Abstract
Cathinone is a plant alkaloid found in khat leaves of perennial shrubs grown in East Africa. Similar to cocaine, cathinone elicits psychostimulant effects which are in part attributed to its amphetamine-like structure. Around 2010, home laboratories began altering the parent structure of cathinone to synthesize derivatives with mechanisms of action, potencies, and pharmacokinetics permitting high abuse potential and toxicity. These “synthetic cathinones” include 4-methylmethcathinone (mephedrone), 3,4-methylenedioxypyrovalerone (MDPV), and the empathogenic agent 3,4-methylenedioxymethcathinone (methylone) which collectively gained international popularity following aggressive online marketing as well as availability in various retail outlets. Case reports made clear the health risks associated with these agents and, in 2012, the Drug Enforcement Agency of the United States placed a series of synthetic cathinones on Schedule I under emergency order. Mechanistically, cathinone and synthetic derivatives work by augmenting monoamine transmission through release facilitation and/or presynaptic transport inhibition. Animal studies confirm the rewarding and reinforcing properties of synthetic cathinones by utilizing self-administration, place conditioning, and intracranial self-stimulation assays and additionally show persistent neuropathological features which demonstrate a clear need to better understand this class of drugs. This Review will thus detail (i) historical context of cathinone use and the rise of “dark” synthetic derivatives, (ii) structural features and mechanisms of synthetic cathinones, (iii) behavioral effects observed clinically and in animals under controlled laboratory conditions, and (iv) neurotransmitters and circuits that may be targeted to manage synthetic cathinone abuse in humans.
Keywords: Addiction, cathinone, designer drugs, dopamine, novel psychoactive substance(s), reward, synthetic cathinone(s)
Graphical abstract
CATHINONE AND THE RISE OF SYNTHETIC DERIVATIVES
Cathinone is a phenylalkylamine derived from leaves of the khat perennial shrub (Catha edulis) that produces psychostimulant effects. Khat leaves are chewed by locals residing in East Africa and the Arabian Peninsula for ability to allay fatigue similarly to how cocaine-containing coca leaves of the C. erythroxylaceae shrub are chewed along trails of the Andes Mountains; leaf chewing in this manner is culturally normative and seen as relatively nonproblematic. Khat leaves appear to have a written history of being chewed by natives beginning in the 14th century, and the custom remains commonplace to date.1 Based on self-reports, khat leaves are chewed for subjective improvements in concentration and libido.2 In an analysis of 8900 responses from secondary school and college-aged students in Saudi Arabia, over one-fifth reported having chewed khat leaves with the majority attributed to male respondents (37.7%) compared to females (3.8%).3 Although (−)-cathinone is self-administered by rodents,4 purified forms of cathinone do not appear to be widely abused in humans, likely as a result of its moderate potency and rapid degradation to cathine during storage.5 These unfavorable characteristics may have played a role in the development of the first synthetic cathinone analogue methcathinone (ephedrone), which was originally synthesized by Hyde and colleagues6 and subsequently marketed as an antidepressant in the Soviet Union and a CNS stimulant in the United States7 Reports of methcathinone abuse surfaced in the United States and elsewhere in the early 1990s,8,9 leading to its placement (along with its parent compound cathinone) into Schedule I controlled substance status in the United States.
Structurally, cathinone possesses striking resemblance to the classically abused agent phenylisopropylamine (amphetamine) and the empathogenic amphetamine derivative 3,4-methylene-dioxymethamphetamine (MDMA). Capitalizing on comparable structures, clandestine laboratories altered motifs of cathinone to produce synthetic agents which have been classified under the umbrella of “novel psychoactive substances” (NPS). Many synthetic cathinones are highly abused with evident toxicity conferring their “dark” nature. Common modifications of cathinone’s parent structure include alkyl or halogen substitutions at points along the aromatic ring (e.g., 4-methylmethcathinone [4-MMC; mephedrone], 4-fluoromethcathinone [flephedrone]; Figure 1) as well as methylenedioxy substitutions (e.g., 3,4-methylenedioxy-N-methylcathinone [methylone]; Figure 2) and pyrrolidinyl substitutions (e.g., α-pyrrolidinovalerophenone [α-PVP]) or a combination therein (e.g., 3,4-methylenedioxypyrovalerone [MDPV]; Figure 3). The United Nations’ Convention on Psychotropic Substances recognized cathinone and its methylated analogue (methcathi-none, the first synthetic cathinone) as potentially harmful and abuse-prone, leading to Schedule I placement in 1971.
Figure 1.
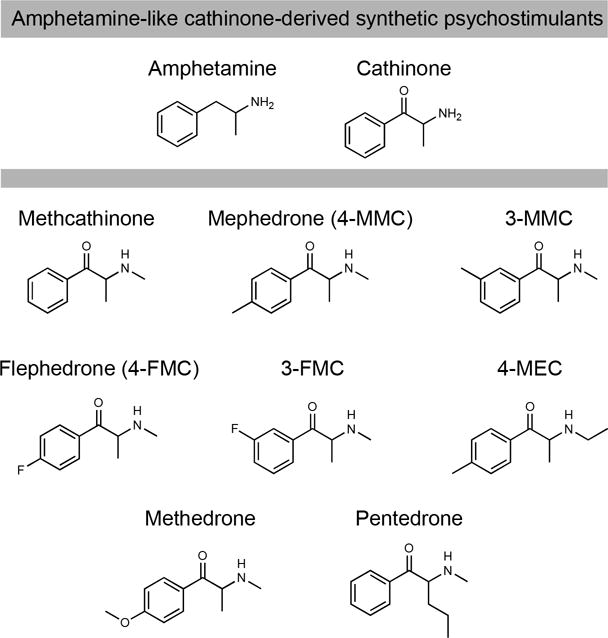
Amphetamine-like cathinone-derived synthetic psychostimulants including methcathinone, “first-generation” mephedrone and its positional isomer 3-MMC, flephedrone and 3-FMC, 4-MEC, methedrone, and pentedrone. Typically, synthetic cathinones in this class possess substitutions along cathinone’s benzene ring.
Figure 2.
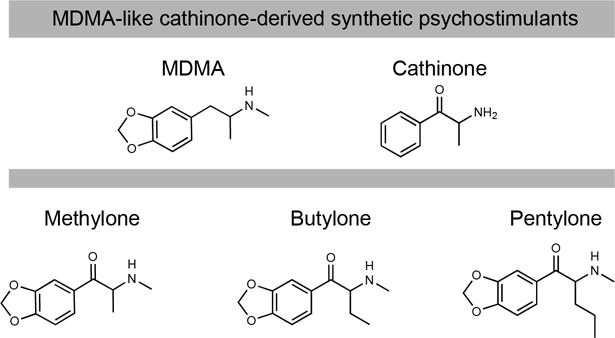
MDMA-like cathinone-derived synthetic psychostimulants including methylone, butylone, and pentylone. Typically, synthetic cathinones in this class contain a methylenedioxy-substitution adjacent to cathinone’s benzene ring.
Figure 3.
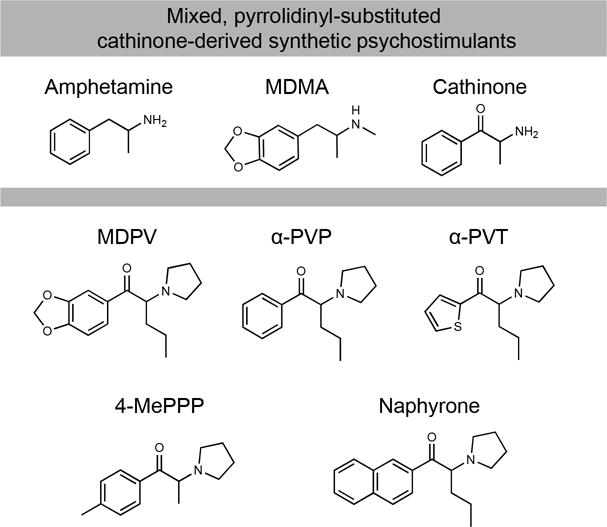
Mixed amphetamine- and MDMA-like cathinone-derived synthetic psychostimulants including MDPV, α-PVP and -PVT, 4-MePPP, and naphyrone. Synthetic cathinones in this class share a benzene ring substitution (methylenedioxy or other) as well as a pyrrolidinyl group along cathinone’s tertiary carbon atom.
The rise in synthetic cathinone production and aggressive marketing arrived on an international scale beginning around 2010. Around then, synthetic cathinones were advertised and sold online and as fictitious products such as “plant food” and “bath salts”, and marked “not for human consumption” to bypass regulations imposed by the Food and Drug Administration. By 2013, over 600 “dark web” sites had been identified in Europe that allowed traffickers and users to purchase these drugs anonymously with the use of untraceable digital currencies.10 In addition, online drug forums, social media plaftorms, and even drug retailers themselves provided users and customers with detailed information on specific product names, availability, slang terminology, packaging imagery, drug effects, efficacy, dosages, and legal status.11,12 In 2011 alone, 6138 calls were made to the Poison Control Centers from emergency departments related to synthetic cathinone (“bath salt”) products.13 As specific chemical structures became scheduled, new compounds tended to rise in popularity to avoid risk of judicial prosecution. Specifically, whereas the National Forensic Laboratory Information System reported 21 analytically confirmed synthetic cathinone drugs in 2011, 42 different synthetic cathinone drugs were found in 2014.14 A Poland-based report documented that, while methyl-and methylenedioxy-substituted cathinones (e.g., mephedrone and methylone) were most abundant in seized samples from 2010, “simple cathinone” packages including α-methylaminovalerophenone (pentedrone) as well as positional isomers of former agents (e.g., 3-methylmethcathinone [3-MMC]) were analytically detected in greatest abundance from 2014 to 2015.15 Like cathinone itself, nearly all synthetic cathinones produce behavioral effects by augmenting monoamine transmission in central nervous system sites of action (i.e., dopamine [DA], noradrenaline [NA], serotonin [5-HT]) through amphetamine-like release facilitation and cocaine-like reuptake inhibition mechanisms. Euphoria and hallucination are met with adverse sympathomimetic effects which collectively make many synthetic cathinone drugs dangerously rewarding and reinforcing “dark” agents.
BIOCHEMICAL STRUCTURES AND NEUROPHYSIOLOGY OF SYNTHETIC CATHINONES
Structural Features
Cathinone’s striking resemblance to amphetamine diverges with the addition of a ketone body (carbon–oxygen double bond) at the β-carbon atom. The carbon atom adjacent to amphetamine’s (and cathinone’s) primary amine is stereogenic thus giving rise to two enantiomers with unique methyl group positional conformations along the z-plane. As such, many synthetic cathinones including mephedrone exist as pharmacologically distinct enantiomers, although racemic mixtures predominate illicit formulations. The backbone of amphetamine can be modified by replacing its hydrogen atoms, methyl groups, or amine groups with substituents=cathinone, in this way, is considered a substituted amphetamine (β-ketone-amphetamine). Also included in the domain of substituted amphetamines are methamphetamine and ephedrine as well as the psychedelics MDMA and 2,5-dimethoxy-4-methylamphetamine (DOM).16 Structural features of synthetic cathinones include substitutions with methyl, ethyl, butyl, isopropyl, methylenedioxy, pyrrolidinyl, fluoro, and benzyl groups against the cathinone backbone (for reviews, see refs 7 and 17). Many synthetic cathinones readily permeate the blood-brain barrier as determined in vitro with alarming high permeability noted for mephedrone and MDPV.18
Effects of Cathinone and Synthetic Derivatives on Brain Monoamine Transmission
Consistent with structural resemblance to amphetamine, cathinone and synthetic derivatives exert psychosomatic effects through augmentation of brain monoamine transmission. Cathinone was first shown to augment extracellular DA content by Peter Kalix in the early 1980s.19,20 Thereafter, cathinone was found to block synaptosomal DA uptake sites (i.e., dopamine transporters [DAT]) at comparable efficacy compared to d-amphetamine (Adderall) while decreasing DAT content following chronic exposure.21 When examining enantiomeric differences, (−)-cathinone was found to possess approximately 3-fold greater potency at blocking central DATs compared to (+)-cathinone whereas both enantiomers significantly enhanced extracellular NA in peripheral tissues.22,23 Pehek and colleagues24 were the first to use in vivo microdialysis to confirm that cathinone produced amphetamine-like elevations in extracellular DA content within the nucleus accumbens (NAcc) of ventral striatum—a central site of action for psychostimulant-associated reward and reinforcement implicated in the development of drug dependence. Cathinone appreciably elevates striatal DA and NA in an enantiomer-sensitive manner which likely drives, at least in part, the pervasiveness of khat leaf chewing in/around East Africa and the Arabian Peninsula.
Synthetic cathinones share similar mechanistic features as the parent compound. Cathinone’s methylated analogue, methcathinone, potently augments extracellular DA content at rates comparable to methamphetamine.25 Other work finds that systemic mephedrone significantly decreases synaptic uptake of striatal DA and, with greater persistence, hippocampal 5-HT content.26 In vivo, both mephedrone and the MDMA-like agent methylone significantly elevate ventral striatal DA and 5-HT.27 Similarly, intravenous MDPV was observed to significantly elevate DA and NA in vivo with approximately 10-fold greater potency compared to intravenous cocaine.28 Electrophysiological study additionally finds that bath-applied MDPV inhibits clearance of DA within mouse striatal slices after delivery of 5–25 Hz electrical pulses. MDPV also enhances DAT currents in both magnitude and duration.29 The “second generation” synthetic cathinone α-PVP also elicits elevations in striatal DA in vivo.30 Many first- and second-generation synthetic cathinones, like the parent compound itself, augment central DA content albeit at varying potencies which likely contributes to differential rates of abuse.
The MDMA-like agents methylone, β-keto-N-methylbenzo-dioxolylbutanamine (butylone), and β-keto-N-methylbenzodioxolylpentanamine (pentylone) induce inward currents at serotonin transporters (SERTs) and dissociate slowly.31 MDMA-like synthetic cathinones are suspected to produce persistent leak currents at SERT comparable to effects of amphetamine on DATs.32 Described later in this review, MDMA-like agents typically show relatively weak reinforcing effects compared to amphetamine-like comparator agents which is likely due to DAT vs SERT selectivity.
It has been proposed that synthetic cathinones including mephedrone activate DATs and in turn induce depolarizing currents that trigger vesicle fusion and DA release similarly to mechanisms ascribed to amphetamine and cocaine (for review, see ref 9). This “DAT hypothesis” suggests that mephedrone, like amphetamine, binds directly to DAT to activate the catecholamine transport pore and, in turn, allows the bound agent (and sodium) to enter the presynaptic terminal to facilitate vesicular fusion and DA release. However, because studies measuring extracellular DA content show that mephedrone-elicited elevations are lesser than those compared to amphetamine, we suggest that mephedrone may have a relatively weaker affinity for DAT although to our knowledge these protein–protein interactions (i.e., mephedrone/amphetamine-DAT) have not been directly compared.
BEHAVIORAL EFFECTS OF SYNTHETIC CATHINONES DETERMINED FROM CASE REPORTS AND CONTROLLED LABORATORY EXPERIMENTS
As indicated above, cathinone and synthetic derivatives structurally and mechanistically resemble psychostimulants amphetamine and cocaine as well as the empathogen MDMA. Accordingly, euphorigenic effects are typically produced following synthetic cathinone use but are experienced alongside agitation, anxiety, and aggression typified by psychostimulant abuse. The following subsections will detail behavioral effects of cathinone and synthetic derivatives as have been captured in case reports. Thereafter, descriptions of behavioral assays used in animal studies to probe cathinone-related reward and reinforcement under controlled laboratory conditions, as well as effects captured in those studies, will be provided.
Clinical Features and Case Studies
Khat leaf chewing often occurs in social settings which tends to buffer otherwise negative effects including anxiety, irritability, and aggressiveness. Somatically, khat leaf chewing can lead to tachycardia, tachypnea, mydriasis, flushing, and headache as a result of cathinone’s sympathomimetic properties. Of perceived benefit, khat leaf chewing reportedly produces euphoria, elation, allayed fatigue, and increased alertness and is generally believed to be a mood elevator. In three vignettes provided by Granek and colleagues,33 clinically diagnosed hypnagogic hallucinations from khat leaf chewers of 25+ years were described, consistent with earlier anecdotal links between khat leaf chewing and schizophreniform psychosis (e.g., ref 34). The authors reason that, while acute effects of khat leaf chewing are relatively innocuous, chronic use permits greater risk of psychotic episodes warranting clinical attention. Separately, stimulant-like effects of khat leaf chewing can be followed by debilitating rebound effects including lethargy and sleepiness. One case report described the danger of utilizing prescription sedatives (diazepam, in this case) to combat insomnia following episodes of khat leaf chewing in chronic users.35 In this report, a 35-year-old man was admitted to an emergency department following a midafternoon comatose state attributed to the combination of excessive khat leaf chewing and diazepam which, in turn, was suspected to lead to aspiration pneumonia followed by acute respiratory distress syndrome (ARDS). Khat leaf chewing is not without risk, but these risks are far less severe relative to those associated with synthetic cathinone use.
Administering synthetic cathinones can produce adverse risks ranging from local tissue injury to death following multiorgan failure. Injection-related soft tissue complications are summarized by Dorairaj and colleagues36 and include cellulitis, vein clotting, abscess formation, and muscle necrosis which can be compounded by poor hygiene, nonsterile injection practices, and lack of injection site asepsis. Injection-related skin infections can often be managed by antibiotic treatment; in extreme cases, surgical debridement may be needed. Administration of synthetic cathinones can produce subjective effects including delusional thoughts, hallucinations, agitation, and aggression as well as, at high doses, severe somatic symptoms resembling cocaine and MDMA overdose including sympathomimetic toxicity and serotonin syndrome. In a recent online survey of self-reported synthetic cathinone users within the United States, subjective effects of greatest frequency included feelings of stimulation/energy, euphoria, improved focus, suppressed appetite, and enhanced sex drive.37 Additionally, self-reported undesired effects included elevated heartrate, excess sweating, nervousness/anxiety, and paranoia.
In one of the first case reports related to synthetic cathinone use, Belhadj-Tahar and Sadeg38 describe the misfortune of a 29-year-old woman who was admitted to the emergency department following a toxicologically verified methcathinone-potentiated coma presented with mydriasis and hyperpnea. The first “pure” medical case report of mephedrone toxicity was published in 2010 and described the emergence of palpitations, sweating, chest pressure, blurred vision, and sympthaomimetic toxicity following intramuscular injection of 3.8 g of mephedrone in a 22-year-old man.39 Consumption of just 100 mg of naphthylpyrovalerone (naphyrone) led to clinical diagnosis of sympathomimetic toxidrome in a 31-year-old patient who acutely suffered from insomnia, hallucinations, and “mortal fear”.40 Methylone was linked directly to sudden cardiac death in a 19-year-old user41 consistent with prior MDMA-related fatality from serotonin syndrome, disseminated intravascular coagulopathy, and anoxic encephalopathy.
Abuse of MDPV was reported in a case report documenting a 25-year-old who was “markedly combative and foaming at the mouth”.42 The patient was hyperthermic, hypertensive and mydriatic upon arrival to the emergency department and subsequently developed renal failure and rhabdomyolysis indicative of multi organ failure although eventually recovered after a prolonged hospital stay. In addition to violent behaviors, MDPV has also been reported to lead to paranoid delusions and hallucinatory delirium which present management obstacles as methods to control erratic behaviors including physical restraints, tasers, and antipsychotics can worsen somatic toxicity.43 Many users who begin using synthetic cathinones following a history of cocaine and methamphetamine use tend to experience intensified neurological/psychiatric conditions.44 Indeed, a 39-year-old man with a history of drug and alcohol use was taken to the emergency department after MDPV use in a hyperthermic (peak fever recorded at 107.1 °F) and tachycardic state; after cooling efforts, he became severely bradycardic and died ∼12 h after admission.45 Collectively, these studies illustrate lethal risk of synthetic cathinone use in human users. To more precisely characterize which domains of behavior synthetic cathinones influence, we turn our attention to animal studies conducted under controlled laboratory conditions.
Behavioral Effects of Synthetic Cathinones in Animal Subjects
Numerous behavioral assays are utilized by preclinical investigators to probe the rewarding and reinforcing effects of drugs that are abused by humans. These behavioral tools in nonhuman animals, including measurement of locomotor activity, place conditioning, drug discrimination, intracranial self-stimulation (ICSS), and intravenous self-administration, help to comprehensively model substance use disorders as observed in human users. Equipped with these tools, preclinical investigators can evaluate neurobiological features that underlie the development to drug dependence. Additionally, intervention strategies including pharmacotherapy can be tested in effort to gauge therapeutic efficacy. This section will briefly describe aforementioned behavioral tools leading into detailed accounts of effects found from experimenter- and self-administered synthetic cathinones.
Locomotor Stimulation and Sensitization
Typical of sympathomimetic drugs, cathinone and synthetic derivatives elevate ambulatory/exploratory behavior as well as “stereotypy” which is often measured as vertical photobeam breaks in a testing arena. Repeated movements, whereby the test subject shuttles between two horizontal locations in a back-and-forth pattern, can additionally be measured. These measures provide simple, targetable behaviors to evaluate the ability of novel psychoactive agents to act in a psychostimulant-like manner. Work in cocaine-injected rats demonstrates a clear, positive relationship between cocaine-stimulated locomotor activity and elevations in ventral striatal DA content;46 follow-up work revealed that direct injection of cocaine within ventral striatum was sufficient to elevate locomotor activity.47 Early work also shows that khat plant extract and purified cathinone acutely elevate locomotor activity in laboratory animals, and that (−)-cathinone is the more potent locomotor-activating enantiomer.19,48 Cathinone-elicited hyperlocomotion is mediated in part by effects on central nervous system sites of action as intracerebroventricular injection of cathinone also significantly elevated ambulation.49
In testing a series of synthetic cathinones, Gatch and colleagues50 showed hyperlocomotor effects in mice after injection of mephedrone, MDPV, and methylone as well as “newer” agents flephedrone, naphyrone, and butylone: robust and persistent effects were noted across the 8 h testing session following injections of MDPV and naphyrone. Gregg and colleagues51 reported that R-mephedrone more potently elevated locomotor activity compared to its racemate S-mephedrone. Significant elevations in locomotor activity were additionally found following injection of 3-fluoromethcathinone (3-FMC) and 4-methoxymethcathinone (methedrone) with greater potency ascribed to the former psychoactive agent.52 Fantegrossi and colleagues53 reported significant locomotor-stimulating effects of systemic MDPV in mice but only under relatively warm ambient conditions (∼28 °C). In a different study, systemic injection of MDPV (1.0 mg/kg, IP) elicited comparable elevations in locomotor activity compared to methamphetamine-injected rats but failed to appreciably elevate core body temperature.54 Other synthetic cathinones including α-PVP and pentedrone were shown to significantly elevate locomotor activity.30,55 Synthetic cathinones appear thus to invariably elevate motor activity in animal subjects albeit with different potencies.
Repeated injections of a psychostimulant often lead to the development of a sensitized behavioral response, typically measured by locomotor activity (ambulation), after many injections compared to initial responses. Sensitization to locomotor-stimulating effects of cathinone and mephedrone were reported from rats receiving a multiday repeated injection regimen.56,57 Mephedrone was shown to produce sensitization of repetitive movements (i.e., consecutive photobeam breaks) using a 7 day variable-dose injection regimen, and greater potency in inducing behavioral sensitization was attributed to mephedrone’s R-enantiomer.51,58 MDPV also produces sensitization to repetitive movements using the variable-dose injection regimen.59 Other studies find that mephedrone- or MDPV-injected animal subjects ambulate at appreciably greater rates when challenged subsequently with either cocaine or methamphetamine suggesting psychostimulant cross-sensitization.60,61 The development of locomotor sensitization is characteristic of cocaine and amphetamine, and the above-mentioned experiments additionally support that synthetic cathinones produce this behavioral signature.
Reward: Effects on Affect/Mood
Psychostimulants “prime” brain reward function during assessment of intracranial self-stimulation (ICSS) in animal subjects. Briefly, ICSS utilizes an intracranially implanted stimulating electrode lowered to a structure or fiber bundle known to support operant reinforcement. Stimulation current required to support self-stimulation is often taken as a metric of hedonic state (“reward threshold”) whereas other teams quantify the rate of responding at a fixed current. Early evidence finds that systemic mephedrone (10 mg/kg, IP) promotes ICSS in mice at lower current intensities relative to cocaine-elicited responding at 15–30 min post-injection.62 This study supports that mephedrone is capable of priming brain reward function but may take a relatively longer time to reach central sites of action relative to cocaine. Not long thereafter, Watterson and colleagues63 demonstrated significant facilitation of ICSS following MDPV injection (0.1 to 2.0 mg/kg, IP) in rats. Indeed, numerous other synthetic cathinones including methcathinone and methylone were shown to facilitate self-stimulation upon acute injection [ref 64, but see ref 65]. Interestingly, high-dose methylone and mephedrone (10 mg/kg, IP) were shown to reduce response rates below vehicle-injected control levels suggesting a suppression of brain reward function. Taken together, synthetic cathinones generally promote electrical self-stimulation behavior in rodents supporting their ability to facilitate brain reward function.
Place conditioning can also be used to probe conditioned reward processes attributed to an experimental treatment. Typically, a two-chamber shuttle apparatus is used whereby each chamber is distinguished by a constellation of tactile, visual and olfactory cues. An experimental treatment, such as synthetic cathinone injection, is paired with one chamber and a control treatment such as saline is paired with the other chamber. A preference score is then determined by measuring the amount of time tested subjects spend on each of the two chambers when subjects are provided a choice between the two chambers in a drug-free state. Only a few reports describe the ability of cathinone and synthetic derivatives to induce place preference in animal subjects. The first study was provided by Schechter66 and showed that systemic injections of (−)-cathinone every other day across an 8 day injection regimen induced significant place preference in rats compared to the saline-paired alternative context. Mephedrone induces place preference which appears to be attributed to the R-enantiomer as rats did not show preference toward a context paired with S-mephedrone.51 MDPV (2 and 5 mg/kg, IP) produced place preference across a 4-day injection schedule.59 Other reports find that pentedrone (3.0 and 10.0 mg/kg, IP) elicits significant place preference at rates comparable to methamphetamine (1 mg/kg, IP). Significance place conditioning was additionally observed for the phenyl/thiophene substitute of α-PVP, α-pyrrolidinopentiothiophenone (α-PVT).67
In an alternative test design, drug discrimination is used to compare the interoceptive stimulus effects of injected drugs by measuring the extent to which a new drug is responded for relative to a comparator agent (often cocaine, amphetamine, or MDMA). Drug discrimination can further apply to stereoisomers of a given agent; for example, l-amphetamine discriminates for d-amphetamine at high doses whereas nicotine, mescaline, and fenfluramine fail to produce stimulus discrimination effects.68 Kalix and Glennon69 were among the first to show that (−)-cathinone fully substitutes for systemic amphetamine unlike other tested aminophenones. Methcathinone fully and potently substitutes against racemic amphetamine; in a follow-up study examining enantiomeric differences, S-methcathinone was revealed as the potent enantiomer producing amphetamine-like stimulus discrimination.25,70 Kohut and colleagues71 observed that methcathinone possesses cocaine-like potency in rhesus monkeys trained in drug discrimination. Methylone failed to fully substitute for amphetamine but retained MDMA-like discriminative stimulus effects.72 Comparing across drug types, Fantegrossi and colleagues53 observed that MDPV fully substituted for both methamphetamine and MDMA with 50% efficacy (ED50) achieved ∼0.03 mg/kg across both comparator drugs. “Second-generation” synthetic cathinones α-PVP, α-PVT and 4-methyl-α-pyrrolidinopropiophenone (4-MePPP) fully substituted for methamphetamine whereas 4-methyl-N-ethylcathinone (4-MEC) failed to discriminate suggesting a unique interoceptive experience relative to methamphetamine.67,73 A second report revealed substitution of 4-MEC for methamphetamine but at appreciably higher doses (50.0 mg/kg, IP) compared to those used by Naylor and colleagues (2015; 1.0–8.0 mg/kg, IP).74 Collectively, drug discrimination has aided our understanding as to the degrees to which novel synthetic psychostimulants resemble the subjective experience attributed to classically abused agents such as cocaine, amphetamine and MDMA.
In effort to capture additional measures noninvasively in existing behavioral experiments, ultrasonic vocalizations (USVs) have proven of great utility in psychostimulant research (for review, see ref 75). USVs are high-pitched vocal emissions produced by constriction and stabilization of the larynx. Rats typically elicit USVs as an intraspecies communication tool which signal “avoid-approach” behaviors. Notably, USVs are often dichotomized based on frequency of emission: 22 kHz USVs are often long, monotonous and are emitted under experimental conditions associated with affective distress including presentation of a predator, electrical foot-shock, and withdrawal from abused drugs including cocaine.76–78 Conversely, 50 kHz USVs are often shorter, acoustically varied and are emitted under appetitive experimental conditions including electrical brain stimulation, mating opportunity, and acute administration of psychostimulants including cocaine [e.g., refs 79–81]. In an initial demonstration, our team found that systemic MDPV elicits comparable yet more persistent rates of 50 kHz USVs compared to cocaine at one-tenth the dose.82 As is mentioned below, intravenous self-administration of MDPV additionally elicits 50-kHz USVs at robust rates including from exposure to the MDPV-paired context in the absence of drug receipt.
Finally, several teams have interrogated effects of synthetic cathinones on anxiety-like behavior in rodents. den Hollander and colleagues83 failed to observe appreciable effects of binge-like mephedrone or methylone on metrics interpreted as reflecting anxiety-like behavior (i.e., % time in open arms of an elevated plus- or zero-maze). Repeated injections of MDPV, however, produced an anxiogenic effect in rats tested 72 h following the last injection.84 Thus, the negative emotional states emerging following synthetic cathinone use may be mediated by dynamic fluctuations in DA/NA levels which MDPV most robustly influences. Indeed, the lasting negative affective states produced by psychostimulants are commonly attributed to alterations in mesolimbic DA activity and effects therein on reward processing [e.g., ref 85].
Reinforcement As Assessed by Intravenous Self-Administration
Intravenous drug self-administration remains a model with face and construct validity that measures abuse potential and captures the volitional aspect of drug-taking. Nearly all drugs that are abused in humans are reinforcing and self-administered intravenously in laboratory subjects including nonhuman primates and rats. In an early comparison study, Johanson and Schuster86 observed greater reinforcing effects of l-cathinone against both the racemic mixture and d-amphetamine in intravenously self-administering monkeys. Both forms of cathinone showed broader inverted U-shaped dose–response curves and greater peak responding compared to d-amphetamine in tested monkeys. Similar work performed by Woolverton and Johanson87 supports the ability of intravenous cathinone to function as a positive reinforcer even when an intravenous cocaine option is presented. Gosnell and colleagues4 provided the first evidence that cathinone is actively self-administered in rats, and that the inverted U-shaped dose–response function was approximately 2-fold leftward shifted relative to intravenous cocaine, suggesting greater potency. Qualitative analysis on patterns of drug-taking revealed that, while intravenous cocaine was self-administered at relatively evenly distributed interinfusion intervals throughout sessions in well-trained rats, intravenous cathinone tended to be rapidly responded for early in sessions suggesting a pattern of use characterized by rapid “load-up” injections followed by even interinfusion intervals to titrate drug levels.
Effects of synthetic cathinones on operational reinforcement were investigated beginning in the early 1990s. Methcathinone was the first cathinone-derived synthetic agent shown to be intravenously self-administered at doses ranging from 0.1 to 1.0 mg/kg/inf.88 In this study, rates of responding were comparable to those performed for self-administered equipotent cocaine. Hadlock and colleagues26 showed that rats actively self-administer mephedrone (∼0.80 mg/kg/inf) commensurate with elevations in core body temperature and with significantly greater infusions earned across days 5–8 of daily 4 h self-administration sessions compared to saline infusions. Thereafter, Aarde and colleagues54 determined the half-life of mephedrone after ∼1.00 mg/kg/inf injection (IV) from plasma to be ∼1-h, and other work from our team observed greater reinforcement associated with R-mephedrone compared to its racemate.89
The MDMA-like agent methylone is self-administered by rats across a range of doses (0.05–0.50 mg/kg/inf), yet, unlike with intravenous cocaine, rats self-administer appreciably greater infusions of high-dose methylone compared to lower doses under low-effort, fixed-ratio 1 (FR-1) access conditions whereby one operant response performed during the availability period is rewarded with an intravenous drug injection.65 Watterson and colleagues63 additionally observed potently reinforcing effects of self-administered MDPV in rats at doses as low as 0.05 mg/kg/inf. Also captured in this study is a remarkably high motivational drive for intravenous MDPV as evidenced by rats responding 100+ times for single injections of MDPV under progressive-ratio (PR) access conditions, a schedule of reinforcement in which exponentially greater operant responses are needed to yield intravenous drug receipt. Compared to self-administered cocaine, MDPV demonstrated comparable reinforcing efficacy at one-tenth the dose, and the latency to begin MDPV self-administration was appreciably shorter compared to cocaine.82 Methylone possesses relatively weak reinforcing efficacy during intravenous self-administration compared to MDPV likely due to concomitant elevations in ventral striatal DA and 5-HT. In one of the only studies to demonstrate synthetic cathinone reinforcement in female test subjects, self-administered methylone was shown to have significantly lower reinforcing value compared to response rates for intravenous mephedrone.90 A second report by the same team91 additionally found that mephedrone is a more effective reinforcer compared to MDMA and methylone in self-administering male rats.
The “second-generation” α-alkyl derived synthetic cathinone α-PVP showed similar reinforcing efficacy during intravenous self-administration compared to MDPV.92 Moreover, α-PVP was shown to have greater reinforcing efficacy compared to pentedrone and pentylone although all tested agents were more reinforcing than methylone.93 In a different study, only one of four tested doses of pentedrone (0.3 mg/kg/inf) was appreciably self-administered by rats,55 suggesting a narrow dose–response function. 4-MePPP, a different pyrrolidinyl substituent synthetic cathinone, showed comparable reinforcing efficacy as α-PVP although with less potency.94 In this study, both α-PVP and 4-MePPP were more reinforcing than 4-MEC yet only α-PVP was revealed as more potent than methamphetamine. α-PVT showed cocaine-like reinforcing efficacy, reinforcing doses ranging from 0.1 to 1.0 mg/kg/inf in an inverted-U dose–response manner, in self-administering mice.67 Self-administration remains an excellent tool to capture the volitional aspect of drug-taking as is observed in humans and provides insight on potency and reinforcing efficacy of new synthetic drugs.
Cognitive Effects Associated with Synthetic Cathinone Use
Chronic use of psychostimulants such as cocaine, methamphetamine, or MDMA is associated with cognitive dysfunction. Similar dysfunction appears to manifest following use of cathinone and synthetic derivatives although results tend to vary by drug type, dosing, and duration of use. Studies on habitual khat users demonstrate deficits in several aspects of learning and cognitive set-shifting.95,96 Chronic users of the synthetic cathinone mephedrone show impaired prose recall, verbal learning and fluency, working memory, and cognitive flexibility,97,98 although readers should note a high incidence of polydrug use in these cited studies. Recent controlled laboratory studies in humans have confirmed that acute administration of mephedrone (200 mg) impairs short-term spatial memory, although divided attention appears unaffected.99 Taken together, these studies support that cathinone and synthetic derivatives are capable of contributing to deficits in cognitive functions.
Corroborating with work performed in humans, acute mephedrone (0.56–10 mg/kg) was shown to produce dose-dependent rate-decreasing and error-increasing effects in an operant-based response test in rodent subjects.100 In nonhuman primates, interestingly, acute mephedrone injection produced improvements in visuospatial attention101 perhaps due to enhancements in vigilance and concentration commonly reported in human users. A single injection of the potent cocaine-like synthetic cathinone MDPV can induce widespread reductions in functional connectivity between frontal and subcortical structures.102 Moreover, cognitive deficits associated with acute synthetic cathinone additionally appear sex-dependent and mediated by circulating ovarian hormones.103 Acute synthetic cathinone use thus appears to produce mixed effects in assessments of cognitive functioning; however, drug abusers (including those who abuse synthetic cathinones) infrequently administer a single, isolated dose of drug.
Several teams have sought to explore how chronic synthetic cathinone use, as is observed in many human users, impacts short- and long-term cognitive functioning. Motbey and colleagues104 observed object recognition deficits when subjects were tested 35 days after a chronic mephedrone dosing regimen (30 mg/kg/day, 10 days). A separate study compared long-term effects of chronic mephedrone to those of the empathogenic agent methylone. Specifically, den Hollander and colleagues83 found that mice treated with mephedrone (30 mg/kg/day, 2×/day, 4 days) showed deficient alternating T-maze task performance when assessed 3 weeks after drug injections whereas methylone-injected mice remained unimpaired. In a follow-up assessment, mephedrone-injected mice returned to vehicle-injected control levels and, curiously, methylone-injected mice showed improvements in reversal learning test performance. In contrast, other reports detail significant deficits in reference memory as assessed by either Morris water maze or a land-based Y-maze following repeated methylone injections.105,106 Collectively, long-term synthetic cathinone use generally associates with deficient cognitive functioning which may relate to neurobiological changes in response to repeated drug use.
It should be noted that the aforementioned studies invariably utilized noncontingent “bingelike” passive exposure of subjects to the cathinone-related drug of interest which may produce pharmacokinetic and pharmacodynamic effects that are not necessarily the same as those occurring following voluntary intake. To achieve improved validity in rodent models, we recently demonstrated that long-term access to self-administered MDPV (96 h access periods alternating with 72 h periods of abstinence for a total of 5 weeks) resulted in significant deficits in novel object recognition memory but not object placement memory when tested 3 weeks following the last drug access session.107 The lack of effect on object placement suggests some sparing of spatial memory function in opposition to findings summarized previously with repeated noncontingent administration. It is therefore of great interest to determine which cognitive deficits are observed following prolonged voluntary access, their dose- and sex-dependency, and the duration of impairment.
NEURAL STRUCTURES AND TRANSMITTER SYSTEMS PROMOTING PHARMACOTHERAPEUTIC INTERVENTION STRATEGIES TO MANAGE SYNTHETIC CATHINONE ABUSE
To date, no pharmacotherapeutic medications are approved for treating stimulant use disorders including to cocaine, amphetamine, and synthetic cathinones. Within this section, we describe new advances from preclinical research teams on our developing understanding of the circuits and transmitters that underlie reward associated with synthetic cathinone use. Exciting progress has supported the targeting of monoaminergic transmission systems as well as glutamatergic, neuropeptide, and central chemokine transmission systems. New “preventative medicine” avenues include CNS-permeable drug-conjugate vaccinations to suppress synthetic cathinone abuse.108
Regulation of Monoamine Transmission
In effort to suppress artificially augmented surges in DA content, several studies have incorporated the use of agents acting on D1- and D2-like receptors. For example, Young and Glennon70 observed that the discriminative stimulus effects of S-methcathinone against an amphetamine-paired lever are effectively shifted (made less effective) by pretreatment with haloperidol, a D2-like receptor antagonist. Seminal work additionally reveals that haloperidol can reduce cathinone-elicited hyperthermia in rabbits19 supporting that cathinone-elicited somatic effects are, at least in part, DA-mediated. Systemic injections of either the D1-like receptor antagonist SCH23990 or the D2-like receptor antagonist sulpiride significantly decreases hyperlocomotor activity following α-PVP injection.30 Other work, however, finds that pretreatment with SCH23390 increases self-administered cathinone infusions relative to vehicle pretreatment levels:4 the increase in self-administered infusions may reflect effort to reach the subjectively positive experience occurring in sessions prior to DA receptor blockade.
Reinforcing efficacy of abused drugs is largely attributed to augmented DA levels; however, pharmacological agents that regulate 5-HT content show some preclinical efficacy. Gannon and colleagues109 recently reported on the ability of clinically available lorcaserin, an obesity medication working in part via 5-HT2A activation, to reduce motivated responding for both cocaine and MDPV in self-administering rats. Targeting 5-HT2 receptors was additionally highlighted by López-Arnau and colleagues110 who found that ketanserin, a nonselective 5-HT2 antagonist, decreased mephedrone- and methylone-elicited elevations in locomotor activity which corroborates prior work linking 5-HT to MDMA-elicited locomotion. It thus appears that stimulation of 5-HT2A receptors produces therapeutically favorable effects against the reinforcing effects of psychostimulants, but that other 5-HT receptor subtypes may contribute to locomotor-stimulating effects following synthetic cathinone use.
Regulation of Glutamate Transmission
Receptor-Based Targeting Strategy
Targeting presynaptic metabotropic glutamate 2 and 3 receptors (mGluR2/3s) also appears as a promising pharmacotherapeutic strategy to manage psychostimulant addiction (for review, see111). Animal studies show enduring neuroadaptations in mGluR2/3 receptors following repeated psychostimulant exposure, which contributes to synaptic plasticity in glutamate and DA levels that drives drug-taking and drug-seeking behavior.112–116 Accordingly, systemic administration of mGluR2/3 agonists can attenuate cocaine and methamphetamine self-administration as well as reinstatement behaviors.117–121 Moreover, the glutamate carboxypeptidase II (GCPII) inhibitor 2-(phosphonomethyl)-pentanedioic acid (2-PMPA), which is an indirect mGluR2/3 agonist, attenuates cocaine place preference,122 cocaine self-administration, and cocaine relapse.123,124
Recently, we showed that mGluR2/3 receptors may also possess pharmacotherapeutic potential for the treatment of synthetic cathinone abuse.125 In our study, we found that 2-PMPA and N-acetylaspartylglutamate (NAAG) dose-dependently reduced the expression of MDPV place preference. We confirmed that these effects were mediated via mGluR2/3 receptors, as the inhibitory effects of NAAG were blocked by pretreatment with the mGluR2/3 antagonist LY341495. Currently, we are investigating whether the effects of NAAG and 2-PMPA extend to MDPV self-administration and reinstatement of MDPV-seeking behavior.
Targeting Glutamate Transporters and Clearance Efficiency
A strategy of targeting glutamate transport may offer possible advantages over conventional receptor-based approaches in managing psychostimulant abuse. Since multiple glutamate receptor subtypes contribute to psychostimulant addiction, a putative medication that blocks (e.g., NMDA) or activates (e.g., mGluR2/3) only a single receptor is unlikely to produce lasting efficacy. The glutamate transport system offers a viable target because chronic cocaine regimens disrupt its ability to maintain glutamate homeostasis, and this action leads to glutamate dysregulation that contributes to cocaine reinforcement and seeking.126–128 Activation of the glutamate transport system shapes activity at multiple glutamate receptor subtypes. For example, activation of cystine-glutamate exchange (system Xc) increases glutamatergic tone onto mGluR2/3 receptors and decreases synaptic glutamate release probability.129 Activation of system Xc leads to activation of presynaptic mGluR2/3 receptors that reduces reinstatement of cocaine seeking by normalizing the reduction in extrasynaptic glutamate following chronic cocaine administration and preventing the increased synaptic glutamate release that drives drug seeking.130 Activation of glutamate transporter subtype 1 (GLT-1) by the β-lactam antibiotic ceftriaxone reduces reinforcing effects of cocaine in mice and the behavioral-sensitizing effects of amphetamine, counteracts deficits in GLT-1 expression and myelin-related proteins in the NAcc of cocaine-withdrawn mice, and inhibits relapse to cocaine seeking in rat self-administration models of reinstatement.131–133 Notably, chronic MDPV administration downregulates NAcc GLT-1 expression, and pretreatment with ceftriaxone normalizes GLT-1 levels and suppresses MDPV-associated reward.59
Neuropeptides: Hypocretin/Orexin and Dynorphin
Neurotransmitters and peptides that influence mesolimbic DA transmission may additionally be targeted to modulate reward and reinforcement associated with synthetic cathinone abuse. As an example, chronic mephedrone was shown to upregulate neuropeptide dynorphin content in rat striatum;134 dynorphin is the endogenous ligand of the kappa opioid receptor (KOR) which associates with subjective states of negative affect and negatively regulates DA-producing cellular physiology [e.g., refs 135 and 136]. While KOR blockade failed to appreciably alter cocaine self-administration in nonhuman primates,137 KORs were observed to regulate cocaine-seeking behavior stimulated by activation of hypocretin/orexin (hcrt/ox) receptors,138 which is discussed in detail in the following section. It reasons, then, that elevated dynorphin levels may contribute to negative affect associated with cessation of many synthetic cathinone drugs including mephedrone, and that the reinforcing effects of psychostimulants may be mediated by synergistic interactions between KORs and other neuropeptide receptors at the cellular level.
Converging lines of anatomical and behavioral studies support that the hypothalamic peptide hypocretin/orexin (hcrt/ox), which transmits via two excitatory postsynaptic G-protein coupled receptors (GPCRs) (OX1R and OX2R), densely innervates DA-producing cellular populations and regulates psychostimulant-associated reinforcement [refs 139 and 140; for review, see, ref 141]. Notably, OX1R blockade decreases operant responses for intravenous cocaine (as well as for sucrose pellets) but fails to appreciably alter responding for normal food chow suggesting a selective role for hcrt/ox transmission via OX1Rs in the seeking/retrieval of palatable reinforcers.142 Muschamp and colleagues136 soon revealed VTA OX1Rs as direct contributors to motivated cocaine-taking. Complementary work finds that hcrt/ox transmission to VTA mediates the reinforcing effects of self-administered cocaine and positively regulates mesolimbic DA transmission.143,144
In a recent study from our team, we found that suvorexant, a clinically available hcrt/ox receptor antagonist, reduces motivated cocaine-taking without suppressing motor activity of tested rats.145 We later observed that suvorexant significantly suppresses 50 kHz USVs associated with anticipation and self-administration of MDPV in rats.146 However, we did not observe significant reductions in the number of self-administered MDPV infusions which was likely due to the employment of a low-effort schedule of reinforcement and a selective role of hcrt/ox in motivational action. Thus, targeting hcrt/ox transmission may favorably normalize pathological motivation including behaviorally activated states associated with the procurement of synthetic cathinone drugs of abuse.
S-Mephedrone: An Enantiomer with Antiaddictive Potential
We have unexpectedly found that the stereo-chemical effects of racemic mephedrone are dramatic. In preclinical assays, S-mephedrone blocks anxiogenic and depressant effects during cocaine withdrawal and displays efficacy against neuropathic pain.84 Importantly, S-mephedrone does not cause extensive amphetamine-like rewarding, reinforcing, motivational, and locomotor-enhancing effects in rats, as does racemic mephedrone and the R-enantiomer.51 Mechanistically, S-mephedrone is a “triple monoamine transporter substrate” that enhances release of 5-HT, DA, and NA27,28,147 but with 50-fold greater potency in releasing 5-HT versus DA.51 The use of candidate medications for drug addictions that target the same transporters or receptors as the primary drug of abuse is a proven strategy for treating substance abuse disorders, as exemplified by efficacious, approved treatments for cigarette smoking (e.g., nicotine patch) and opioid dependence (e.g., methadone, buprenorphine).
There is strong scientific premise for studying triple 5-HT/DA/NA transporter substrates with preferential 5-HT-releasing effects for managing psychostimulant addiction. Cocaine withdrawal produces a dual deficit of synaptic DA and 5-HT in the brain of rats and humans, indicating the advantage of developing medications that normalize dysregulation of both neurotransmitter systems during cocaine abstinence. Specific evidence indicates that (i) withdrawal from abused stimulants produces a 5-HT deficit that resembles major depressive disorder in humans, (ii) administration of DA and 5-HT-releasing agents alone or together decreases drug-seeking in animals, (iii) increases in extracellular 5-HT in brain antagonize psychomotor activation by DA releasers, and (iv) a single molecule that releases both DA and 5-HT could suppress cocaine self-administration while having low abuse liability [e.g., refs 148 and 149]. A limitation of using amphetamines and preferential DA releasers as medications is a high abuse potential due to increased mesolimbic DA output. Since increases in synaptic 5-HT counteract stimulant and reinforcing effects caused by increased DA transmission, monoamine transporter substrates that release both DA and 5-HT, such as the compound PAL287,150 are hypothesized to have lower abuse potential while maintaining an ability to antagonize withdrawal symptomatology and relapse. Although S-mephedrone does interact with 5-HT2A, 5-HT2B, and 5-HT2C receptors, it lacks agonist activity at any of them.84 As such, potential advantages of S-mephedrone are reduced risks of hallucinogenic effects associated with 5-HT2A activation,18 reduced cardiovascular effects related to 5-HT2B activation,151 and reduced sensitivity of 5-HT2A and 5-HT2C receptors which may otherwise promote relapse.152
Since S-mephedrone suffers a slow but characterized racemization to mephedrone, S-mephedrone itself is unsuited to be considered as a promising therapeutic agent for treating psychostimulant abuse. The in vivo metabolism of mephedrone is nearly exclusively Cyp2D6-mediated oxidation of the tolyl methyl to hydroxymethyl 7 and oxidative demethylation to normephedrone 8.153,154 In summary, preclinical evidence with S-mephedrone suggests that future work be directed toward identification of stereochemically and metabolically stable S-mephedrone analogs as potential treatments for psychostimulant abuse and perhaps neuropathic pain due to its dual enhancement of both serotonergic and adrenergic transmission.
Chemokine Modulation of Reward and Reinforcement
A separate body of work has positioned the chemokine receptor–ligand pair, CXCR4-CXCL12, as a modulator of mesolimbic DA activity and as a contributor to the behavioral effects of psychostimulants. CXCL12, for example, is acutely elevated in humans and rodents following cocaine use.155 The receptor for CXCL12, CXCR4, is a constitutively expressed GPCR localized to adult neurons and glia in reward- and emotion-governing structures including prefrontal cortex, VTA and NAcc.156,157 While CXCR4 is coupled to Gαi, calcium influx elicited by CXCL12 tends to favor neuroexcitatory effect associated with the ligand binding [e.g., ref 158]. In the midbrain, CXCR4 activation by intranigral CXCL12 injection depolarizes ipsilateral neurons residing in substantia nigra leading to DA elevations in dorsal striatal targets commensurate with increased contralateral turning behavior.159 Moreover, bilateral intra-VTA CXCL12 potentiates cocaine-induced hyperlocomotion, an effect that is blocked by systemic CXCR4 antagonism with the clinically available bone marrow stimulant agent AMD3100 (Plerixafor).157 In vitro, CXCL12 increases putative DA-producing cellular firing and extracellular DA content in a CXCR4-activation-dependent manner.158 In rodents, repeated administration of cocaine increases CXCL12 mRNA in the VTA.160 Notably, CXCR4 inhibition with AMD3100 has proven efficacious in reducing locomotor-stimulating and rewarding effects of MDPV in rats. More recently, we demonstrated that AMD3100 pretreatment blocks place conditioning for an MDPV-paired context and normalizes MDPV-elicited 50 kHz USVs.161 Together, these findings implicate CXCR4 inhibition with AMD3100 as an effective therapeutic strategy to normalize MDPV-associated reward as well as to suppress protracted anxiogenic effects following chronic MDPV use.
Neurotoxic and Neuroinflammatory Effects: A Role for Anti-Inflammatory Agents?
Given their primary pharmacological sites of action, presynaptic monoaminergic terminals in striatum and other forebrain regions are frequently investigated as potential substrates of possible neurotoxic effects following psychostimulant use [e.g., refs 162 and 163]. It is thus surmised that synthetic cathinones, through comparable central sites of action, may exert similar neurotoxic effects on monoaminergic neuron function.164 However, studies examining the effects of synthetic cathinones on monoamine transmission in the cortex, striatum, and hippocampus have yielded mixed results [for reviews, see refs 165 and 166]. Specifically, some reports indicate that repeated administration of mephedrone or methylone produces transient or minimal changes in central monoamine content (DA and 5-HT) as well as turnover ratios.57,83,104,167–171 Robust and persistent depletions in tissue monoamine content and/or reduced monoamine terminal markers have been reported following administration of higher doses of these synthetic cathinones at increased frequency (i.e., 20–25 mg/kg, 3–4×/day).105,170,172,173 Still, other investigators have failed to observe any effects of mephedrone, MDPV, or methylone on tissue monoamine content [e.g., refs 174 and 175].
Psychostimulants promote inflammatory processes such as the release of proinflammatory cytokines and activation of microglia, which in turn may result in neurotoxicity and/or cognitive dysfunction.163,176,177 There is relatively unconvincing evidence, however, that repeated injections of mephedrone or methylone produce microgliosis despite high doses and frequent injection.104,105,172 It should be noted that some of these reports have observed increases in glial fibrillary acidic protein (GFAP) immunoreactivity following synthetic cathinone injections.105,170 A separate body of work has begun uncovering cytotoxic effects of mephedrone, MDPV, and methylone largely derived from in vitro preparations (cultured neurons, endothelial cells, DA-producing SH-SY5Y cells, hepatocytes, and upper airway epithelial cells).170,178–181
Recent evidence of neurotoxic effects of long-term MDPV self-administration in object recognition circuitry (e.g., entorhinal and perirhinal cortices) was provided by our laboratory,107 although no evidence of neurodegeneration was observed in monoamine-populated structures including pre-frontal cortex, striatum and hippocampus. However, it was recently observed that repeated administration of high doses of mephedrone (50 mg/kg/day fpr 14 days or 3× daily for 7 days) to pregnant female mice during gestational days 5–18 produced offspring with reduced body weight and increased rate of stillborn birth.182 In addition, mice exposed to mephedrone in utero displayed significant evidence apoptosis and reduced cell proliferation in the hippocampus. Thus, some synthetic cathinone may induce hippocampal neurotoxic and teratological effects. Consequently, we surmise that additional research is needed to examine multiple forms of toxicity in the central nervous system following prolonged synthetic cathinone exposure or use. Specifically, toxicity-associated measures should include necrosis, apoptosis, necroptosis, autophagy, oxidative stress, excitotoxicity, and activation of inflammatory and cell death signaling pathways. Elucidation of these pathways and mechanisms is needed to understand and potentially mitigate inflammatory and/or cytotoxic effects of synthetic cathinones which may underlie their ability to induce cognitive Dysfunction and abuse propagation.
CONCLUDING REMARKS ON THE “DARK” NATURE OF CATHINONE-DERIVED SYNTHETIC PSYCHOSTIMULANTS
An appreciable body of work has corroborated to make clear the lethal risk of single-motif structural alterations to cathinone’s parent structure through case studies, self-reports, and controlled laboratory experiments detailing effects of synthetic cathinone abuse. For a detailed account corroborating preclinical and clinical reports related to synthetic cathinone abuse, readers are referred to ref 183. Legislation has adapted to prosecute those possessing and manufacturing certain synthetic drugs, but novel formulations often, to societal detriment, avoid legal risk. Our understanding of how synthetic cathinones affect transmitters and circuits within the CNS has afforded multiple avenues for pharmacotherapeutic intervention testing—notably, some preclinical efforts reveal effective therapeutics from clinically available medications. Investigating the neurotoxic effects of synthetic cathinones, acutely and protractedly, promotes the investigation of anti-inflammatory agents in efforts to normalize long-term impairments in cognitive and emotional health. The rise of synthetic cathinone abuse occurs alongside the emergences of synthetic cannabinoids and opiates. Collectively, these designer drugs create a public health epidemic unlike those the United States has experienced for “pure” agents such as cocaine and heroin. Our teams and others, however, remain optimistic in working to comprehensively understand the behavioral features and mechanisms of cathinone-derived psychostimulants in hopes to send one or more therapeutic agents to clinics in ultimate efforts to manage their pervasive abuse.
Acknowledgments
Funding
The authors acknowledge generous support from the National Institute on Drug Abuse (R01 DA039139 awarded to S.M.R. and R01 DA043172 awarded to M.F.O.).
ABBREVIATIONS
- DOM
2,5-dimethoxy-4-methylamphetamine
- two PMPA
2-(phosphonomethyl)-pentanedioic acid
- 3-FMC
3-fluoromethcathinone
- 3-MMC
3-methylmethcathinone
- methylone
3,4-methylenedioxy-N-methylcathinone
- MDMA
3,4-methylenedioxymethamphetamine
- MDPV
3,4-methylenedioxypyrovalerone
- 4-FMC (flephedrone)
4-fluoromethcathinone
- methedrone
4-methoxymethcathinone
- 4-MePPP
4-methyl-α-pyrrolidinopropiophenone
- 4-MEC
4-methyl-N-ethylcathinone
- 4-MMC (mephedrone)
4-methylmethcathinone
- pentedrone
α-methylaminovalerophenone
- α-PVT
α-pyrrolidinopentiothiophenone
- α-PVP
α-pyrrolidinovalerophenone
- butylone
β-keto-N-methylbenzodioxolylbutanamine
- pentylone
β-keto-N-methylbenzodioxolylpentanamine
- ARDS
acute respiratory distress syndrome
- DA
dopamine
- DAT
dopamine transporter
- GPCR
G-protein coupled receptor
- GFAP
glial fibrillary acidic protein
- GCPII
glutamate carboxypeptidase II
- hcrt/ox
hyporcretin/orexin
- OX1R
hypocretin/orexin receptor subtype-1
- OX2R
hypocretin/orexin receptor subtype-2
- ICSS
intracranial self-stimulation
- KOR
kappa opioid receptor
- LHb
lateral habenula
- mGluR
metabotropic glutamate receptor
- MAO
monoamine oxidase
- NAAG
N-acetylaspartylglutamate
- naphyrone
naphthylpyrovalerone
- NA
noradrenaline
- NAT
noradrenaline transporter
- NPS
novel psychoactive substance
- NAcc
nucleus accumbens
- amphetamine
phenylisopropylamine
- 5-HT
serotonin
- SERT
serotonin transporter
- USV
ultrasonic vocalization
- VTA
ventral tegmental area
Footnotes
ORCID
Steven J. Simmons: 0000-0002-6982-4740
Callum Hicks: 0000-0002-6144-8883
Author Contributions
S.J.S. outlined, contributed to, corroborated, and edited the manuscript under direction of S.M.R. and M.F.O. J.M.L.-J., C.F.O., C.H., S.M.R., and M.F.O. contributed written sections to the manuscript. J.W.M. composed graphics for the manuscript.
Notes
The authors declare no competing financial interest.
References
- 1.Alles GA, Fairchild MD, Jensen M, Alles A. Chemical pharmacology of Catha edulis. J Med Pharm Chem. 1961;3(2):323–352. doi: 10.1021/jm50015a010. [DOI] [PubMed] [Google Scholar]
- 2.Numan N. Exploration of adverse psychological symptoms in Yemeni khat users by the Symptoms Checklist-90 (SCL-90) Addiction. 2004;99(1):61–65. doi: 10.1111/j.1360-0443.2004.00570.x. [DOI] [PubMed] [Google Scholar]
- 3.Ageely HM. Prevalence of Khat chewing in college and secondary (high) school students of Jazan region, Saudi Arabia. Harm Reduction Journal. 2009;6(1):11. doi: 10.1186/1477-7517-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gosnell BA, Yracheta JM, Bell SM, Lane KE. Intravenous self-administration of cathinone by rats. Behav Pharmacol. 1996;7(6):526–531. [PubMed] [Google Scholar]
- 5.Kalix P. Pharmacological properties of the stimulant khat. Pharmacol Ther. 1990;48:397–416. doi: 10.1016/0163-7258(90)90057-9. [DOI] [PubMed] [Google Scholar]
- 6.Hyde JF, Browning E, Adams R. Synthetic homologs of D, L-ephedrine. J Am Chem Soc. 1928;50:2287–2292. [Google Scholar]
- 7.Kelly JP. Cathinone derivatives: a review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3(7–8):439–453. doi: 10.1002/dta.313. [DOI] [PubMed] [Google Scholar]
- 8.Emerson TS, Cisek JE. Methcathinone: a Russian designer amphetamine infiltrates the rural midwest. Ann Emerg Med. 1993;22:1897–1903. doi: 10.1016/s0196-0644(05)80419-6. [DOI] [PubMed] [Google Scholar]
- 9.De Felice LJ, Glennon RA, Negus SS. Synthetic cathinones: chemical phylogeny, physiology, and neuropharmacology. Life Sci. 2014;97(1):20–26. doi: 10.1016/j.lfs.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EMCDDA. European Drug Report 2015: trends and developments. 2015 http://www.emcdda.europa.eu/attachements.cfm/att_239505_EN_TDAT15001ENN.pdf.
- 11.Meyers K, Kaynak O, Bresani E, Curtis B, McNamara A, Brownfield K, Kirby KC. The availability and depiction of synthetic cathinones (bath salts) on the Internet: Do online suppliers employ features to maximize purchases? Int J Drug Policy. 2015;26:670–674. doi: 10.1016/j.drugpo.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashrafioun L, Bonadio FA, Baik KD, Bradbury SL, Carhart VL, Cross NA, Davis AK, Feuille M, Harper AR, Lackey JH, Lang B, Lauritsen KJ, Leith J, Osborn LA, Rosenberg H, Stock J, Zaturenskaya M. Patterns of use, acute subjective experiences, and motivations for using synthetic cathinones (″bath salts″) in recreational users. J Psychoact Drugs. 2016;48:336–343. doi: 10.1080/02791072.2016.1229875. [DOI] [PubMed] [Google Scholar]
- 13.National Drug Early Warning System (NDEWS) Poison Control Center Statistics: Facts About “Bath Salts”. 2012 https://aapcc.s3.amazonaws.com/pdfs/topics/Bath_Salts_6.2012.pdf, accessed 19 April 2018.
- 14.National Forensic Laboratory Information System. Special Report: Synthetic Cathinones Reported in NFLIS 2013–2015. 2015 https://www.nflis.deadiversion.usdoj.gov/DesktopModules/ReportDownloads/Reports/NFLIS-SR-SynthCannabinoidCathinone.pdf, accessed 24 April 2018.
- 15.Maciów-Głąb M, Kula K, Kłys M, Rojek SD. New psychoactive substances in substantive evidence in expert practice of the Department of Forensic Medicine, UJCM in the years 2010–2015. Arch Med Sadowej Kryminol. 2017;67(3):178–200. doi: 10.5114/amsik.2017.71449. [DOI] [PubMed] [Google Scholar]
- 16.Hagel JM, Krizevski R, Marsolais F, Lewinsohn E, Facchini PJ. Biosynthesis of amphetamine analogs in plants. Trends Plant Sci. 2012;17(7):404–412. doi: 10.1016/j.tplants.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Majchrzak M, Celinśki R, Kuś P, Kowalska T, Sajewicz M. The newest cathinone derivatives as designer drugs: an analytical and toxicological review. Forensic Toxicol. 2018;36:33–50. doi: 10.1007/s11419-017-0385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. British journal of pharmacology. 2013;168(2):458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalix P. A constituent of khat leaves with amphetamine-like releasing properties. Eur J Pharmacol. 1980;68(2):213–215. doi: 10.1016/0014-2999(80)90326-x. [DOI] [PubMed] [Google Scholar]
- 20.Kalix P. The amphetamine-like releasing effect of the alkaloid (−) cathinone on rat nucleus accumbens and rabbit caudate nucleus. Prog Neuro-Psychopharmacol Biol Psychiatry. 1982;6:43. doi: 10.1016/s0364-7722(82)80106-9. [DOI] [PubMed] [Google Scholar]
- 21.Wagner GC, Preston K, Ricaurte GA, Schuster CR, Seiden LS. Neurochemical similarities between d, l-cathinone and d-amphetamine. Drug Alcohol Depend. 1982;9(4):279–284. doi: 10.1016/0376-8716(82)90067-9. [DOI] [PubMed] [Google Scholar]
- 22.Gugelmann R, Von Allmen M, Brenneisen R, Porzig H. Quantitative differences in the pharmacological effects of (+)-and (−)-cathinone. Experientia. 1985;41(12):1568–1571. doi: 10.1007/BF01964811. [DOI] [PubMed] [Google Scholar]
- 23.Kalix P. The releasing effect of the isomers of the alkaloid cathinone at central and peripheral catecholamine storage sites. Neuropharmacology. 1986;25(5):499–501. doi: 10.1016/0028-3908(86)90174-7. [DOI] [PubMed] [Google Scholar]
- 24.Pehek EA, Schechter MD, Yamamoto BK. Effects of cathinone and amphetamine on the neurochemistry of dopamine in vivo. Neuropharmacology. 1990;29(12):1171–1176. doi: 10.1016/0028-3908(90)90041-o. [DOI] [PubMed] [Google Scholar]
- 25.Glennon RA, Yousif M, Naiman N, Kalix P. Methcathinone: a new and potent amphetamine-like agent. Pharmacol, Biochem Behav. 1987;26(3):547–551. doi: 10.1016/0091-3057(87)90164-x. [DOI] [PubMed] [Google Scholar]
- 26.Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Hoonakker AJ, et al. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339(2):530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Cozzi NV, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37(5):1192. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Brandt SD, et al. Powerful cocaine-like actions of 3, 4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38(4):552. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolanos R, Solis E, Jr, Sakloth F, De Felice LJ, Glennon RA. Deconstruction” of the abused synthetic cathinone methylenedioxypyrovalerone (MDPV) and an examination of effects at the human dopamine transporter. ACS Chem Neurosci. 2013;4(12):1524–1529. doi: 10.1021/cn4001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaizaki A, Tanaka S, Numazawa S. New recreational drug 1-phenyl-2-(1-pyrrolidinyl)-1-pentanone (alpha-PVP) activates central nervous system via dopaminergic neuron. J Toxicol Sci. 2014;39(1):1–6. doi: 10.2131/jts.39.1. [DOI] [PubMed] [Google Scholar]
- 31.Dolan SB, Chen Z, Huang R, Gatch MB. Ecstasy” to addiction: Mechanisms and reinforcing effects of three synthetic cathinone analogs of MDMA. Neuropharmacology. 2018;133:171. doi: 10.1016/j.neuropharm.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Menchaca AA, Solis E, Jr, Cameron K, De Felice LJ. S (+) amphetamine induces a persistent leak in the human dopamine transporter: molecular stent hypothesis. Br J Pharmacol. 2012;165(8):2749–2757. doi: 10.1111/j.1476-5381.2011.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granek M, Shalev A, Weingarten AM. Khat-induced hypnagogic hallucinations. Acta Psychiatr Scand. 1988;78(4):458–461. doi: 10.1111/j.1600-0447.1988.tb06367.x. [DOI] [PubMed] [Google Scholar]
- 34.Giannini JA, Castellani S. A manic-like psychosis due to Khat Catha edulis Forsk. J Toxicol, Clin Toxicol. 1982;19(5):455–459. doi: 10.3109/15563658208992500. [DOI] [PubMed] [Google Scholar]
- 35.Wewalka M, Drolz A, Staufer K, Scherzer TM, Fuhrmann V, Zauner C. Development of ARDS after excessive kath consumption: A case report. Case Rep Crit Care. 2011:291934. doi: 10.1155/2011/291934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorairaj JJ, Healy C, McMenamin M, Eadie PA. The untold truth about “bath salt” highs: a case series demonstrating local tissue injury. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2012;65(2):e37–e41. doi: 10.1016/j.bjps.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Johnson PS, Johnson MW. Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoact Drugs. 2014;46(5):369–378. doi: 10.1080/02791072.2014.962717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belhadj-Tahar H, Sadeg N. Methcathinone: a new postindustrial drug. Forensic Sci Int. 2005;153(1):99–101. doi: 10.1016/j.forsciint.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 39.Wood DM, Davies S, Puchnarewicz M, Button J, Archer R, Ovaska H, Dargan PI, et al. Recreational use of mephedrone (4-methylmethcathinone, 4-MMC) with associated sympathomimetic toxicity. J Med Toxicol. 2010;6(3):327–330. doi: 10.1007/s13181-010-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Derungs A, Schietzel S, Meyer MR, Maurer HH, Krähenbühl S, Liechti ME. Sympathomimetic toxicity in a case of analytically confirmed recreational use of naphyrone (naphthylpyrovalerone) Clin Toxicol. 2011;49(7):691–693. doi: 10.3109/15563650.2011.592838. [DOI] [PubMed] [Google Scholar]
- 41.Carbone PN, Carbone DL, Carstairs SD, Luzi SA. Sudden cardiac death associated with methylone use. American journal of forensic medicine and pathology. 2013;34(1):26–28. doi: 10.1097/PAF.0b013e31827ab5da. [DOI] [PubMed] [Google Scholar]
- 42.Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3, 4-methylenedioxypyrovalerone. Annals of emergency medicine. 2012;60(1):103–105. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Penders TM, Gestring RE, Vilensky DA. Intoxication delirium following use of synthetic cathinone derivatives. Am J Drug Alcohol Abuse. 2012;38(6):616–617. doi: 10.3109/00952990.2012.694535. [DOI] [PubMed] [Google Scholar]
- 44.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs”(synthetic cathinones) in the United States. Clin Toxicol. 2011;49(6):499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- 45.Kesha K, Boggs CL, Ripple MG, Allan CH, Levine B, Jufer-Phipps R, Fowler DR. Methylenedioxypyrovalerone (“bath salts”), related death: case report and review of the literature. J Forensic Sci. 2013;58(6):1654–1659. doi: 10.1111/1556-4029.12202. [DOI] [PubMed] [Google Scholar]
- 46.Hurd YL, Weiss F, Koob GF, And NE, Ungerstedt U. Cocaine reinforcement and extracellular dopamine overflow in rat nucleus accumbens: an in vivo microdialysis study. Brain Res. 1989;498(1):199–203. doi: 10.1016/0006-8993(89)90422-8. [DOI] [PubMed] [Google Scholar]
- 47.Delfs JM, Schreiber L, Kelley AE. Microinjection of cocaine into the nucleus accumbens elicits locomotor activation in the rat. J Neurosci. 1990;10(1):303–10. doi: 10.1523/JNEUROSCI.10-01-00303.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knoll J. Studies on the central effects of (−) cathinone. NIDA Res Monogr. 1979;27:322–323. [PubMed] [Google Scholar]
- 49.Calcagnetti DJ, Schechter MD. Increases in the locomotor activity of rats after intracerebral administration of cathinone. Brain Res Bull. 1992;29(6):843–846. doi: 10.1016/0361-9230(92)90153-o. [DOI] [PubMed] [Google Scholar]
- 50.Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of “bath salt” cathinones. Behav Pharmacol. 2013;24:437. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gregg RA, Baumann MH, Partilla JS, Bonano JS, Vouga A, Tallarida CS, Negus SS, et al. Stereochemistry of mephedrone neuropharmacology: enantiomer-specific behavioural and neurochemical effects in rats. Br J Pharmacol. 2015;172(3):883–894. doi: 10.1111/bph.12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marusich JA, Grant KR, Blough BE, Wiley JL. Effects of synthetic cathinones contained in “bath salts” on motor behavior and a functional observational battery in mice. NeuroToxicology. 2012;33(5):1305–1313. doi: 10.1016/j.neuro.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3, 4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38(4):563. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3, 4-methylenediox-ypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hwang JY, Kim JS, Oh JH, Hong SI, Ma SX, Jung YH, Jang CG, et al. The new stimulant designer compound pentedrone exhibits rewarding properties and affects dopaminergic activity. Addict Biol. 2017;22(1):117–128. doi: 10.1111/adb.12299. [DOI] [PubMed] [Google Scholar]
- 56.Banjaw MY, Schmidt WJ. Catha edulis extract and its active principle cathinone induce ipsilateral rotation in unilaterally lesioned rats. Behav Pharmacol. 2006;17(7):615–620. doi: 10.1097/01.fbp.0000236273.10418.2b. [DOI] [PubMed] [Google Scholar]
- 57.Shortall SE, Macerola AE, Swaby RT, Jayson R, Korsah C, Pillidge KE, Wigmore PM, Ebling FJ, Green AR, Fone KC, King MV. Behavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the rat. Eur Neuropsychopharmacol. 2013;23:1085–1095. doi: 10.1016/j.euroneuro.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Gregg RA, Tallarida CS, Reitz A, McCurdy C, Rawls SM. Mephedrone (4-methylmethcathinone), a principal constituent of psychoactive bath salts, produces behavioral sensitization in rats. Drug Alcohol Depend. 2013;133(2):746–750. doi: 10.1016/j.drugalcdep.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gregg RA, Hicks C, Nayak SU, Tallarida CS, Nucero P, Smith GR, Reitz AB, Rawls SM. Synthetic cathinone MDPV downregulates glutamate transporter subtype I (GLT-1) and produces rewarding and locomotor-activating effects that are reduced by a GLT-1 activator. Neuropharmacology. 2016;108:111–119. doi: 10.1016/j.neuropharm.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berquist MD, Traxler HK, Mahler AM, Baker LE. Sensitization to the locomotor stimulant effects of “bath salt” constituents, 4-methylmethcathinone (4-MMC) and 3, 4-methylenedioxypyrovalerone (MDPV), in male Sprague-Dawley rats. Drug Alcohol Depend. 2016;164:128–134. doi: 10.1016/j.drugalcdep.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watterson LR, Kufahl PR, Taylor SB, Nemirovsky NE, Olive MF. Sensitization to the motor stimulant effects of 3, 4-methylenedioxypyrovalerone (MDPV) and cross-sensitization to methamphetamine in rats. J Drug Alcohol Res. 2016;5:1. doi: 10.4303/jdar/235967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robinson JE, Agoglia AE, Fish EW, Krouse MC, Malanga CJ. Mephedrone (4-methylmethcathinone) and intracranial self-stimulation in C57BL/6J mice: comparison to cocaine. Behav Brain Res. 2012;234(1):76–81. doi: 10.1016/j.bbr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, Marusich JA, Wegner S, Olive MF. Potent rewarding and reinforcing effects of the synthetic cathinone 3, 4-methylenedioxypyrovalerone (MDPV) Addiction biology. 2014;19(2):165–174. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology. 2014;231(1):199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watterson LR, Hood L, Sewalia K, Tomek SE, Yahn S, Johnson CT, Olive MF. The reinforcing and rewarding effects of methylone, a synthetic cathinone commonly found in “bath salts. J Addict Res Ther. 2012;2 doi: 10.4172/2155-6105.S9-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schechter MD. Rats become acutely tolerant to cathine after amphetamine or cathinone administration. Psychopharmacology. 1990;101(1):126–131. doi: 10.1007/BF02253729. [DOI] [PubMed] [Google Scholar]
- 67.Cheong JH, Choi MJ, Jang CG, Lee YS, Lee S, Kim HJ, Yoon SS. Behavioral evidence for the abuse potential of the novel synthetic cathinone alpha-pyrrolidinopentiothiophenone (PVT) in rodents. Psychopharmacology. 2017;234(5):857–867. doi: 10.1007/s00213-017-4526-8. [DOI] [PubMed] [Google Scholar]
- 68.Schechter MD, Rosecrans JA. D-amphetamine as a discriminative cue: drugs with similar stimulus properties. Eur J Pharmacol. 1973;21(2):212–216. doi: 10.1016/0014-2999(73)90228-8. [DOI] [PubMed] [Google Scholar]
- 69.Kalix P, Glennon RA. Further evidence for an amphetamine-like mechanism of action of the alkaloid cathinone. Biochem Pharmacol. 1986;35(18):3015–3019. doi: 10.1016/0006-2952(86)90380-1. [DOI] [PubMed] [Google Scholar]
- 70.Young R, Glennon RA. Discriminative stimulus effects of S (−)–methcathinone (CAT): a potent stimulant drug of abuse. Psychopharmacology. 1998;140(3):250–256. doi: 10.1007/s002130050765. [DOI] [PubMed] [Google Scholar]
- 71.Kohut SJ, Fivel PA, Blough BE, Rothman RB, Mello NK. Effects of methcathinone and 3-Cl-methcathinone (PAL-434) in cocaine discrimination or self-administration in rhesus monkeys. Int J Neuropsychopharmacol. 2013;16(9):1985–1998. doi: 10.1017/S146114571300059X. [DOI] [PubMed] [Google Scholar]
- 72.Dal Cason TA, Young R, Glennon RA. Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs. Pharmacol, Biochem Behav. 1997;58(4):1109–1116. doi: 10.1016/s0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- 73.Naylor JE, Freeman KB, Blough BE, Woolverton WL, Huskinson SL. Discriminative-stimulus effects of second generation synthetic cathinones in methamphetamine-trained rats. Drug Alcohol Depend. 2015;149:280–284. doi: 10.1016/j.drugalcdep.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gatch MB, Rutledge MA, Forster MJ. Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology. 2015;232(7):1197–1205. doi: 10.1007/s00213-014-3755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barker DJ, Simmons SJ, West MO. Ultrasonic vocalizations as a measure of affect in preclinical models of drug abuse: a review of current findings. Current neuropharmacology. 2015;13(2):193–210. doi: 10.2174/1570159X13999150318113642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50(5):967–972. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- 77.Mutschler NH, Miczek KA. Withdrawal from IV cocaine “binges” in rats: ultrasonic distress calls and startle. Psychopharmacology. 1998;135(2):161–168. doi: 10.1007/s002130050497. [DOI] [PubMed] [Google Scholar]
- 78.Taylor JO, Urbano CM, Cooper BG. Differential patterns of constant frequency 50 and 22 khz usv production are related to intensity of negative affective state. Behav Neurosci. 2017;131(1):115. doi: 10.1037/bne0000184. [DOI] [PubMed] [Google Scholar]
- 79.Matochik JA, Barfield RJ. Hormonal control of precopulatory sebaceous scent marking and ultrasonic mating vocalizations in male rats. Horm Behav. 1991;25(4):445–460. doi: 10.1016/0018-506x(91)90013-8. [DOI] [PubMed] [Google Scholar]
- 80.Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114(2):320. [PubMed] [Google Scholar]
- 81.Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behav Brain Res. 2010;212(1):109–114. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simmons SJ, Gregg RA, Tran FH, Mo L, Weltin E, Barker DJ, Muschamp JW, et al. Comparing rewarding and reinforcing properties between ‘bath salt’ 3, 4-methylenedioxypyrovalerone (MDPV) and cocaine using ultrasonic vocalizations in rats. Addict Biol. 2018;23(1):102–110. doi: 10.1111/adb.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.den Hollander B, Rozov S, Linden AM, Uusi-Oukari M, Ojanperä I, Korpi ER. Long-term cognitive and neurochemical effects of “bath salt” designer drugs methylone and mephedrone. Pharmacol, Biochem Behav. 2013;103(3):501–509. doi: 10.1016/j.pbb.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 84.Philogene-Khalid HL, Hicks C, Reitz AB, Liu-Chen LY, Rawls SM. Synthetic cathinones and stereochemistry: S enantiomer of mephedrone reduces anxiety-and depressant-like effects in cocaine-or MDPV-abstinent rats. Drug Alcohol Depend. 2017;178:119–125. doi: 10.1016/j.drugalcdep.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, Comings DE. The reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive and compulsive behaviors. J Psychoact Drugs. 2000;32(sup1):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- 86.Johanson CE, Schuster CR. A comparison of the behavioral effects of l-and dl-cathinone and d-amphetamine. J Pharm Exp Ther. 1981;219(2):355–362. [PubMed] [Google Scholar]
- 87.Woolverton WL, Johanson CE. Preference in rhesus monkeys given a choice between cocaine and d, l-cathinone. J Exp Anal Behav. 1984;41(1):35–43. doi: 10.1901/jeab.1984.41-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaminski BJ, Griffiths RR. Intravenous self-injection of methcathinone in the baboon. Pharmacol, Biochem Behav. 1994;47(4):981–983. doi: 10.1016/0091-3057(94)90307-7. [DOI] [PubMed] [Google Scholar]
- 89.Philogene-Khalid HL, Simmons SJ, Nayak S, Martorana RM, Su SH, Caro Y, Murad A. Stereoselective differences between the reinforcing and motivational effects of cathinone-derived 4-methylmethcathinone (mephedrone) in self-administering rats. ACS Chem Neurosci. 2017;8:2648–2654. doi: 10.1021/acschemneuro.7b00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Creehan KM, Vandewater SA, Taffe MA. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology. 2015;92:90–97. doi: 10.1016/j.neuropharm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vandewater SA, Creehan KM, Taffe MA. Intravenous self-administration of entactogen-class stimulants in male rats. Neuropharmacology. 2015;99:538–545. doi: 10.1016/j.neuropharm.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3, 4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology. 2015;232(16):3045–3055. doi: 10.1007/s00213-015-3944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Javadi-Paydar M, Nguyen JD, Vandewater SA, Dickerson TJ, Taffe MA. Locomotor and reinforcing effects of pentedrone, pentylone and methylone in rats. Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huskinson SL, Naylor JE, Townsend EA, Rowlett JK, Blough BE, Freeman KB. Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology. 2017;234(4):589–598. doi: 10.1007/s00213-016-4492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hoffman R, Al’Absi M. Khat use and neurobehavioral functions: suggestions for future studies. J Ethnopharmacol. 2010;132:554–563. doi: 10.1016/j.jep.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ismail AA, El Sanosy RM, Rohlman DS, El-Setouhy M. Neuropsychological functioning among chronic khat users in Jazan Region, Saudi Arabia. Substance abuse. 2014;35(3):235–244. doi: 10.1080/08897077.2013.832469. [DOI] [PubMed] [Google Scholar]
- 97.Freeman TP, Morgan CJ, Vaughn-Jones J, Hussain N, Karimi K, Curran HV. Cognitive and subjective effects of mephedrone and factors influencing use of a ‘new legal high’. Addiction. 2012;107:792–800. doi: 10.1111/j.1360-0443.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- 98.Herzig DA, Brooks R, Mohr C. Inferring about individual drug and schizotypy effects on cognitive functioning in polydrug using mephedrone users before and after clubbing. Hum Psychopharmacol. 2013;28:168–182. doi: 10.1002/hup.2307. [DOI] [PubMed] [Google Scholar]
- 99.de Sousa Fernandes Perna EB, Papaseit E, Pérez-Mañá C, Mateus J, Theunissen EL, Kuypers KPC, Ramaekers JG, et al. Neurocognitive performance following acute mephedrone administration, with and without alcohol. J Psychopharmacol. 2016;30(12):1305–1312. doi: 10.1177/0269881116662635. [DOI] [PubMed] [Google Scholar]
- 100.Varner KJ, Daigle K, Weed PF, Lewis PB, Mahne SE, Sankaranarayanan A, Winsauer PJ. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology. 2013;225:675–685. doi: 10.1007/s00213-012-2855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wright MJ, Jr, Vandewater SA, Angrish D, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) and d-methamphetamine improve visuospatial associative memory, but not spatial working memory, in rhesus macaques. Br J Pharmacol. 2012;167:1342–1352. doi: 10.1111/j.1476-5381.2012.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Colon-Perez LM, Tran K, Thompson K, Pace MC, Blum K, Goldberger BA, Gold MS, Bruijnzeel AW, Setlow B, Febo M. The psychoactive designer drug and bath salt constituent MDPV causes widespread disruption of brain functional connectivity. Neuropsychopharmacology. 2016;41:2352–2365. doi: 10.1038/npp.2016.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weed PF, Leonard ST, Sankaranarayanan A, Winsauer PJ. Estradiol administration to ovariectomized rats potentiates mephedrone-induced disruptions of nonspatial learning. J Exp Anal Behav. 2014;101:303–315. doi: 10.1002/jeab.72. [DOI] [PubMed] [Google Scholar]
- 104.Motbey CP, Karanges E, Li KM, Wilkinson S, Winstock AR, Ramsay J, Hicks C, Kendig MD, Wyatt N, Callaghan PD, McGregor IS. Mephedrone in adolescent rats: residual memory impairment and acute but not lasting 5-HT depletion. PLoS One. 2012;7:e45473. doi: 10.1371/journal.pone.0045473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J. Serotonergic impairment and memory deficits in adolescent rats after binge exposure of methylone. J Psychopharmacol. 2014;28:1053–1063. doi: 10.1177/0269881114548439. [DOI] [PubMed] [Google Scholar]
- 106.Daniel JJ, Hughes RN. Increased anxiety and impaired spatial memory in young adult rats following adolescent exposure to methylone. Pharmacol, Biochem Behav. 2016;146–147:44–49. doi: 10.1016/j.pbb.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 107.Sewalia K, Watterson LR, Hryciw A, Belloc A, Ortiz JB, Olive MF. Neurocognitive dysfunction following repeated binge-like self-administration of the synthetic cathinone 3, 4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2017 doi: 10.1016/j.neuropharm.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nguyen JD, Bremer PT, Ducime A, Creehan KM, Kisby BR, Taffe MA, Janda KD. Active vaccination attenuates the psychostimulant effects of α-PVP and MDPV in rats. Neuropharmacology. 2017;116:1–8. doi: 10.1016/j.neuropharm.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gannon BM, Sulima A, Rice KC, Collins GT. Inhibition of Cocaine and 3, 4-Methylenedioxypyrovalerone (MDPV) Self-Administration by Lorcaserin Is Mediated by 5-HT2C Receptors in Rats. J Pharmacol Exp Ther. 2018;364(2):359–366. doi: 10.1124/jpet.117.246082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.López-Arnau R, Martínez-Clemente J, Pubill D, Escubedo E, Camarasa J. Comparative neuropharmacology of three psychostimulant cathinone derivatives: butylone, mephedrone and methylone. British journal of pharmacology. 2012;167(2):407–420. doi: 10.1111/j.1476-5381.2012.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Allain F, Roberts DC, Lévesque D, Samaha AN. Intermittent intake of rapid cocaine injections promotes robust psychomotor sensitization, increased incentive motivation for the drug and mGlu2/3 receptor dysregulation. Neuropharmacology. 2017;117:227–237. doi: 10.1016/j.neuropharm.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 113.Beveridge T, Smith H, Nader M, Porrino L. Group II metabotropic glutamate receptors in the striatum of nonhuman primates: dysregulation following chronic cocaine self-administration. Neurosci Lett. 2011;496:15–19. doi: 10.1016/j.neulet.2011.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghasemzadeh M, Mueller C, Vasudevan P. Behavioral sensitization to cocaine is associated with increased glutamate receptor trafficking to the postsynaptic density after extended withdrawal period. Neuroscience. 2009;159:414–426. doi: 10.1016/j.neuroscience.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 115.Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- 116.Xie X, Steketee JD. Effects of repeated exposure to cocaine on group II metabotropic glutamate receptor function in the rat medial prefrontal cortex: behavioral and neurochemical studies. Psychopharmacology. 2009;203:501–510. doi: 10.1007/s00213-008-1392-4. [DOI] [PubMed] [Google Scholar]
- 117.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry. 2010;68:240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cannella N, Halbout B, Uhrig S, Evrard L, Corsi M, Corti C, Deroche-Gamonet V, Hansson AC, Spanagel R. The mGluR2/3 agonist LY379268 induced anti-reinstatement effects in rats exhibiting addiction-like behavior. Neuropsychopharmacology. 2013;38:2048–2056. doi: 10.1038/npp.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Crawford JT, Roberts DC, Beveridge TJ. The group II metabotropic glutamate receptor agonist, LY379268, decreases methamphetamine self-administration in rats. Drug Alcohol Depend. 2013;132:414–419. doi: 10.1016/j.drugalcdep.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Karkhanis AN, Beveridge TJ, Blough BE, Jones SR, Ferris MJ. The individual and combined effects of phenmetrazine and mgluR2/3 agonist LY379268 on the motivation to self-administer cocaine. Drug Alcohol Depend. 2016;166:51–60. doi: 10.1016/j.drugalcdep.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Slusher BS, Thomas A, Paul M, Schad CA, Ashby CR. Expression and acquisition of the conditioned place preference response to cocaine in rats is blocked by selective inhibitors of the enzyme N-acetylated-α-linked-acidic dipeptidase (NAALA-DASE) Synapse. 2001;41:22–28. doi: 10.1002/syn.1056. [DOI] [PubMed] [Google Scholar]
- 123.Xi ZX, Kiyatkin M, Li X, Peng XQ, Wiggins A, Spiller K, Li J, Gardner EL. N-acetylaspartylglutamate (NAAG) inhibits intravenous cocaine self-administration and cocaine-enhanced brain-stimulation reward in rats. Neuropharmacology. 2010;58:304–313. doi: 10.1016/j.neuropharm.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xi ZX, Li X, Peng XQ, Li J, Chun L, Gardner EL, Thomas AG, Slusher BS, Ashby CR., Jr Inhibition of NAALADase by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. J Neurochem. 2010;112:564–576. doi: 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hicks C, Gregg RA, Nayak SU, Cannella LA, Schena GJ, Tallarida CS, Reitz AB, Smith GR, Rawls SM. Glutamate carboxypeptidase II (GCPII) inhibitor 2-PMPA reduces rewarding effects of the synthetic cathinone MDPV in rats: a role for N-acetylaspartylglutamate (NAAG) Psychopharmacology. 2017;234:1671–1681. doi: 10.1007/s00213-017-4568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16(10):974–86. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.D’Souza MS. Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci. 2015;9:404. doi: 10.3389/fnins.2015.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bachtell RK, Jones JD, Heinzerling KG, Beardsley PM, Comer SD. Glial and neuroinflammatory targets for treating substance use disorders. Drug Alcohol Depend. 2017;180:156–170. doi: 10.1016/j.drugalcdep.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miguéns M, Crespo JA, Del Olmo N, Higuera-Matas A, Montoya GL, García-Lecumberri C, Ambrosio E. Differential cocaine-induced modulation of glutamate and dopamine transporters after contingent and non-contingent administration. Neuropharmacology. 2008;55:771–779. doi: 10.1016/j.neuropharm.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 131.Ward SJ, Rasmussen BA, Corley G, Henry C, Kim JK, Walker EA, Rawls SM. Beta-lactam antibiotic decreases acquisition of and motivation to respond for cocaine, but not sweet food, in C57Bl/6 mice. Behav Pharmacol. 2011;22:370–373. doi: 10.1097/FBP.0b013e3283473c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kovalevich J, Corley G, Yen W, Rawls SM, Langford D. Cocaine-induced loss of white matter proteins in the adult mouse nucleus accumbens is attenuated by administration of a β-lactam antibiotic during cocaine withdrawal. Am J Pathol. 2012;181:1921–1927. doi: 10.1016/j.ajpath.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.German CL, Alburges ME, Hoonakker AJ, Fleckenstein AE, Hanson GR. Mephedrone alters basal ganglia and limbic dynorphin systems. Synapse. 2014;68(12):634–640. doi: 10.1002/syn.21778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA. The kappa-opioid agonist U69, 593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64(11):982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Carlezon WA, et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111(16):E1648–E1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hutsell BA, Cheng K, Rice KC, Negus SS, Banks ML. Effects of the kappa opioid receptor antagonist nor-binaltorphimine (nor-BNI) on cocaine versus food choice and extended-access cocaine intake in rhesus monkeys. Addict Biol. 2016;21(2):360–73. doi: 10.1111/adb.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Matzeu A, Kallupi M, George O, Schweitzer P, Martin-Fardon R. Dynorphin Counteracts Orexin in the Paraventricular Nucleus of the Thalamus: Cellular and Behavioral Effects. Neuropsychopharmacology. 2018;43(5):1010–20. doi: 10.1038/npp.2017.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Peyron C, Tighe DK, Van Den Pol AN, De Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fadel J, Deutch AY. Anatomical substrates of orexin–dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 141.Gentile TA, Simmons SJ, Muschamp JW. Hypocretin (Orexin) in Models of Cocaine Addiction. Neuroscience of Cocaine. 2017:235–245. [Google Scholar]
- 142.Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Bonci A, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29(36):11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.España RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2011;214(2):415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Prince CD, Rau AR, Yorgason JT, España RA. Hypocretin/orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci. 2015;6(1):138–146. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gentile TA, Simmons SJ, Barker DJ, Shaw JK, España RA, Muschamp JW. Suvorexant, an orexin/hypocretin receptor antagonist, attenuates motivational and hedonic properties of cocaine. Addiction biology. 2018;23(1):247–255. doi: 10.1111/adb.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Simmons SJ, Martorana R, Philogene-Khalid H, Tran FH, Gentile TA, Xu X, Muschamp JW, et al. Role of hypocretin/orexin receptor blockade on drug-taking and ultrasonic vocalizations (USVs) associated with low-effort self-administration of cathinone-derived 3, 4-methylenedioxypyrovalerone (MDPV) in rats. Psychopharmacology. 2017;234(21):3207–3215. doi: 10.1007/s00213-017-4709-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cameron KN, Kolanos R, Solis E, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol. 2013;168:1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Daw ND, Kakade S, Dayan P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002;15:603–616. doi: 10.1016/s0893-6080(02)00052-7. [DOI] [PubMed] [Google Scholar]
- 149.Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2004;29:660–668. doi: 10.1038/sj.npp.1300346. [DOI] [PubMed] [Google Scholar]
- 150.Rothman RB, Blough BE, Baumann MH. Dual dopamine-5-HT releasers: potential treatment agents for cocaine addiction. Trends Pharmacol Sci. 2006;27:612–618. doi: 10.1016/j.tips.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 151.Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–41. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 152.Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology (Berl) 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- 153.Pedersen AJ, Reitzel LA, Johansen SS, Linnet K. In vitro metabolism studies on mephedrone and analysis of forensic cases. Drug Test Anal. 2013;5:430–438. doi: 10.1002/dta.1369. [DOI] [PubMed] [Google Scholar]
- 154.Mayer FP, Wimmer L, Dillon-Carter O, et al. Phase I metabolites of mephedrone display biological activity as substrates at monoamine transporters. Br J Pharmacol. 2016;173:2657–2668. doi: 10.1111/bph.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Araos P, Pedraz M, Serrano A, Lucena M, Barrios V, García-Marchena N, Baixeras E, et al. Plasma profile of pro-inflammatory cytokines and chemokines in cocaine users under outpatient treatment: influence of cocaine symptom severity and psychiatric co-morbidity. Addict Biol. 2015;20(4):756–772. doi: 10.1111/adb.12156. [DOI] [PubMed] [Google Scholar]
- 156.Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostène W, Mélik Parsadaniantz S. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. European Journal of Neuroscience. 2002;16(9):1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- 157.Trecki J, Brailoiu GC, Unterwald EM. Localization of CXCR4 in the forebrain of the adult rat. Brain Res. 2010;1315:53–62. doi: 10.1016/j.brainres.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guyon A, Skrzydelski D, Rovere C, Apartis E, Rostene W, Kitabgi P, Melik-Parsadaniantz S, Nahon JL. Stromal-cell-derived factor 1α/CXCL12 modulates high-threshold calcium currents in rat substantia nigra. Eur J Neurosci. 2008;28(5):862–870. doi: 10.1111/j.1460-9568.2008.06367.x. [DOI] [PubMed] [Google Scholar]
- 159.Skrzydelski D, Guyon A, Dauge V, Rovere C, Apartis E, Kitabgi P, Parsadaniantz SM, et al. The chemokine stromal cell-derived factor-1/CXCL12 activates the nigrostriatal dopamine system. J Neurochem. 2007;102(4):1175–1183. doi: 10.1111/j.1471-4159.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- 160.Kim J, Connelly KL, Unterwald EM, Rawls SM. Chemokines and cocaine: CXCR4 receptor antagonist AMD3100 attenuates cocaine place preference and locomotor stimulation in rats. Brain, Behav, Immun. 2017;62:30–34. doi: 10.1016/j.bbi.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Oliver CF, Simmons SJ, Nayak SU, Smith GR, Reitz AB, Rawls SM. Chemokines and ‘bath salts’: CXCR4 receptor antagonist reduces rewarding and locomotor-stimulant effects of the designer cathinone MDPV in rats. Drug Alcohol Depend. 2018;186:75. doi: 10.1016/j.drugalcdep.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pereira RB, Andrade PB, Valentao P. A comprehensive view of the neurotoxicity mechanisms of cocaine and ethanol. Neurotoxic Res. 2015;28:253–267. doi: 10.1007/s12640-015-9536-x. [DOI] [PubMed] [Google Scholar]
- 164.Tyrkko E, Andersson M, Kronstrand R. The toxicology of new psychoactive substances: synthetic cathinones and phenylethylamines. Ther Drug Monit. 2016;38:190–216. doi: 10.1097/FTD.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 165.Angoa-Perez M, Anneken JH, Kuhn DM. Neurotoxicology of synthetic cathinone analogs. Curr Top Behav Neurosci. 2016;32:209–230. doi: 10.1007/7854_2016_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pantano F, Tittarelli R, Mannocchi G, Pacifici R, di Luca A, Paolo Busardò F, Marinelli E. Neurotoxicity induced by mephedrone: an up-to-date review. Curr Neuropharmacol. 2017;15(5):738–749. doi: 10.2174/1570159X14666161130130718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Angoa-Perez M, Kane MJ, Francescutti DM, Sykes KE, Shah MM, Mohammed AM, Thomas DM, Kuhn DM. Mephedrone, an abused psychoactive component of ‘bath salts’ and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J Neurochem. 2012;120:1097–1107. doi: 10.1111/j.1471-4159.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Angoa-Perez M, Kane MJ, Briggs DI, Francescutti DM, Sykes CE, Shah MM, Thomas DM, Kuhn DM. Mephedrone does not damage dopamine nerve endings of the striatum, but enhances the neurotoxicity of methamphetamine, amphetamine, and MDMA. J Neurochem. 2013;125:102–110. doi: 10.1111/jnc.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Angoa-Perez M, Kane MJ, Herrera-Mundo N, Francescutti DM, Kuhn DM. Effects of combined treatment with mephedrone and methamphetamine or 3, 4-methylenedioxymethamphetamine on serotonin nerve endings of the hippocampus. Life Sci. 2014;97:31–36. doi: 10.1016/j.lfs.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Martinez-Clemente J, Lopez-Arnau R, Abad S, Pubill D, Escubedo E, Camarasa J. Dose and time-dependent selective neurotoxicity induced by mephedrone in mice. PLoS One. 2014;9:e99002. doi: 10.1371/journal.pone.0099002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pail PB, Costa KM, Leite CE, Campos MM. Comparative pharmacological evaluation of the cathinone derivatives, mephedrone and methedrone, in mice. NeuroToxicology. 2015;50:71–80. doi: 10.1016/j.neuro.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 172.Lopez-Arnau R, Martinez-Clemente J, Abad S, Pubill D, Camarasa J, Escubedo E. Repeated doses of methylone, a new drug of abuse, induce changes in serotonin and dopamine systems in the mouse. Psychopharmacology. 2014;231:3119–3129. doi: 10.1007/s00213-014-3493-6. [DOI] [PubMed] [Google Scholar]
- 173.Ciudad-Roberts A, Duart-Castells L, Camarasa J, Pubill D, Escubedo E. The combination of ethanol with mephedrone increases the signs of neurotoxicity and impairs neurogenesis and learning in adolescent CD-1 mice. Toxicol Appl Pharmacol. 2016;293:10–20. doi: 10.1016/j.taap.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 174.Anneken JH, Angoa-Perez M, Kuhn DM. 3,4-Methylenedioxypyrovalerone prevents while methylone enhances methamphetamine-induced damage to dopamine nerve endings: beta-ketoamphetamine modulation of neurotoxicity by the dopamine transporter. J Neurochem. 2015;133:211–222. doi: 10.1111/jnc.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Anneken JH, Angoa-Perez M, Sati GC, Crich D, Kuhn DM. Dissecting the influence of two structural substituents on the differential neurotoxic effects of acute methamphetamine and mephedrone treatment on dopamine nerve endings with the use of 4-methylmethamphe tamine and methcathinone. J Pharmacol Exp Ther. 2017;360:417–423. doi: 10.1124/jpet.116.237768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotoxic Res. 2013;23:174–188. doi: 10.1007/s12640-012-9334-7. [DOI] [PubMed] [Google Scholar]
- 177.Lacagnina MJ, Rivera PD, Bilbo SD. Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharmacology. 2017;42:156–177. doi: 10.1038/npp.2016.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rosas-Hernandez H, Cuevas E, Lantz SM, Imam SZ, Rice KC, Gannon BM, Fantegrossi WE, Paule MG, Ali SF. 3,4-methylenedioxypyrovalerone (MDPV) induces cytotoxic effects on human dopaminergic SH-SY5Y cells. J Drug Alcohol Res. 2016;5:235991. [Google Scholar]
- 179.Rosas-Hernandez H, Cuevas E, Lantz SM, Rice KC, Gannon BM, Fantegrossi WE, Gonzalez C, Paule MG, Ali SF. Methamphetamine, 3,4-methylenedioxymethamphet-amine (MDMA) and 3,4-methylenedioxypyrovalerone (MDPV) induce differential cytotoxic effects in bovine brain microvessel endothelial cells. Neurosci Lett. 2016;629:125–130. doi: 10.1016/j.neulet.2016.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wojcieszak J, Andrzejczak D, Woldan-Tambor A, Zawilska JB. Cytotoxic activity of pyrovalerone derivatives, an emerging group of psychostimulant designer cathinones. Neurotoxic Res. 2016;30:239–250. doi: 10.1007/s12640-016-9640-6. [DOI] [PubMed] [Google Scholar]
- 181.Valente MJ, Bastos ML, Fernandes E, Carvalho F, Guedes de Pinho P, Carvalho M. Neurotoxicity of β-keto amphetamines: deathly mechanisms elicited by methylone and MDPV in human dopaminergic SH-SY5Y cells. ACS Chem Neurosci. 2017;8:850–859. doi: 10.1021/acschemneuro.6b00421. [DOI] [PubMed] [Google Scholar]
- 182.Naseri G, Fazel A, Golalipour MJ, Haghir H, Sadeghian H, Mojarrad M, Ghorbani A, et al. Exposure to mephedrone during gestation increases the risk of stillbirth and induces hippo-campal neurotoxicity in mice offspring. Neurotoxicol Teratol. 2018;1928:2287–92. doi: 10.1016/j.ntt.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 183.Simmons SJ, Kim E, Gentile TA, Murad A, Muschamp JW, Rawls SM. Synthetic Cathinones. Springer; Cham: 2018. Behavioral Profiles and Underlying Transmitters/Circuits of Cathinone-Derived Psychostimulant Drugs of Abuse; pp. 125–152. [Google Scholar]


