormal emotional and motivational regulation in MDD [[30], [31], [32]]. The vmOFC, via top-down regulation of the amygdala and ventral striatum (e.g., caudate), mediates emotion processing [33,34]. Increased activation in the vmOFC and amygdala was simultaneously observed in adult patients with MDD during an emotional-regulation task [35,36]. These findings suggest that depression is associated with sustained activation in brain areas responsible for top-down emotional regulation. In addition, decreased connectivity between the amygdala and the caudate has been reported to be strongly implicated in hopelessness and anhedonia, the debilitating symptoms of MDD [32].
Increased amygdala-hippocampus/parahippocampus rsFC was demonstrated in adult patients compared to HC. The amygdala is adjacent to the hippocampus within the medial temporal lobe, and they are richly connected with each other [37,38]. A previous study suggested that amygdala-hippocampal connections facilitate several emotional-behavioral functions, especially emotional memory [39]. A study using a memory task showed greater amygdala-hippocampal connectivity in adult MDD patients than in controls during negative emotional memory encoding, but no group differences were found with neutral or positive memories [40]. Based on this research, our findings may be further proven that amygdala-hippocampal hyperconnectivity is related to the excessive concern of negative events in adult MDD patients.
Adult patients also demonstrated significantly decreased amygdala-insula rsFC compared to HC. Apart from its role in AN, the insula is considered a hub of salience network that detects salient events and interacts with other neural networks to generate appropriate response to salient stimuli [41,42]. Reduced amygdala rsFC with insula has been observed in adults with MDD and is related to disrupted bottom-up salience processing of negative emotion. This type of disruption may result in weak self-awareness of negative feelings, thus leading to negative bias in MDD [17,38].
4.2. Adolescent-specific abnormal amygdala rsFC in the CCN and DMN
Decreased amygdala rsFC within the cognitive control network (CCN, a network involved in cognitive and executive functions [42]) and incoordinate amygdala rsFC (both increased and decreased) within the DMN (a network involved in inner attention [43]) were observed in adolescents with MDD. These findings converge with a theoretical model in which adolescents with depression are inclined to become trapped in cognitive dysregulation and negative rumination [12]. Prior studies have centrally indicated the DLPFC, part of the CCN, in “top-down” cognitive control, from attention to emotion [44,45]. DLPFC hypoactivation has been observed in adolescents with MDD when exerting cognitive inhibition during emotional processing, suggesting their cognitive vulnerability to depression [46]. Critically, decreased amygdala rsFC with CCN has been suggested as dysregulated top-down cognitive control from DLPFC to amygdala, standing for aberrant affective cognition processing among depressed adolescents [9].
The precuneus, which was hyperconnected with the amygdala in our findings, is linked to reflective self-awareness [43] and is reliably activated when instructed with self-related information in adolescents with MDD [47]. Critically, increased amygdala rsFC with precuneus has been frequently reported in MDD adolescents [10,48], and this pattern has been related to rumination, a recursive self-referential thinking pattern with more responding to negative materials [49]. The ITG of DMN has also been implicated in social cognition and processing of perception and emotion [9,50]. A prior study discovered that adolescent MDD patients demonstrated ITG structural deficits compared to HC [51]. Deactivated ITG has also been observed in depressed adolescents during facial emotion identification, and this is feature correlated with greater perceptual processing [48]. The up-mentioned patterns reflected biases toward internal thoughts that were more sensitive to external perception.
In summary, abnormal amygdala rsFC within networks implicated in cognitive control may underlie the characteristic affective-cognition abnormalities in adolescents with MDD. Because the maturation of brain regions supporting cognitive processes is protracted, these immature brain regions might be susceptible to psychiatric disorders, causing poor cognitive function in adolescent patients [52]. Evidence from a previous study suggests that cognitive dysfunction is a feature of adolescent MDD [53], which would be an important implication for the mechanism, prevention, and treatment of MDD in adolescents, or this reason that cognitive-behavioral therapy (CBT) has been recommended by clinical trial [54] and National Institute for Health and Care Excellence (NICE) guidelines in the United Kingdom [55] to be the first-line treatment for adolescents with MDD.
4.3. Differences in abnormal amygdala rsFC between adult and adolescent MDD patients
The current study reveals two main significant differences in amygdala rsFC between adults and adolescents. First, the amygdala was hyperconnected to the hippocampus/parahippocampus and hypoconnected to the insula among adult patients with MDD compared with adolescent patients with MDD. The hypoconnectivity between the amygdala and hippocampus/parahippocampus has been reported in adolescent MDD patients, and this abnormality was associated with an increased level of general depression and a reduced sense of well-being [10]. Therefore, observed negative synchrony between these regions in adolescent patients compared with that in adults may fail to suppress spontaneously emerged negative encoding during rest in a more severe way. However, reduced amygdala rsFC with insula in adults with MDD compared to adolescent MDD may indicate more disruptions of bottom-up salience process and severe negative bias in adult patients [17,38].
Second, our meta-regression analysis demonstrated that age-related differences in the two groups showed that increased age was correlated with decreased amygdala rsFC with the right insula in adult patients, while no such association between amygdala rsFC abnormalities and age was detected in adolescent MDD patients. This association may be because the human brain exhibits myelination and prolonged neural pruning well into young adulthood, and adolescence is a period with continued neural development [56], during which the networks may still be in an unstable state.
These differences indicated that adult and adolescent MDD involve different brain network abnormalities, which may provide insights into possible clinical treatment insight into the two groups. Alterations in adult patients are localized within the AN, which is also the target of standard antidepressants [57]; thus, adult patients might be more sensitive than adolescents to pharmacological treatments. Alterations in adolescent patients are especially prominent in networks involved in cognitive control, which give neural bases for the effect of CBT in this particular population [58].
4.4. Functional lateralization of the left vs. right amygdala
An additional finding of our study is the functional lateralization of amygdala connectivity in both adults and adolescents with MDD. Subgroup results from adults demonstrated that the left amygdala seed has rsFC with prefrontal-limbic regions, whereas the right amygdala seed demonstrated connectivity primarily with subcortical regions and occipital lobe. For adolescents with MDD, the left amygdala seed demonstrated connectivity mainly with IFG and precuneus/cuneus, while the right amygdala showed connectivity with widely distributed regions, including DLPFC, ACC, ITG, and lingual gyrus. Lateralization of amygdala activity has been explored in previous task-based meta-analyses [4,59,60]. In Bass's meta-analysis, more activity of the left amygdala than the right was observed during emotion processing [59]. Wager et al. also reported a pattern of amygdala activity lateralization, particularly under negative-value emotional stimuli [60]. While another task fMRI about adolescents found that only the right amygdala was activated when encoding emotional stimulus [61]. In addition, it has been reported that top-down regulation may involve only the left amygdala, while bottom-up response modulates both left and right [44]. Using only the left or the right amygdala as the seed or simply combining the results from both sides might overlook the potential difference in rsFC of unilateral amygdala, as discovered by our current study. Thus, we suggested that when performing seed-based FC analysis, the seeds on both the left and right hemispheres should be investigated simultaneously and using statistical methods to exclude hemisphere effects before combining the results.
4.5. Future directions
Several challenges need to be noted when a new study is conducted in the future. First, there were too few studies probing treatment effects among MDD patients. Research has shown that pharmacological intervention may influence cerebral activity [62]; thus, future studies examining a cohort before and after treatment would be helpful to clarify the effects of treatment on amygdala rsFC and its possible association with treatment response. Second, only a few studies have examined the relationship between amygdala rsFC aberrations and depressive symptom severity. It will be important to explore the interaction between brain networks and clinical symptom severity of this disorder, especially in different age groups, to explore the phenomenon from the viewpoint of neuroplasticity point of view. Third, we found that most patients in previous studies had comorbid anxiety disorders, such as posttraumatic stress disorder or phobia; therefore, further studies should explore how this comorbidity might influence connectivity.
5. Conclusions
In conclusion, we provided the first meta-analytic evidence that adult and adolescent patients with MDD have specific patterns of amygdala-centered rsFC abnormalities, which may also reflect the corresponding network dysfunction in MDD. Adult MDD-specific amygdala rsFC abnormalities within AN presumably reflected emotional dysregulation in MDD, whereas adolescent-specific amygdala rsFC abnormalities in networks involved in cognitive control might be relevant to affective cognition deficiency that is characteristic in adolescent MDD. Future studies that directly compare the adult and adolescent patient groups are needed to test our findings, examine changes in amygdala rsFC over development, and relate these changes to more specific patterns such as functional lateralization, comorbidities, symptom severity and treatment outcomes.
Acknowledgments
Acknowledgements
The authors would like to thank their tutors and classmates for providing valuable help.
Funding sources
This study was supported by a grant from the National Natural Science Foundation of China (81671669) and by a Sichuan Provincial Youth Grant (2017JQ0001). The funding sources had no involvement in the study design, collection, analysis, or interpretation of data.
Declaration of interests
The authors report no potential conflicts of interest.
Author contributions
Shi Tang and Lu Lu designed the study, collected data and performed analyses; Lianqing Zhang, Xinyu Hu, Xuan Bu, Hailong Li, Xiaoxiao Hu, Yingxue Gao and Zirui Zeng provided helpful suggestions; Shi Tang drafted the main article; and Qiyong Gong and Xiaoqi Huang critically reviewed the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebiom.2018.09.010.
Contributor Information
Qiyong Gong, Email: qiyonggong@hmrrc.org.cn.
Xiaoqi Huang, Email: julianahuang@163.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Smith K. Mental health: a world of depression. Nature. 2014;515(7526):181. doi: 10.1038/515180a. [DOI] [PubMed] [Google Scholar]
- 2.Anthes E. Depression: a change of mind. Nature. 2014;515(7526):185–187. doi: 10.1038/515185a. [DOI] [PubMed] [Google Scholar]
- 3.Morris J.S., Ohman A., Dolan R.J. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393(6684):467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- 4.Sergerie K., Chochol C., Armony J.L. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32(4):811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 6.Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X., Zhu X., Wang X. First-episode medication-naive major depressive disorder is associated with altered resting brain function in the affective network. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Y., Kong L., Wu F. Decreased functional connectivity between the amygdala and the left ventral prefrontal cortex in treatment-naive patients with major depressive disorder: a resting-state functional magnetic resonance imaging study. Psychol Med. 2013;43(9):1921–1927. doi: 10.1017/S0033291712002759. [DOI] [PubMed] [Google Scholar]
- 9.Pannekoek J.N., van der Werff S.J.A., Meens P.H.F. Aberrant resting-state functional connectivity in limbic and salience networks in treatment-naïve clinically depressed adolescents. J Child Psychol Psychiatry. 2014;55(12):1317–1327. doi: 10.1111/jcpp.12266. [DOI] [PubMed] [Google Scholar]
- 10.Cullen K.R., Westlund M.K., Klimes-Dougan B. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiat. 2014;71(10):1138. doi: 10.1001/jamapsychiatry.2014.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S.M., Park S.Y., Kim Y.I. Affective network and default mode network in depressive adolescents with disruptive behaviors. Neuropsychiatr Dis Treat. 2015:49. doi: 10.2147/NDT.S95541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerestes R., Davey C.G., Stephanou K., Whittle S., Harrison B.J. Functional brain imaging studies of youth depression: a systematic review. Neuroimage Clin. 2014;4:209–231. doi: 10.1016/j.nicl.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmaal L., Hibar D.P., Samann P.G. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Mol Psychiatry. 2017;22(6):900–909. doi: 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Connolly M.E., Gollan J.K., Cobia D., Wang X. Reduced striatal activation in females with major depression during the processing of affective stimuli. J Psychiatr Res. 2015;68:384–391. doi: 10.1016/j.jpsychires.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Nixon N.L., Liddle P.F., Worwood G., Liotti M., Nixon E. Prefrontal cortex function in remitted major depressive disorder. Psychol Med. 2013;43(6):1219–1230. doi: 10.1017/S0033291712002164. [DOI] [PubMed] [Google Scholar]
- 16.Miller C.H., Hamilton J.P., Sacchet M.D., Gotlib I.H. Meta-analysis of functional neuroimaging of major depressive disorder in youth. JAMA Psychiat. 2015;72(10):1045–1053. doi: 10.1001/jamapsychiatry.2015.1376. [DOI] [PubMed] [Google Scholar]
- 17.Ramasubbu R., Konduru N., Cortese F., Bray S., Gaxiola-Valdez I., Goodyear B. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front Psych. 2014;5:17. doi: 10.3389/fpsyt.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye J., Shen Z., Xu X. Abnormal functional connectivity of the amygdala in first-episode and untreated adult major depressive disorder patients with different ages of onset. Neuroreport. 2017;28(4):214–221. doi: 10.1097/WNR.0000000000000733. [DOI] [PubMed] [Google Scholar]
- 19.Fox P.T., Lancaster J.L., Laird A.R., Eickhoff S.B. Meta-analysis in human neuroimaging: computational modeling of large-scale databases. Annu Rev Neurosci. 2014;37:409–434. doi: 10.1146/annurev-neuro-062012-170320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller V.I., Cieslik E.C., Laird A.R. Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev. 2018;84:151–161. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radua J., Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195(5):393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- 22.Radua J., Romeo M., Mataix-Cols D., Fusar-Poli P. A general approach for combining voxel-based meta-analyses conducted in different neuroimaging modalities. Curr Med Chem. 2013;20(3):462–466. [PubMed] [Google Scholar]
- 23.Knobloch K., Yoon U., Vogt P.M. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39(2):91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Cipriani A., Zhou X., Del Giovane C. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388:881–890. doi: 10.1016/S0140-6736(16)30385-3. [DOI] [PubMed] [Google Scholar]
- 25.Radua J., Mataix-Cols D., Phillips M.L. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry. 2012;27(8):605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Radua J., Rubia K., Canales E., Pomarol-Clotet E., Fusar-Poli P., Mataix-Cols D. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psych. 2014;5(13) doi: 10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radua J., van den Heuvel O.A., Surguladze S., Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 2010;67(7):701–711. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- 28.Sterne Jonathan A.C., Egger Matthias. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 29.Kaiser R.H., Andrews-Hanna J.R., Wager T.D., Pizzagalli D.A. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiat. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand A., Li Y., Wang Y. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry. 2005;57(10):1079–1088. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Mayberg H.S. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 32.Tye K.M., Mirzabekov J.J., Warden M.R. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493(7433):537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tye K.M., Stuber G.D., de Ridder B., Bonci A., Janak P.H. Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature. 2008;453(7199):1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegle G.J., Steinhauer S.R., Thase M.E., Stenger V.A., Carter C.S. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychol. 2002;51(9):693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janak P.H., Tye K.M. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tahmasian M., Knight D.C., Manoliu A. Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Front Hum Neurosci. 2013;7:639. doi: 10.3389/fnhum.2013.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labar K.S., Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton J.P., Gotlib I.H. Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychol. 2008;63(12):1155–1162. doi: 10.1016/j.biopsych.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veer I.M., Beckmann C.F., van Tol M.J. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeley W.W., Menon V., Schatzberg A.F. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kjaer T.W., Nowak M., Lou H.C. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage. 2002;17(2):1080–1086. [PubMed] [Google Scholar]
- 44.Ochsner K.N., Ray R.R., Hughes B. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol Sci. 2009;20(11):1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Mathews A., MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- 47.Quevedo K., Ng R., Scott H. The neurobiology of self-face recognition in depressed adolescents with low or high suicidality. J Abnorm Psychol. 2016;125(8):1185–1200. doi: 10.1037/abn0000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho T.C., Zhang S., Sacchet M.D. Fusiform gyrus dysfunction is associated with perceptual processing efficiency to emotional faces in adolescent depression: a model-based approach. Front Psych. 2016;7:40. doi: 10.3389/fpsyg.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooney R.E., Joormann J., Eugène F., Dennis E.L., Gotlib I.H. Neural correlates of rumination in depression. Cogn Affect Behav Neurosci. 2010;10(4):470–478. doi: 10.3758/CABN.10.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goulden N., McKie S., Thomas E.J. Reversed frontotemporal connectivity during emotional face processing in remitted depression. Biol Psychol. 2012;72(7):604–611. doi: 10.1016/j.biopsych.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramezani M., Johnsrude I., Rasoulian A. Temporal-lobe morphology differs between healthy adolescents and those with early-onset of depression. NeuroImage. 2014;6:145–155. doi: 10.1016/j.nicl.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gogtay N., Giedd J.N., Lusk L. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allott K., Fisher C.A., Amminger G.P., Goodall J., Hetrick S. Characterizing neurocognitive impairment in young people with major depression: state, trait, or scar? Brain Behav. 2016;6(10) doi: 10.1002/brb3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glass R.M. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. J Pediatr. 2005;146(1):145. doi: 10.1016/j.jpeds.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 55.NICE Depression in children and young people. Clin Guidel. 2015;28:1–271. [Google Scholar]
- 56.Gabard-Durnam L.J., Flannery J., Goff B. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopez J.F., Chalmers D.T., Little K.Y., Watson S.J., A.E. Bennett Research Award Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychol. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 58.March J., Silva S., Petrycki S., Curry J., Wells K., Fairbank J. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 59.Baas D., Aleman A., Kahn R.S. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Brain Res Rev. 2004;45(2):96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Wager T.D., Phan K.L., Liberzon I., Taylor S.F. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19(3):513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 61.Vasa R.A., Pine D.S., Thorn J.M. Enhanced right amygdala activity in adolescents during encoding of positively valenced pictures. Dev Cogn Neurosci. 2011;1(1):88–99. doi: 10.1016/j.dcn.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma Y. Neuropsychological mechanism underlying antidepressant effect: a systematic meta-analysis. Mol Psychiatry. 2015;20(3):311–319. doi: 10.1038/mp.2014.24. [DOI] [PubMed] [Google Scholar]
- 63.Peters A.T., Burkhouse K., Feldhaus C.C., Langenecker S.A., Jacobs R.H. Aberrant resting-state functional connectivity in limbic and cognitive control networks relates to depressive rumination and mindfulness: a pilot study among adolescents with a history of depression. J Affect Disord. 2016;200:178–181. doi: 10.1016/j.jad.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 64.Chattopadhyay S., Tait R., Simas T. Cognitive behavioral therapy lowers elevated functional connectivity in depressed adolescents. EBioMedicine. 2017;17:216–222. doi: 10.1016/j.ebiom.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Connolly C.G., Ho T.C., Blom E.H. Resting-state functional connectivity of the amygdala and longitudinal changes in depression severity in adolescent depression. J Affect Disord. 2017;207:86–94. doi: 10.1016/j.jad.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cullen K.R., Gee D.G., Klimes-Dougan B. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009;460(3):227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Straub J., Metzger C.D., Plener P.L., Koelch M.G., Groen G., Abler B. Successful group psychotherapy of depression in adolescents alters fronto-limbic resting-state connectivity. J Affect Disord. 2017;209:135–139. doi: 10.1016/j.jad.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 68.Jacobs R.H., Barba A., Gowins J.R. Decoupling of the amygdala to other salience network regions in adolescent-onset recurrent major depressive disorder. Psychol Med. 2016;46(5):1055–1067. doi: 10.1017/S0033291715002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Altinay M., Karne H., Beall E., Anand A. Quetiapine extended release open-label treatment associated changes in amygdala activation and connectivity in anxious depression an fMRI study. J Clin Psychopharmacol. 2016;36(6):562–571. doi: 10.1097/JCP.0000000000000600. [DOI] [PubMed] [Google Scholar]
- 70.Deligiannidis K.M., Sikoglu E.M., Shaffer S.A. GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: a preliminary study. J Psychiatr Res. 2013;47(6):816–828. doi: 10.1016/j.jpsychires.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lui S., Wu Q., Qiu L. Resting-state functional connectivity in treatment-resistant depression. Am J Psychiatry. 2011;168(6):642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y.L., Yang S.Z., Sun W.L., Shi Y.Z., Duan H.F. Altered functional interaction hub between affective network and cognitive control network in patients with major depressive disorder. Behav Brain Res. 2016;298(Pt B):301–309. doi: 10.1016/j.bbr.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 73.Yang J., Yin Y., Svob C. Amygdala Atrophy and Its Functional Disconnection with the Cortico-Striatal-Pallidal-Thalamic Circuit in Major Depressive Disorder in Females. PloS one. 2017;12(1):e0168239. doi: 10.1371/journal.pone.0168239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material