Abstract
Background:
Understanding the mechanisms behind exerting self-control may reveal why health behaviors are resistant to change. Activity in the right inferior frontal gyrus (rIFG) plays a role in self-control processes and may be modulated using transcranial direct current stimulation (tDCS).
Objective:
In this early phase behavioral research study, we investigated whether anodal stimulation over the rIFG with cathodal stimulation over the left IFG (versus sham) reduced chocolate consumption.
Methods:
Twenty-three healthy females (ages 18–35) completed two tDCS sessions (2.0 mA vs. sham; order counterbalanced) in a within-subject, double-blind, randomized design with a 4-week washout. Participants were self-reported “chocolate cravers” and restrained eaters. Self-report assessments on disinhibited eating were completed at intake. Delay discounting and inhibitory control were assessed at the remaining visits. During stimulation, participants completed an inhibitory control training task (chocolate go/no-go task) and were randomized to the chocolate no-go condition (inhibit all responses to chocolate cues) or the control condition (inhibit responses to chocolate cues on half the trials). Following stimulation, participants completed a 15-minute chocolate “taste test” with chocolate rating forms. Afterwards, staff measured the remaining chocolate to determine total consumption.
Results:
Contrary to our hypotheses, active tDCS significantly increased chocolate consumption vs. sham (mean=43.2 vs. 32.2, p=0.005) in both task conditions, but had no effect on chocolate ratings (ps>0.05). Higher delay discounting and self-reported disinhibited eating predicted greater consumption (ps<0.05).
Conclusions:
The results suggest widespread activation of the prefrontal cortex may reduce the ability to resist chocolate. Our data highlights important methodological considerations for conducting tDCS studies to target health behaviors.
Keywords: Brain stimulation, inhibitory control, food intake, inferior frontal gyrus, chocolate
Introduction
The CDC estimated 7 out of 10 deaths in 2014 were due to chronic diseases, which account for 86% of health care costs in the U.S. (2015; Gerteis, et al., 2014). Health risk behaviors, such as tobacco and alcohol consumption, unhealthy diet, physical inactivity, and unhealthy sleep contribute to many of these chronic conditions. However, modifying health behavior – including decisions to diet or exercise – is often resistant to change because it involves prioritizing the long-term gain of better health over the short-term costs of discomfort or inconvenience. Therefore, there is a critical need to better understand the mechanisms which underlie health risk behaviors and develop more effective strategies to prevent and manage these chronic conditions.
Advances in the neuroimaging field may advance our understanding of the brain activity patterns that influence risky health behaviors. Multiple labs, including our own, have shown that neural activity in brain regions associated with cognitive control and goal-directed behavior are important in successful smoking cessation (Froeliger, et al., 2017; Janes, Pizzagalli, & Richardt, 2010; Loughead, et al., 2015), and several of these brain regions are also critical in controlling eating behavior (Berridge, Ho, Richard, & DiFeliceantonio, 2010; Frankort, et al., 2015; Kishinevsky, et al., 2012). For example, inhibitory control processes that are important for regulating self-control are associated with activation in the right inferior frontal gyrus [rIFG; (Aron, Robbins, & Poldrack, 2014; Hampshire, Chamberlain, Monti, Duncan, & Owen, 2010; Luijten, et al., 2014; Swick, Ashley, & Turken, 2011; van Belle, Vink, Durston, & Zandbelt, 2014)]. Likewise, activation in the IFG has been associated with response to food cues (Hare, Camerer, & Rangel, 2009) and successful weight loss (DelParigi, et al., 2007; McCaffery, et al., 2009). It seems plausible that modulating activity in brain regions associated with self-control may promote healthy behavior changes.
A growing body of evidence supports the safety and potential utility of transcranial direct current stimulation (tDCS) for improving cognitive control (Demirtas-Tatlidede, Vahabzadeh-Hagh, & Pascual-Leone, 2013). Initial data suggest a single session of tDCS targeting the rIFG can improve inhibitory control processes without any adverse side effects (Cai, et al., 2016; Cunillera, Brignani, Cucurell, Fuentemilla, & Miniussi, 2016; Stramaccia, et al., 2015). Significant reductions in inhibitory control using a tDCS montage similar to that of the current study have been observed (Jacobson, Javitt, & Lavidor, 2011). Studies using another similar montage (i.e., electrodes placed directly on F3 and F4) have found tDCS effects on various domains, including reducing initial action latency (Heinze, et al., 2014), risk-taking behaviors (Fecteau, et al., 2007), and responses to desirable visual cues (i.e., formally abused drugs) (Conti & Nakamura-Palacios, 2014). In contrast, a single session of tDCS over the right dorsolateral prefrontal cortex (DLPFC) had no effect on food consumption, although self-reported impulsivity was associated with eating behavior (Georgii, Goldhofer, Meule, Richard, & Blechert, 2017). In light of these discrepant findings and potential heterogeneity in response to tDCS, more research is required to demonstrate whether tDCS treatment will facilitate health behavior changes.
One explanation for the heterogeneity of tDCS effects involves methodological differences in what subjects do during stimulation. Whether tasks are administered during or after the stimulation and the type and difficulty of the task (e.g., working memory vs. inhibitory control) may contribute to the differences across studies (Dedoncker, Brunoni, Baeken, & Vanderhasselt, 2016). While the neurobiological mechanisms of these tDCS effects have yet to be fully elucidated, the brief application of direct current may increase the likelihood of neural activity by altering membrane potentials. Combining tDCS with inhibitory control training may have synergistic effects on neuroplasticity (i.e., increase neural efficiency), which may yield improved performance on cognitive control measures. Future research must examine whether inhibitory control is associated with response to behavior change intervention, and if so, whether neuromodulation will modify inhibitory control. The strong body of evidence linking inhibitory control with health risk behaviors (i.e., disordered eating, chronic drug abuse; Houben, Nederkoorn, & Jansen, 2014; Jasinska, et al., 2012; Jentsch & Pennington, 2014; Smith, Mattick, Jamadar, & Iredale, 2014) suggests that combining tDCS with an inhibitory control training task may promote greater control over health risk behaviors.
In this early phase behavioral research study, we investigated whether tDCS over the rIFG, combined with an inhibitory control training task (Houben & Jansen, 2011), would reduce chocolate consumption— a highly palatable snack food— by using an ad-lib “taste test” paradigm in healthy young-adult females. We hypothesized that active tDCS, compared to sham stimulation, would reduce chocolate consumption and that these effects would be strongest among individuals trained to inhibit responses to chocolate cues.
Materials and Methods
Participants
All procedures were approved by the University of Pennsylvania Institutional Review Board and carried out in accordance with the Declaration of Helsinki. Participants were healthy young adult females between the ages of 18 and 35 who self-reported being a “chocolate craver”. Chocolate cravers are those who reported to (a) like chocolate; (b) be “very bad”/“bad”/“somewhat bad” at postponing a chocolate craving; (c) “moderately”/“very much like” to gain more control over their chocolate craving; and (d) find it “neutral”/‘somewhat difficult”/“very difficult” to gain more control over their chocolate craving (Meule & Hormes, 2015; Van Gucht, Soetens, Raes, & Griffith, 2014; Van Gucht, et al., 2008). Based on evidence suggesting restrained eaters may respond differently to inhibitory control training (Houben & Jansen, 2011), only females with scoring 15 or higher on a Cognitive Restraint scale (Karlsson, Persson, Sjostrom, & Sullivan, 2000) were eligible. Participation was restricted to females between the ages of 18 and 35 because chocolate is the most frequently craved food among women and women are more likely to report chocolate craving (Benton, Greenfield, & Morgan, 1998; Muller, Dettmer, & Macht, 2008; Pelchat, 1997; Van Gucht, et al., 2014).
All participants provided written informed consent and completed an in-person eligibility screen, including a breath alcohol test and a urine pregnancy test. Additional exclusion criteria included: planned pregnancy or breastfeeding; history of brain injury or seizures; presence of metal (other than dental apparatus) in the head; low or borderline intelligence [estimated IQ <90 on Shipley Institute of Living Scale (Zachary, 2000)]; and any impairment that would prevent task performance.
Procedures
Participants in this within-subject cross-over investigation completed two identical laboratory sessions (one involving active stimulation and the other involving sham stimulation). Session order was double-blind, randomized and counterbalanced, and sessions were scheduled between 9am and 5pm. Times were consistent within-subject (+/− two hours). Because menstrual cycle phase may influence food cravings (Hormes & Timko, 2011; McVay, Copeland, Newman, & Geiselman, 2012), sessions were scheduled approximately 28 days apart so that menstrual cycle phase was consistent within participant. During each 4-hour session, weight was measured and a food recall interview was conducted to assess food consumed since waking. Hunger ratings were collected upon arrival, and blood glucose (Abbott Diabetes Care Freestyle Freedom Lite Blood Glucose Monitoring System, Alameda, CA, USA) was measured upon arrival and again after the 2-hour waiting period prior to stimulation (included to control time since last meal). Participants completed self-report questionnaires and computerized inhibitory control tasks during the 2-hour waiting period (see below for task descriptions). Once tasks were complete, participants were permitted to read provided magazines (with images of food removed), but asked to refrain from using electronic devices and any other products that may affect appetite (e.g., chewing gum, snacks). After the waiting period, participants received active or sham stimulation while performing the inhibitory control training task. Following stimulation, the participants completed the ad-libitum chocolate “taste test”.
Inhibitory Control Training Task
Participants completed a training task while receiving tDCS. The task was based on a go/no-go task used in prior research and selected based on evidence that it is sensitive to differential responses to chocolate cues for non-chocolate food cues (Houben, Havermans, Nederkoorn, & Jansen, 2012; Houben & Jansen, 2011). The current task consisted of 2 blocks of 288 trials (576 trials total). During each block, participants were presented with pictures and instructed to press the space bar when a “go” cue was displayed, and to refrain from responding when a “no-go” cue was displayed. The go/no-go cues were the letters ‘p’ and ‘f’ (counterbalanced across participants), which were displayed randomly in one of four corners in each picture. Stimuli were four pictures of chocolate, four neutral pictures of non-food related household items (e.g., books, candle), and eight filler pictures (snack foods; e.g., cookies, candy, nuts). Filler stimuli were included to mask the goal of the study in order to avoid demand characteristics. Although the filler stimuli had the potential to affect participant appetite, the task previously demonstrated sensitivity to chocolate-specific cues, which was fundamental to our study based on our sample of “chocolate cravers”. All stimuli were drawn from the FoodPics database (Blechert, Meule, Busch, & Ohla, 2014) and were matched on contrast, complexity, valence, and arousal. All food-related stimuli were also matched on craving level reported in the database. Each trial simultaneously presented a picture and a go/no-go cue (1500 ms). During an initial practice block, a green circle is displayed after a correct inhibition/response (500 ms), and a red cross after an incorrect inhibition/response (500 ms).
For the between-subjects manipulation, participants were randomly assigned to one of two conditions: the “control condition” or the “chocolate no-go condition”. For both conditions, the “no-go” cues were presented on 25% of the trials (144 out of 576). In the “control condition”, the “no-go” cues were evenly divided between chocolate and neutral stimuli whereas, in the “chocolate/no-go condition”, all “no-go” cues were paired with chocolate stimuli. Table 1 depicts the number of “go” and “no-go” cues by stimulus type across both conditions. “Go” and “no-go” trials were always presented in random order. Because the two conditions had different numbers of chocolate and neutral stimuli, the primary outcome was the percentage of correct inhibitions for each type of stimuli.
Table 1.
No-go and Go cues by stimulus type for the inhibitory control training task
| Chocolate No-go Condition | Control Condition | |||
|---|---|---|---|---|
| # Go cues | # No-go cues | # Go cues | # No-go cues | |
| Chocolate | 0 | 72 | 108 | 36 |
| Neutral | 216 | 0 | 108 | 36 |
| Filler | 216 | 72 | 216 | 72 |
| Total Trials | 432 | 154 | 432 | 154 |
Ad-libitum Chocolate Consumption
During the ad-libitum chocolate “taste test”, participants were presented with three bowls, each containing 100g of a different type of chocolate (milk, semi-sweet, dark), and were told to eat “as much or as little” as they liked while completing assessments. Participants rated each type on the following characteristics using visual analog scales (VAS): smell, taste, intensity, appearance, taste persistence, and sweetness. After 15 minutes, research staff retrieved the bowls and weighed each one separately using a scale calibrated to 0.05 g (VWR® P-Series Portable Balance, [VWR, Radnor, PA]). Research staff were not present during the “taste test”, participants were not informed that chocolate consumption would be measured, and the verbal instructions delivered to participants were intentionally vague to avoid altering eating behavior due to demand characteristics.
Baseline Measures of Inhibitory Control
Stop Signal Task (SST):
In this task, participants view a series of arrows presented sequentially on a screen and are instructed to press labeled keyboard keys as quickly and as accurately as possible to indicate the direction the arrow faced. Following a 32-trial practice, audio stop signals are presented on 25% of trials for a 32-trial practice and three task blocks of 64 trials each. The initial stop delay in each block is 250 ms and adjusts by 50 ms increments depending on whether the participant is able to successfully inhibit a response (Logan, 1994; Logan, Schachar, & Tannock, 1997). The adjusting stop delay allows the determination of the delay at which inhibition occurs on ~50% of trials. Trials consist of a 500 ms warning stimulus followed by a 1000 ms go signal (left- and right-facing arrows) and a 1000 ms blank screen intertrial interval. The primary outcome is the stop signal reaction time (SSRT), which is the difference in mean reaction time on successful go trials and the mean stop delay on successful inhibition trials.
Food Cue Stop Task:
This task is a modified version of the Stop Signal Task described above. In this version, participants are instructed to respond as fast as possible to food-related pictures and filler stimuli (pictures of office items) by pressing keyboard keys (e.g., “z” for food-related pictures and “/” for pictures of office items; instructions are counterbalanced across participants). When the auditory stop signal is presented (25% of the trials), they must inhibit their response. Similar to the standard SST, stop delay adjusts in increments of 50 ms depending on whether the participant is able to inhibit the response. The task consists of 4 blocks of 64 trials. Two blocks contain pictures of high-calorie, palatable foods (e.g., ice cream, cookies, French fries) and two blocks contain pictures of low calorie, less palatable foods (e.g., broccoli, oranges, lettuce). During each block, 32 trials present food-related pictures and 32 trials present a neutral stimulus and the stop signals are distributed equally across stimulus type. SSRT to each stimulus type (high calorie, low calorie, and neutral) is the primary dependent variable and is calculated by subtracting the mean stop delay from the mean RT on go-trials for each trial type (task duration: ~15 minutes).
Delay Discounting Task:
In this paradigm, participants choose between a smaller hypothetical reward available immediately (e.g., $20 today) and a larger hypothetical reward available after a delay (e.g., $40 in a month). As in previous work, the immediate reward is fixed and the magnitude and delay of the larger reward varies from trial to trial. The primary behavioral outcome is the subject’s discount rate, estimated with a logistic regression that assumes a person’s decisions are a stochastic function of the difference in subjective value between the two options (Wileyto, Audrain-McGovern, Epstein, & Lerman, 2004). Keeping with standard behavioral findings (Kable & Glimcher, 2007, 2010; Kirby & Santiesteban, 2003; Mazur, 1987), we assume the subjective value (SV) is a hyperbolic function of the reward amount (A) and delay (D): SV=A/(1+kD), where k is the participant’s discount rate. Larger values of k indicate a greater degree of discounting future rewards.
tDCS procedures
A neuroConn Channel DC Stimulator Plus was used to apply a constant direct current via two 5cm × 5cm electrodes covered in saline-soaked sponges. Electrode placement was determined using the international 10–20 system developed for EEG. For anodal stimulation over the rIFG, the anodal electrode was placed between F4 and F8 and the cathodal electrode was placed between F3 and F7 as in prior work (Jacobson, et al., 2011). We employed finite-element modeling using the HD-Explore software (Soterix, NYC; Figure 1). By assigning different conductances to the various tissues and fluids of the head (e.g. skin, bone, CSF, brain, etc.) this software enables the creation of a model representing the likely distribution of current flow in the brain when tDCS is applied employing the stated parameters. This modeling software has been successfully employed in multiple prior studies (Bender, Filmer, & Dux, 2017; Frings, Brinkmann, Friehs, & van Lipzig, 2018; van ‘t Wout & Silverman, 2017). During the active condition, current was ramped up to 2.0 mA over 30s, maintained for 19 minutes and then ramped down over 30s (total stimulation period 20 minutes). During sham tDCS, current was ramped up to 2.0 mA and then down to 0mA during the beginning and end 30s of the 20-minute period. This process mimics the skin sensations experienced during active stimulation; when asked, most participants cannot distinguish between real and sham tDCS using this procedure (Gandiga, Hummel, & Cohen, 2006). Double-blinding was guaranteed via input codes on the stimulation device, which were generated from a list of active and sham stimulation codes, non-accessible to tDCS administrators.
Figure 1.
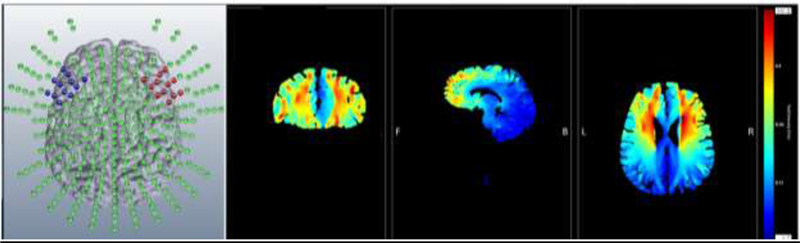
Computer-generated finite element models of the distribution of current using the stimulation parameters employed in the current experiment (Soterix, NYC). The figure on the left shows all possible stimulation sites that can be modeled by the software. Red (anode) and blue (cathode) dots represent the approximate size and shape of the regions covered by pad electrodes employed in the current study. The right three images show the estimated distribution of current flow in the coronal, sagittal, and axial planes, respectively. The color bar (far right) represents electric field strength in volts per meter (V/m).
Outcomes:
The primary outcome was the total chocolate consumption (in g) during the ad libitum period. Secondary outcomes included subjective chocolate ratings.
Baseline measures:
The Three Factors Eating Questionnaire (TFEQ) was administered at Intake to assess dietary habits. The 51-item TFEQ contains 36 items with a true/false response format, 14 items on a 1–4 response scale, and one item on a 0–5 rating scale. These items were aggregated into three scales: 1) cognitive restraint, 2) disinhibition and 3) hunger (Stunkard & Messick, 1985). These scales are valid and reliable across obese and non-obese populations (Bond, McDowell, & Wilkinson, 2001; Karlsson, et al., 2000). Prior to each stimulation session, chocolate craving was measured with the state version of the Food Craving Questionnaire (FCQ-S), adapted to specifically probe for chocolate craving. The FCQ-S is a 15-item scale that measures current desire, expectation of positive effects from eating, and anticipation of relief from negative mood after eating (Meule & Hormes, 2015). Response categories were 1 (strongly disagree) to 5 (strongly agree).
Treatment-related measures:
Side effects of tDCS and blinding success were assessed at the end of each session (Kessler, Turkeltaub, Benson, & Hamilton, 2012). Participants rated to what extent they experienced 10 potential side effects of tDCS during and after stimulation (headache, difficulty concentrating, acute mood changes, changes in visual perception, tingling, itching, burning, pain, fatigue, and nervousness) on a scale from 0 (not at all) to 10 (severe). Following each session, participants were asked whether they thought they had received active or sham stimulation to assess fidelity of the blind.
Analysis
Descriptive statistics were obtained for all variables. Effects of stimulation on chocolate consumption and subjective ratings were modeled using regression with subject-level random effects, and estimated using maximum likelihood techniques (Stata xt-reg; Stata Corporation, College Station, TX, USA) with linear mixed effects models. Session (active vs. sham) was a within-subject factor and task condition (“control” vs. “chocolate no-go”) and session order (active 1st vs. sham 1st) were between-subjects factors. Pearson correlations were used to identify potential covariates and variables correlated with the dependent variable (e.g., chocolate consumption or task performance) at p<0.1 or baseline variables that differed between sessions were included in initial models; variables with p-values>0.1 in the regression models were allowed to drop.
Results
Participant Characteristics
Twenty-four participants completed both stimulation sessions. One participant’s data was withdrawn from analysis due to an equipment malfunction in session 1 which resulted in unblinding, leaving 23 subjects included in the analysis. The sample was predominantly Caucasian (n=15, 65%) and nearly all completed at least some college (n=21, 91%). On average, participants were 24.7 years old (SD=4.2), had a Shipley IQ score of 112.2 (SD=6.8), and a body mass index (BMI) of 24.3 (SD=4.3). For the TFEQ, scores on the Restraint, Disinhibition, and Hunger subscales were 10.8 (SD=5.8), 9.1 (SD=3.1), and 6.9 (SD=2.3), respectivelyi. Except for TFEQ Hunger, which was higher among those who received sham first [mean=8 (SD=2) vs active first mean=5.6 (SD=2.1), p=0.01], there were no between-subject differences for session order or task condition (all ps>0.25).
Data from blood glucose readings, hunger ratings, and chocolate craving are presented in Table As expected, there was a significant decrease in blood glucose from baseline to the end of the 2-hour waiting period during both sham (p<0.001) and active conditions (p=0.003). Because this decrease was larger during the sham, versus the active condition (mean difference=22.7 vs 3, p=0.003), the difference score was included as a covariate. There were no effects of session order or task condition on blood glucose (ps>0.2) and no differences in baseline hunger ratings or craving subscales between sessions, session order, or task condition (all ps>0.2). The blood glucose change score, FCQS Control subscale (averaged across sessions), TFEQ Restraint, and TFEQ Hunger were correlated at p<0.1 with chocolate consumption and were included as covariates.
Baseline Inhibitory Control Tasks
Descriptive statistics for the baseline stop signal task, food cue stop signal task, and delay discounting task are shown in Table 2. For the Food Cue Stop Task, stop signal reaction time (SSRT) following images of high calorie food was significantly slower than SSRT following images of low calorie food, indicating difficulty inhibiting responses to highly palatable food cues (active, p=0.01; sham, p=0.06). This difference did not vary between sessions (p=0.27). There were no between-subject differences for session order or task condition on any task (all ps>0.3). Only delay discounting and Standard SSRT were associated with chocolate consumption at p<0.1 and were included as potential covariates.
Table 2.
Baseline craving and inhibitory control measures
| Sham | Active | p-value | |
|---|---|---|---|
| Basline blood glucose (mg/dl) | 99.1 (3.0) | 91.1 (3.8) | 0.06 |
| 2-h blood glucose (mg/dl) | 76.4 (2.3) | 80.8 (2.9) | 0.23 |
| Hunger (range 0–100) | 34.9 (24.7) | 39.4 (28.3) | 0.38 |
| Baseline Chocolate Craving | |||
| FCQS Desire | 8.7 (2.7) | 8.9 (2.2) | 0.60 |
| FCQS Reinforcement | 7.5 (3.1) | 8.0 (2.6) | 0.40 |
| CQS Relief | 10.7 (2.1) | 10.2 (2.6) | 0.24 |
| FCQS Control | 6.7 (2.4) | 7.1 (2.5) | 0.38 |
| FCQS Physical Craving | 9.8 (2.6) | 9.1 (2.9) | 0.08 |
| Baseline Inhibitory Control | |||
| Stop Signal Reaction Time (ms) | 227.4 (32) | 241.4 (48) | 0.06 |
| Food Cue SSRT Neutral (ms) | 266.0 (53) | 281.6 (62) | 0.13 |
| Food Cue SSRT Low (ms) | 261.7 (52) | 274.4 (44) | 0.05 |
| Food Cue SSRT High (ms) | 274.7 (45) | 300.8 (69) | 0.02 |
| Delay Discounting (log k) | −1.83 (0.5) | −1.81 (0.4) | 0.78 |
Note. Values are mean (SD) unless otherwise noted. FCQS=Food Craving Questionnaire – State version
tDCS Side Effects and Blinding
The most commonly reported side effects during active stimulation (any rating ≥1 on a 10-point scale) were tingling at the site of the electrode (reported by 100% of participants), followed by itching (96%), burning sensation (91%), pain (74%), difficulty concentrating (65%), nervousness (43%), fatigue (35%), mood change (26%), and headache (17%). The means and standard deviations for all side effects are presented in Supplementary Table 1. Although there were no significant main effects of stimulation (all ps>0.07), there were interactions with session order for itching (interaction p=0.02), nervousness (interaction p<0.001), and difficulty concentrating (interaction p=0.04). Among those who received active stimulation first, all three side effects were rated significantly higher during active, compared to sham (ps<0.05). In contrast, among those who received sham first, there were no differences in side effects between conditions (ps>0.15). When participants received active stimulation, 60% correctly identified the stimulation condition. However, when sham stimulation was administered, 78% incorrectly identified the condition as active stimulation. The ability to correctly guess stimulation condition did not vary by session order and was not related to chocolate consumption.
Inhibitory Control Training Task
In addition to stimulation condition, task condition, and session order, the following variables were retained as covariates: pre-task hunger rating, delay discounting, Standard SSRT, change in glucose, and BMI. There were no interactions with task condition or session order (ps>0.25). Therefore, subsequent analyses removed the interaction term and main effects are reported (see Supplementary Table 2). Although there was no effect of tDCS on inhibition to chocolate stimuli (p=0.99), active stimulation significantly improved the ability to inhibit responses to filler stimuli (β=3.3, p=0.01; Figure 2). Pre-task hunger was negatively related to the ability to inhibit responses to chocolate stimuli (β=−0.12, p=0.001), but was only marginally related to filler stimuli (β=−0.07, p=0.08). No other covariates remained significant after correcting for multiple outcomes (ps>0.04). When covariates were removed, the effect of tDCS on inhibition to filler stimuli was marginal (β=1.7, p=0.09).
Figure 2.

The effects of active vs. sham stimulation on inhibitory control during the training task for chocolate and filler stimuli, controlling for covariates. The main effect was significant for filler stimuli (p=0.008), but not for chocolate stimuli (p=0.9).
Chocolate Consumption
The final multiple regression model for chocolate consumption included TFEQ Disinhibition scores, SSRT, and delay discounting (see Supplementary Table 2). Active stimulation significantly increased total chocolate consumption [sham mean=32.7 g (SE=3.3) and active mean=42.7 g (SE=3.4); β=10.0, 95% Confidence Interval (CI) 2.9 to 17.1, p=0.006], controlling for task condition and session order. There were main effects of session order (β=13.2, p=0.03), delay discounting (β=16.1, p=0.01), and TFEQ Disinhibition (β=3.2, p=0.001). Those who received sham first, preferred more immediate rewards, and reported more difficulty controlling eating behavior consumed more chocolate. There were no main effects of task condition or Standard SSRT (ps>0.2), nor were there interactions between any covariate and stimulation condition (ps>0.2). Although the stimulation condition x task condition interaction was not significant (p=0.3), the effect of active stimulation appeared to be strongest among those in the control condition (p=0.005), whereas the effect was attenuated among those trained to inhibit responses to chocolate stimuli (p=0.2; see Figure 3). The model was not substantially changed when covariates were removed. For the regression models examining the effects of tDCS on chocolate ratings, there were no significant effects of stimulation (ps>0.05).
Figure 3.

The effects of active stimulation vs sham in the full sample (left panel) and by each of the task conditions (right panel). During the active condition, participants ate significantly more chocolate (p=0.005). Although the Stimulation × Task Condition interaction was not significant (p=0.2), the effect was strongest in the Control condition.
Secondarily, we evaluated whether performance on the inhibitory control training task predicted chocolate consumption. Using similar regression models as those described above (see Supplementary Table 3), percent inhibition to chocolate and filler stimuli were added to separate models. Although percent inhibition to chocolate stimuli did not significantly predict chocolate consumption (p=0.1), inhibitions to filler stimuli were positively related to chocolate consumption (β=0.63, p=0.05). Interestingly, when inhibition to filler stimuli was added to the model, the tDCS effect was no longer significant (β=7.3, p=0.11), suggesting that performance on the training task may have partially mediated the effect of stimulation on chocolate consumption.
Discussion
The potential for tDCS as an intervention for health risk behaviors has received much attention in recent years. This early phase behavioral research study investigated whether a single session of anodal stimulation over the rIFG with cathodal stimulation over the left IFG (versus sham), administered concurrently with an inhibitory control training task, would enhance inhibitory control, thus reducing consumption of palatable snack food (i.e., chocolate). Contrary to our hypothesis, chocolate consumption increased following active versus sham stimulation. Although our data suggest the tDCS effect was mitigated among subjects trained to inhibit responses to chocolate stimuli, the group by stimulation condition was not significant. Consistent with prior research, we found preferences for immediate, rather than delayed, rewards (i.e., delay discounting) and difficulty inhibiting eating behavior were associated with greater chocolate consumption. While our findings raise questions about the efficacy of modulating IFG via tDCS for altering eating behavior, it adds to our understanding of what parameters may influence the effectiveness of tDCS.
Based on evidence that the rIFG plays a crucial role in regulating self-control processes (Hampshire, et al., 2010; Luijten, et al., 2014; Swick, et al., 2011; van Belle, et al., 2014) and stimulating the rIFG may reduce food craving, we were surprised by the contradictory, yet robust, increased chocolate consumption among participants. A recent review highlighted several methodological considerations for tDCS studies, including electrode placement, lateralization, and task requirements during stimulation— all of which may contribute to inconsistent findings (Brevet-Aeby, Brunelin, Iceta, Padovan, & Poulet, 2016). Subjects in our study received anodal stimulation between F4 and F8 (rIFG) and cathodal stimulation between F3 and F7 (left IFG). These regions may have overlapped with the right and left DLPFC, respectively, which are often stimulated at F4 (right) and F3 (left). Several studies – including work from our labs – have demonstrated that anodal stimulation of the left DLPFC not only improves executive function during difficult tasks (Gill, Shah-Basak, & Hamilton, 2015) but also has beneficial effects on behavior, such as improving the ability to resist cigarettes (Falcone, et al., 2016); however, there is less evidence of efficacy for anodal right-cathodal left stimulation of DLPFC.
Unilateral anodal stimulation of the rIFG has been shown to activate neural pathways associated with inhibitory control, leading to improved response inhibition (Jacobson, et al., 2011). In contrast, cathodal stimulation is hypothesized to have inhibitory effects on neuronal function and therefore cathodal stimulation of left IFG may have had a greater impact than anticipated. A meta-analysis of tDCS studies identified differential effects of unilateral anodal, unilateral cathodal, and bilateral anodal-cathodal stimulation on motor versus cognitive outcomes. Across all methods, tDCS effects were more consistent for motor outcomes compared to cognitive outcomes, and the anodal-cathodal montage had the lowest effect probability (Jacobson, Koslowsky, & Lavidor, 2012). Unilateral anodal stimulation of the rIFG increases inhibitory control, but the effects of bilateral stimulation vary based on the type and difficulty of the cognitive task (Brevet-Aeby, et al., 2016). Thus, our design may have benefited from a unilateral tDCS montage. Nevertheless, the majority of this research has evaluated motor or cognitive outcomes and we believe that an important aspect of our design was the focus on a behavioral outcome (i.e., chocolate consumption) and recommend that future studies explore the effects of tDCS montage on behavioral outcomes.
There is also substantial variation in task requirements within tDCS research. In this study, participants completed a task during stimulation, but also completed a 1-hour cognitive task battery prior to stimulation. Because our primary behavioral outcome was chocolate consumption, we felt it was important to control for time of last food consumption and exposure to food-related cues prior to the ad-libitum chocolate period. While it is possible that fatigue from the cognitive task battery influenced our results, the within subjects design likely minimized these effects. Regarding task administration during tDCS, our previous study of the effect of tDCS on the ability to resist smoking (Falcone, et al., 2016) asked participants to simply try their best to refrain from smoking during stimulation. Recent reviews have found that completing cognitive tasks during, rather than following stimulation, may improve performance accuracy (Dedoncker, et al., 2016; Elmasry, Loo, & Martin, 2015). However, transfer effects were small, if present at all (Elmasry, et al., 2015) and in one review, this effect was observed only among neuropsychiatric patients (Dedoncker, et al., 2016). tDCS research has been conducted predominantly on clinical populations (i.e., patients with chronic medical conditions or psychiatric disorders), and it has typically involve completing tasks during the stimulation. Important questions regarding subject characteristics, optimal timing of cognitive outcomes, and transfer of skills to health behavior should be addressed by future research. When asking these questions, it is important to consider who administers the stimulation, as well as who measures the outcome evaluation. In the current study, the same person administered the tDCS and outcome evaluation (i.e., presenting and measuring chocolate). Although an optimal design could separate the tDCS administration from outcome evaluation, we believe the double-blind procedures minimized influence on the subject’s behavior.
Although we focused on the rIFG, nearby brain regions like the anterior insula may have also been stimulated, producing unanticipated consequences. Activation of the anterior insula has been associated with increased cue salience (Craig, 2009; Janes, Farmer, Peechatka, Frederick Bde, & Lukas, 2015; Wiech, et al., 2010). Therefore, it is possible that excitation of this region facilitated increased salience of the chocolate cues. It is also possible that the two conditions of the inhibitory control training task altered the cue salience. When the nature of the food cues was uninformative to the go/no-go signal (i.e., control condition), stimulation improved inhibitory control. In contrast, during the chocolate no-go condition there was no effect of tDCS on inhibitory control, suggesting that consistent pairing of the cue with the response (i.e., always inhibit) may have increased the salience of the cue. Novel techniques such as MRI-guided neuronavigation may enhance the ability to localize tDCS montages and determine the effects of modulating nearby brain regions (De Witte, et al., 2018).
The fact that we selected “chocolate cravers” who also reported being restrained eaters, may partially explain why we did not observe changes in inhibition to chocolate cues between active and sham conditions. However, during active vs sham stimulation, participants exhibited an increased ability to inhibit responding to filler stimuli. Because we specifically selected individuals who craved chocolate and were predisposed to have difficulty inhibiting chocolate consumption, the ability to inhibit responses to chocolate stimuli may have been less malleable than inhibition to other palatable foods (even when specifically trained not to respond to chocolate stimuli during the task). Importantly, our data also suggested that the ability to inhibit responses to palatable foods (other than chocolate) may partially mediate the effects of tDCS on chocolate consumption. In addition, subjects were asked about chocolate-specific craving prior to stimulation, which may have primed chocolate-craving participants to sustain their chocolate inhibitions, but remain flexible with other food-related cues. Future research should consider findings from both the tDCS and fMRI fields. Previous fMRI research demonstrates a correlation between neural sensitivity towards high-calorie (vs. low-calorie) foods and restrained eating, in addition to significant differences in activation levels across visual- and attention-focused brain regions. Specifically, individuals classified as restrained eaters showed hyperactivity in neural regions associated with reward when presented with highly palatable (i.e., high calorie, chocolate) food cues (Dong, et al., 2016; Wang, et al., 2016). Because we recruited subjects who self-identified as being restrained eaters, it is possible that stimulating an already hyperactive brain region may have contributed to our unexpected results. Perhaps a more comprehensive profile of food cravings and personalized cues, while also considering the impact of a montage that may activate potentially hyperactive brain regions, would have yielded different results.
Outside of food preference profiles, future research should more heavily consider whether different menstrual cycle phases, both within and across participants, impact the effects of tDCS on eating behavior. Due to a small sample size, scheduling and staff availability constraints, the presented study could not examine this question; however, it is an important question to consider. In addition, it is unknown whether participants had previously received brain stimulation via tDCS or TMS. Per university practices for brain stimulation studies, each study is required to enter participant stimulation session dates and initials into a university-wide, password-protected spreadsheet to avoid any research participant from receiving multiple stimulations within a week. While this method informs us that no participants were participating in concurrent tDCS studies during their active participation in our study, it does not provide information on participants were tDCS naïve.
Evidence supporting tDCS as a potential tool for behavior change has become increasingly robust, but our findings suggest its efficacy might be influenced by methodological factors that have not been widely studied. Electrode montage, concurrent task and tDCS administration, and participant characteristics (i.e.,clinical versus non-clinical) may contribute to discrepancies across tDCS studies. Although a recent meta-analysis concluded tDCS had no effect on cognitive outcomes (Horvath, Forte, & Carter, 2015), several methodological issues regarding this review were raised suggesting these findings were not conclusive (Price & Hamilton, 2015). Through this line of research, we aim to gain a better understanding of the parameters under which tDCS may produce health behavior change and reduce risk behaviors (i.e., tobacco use, overconsumption of sugar) which have far-reaching public health consequences.
Supplementary Material
Acknowledgements:
This research was supported by grants from the National Institutes of Health (R35 CA197461 and K23 DA035295). The funding source had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None.
Although these scores are significantly less than those of very restrained eaters (“dieters”), the Restraint and Disinhibition scores are significantly higher in our sample than in unrestrained eaters(“free eaters”). The Hunger scores are similar across all subject groups [42].
References
- Aron AR, Robbins TW, & Poldrack RA (2014). Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci, 18, 177–185. [DOI] [PubMed] [Google Scholar]
- Bender AD, Filmer HL, & Dux PE (2017). Transcranial direct current stimulation of superior medial frontal cortex disrupts response selection during proactive response inhibition. Neuroimage, 158, 455–465. [DOI] [PubMed] [Google Scholar]
- Benton D, Greenfield K, & Morgan M (1998). The development of the attitudes to chocolate questionnaire. Personality and Individual Differences, 24, 513–520. [Google Scholar]
- Berridge KC, Ho CY, Richard JM, & DiFeliceantonio AG (2010). The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res, 1350, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Meule A, Busch NA, & Ohla K (2014). Food-pics: an image database for experimental research on eating and appetite. Frontiers in Psychology, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond MJ, McDowell AJ, & Wilkinson JY (2001). The measurement of dietary restraint, disinhibition and hunger: an examination of the factor structure of the Three Factor Eating Questionnaire (TFEQ). Int J Obes Relat Metab Disord, 25, 900–906. [DOI] [PubMed] [Google Scholar]
- Brevet-Aeby C, Brunelin J, Iceta S, Padovan C, & Poulet E (2016). Prefrontal cortex and impulsivity: Interest of noninvasive brain stimulation. Neurosci Biobehav Rev, 71, 112–134. [DOI] [PubMed] [Google Scholar]
- Cai Y, Li S, Liu J, Li D, Feng Z, Wang Q, Chen C, & Xue G (2016). The Role of the Frontal and Parietal Cortex in Proactive and Reactive Inhibitory Control: A Transcranial Direct Current Stimulation Study. J Cogn Neurosci, 28, 177–186. [DOI] [PubMed] [Google Scholar]
- Conti CL, & Nakamura-Palacios EM (2014). Bilateral Transcranial Direct Current Stimulation Over Dorsolateral Prefrontal Cortex Changes the Drug-cued Reactivity in the Anterior Cingulate Cortex of Crack-cocaine Addicts. Brain Stimulation, 7, 130–132. [DOI] [PubMed] [Google Scholar]
- Control, C. f. D., & Prevention. (2015). Leading causes of death and numbers of deaths, by sex, race, and Hispanic origin: United States, 1980 and 2014 (Table 19) Health, United States. [Google Scholar]
- Craig AD (2009). How do you feel — now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10, 59. [DOI] [PubMed] [Google Scholar]
- Cunillera T, Brignani D, Cucurell D, Fuentemilla L, & Miniussi C (2016). The right inferior frontal cortex in response inhibition: A tDCS-ERP co-registration study. Neuroimage, 140, 66–75. [DOI] [PubMed] [Google Scholar]
- De Witte S, Klooster D, Dedoncker J, Duprat R, Remue J, & Baeken C (2018). Left prefrontal neuronavigated electrode localization in tDCS: 10–20 EEG system versus MRI-guided neuronavigation. Psychiatry Research: Neuroimaging, 274, 1–6. [DOI] [PubMed] [Google Scholar]
- Dedoncker J, Brunoni AR, Baeken C, & Vanderhasselt M-A (2016). A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters. Brain Stimulation, 9, 501–517. [DOI] [PubMed] [Google Scholar]
- DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, & Tataranni PA (2007). Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond), 31, 440–448. [DOI] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, & Pascual-Leone A (2013). Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology, 64, 566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Wang Y, Jackson T, Chen S, Wang Y, Zhou F, & Chen H (2016). Impulse control and restrained eating among young women: Evidence for compensatory cortical activation during a chocolate-specific delayed discounting task. Appetite, 105, 477–486. [DOI] [PubMed] [Google Scholar]
- Elmasry J, Loo C, & Martin D (2015). A systematic review of transcranial electrical stimulation combined with cognitive training. Restorative Neurology & Neuroscience, 33, 263–278. [DOI] [PubMed] [Google Scholar]
- Falcone M, Bernardo L, Ashare RL, Hamilton R, Faseyitan O, McKee SA, Loughead J, & Lerman C (2016). Transcranial Direct Current Brain Stimulation Increases Ability to Resist Smoking. Brain Stimulation, 9, 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, & Pascual-Leone A (2007). Diminishing Risk-Taking Behavior by Modulating Activity in the Prefrontal Cortex: A Direct Current Stimulation Study. The Journal of Neuroscience, 27, 12500–12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankort A, Roefs A, Siep N, Roebroeck A, Havermans R, & Jansen A (2015). Neural predictors of chocolate intake following chocolate exposure. Appetite, 87, 98–107. [DOI] [PubMed] [Google Scholar]
- Frings C, Brinkmann T, Friehs MA, & van Lipzig T (2018). Single session tDCS over the left DLPFC disrupts interference processing. Brain Cogn, 120, 1–7. [DOI] [PubMed] [Google Scholar]
- Froeliger B, McConnell PA, Bell S, Sweitzer M, Kozink RV, Eichberg C, Hallyburton M, Kaiser N, Gray KM, & McClernon FJ (2017). Association Between Baseline Corticothalamic-Mediated Inhibitory Control and Smoking Relapse Vulnerability. JAMA Psychiatry, 74, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, & Cohen LG (2006). Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol, 117, 845–850. [DOI] [PubMed] [Google Scholar]
- Georgii C, Goldhofer P, Meule A, Richard A, & Blechert J (2017). Food craving, food choice and consumption: The role of impulsivity and sham-controlled tDCS stimulation of the right dlPFC. Physiol Behav, 177, 20–26. [DOI] [PubMed] [Google Scholar]
- Gerteis J, Izrael D, Deitz D, LeRoy L, Ricciardi R, Miller T, & Basu J (2014). Multiple chronic conditions chartbook Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- Gill J, Shah-Basak PP, & Hamilton R (2015). It’s the thought that counts: examining the task-dependent effects of transcranial direct current stimulation on executive function. Brain Stimul, 8, 253–259. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, & Owen AM (2010). The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage, 50, 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, & Rangel A (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science, 324, 646–648. [DOI] [PubMed] [Google Scholar]
- Heinze K, Ruh N, Nitschke K, Reis J, Fritsch B, Unterrainer JM, Rahm B, Weiller C, & Kaller CP (2014). Transcranial direct current stimulation over left and right DLPFC: Lateralized effects on planning performance and related eye movements. Biol Psychol, 102, 130–140. [DOI] [PubMed] [Google Scholar]
- Hormes JM, & Timko CA (2011). All cravings are not created equal. Correlates of menstrual versus non-cyclic chocolate craving. Appetite, 57, 1–5. [DOI] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, & Carter O (2015). Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-session Transcranial Direct Current Stimulation (tDCS). Brain Stimul, 8, 535–550. [DOI] [PubMed] [Google Scholar]
- Houben K, Havermans RC, Nederkoorn C, & Jansen A (2012). Beer a no-go: learning to stop responding to alcohol cues reduces alcohol intake via reduced affective associations rather than increased response inhibition. Addiction, 107, 1280–1287. [DOI] [PubMed] [Google Scholar]
- Houben K, & Jansen A (2011). Training inhibitory control. A recipe for resisting sweet temptations. Appetite, 56, 345–349. [DOI] [PubMed] [Google Scholar]
- Houben K, Nederkoorn C, & Jansen A (2014). Eating on impulse: the relation between overweight and food-specific inhibitory control. Obesity (Silver Spring), 22, E6–8. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Javitt DC, & Lavidor M (2011). Activation of inhibition: diminishing impulsive behavior by direct current stimulation over the inferior frontal gyrus. J Cogn Neurosci, 23, 3380–3387. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M, & Lavidor M (2012). tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res, 216, 1–10. [DOI] [PubMed] [Google Scholar]
- Janes AC, Farmer S, Peechatka AL, Frederick Bde B, & Lukas SE (2015). Insula-Dorsal Anterior Cingulate Cortex Coupling is Associated with Enhanced Brain Reactivity to Smoking Cues. Neuropsychopharmacology, 40, 1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, & Richardt S (2010). Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry, 67, 722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Yasuda M, Burant CF, Gregor N, Khatri S, Sweet M, & Falk EB (2012). Impulsivity and inhibitory control deficits are associated with unhealthy eating in young adults. Appetite, 59, 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, & Pennington ZT (2014). Reward, interrupted: Inhibitory control and its relevance to addictions. Neuropharmacology, 76 Pt B, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, & Glimcher PW (2007). The neural correlates of subjective value during intertemporal choice. Nat Neurosci, 10, 1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, & Glimcher PW (2010). An “as soon as possible” effect in human intertemporal decision making: behavioral evidence and neural mechanisms. J Neurophysiol, 103, 2513–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson J, Persson LO, Sjostrom L, & Sullivan M (2000). Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord, 24, 1715–1725. [DOI] [PubMed] [Google Scholar]
- Kessler SK, Turkeltaub PE, Benson JG, & Hamilton RH (2012). Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimulation, 5, 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, & Santiesteban M (2003). Concave utility, transaction costs, and risk in measuring discounting of delayed rewards. J Exp Psychol Learn Mem Cogn, 29, 66–79. [PubMed] [Google Scholar]
- Kishinevsky FI, Cox JE, Murdaugh DL, Stoeckel LE, Cook EW 3rd, & Weller RE (2012). fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite, 58, 582–592. [DOI] [PubMed] [Google Scholar]
- Logan GD (1994). On the Ability to Inhibit Thought and Action: A User’s Guide to the Stop Signal Paradigm. Dagenbach D & Carr TH (Eds.), Inhibitory Processes in Attention, Memory, and Language (pp. 189–238). San Diego: Academic Press. [Google Scholar]
- Logan GD, Schachar RJ, & Tannock R (1997). Impulsivity and inhibitory control. Psychol Sci, 8, 60–66. [Google Scholar]
- Loughead J, Wileyto EP, Ruparel K, Falcone M, Hopson R, Gur R, & Lerman C (2015). Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology, 40, 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten M, Machielsen MW, Veltman DJ, Hester R, de Haan L, & Franken IH (2014). Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J Psychiatry Neurosci, 39, 149–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE (1987). An adjusting procedure for studying delayed reinforcement. Quantitative Analysis of Behavior: The Effects of Delay and Intervening Events on Reinforcement Value (Commons ML, Mazur JE, Nevin JA, Rachlin H, eds), pp 55–73. Hillsdale, Nj: Lawrence Erlbaum Associates, Publishers. [Google Scholar]
- McCaffery JM, Haley AP, Sweet LH, Phelan S, Raynor HA, Del Parigi A, Cohen R, & Wing RR (2009). Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am J Clin Nutr, 90, 928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay MA, Copeland AL, Newman HS, & Geiselman PJ (2012). Food cravings and food cue responding across the menstrual cycle in a non-eating disordered sample. Appetite, 59, 591–600. [DOI] [PubMed] [Google Scholar]
- Meule A, & Hormes JM (2015). Chocolate versions of the Food Cravings Questionnaires. Associations with chocolate exposure-induced salivary flow and ad libitum chocolate consumption. Appetite, 91, 256–265. [DOI] [PubMed] [Google Scholar]
- Muller J, Dettmer D, & Macht M (2008). The Attitudes to Chocolate Questionnaire: psychometric properties and relationship to dimensions of eating. Appetite, 50, 499–505. [DOI] [PubMed] [Google Scholar]
- Pelchat ML (1997). Food cravings in young and elderly adults. Appetite, 28, 103–113. [DOI] [PubMed] [Google Scholar]
- Price AR, & Hamilton RH (2015). A Re-evaluation of the Cognitive Effects From Single-session Transcranial Direct Current Stimulation. Brain Stimul, 8, 663–665. [DOI] [PubMed] [Google Scholar]
- Smith JL, Mattick RP, Jamadar SD, & Iredale JM (2014). Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend, 145, 1–33. [DOI] [PubMed] [Google Scholar]
- Stramaccia DF, Penolazzi B, Sartori G, Braga M, Mondini S, & Galfano G (2015). Assessing the effects of tDCS over a delayed response inhibition task by targeting the right inferior frontal gyrus and right dorsolateral prefrontal cortex. Exp Brain Res, 233, 2283–2290. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, & Messick S (1985). The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res, 29, 71–83. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, & Turken U (2011). Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage, 56, 1655–1665. [DOI] [PubMed] [Google Scholar]
- van ‘t Wout M, & Silverman H (2017). Modulating what is and what could have been: The effect of transcranial direct current stimulation on the evaluation of attained and unattained decision outcomes. Cogn Affect Behav Neurosci, 17, 1176–1185. [DOI] [PubMed] [Google Scholar]
- van Belle J, Vink M, Durston S, & Zandbelt BB (2014). Common and unique neural networks for proactive and reactive response inhibition revealed by independent component analysis of functional MRI data. Neuroimage, 103, 65–74. [DOI] [PubMed] [Google Scholar]
- Van Gucht D, Soetens B, Raes F, & Griffith JW (2014). The Attitudes to Chocolate Questionnaire. Psychometric properties and relationship with consumption, dieting, disinhibition and thought suppression. Appetite, 76, 137–143. [DOI] [PubMed] [Google Scholar]
- Van Gucht D, Vansteenwegen D, Beckers T, Hermans D, Baeyens F, & Van den Bergh O (2008). Repeated cue exposure effects on subjective and physiological indices of chocolate craving. Appetite, 50, 19–24. [DOI] [PubMed] [Google Scholar]
- Wang Y, Dong D, Todd J, Du J, Yang Z, Lu H, & Chen H (2016). Neural correlates of restrained eaters’ high susceptibility to food cues: An fMRI study. Neurosci Lett, 631, 56–62. [DOI] [PubMed] [Google Scholar]
- Wiech K, Lin C. s., Brodersen KH, Bingel U, Ploner M, & Tracey I (2010). Anterior Insula Integrates Information about Salience into Perceptual Decisions about Pain. The Journal of Neuroscience, 30, 16324–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileyto EP, Audrain-McGovern J, Epstein LH, & Lerman C (2004). Using logistic regression to estimate delay-discounting functions. Behav Res Methods Instrum Comput, 36, 41–51. [DOI] [PubMed] [Google Scholar]
- Zachary RS (2000). Shipley Institute of Living Scale - Revised Manual: Western Psychological Services. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


