Abstract
γδ T‐cells perform a wide range of tissue‐ and disease‐specific functions that are dependent on the effector cytokines produced by these cells. However, the aggregate signals required for the development of interferon‐γ (IFNγ) and interleukin‐17 (IL‐17) producing γδ T‐cells remain unknown. Here, we define the cues involved in the functional programming of γδ T‐cells, by examining the roles of T‐cell receptor (TCR), Notch, and cytokine‐receptor signaling. KN6 γδTCR‐transduced Rag2 −/− T‐cell progenitors were cultured on stromal cells variably expressing TCR and Notch ligands, supplemented with different cytokines. We found that distinct combinations of these signals are required to program IFNγ versus IL‐17 producing γδ T‐cell subsets, with Notch and weak TCR ligands optimally enabling development of γδ17 cells in the presence of IL‐1β, IL‐21 and IL‐23. Notably, these cytokines were also shown to be required for the intrathymic development of γδ17 cells. Together, this work provides a framework of how signals downstream of TCR, Notch and cytokine receptors integrate to program the effector function of IFNγ and IL‐17 producing γδ T‐cell subsets.
Keywords: Cytokines, Notch, T‐cell receptor
Introduction
γδ T‐cells are able to perform a wide range of species, tissue‐ and disease‐specific functions that include tumor surveillance, tissue healing, pathogen clearance and acting as the interface between the innate and adaptive immune responses.1, 2 Depending on the context, the same effector functions that make γδ T‐cells an important arm of the immune system can alternatively contribute to the development, progression and exacerbation of various diseases. These harmful effects include roles in the pathogenesis of autoimmune conditions such as psoriasis, multiple sclerosis, and arthritis, as well as contributions to breast cancer metastasis and bone loss.3, 4, 5, 6, 7 Given that the beneficial and deleterious effects of γδ T‐cells are largely attributable to the ability of these cells to rapidly produce high levels of inflammatory cytokines, it is important to understand the development and function of the major effector subtypes.
The most commonly studied effector subsets of mouse γδ T‐cells fall into the interferon‐γ (IFNγ) or IL‐17 producing (γδ17) categories. Although it is generally accepted that both subtypes are programmed in the thymus, the exact set of events leading to their functional differentiation remains controversial. In particular, several distinct, and sometimes contradictory, mechanisms have been proposed as important for γδ17 cell differentiation.8 Initial reports suggested that antigen experienced γδ T‐cells, which had received ligand‐dependent TCR signals, mature as IFNγ producers, while antigen naïve cells differentiate toward the γδ17 lineage.9 Furthermore, in the past decade, there have been several reports debating the importance of different degrees of TCR signaling in the generation of γδ17 cells.10, 11, 12, 13 The signal strength hypothesis asserts that the quantity of the signal downstream of the TCR can direct lineage choice.8 TCR signaling activates the ERK‐Egr3 pathway, which results in the upregulation of Id3 in direct proportion to the strength of signal.14 Moreover, high ERK signaling has been shown to repress the development of γδ17 cells,15 and Id3 activity can repress the activity of HEB, which is required for γδ17 development.16 However, very few γδTCR ligands are known, complicating attempts to control γδTCR signal strength in order to investigate the extent its influence on γδ T‐cell effector programming.
Another subject of controversy with regard to γδ17 development is the involvement of cytokines. Early reports by Lochner et al. suggested that the mechanism of γδ17 programming differed from that of Th17, as it did not rely on IL‐6.17 Noting the relevance of IL‐23 in γδ17 mediated autoimmune pathogenesis, Sutton and colleagues demonstrated that IL‐1β and IL‐23 could induce the production of IL‐17 by innate‐like γδ T‐cells in the periphery.18 However, other reports have shown that IL‐17 production by γδ T‐cells in the intestinal mucosa is IL‐23 independent, despite these cells being IL‐23R+.8 Furthermore, Nurieva et al. noticed an almost complete absence of IL‐17 production by γδ T‐cells in the periphery of IL‐21−/− mice.19 Although these studies did not demonstrate relevance of these cytokines to thymic programming of γδ17 cells, they provided a rationale for assessing this possibility.
Notch signaling has also been implicated as a driver of γδ17 differentiation. Evidence for the involvement of the Notch1 pathway has been ascribed to various aspects of IL‐17 producing cell differentiation, such as regulation of Rorc (RORγt) and IL‐23R expression; it has also been shown to act as a metabolic regulator in Th17 cells.20, 21, 22 The main evidence for a role of Notch signaling in γδ17 T‐cells is derived from work by Yoshikai and colleagues, who observed that the downstream target of Notch signaling Hes1 was induced in γδ17 cells and appears to be the main factor responsible for the development of these cells, rather than the RORγt or STAT3 pathways that operate in Th17 development.23, 24 These studies support the involvement of the Notch pathway in supporting the γδ17 lineage fate.
The thymic microenvironment also provides a wide range of tightly controlled cues that direct the development of functionally distinct T‐cells. Most in vivo studies can only focus on modulating a few of these factors at a time, and it is difficult to control their timing and duration. Here, we have taken an alternative approach toward understanding the potentially collaborative roles of TCR, Notch, and cytokine signals in γδ17 development. To evaluate the impact of these factors at precisely the time that they acquire access to γδTCR‐mediated programming, we have used Rag2 −/− T‐cell progenitors transduced to express the KN6 γδTCR. Both weak and strong ligands are known for the KN6 γδTCR, providing an ideal system to modulate TCR signal strength.25 We engineered stromal cells to express the Notch ligand Dll4 and/or the KN6‐TCR ligands, and co‐cultured them with the KN6‐expressing Rag2 −/− progenitors. In addition, we supplemented the cultures with different permutations of the cytokines IL‐1β, IL‐7, IL‐21 and IL‐23 to provide a matrix of conditions designed to reveal their combinatorial inputs into γδ17 development. These studies allowed us to define the signals that enable the development of IL‐17 producing KN6 γδ T‐cells, and to illuminate how Notch, TCR and cytokine receptor signals integrate to allow and drive the programming of γδ T‐cells.
Results
In vitro‐derived γδ T‐cells mature as IFNγ CD27+ cells in the presence of strong γδTCR ligand
The KN6 (Vγ4Vδ5) TCR was initially cloned from IFNγ producing γδ T‐cells that recognize the nonclassical MHC1b molecules T10 and T22.25, 26 As both strong (T22) and weak (T10) ligands are known for the KN6 γδTCR, it is a very useful model for understanding the impact of TCR signal strength on γδ T‐cell development and functional maturation.27, 28, 29 Previous work suggested that Notch1 signaling can drive the development of γδ17 cells, but did not address the role of TCR signal strength.23 Therefore, we first assessed the ability of KN6 TCR+ cells to develop toward the γδ17‐lineage in the presence or absence of the Notch ligand Dll4. Rag2 −/− DN3 cells were transduced to express the KN6 γδTCR and cultured with IL‐7 on OP9 cells or OP9‐DL cells, which express the Dll4 Notch ligand (Supplementary figure 1a). OP9 cells are derived from the op/op mice, which have an H2K haplotype and thus express both T22 and T10 alleles. Based on our previous studies in which we showed that co‐expressed TCRs of different strength have an additive effect on lineage choice, we predicted that the strong TCR signal would predominate under these conditions.30
Analysis of co‐cultures on Day 4 revealed that the provision of KN6‐TCR allowed for increased expansion of transduced Rag2 −/− DN3 cells compared to control (MIY) transduced DN3s. Furthermore, OP9‐DL cells were more supportive of expansion than OP9 cells (Supplementary figure 1b). We assessed cytokine production by flow cytometric analysis and ELISA, which revealed that in vitro‐derived KN6 cells co‐cultured on OP9 or OP9‐DL cells were efficient producers of IFNγ but not IL‐17 (Supplementary figure 1c). Therefore, the provision of Dll4 under these conditions was insufficient to support differentiation of γδ17 cells. Interestingly, Dll4 did not inhibit the ability of the KN6 cells to produce IFNγ (Supplementary figure 1c). By Day 4, most KN6 cells cultured on either OP9 or OP9‐DL cells had differentiated into mature γδ T‐cells, as defined by the downregulation of CD24 (Supplementary figure 1d). Notably, regardless of exposure to Dll4, the KN6 cells maintained high CD27 expression, a phenotype that is associated with IFNγ‐producing γδ T‐cells31 (Supplementary figure 1d). Thus, Notch1 signaling alone was not sufficient to allow the development of γδ17 cells in the presence of T22.
Role of γδ TCR signal strength and Notch signaling for cytokine production of KN6+ cells
The expression of both T22 and T10 on OP9 cells precluded us from directly testing weak versus strong TCR signals in concert with presence or absence of Notch signals in this system. We therefore used primary mouse embryonic fibroblasts (MEF) derived from BALB/c mice (H2d haplotype, T10+ T22−)26 to generate T10, T10 + DL4, T10 + T22 and T10 + T22 + DL4 cell lines (Supplementary figure 2). KN6‐transduced Rag2 −/− DN3 cells were cultured on the four MEF lines (Figure 1a), and on Day 4 of co‐culture, cellularity of KN6 cells was assessed (Figure 1b). KN6 cells cultured on MEFs expressing Dll4 had higher cellularity than those cultured in the absence of Notch ligand, as also had been observed in the OP9/OP9‐DL4 co‐cultures. In addition, the presence of T22 resulted in greater cellularity of KN6 cells than those cultured with T10, although this effect was not as pronounced as that observed with exposure to Dll4 (Figure 1b). KN6 cells co‐cultured on T22+ MEFs had significantly higher expression of Id3 when compared to KN6 cells co‐cultured on T10+ MEFs, while MIY‐transduced DN3 cells failed to induce detectable Id3 levels (Figure 1c). This observation is consistent with Id3 levels being directly affected by γδTCR ligand exposure to weak or strong ligands.14 A differential impact of T22 and T10 was also seen in KN6 cell maturation, in that KN6 cells co‐cultured on T22+ MEFs showed a more efficient downregulation of CD24, with a concomitant upregulation of CD73, indicating a role for TCR signal strength in γδ T‐cell maturation as well as fate determination (Figure 1d).
Figure 1.
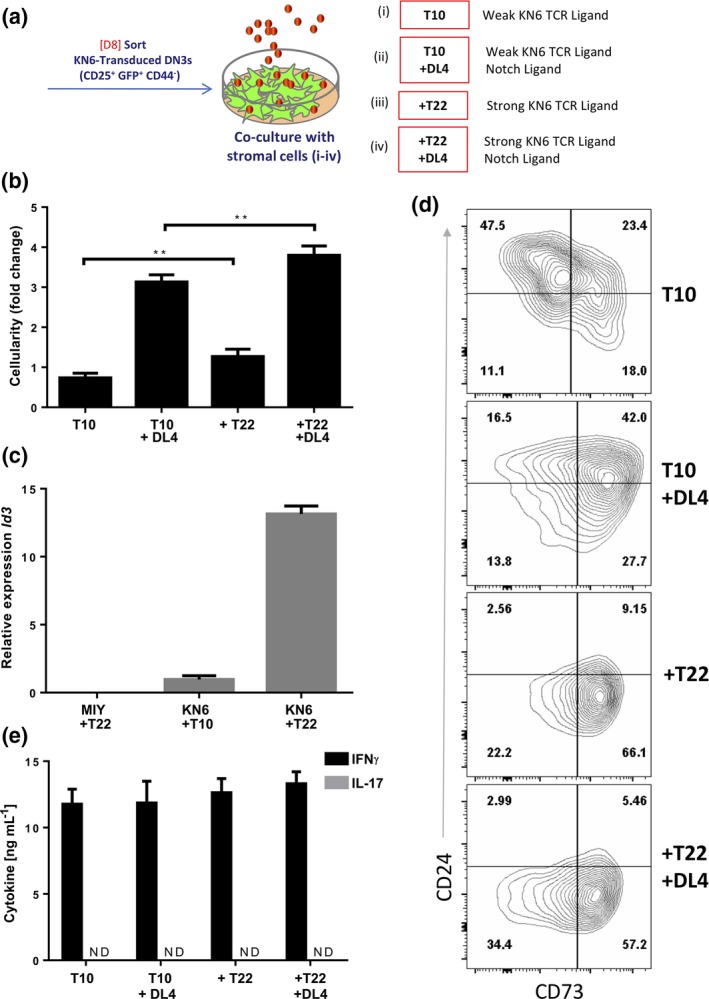
Provision of weak binding KN6 γδTCR ligand T10 and/or Notch ligand DL4 supports KN6 maturation and is sufficient for the development of IFNγ but not IL‐17 producing KN6 γδ T‐cells. (a) D8 in vitro‐derived Rag2 −/− DN3 cells retrovirally transduced to express the γδTCR (KN6) or MIY were cultured with IL‐7 on the indicated stromal cells for 4 d. (b) Total cellularity displayed as fold increase/input of 4 d cultures as indicated. (c) qPCR quantification of Id3 mRNA from Day 4 KN6 or DN3 cells co‐cultured on indicated stromal cells. (d) Day 4 co‐culture flow cytometric analysis for maturation markers CD24 and CD73. (e) IFNγ and IL‐17 production as measured by ELISA. Cells were sorted on Day 4 and stimulated for 36 h with PMA/Ionomycin. Flow cytometric analyzed samples are gated as CD45+ DAPI − and γδ‐TCR + for samples transduced with KN6. Results represent at least five independent and separately run experiments. The data and error bars are presented as standard error of the mean (s.e.m.). Statistical significance was determined using one‐way analysis of variance as P < 0.05 (**P < 0.01).
To determine whether differential TCR and Notch signals could influence the production of IFNγ and IL‐17 by KN6 cells, we stimulated them on Day 4 of co‐culture and measured cytokine production (Figure 1e). KN6 cells were able to produce high levels of IFNγ, but were unable to produce IL‐17, regardless of the stromal cells used for their differentiation. These results suggest that the provision of weak γδ TCR ligand modulates the maturation status of KN6 cells, but does not lead to the development of γδ17 cells in cultures, regardless of Notch ligand Dll4 exposure.
Cellularity of KN6 cells exposed to IL‐1β, IL‐21 and IL‐23 is dependent on Notch signaling
Thus far, our results indicated that provision of TCR and Notch ligands in culture were not sufficient to direct γδ17 differentiation. Therefore, we next investigated whether cytokines known to induce IL‐17 production in mature γδ T‐cells also participate in the programming of the γδ17 effector fate. Previous work suggested that γδ17 cells differ from their Th17 counterparts in terms of cytokine requirements, with IL‐1β, IL‐21 and IL‐23 implicated as drivers of IL‐17 production by γδ17 cells, whereas IL‐6 is a key cytokine for Th17 cells.17, 18, 19, 32, 33, 34, 35 Therefore, we assessed whether adding combinations of IL‐1β, IL‐21 and IL‐23 to the KN6/MEF co‐cultures could result in the functional programming of the γδ17‐effector subtype.
KN6‐transduced Rag2 −/− DN3 cells (KN6 cells) were co‐cultured on the four MEF lines (+IL‐7) in the presence or absence of added IL‐1β, IL‐21 and IL‐23 (+CK). (Figure 2a). It was immediately apparent at Day 4 of culture that expansion was differentially affected by cytokines in the presence and absence of Dll4. KN6 cells co‐cultured on stromal cells expressing Dll4 (T10 + DL4 or T10 + T22 + DL4) showed greater cellularity than cultures without Dll4. Moreover, +DL4 culture cellularity was not affected by the presence or absence of CK (Figure 2a). However, in the absence of Dll4, provision of CK greatly reduced the cellularity of KN6 cells compared with cultures supplemented with IL‐7 alone. Therefore, Dll4 not only aided in expansion of the KN6 cells, but also protected them from the deleterious effects of CK addition.
Figure 2.
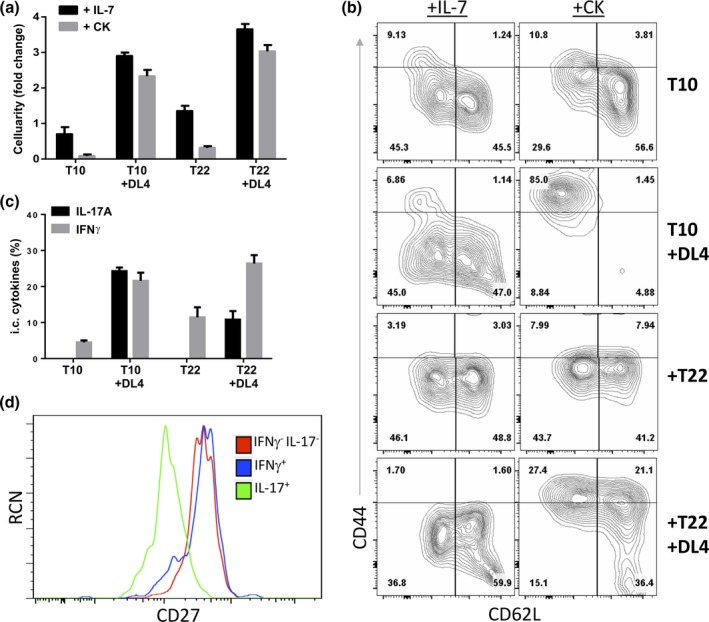
KN6 γδ T‐cells supplemented with IL‐1β, IL‐21 and IL‐23 differentiate toward γδ17 cells in cultures supplemented with Notch1 Ligand Dll4. (a) Day 4 cellularity of KN6 cells on the indicated stromal cells and supplemented with cytokines as shown. (b) Day 4 flow cytometric analysis of γδ17 associated surface markers CD44 and CD62L. (c, d) KN6 cells were stimulated for 6 h with PMA/Ionomycin +CK and (c) analyzed by flow cytometry for cytokine production by KN6 cells. (d) Flow cytometric analysis for CD27 expression on the indicated KN6 γδ T‐cell cytokine expressing subsets. Data shown represent at least 3 independent experiments. KN6 cells were supplemented with the indicated cytokines and cultured on the indicated stromal lines (+CK denotes addition of IL‐7, IL‐1β, IL‐21, IL‐23 on D0). KN6 cells were pre‐gated for DAPI − CD45+and TCRγδ+. The data and error bars are presented as standard error of the mean (s.e.m.). Statistical significance was determined using a two‐tailed unpaired Student's t‐test as P < 0.05 (**P < 0.01).
To elucidate which cytokines were responsible for the loss of cellularity in the absence of Dll4, we added different combinations of cytokines to KN6 co‐cultures (Supplementary figure 3a). All cultures containing IL‐1β showed greatly decreased cellularity in the absence of Dll4, and this appeared to be exacerbated by the addition of IL‐21. To understand the mechanism by which IL‐1β affected KN6 cellularity, we analyzed the levels of IL‐6, an inflammatory cytokine induced by IL‐1β. IL‐6 has been shown to inhibit the expansion of hematopoietic stem cells, in a manner which is blocked by Notch signaling,36 bringing up the possibility that a similar mechanism could be at play in KN6/MEF co‐cultures. Indeed, we found that any culture supplemented with IL‐1β also contained high amounts of IL‐6 protein, as assessed by ELISA. Moreover, IL‐6 levels were increased under conditions that also included IL‐21 (Supplementary figure 3b). We also detected elevated IL‐6 mRNA in KN6 cells cultured on MEF cell lines supplemented with CK, as measured by qPCR (Supplementary figure 3c), validating that the KN6 cells themselves were being induced to produce IL‐6.
These results raised the question of how Notch signaling might protect KN6 cells from the deleterious effects of IL1β‐induced IL‐6 expression. Earlier work by Csaszar et al. demonstrated that Notch can regulate IL‐6 signaling in HSCs by increasing the cleavage of IL‐6R from the cell surface.36 To assess whether this could account for the ability of Dll4 to allow KN6 expansion in the presence of CK, we measured soluble (s)IL‐6R by ELISA, and found that Dll4‐expressing cultures had higher levels of sIL‐6r than cultures lacking Dll4 (Supplementary figure 3d). In addition, qPCR analysis revealed that Notch signaling suppressed Il6r mRNA levels (Supplementary figure 3e). To directly test the causal role of IL‐6 in reducing cellularity, we blocked IL‐6R signaling using a combination of αIL‐6 and αIL‐6R neutralizing antibodies, and found that blocking IL‐6R signaling significantly improved the cellularity of KN6 cells exposed to CK in the absence of Dll4 (Supplementary figure 3f). Therefore, the poor cellularity of KN6 cells in the presence of CK could be at least partially attributed IL‐6 signaling, which was inhibited at both the transcriptional and post‐translational levels in the presence of Notch signaling.
TCR, Notch and cytokine receptor signals integrate to promote the differentiation of γδ17 T‐cells
We next analyzed the ability of KN6 cells to differentiate toward the γδ17 lineage under conditions of varied TCR, Notch, and cytokine signals. γδ17 cells are characterized by high levels of CD44 and low levels of CD62L and CD27.31 We therefore assessed the expression of these cell surface markers in control (+IL‐7) versus CK supplemented cultures. Provision of CK dramatically increased the CD44hi CD62Llo population in KN6 cultures in the presence of Dll4 (Figure 2b), with the T10 + DL4 co‐cultures exclusively giving rise to CD44hi CD62Llo KN6 cells. In addition, CD27lo KN6 cells were significantly increased in cultures with Dll4 and CK relative to the other culture conditions, except when IL‐21 was excluded from the CK cocktail (xSupplementary figure 4). This result suggests that IL‐21 is indispensable for the downregulation of CD27, which has been shown to play a co‐stimulatory role in development of IFNγ‐producing γδ T‐cells.37
To analyze the functionality of the KN6 cells generated under these different conditions, we assessed IL‐17 and IFNγ production by flow cytometry 6 h after stimulation. Strikingly, IL‐17A+ cells were only present in +DL4 cultures supplemented with CK, while IFNγ+ cells were present throughout (Figure 2c). Furthermore, gating on the cytokine producing subsets revealed that IL‐17+ KN6 cells were primarily CD27lo, consistent with development of γδ17 cells rather than aberrant expression of IL‐17 (Figure 2d). We next performed a multiplex cytokine analysis by ELISA on co‐culture supernatants following 36 h of stimulation with PMA/Ionomycin (Figure 3). Our results showed that all cultures contained KN6 cells that could produce copious amounts of IFNγ, although cells co‐cultured without any cytokines, or supplemented with CK in the absence of Dll4, produced less IFNγ. Consistent with our flow cytometry analysis, IL‐17A production was restricted to T10 + DL4 and T22 + DL4 KN6 co‐cultures supplemented with CK; in particular, IL‐1β and IL‐21, or IL‐1β, IL‐21 and IL‐23. Importantly, KN6 cells cultured on T10 + DL4 and supplemented with CK were the most efficient IL‐17A producers. Moreover, IL‐17 levels did not change in the presence of anti‐IL‐6/IL‐6R antibodies (CK+αIL‐6), indicating that IL‐6 affected cellularity but not effector function programming.
Figure 3.
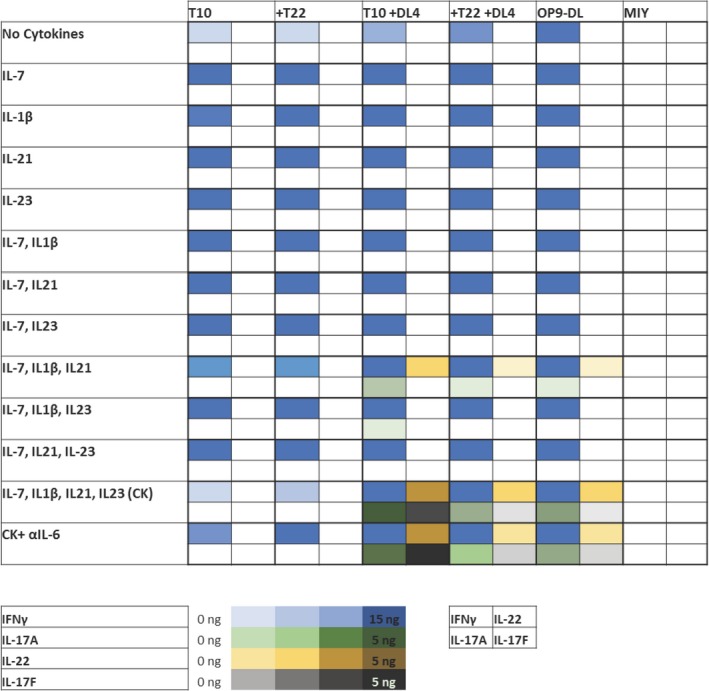
Cytokine production profile of in vitro‐derived KN6 γδ T cells. Quantification of IFNγ, IL‐17A, IL‐17F and IL‐22 production by Day 4 sorted KN6 γδ T‐cells cultured as indicated. αIL‐6 denotes supplementation of co‐cultures with neutralizing antibodies for IL‐6 and IL‐6R. KN6 cells were stimulated with PMA/Ionomycin for 36 h and the cytokine concentrations in the supernatant were quantified by ELISA. This figure shows the mean cytokine levels expressed by KN6 cells from at least 3 separate experiments.
We also evaluated production of the type 17 cytokines IL‐22 and IL‐17F. Expression of IL‐22 closely followed the pattern for IL‐17A, in agreement with previous reports stating that Notch signaling is necessary for IL‐22 production.38, 39, 40 Furthermore, IL‐1β and IL‐21 were sufficient for IL‐22 production in T10 + DL4 co‐cultures, as has been previously observed in CD4+ cells.41 IL‐17F was optimally produced by KN6 cells co‐cultured on T10 + DL4 cells supplemented with CK, but lower levels of IL‐22 and IL‐17F were also produced by KN6 cells in T22 + DL4 + CK co‐cultures. These results suggest that weaker TCR signaling enhances γδ17 programming, but only when the other required inputs are available.
We also used qPCR to measure expression of cytokine mRNAs in KN6 cells cultured under these different conditions. All cultures supplemented with CK expressed Ifng mRNA, while expression of Il17a and Il22 transcripts were only detected in Dll4‐expressing co‐cultures (Supplementary figure 5). Of note, only T10 + DL4 cultures supplemented with CK showed detectable expression of Il17f mRNA. As a control, analysis of the starting population (untransduced in vitro‐derived Rag2 −/− DN3 cells) did not show detectable levels of these cytokine genes, indicating that γδTCR signaling was required for their induction. Taken together, these data suggest that KN6 cells exposed to a weak TCR ligand, Dll4, and the cytokines IL‐1β, IL‐21 and IL‐23, are efficiently directed into the γδ17 cell lineage. Intriguingly, the provision of Dll4 and CKs enabled γδ17 programming even in the presence of a strong TCR ligand (T22), albeit at a lower frequency. However, IFNγ production was not significantly affected, even under the culture conditions that best promoted γδ17 development. These results indicate that cytokine signaling directly enables γδ17 T‐cell programming, without causing a loss of access to IFNγ induction.
Transcriptional programming of γδ17 cells by combinatorial signals from TCR, Notch and cytokine receptors
Having established the optimal culture conditions for generating γδ17 cells, we set out to define the molecular consequences of the integration of these signals by measuring gene expression. RORγt (Rorc) is a prototypical transcription factor associated with IL‐17 producing cells, whereas T‐bet (Tbx21) is diagnostic of IFNγ‐producing cells. KN6 cells cultured in the presence of Dll4 expressed higher RORγt mRNA levels than those without Dll4, irrespective of the TCR ligand used (Figure 4a). However, the levels of Il23r and Il21r mRNA were sensitive to the TCR ligand, as they were higher in T10 + DL4 + CK cultures than in T22 + DL4 + CK cultures (Figure 4b). Tbx21 mRNA was observed in all subsets that were supplemented with CK (Figure 4a). Interestingly, KN6 cells cultured on T22 + MEFs expressed high levels of Tbx21 when supplemented with IL‐7 alone, suggesting that exposure to strong ligand is enough to promote development along the IFNγ (γδ1) lineage. Considering the proposed role of Id3 downstream of TCR signal strength in effector fate determination, we next measured Id3 mRNA levels. As previously observed, Id3 mRNA expression was higher in KN6 cells cultured on MEFs bearing a strong TCR ligand, when supplemented with IL‐7 alone (Figure 4c). However, provision of IL‐1β, IL‐23 and IL‐21 correlated with significantly reduced Id3 mRNA levels in KN6 cells exposed to strong ligand. These results are consistent with a requirement for cytokine‐induced dampening of TCR signal in the differentiation of γδ T‐cell precursors toward the γδ17 effector fate in the presence of a strong TCR ligand.
Figure 4.
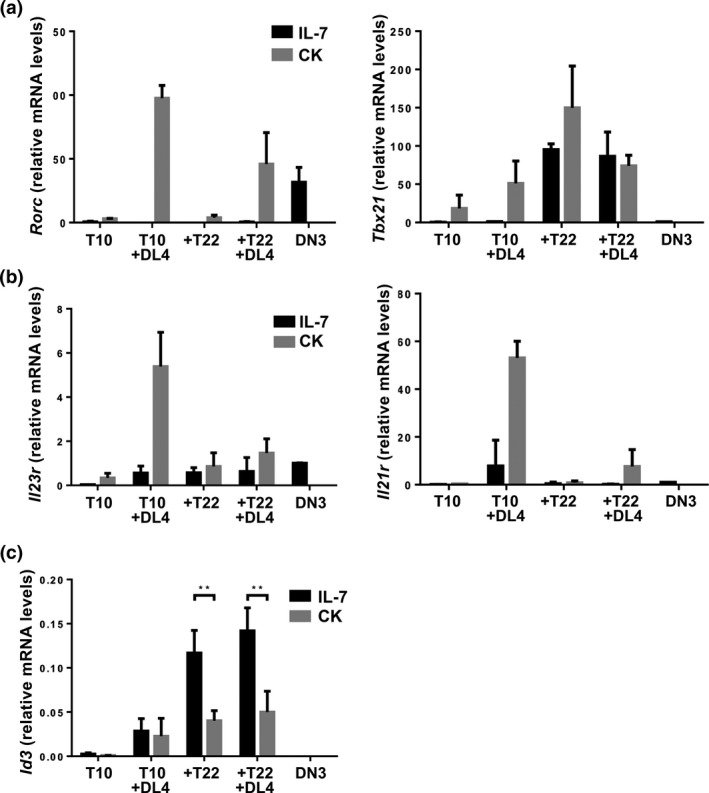
KN6 γδ T‐cells supplemented with IL‐1β, IL‐21, and IL‐23 as well as Notch ligand Dll4, express γδ17 lineage hallmark transcription factors, type‐17 cytokine receptors, and lower levels of Id3. (a) Quantification of Rorc (encoding RORγt) and Tbx21 (encoding Tbet) mRNA expression by qPCR. (b) Quantification of Il21r and Il23r mRNA by qPCR. (c) Quantification of Id3 mRNA by qPCR. Data shown are representative of at least 3 independent experiments harvested on Day 4. The data and error bars are presented as standard error of the mean (s.e.m.). Statistical significance was determined using a two‐tailed unpaired Student's t‐test as P < 0.05 (**P < 0.01). ns = nonsignificant.
Intrathymic requirement for cytokine receptor signaling during γδ17 programming
Having established that γδTCR ligands, Dll4 and cytokines (IL‐1β, IL‐23, IL‐21) are sufficient to induce effective γδ17 differentiation in vitro, we turned to the question of whether these cytokines play a role during the normal thymic development of γδ T‐cells. Several lines of evidence indicate that these cytokines are available to developing γδ T‐cells in the thymus. Previous work by Petrie and colleagues showed that IL‐1β and IL‐23 are expressed by mTECs at comparable levels to IL‐7.42 Moreover, specific subsets of thymic γδ T‐cells and a population of CD4+ T‐cells express high levels of IL‐21R, during the appropriate ontological window of time to affect γδ17 development (http://www.immgen.org),43 plus a subset of IL‐21 expressing thymocytes was recently reported.44 Therefore, we assessed whether inhibiting IL‐1β, IL‐21 and IL‐23 inputs would interfere with the generation of γδ17 cells in the context of wild‐type progenitors in the fetal thymus.
To address this question, we treated fetal thymic organ cultures (FTOCs) with antibodies that neutralize IL‐1β, IL‐21 and IL‐23 cytokine receptors (αCK), or with anti‐IL‐1R alone as a control. FTOCs in which all three cytokine receptors were blocked showed a higher frequency of γδ T‐cells, although the total cell number appeared approximately the same (Supplementary figure 6a). However, analysis of cytokine production by intracellular staining following stimulation showed that there were significantly fewer IL‐17 producing cells, while IFNγ production was not affected (Supplementary figure 6b). Interestingly, the remaining γδ17 cells from the αCK group had a lower mean fluorescence intensity for IL‐17 (Supplementary figure 6c). Furthermore, in agreement with published reports,45, 46 we found that control IL‐17 producing cells had a significantly higher CD44 mean fluorescence intensity than IFNγ producing cells (Supplementary figure 6d). This is consistent with an impact on γδ17 programming rather than just on IL‐17 production.
To address the possibility that γδ17‐committed cells were already present in the FTOCs before they were harvested and exposed to antibodies, we took advantage of hanging drop cultures, in which FTOCs are depleted of lymphocytes and then reconstituted with fetal liver‐derived progenitors.47, 48 Following FTOC reconstitution, the experimental group was supplemented with the αCK cocktail, and the cultures were harvested on Day 14 to allow sufficient time for the development of γδ T‐cells from the pre‐thymic progenitors. Again, we observed a higher frequency of γδ T‐cells in the αCK group, without significantly different γδ T‐cell numbers (Figure 5a). Remarkably, however, IL‐17 producing γδ T‐cells were nearly absent in αCK treated FTOCs. IFNγ production, by contrast, was not significantly different between the two groups (Figure 5b). Taken together, these data indicate that endogenous signals derived from thymic‐derived IL‐1β, IL‐21 and IL‐23 play a role in the intrathymic development of γδ17 cells.
Figure 5.
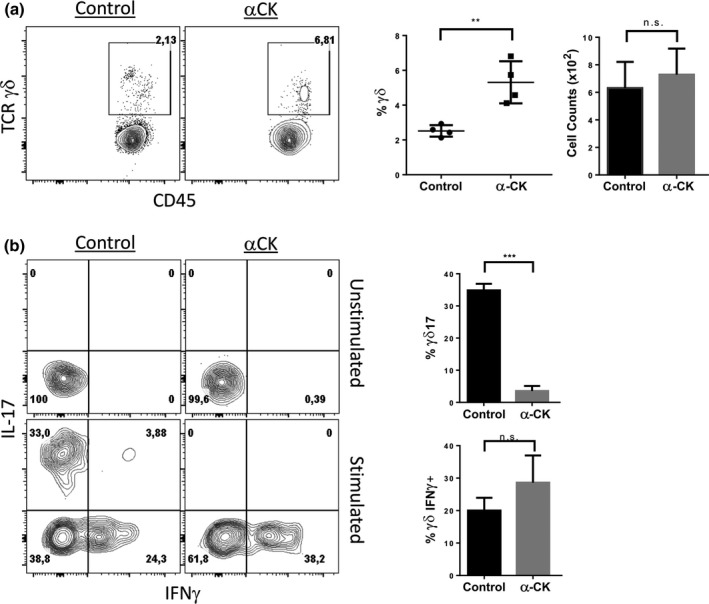
γδ17 cells fail to develop in anti IL‐1βR, IL‐21R and IL‐23R treated hanging drop FTOCs. Embryonic Day 14 WT fetal thymic lobes were reconstituted with Embryonic Day 14 WT fetal livers on Day 7 using the hanging drop methodology. FTOCs were treated with control or anti IL‐1βR, IL‐21R and IL‐23R (αCK) containing media for 14 days prior to analysis of: (a) γδ T‐cell frequency by flow cytometry and cellularity on Day 14. (b) Frequency of IL‐17 and IFN‐γ producing γδ T‐cells. Data are representative of at least 3 experiments. The data and error bars are presented as standard error of the mean (s.e.m.). Statistical significance was determined using a two‐tailed unpaired Student's t‐test as P < 0.05 (**P < 0.01, ***P < 0.001); n.s. = nonsignificant.
Discussion
γδ17 cell fate choice relies on the induction of a gene network downstream of specific sets of environmental signals, but the nature of these signals and how they work together to drive the γδ17 fate has been unclear. Here, we used a tightly controlled experimental system to investigate how TCR, Notch, and cytokine signaling are integrated to control γδ T‐cell effector programming. We found that γδ TCR‐expressing cells can functionally mature toward the IFNγ producing phenotype under most conditions, whereas γδ17 development requires Notch signaling and cytokines in addition to TCR signaling, both in vitro and in vivo. Furthermore, we observed that while weak TCR‐ligand interactions are required for optimal γδ17 development, Notch and cytokines can also divert cells experiencing strong TCR signals toward the γδ17 fate. Importantly, we have shown that cytokine receptor signaling in response to IL‐1β, IL‐23 and IL‐21 is critical for γδ17 development in the fetal thymus, in addition to its role in inducing IL‐17 expression in mature peripheral γδ17 cells. Our results provide unique insights into the combinatorial inputs that are required for γδ17 development, and how they are integrated to provide access to the γδ17 gene network.
Whether or not there is a role for γδTCR signaling in determining effector differentiation and cytokine production has been controversial, with evidence existing for both sides of the argument.13, 15, 49, 50 Early reports suggested that “antigen‐naïve” γδ T‐cells gave rise to γδ17 cells, whereas “antigen‐experienced” γδ T‐cells became IFNγ‐producers.9 However, a series of elegant experiments by Munoz‐Ruiz et al. suggested that signals downstream of the TCR play an important role in the maturation and effector programming of γδ T‐cells of both effector fates.12 Other work showed that lowering of the γδTCR signal below certain thresholds resulted in a severe loss of γδ17 effector subtype.11 In our studies, we observed that exposure to a strong TCR ligand accelerated γδ T‐cell maturation, as assessed by CD24 downregulation, relative to weak TCR ligand. Furthermore, we showed that TCR signals support IFNγ producing effector differentiation regardless of ligand strength, whereas weak TCR ligands are optimal for γδ17 development. Therefore, our work provides support for the need for TCR signaling in γδ17 development, and for an impact of TCR signal strength on effector fate choice. However, these studies also reveal that the decisive signals for γδ17 programming are provided by additional environmental cues, in the presence of the TCR signal.
Although TCR signals are clearly required for development and effector programming of γδ17s, our results also point to a need for “dampening” of TCR signaling during γδ17 development. This is supported by our observation that a subpopulation of γδ T‐cells exposed to strong ligand to also commit to the γδ17 effector fate. One critical consequence of TCR signal dampening is a decrease in Id3 upregulation in the presence of cytokines. Id3 inhibits the activity of the E protein HEB (Tcf12), which is required for γδ17 programming upstream of Rorc expression.51 Therefore, cytokine‐driven suppression of Id3 upregulation in response to TCR would be expected to enable HEB activity and allow activation of the γδ17 network. This is supported by the selective upregulation of Rorc, Il23r and Il21r only in cultures that display low levels of Id3. However, the impact of strong TCR signaling on Il23r expression occurs even in the presence of Dll4 and cytokines, indicating that there are likely additional determinants of γδ17 differentiation that can be disrupted by strong TCR signals regardless of Id3 levels.
In contrast, Tbx21 upregulation correlates best with strong TCR signals, regardless of Dll4 or cytokines. This model aligns with that proposed by Turchinovich et al., 52 whereby the activation of a gene regulatory network downstream of TCR signaling in the form of Egr3, NFAT and NFkB is directly associated with the upregulation of Tbx21. Hence, these findings lend support to the quantitative signal strength model extending to functional programming, as the amount of signals accumulated downstream of the TCR, such as Id3, directly correlate with differentiation into distinct functional subtypes. It will be important to further test this notion by making use of models that enable more quantitative measurement of TCR signals, and the levels at which they can be modulated by other signals. Those models will allow us to uncover the thresholds that may be required for differentiation into distinct γδ T‐cell effector subsets, how those thresholds are altered by cytokine signals and Dll4, including the downstream molecular consequences.
Notch1 signaling has been directly implicated in the development and homeostasis of γδ17 T‐cells in mice,23, 24 but determining the specific roles of Notch in γδ17 development has been complicated by its other essential roles during T‐cell development. Our culture system allowed us to precisely regulate Notch ligand accessibility at the initiation of γδ T‐cell development, without disrupting earlier developmental events. We observed that Notch signaling was not sufficient for the development of γδ17 cells, regardless of TCR signal strength. However, Notch was clearly essential for the development of this subset, as KN6 cells co‐cultured without Dll4 were unable to produce IL‐17 or associated cytokines. The mechanism by which Notch enables γδ17 development appears complex and may involve several distinct functions of the Notch signaling pathway. One key role of Notch signaling in γδ17 development appears to be enhancing expansion and survival of γδ T‐cell precursors, consistent with previous reports.14, 53 Specifically, Notch signaling appears to provide protection from the deleterious effects of cytokine signaling by inhibiting Il6r expression and inducing IL‐6R shedding. In agreement with previous reports,17 blocking IL‐6 signaling in these cultures did not affect IL‐17 production, consistent with IL‐6 interference providing one mechanism by which Notch signaling sustains developing γδ17 cells. Additional studies will be required to assess whether there are direct inputs of Notch into the expression of key genes such as Tcf12 and Rorc during γδ17 development, as recently suggested.51
Because of their sites of effector function and ability to respond quickly to environmental cues, γδ T‐cells are often labeled as innate lymphocytes.54, 55 Previous reports have suggested that Type 17 skewing cytokines or TGF‐β1 are sufficient for IL‐17 production by mature γδ T‐cells in the absence of TCR signals.18, 56 However, these reports failed to assess how TCR signals in addition to other environmental cues may be involved in the early programming of γδ17 T‐cells. Of note, the presence of Dll4 did not significantly hamper IFNγ production, even when supplemented with IL‐1β, IL‐21 and IL‐23. Moreover, both RORγt (Rorc) and T‐bet (Tbx21) were upregulated in cultures exposed to Dll4, indicating that activation of the γδ17 program did not preclude access to factors required for IFNγ production. Furthermore, the addition of cytokines was shown to result in higher expression of Il21r and Il23r when supplemented with Dll4, suggesting the activation of a positive feedback loop that enforces γδ17 programming and cytokine responsiveness during development.
Finally, we have addressed the question of whether the requirement for IL‐1β, IL‐21 and IL‐23 for γδ17 development in our culture system is recapitulated in the normal thymic environment. We found that generation of γδ17 cells in precursors developing in FTOC was significantly reduced in the presence of antibodies against these three cytokine receptors, strongly indicating their physiological relevance. Hence, signals from IL‐1β, IL‐21 and IL‐23 provide important cues necessary for the development of γδ17 cells not only in vitro but also intrathymically.
In summary, we have shown using the KN6 γδ T‐cell model of development that γδ T‐cell precursors optimally differentiate toward the γδ17‐effector subtype when exposed to the Notch ligand (Dll4), weak TCR ligand (T10), and cytokine cues provided by IL‐1β, IL‐21 and IL‐23. Taken together, this work provides a framework for understanding the integration of signals downstream of the Notch, TCR, and γδ17 associated cytokine receptors. Specifically, cytokines are required to dampen the TCR signal, in part by lowering Id3, which permits the HEB‐dependent elaboration of the γδ17 network.51 Notch signals are concurrently required to protect developing γδ17 cells from the negative effects of IL‐1β‐mediated IL‐6 activity triggered by the provision of these cytokines. Further work in this area will focus on defining the quantitative signal thresholds required for γδ17 cells versus other γδ T‐cell effector subsets, such as Vγ1/Vδ6.3 and Vγ5/Vδ1 cells. It will also be important to address the roles of other cytokines including TGF‐β, IL‐15 and IL‐18 in these processes. In conclusion, our studies provide important new insights into the generation of a subset of γδ T‐cells which play a significant role in regulating health and disease. Furthermore, they have revealed the minimal requirement for producing γδ17 cells in vitro, which could enable their use in clinical settings.
Methods
Mice
Rag2‐deficient mice57 were bred and maintained in the Sunnybrook Research Institute Comparative Research facility in specific pathogen‐free conditions. C57BL/6 and BALB/c wild‐type mice were obtained from Jackson Laboratories. All animal procedures were approved by the Sunnybrook Research Institute Animal Care Committee (Toronto, Ontario, Canada).
Retroviral transduction and cultures
Retroviral constructs were generated, as previously described,14, 53, 58 by subcloning the cDNAs of interest into the pMigR1 or pMIY plasmids, upstream of the internal ribosomal entry site.59, 60 Stable retroviral‐producing GP+E.86 packaging cell lines were generated for each construct. The KN6 TCRγ and TCRδ subunits were cloned into pMiY as a fusion protein linked by the 2A Tescovirus linker peptide, as described previously.14 OP9‐DL1, OP9‐DL4 and OP9‐Ctrl cells were produced and maintained as previously described,59, 60 and co‐cultures were supplemented with 1 ng mL−1 mouse recombinant IL‐7 and 5 ng mL−1 human recombinant Flt‐3L (Peprotech). Fetal livers were obtained from timed‐pregnant Rag2 −/− female mice on Day 14 of gestation. Single‐cell suspensions were generated by disruption through a 40‐μm nylon mesh screen using a syringe plunger, and fetal livers cells were co‐cultured with OP9‐DL1 cells as previously described to produce DN3 cells.58 For retroviral transduction of DN3 cells, cells from Day 7 FL/OP9‐DL1 co‐cultures were passaged for an overnight co‐culture with stable retrovirus‐producing GP+E.86 packaging cells. Following this step, the transduced (GFP+ and YFP+) CD44− CD25+ DN3 cells were purified by cell sorting and placed back onto stromal cell co‐cultures, as previously described with or without combinations of recombinant IL‐1β (5 ng mL−1), IL‐7 (1 ng mL−1), IL‐21 (20 ng mL−1), IL‐23 (10 ng mL−1).58
Stimulation and cytokine analysis
Indicated populations were sorted and stimulated for 5 h (for flow cytometry) or 36 h (for ELISA) with 50 ng mL−1 PMA (Sigma) and 500 ng mL−1 Ionomycin (Sigma) or 10 μg mL−1 anti‐mouse CD3. CD45+ GFP+ cells were sorted from transduced DN3s cultured for 4 days on indicated cells. Sorted cells (5 × 104 cells/well of a 96‐well plate) were subsequently cultured with 50 ng mL−1 PMA (Sigma) and 500 ng mL−1 Ionomycin (Sigma) in OP9 medium. Where indicated, instead of PMA/Ionomycin, cells were incubated in wells coated with 10 μg mL−1 anti‐mouse CD3.
For assessing cytokine secretion by ELISA, supernatants were harvested after 36 h, and IFNγ, IL‐17, IL‐6, IL‐22 levels were quantified using the DuoSet ELISA Development System (R&D Systems) according to the manufacturer's protocol. For assessing cytokine secretion by multiplex analysis, supernatants were harvested after 36 h, and cytokine levels were quantified using the Mouse Th17 Magnetic Bead Panel (Milliplex) or Mouse Procartaplex Immunoassay (e‐Bioscience) according to the manufacturer's protocol. For assessing intracellular staining, cells were incubated for 5 h with the indicated concentrations of PMA/Ionomycin or plate bound anti‐CD3 in the presence of 1x Brefeldin A solution (e‐Bioscience). Cells were stained with the indicated commercially available antibodies (BioLegend, and BD Biosciences) for intracellular cytokines, following permeabilization by the Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Biosciences). The cells were analyzed with a BD‐LSRII flow cytometer, using FlowJo software (Treestar, Inc.).
Fetal thymus organ culture
Fetal thymus organ cultures were prepared as previously described.61, 62 1 × 1 cm squares of absorbable gelatin sponge Gelfoam (Pharmacia & Upjohn) were cut and placed overnight in 1 mL of OP9 media in individual wells of a 12‐well plate. Day 14 timed pregnant C57BL/6 mice were sacrificed and fetuses were separated. Thymic lobes were harvested from the embryos and, after removing excess blood by washing with PBS, were placed on membrane raft Nuclepore Track‐etch Membrane 0.8 μm (Whatman) that was placed on top of the Gelfoam.61 The lobes were cultured with or without IL‐1, IL‐21 and IL‐23 cytokine receptor inhibitors at 400 ng mL−1 (InVivoMAb: αCD121a Jama‐147 and α4A9, R&D Systems: αIL‐23R 258010) at the start of cultures and supplemented with fresh media every 4 days. The FTOCs were harvested at indicated time‐points and prepared for analysis by flow cytometry.
FTOC reconstitutions via hanging drop
Embryonic Day 14 FTOCs were depleted of thymocytes using deoxyguanosine for 6 days prior to reconstitution with fetal liver derived LSK cells.
Flow cytometry
All single‐cell suspensions were stained with the indicated and commercially available antibodies (listed in Supplementary table 1) and analyzed with a BD‐LSRII flow cytometer, using Flowjo software (Treestar, Inc.). Fetuses were harvested from Day 14 timed‐pregnant C57BL/6 mice and thymic lobes were cultured on a membrane raft Nuclepore Track‐etch Membrane 0.8 μm (Whatman), which was placed on top of the described Gelfoam (Pharmacia & Upjohn). The cultures were supplemented with OP9 media containing 1.1 mmol L−1 deoxyguanosine, and lobes were treated for 6 days. Following this period, lobes were taken off the rafts, washed with PBS, and transferred to fresh rafts where they were allowed to recover for 24 h. Single lobes were added to individual wells of a Terasaki plate that each contained 38 μL media plus 2 × 103 LSK cells isolated from Day 14 timed‐pregnant C57BL/6 fetal livers. Terisaki plates were inverted prior to their placement in the incubator, after ensuring that the lobes were hanging at the center of the droplets. After 24 h, the lobes were washed in media and transferred back to rafts and as with the FTOCs, cultured with or without IL‐1β, IL‐21 and IL‐23 cytokine receptor inhibitors at 400 ng mL−1 (InVivoMAb, R&D Systems). Fresh media was supplemented every 4 days and the FTOCs were harvested at indicated time‐points and prepared for analysis by flow cytometry.
Please refer to Supplementary table 1 for the list of antibodies used. Fixation/Permeabilization Solution Kit (BD) was used for intracellular staining following 5 h of incubation with cell stimulating cocktail and Brefeldin A (e‐Bioscience). Dead cells were excluded from the analyses using DAPI gating or Fixable Viability Dye eFluor 450 (e‐Bioscience) in the case of fixed cells.
Quantitative real‐time PCR
Thymocyte populations were purified by flow cytometry following selection using magnetic anti‐CD45 beads (Miltenyi Biotech). Total RNA was extracted using TRIzol (Invitrogen) and converted to cDNA using Quantitect Reverse Transcription Kit (Qiagen) according to manufacturer's instructions. Expression of the indicated genes was measured by quantitative real‐time PCR using SYBR GreenER (Invitrogen). Primer sequences are indicated in Supplementary table 2. β‐actin was used to normalize cycle thresholds.
Generation of BALB/c mouse embryonic fibroblasts
Mouse embryonic fibroblast (MEF) lines were generated from embryonic Day 14 fetuses.60, 61, 62 Embryonic Day 14 fetuses were harvested from timed pregnant BALB/c mice and placed in a 10‐cm dish containing PBS to wash the embryos before moving them to another 10 cm plate containing PBS. Forceps and a razor blade were used to remove the internal organs and head of the embryo, respectively. The remainder of the embryo was minced well using a razor blade and incubated in a 15‐mL conical tube with 5 mL of 2x Trypsin/PBS for 15 min at 37°C. Following the incubation, the content of the tube was mixed by pipetting prior to being spun down at 300 g for 5 min. 250 μL of the supernatant was then separated and mixed with 750 μL of medium (DMEM supplemented with 10% FBS) and used to coat wells of Falcon 6‐well plates containing a microscope slide cover. The individual wells were monitored and passaged as the cells grew out from under the slide covers. The cells were then harvested, pelleted by centrifugation, and re‐seeded to make a monolayer. Supernatants from retroviral‐producing GP+E.86 packaging cell lines transfected with pMigR1, pMigR1‐DL1 or pMigR1‐DL4 were used to transduce the BALB/c (B/c) fibroblasts and generate stable B/c‐DL1, B/c‐DL4, and B/c‐Ctrl cell lines, as previously described.60, 63, 64 Subsequently, BALB/c‐DL4 and BALB/c‐Ctrl lines were each retrovirally transduced to express pMiCherry or pT22b‐MiCherry64 creating the four new mouse embryonic fibroblast cell lines (MEFs) BALB/c‐Ctrl, BALB/c‐T22, BALB/c‐DL4 and BALB/c‐DL4‐T22.
Statistical analysis
The data and error bars are presented as standard error of the mean (s.e.m.). To determine statistical significance, a two‐tailed unpaired Student's t‐test was used for comparison between two experimental groups, using Prism software. In comparisons of 3 or more groups, the significance was determined using one‐way analysis of variance (one‐way ANOVA). Statistical significance was determined as P < 0.05 (*P < 0.05; **P < 0.01; ***P < 0.001) and, n.s. as nonsignificant.
Conflict of Interest
The authors have no competing interests to declare.
Supporting information
Acknowledgments
We are thankful to Geneve Awong, Gisele Knowles and Courtney McIntosh for their expertise and assistance in flow cytometry and cell sorting, Sean Oh for technical support, and Lisa Wells and Christina Lee for expert assistance with animal care. PZ was supported by an Ontario Graduate Scholarship award. This work was supported by grants from the Canadian Institutes of Health Research (CIHR) MOP‐119538, MOP‐42387 and FND‐154332, National Institutes of Health NIH‐1P01AI102853‐01, and the Krembil Foundation. JCZP is supported by a Canada Research Chair in Developmental Immunology.
References
- 1. Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 2010; 10: 467–478. [DOI] [PubMed] [Google Scholar]
- 2. Rei M, Pennington DJ, Silva‐Santos B. The emerging Protumor role of gammadelta T lymphocytes: implications for cancer immunotherapy. Cancer Res 2015; 75: 798–802. [DOI] [PubMed] [Google Scholar]
- 3. Ramirez‐Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vgamma4+ gammadeltaT17 memory‐like cells that travel to distant skin and accelerate secondary IL‐17‐driven responses. Proc Natl Acad Sci USA 2015; 112: 8046–8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andersson A, Grahnemo L, Engdahl C, et al IL‐17‐producing gammadeltaT cells are regulated by estrogen during development of experimental arthritis. Clin Immunol 2015; 161: 324–3232. [DOI] [PubMed] [Google Scholar]
- 5. Malik S, Want MY, Awasthi A. The emerging roles of gamma‐delta T cells in tissue inflammation in experimental autoimmune encephalomyelitis. Front Immunol 2016; 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coffelt SB, Kersten K, Doornebal CW, et al IL‐17‐producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015; 522: 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uluckan O, Jimenez M, Karbach S, et al Chronic skin inflammation leads to bone loss by IL‐17‐mediated inhibition of Wnt signaling in osteoblasts. Sci Transl Med 2016; 8: 237–330. [DOI] [PubMed] [Google Scholar]
- 8. Zarin P, Chen EL, In TS, et al Gamma delta T‐cell differentiation and effector function programming, TCR signal strength, when and how much? Cell Immunol 2015; 296: 70–75. [DOI] [PubMed] [Google Scholar]
- 9. Jensen KD, Su X, Shin S, et al Thymic selection determines gammadelta T cell effector fate: antigen‐naive cells make interleukin‐17 and antigen‐experienced cells make interferon gamma. Immunity 2008; 29: 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barbee SD, Woodward MJ, Turchinovich G, et al Skint‐1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci USA 2011; 108: 3330–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wencker M, Turchinovich G, Di Marco Barros R, et al Innate‐like T cells straddle innate and adaptive immunity by altering antigen‐receptor responsiveness. Nat Immunol 2014; 15: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Munoz‐Ruiz M, Ribot JC, Grosso AR, et al TCR signal strength controls thymic differentiation of discrete proinflammatory gammadelta T cell subsets. Nat Immunol 2016; 17: 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chien YH, Zeng X, Prinz I. The natural and the inducible: interleukin (IL)‐17‐producing gammadelta T cells. Trends Immunol 2013; 34: 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lauritsen JP, Wong GW, Lee SY, et al Marked induction of the helix‐loop‐helix protein Id3 promotes the gammadelta T cell fate and renders their functional maturation Notch independent. Immunity 2009; 31: 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sumaria N, Grandjean CL, Silva‐Santos B, et al Strong TCRgammadelta signaling prohibits thymic development of IL‐17A‐secreting gammadelta T cells. Cell Rep 2017; 19: 2469–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ashouri JF, Weiss A. Endogenous Nur77 is a specific indicator of antigen receptor signaling in human T and B cells. J Immunol 2017; 198: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lochner M, Peduto L, Cherrier M, et al In vivo equilibrium of proinflammatory IL‐17+ and regulatory IL‐10+ Foxp3+ RORgamma t+ T cells. J Exp Med 2008; 205: 1381–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sutton CE, Lalor SJ, Sweeney CM, et al Interleukin‐1 and IL‐23 induce innate IL‐17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009; 31: 331–341. [DOI] [PubMed] [Google Scholar]
- 19. Nurieva R, Yang XO, Martinez G, et al Essential autocrine regulation by IL‐21 in the generation of inflammatory T cells. Nature 2007; 448: 480–483. [DOI] [PubMed] [Google Scholar]
- 20. Meyer Zu Horste G, Wu C, Wang C, et al RBPJ controls development of pathogenic Th17 cells by regulating IL‐23 receptor expression. Cell Rep 2016; 16: 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coutaz M, Hurrell BP, Auderset F, et al Notch regulates Th17 differentiation and controls trafficking of IL‐17 and metabolic regulators within Th17 cells in a context‐dependent manner. Sci Rep 2016; 6: 39117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mukherjee S, Schaller MA, Neupane R, et al Regulation of T cell activation by Notch ligand, DLL4, promotes IL‐17 production and Rorc activation. J Immunol 2009; 182: 7381–7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shibata K, Yamada H, Sato T, et al Notch‐Hes1 pathway is required for the development of IL‐17‐producing gammadelta T cells. Blood 2011; 118: 586–593. [DOI] [PubMed] [Google Scholar]
- 24. Nakamura M, Shibata K, Hatano S, et al A genome‐wide analysis identifies a notch‐RBP‐Jkappa‐IL‐7Ralpha axis that controls IL‐17‐producing gammadelta T cell homeostasis in mice. J Immunol 2015; 194: 243–251. [DOI] [PubMed] [Google Scholar]
- 25. Bonneville M, Ishida I, Mombaerts P, et al Blockage of alpha beta T‐cell development by TCR gamma delta transgenes. Nature 1989; 342: 931–934. [DOI] [PubMed] [Google Scholar]
- 26. Felix R, Cecchini MG, Hofstetter W, et al Impairment of macrophage colony‐stimulating factor production and lack of resident bone marrow macrophages in the osteopetrotic op/op mouse. J Bone Miner Res 1990; 5: 781–789. [DOI] [PubMed] [Google Scholar]
- 27. Schild H, Mavaddat N, Litzenberger C, et al The nature of major histocompatibility complex recognition by gamma delta T cells. Cell 1994; 76: 29–37. [DOI] [PubMed] [Google Scholar]
- 28. Adams EJ, Chien YH, Garcia KC. Structure of a gammadelta T cell receptor in complex with the nonclassical MHC T22. Science 2005; 308: 227–231. [DOI] [PubMed] [Google Scholar]
- 29. Langrish CL, Chen Y, Blumenschein WM, et al IL‐23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zarin P, Wong GW, Mohtashami M, et al Enforcement of gammadelta‐lineage commitment by the pre‐T‐cell receptor in precursors with weak gammadelta‐TCR signals. Proc Natl Acad Sci USA 2014; 111: 5658–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ribot JC, deBarros A, Pang DJ, et al CD27 is a thymic determinant of the balance between interferon‐gamma‐ and interleukin 17‐producing gammadelta T cell subsets. Nat Immunol 2009; 10: 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Caccamo N, La Mendola C, Orlando V, et al Differentiation, phenotype, and function of interleukin‐17‐producing human Vgamma9Vdelta2 T cells. Blood 2011; 118: 129–138. [DOI] [PubMed] [Google Scholar]
- 33. Sutton C, Brereton C, Keogh B, et al A crucial role for interleukin (IL)‐1 in the induction of IL‐17‐producing T cells that mediate autoimmune encephalomyelitis. J Exp Med 2006; 203: 1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cai Y, Xue F, Fleming C, et al Differential developmental requirement and peripheral regulation for dermal Vgamma4 and Vgamma6T17 cells in health and inflammation. Nat Commun 2014; 5: 3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lalor SJ, Dungan LS, Sutton CE, et al Caspase‐1‐processed cytokines IL‐1beta and IL‐18 promote IL‐17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol 2011; 186: 5738–5748. [DOI] [PubMed] [Google Scholar]
- 36. Csaszar E, Wang W, Usenko T, et al Blood stem cell fate regulation by Delta‐1‐mediated rewiring of IL‐6 paracrine signaling. Blood 2014; 123: 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ribot JC, Silva‐Santos B. Differentiation and activation of gammadelta T Lymphocytes: focus on CD27 and CD28 costimulatory receptors. Adv Exp Med Biol 2013; 785: 95–105. [DOI] [PubMed] [Google Scholar]
- 38. Alam MS, Maekawa Y, Kitamura A, et al Notch signaling drives IL‐22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci USA 2010; 107: 5943–5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhuyan ZA, Asanoma M, Iwata A, et al Abrogation of Rbpj attenuates experimental autoimmune uveoretinitis by inhibiting IL‐22‐producing CD4+ T cells. PLoS One 2014; 9: e89266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wei X, Wang JP, Hao CQ, et al Notch signaling contributes to liver inflammation by regulation of interleukin‐22‐producing cells in hepatitis B virus infection. Front Cell Infect Microbiol 2016; 6: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yeste A, Mascanfroni ID, Nadeau M, et al IL‐21 induces IL‐22 production in CD4+ T cells. Nat Commun 2014; 5: 3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Griffith AV, Fallahi M, Nakase H, et al Spatial mapping of thymic stromal microenvironments reveals unique features influencing T lymphoid differentiation. Immunity 2009; 31: 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heng TS, Painter MW; Immunological Genome Project C . The immunological genome project: networks of gene expression in immune cells. Nat Immunol 2008; 9: 1091–1094. [DOI] [PubMed] [Google Scholar]
- 44. Marnik EA, Wang X, Sproule TJ, et al Precocious interleukin 21 expression in naive mice identifies a natural helper cell population in autoimmune disease. Cell Rep 2017; 21: 208–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lombes A, Durand A, Charvet C, et al Adaptive immune‐like gamma/delta T lymphocytes share many common features with their alpha/beta T cell counterparts. J Immunol 2015; 195: 1449–1458. [DOI] [PubMed] [Google Scholar]
- 46. Haas JD, Gonzalez FH, Schmitz S, et al CCR6 and NK1.1 distinguish between IL‐17A and IFN‐gamma‐producing gammadelta effector T cells. Eur J Immunol 2009; 39: 3488–3497. [DOI] [PubMed] [Google Scholar]
- 47. Jenkinson EJ, Franchi LL, Kingston R, et al Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: application in the production of chimeric thymus rudiments. Eur J Immunol 1982; 12: 583–587. [DOI] [PubMed] [Google Scholar]
- 48. Anderson G, Jenkinson EJ. Fetal thymus organ culture. CSH Protoc 2007; 2007: pdb‐prot4808. [DOI] [PubMed] [Google Scholar]
- 49. Prinz I, Silva‐Santos B, Pennington DJ. Functional development of gammadelta T cells. Eur J Immunol 2013; 43: 1988–1994. [DOI] [PubMed] [Google Scholar]
- 50. Fahl SP, Coffey F, Kain L, et al Role of a selecting ligand in shaping the murine gamma delta‐TCR repertoir. Proc Natl Acad Sci USA 2018; 115: 1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. In TSH, Trotman‐Grant A, Fahl S, et al HEB is required for the specification of fetal IL‐17‐producing gammadelta T cells. Nat Commun 2017; 8: 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Turchinovich G, Hayday AC. Skint‐1 identifies a common molecular mechanism for the development of interferon‐gamma‐secreting versus interleukin‐17‐secreting gammadelta T cells. Immunity 2011; 35: 59–68. [DOI] [PubMed] [Google Scholar]
- 53. Ciofani M, Knowles GC, Wiest DL, et al Stage‐specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity 2006; 25: 105–116. [DOI] [PubMed] [Google Scholar]
- 54. Ferreira LM. Gammadelta T cells: innately adaptive immune cells? Int Rev Immunol 2013; 32: 223–248. [DOI] [PubMed] [Google Scholar]
- 55. Sutton CE, Mielke LA, Mills KH. IL‐17‐producing gammadelta T cells and innate lymphoid cells. Eur J Immunol 2012; 42: 2221–2231. [DOI] [PubMed] [Google Scholar]
- 56. Do JS, Fink PJ, Li L, et al spontaneous development of IL‐17‐producing gamma delta T cells in the thymus occurs via a TGF‐beta 1‐dependent mechanism. J Immunol 2010; 184: 1675–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shinkai Y, Rathbun G, Lam KP, et al RAG‐2‐deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 1992; 68: 855–867. [DOI] [PubMed] [Google Scholar]
- 58. Ciofani M, Schmitt TM, Ciofani A, et al Obligatory role for cooperative signaling by pre‐TCR and Notch during thymocyte differentiation. J Immunol 2004; 172: 5230–5239. [DOI] [PubMed] [Google Scholar]
- 59. Schmitt TM, Zuniga‐Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta‐like‐1 in vitro . Immunity 2002; 17: 749–756. [DOI] [PubMed] [Google Scholar]
- 60. Mohtashami M, Shah DK, Nakase H, et al Direct comparison of Dll1‐ and Dll4‐mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. J Immunol 2010; 185: 867–876. [DOI] [PubMed] [Google Scholar]
- 61. Hogquist KA. Assays of thymic selection. Fetal thymus organ culture and in vitro thymocyte dulling assay. Methods Mol Biol 2001; 156: 219–232. [DOI] [PubMed] [Google Scholar]
- 62. Haks MC, Lefebvre JM, Lauritsen JP, et al Attenuation of gammadeltaTCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity 2005; 22: 595–606. [DOI] [PubMed] [Google Scholar]
- 63. Mohtashami M, Shah DK, Kianizad K, et al Induction of T‐cell development by Delta‐like 4‐expressing fibroblasts. Int Immunol 2013; 25: 601–611. [DOI] [PubMed] [Google Scholar]
- 64. Coffey F, Lee SY, Buus TB, et al The TCR ligand‐inducible expression of CD73 marks gammadelta lineage commitment and a metastable intermediate in effector specification. J Exp Med 2014; 211: 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


