Abstract
Background & Aims
Many pediatric patients with acute liver failure (PALF) do not receive a specific diagnosis (such as herpes simplex virus or Wilson disease or fatty acid oxidation defects)—they are left with an indeterminate diagnosis and are more likely to undergo liver transplantation, which is contraindicated for some disorders. Strategies to facilitate complete diagnostic testing should increase identification of specific liver diseases and might reduce liver transplantation. We investigated whether performing recommended age-specific diagnostic tests (AS-DTs) at the time of hospital admission reduces the percentage PALFs with an indeterminate diagnosis.
Methods
We performed a multinational observational cohort study of 658 PALF participants in the United States and Canada, enrolled at 10 medical centers, during 3 study phases from December 1999 through December 2014. A learning collaborative approach was used to implement AS-DT using an electronic medical record admission order set at hospital admission in phase 3 of the study. Data from 10 study sites participating in all 3 phases were compared before (phases 1 and 2) and after (phase 3) diagnostic test recommendations were inserted into electronic medical record order sets.
Results
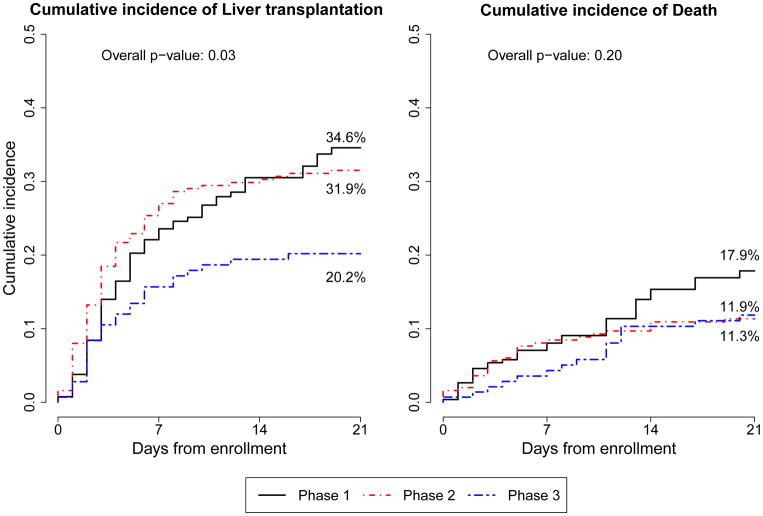
The percentage of subjects with an indeterminate diagnosis decreased significantly between phases 1–2 (48.0%) and phase 3 (to 30.8%) (P=.0003). The 21-day cumulative incidence rates for liver transplantation were significantly different among phase 1 (34.6%), phase 2 (31.9%), and phase 3 (20.2%) (P=.030). The 21-day cumulative incidence rates for death did not differ significantly among phase 1 (17.9%), phase 2 (11.9%), and phase 3 (11.3%) (P=.20).
Conclusion
In a multinational study of children with acute liver failure, we found that incorporating diagnostic test recommendations into electronic medical record order sets accessed at time of admission reduced the percentage with an indeterminate diagnosis that may have reduced liver transplants without increasing mortality. Widespread use of this approach could significantly enhance care of acute liver failure in children.
Keywords: management, hepatic, genetic disorder, early detection
Introduction
Acute liver failure (ALF) is a rare syndrome in which abrupt liver injury severely impairs liver function in a previously healthy individual.1 A preceding non-specific prodrome may last days or weeks, but once features of ALF are established, the clinical course is dynamic, unpredictable, and sometimes rapidly progressive.2, 3 Interventions are largely supportive, although specific life-saving therapy is initiated if a treatable diagnosis is promptly identified.4–6 A specific diagnosis may also suggest liver transplantation (LT) is contraindicated. Unfortunately, a diagnosis is not established (i.e. is indeterminate) in 49% of children 5 and death or LT can occur within days following initial hospitalization. As children with indeterminate pediatric acute liver failure (PALF) are more likely to receive LT than those with an established diagnosis, enhanced diagnostic specificity may impact LT decisions.1
The Indeterminate cohort is heterogenous as it is composed of children whose more specific diagnosis was not established for reasons such as an incomplete diagnostic evaluation due to death, LT, or clinical improvement, an incomplete differential diagnosis, immune dysregulation defying discrete diagnostic testing, or novel metabolic or infectious conditions.5 Narkewicz et. al. examined 703 PALF study participants and found only 55% had complete testing for autoimmune hepatitis.5 Testing for other conditions, such as Wilson disease, fatty acid oxidation defects and herpes simplex virus (HSV) was also incomplete with significant variations in diagnostic testing among sites.5 Given evidence of incomplete diagnostic testing and a rapid clinical course for some participants, PALF investigators established a process to improve diagnostic testing frequency using a learning collaborative strategy 7 adopted by others to reduce clinical variability and improve outcome.8, 9
Here, the PALF cohort is characterized before and after investigators incorporated age-specific diagnostic testing (AS-DT) recommendations into the electronic medical record (EMR) to determine if enhanced diagnostic testing occurred and whether this intervention was followed by a decrease in the frequency of an indeterminate diagnosis.
Materials and Methods
This observational cohort study was conducted by the PALF study group funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; UO1-DK072146). Patients < 18 years of age were eligible for enrollment if they met the following criteria: 1) no prior evidence of chronic liver disease, 2) biochemical evidence of acute liver injury, and 3) hepatic insufficiency characterized by prothrombin time (PT) ≥ 20 seconds or international normalized ratio (INR) ≥ 2.0 (not correctable with vitamin K) OR by a PT ≥ 15 seconds or INR ≥ 1.5 in the presence of encephalopathy (EN). Two clinical EN grade scales were used depending on participant age 1, the Whitington scale 10 for subjects up to 3 years of age (Supplemental Table 1) and West Haven score 11 for those 4 years and older. EN assignment was by the same investigator, if possible, throughout the data collection period. Diagnostic evaluation, medical management and assigning the final diagnosis were directed by the attending physician(s) and consistent with the standard of care at each site as previously reported.1 Participants were enrolled between December 1999 and December 2014 during 3 study phases determined by funding periods. Entry criteria were never altered. Study approval was by Institutional Review Boards of all institutions and NIDDK provided a Certificate of Confidentiality. A Data and Safety Monitoring Board, appointed by the NIDDK, provided study oversight. Informed consent was obtained from parents or guardians.
Phase 1 (P1) began in December 1999. Demographic and clinical data were recorded daily for up to seven days. The first outcome of death, LT, or hospital discharge with native liver within 21 days following enrollment was recorded. Participants discharged prior to 21 days following enrollment without undergoing LT received follow-up to confirm participant status of “alive”, “dead”, or “liver transplant” by day 21. Initial (e.g., at enrollment) and final diagnoses were determined by the site principal investigator based on study guidelines. A diagnosis of neonatal iron storage disease was later revised to gestational alloimmune liver disease (GALD) to reflect pathophysiological advances.12, 13 An indeterminate diagnosis was registered in the absence of evidence for a specific diagnosis.
Phase 2 (P2) began in 2006. Data elements collected over the 7 days following enrollment were similar to P1. Modifications for the P2 protocol included outcome data extended from 21 days to 1 year from study entry. Data obtained 1 year following study entry were collected in clinic or by telephone and included vital status, change in diagnosis, and medical or surgical intervention including LT. All outcomes within 1 year of study entry were recorded in P2, not just by the first event as in P1.
In transition from P2 to P3, enrolling sites decreased from 20 to 12 due to factors that included enrollment targets, funding restrictions, site and consortium resources, data quality, and initiating broader and more detailed data collection, 10 of the 12 sites participated in all phases of PALF. Data elements collected in P1 and P2 were carried forward, including participant outcomes at 1 year. Daily data collection was extended to the entire enrollment hospitalization.
Enrollment into P3 was briefly delayed to adapt data collection tools to incorporate granular patient and management detail. Investigators also engaged in collaborative discussions to improve frequency of diagnostic testing using data collected during P1 and P2.1, 5, 14, 15 The product of this learning collaborative was to recommend AS-DT at the time of hospital admission with the goals of increasing the frequency of testing for age appropriate diagnoses identified in P1 and P2, and reducing the frequency of indeterminate diagnosis, regardless of participation in the PALF study. Factors influencing test selection included blood volume restrictions, final diagnoses within age groups, availability of the test, and likelihood that a positive test would be clinically available in time to either establish the diagnosis (e.g, viral PCR) or lead to a more complete, focused diagnostic evaluation (e.g. lactate:pyruvate ratio, ceruloplasmin). Recommended AS-DT was incorporated into EMR-based order sets at all P3 sites easily accessed easily by the admitting physician by typing “acute liver failure” into the search function. Each test was within the standard of care at each site and defaulted to be ordered at hospital admission regardless of participation in the PALF study. Diagnostic testing and biochemical testing was performed by the local laboratory or its affiliate. Central testing was not performed. Diagnostic tests in the order sets were not incorporated into the research protocol, but served as a tool to promote safe, efficient, and evidence-based patient care. The attending physician was responsible for ordering individual tests, which could be selectively removed or added depending upon the clinical circumstances. Diagnostic criteria for known diagnoses were outlined in the PALF Manual of Operations and served to guide the investigator in establishing the final diagnosis.
Statistical analysis
Baseline demographic, and laboratory data for all sites and for those included in all phases of PALF are reported. Study entry labs include measurements up to 3 days before enrollment with preference given to the enrollment day and then those closest to enrollment day. Etiology category and specifics within category are shown. If the category was suspected but the specific etiology within the category was not, etiology was categorized as indeterminate. Continuous variables are described by median and 25th and 75th percentiles. Categorical variables are described by frequencies and percentages. Comparative data analyses were performed from 10 study sites participating in all 3 phases before (P1+P2) and after (P3) initiating testing recommendations. Wilcoxon rank sum statistics were used to test for differences in distributions of continuous variables between the study phases before and after AS-DT implementation (P1+P2 vs P3) using data from the 10 clinical sites participating in all phases of PALF. Pearson’s or exact chi-square statistics were used to test for differences in percentages of categorical variables before and after AS-DT implementation and among the age groups in combined P1+P2. 21-day cumulative incidence rates for liver transplantation and death were calculated and reported for those sites included in all PALF phases. Death was considered a competing risk for LT. Post-LT death was excluded by treating LT as a competing risk for death. Gray’s test was used to compare the cumulative incidence functions among the phases. P-values less than 0.05 are considered statistically significant. The data were analyzed with SAS 9.4 and R 2013 was used to create figures.
Results
Twenty-four sites in the PALF consortium enrolled participants in at least 1 phase while 10 participated in all three phases (Supplemental Table 2). Demographic, diagnostic and laboratory data in the overall PALF cohort (n=1144) and the 10-site sub-cohort [n=658: P1+P2 (n=515), and P3 (n=143)] are reported (Table 1). P3 participants were younger, more likely to be male, and had similar total bilirubin and alanine aminotransferase levels as those in combined phases 1&2. Differences in INR and creatinine are statistically different, but clinically similar. Assessable peak encephalopathy (EN) was less common in P3 than P1 and P2. The cohort deemed not assessable (e.g., on ventilator) likely included participants with EN stage III and IV.
Table 1.
Demographic and laboratory data in the overall PALF cohort (n=1144) and the 10-site sub-cohort (N=658).
| Characteristic | All Sites (n = 1144) | Sites in all 3 phases Total (n=658) | Phase 1 & 2 12/99–12/10 (n=515) | Phase 3 5/12 – 12/14 (n=143) | Phase comparison p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age(yrs), | 0.03 | ||||||||
| median, | 4.5, | 4.5, | 5.0, | 2.9, | |||||
| Q1–Q3 | 0.8 – 13.4 | 1.0 – 13.9 | 1.2 – 14.1 | 0.3 – 13.7 | |||||
| Age at enrollment, n % | |||||||||
| ≤ 28 days | 156 | 13.6 | 79 | 12.0 | 50 | 9.7 | 29 | 20.3 | 0.003 |
| 29–90 days | 64 | 5.6 | 34 | 5.2 | 28 | 5.4 | 6 | 4.2 | |
| 91 days to < 1 year | 99 | 8.7 | 54 | 8.2 | 44 | 8.5 | 10 | 7.0 | |
| 1–3 years | 224 | 19.6 | 144 | 21.9 | 108 | 21.0 | 36 | 25.2 | |
| 4–12 years | 297 | 26.0 | 153 | 23.3 | 132 | 25.6 | 21 | 14.7 | |
| 13–17 years | 304 | 26.6 | 194 | 29.5 | 153 | 29.7 | 41 | 28.7 | |
| Male, n % | 585 | 51.1 | 332 | 50.5 | 249 | 48.4 | 83 | 58.0 | 0.04 |
| Race, n % | |||||||||
| White | 812 | 72.6 | 463 | 72.7 | 368 | 73.5 | 95 | 69.9 | 0.40 |
| Non-White | 307 | 27.4 | 174 | 27.3 | 133 | 26.5 | 41 | 30.1 | |
| Unknown, n % of total | 25 | 2.2 | 21 | 3.2 | 14 | 2.7 | 7 | 4.9 | |
| Study Entry Labs (includes values up to 3 days before study entry) | |||||||||
| INR, | 0.02 | ||||||||
| Median | 2.6, | 2.6, | 2.5, | 2.7, | |||||
| Q1–Q3 | 2.1 – 3.8 | 2.1 – 3.7 | 2.0 – 3.7 | 2.2 – 3.9 | |||||
| Missing, n % of total | 97 | 8.5 | 30 | 4.6 | 30 | 11.3 | 0 | 0.0 | |
| Total Bilirubin (mg/dL), | 0.11 | ||||||||
| median, | 9.1, | 7.9, | 8.7, | 6.1, | |||||
| Q1–Q3 | 2.8 – 17.0 | 2.7 – 16.3 | 2.7 – 16.6 | 2.5 – 14.2 | |||||
| Missing, n % of total | 103 | 9.0 | 81 | 12.3 | 58 | 11.3 | 23 | 16.1 | |
| ALT (IU/L) | 0.45 | ||||||||
| median, | 1545, | 1735, | 1693, | 1858, | |||||
| Q1–Q3 | 386 – 3393 | 509 – 3932 | 513 – 3754 | 490 – 4708 | |||||
| Missing, n % of total | 94 | 8.2 | 12 | 1.8 | 10 | 1.9 | 2 | 1.4 | |
| Creatinine (mg/dL) | 0.0001 | ||||||||
| median, | 0.5, | 0.5, | 0.5, | 0.4 | |||||
| Q1–Q3 | 0.3 – 0.8 | 0.3 – 0.7 | 0.3 – 0.8 | 0.2 – 0.7 | |||||
| Missing, n % of total | 18 | 1.6 | 12 | 1.8 | 11 | 2.1 | 1 | 0.7 | |
|
| |||||||||
| Encephalopathy Grade peak up to 7 days past enrollment, n % | 0.03 | ||||||||
| 0 | 435 | 39.9 | 242 | 38.7 | 184 | 36.8 | 58 | 46.0 | |
| 1 | 238 | 21.9 | 133 | 21.3 | 100 | 20.0 | 33 | 26.2 | |
| 2 | 172 | 15.8 | 105 | 16.8 | 88 | 17.6 | 17 | 13.5 | |
| 3 | 134 | 12.3 | 73 | 11.7 | 64 | 12.8 | 9 | 7.1 | |
| 4 | 110 | 10.1 | 73 | 11.7 | 64 | 12.8 | 9 | 7.1 | |
| Not assessable, n % of total | 53 | 4.6 | 30 | 4.6 | 15 | 2.9 | 15 | 10.5 | |
| Not done, n % of total | 2 | 0.2 | 2 | 0.3 | 0 | 0.0 | 2 | 1.4 | |
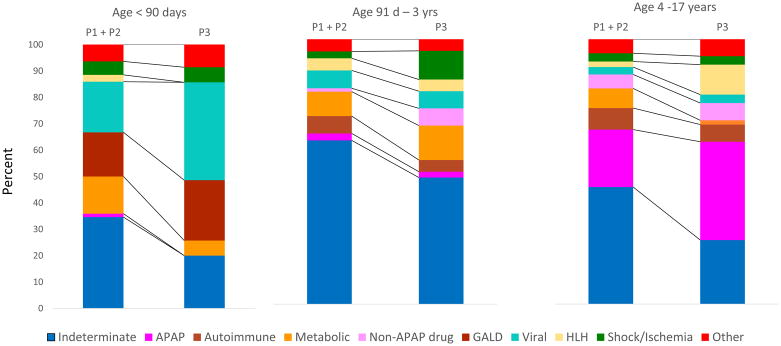
Age-specific diagnoses reported in the combined P1 and P2 cohort (Supplemental Table 3) of all participants determined priorities for AS-DT recommendations for P3. In P1+P2 (n=986), indeterminate PALF was the most common final diagnosis (444/986; 45%), accounting for most children age 91 days through 3 years (162/274; 59%). The most common diagnoses among participants younger than 91 days of age were HSV, gestational alloimmune liver disease (GALD), and metabolic conditions including galactosemia, and mitochondrial/respiratory chain defects. No participant <91 days had a diagnosis of other causes of viral hepatitis (e.g., Epstein Barr virus, hepatitis A, B, C, or E) or autoimmune hepatitis. Thus, AS-DT in these youngest participants included selected viruses, metabolic disease, and GALD, but did not include autoantibody testing or viral diseases not previously identified, other than confirming maternal hepatitis B serology to identify newborns at-risk for vertical transmission. For participants, older than 90 days old, autoimmune hepatitis and acetaminophen or other drug-related liver diseases were identified. Metabolic diseases, including mitochondrial, were distributed throughout older participants, but Wilson disease was diagnosed only in participants older than 3 years. Recommended AS-DT for children < 90 days, 91 days through 3 years, and 4 years up to 18 years are in Table 2.
Table 2.
Recommendations for Minimal Diagnostic Evaluation by Age in Pediatric Acute Liver Failure
| Recommended Tests | Indication | Recommended Age of Diagnostic Testing | |||
|---|---|---|---|---|---|
| Blood and Urine Tests | <3 mo. | 3 mo. to 3 yrs. | 3 mo. to 18yr. | 4 to 18 yr. | |
| Herpes blood PCR | Systemic Herpes infection | X | X | ||
| Serum amino acid profile | Urea cycle; other metabolic defects | X | X | ||
| Ferritin | GALD screen | X | |||
| Lactate, pyruvate | Mitochondrial screen | X | X | ||
| Plasma acylcarnitine profile | FAO defects | X | X | ||
| Urine succinylacetone | Tyrosinemia | X | |||
| Enterovirus blood PCR | Systemic Enterovirus infection | X | X | ||
| Acetaminophen level | Acetaminophen exposure | X | |||
| Hepatitis A virus IgM | Hepatitis A | X | |||
| Hepatitis B Surface antigen | Hepatitis B | X | |||
| EBV VCA IgM or PCR | EBV infection | X | |||
| Antinuclear antibody | Autoimmune disease screen | X | |||
| Anti-smooth muscle ab | Autoimmune disease screen | X | |||
| Liver kidney microsomal ab | Autoimmune disease screen | X | |||
| Immunoglobulin G | Autoimmune disease screen | X | |||
| Ceruloplasmin | Wilson disease screen | X | |||
| 24-hour Urine copper | Wilson disease screen | X | |||
| Historical Information | |||||
| Drug history | APAP other drug or HDS exposure | X | X | ||
| Confirm newborn screen results | Galactosemia and tyrosinemia | X | |||
| Confirm maternal Hepatitis B serology | Hepatitis B in newborn | X | |||
| Procedures | |||||
| Abdominal ultrasound with doppler | Vascular anomalies | X | X | ||
| Echocardiogram | Cardiac dysfunction | X | X | ||
| Optional Diagnostic screening | |||||
| Blood culture | Sepsis | ||||
| Viral testing for adenovirus, enterovirus, HHV-6, parvovirus, influenza | Viral infection | ||||
| Hepatitis E IgM | Hepatitis E | ||||
| Soluble IL2R, ferritin, triglyceride level | HLH | ||||
| Liver copper, Wilson gene mutation analysis | Wilson disease | ||||
| MRI for extrahepatic iron deposition | GALD | ||||
| Urine orotic acid | Urea cycle defects | ||||
Ab: antibody, APAP: acetaminophen, EBV: Epstein Barr Virus, FAO: fatty acid oxidation defects, GALD: gestational alloimmune liver disease, HHV-6: Human Herpes Virus-6, HLH: hemophagocytic lymphohistiocytosis, HDS: herbal, dietary supplement, IgM: immunoglobulin M, IL2R: interleukin 2 receptor, MRI: Magnetic resonance imaging, PCR: polymerase chain reaction, VCA: viral capsule antigen
Changes in the pattern of diagnostic testing before implementing the recommendations (P1+P2) and after (P3) are depicted in Table 3. In concordance with AS-DT recommendations, participants < 90 days demonstrated an increase in diagnostic testing for HSV (p=0.006), enterovirus (p<0.0001), lactate (p=0.03), and pyruvate (p=0.02). Children over 90 days experienced a significant increase in diagnostic testing for all three autoantibodies, enterovirus, serum amino acids, acylcarnitine profile, lactate, pyruvate, and APAP (all with p<0.0001), ferritin (p=0.0001), ANA (p=0.0004), and HSV (p=0.006). While ferritin was not recommended for older participants, its inclusion in diagnostic criteria for hemophagocytic lymphohistiocytosis, more frequently diagnosed in P3, likely influenced testing. Modification of AS-DT recommendations for individual circumstances, such as having an established diagnosis at hospital admission (e.g., acetaminophen toxicity), was not captured.
Table 3.
Frequency of diagnostic tests performed before and after age-specific testing recommendations
| Test performed | All PALF participants (N=1144) | Total (N=658) | Phases 1 & 2 (N=515) | Phase 3 (N=143) | Age ≤ 90 days p-value | Age > 90 days p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age ≤ 90 days (n=78) | Age > 90 days (n=437) | Age ≤ 90 days (n=35) | Age > 90 days (n=108) | |||||||||||
| ANA, n % | 678 | 59.3 | 380 | 57.8 | 8 | 10.3 | 282 | 64.5 | 1 | 2.9 | 89 | 82.4 | 0.27 | 0.0004 |
| ASMA, n % | 667 | 58.3 | 378 | 57.4 | 5 | 6.4 | 288 | 65.9 | 2 | 5.7 | 83 | 76.9 | 1.00 | 0.03 |
| ALKM, n % | 614 | 53.7 | 341 | 51.8 | 3 | 3.9 | 251 | 57.4 | 1 | 2.9 | 86 | 79.6 | 1.00 | <0.0001 |
| AI all 3, n % | 559 | 48.9 | 309 | 47.0 | 2 | 2.6 | 227 | 52.0 | 1 | 2.9 | 79 | 73.1 | 1.00 | <0.0001 |
| HSV*, n % | 426 | 37.2 | 306 | 46.5 | 36 | 46.2 | 183 | 41.9 | 26 | 74.3 | 61 | 56.5 | 0.006 | 0.006 |
| Enterovirus*, n % | 155 | 13.5 | 138 | 21.0 | 12 | 15.4 | 49 | 11.2 | 29 | 82.9 | 48 | 44.4 | <0.0001 | <0.0001 |
| Serum AA, n % | 412 | 36.0 | 265 | 40.3 | 45 | 57.7 | 127 | 29.1 | 27 | 77.1 | 66 | 61.1 | 0.047 | <0.0001 |
| Acylcarnitine profile, n % | 358 | 31.3 | 247 | 37.5 | 37 | 47.4 | 111 | 24.4 | 23 | 65.7 | 76 | 70.4 | 0.07 | <0.0001 |
| Urine succinylacetone, | 249 | 21.8 | 154 | 23.4 | 42 | 53.9 | 69 | 15.8 | 24 | 68.6 | 19 | 17.6 | 0.14 | 0.65 |
| Hepatitis A, n % | 785 | 68.6 | 465 | 70.7 | 35 | 44.9 | 330 | 75.5 | 9 | 25.7 | 91 | 84.3 | 0.054 | 0.052 |
| Hepatitis B, n % | 893 | 78.1 | 525 | 79.8 | 43 | 55.1 | 374 | 85.6 | 14 | 40.0 | 94 | 87.0 | 0.14 | 0.70 |
| Ferritin, n % | 476 | 41.6 | 256 | 38.9 | 43 | 55.1 | 133 | 30.4 | 26 | 74.3 | 54 | 50.0 | 0.054 | 0.0001 |
| Ceruloplasmin*, n % | 538 | 47.0 | 332 | 50.5 | 5 | 6.4 | 257 | 58.8 | 2 | 5.7 | 68 | 63.0 | 1.00 | 0.43 |
| Urine copper, n % | 195 | 17.0 | 99 | 15.0 | 0 | 0.0 | 74 | 16.9 | 0 | 0.0 | 25 | 23.1 | -- | 0.13 |
| Lactate, n % | 751 | 65.6 | 398 | 60.5 | 54 | 69.2 | 218 | 49.9 | 31 | 88.6 | 95 | 88.0 | 0.03 | <0.0001 |
| Pyruvate, n % | 254 | 22.2 | 213 | 32.4 | 31 | 39.7 | 93 | 21.3 | 22 | 62.9 | 67 | 62.0 | 0.02 | <0.0001 |
| Lactate/Pyruvate on same day, n % | 225 | 19.7 | 187 | 28.4 | 27 | 34.6 | 77 | 17.6 | 20 | 57.1 | 63 | 58.3 | 0.03 | <0.0001 |
| Acetaminophen level, n % | 487 | 42.6 | 325 | 49.4 | 8 | 10.3 | 231 | 52.9 | 4 | 11.4 | 82 | 75.9 | 1.00 | <0.0001 |
There was less viral and ceruloplasmin testing in Phase 1 than in Phase 2.
BOLD: Expected to increase based on recommended age-specific diagnostic tests. ITALICS: Expected to decrease based on recommended age-specific diagnostic tests
Change in distribution of diagnoses after implementing AS-DT recommendations in P3 is reflected in Table 4. The difference in the percentage of participants with an indeterminate diagnosis decreased significantly between P1+P2 (48.0%) and P3 (30.8%); p=0.0003. The percentage with an indeterminate diagnosis declined in each age group, but most significantly in the oldest age group [P1+P2 (44.2%) vs P3 (24.2%); p=0.004, Figure 1].
Table 4.
Changes in the distributions of diagnoses between each PALF among the 10 sites participating in all phases of PALF.
| Characteristic | All Participants (n= 1144) | Sites in all 3 phases Total (N=658) | Phases 1 & 2 12/99–12/10 (N=515) | Phase 3 5/12 – 12/14 (N=143) | Phase comparison p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Final Diagnosis, n % | 0.002* | ||||||||
| Indeterminate | 491 | 42.9 | 291 | 44.2 | 247 | 48.0 | 44 | 30.8 | |
| APAP | 152 | 13.3 | 91 | 13.8 | 67 | 13.0 | 24 | 16.8 | |
| AutoAB(+)/Autoimmune | 75 | 6.6 | 39 | 5.9 | 33 | 6.4 | 6 | 4.2 | |
| Metabolic | |||||||||
| Wilson Disease | 36 | 3.2 | 21 | 3.2 | 21 | 4.1 | 0 | 0.0 | |
| Mitochondrial Disease | 17 | 1.5 | 9 | 1.4 | 4 | 0.8 | 5 | 3.5 | |
| Galactosemia | 15 | 1.3 | 6 | 0.9 | 4 | 0.8 | 2 | 1.4 | |
| Other Metabolic | 42 | 3.7 | 19 | 2.9 | 17 | 3.3 | 2 | 1.4 | |
| Non-APAP drug | 37 | 3.2 | 24 | 3.7 | 17 | 3.3 | 7 | 4.9 | |
| Gestational alloimmune liver disease | 37 | 3.2 | 21 | 3.2 | 13 | 2.5 | 8 | 5.6 | |
| Viral | |||||||||
| Herpes/Enterovirus | 53 | 4.6 | 31 | 4.7 | 18 | 3.5 | 13 | 9.1 | |
| Other Viral | 43 | 3.8 | 20 | 3.0 | 15 | 2.9 | 5 | 3.5 | |
| Hemophagocytic lymphohistiocytosis | 34 | 3.0 | 24 | 3.6 | 15 | 2.9 | 9 | 6.3 | |
| Shock/Ischemia | 40 | 3.5 | 26 | 4.0 | 17 | 3.3 | 9 | 6.3 | |
| Other | 72 | 6.3 | 36 | 5.5 | 27 | 5.2 | 9 | 6.3 | |
| Indeterminate Final Diagnosis | 491 | 42.9 | 291 | 44.2 | 247 | 48.0 | 44 | 30.8 | 0.0003 |
p-value for final diagnoses listed in bold
Figure 1.
Final diagnosis by age and phase before (P1+P2) and after (P3) age specific diagnostic testing.
Cumulative incidence rate for LT at 21 days was significantly different (p=0.030) among P1 (34.6%), P2 (31.9%) and P3 (20.2%) (Figure 2). The hazard ratio for LT in P3 compared to the combined P1&2 is 0.59 (p=0.01) and remained similar after adjusting for participant age and clinical center with a hazard ratio of 0.60 (p=0.02). In contrast, 21-day cumulative incidence rate for death was not significantly different (p=0.20) among P1 (17.9%), P2 (11.9%) and P3 (11.3%). Outcomes 1 year following enrollment were available only in P2 and P3. The cumulative incidence for LT did not differ significantly in P3 (26.1%) compared to P2 (36.1%) (p=0.07). (Supplemental Figure 1)
Figure 2.
Comparing the cumulative incidence of liver transplantation and death among the 3 phases of PALF.
Discussion
Using principles of collaborative learning, PALF investigators implemented recommendations that impacted diagnostic testing, final diagnosis and outcome.7, 8 Following integration of AS-DT into admission EMR-based order sets, diagnostic testing increased and percentage of participants with specified diagnosis increased, while percentage of both indeterminate diagnosis and LT decreased. LT utilization was reduced without increasing mortality. These efforts affirm the report from the Institute of Medicine on “Improving Diagnosis in Health Care” that asserted errors in establishing the correct diagnosis occur and may have lasting consequences, and efforts to improve the diagnostic process should be implemented.16
An established diagnosis enables caregivers to focus on the child’s disease, it’s treatment, and associated outcome. Yet efforts to confirm a correct diagnosis remain imperfect. Diagnostic error rates have never been reported in ALF in children or adults, but can range from 1–20% when autopsy findings or simulated patients are anonymously evaluated.17 Errors in diagnosis result from a failure in one or several steps including incomplete or incorrect interpretation of medical records or history, inaccuracies in physical findings, deficient diagnostic considerations, flawed communication or clinical reasoning skills, and misinterpreting results of clinical, radiological or histopathological tests.18–21 Mild to moderate patient harm resulted as a consequence of diagnostic delay or additional diagnostic testing in up to 50% of diagnostic errors in adults with cancer.21 Improved diagnostic accuracy in PALF may yield opportunities for disease specific therapy, identify contraindications to LT, and prevent the harmful consequences of unnecessary LT.
PALF participants with an indeterminate diagnosis are without a reliable distinguishing clinical feature.5 Uncertainties regarding treatment and clinical course embedded in the indeterminate cohort may influence management decisions to err on the side of LT to avoid death or irreversible morbidities. In fact, Kings College Criteria include non-A, non-B hepatitis (e.g., indeterminate) among the risk factors associated with increased mortality in non-acetaminophen ALF.22 Thus, it is not surprising that LT is more likely to occur in patients with an indeterminate vs specific established diagnosis.5 Given these unique clinical circumstances associated with PALF, the importance and urgency to establishing a diagnosis is apparent.
PALF investigators were successful in decreasing the percentage of participants with an indeterminate diagnosis in part due to a greater percentage of participants with diagnoses of HSV, enterovirus, mitochondrial disease, HLH and GALD following integration of diagnostic recommendations. Early identification of HSV GALD, HLH, or acute acetaminophen toxicity leads to potentially life-saving targeted medical interventions and treatments.14, 23–26 Conversely, a diagnosis of mitochondrial hepatopathy with systemic manifestations, currently a relative contraindication to LT, represents proper stewardship of a scarce resource.27, 28 While our data cannot confirm a change in treatment or intervention followed the increase in known diagnoses, we assume the investigator would initiate specific treatment for an established diagnosis which would potentially impact patient outcome.
While a specific diagnosis may be associated with a good (e.g., acetaminophen toxicity) or poor (e.g., neonatal HSV) outcome, diagnosis alone is insufficient to predict outcome, as survival with native liver, death, and liver transplantation occur within all diagnostic categories. We know other factors such as encephalopathy 29 as well as immune and inflammatory responses provoked by inciting events 30, 31 participate in determining outcomes. Differences in patient management, variability of LT decisions, and organ availability also impact outcome.32 On June 18, 2013, United Network Organ Sharing policy entitled “Share 35” was implemented to improve access to deceased donor organs. The brief overlap of 18 months between implementation of Share 35 and the end of PALF follow-up precludes assessment of its impact on outcome. However, this policy should have made deceased donor organs more available with the potential to increase LT among the sickest patients, yet the percentage who underwent LT in P3 was less than in earlier phases. While changes in outcome identified in this analysis cannot be solely attributed to implementing AS-DT recommendations, reducing the prevalence of an indeterminate diagnosis may have made some impact on LT, without adversely affecting mortality.
A prioritized approach to diagnosis is a core principle in clinical medicine.33, 34 However, as knowledge and experience evolve, modifying and interpreting what constitutes best clinical practice is expected; interpreting early diagnostic testing is no exception.34 For example, subsequent to initiating AS-DT elements, positive tests for autoantibodies were determined to be not specific for autoimmune hepatitis in PALF.35 This is not to say autoantibody testing should be abandoned. Rather, in the absence of a gold standard diagnosis for autoimmune hepatitis in the setting of PALF, results need to be placed into clinical context that might include histologic findings or elevated immunoglobulins. An elevated serum lactate > 2.5 mmol/L or, more specifically, a molar ratio of lactate:pyruvate of at least 25 were established screening tests for mitochondrial hepatopathies when AS-DT was implemented.36–38 However, an increased lactate:pyruvate ratio was also found to be non-specific for mitochondrial disorders among PALF participants.39 Possible reasons for this finding include secondary disruption of respiratory chain function in the setting of ALF, presence of an undiagnosed mitochondrial disorder concomitant with development of ALF due to another known pathogenic condition, or altered fluid status and tissue perfusion associated with critically ill patients. In addition, integration of genetic testing using targeted next generation sequencing into AS-DT algorithms will be transformational as cost and turn-around time for results decrease.40 Therefore, AS-DT of children with ALF must not be static or inflexible, but should adapt to changes and improvements in diagnostic testing and assessed in the context of clinical expertise that may defy characterization by algorithms.
Limitations associated with studying a long-term observational cohort such as this one are unavoidable. Clinical, procedural, and LT decisions as well as designation of the final diagnosis were site and investigator dependent and subject to differences in clinical practice, patient referral patterns, consultant recommendations, organ availability, and other factors. Decisions to exclude, include, or expand elements of the recommended minimal age-specific diagnostic evaluation were site- and investigator-dependent and not protocol-driven. Improvements in, or availability of, diagnostic tests as well as maturation of clinical reasoning likely occurred over the study period, which may contribute to an ascertainment bias. Changes in practice at the enrolling sites may have an impact on the nature of the cohort including baseline demographics and changes in the duration of follow-up may impact outcome determinates. As the comparative study was limited to participants in North America, these findings may not be generalizable to other regions of the world.
In conclusion, within this cohort of PALF participants, integrating EMR-based AS-DT at hospital admission was associated with enhanced diagnostic specificity and a commensurate reduction in indeterminate diagnoses. The percentage of participants undergoing LT within 21 days decreased without a change in mortality. Widespread utilization of EMR-based AS-DT in PALF may improve outcomes in PALF and enhance the utilization of an invaluable limited resource, the donor liver.
Supplementary Material
Supplemental Table 1. Whitington Scale for encephalopathy in children less than 3 years of age.
Supplemental Table 2. Pediatric Acute Liver Failure Study Group Clinical Sites
Acknowledgments
Funding for the project is provided by the National Institutes of Health (NIH-NIDDK U01 DK072146). Key individuals who have actively participated in the PALF studies include (by site): Current Sites, Principal Investigators and Coordinators – Robert H. Squires, MD, Kathryn Bukauskas, RN, CCRC, Madeline Schulte, RN, BSN, Clinical Research Coordinator (Children’s Hospital of Pittsburgh of UPMC, Pittsburgh, Pennsylvania); Michael R. Narkewicz, MD, Michelle Hite, MA, CCRC (Children’s Hospital Colorado, Aurora, Colorado); Kathleen M. Loomes, MD, Elizabeth B. Rand, MD, David Piccoli, MD, Deborah Kawchak, MS, RD, Christa Seidman, Clinical Research Coordinator (Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania); Rene Romero, MD, Saul Karpen, MD, PhD, Liezl de la Cruz-Tracy, CCRC (Emory University, Atlanta, Georgia); Vicky Ng, MD, Kelsey Hunt, Clinical Research Coordinator (Hospital for Sick Children, Toronto, Ontario, Canada); Girish C. Subbarao, MD, Ann Klipsch, RN, Sarah Munson, Clinical Research Coordinator (Indiana University Riley Hospital, Indianapolis, Indiana); Estella M. Alonso, MD, Lisa Sorenson, PhD, Susan Kelly, RN, BSN, Katie Neighbors, MPH, CCRC (Lurie Children’s Hospital of Chicago, Chicago, Illinois); Philip Rosenthal, MD, Shannon Fleck, Clinical Research Coordinator (University of California San Francisco, San Francisco, California); Mike A. Leonis, MD, PhD, John Bucuvalas, MD, Tracie Horning, Clinical Research Coordinator (University of Cincinnati, Cincinnati, Ohio); Norberto Rodriguez Baez, MD, Shirley Montanye, RN, Clinical Research Coordinator, Margaret Cowie, Clinical Research Coordinator (University of Texas Southwestern, Dallas, Texas); Simon P. Horslen, MD, Karen Murray, MD, Melissa Young, Clinical Research Coordinator, Heather Nielson, Clinical Research Coordinator, Jani Klein, Clinical Research Coordinator (University of Washington, Seattle, Washington); David A. Rudnick, MD, PhD, Ross W. Shepherd, MD, Kathy Harris, Clinical Research Coordinator (Washington University, St. Louis, Missouri).
Previous Sites, Principal Investigators and Coordinators – Saul J. Karpen, MD, PhD, Alejandro De La Torre, Clinical Research Coordinator (Baylor College of Medicine, Houston, Texas); Dominic Dell Olio, MD, Deirdre Kelly, MD, Carla Lloyd, Clinical Research Coordinator (Birmingham Children’s Hospital, Birmingham, United Kingdom); Steven J. Lobritto, MD, Sumerah Bakhsh, MPH, Clinical Research Coordinator (Columbia University, New York, New York); Maureen Jonas, MD, Scott A. Elifoson, MD, Roshan Raza, MBBS (Harvard Medical School, Boston, Massachusetts); Kathleen B. Schwarz, MD, Wikrom W. Karnsakul, MD, Mary Kay Alford, RN, MSN, CPNP (Johns Hopkins University, Baltimore, Maryland); Anil Dhawan, MD, Emer Fitzpatrick, MD (King’s College Hospital, London, United Kingdom); Benjamin L. Shneider, MD, Nanda N. Kerkar, MD, Brandy Haydel, CCRC, Sreevidya Narayanappa, Clinical Research Coordinator (Mt. Sinai School of Medicine, New York, New York); M. James Lopez, MD, PhD, Victoria Shieck, RN, BSN (University of Michigan, Ann Arbor, Michigan). The authors are also grateful for support from the National Institutes of Health (Edward Doo, MD, Director Liver Disease Research Program, and Averell H. Sherker, MD, Scientific Advisor, Viral Hepatitis and Liver Diseases, DDDN-NIDDK) and for assistance from members of the Data Coordinating Center at the University of Pittsburgh (directed by Steven H. Belle, PhD, MScHyg).
Grant support: National Institutes of Health (NIH-NIDDK U01 DK072146).
Abbreviations
- APAP
acetaminophen
- ALF
acute liver failure
- AS-DT
age-specific diagnostic testing
- ALKM
anti-liver kidney Microsomal antibody
- ANA
antinuclear antibody
- ASMA
anti-smooth muscle antibody
- AA
amino acids
- CSF
cerebral spinal fluid
- CIR
cumulative incidence rates
- DNA
deoxyribonucleic acid
- EMR
electronic medical record
- EN
encephalopathy
- EBV
Epstein Barr Virus
- FAO
fatty acid oxidation defects
- GALD
gestational alloimmune liver disease
- HAV
hepatitis A virus
- HBsAg
hepatitis B surface antigen
- HSV
herpes simplex virus
- HLH
hemophagocytic lymphohistiocytosis
- HHV-6
human herpes virus-6
- IL2R
interleukin-2 receptor
- INR
international normalized ratio
- IgG
Immunoglobulin G
- IgM
Immunoglobulin M
- LT
liver transplantation
- MRI
magnetic resonance imaging
- NIDDK
National Institute of Diabetes and Digestive and Kidney Disorders
- P1
phase 1
- P2
phase 2
- P3
phase 3
- PALF
pediatric acute liver failure
- PCR
polymerase chain reaction
- PT
prothrombin time
- VCA
viral capsule antigen
Footnotes
Disclosures:
Michael R Narkewicz: No conflicts
Simon Horslen: No conflicts
Regina M Hardison: No conflicts
Benjamin L Shneider:
Norberto Rodriguez-Baez:
Estella Alonso: No conflicts
Vicky Ng: No conflicts
Mike Leonis: No conflicts
Kathy Loomes: No conflicts
David Rudnick: No conflicts
Phil Rosenthal: No conflicts for this study; Gilead: Consulting, Research Grants; Abbvie: Consulting, Research Grants; BMS: Research Grant; Roche/Genentech: Consulting, Research Grant; Intercept: Consulting; Alexion: Consulting; Retrophin: Consulting, Speakers’ bureau; Albireo: Consulting; Audentes: Consulting
Rene Romero: No conflicts for this study; Gilead: Consulting
Girish C Subbarao: No conflicts
Ruosha Li: No conflicts
Steven H Belle: No conflicts
Robert H. Squires: No conflicts for this manuscript; Up-To-Date, Royalty
Author Contributions:
Study concept and design + data acquisition + analysis and interpretation + drafting manuscript + critical revision + obtained funding + administrative: Robert H. Squires, MD
Study concept and design + data acquisition + analysis and interpretation + drafting manuscript + critical revision: Michael R. Narkewicz, Norberto Rodriguez-Baez
Data acquisition + analysis and interpretation + drafting manuscript + critical revision: Simon Horslen, Benjamin Shneider, Estella Alonso
Data acquisition + analysis and interpretation + critical revision: Vicky Ng, Mike Leonis, Kathy Loomes, David Rudnick, Phil Rosenthal, Rene Romero, Girish C. Subbarao
Analysis and interpretation + drafting manuscript + critical revision + statistical analysis + administrative: Steven H Belle
Analysis and interpretation + drafting manuscript + critical revision + statistical analysis: Regina M. Hardison, Ruosha Li
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- 1.Squires RH, Jr, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R, Belle SH, Horslen S, et al. Clinical Course among Cases of Acute Liver Failure of Indeterminate Diagnosis. J Pediatr. 2016;171:163–70. e1–3. doi: 10.1016/j.jpeds.2015.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu BR, Zhang S, Narkewicz MR, et al. Evaluation of the liver injury unit scoring system to predict survival in a multinational study of pediatric acute liver failure. J Pediatr. 2013;162:1010–1016. e4. doi: 10.1016/j.jpeds.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moldovan B, Mentha G, Majno P, et al. Demographics and outcomes of severe herpes simplex virus hepatitis: a registry-based study. J Hepatol. 2011;55:1222–6. doi: 10.1016/j.jhep.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Narkewicz MR, Dell Olio D, Karpen SJ, et al. Pattern of diagnostic evaluation for the causes of pediatric acute liver failure: an opportunity for quality improvement. J Pediatr. 2009;155:801–806. e1. doi: 10.1016/j.jpeds.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marzullo L. An update of N-acetylcysteine treatment for acute acetaminophen toxicity in children. Curr Opin Pediatr. 2005;17:239–45. doi: 10.1097/01.mop.0000152622.05168.9e. [DOI] [PubMed] [Google Scholar]
- 7.Wolf MJ, Lee EK, Nicolson SC, et al. Rationale and methodology of a collaborative learning project in congenital cardiac care. Am Heart J. 2016;174:129–37. doi: 10.1016/j.ahj.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahle WT, Nicolson SC, Hollenbeck-Pringle D, et al. Utilizing a Collaborative Learning Model to Promote Early Extubation Following Infant Heart Surgery. Pediatr Crit Care Med. 2016;17:939–947. doi: 10.1097/PCC.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider CL, Cobb WS, Carbonell AM, et al. A collaborative approach reduces the learning curve and improves outcomes in laparoscopic nephrectomy. Surg Endosc. 2011;25:182–5. doi: 10.1007/s00464-010-1153-4. [DOI] [PubMed] [Google Scholar]
- 10.Whitington PFAE. Fulminant hepatitis and acute liver failure. In: DAK, editor. Paediatric Liver Disease. Oxford: Blackwell; 2003. pp. 107–26. [Google Scholar]
- 11.Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–21. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 12.Knisely AS, Mieli-Vergani G, Whitington PF. Neonatal hemochromatosis. Gastroenterol Clin North Am. 2003;32:877–89. vi–vii. doi: 10.1016/s0889-8553(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 13.Bonilla S, Prozialeck JD, Malladi P, et al. Neonatal iron overload and tissue siderosis due to gestational alloimmune liver disease. J Hepatol. 2012;56:1351–5. doi: 10.1016/j.jhep.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz KB, Dell Olio D, Lobritto SJ, et al. Analysis of viral testing in nonacetaminophen pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2014;59:616–23. doi: 10.1097/MPG.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundaram SS, Alonso EM, Narkewicz MR, et al. Characterization and outcomes of young infants with acute liver failure. J Pediatr. 2011;159:813–818. e1. doi: 10.1016/j.jpeds.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Improving dignosis in health care. 2015;2017 [Google Scholar]
- 17.Graber ML. The incidence of diagnostic error in medicine. BMJ Qual Saf. 2013;22(Suppl 2):ii21–ii27. doi: 10.1136/bmjqs-2012-001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGlynn EA, McDonald KM, Cassel CK. Measurement Is Essential for Improving Diagnosis and Reducing Diagnostic Error: A Report From the Institute of Medicine. JAMA. 2015;314:2501–2. doi: 10.1001/jama.2015.13453. [DOI] [PubMed] [Google Scholar]
- 19.Singh H, Graber ML. Improving Diagnosis in Health Care--The Next Imperative for Patient Safety. N Engl J Med. 2015;373:2493–5. doi: 10.1056/NEJMp1512241. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald R. Error in radiology. Clin Radiol. 2001;56:938–46. doi: 10.1053/crad.2001.0858. [DOI] [PubMed] [Google Scholar]
- 21.Raab SS, Grzybicki DM. Quality in cancer diagnosis. CA Cancer J Clin. 2010;60:139–65. doi: 10.3322/caac.20068. [DOI] [PubMed] [Google Scholar]
- 22.O’Grady JG, Alexander GJ, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439–45. doi: 10.1016/0016-5085(89)90081-4. [DOI] [PubMed] [Google Scholar]
- 23.Rand EB, Karpen SJ, Kelly S, et al. Treatment of neonatal hemochromatosis with exchange transfusion and intravenous immunoglobulin. J Pediatr. 2009;155:566–71. doi: 10.1016/j.jpeds.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Stefanou C, Tzortzi C, Georgiou F, et al. Combining an antiviral with rituximab in EBV-related haemophagocytic lymphohistiocytosis led to rapid viral clearance; and a comprehensive review. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-216488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackford MG, Felter T, Gothard MD, et al. Assessment of the clinical use of intravenous and oral N-acetylcysteine in the treatment of acute acetaminophen poisoning in children: a retrospective review. Clin Ther. 2011;33:1322–30. doi: 10.1016/j.clinthera.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Alonso EM, Horslen SP, Behrens EM, et al. Pediatric acute liver failure of undetermined cause: A research workshop. Hepatology. 2017;65:1026–1037. doi: 10.1002/hep.28944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mindikoglu AL, King D, Magder LS, et al. Valproic acid-associated acute liver failure in children: case report and analysis of liver transplantation outcomes in the United States. J Pediatr. 2011;158:802–7. doi: 10.1016/j.jpeds.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Squires RH, Ng V, Romero R, et al. Evaluation of the pediatric patient for liver transplantation: 2014 practice guideline by the American Association for the Study of Liver Diseases, American Society of Transplantation and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Hepatology. 2014;60:362–98. doi: 10.1002/hep.27191. [DOI] [PubMed] [Google Scholar]
- 29.Ng VL, Li R, Loomes KM, et al. Outcomes of Children With and Without Hepatic Encephalopathy From the Pediatric Acute Liver Failure Study Group. J Pediatr Gastroenterol Nutr. 2016;63:357–64. doi: 10.1097/MPG.0000000000001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bucuvalas J, Filipovich L, Yazigi N, et al. Immunophenotype predicts outcome in pediatric acute liver failure. J Pediatr Gastroenterol Nutr. 2013;56:311–5. doi: 10.1097/MPG.0b013e31827a78b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamora R, Vodovotz Y, Mi Q, et al. Data-Driven Modeling for Precision Medicine in Pediatric Acute Liver Failure. Mol Med. 2016:22. doi: 10.2119/molmed.2016.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Squires JE, Rudnick DA, Hardison RM, et al. Liver Transplantation in Pediatric Acute Liver Failure (PALF): Practices and Patient Characteristics. Hepatology. 2017 [Google Scholar]
- 33.Bowen JL. Educational strategies to promote clinical diagnostic reasoning. N Engl J Med. 2006;355:2217–25. doi: 10.1056/NEJMra054782. [DOI] [PubMed] [Google Scholar]
- 34.Norman G. Research in clinical reasoning: past history and current trends. Med Educ. 2005;39:418–27. doi: 10.1111/j.1365-2929.2005.02127.x. [DOI] [PubMed] [Google Scholar]
- 35.Narkewicz MR, Horslen S, Belle SH, et al. Prevalence and Significance of Autoantibodies in Children With Acute Liver Failure. J Pediatr Gastroenterol Nutr. 2017;64:210–217. doi: 10.1097/MPG.0000000000001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helbling D, Buchaklian A, Wang J, et al. Reduced mitochondrial DNA content and heterozygous nuclear gene mutations in patients with acute liver failure. J Pediatr Gastroenterol Nutr. 2013;57:438–43. doi: 10.1097/MPG.0b013e31829ef4b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lane M, Boczonadi V, Bachtari S, et al. Mitochondrial dysfunction in liver failure requiring transplantation. J Inherit Metab Dis. 2016;39:427–36. doi: 10.1007/s10545-016-9927-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Debray FG, Mitchell GA, Allard P, et al. Diagnostic accuracy of blood lactate-to-pyruvate molar ratio in the differential diagnosis of congenital lactic acidosis. Clin Chem. 2007;53:916–21. doi: 10.1373/clinchem.2006.081166. [DOI] [PubMed] [Google Scholar]
- 39.Feldman AG, Sokol RJ, Hardison RM, et al. Lactate and Lactate: Pyruvate Ratio in the Diagnosis and Outcomes of Pediatric Acute Liver Failure. J Pediatr. 2017;182:217–222. e3. doi: 10.1016/j.jpeds.2016.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicastro E, D’Antiga L. Next generation sequencing in pediatric hepatology and liver transplantation. Liver Transpl. 2018;24:282–293. doi: 10.1002/lt.24964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Whitington Scale for encephalopathy in children less than 3 years of age.
Supplemental Table 2. Pediatric Acute Liver Failure Study Group Clinical Sites