Abstract
Autonomic nervous system (ANS) activity is a core component of emotion processing. The limbic system and medial prefrontal cortex play important roles in the regulation of ANS activity. However, the integration of brain activity and ANS activity has yet to be investigated in adolescents despite independent evidence of adolescents’ heightened neural and physiological sensitivity to emotional stimuli. The present study examined the relations of ANS activity in the parasympathetic nervous system (PNS) and sympathetic nervous system (SNS) with brain activity during emotional face processing in adolescents. 135 adolescents (65 female; M = 17.15 yr, SD = 0.42) completed an emotional faces task during an fMRI scan while electrocardiography and skin conductance were recorded simultaneously. Using linear mixed-effect modelling, we tested the effect of change in respiratory sinus arrhythmia (RSA), a measure of PNS activity, and number of skin conductance responses (SCRs), a measure of SNS activity, on neural activity while adolescents viewed emotional faces. Greater RSA withdrawal, indicating decreased PNS activity, was associated with increased activation in the ventromedial prefrontal cortex (vmPFC). More SCRs, indicating greater SNS activity, were associated with decreased activation in several regions including the dorsomedial prefrontal cortex and increased activation in the left hippocampus. Left hippocampus-SCR coupling and vmPFC-RSA coupling predicted baseline SCR and RSA respectively. These findings implicate the hippocampus for potentiating SNS activity, document that regulation of SNS and PNS activity are coordinated with distinct regions of the medial prefrontal cortex, and suggest potential developmental differences in vmPFC regulation of PNS activity between adolescents and adults.
Keywords: neurovisceral integration, somatic marker, sympathetic, parasympathetic, fMRI
1. Introduction
1.1. Background
Adolescence is a period of increased emotional volatility and intensity (Arnett, 1999; Guyer, Silk, & Nelson, 2016; Larson, Moneta, Richards, & Wilson, 2002). Autonomic nervous system (ANS) activity is a core component of emotional arousal and experience. Understanding the neural mechanisms through which ANS activity is regulated and integrated can therefore provide valuable insight into emotional health in adolescence. In the present study, we investigated the neural mechanisms involved in the production and detection of the visceral aspects of adolescents’ emotion processing. Mechanistic theories of coordinated activity between the brain and the ANS propose that a network of interconnected brain regions provide tonic maintenance of physiological arousal (i.e., constant excitatory or inhibitory influence to maintain baseline levels), initiate phasic changes in arousal (i.e., reactive changes in activity that lead to temporary perturbations), and detect those changes through interoceptive feedback, all of which contribute to the initiation, physiological expression, and appraisal of affective states (Smith, Thayer, Khalsa, & Lane, 2017). Research on adults has provided support for these “neurovisceral” or “somatic marker” models of emotion, but it remains an open question whether they function similarly for adolescents given their heightened emotional reactivity and the ongoing maturation of prefrontal cortex and limbic circuitry (Casey, Jones, & Hare, 2008). Identifying the neural mechanisms involved in the coordination of adolescents’ ANS activity could facilitate a more precise understanding of their emotional health. To our knowledge, this is the first study to examine the coordination between brain function and autonomic activity in adolescents as well as the first to examine coordinated activity of the brain with the SNS and PNS simultaneously.
In their model of neurovisceral integration, Thayer and Lane (2000, 2009) articulated the brain mechanisms contributing to the maintenance and modulation of parasympathetic nervous system (PNS) activity. This model builds on Porges’ polyvagal theory (Porges, 2007; Porges, Doussard-Roosevelt, & Maiti, 1994), which suggests that physiological states serve adaptive functions for facilitating specific behavior patterns. In particular, activity of the myelinated vagus nerve is thought to allow for flexible modulation of arousal in the social environment. High baseline vagal activity, reflecting greater tonic PNS influence, allows for a greater range of possible physiological responses, which supports more flexible emotional reactivity and regulation (Porges et al., 1994). For example, mild to moderate decreases in PNS influence (i.e., vagal withdrawal) support orienting and preparedness to respond by increasing overall arousal with or without sympathetic nervous system (SNS) activation, while stronger vagal withdrawal permits SNS influences to further increase arousal and therefore facilitate defensive (fight or flight) responding. Mild to moderate vagal augmentation connotes perception of the immediate context as safe and facilitates calm social engagement (Hastings, Kahle, & Han, 2014). Meanwhile, vagal afferents convey interoceptive information to the brain about physiological state to guide the interpretation of environmental context. Cardiac vagal tone, as indexed by high frequency heart rate variability (HRV-HF), has therefore been theorized to index the regulation of attention to environmental input and emotion through coordinated activity of the brain and PNS (Thayer & Lane, 2000).
Research using neuroimaging and pharmacological blockade has found that neural activity in regions in the medial prefrontal cortex (mPFC) is associated with HRV-HF (Thayer, Åhs, Fredrikson, Sollers, & Wager, 2012; Thayer & Lane, 2009). Specifically, greater activity in the ventromedial prefrontal cortex (vmPFC), including parts of the anterior cingulate cortex (ACC), coupled with lower amygdala activity, has been associated with higher HRV and lower heart rates. The mPFC is thought to inhibit the amygdala (Gee et al., 2013; Kim et al., 2011; Thayer & Lane, 2009). Amygdala activation leads to heart rate increases and HRV decreases through activation or disinhibition of sympathoexcitatory neurons in the rostral ventrolateral medulla, and inhibition of vagal activity through the nucleus ambiguus (Thayer & Lane, 2009).
Thayer and Lane’s (2009) model of mPFC regulation of autonomic states through the PNS is generally consistent with Damasio’s somatic marker hypothesis involving the SNS (Damasio, Everitt, & Bishop, 1996). Based primarily on studies of skin conductance responses (SCRs), one index of SNS activity, in patients with vmPFC damage, Damasio and colleagues (1996) proposed that both the initiation and interoception of autonomic responses via a feedback loop to and from the body and vmPFC is central to both emotion processing and decision-making. Neuroimaging studies have found vmPFC activity to be negatively correlated with task-independent fluctuations in skin conductance level (Nagai, Critchley, Featherstone, Trimble, & Dolan, 2004; Patterson, Ungerleider, & Bandettini, 2002), suggesting tonic inhibition. In fact, one study has found vmPFC activity to have a negative causal influence on skin conductance through Granger causality (Zhang et al., 2014). Through a series of studies, Critchley (2005, 2009) has consistently found activity in the dorsal ACC to positively relate to phasic SCRs and other indicators of SNS activity, suggesting that dorsal ACC activity may initiate SNS responses. Anterior insula activity is often coupled with dorsal ACC activity and involved in the interoceptive processing of physiological states (Critchley, 2005). Closing the feedback loop, emotional self-awareness, facilitated by interoception, and mediated through mPFC activity, may attenuate one’s own physiological and emotional reactivity through increased PNS activity (Lane, 2008). Further, the association of mPFC with ANS activity is complex and context-dependent, perhaps reflecting its role in both regulating ANS activity in response to external and cognitive cues and integrating interoceptive feedback. For example, one study found dorsal ACC activity to be specifically associated with stimulus-elicited SCRs but not endogenous (or nonspecific) SCRs (Zhang et al., 2012).
Taken together, the neurovisceral integration model, somatic marker hypothesis, and work by Critchley and others converge on a network of brain regions with opponent processes and built-in feedback loops. The dorsal ACC and amygdala increase somatic arousal in response to external emotional stimuli by triggering SNS activity and inhibiting PNS activity (vagal withdrawal), while vmPFC tonically inhibits the SNS and promotes PNS activity, but may also be involved in integrating cognitive and interoceptive information. Meanwhile, the resulting autonomic responses are detected through interoceptive processes involving the anterior insula. Given that these same brain regions are implicated in the processing of internal and external emotion cues, variation in the coordination of these neurophysiological processes may be involved in individual differences in emotional reactivity and regulation.
1.2. Brain and ANS activity in adolescence
Adolescence is a particularly relevant time to investigate the brain’s processing of emotion and regulation of arousal, as the brain regions implicated in these processes have been found to undergo considerable functional specialization across adolescence (Blakemore & Mills, 2014; Eric E. Nelson, Jarcho, & Guyer, 2016). For example, decreases in amygdala reactivity and increases in mPFC activity when attending to emotions depicted in facial expressions are seen across adolescence (Del Piero, Saxbe, & Margolin, 2016). Within the mPFC, in response to viewing negative emotional pictures, activity in the vmPFC has been found to decrease from childhood to adulthood, whereas activity in the dmPFC has been found to increase (Silvers et al., 2017). Inhibition of the amygdala by the mPFC (rostral ACC) while viewing fearful faces has been found to emerge in early adolescence and increase into adulthood, as indicated by a switch from positive to negative functional connectivity (Gee et al., 2013). Conversely, functional connectivity between the amygdala and the vmPFC when viewing negative emotional pictures compared with neutral pictures has been found to increase linearly across adolescence (Vink, Derks, Hoogendam, Hillegers, & Kahn, 2014). Taken together, these results suggest that during emotion processing, amygdala reactivity declines across adolescence, corresponding to an increasing inhibitory influence over the amygdala by dorsal regions of the mPFC, whereas vmPFC activity decreases and becomes more positively coupled with amygdala activity. Given the centrality of mPFC-amygdala circuitry to the neurovisceral integration model (Thayer & Lane, 2009), these developmental shifts call into question whether the mechanisms of ANS regulation are consistent from adolescence into adulthood.
PNS indices, such as respiratory-sinus arrhythmia (RSA), a measure of HRV within the frequency spectrum of the respiratory cycle, also change across development. Baseline RSA increases from childhood to adolescence (El-Sheikh, 2005; Gentzler, Rottenberg, Kovacs, George, & Morey, 2012; Hinnant, Elmore-Staton, & El-Sheikh, 2011) and decreases from adolescence through adulthood (Hollenstein, McNeely, Eastabrook, Mackey, & Flynn, 2012; Pfeifer et al., 1983). Meanwhile, the magnitude of RSA withdrawal in response to stressors has been found to decrease between the ages of 4 and 17 (El-Sheikh, 2005; Shader et al., 2018) but to increase between the ages of 12 and 23 (Hollenstein et al., 2012). This suggests that mid to late adolescence marks a key developmental inflection point in the trajectory of parasympathetic regulation. This inflection point in both baseline RSA and RSA reactivity may reflect developmental shifts in activation and connectivity of the mPFC, particularly the vmPFC. Developmental shifts in vmPFC activity occur over the same time period (Del Piero et al., 2016; Gee et al., 2013; Vink et al., 2014), and vmPFC is known to influence both the tonic maintenance of and phasic changes in PNS activity (Thayer & Lane, 2009).
With regard to the SNS, baseline SNS activity, whether indexed by skin conductance level or the number of nonspecific SCRs, has been found to be quite stable from childhood to adolescence and into adulthood (El-Sheikh, 2007; Venables & Mitchell, 1996). However, children and adolescents show higher amplitude SCRs than adults in response to emotional stimuli (Shields, 1983), and adolescents habituate more slowly when repeatedly viewing negative emotional pictures depending on how many stimuli they view before the absence of a SCR occurs following the stimulus (Miller & Shields, 1980). These findings suggest that tonic SNS activity is relatively stable, and phasic SNS responding decreases across adolescence, becoming more regulated from adolescence to adulthood. Thus, given these developmental changes in SNS and PNS activity, and the still maturing connectivity of the mPFC and amygdala, it is important to determine whether the coupling of the mPFC with the ANS in adolescents is similar to, or different than, what has been observed in adults.
1.2. Goals and Hypotheses
The present study investigated emotion-relevant brain mechanisms that coordinate autonomic activity in late adolescence, a key inflection point in the trajectory of PNS functioning and the development of prefrontal-limbic circuitry. We sought to identify region where brain activity during affective processing corresponded with PNS activity, as measured by RSA, and SNS activity, as measured by the number of SCRs. Based on studies of brain-HRV coupling in adults (Thayer & Lane, 2009) activity in the vmPFC would be expected to be positively associated with RSA and negatively associated with the number of SCRs. Furthermore, amygdala, dorsal ACC, and insula activation would be expected to be inversely related to RSA changes and positively associated with SCR changes (Critchley, 2009). However, because the functional reactivity and connectivity of mPFC and limbic structures undergo significant changes during adolescence, we anticipated that the associations between brain and ANS functioning in adolescents might be qualitatively distinct from reported observations in adults. Finally, we tested whether individual differences in ANS activity at rest related to individual differences in the strength of brain-ANS coupling. Significant positive associations between baseline ANS activity at rest and brain-ANS coupling would suggest that these brain regions maintain a tonic regulatory influence on the homeostatic set points of ANS activity. Conversely, the absence of such associations would indicate that the covariation between brain and ANS activity relates to the brain’s initiation and/or interoception of phasic changes in ANS activity, but that the baseline level of activity is attributable to other individual physiological differences.
2. Method
2.1. Participants
As described in prior work (Weissman et al., 2017) participants were 229 Mexican-American adolescents (MAge=17.16 years, SD=.44, 110 female) enrolled in a neurobiology sub-study of the California Families Project (CFP), a 10-year, prospective, longitudinal study. CFP participants include 674 Mexican-American families with a fifth grade child (MAge=10.4, SD =.61, 50% female) drawn at random from school rosters during the 2006–2007 and 2007–2008 school years. The sub-study was designed to examine neurobiological mechanisms in the etiology and development of depression in the transition to early adulthood. Therefore, youths with elevated but sub-clinical depressive problems were oversampled from the CFP, using counts of adolescents’ self-reported symptoms in 9th grade (age 14–15) based on the Diagnostic Interview Schedule for Children-IV (DISC-IV; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000) and the Anhedonic Depression and General Distress subscales of the Mood and Anxiety Symptom Questionnaire (Watson & Clark, 1991). The sample consisted of adolescents whose symptom scores ranged above the median on all 3 measures of depression (N=43), on 2 measures (N=64), on 1 measure (N=68), and adolescents who were at or below the median on all 3 measures (N=54), ensuring variability in symptoms. No participants met diagnostic criteria for major depressive disorder based on the CDISC-IV at the time of sample selection.
2.2. Procedures
At age 16–17, adolescents and one parent visited a hospital research facility, where they participated in a functional magnetic resonance imaging (fMRI) scan with simultaneous electrocardiogram (ECG) and skin conductance recording. Baseline ECG and skin conductance measurements were collected before the scan while participants were lying on the scanner bed, outside of the scanner for 3 minutes. Adolescents then completed a structural scan and three fMRI tasks. The emotional faces task was the third and final fMRI task. All participants also completed a reward task and a social exclusion task, as part of the approximately 90–120 minute scan protocol. As such, 36 participants did not complete the faces task due to time constraints.
2.3. The Emotional Faces Task
As described in other work (Vilgis et al., 2018; Weissman et al., 2017), the emotional faces task (Guyer, Choate, Grimm, Pine, & Keenan, 2011) was used to examine neural response to facial expressions of emotion while attentional focus was constrained in two different ways (Figure 1). Stimuli were assembled from several validated sets of greyscale emotional face images (Ebner, Riediger, & Lindenberger, 2010; Goeleven, De Raedt, Leyman, & Verschuere, 2008; Minear & Park, 2004; E. E. Nelson, 2004; Tone, Erin B., Schmidt, S. M., Davis, n.d.; Tottenham, Borscheid, Ellertsen, Marcus, & Nelson, 2002). Participants viewed 12 sad, 12 angry, 12 happy, and 12 neutral faces portrayed by 48 unique actors. While viewing each picture, participants responded using their fingers on one hand to one of two questions: “How sad does this person make you feel?” or “How wide is the nose?” (1=Not at all to 5=Very much so). Each actor portraying the faces was presented only once to each participant, displaying one of the four emotions at random, but across participants all actors were displayed with all four expressions. The task consisted of 12 blocks of 44 seconds each, which began with instructions (displayed for 4000 ms) directing participants to rate sadness or nose width for all faces presented in that block. Ten stimuli (displayed for 3000 ms each) were then presented in pseudorandom order and 2 of which were crosshair fixation points (i.e., “+”). Thus, each block included 2 presentations of each of the 4 emotions. An inter-trial interval of 750–1250 milliseconds occurred between blocks, with an average of 1000 milliseconds. With jittered inter-trial intervals, each block varied in length from 43 to 45 seconds with an average length of 44 seconds across blocks for all participants. Blocks were presented in random order. The 12 trial blocks were completed in 9 minutes and 20 seconds.
Figure 1: The Emotional Faces Task.
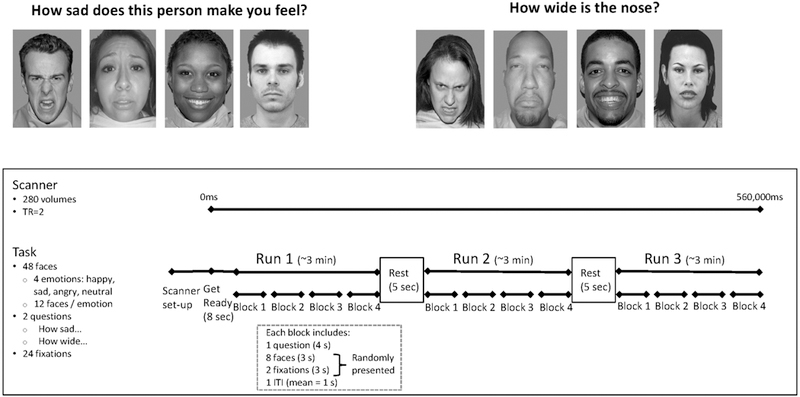
The task consisted of 12 blocks separated into 3 runs with 5 seconds of rest in between. Each block consisted of one question, either “How sad does this person make you feel?” or “How wide is the nose?” followed by 2 fixation crosses and 8 faces, 2 displaying each of the 4 emotions: sad, angry, happy, and neutral. The task lasted 560 seconds, during which 280 volumes were recorded, each collected over the course of 2 seconds.
2.4. FMRI image acquisition and preprocessing
A fMRI scan was conducted on a Siemens 3T TIM Trio MRI scanner with a 32-channel head coil (voxel size=3.5×3.5×3.5 mm, slices=35, slice thickness=3.5 mm, repetition time=2000 ms, echo time=27 ms, flip angle=80 degrees, interleaved slice geometry, field of view=224 mm). Images were T2-weighted. The first three acquired volumes were discarded to ensure magnet stabilization. Preprocessing was conducted using the FMRIB Software Library (FSL; Smith et al., 2004) and Analysis of Functional NeuroImaging (AFNI) software (Cox, 1996). Each participant’s functional data were co-registered with their brain-extracted structural images and normalized to Montreal Neurological Institute (MNI) stereotaxic space using FSL’s two-stage registration method via FLIRT. Alignment was visually confirmed for all participants. One participant was excluded from further analysis due to incomplete acquisition that resulted in no coverage of dorsal cortical regions. Normalized data then underwent preprocessing and first-level analysis using AFNI’s afni_proc.py. Preprocessing consisted of slice timing correction, rigid body motion correction with six degrees of freedom, spatial smoothing with a 6 mm half-maximum Gaussian kernel, and censoring of volumes with head motion greater than 1 mm from the previous volume. For 9 participants, this resulted in more than 25% of their data being censored. These participants were excluded from further analyses.
2.5. ECG data acquisition and processing
ECG data were collected simultaneously with the fMRI scan using three electrodes on the chest connected with Biopac fMRI compatible wireless signal logging (Biopac Systems, USA) through Siemens’ telnet MPCU, with a sampling frequency of 400 Hz. Due to human error or equipment malfunction, ECG data were completely missing for 27 participants. These participants were excluded from further analyses. Equipment malfunction resulted in premature termination of ECG data collection during the emotional faces task for eight participants. Only blocks in which these participants had ECG data were included in later analyses. Data were converted into an ASCII formatted string of amplitude values that was then fed into Mindware HRV software (Mindware Technologies, Gahanna, OH). The inter-beat-interval sequence was used to calculate RSA. Inter-beat intervals were measured by the elapsed time between subsequent local maxima in the QRS complex (R-spikes) (Berntson et al., 1997). ECG data were inspected visually for appropriate identification of R-spikes by trained research assistants who edited the data when the automated software misidentified the R-spikes. Due to high frequency noise resulting from MRI interference, data from six participants were too noisy to identify R-spikes. These participants were excluded from further analyses, resulting in 33 excluded for inadequate RSA data. The specific frequency band used to quantify RSA was 0.12–0.4, an appropriate respiratory frequency band for 16–17 year olds (Shader et al., 2018). Mindware computes RSA as the natural log of spectral power in this frequency band. Baseline RSA was computed in 30-second epochs, which were then averaged within each of the baselines. 30 second epochs provide sufficient respiratory cycles for computation of HRV-HF. Having multiple 30 sec epochs within a baseline period, which are then averaged, serves to minimize the effects of marginal outliers that fall within the individual’s range of values and therefore are not removed during the editing process, yet may slightly bias any given epoch toward lower or higher HRV-HF calculations (Lewis, Furman, McCool, & Porges, 2012).
RSA during the task was calculated within each of the 40s blocks of the Faces trial, during which adolescents viewed 8 faces depicting 4 different emotions and rated them on either subjective sadness or nose width. For analyses of relations between RSA and MRI activity, RSA across the 12 blocks of the task was within-subject mean-centered in order to look at individual variability of RSA across the blocks in relation to individual variability in brain activity across the blocks.
2.6. Skin conductance data acquisition and preprocessing
Skin conductance data were acquired simultaneously with the scan from two electrodes on the base of the palm of the nondominant hand, using a Biopac MP150 system and AcqKnowledge 4.1 software (Biopac Systems, USA). The gain was 10 microsiemens. Due to human error or equipment malfunction, data were completely missing for 23 participants. Using Mindware EDA software (Mindware Technologies, Gahanna, OH), a rolling filter was applied to account for high frequency noise from the fMRI signal. The number of nonspecific SCRs, thresholded at an amplitude change of .05 microsiemens, was counted for each block (Braithwaite, Watson, Robert, & Mickey, 2013). These counts were then within-subject mean-centered. The frequency of nonspecific SCRs, sometimes referred to as electrodermal lability, is a valid measure of SNS activity, associated with subjective arousal, negative emotion, and cardiac measures of SNS activity (Kelsey, 1991; Nikula, 1991). It was selected as an index of SNS activity, because the frequency of nonspecific SCRs does not have a tendency toward low frequency drift across long tasks (Braithwaite et al., 2013), unlike both skin conductance level (Braithwaite et al., 2013) and the blood oxygen level dependent signal (Smith et al., 1999), which do, risking spurious associations with brain regions with high spatial intensity gradients. Number of SCRs were counted within each of the 40s blocks of the Faces trial, during which adolescents viewed 8 faces depicting 4 different emotions and rated them on either subjective sadness or nose width. These counts were then centered around the mean for each subject.
2.7. Analyses
After excluding participants from the 229 enrolled for not completing the fMRI task (N=36), problems with the fMRI (N=10) and for problems with the ECG data (N=33) and/or skin conductance (N=23) signals, the final sample consisted of 135 adolescents (MAge = 17.13, SDAge = .44, 65 female), 127 of whom had complete data. Partial data (7–8 blocks) were included for the other eight participants. We modeled the faces task as a block design with independent regression coefficients calculated for each of the 12 blocks using AFNI’s 3dDeconvolve. To identify the neural mechanisms associated with within-person fluctuations in RSA and SC, mixed-effects modelling was conducted using AFNI’s 3dlme (Chen, Saad, Britton, Pine, & Cox, 2013). Within-subject effects on neural activity were determined for RSA and SCR fluctuations, attention condition (i.e., ratings: “How sad…” or “How wide…”), and their interaction (code included in Supplemental Materials). Group analyses were conducted within a region of interest (ROI) created by combining the following bilateral regions from the TT Daemon atlas in AFNI: medial frontal gyrus, rectal gyrus, anterior cingulate, insula, and amygdala (16,855 voxels; Figure 2), as well as at the whole brain level. The atlas-based ROI encompasses the non-brainstem regions that have been most commonly theorized and empirically shown to regulate and perceive ANS activity (e.g. Critchley, 2009; Thayer & Lane, 2009). Cluster thresholding was determined using AFNI’s 3dClustSim program (updated 7/2016; Cox, Chen, Glen, Reynolds, & Taylor, 2017), which generates Monte Carlo simulations to determine appropriate cluster sizes, and AFNI’s 3dFWHMx program, which accounts for the number of voxels and the intrinsic spatial autocorrelation in the data residuals. Based on output from these programs, a voxel-wise threshold of t = 3.280 (p = 0.001) with a minimum cluster size of 28 voxels within the ROI. At the whole brain level, a minimum cluster size of 90 voxels was used, to set the overall alpha in both cases at .05.
Figure 2: Region of interest (ROI).
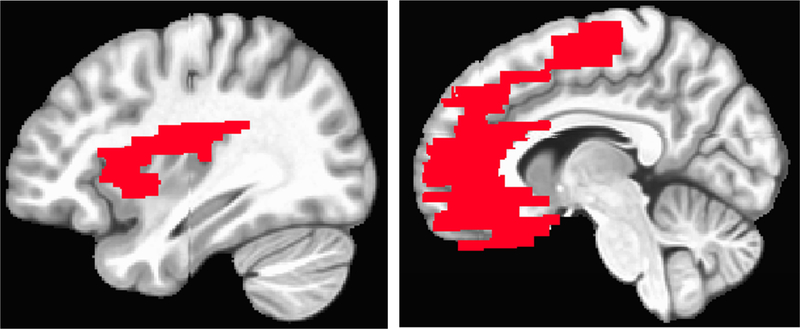
A region of interest made up of brain regions previously associated with autonomic activity was created by combining the following bilateral regions from the TT Daemon atlas in AFNI: medial frontal gyrus, rectal gyrus, anterior cingulate, insula, and amygdala (16,855 voxels).
To test whether individual differences in ANS activity at rest related to the strength of brain-ANS coupling, regression coefficients were extracted from the regions that demonstrated a significant relation between their activity and within-person fluctuations in RSA and the number of SCRs. Multilevel structural equation modelling in MPlus (Muthén & Muthén, 2017) was then used to identify the random component of the relation between mean-centered activity in each significant cluster and its corresponding autonomic indicator. The relation between this random slope and baseline ANS activity was then evaluated for each region (example code included in Supplemental Materials). These models were intended to probe the nature of the already-identified significant associations between each brain region and ANS activity. Both Bonferroni corrected and uncorrected significance thresholds are therefore used for each model.
3. Results
3.1. Autonomic Activity
PNS.
RSA did not differ significantly between baseline and the task (t = 1.48, n.s.). RSA also did not differ on average when participants rated “How sad does this person make you feel?” compared to “How wide is the nose?” (t = .62, n.s.). Within-person variance accounted for a significant 27% of the total variance in RSA (χ2 = 2158, df = 1570, p < .01). After within-subject mean centering, the SD of RSA across all blocks and all subjects was .61.
SNS.
The number of SCRs per minute was significantly greater at baseline than during the task (t = −3.27, p < .01; Table 1). During the task, the number of SCRs per minute did not differ on average during the task when participants rated “How sad does this person make you feel?” compared to “How wide is the nose?” (t = .58, n.s.). Between-person variance accounted for a significant 36% of the total variance in SCRs (χ2 = 2441, df = 1570, p < .01). After within-subject mean centering, the SD of the number of SCRs across all blocks for all subjects was 1.83.
Table 1:
Descriptive statistics of demographic characteristics and study variables
| Variable | M | SD |
|---|---|---|
| Gender (1=female; 0=male) | 0.48 | - |
| Age at scan (years) | 17.13 | .44 |
| RSA baseline | 6.78 | 1.01 |
| RSA task (How sad…) | 6.56 | 1.06 |
| RSA task (How wide…) | 6.57 | 1.07 |
| SCR/minute baseline | 4.97 | 4.07 |
| SCR/minute task (How sad…) | 3.83 | 3.34 |
| SCR/minute task (How wide…) | 3.75 | 3.43 |
Note: N = 135. RSA = respiratory sinus arrhythmia, measured in ln(msec)2. SCR = skin conductance response; Skin conductance was measured in microsiemens with SCR defined as an increase exceeding .05 microsiemens.
3.2. Attention-Specific Brain Activity
On average, participants responded to the question, “How sad does this person make you feel?” with a rating of 1.69 out of 5 (SD = .51) and to the question, “How wide is the nose?” with a rating of 2.28 out of 5 (SD = .32). When adolescents rated their subjective emotional state (i.e., “How sad does this person make you feel?”), they exhibited greater activation in the left superior temporal gyrus (i.e. temporoparietal junction) compared to when they rated a non-emotional feature of the face (i.e., “How wide is the nose?”; see Supplemental Materials).When adolescents judged nose width relative to their subjective emotional state, they exhibited greater activation in five clusters with their peak activations in right superior parietal lobule, left inferior parietal lobule, left precuneus, left middle occipital gyrus, and right inferior temporal gyrus (see Supplemental Materials).
3.3. Brain-Autonomic Coupling
RSA across the emotional faces fMRI task was inversely related to activity in a cluster located in the vmPFC (Table 2, Figure 3) in the ROI analysis. Whole-brain analyses revealed no other regions demonstrating significant associations with RSA. The number of SCRs across the emotional faces fMRI task was inversely related to activity in the dmPFC (Table 2, Figure 3) in the ROI analysis. Two larger clusters in the dmPFC were also identified in whole-brain analyses. Additionally, whole-brain analyses revealed a significant positive relation involving within-subject change in the number of SCRs and activity in a cluster in the left parahippocampal gyrus, including the left hippocampus, and a significant negative relation with activity in clusters located in the right ventral precuneus, right middle temporal gyrus, and right inferior parietal lobule. There were no significant interactions between what participants judged in a given task condition and either RSA or number of SCRs in relation to activity in the ROIs. Supplemental materials include results indicating significant interactions in whole-brain analyses.
Table 2:
Regions with significant within-subject relations between autonomic and brain activity
| Voxels | Peak (x, y, z) | Region | BA | Peak voxel t-score |
|---|---|---|---|---|
| RSA (ROI) | ||||
| 50 | 0, 52, −14 | Left Medial Frontal Gyrus | 11 | −3.96 |
| SCR (ROI) | ||||
| 95 | ‒2, 50, 42 | Left Medial Frontal Gyrus | 9 | ‒4.51 |
| SCR (Whole Brain) | ||||
| 172 | ‒24, −40, 6 | Left Parahippocampal Gyrus | 30 | 3.98 |
| 156 | ‒4, 68, 18 | Left Medial Frontal Gyrus | 10 | ‒3.86 |
| 135 | ‒2, 50, 44 | Left Medial Frontal Gyrus | 8 | ‒4.14 |
| 96 | 2, −70, 28 | Right Precuneus | 31 | ‒3.99 |
| 104 | 60, −62, −4 | Right Middle Temporal Gyrus | 21 | ‒4.02 |
| 91 | 44, −42, 60 | Right Inferior Parietal Lobule | 40 | ‒3.34 |
Note: Peak (x, y, z) = MNI coordinates for the voxel with the highest coefficient within each cluster; BA = Brodmann’s area; ROI = region of interest; N = 135, Voxel-wise threshold: t = 3.280, p = 0.001, Minimum cluster (ROI) = 28 voxels, Minimum cluster (Whole Brain) = 90 voxels; alpha <0.05.
Figure 3: Brain regions with activity corresponding to within-person fluctuations in RSA and number of SCRs.
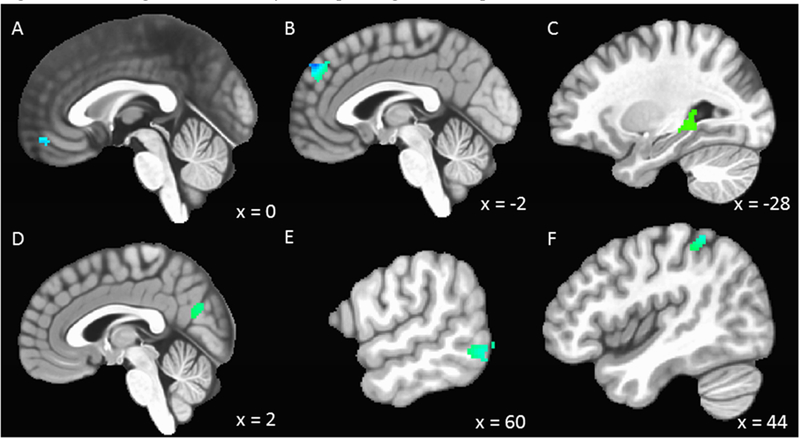
N=135. RSA = respiratory sinus arrhythmia; SCRs = skin conductance responses. (A) Increases and decreases in RSA were inversely related to activity in the ventral medial frontal gyrus. (B) Increases and decreases in the number of SCRs were inversely related to activity in the dorsal medial frontal gyrus. Whole brain analyses revealed a positive relation between change in the number of SCRs and activity in the left parahippocampal gyrus (C) and a negative relation with activity in the precuneus (D), right middle temporal gyrus (E), and right inferior parietal lobule (F). Voxel-wise threshold: t = 3.280, p = 0.001, Minimum cluster (ROI) = 28 voxels, Minimum cluster (Whole Brain) = 90 voxels; alpha <0.05.
3.4. Relations between baseline autonomic activity and brain-autonomic coupling
Figure 4 depicts the results of multilevel structural equation modelling to identify the relation between baseline ANS activity and brain-ANS coupling. There was a significant positive relation between baseline RSA and the random slope (S) of the relation between vmPFC activity and RSA (B = 3.29, S.E. = 1.66, p < .05, R2 = .097; see Figure 4A), but only without the application of Bonferroni correction for examining six models. This means that adolescents whose vmPFC-RSA coupling was more negative had lower baseline RSA, whereas adolescents whose vmPFC-RSA coupling was more positive had higher baseline RSA. Coupling between left hippocampus-SCR was significantly related to baseline SCR/min, including with the application of Bonferroni correction for the six models (B = .920, S.E. = .305, p < .008, R2=.224; see Figure 4C). Adolescents whose hippocampus-SCR coupling was more positive had more SCRs/min at baseline. Neither dmPFC-SCRs (Figure 4B), precuneus-SCRs (Figure 4D), right middle temporal gyrus-SCRs (Figure 4E), nor right inferior parietal lobule-SCRs coupling (Figure 4F) were significantly related to baseline SCRs/min.
Figure 4: Multilevel structural equation model testing the relation between brain-autonomic coupling and baseline autonomic activity.
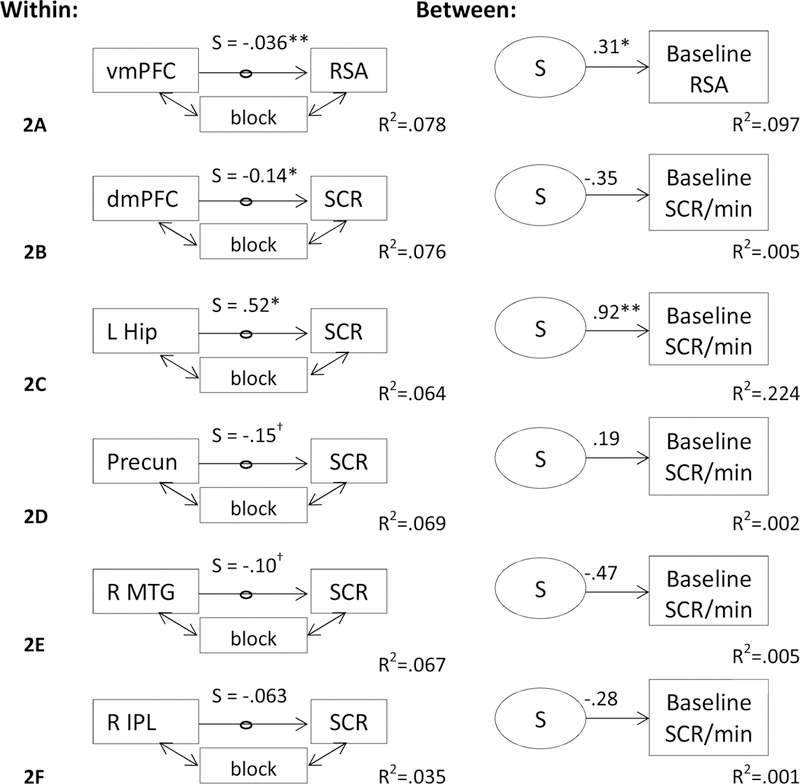
Betas were extracted from the significant clusters yielding significant (cluster α < .05) brain-autonomic coupling. Within subjects, latent variables were then created based on the regression coefficients of the relation between brain and autonomic activity, controlling for the effects of task block (i.e., detrending). The relation between ventromedial prefrontal cortex (vmPFC) activity and RSA was significant and significantly predicted baseline RSA. The relation between left hippocampus (L Hip) activity and SCR was significant and significantly predicted baseline SCR/min; Precun = precuneus, R MTG = right middle temporal gyrus, R IPL = right inferior parietal lobule. All coefficients are unstandardized. †p<.10, *p<.05, **p<.008 (Bonferroni correction). N=135.
4. Discussion
In the current study, we investigated brain mechanisms involved in the coordination of autonomic activity during emotion processing in adolescents. Activity in the vmPFC was inversely related to PNS activity, such that increased brain activity was associated with decreased parasympathetic activity. This suggests either that the vmPFC has an inhibitory influence on PNS activity or that through interoceptive inputs, vmPFC registers visceral manifestations of PNS withdrawal. Similarly, activity in the dmPFC was inversely related to SNS activity, such that increased brain activity was associated with decreased sympathetic activity. Activity in the ventral precuneus, right middle temporal gyrus, and right inferior parietal lobule also increased when SNS activity decreased. This suggests that activity in these regions may have an inhibitory influence on the SNS. Hippocampus activity was positively associated with SNS activity, suggesting an excitatory influence of the hippocampus on the SNS.
It is notable that activity of distinct regions of the mPFC was inversely associated with PNS versus SNS activity. Activity in the mPFC has been consistently observed in studies of neurovisceral integration (Critchley, 2005; Thayer et al., 2012), but studies with adults have typically found vmPFC activity to be positively associated with HRV (Thayer & Lane, 2009) and negatively associated with skin conductance level (Nagai et al., 2004). This discrepancy could be attributable to 1) changes in prefrontal-limbic regulatory circuitry occurring between adolescence to adulthood, 2) changes in the functional role of the vmPFC with regard to regulating the ANS and integrating cognitive and interoceptive information, or 3) a manifestation of differences in task and analytic approach that are not developmental in nature.
Amygdala-mPFC connectivity has been found to shift from positive to negative during development (Gee et al., 2013). Although this shift has been estimated to occur early in adolescence, around age 10 on average, many adolescents over the age of 10 demonstrate positive amygdala-mPFC connectivity, and adults’ show significantly more negative amygdala-mPFC connectivity on average relative to adolescents (Gee et al., 2013). It is therefore plausible that vmPFC-PNS coupling undergoes a similar shift from negative to positive.
The vmPFC is thought to serve as a hub of multifaceted functionality involved in the generation of affective meaning (Roy, Shohamy, & Wager, 2012), including approach and avoidance valuation, visceromotor control, and interoception. A shift from negative to positive vmPFC coupling with PNS activity may therefore reflect a shift in the vmPFC’s role from a bottom-up interoceptive processing of emotional arousal, to a more top-down visceromotor influence. Although bottom-up interoceptive processing might promote the emotionally reactive phenotype of adolescence, a stronger top-down visceromotor influence may promote a flexible, efficient, regulated, and recovery-oriented adult phenotype through positive mobilization of the PNS.
The negative association we documented here between RSA and vmPFC activity during emotion processing contrasts with previous results (Gianaros, Van der Veen, & Jennings, 2004; Lane et al., 2009), but the difference may be attributable to developmental differences in autonomic system regulation between adolescents and adults expected to emerge after the age of 16–17. However, differences between our findings and past findings may also be attributable to the task design and analytic approach used in this study. We employed a fully within-person design and used a task that required adolescents to engage in emotion processing. Recent models of neurovisceral integration have proposed that regions of the mPFC support regulation of autonomic functioning based on integration of sensory input and past experiences, with the vmPFC being more involved in integrating interoceptive feedback and the dmPFC being more involved in integrating higher level cognitive information (Smith, Thayer, Khalsa, & Lane, 2017). The observed negative association between RSA and vmPFC activity within-subjects may therefore be indicative of the vmPFC integrating visceral manifestations of PNS withdrawal. However, negative vmPFC-RSA coupling was observed across the entire task, not just during the blocks when adolescents reflected on their own emotions. In addition, the significant positive relation between individual differences in baseline RSA and vmPFC-RSA coupling suggests that the strength of the inhibitory connections between PNS and vmPFC activity is not only a phasic response of registering RSA withdrawal, but also an indicator of the tonic set point of the PNS, independent of context.
The negative association between the number of SCRs and dmPFC activity during emotion processing may reflect cognitive regulation of emotional reactivity resulting in inhibition of sympathoexcitatory mechanisms. Like the dmPFC, the ventral precuneus (Zhang & Li, 2012) and middle temporal gyrus are regions that comprise the default mode network (DMN) and have been found previously to demonstrate inverse activity patterns with skin conductance (Nagai et al., 2004), potentially indicating that DMN activity downregulates fight or flight responding. Importantly, unlike brain-PNS coupling, the patterns of brain-SNS coupling we observed here are more or less consistent with previous reports in adults. Individual differences in coupling of DMN regions with SNS activity were not associated with SCRs/min at rest. Therefore, while regional DMN activity was inversely related to SNS activity, individual differences in the strength of that coupling do not appear to be the source of tonic set points in SNS activity. Rather, activation of regions in the DMN appears to elicit phasic decreases in SNS activity, regardless of the baseline set point. This may reflect the role of the dmPFC and other DMN regions in emotion regulation. Just as dmPFC activity increases to inhibit amygdala reactivity in emotional situations, it may phasically downregulate SNS activity as well.
The positive association between activity in parahippocampal gyrus and SCRs has also been observed previously in adults (Nagai et al., 2004). In addition, viral transneuronal labelling has found SNS circuitry to originate in the rat ventral hippocampus (Westerhaus & Loewy, 2001), and epileptogenic activity in the cat hippocampus is associated with increased SNS activity (Lathers, Schraeder, & Turner, 1993). The hippocampus is central to learning, memory and stress-related feedback loops (Lupien & Lepage, 2001). The association between increased skin conductance responding and hippocampus activity potentially demonstrates the sensitivity of the hippocampus to stress-related signals in the body as markers of affective salience, which may be especially pronounced during adolescence. Moreover, individuals with stronger hippocampus-SCR coupling had more SCRs/min at baseline, suggesting that hippocampus activity plays a role in maintaining baseline SNS arousal.
Notably, significant associations with fluctuations in RSA and SCRs were not observed in the amygdala, anterior insula, or ACC as expected. This may reflect differences in neurovisceral integration between adolescents and adults, or it may be a product of study design, which measured RSA and the number of SCRs across 40 second blocks. Although this approach allowed us to investigate interrelations among brain function, PNS activity, and SNS activity simultaneously over the same time course, it may not have captured the same engagement of brain activation in these regions that is typically associated with more rapid phasic changes in ANS activity.
While this study has many strengths, including a large, adolescent sample, simultaneous measures of RSA, SCR, and fMRI, and a full within-subjects design, it also has some limitations. First, although this study expanded the age range of extant studies on neurovisceral integration, and found differences in vmPFC-PNS coupling compared to what has been found with adults, we could not test for age-related differences within this single-age cohort. Future work could use longitudinal repeated-measures designs, or recruit participants of various age ranges from middle childhood to adulthood, to evaluate developmental changes in neurovisceral integration. Second, while the stimuli used in the fMRI task were emotional in nature, they did not elicit significant increases in autonomic arousal on average, although there was considerable variability in ANS activity, which was necessary for documenting relations with brain activity. Future work could employ more evocative emotional stimuli, such as film clips or social evaluative stressors to elicit stronger changes in autonomic activity and identify associated brain mechanisms.
5. Conclusion
Adolescents’ fluctuations in SNS and PNS activity, as indicated by SCRs and RSA respectively, were associated with specific patterns of brain activity during emotion processing. Activity in the vmPFC was associated with decreases in RSA, indicating a potential developmental difference between adolescents and adults, for whom vmPFC activity is usually associated with increases in RSA, and reflecting the role of this region in regulating PNS activity. Activity in the dmPFC and other regions within the DMN was associated with decreases in the number of SCRs, potentially reflecting the role of this network in downregulating fight or flight responding, as has been documented previously in adults. Activity in the hippocampus was associated with increases in the number of SCRs, consistent with the role of the hippocampus in regulating and integrating stress-related signals from the body. These findings expand our understanding of the neurophysiological mechanisms involved in integrating and regulating the bodily manifestations of emotion processing.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants R01-MH098370 (Guyer, Hastings), R01-DA017902 (Robins, Conger, Widaman), and 5T32MH020006-19, the National Science Foundation (IBSS 1327768), and a U.S. Department of Health and Human Services Poverty Research Center grant. We thank Nicole Welindt, Timothy Bell, Elizabeth Kim, Lucia Leon, and Nilsen Gomez-Tabal for assisting with recruitment and data collection and processing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
None of the authors have any conflicts or competing interests to report.
References
- Arnett JJ (1999). Adolescent storm and stress, reconsidered. American Psychologist, 54(5), 317–326. 10.1037/0003-066X.54.5.317 [DOI] [PubMed] [Google Scholar]
- Berntson GG, Thomas Bigger J, Eckberg DL, Grossman P, Kaufmann PG, Malik M, … Van Der Molen MW. (1997). Heart rate variability: Origins methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. 10.1111/j.1469-8986.1997.tb02140.x [DOI] [PubMed] [Google Scholar]
- Blakemore S-J, & Mills KL (2014). Is Adolescence a Sensitive Period for Sociocultural Processing? Annual Review of Psychology, 65(1), 187–207. 10.1146/annurev-psych-010213-115202 [DOI] [PubMed] [Google Scholar]
- Braithwaite J, Watson D, Robert J, & Mickey R (2013). A Guide for Analysing Electrodermal Activity (EDA) & Skin Conductance Responses (SCRs) for Psychological Experiments … (Vol. 49). Springer: Cambridge Press; http://doi.org/10.1017.S0142716405050034 [Google Scholar]
- Casey BJ, Jones RM, & Hare TA (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124(1), 111–126. 10.1196/annals.1440.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Saad ZS, Britton JC, Pine DS, & Cox RW (2013). Linear mixed-effects modeling approach to FMRI group analysis. NeuroImage, 73, 176–190. 10.1016/J.NEUROIMAGE.2013.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research, 29(3), 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). fMRI clustering and false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 114(17), E3370–E3371. 10.1073/pnas.1614961114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD (2005). Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology, 493(1), 154–166. 10.1002/cne.20749 [DOI] [PubMed] [Google Scholar]
- Critchley HD (2009). Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. International Journal of Psychophysiology, 73(2), 88–94. 10.1016/j.ijpsycho.2009.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, & Dolan RJ (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7(2), 189–195. 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- Damasio AR, Everitt BJ, & Bishop D (1996). The Somatic Marker Hypothesis and the Possible Functions of the Prefrontal Cortex [and Discussion]. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 351(1346). [DOI] [PubMed] [Google Scholar]
- Del Piero LB, Saxbe DE, & Margolin G (2016). Basic emotion processing and the adolescent brain: Task demands, analytic approaches, and trajectories of changes. Developmental Cognitive Neuroscience, 19, 174–189. 10.1016/j.dcn.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Riediger M, & Lindenberger U (2010). FACES-a database of facial expressions in young, middle-aged, and older women and men: Development and validation. Behavior Research Methods, 42(1), 351–362. 10.3758/BRM.42.1.351 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M (2005). Stability of respiratory sinus arrhythmia in children and young adolescents: A longitudinal examination. Developmental Psychobiology, 46(1), 66–74. 10.1002/dev.20036 [DOI] [PubMed] [Google Scholar]
- El-Sheikh M (2007). Children’s skin conductance level and reactivity: Are these measures stable over time and across tasks? Developmental Psychobiology, 49(2), 180–186. 10.1002/dev.20171 [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, … Tottenham N. (2013). A Developmental Shift from Positive to Negative Connectivity in Human Amygdala-Prefrontal Circuitry. Journal of Neuroscience, 33(10), 4584–4593. 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentzler AL, Rottenberg J, Kovacs M, George CJ, & Morey JN (2012). Atypical development of resting respiratory sinus arrhythmia in children at high risk for depression. Developmental Psychobiology, 54(5), 556–567. 10.1002/dev.20614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Van der Veen FM, & Jennings JR (2004). Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology, 41(4), 521–530. 10.1111/1469-8986.2004.00179.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeleven E, De Raedt R, Leyman L, & Verschuere B (2008). The Karolinska directed emotional faces: A validation study. Cognition and Emotion, 22(6), 1094–1118. 10.1080/02699930701626582 [DOI] [Google Scholar]
- Guyer AE, Choate VR, Grimm KJ, Pine DS, & Keenan K (2011). Emerging Depression Is Associated With Face Memory Deficits in Adolescent Girls. Journal of the American Academy of Child & Adolescent Psychiatry, 50(2), 180–190. 10.1016/j.jaac.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Silk JS, & Nelson EE (2016). The neurobiology of the emotional adolescent: From the inside out. Neuroscience & Biobehavioral Reviews, 70, 74–85. 10.1016/j.neubiorev.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Kahle SS, & Han GH-P (2014). Developmental affective psychophysiology: Using physiology to inform our understanding of emotional development. Contributions to Human Development, 26, 13–28. 10.1159/000354347 [DOI] [Google Scholar]
- Hinnant JB, Elmore-Staton L, & El-Sheikh M (2011). Developmental trajectories of respiratory sinus arrhythmia and preejection period in middle childhood. Developmental Psychobiology, 53(1), 59–68. 10.1002/dev.20487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenstein T, McNeely A, Eastabrook J, Mackey A, & Flynn J (2012). Sympathetic and parasympathetic responses to social stress across adolescence. Developmental Psychobiology, 54(2), 207–214. 10.1002/dev.20582 [DOI] [PubMed] [Google Scholar]
- Kelsey RM (1991). Electrodermal Lability and Myocardial Reactivity to Stress. Psychophysiology, 28(6), 619–631. 10.1111/j.1469-8986.1991.tb01005.x [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, & Whalen PJ (2011). The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behavioural Brain Research, 223(2), 403–410. 10.1016/J.BBR.2011.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD (2008). Neural Substrates of Implicit and Explicit Emotional Processes: A Unifying Framework for Psychosomatic Medicine. Psychosomatic Medicine, 70(2), 214–231. 10.1097/PSY.0b013e3181647e44 [DOI] [PubMed] [Google Scholar]
- Lane RD, McRae K, Reiman EM, Chen K, Ahern GL, & Thayer JF (2009). Neural correlates of heart rate variability during emotion. NeuroImage, 44(1), 213–222. 10.1016/J.NEUROIMAGE.2008.07.056 [DOI] [PubMed] [Google Scholar]
- Larson RW, Moneta G, Richards MH, & Wilson S (2002). Continuity, Stability, and Change in Daily Emotional Experience across Adolescence. Child Development, 73(4), 1151–1165. 10.1111/1467-8624.00464 [DOI] [PubMed] [Google Scholar]
- Lathers CM, Schraeder PL, & Turner N (1993). The Effect of Phenobarbital on Autonomic Function and Epileptogenic Activity Induced by the Hippocampal Injection of Penicillin in Cats. The Journal of Clinical Pharmacology, 33(9), 837–844. 10.1002/j.1552-4604.1993.tb01960.x [DOI] [PubMed] [Google Scholar]
- Lewis GF, Furman SA, McCool MF, & Porges SW (2012). Statistical strategies to quantify respiratory sinus arrhythmia: are commonly used metrics equivalent? Biological Psychology, 89(2), 349–64. 10.1016/j.biopsycho.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, & Lepage M (2001). Stress, memory, and the hippocampus: can’t live with it, can’t live without it. Behavioural Brain Research, 127(1–2), 137–158. 10.1016/S0166-4328(01)00361-8 [DOI] [PubMed] [Google Scholar]
- Miller EM, & Shields SA (1980). Skin conductance response as a measure of adolescents’ emotional reactivity. Psychological Reports, Vol 46(2), 587–590. 10.2466/pr0.1980.46.2.587 [DOI] [Google Scholar]
- Minear M, & Park DC (2004). A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, and Computers, 36(4), 630–633. 10.3758/BF03206543 [DOI] [PubMed] [Google Scholar]
- Muthén L, & Muthén B (2017). Mplus user’s guide (8th ed.). Los Angeles: Author; http://doi.org/10.13155/29825 [Google Scholar]
- Nagai Y, Critchley H ., Featherstone E, Trimble M ., & Dolan R . (2004). Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. NeuroImage, 22(1), 243–251. 10.1016/j.neuroimage.2004.01.019 [DOI] [PubMed] [Google Scholar]
- Nelson EE (2004). Adolescent and adult facial expressions stimuli database: Section on development and affective neuroscience Bethesda, MD. [Google Scholar]
- Nelson EE, Jarcho JM, & Guyer AE (2016). Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–127. 10.1016/j.dcn.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikula R (1991). Psychological Correlates of Nonspecific Skin Conductance Responses. Psychophysiology, 28(1), 86–90. 10.1111/j.1469-8986.1991.tb03392.x [DOI] [PubMed] [Google Scholar]
- Patterson JC, Ungerleider LG, & Bandettini PA (2002). Task-Independent Functional Brain Activity Correlation with Skin Conductance Changes: An fMRI Study. NeuroImage, 17(4), 1797–1806. 10.1006/nimg.2002.1306 [DOI] [PubMed] [Google Scholar]
- Pfeifer MA, Weinberg CR, Cook D, Best JD, Reenan A, & Halter JB (1983). Differential changes of autonomic nervous system function with age in man. The American Journal of Medicine, 75(2), 249–258. http://doi.org/0002-9343(83)91201-9 [pii] [DOI] [PubMed] [Google Scholar]
- Porges SW (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. 10.1016/j.biopsycho.2006.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, & Maiti AK (1994). Vagal Tone and the Physiological Regulation of Emotion. Monographs of the Society for Research in Child Development, 59(2/3), 167 10.2307/1166144 [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, & Wager TD (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences, 16(3), 147–156. 10.1016/J.TICS.2012.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shader TM, Gatzke-Kopp LM, Crowell SE, Jamila Reid M, Thayer JF, Vasey MW, … Beauchaine TP. (2018). Quantifying respiratory sinus arrhythmia: Effects of misspecifying breathing frequencies across development. Development and Psychopathology, 30(1), 351–366. 10.1017/S0954579417000669 [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, Differences From Previous Versions, and Reliability of Some Common Diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry, 39(1), 28–38. 10.1097/00004583-200001000-00014 [DOI] [PubMed] [Google Scholar]
- Shields SA (1983). Development of Autonomic Nervous System responsivity in children: A review of the literature. International Journal of Behavioral Development, Vol 6(3), 291– 319. [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin R, … Ochsner KN. (2017). The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-to-dorsal shift in medial prefrontal recruitment. Developmental Cognitive Neuroscience, 25, 128–137. 10.1016/j.dcn.2016.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Lewis BK, Ruttimann UE, Ye FQ, Sinnwell TM, Yang Y, … Frank JA. (1999). Investigation of low frequency drift in fMRI signal. NeuroImage, 9(5), 526–533. 10.1006/nimg.1999.0435 [DOI] [PubMed] [Google Scholar]
- Smith R, Thayer JF, Khalsa SS, & Lane RD (2017). The hierarchical basis of neurovisceral integration. Neuroscience and Biobehavioral Reviews, 75, 274–296. 10.1016/j.neubiorev.2017.02.003 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, … Matthews PM. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, & Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews, 36(2), 747–756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. 10.1016/S0165-0327(00)00338-4 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2009). Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience and Biobehavioral Reviews 10.1016/j.neubiorev.2008.08.004 [DOI] [PubMed]
- Tone Erin B., Schmidt SM, Davis JS. (n.d.). The Georgia State University Diverse Face Stimulus Set Atlanta, GA. [Google Scholar]
- Tottenham N, Borscheid A, Ellertsen K, Marcus DJ, & Nelson CA (2002). Categorization of facial expressions in children and adults: Establishing a larger stimulus set. Journal of Cognitive Neuroscience, 74 Retrieved from https://scholar.google.com/scholar?cluster=12127419444031818341&hl=en&as_sdt=40000005&sciodt=0,22 [Google Scholar]
- Venables PH, & Mitchell DA (1996). The effects of age, sex and time of testing on skin conductance activity. Biological Psychology, 43(2), 87–101. 10.1016/0301-0511(96)05183-6 [DOI] [PubMed] [Google Scholar]
- Vilgis V, Gelardi KL, Helm JL, Forbes EE, Hipwell AE, Keenan K, & Guyer AE (2018). Dorsomedial Prefrontal Activity to Sadness Predicts Later Emotion Suppression and Depression Severity in Adolescent Girls. Child Development 10.1111/cdev.13023 [DOI] [PMC free article] [PubMed]
- Vink M, Derks JM, Hoogendam JM, Hillegers M, & Kahn RS (2014). Functional differences in emotion processing during adolescence and early adulthood. NeuroImage, 91, 70–76. 10.1016/j.neuroimage.2014.01.035 [DOI] [PubMed] [Google Scholar]
- Watson D, & Clark LA (1991). The mood and anxiety symptom questionnaire Unpublished Manuscript, University of Iowa, Department of Psychology, Iowa City. [Google Scholar]
- Weissman DG, Gelardi KL, Conger RD, Robins RW, Hastings PD, & Guyer AE (2017). Adolescent Externalizing Problems: Contributions of Community Crime Exposure and Neural Function During Emotion Introspection in Mexican-Origin Youth. Journal of Research on Adolescence, 28(2). 10.1111/jora.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhaus MJ, & Loewy AD (2001). Central representation of the sympathetic nervous system in the cerebral cortex. Brain Research, 903(1–2), 117–127. 10.1016/S0006-8993(01)02453-2 [DOI] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Ide JS, Luo X, Farr OM, & Li CR (2014). Ventromedial prefrontal cortex and the regulation of physiological arousal. Social Cognitive and Affective Neuroscience, 9(7), 900–8. 10.1093/scan/nst064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Luo X, Farr OM, & Li C shan R. (2012). Cerebral correlates of skin conductance responses in a cognitive task. NeuroImage, 62(3), 1489–1498. 10.1016/j.neuroimage.2012.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, & Li CR (2012). Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage, 59(4), 3548–62. 10.1016/j.neuroimage.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


