Abstract
Brown/beige fat plays a crucial role in maintaining energy homeostasis through non-shivering thermogenesis in response to cold temperature and excess nutrition (adaptive thermogenesis). Although numerous molecular and genetic regulators have been identified, relatively little information is available regarding thermogenic dietary molecules. Recently, a growing body of evidence suggests that high consumption of n-3 polyunsaturated fatty acids (PUFA) or activation of GPR120, a membrane receptor of n-3 PUFA, stimulate adaptive thermogenesis. In this review, we summarize the emerging evidence that n-3 PUFA promote brown/beige fat formation and highlight the potential mechanisms whereby n-3 PUFA require GPR120 as a signaling platform or act independently. Human clinical trials are revisited in the context of energy expenditure. Additionally, we explore some future perspective that n-3 PUFA intake might be a useful strategy to boost or sustain metabolic activities of brown/beige fat at different lifecycle stages of pregnancy and senescence. Given that a high ratio of n-6/n-3 PUFA intake is associated with the development of obesity and type 2 diabetes, understanding the impact of n-6/n-3 ratio on energy expenditure and adaptive thermogenesis will inform the implementation of a novel nutritional strategy for preventing obesity.
Keywords: Fish oil, GPR120, n-6/n-3 ratio, adaptive thermogenesis, brown adipocyte, beige adipocytes, thermogenic diet, UCP1
1. Introduction
Adaptive thermogenesis is an energy-demanding process in which futile uncoupled-respiration releases the mitochondrial proton gradient as heat. It is regulated by a range of intrinsic and extrinsic factors including daily diet [1]. Diet plays a first-line role in regulating energy balance and adiposity. Beyond the obvious strategy of calorie restriction, emerging evidence supports the thermogenic function of several food-derived components. Dietary intervention is more likely a safe approach to modulate brown/beige energetics compared to targeted activation of the β3-adrenergic receptor (ADRB3) by pharmacological interventions due to the increased risk of cardiovascular side effects [2]. Nearly a dozen dietary polyphenolic compounds have been identified as candidate molecules to stimulate brown/beige activation [1, 3]. For example, capsaicin [4], green tea catechins [5–7], resveratrol [8, 9], quercetin [10, 11], flavan-3-ols [12], and berberine [13] are reported to possess brown stimulatory property. However, low bioavailability of dietary polyphenols poses a challenge in launching a readily translatable anti-obesity approach through diet-induced thermogenesis.
One promising dietary factor to promote adaptive thermogenesis is fish oil. The metabolic benefits of fish oil were first identified in the early 1970’s in the Greenlandic Inuit who have adapted to the extremely cold temperature in the Arctic region. Danish researchers reported that the cardio-protective effects of the Greenlandic Inuit diet are attributed to the specialized fish diet enriched with n-3 polyunsaturated fatty acids (PUFA), in particular eicosapentaenoic acid (EPA, C20:5n-3) and docosahexaenoic acid (DHA, C22:6n-3 ) [14]. Fish oil supplementation provides various health benefits in a tissue-specific manner; n-3 PUFA have been shown to increase energy expenditure in muscle [15], decrease inflammation in immune cells [16], promote insulin secretion in pancreatic beta cells [17], and attenuate pro-atherogenic lipoprotein production in the liver [18]. The metabolic benefits derived from fish oil resemble the adaptive metabolic responses upon brown/beige fat activation. This implies that the previously reported metabolic benefits of fish oil may be, at least partly, through adaptive thermogenesis. Very recently, n-3 PUFA intake has gained attention as a dietary regimen to promote thermogenesis. A series of studies published by different groups has revealed the novel functions of fish oil in activating brown fat as well as recruiting beige fat within WAT [19–21].
The prime goal of this review is to compile recent evidence that contributes to our understanding of the thermogenic function of dietary n-3 PUFA. We also define the potential mechanisms underlying the thermogenic function of n-3 PUFA. Given that G-protein coupled receptor 120 (GPR120), also known as free fatty acid receptor 4 (FFAR4), is a well-known membrane receptor for n-3 PUFA [22], we highlight the mechanism whereby GPR120 serves as a signaling platform to regulate transcriptional, immunological, and endocrine function for thermogenic activation of brown/beige fat. We also delineate the GPR120-independent mechanisms by which n-3 PUFA intake stimulates sensory neurons in the gut, activating the sympathetic nervous system (SNS), and n-6/n-3 ratio modulates the thermogenic potential of adipocytes through different eicosanoid formation. Next, we revisit the human clinical trials with fish oil in the context of weight loss and energy expenditure to gain general insights into the thermogenic function of n-3 PUFA in humans. In the last part of this review, we briefly provide physiological perspectives that n-3 PUFA supplementation may improve metabolic activity at different lifecycle states. We discuss the likelihood that maternal n-3 PUFA intake reinforces programming of fetal brown fat development and rejuvenates beige fat thermogenesis in the elderly, helping to attenuate age-related pathology.
2. Proposed mechanism established from animal studies
2.1. Thermogenic function of fish oil through GPR120 activation
G-protein-coupled receptor 120 (GPR120) is a functional receptor/sensor of n-3 fatty acids (FA) [22]. Upon binding of n-3 PUFA, GPR120 activates the heterodimeric Gαq subunit and subsequently induces diverse cellular responses via several second messengers such as [Ca2+]i, cAMP, and diacylglycerol (DAG) (reviewed in [22, 23]). Adipocytes, but not preadipocytes, express high levels of GPR120 in the rank order of BAT≫ iWAT ~ eWAT > mWAT [21]. In this section, we focus on the role of GPR120 activation by fish oil in governing brown/beige fat activation.
A. Activation of brown fat-specific transcriptional program by n-3 PUFA
Humans contain a measurable amount of classical brown fat that expresses constitutively active uncoupling protein 1 (UCP1) compared to beige fat that has temporal and reversible UCP1 activation dependent on environmental stimuli such as cold temperature and excess nutrition [24]. Provided the continuous nature of the thermogenic function, a small increase of BAT mass and/or activity are thought to exert a profound impact on overall energy expenditure. Classical brown fat is found in certain anatomical depots such as the cervical, supraclavicular, perirenal, axillary, and paravertebral areas [25].
Several research groups including our laboratory have tested the possibility that dietary fish oil or GPR120 agonists augment the mass and/or activity of classical brown fat [19, 21, 26, 27]. As an in vitro model of brown adipocytes, Kim et al. isolated brown fat precursor cells from the interscapular BAT (iBAT), and then n-3 PUFA (i.e., EPA and DHA) were added during differentiation and compared with other fatty acids (oleic acid and palmitic acid). Treatment with 100 μM of EPA and DHA, but not oleic and palmitic acid, significantly promoted brown fat-specific transcriptional programming, resulting in an increase of uncoupled respiration [19]. Supporting these results, Pahlavani et al. showed that EPA (50–100 μM) treatment increased thermogenesis in HIB1B cells, brown preadipocytes derived from a murine brown fat tumor [27]. In addition, Kim el al. demonstrated that EPA-induced transcriptional activation of brown adipogenesis relied on microRNA (miRNA)-mediated epigenetic mechanisms in primary murine brown adipocytes. Functional clusters of brown fat-specific miRNAs including miR193b/365, miR30b, and miR378 were induced by EPA treatment [19]. More importantly, transcriptional activation of brown adipogenesis as well as miRNA production were dependent on GPR120 signaling. Stimulation with GW9508, a GPR120 agonist, recapitulated the effects of EPA. Conversely, depleting GPR120 expression by siRNA [19] or genetic deletion [21], attenuated EPA-mediated activation of brown adipogenesis. These data suggest that GPR120 signaling precedes the transcriptional activation of brown adipogenesis, in part through miRNAs-mediated epigenetic mechanisms.
The effects of EPA on BAT thermogenesis were validated in HF diet-fed C57BL/6 mice. EPA supplementation, either provided as pure EPA [27, 28] or fish oil formulation [19–21, 26], reduced HF-diet induced obesity and metabolic dysfunction by increasing thermogenic energy expenditure. In addition, these in vivo metabolic benefits were concurrent with GPR120 activation and brown-specific miRNA production [19]. A further metabolic insight into the role of GPR120 on BAT thermogenesis was found in GPR120 null mice. These mice failed to upregulate cold-induced BAT genes and demonstrated loss of brown fat-specific gene profiles and morphology, and ineffective maintenance of body temperature with cold exposure [29]. Taken together, these results suggest a novel epigenetic signaling axis of EPA/GPR120/miRNAs is involved in regulating brown fat function [19]. In addition, GPR120-dependent FGF21(fibroblast growth factor 21) secretion was proposed to propagate the BAT-driven thermogenic responses to other tissues, e.g., liver and muscle, via autocrine and paracrine signaling of FGF21 [21].
Rosell et al. report the very intriguing intrinsic feature of GPR120 in BAT [30]. Cold treatment itself promotes ~2-fold induction of GPR120 in BAT, but not in WAT [30], suggesting that the intrinsic ligand for GPR120 may be synthesized in brown fat as a part of cold adaptation. From this aspect, it is worthwhile to dissect BAT lipid metabolism during cold adaptation. In fact, n-3 PUFA is endogenously synthesized in brown fat, but not in white fat, in mice and rats [31, 32]. In addition, Inuits, indigenous people of the Canadian Arctic, possess selected alleles of FA desaturases, thereby facilitating endogenous n-3 PUFA synthesis [33]. Moreover, an induction of GPR120 by fish oil intake synergistically promotes thermogenesis compared to cold exposure alone [19, 30]. The elevated levels of n-3 FA in BAT via endogenous biosynthesis as well as high fish consumption would synergistically contribute to keeping Inuits warm against cold temperature by promoting brown thermogenesis. Further research is warranted to understand the exact nature of cold-induced GPR120 induction in BAT in the context of lipid profile changes, n-6/n-3 ratio, membrane fluidity, and thermogenic heat release.
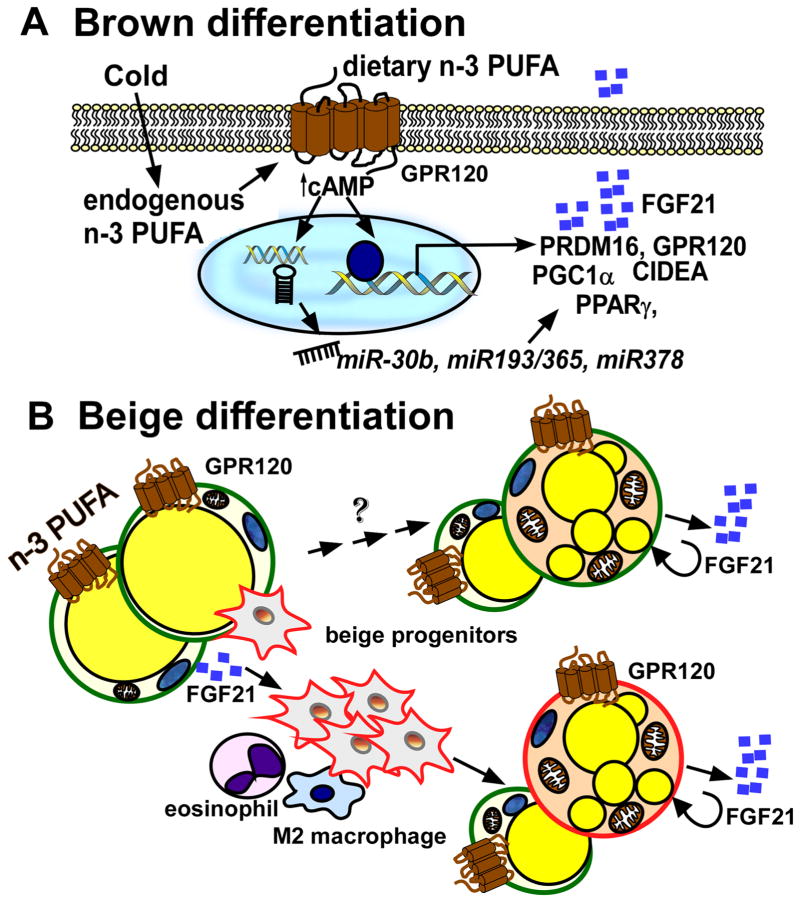
In conclusion, GPR120 activation, either by endogenous synthesis of n-3 PUFA in cold or by dietary n-3 supplementation, plays an essential role in boosting brown fat-specific transcriptional programming including miRNA-mediated epigenetic mechanisms and FGF21 signaling (Figure 1A).
Figure 1. Activation of brown and beige thermogenesis by n-3 PUFA by GPR120-dependent mechanisms.
A. Cold exposure increases endogenous production of n-3 PFUA and GPR120 transcription. Recognized by the GRP120 receptor, n-3 PUFA, either endogenously formed or obtained by dietary intake, trigger signals to potentiate brown thermogenesis in precursor cells through cAMP/miRNA-dependent (miR30b, 193b/365, miR378) transcriptional reprogramming in BAT. FGF21 secretion is augmented by n-3 PUFA in BAT, increasing thermogenic activity. B. In the WAT, the activation of GPR120 by n-3 PUFA increases FGF21 secretion. The autocrine and paracrine effects of FGF21 alter adipose immune responses including recruitment of M2 innate immune responses (e.g., eosinophil and M2 macrophage polarization) and proliferation of beige precursors (red). These collective modifications of endocrine and immune function by n-3 PUFA promote beige adipocyte differentiation from precursor cells rather than trans-differentiation of white adipocytes (green).
B. Role of GPR120 activation in beige fat differentiation by n-3 PUFA
Oh et al. first demonstrated that white adipocytes express high levels of GPR120, but not GPR40 [34]. The stimulation of GPR120 by n-3 PUFA, or its chemical agonist, promotes glucose uptake by sensitizing insulin signaling [22]. Supporting this notion, genetic ablation of GPR120 in mice results in hepatic steatosis and insulin resistance [35]. In humans, lack of GPR120 signaling activity due to a genetic mutation in the GPR120 gene (p.R2700H) is correlated with increased risk of obesity [35]. Despite several controversies surrounding the anti-obesogenic role of GPR120 in humans, evidence derived from experimental animals indicates that GPR120 activation increases lipid combustion and reduces adiposity.
Several groups of scientists provided direct evidence that fish oil promotes beige fat development from adipogenic precursor cells [20, 29, 36–38]. Zhao et al. first reported that 200 μM EPA treatment of stromal vascular (SV) cells isolated from mouse inguinal fat effectively induced beige-specific signature gene profiles, and increased mitochondrial energy expenditure [37]. Laigelesia et al. investigated the metabolic effects of EPA (100–200μM) on human adipogenic stem cells isolated from subcutaneous fat. Incubation with EPA (100–200μM) during adipogenic differentiation promoted mitochondrial biogenesis and induced thermogenic gene expression and specific beige markers such as Cd137 [29]. Similarly, Fleckenstein-Elsen et al. demonstrated that EPA, but not arachidonic acid (ARA, C20:4n-6), promoted beige adipocyte formation from primary hADS [38]. The conversion of uncommitted SV cells into beige fat by fish oil is GPR120 dependent. Treatment with GW9508, a chemical agonist of GPR120, recapitulated the white-to-beige conversion by fish oil [21]. Conversely, beige conversion by EPA was abolished in SV cells prepared from GPR120 null mice or in the presence of AH7614, a chemical antagonist of GPR120 [21]. However, GPR120 activation by EPA in fully-mature white adipocytes does not cause beige conversion, suggesting that EPA acts on beige adipocyte recruitment rather than by promoting trans-differentiation [37].
The involvement of GPR120 in beige adipocyte differentiation was further confirmed in animal studies. Quesada-Lopez et al. demonstrated that feeding adult mice with GW9508 for 7 days, significantly upregulated thermogenic genes (i.e., Ucp1, Pgc1a, Fgf21, Sirt3), and caused browning of inguinal WAT (iWAT) [21]. The simultaneous administration of CL316243, a β3-specific adrenergic receptor agonist, with GW9508 showed a synergistic increase in oxygen consumption rate and browning of WAT. In contrast, genetic ablation of GPR120 completely abolished cold- and β3 agonist-induced WAT browning. This particular study demonstrated that elevated levels of FGF21 are linked with GPR120 activation in both WAT and BAT, suggesting a novel signaling axis in which adipocyte GPR120 links with FGF21, a key endocrine hormone for fatty acid oxidation [21]. Consistent with this study, TUG-891, another GPR120-specific agonist, significantly promotes fat oxidation and adipocyte browning through Gq/α-mediated calcium release, mitochondrial depolarization, and mitochondrial fission [39].
In summary, multiple pathways work in concert to facilitate beige fat induction through GPR120 activation via either dietary n-3 PUFA supplementation or by chemical agonism (Figure 1B).
C. Regulation of innate immunity by n-3 PUFA in beige fat differentiation
White adipose tissue is an important endocrine organ releasing numerous adipokines that can alter inflammatory status and insulin sensitivity. Adipose tissue inflammation is mediated by the inflammatory responses of adipose tissue macrophages (ATMs), Oh et al. demonstrated the anti-inflammatory function of GPR120 in adipose tissue [34]. Activation of GPR120 by n-3 PUFA or chemical agonists preferentially promotes anti-inflammatory M2 macrophage polarization and protects from HF diet-induced metabolic dysfunction [34]. In the context of WAT browning, the physiological relevance of type 2 innate immune responses has been recently highlighted [40]. ADRB3 pathway activation by cold stress initiates type 2 innate immune responses and alternative M2 macrophage polarization, which mediates UCP1-positive beige fat development. M2 macrophages have been shown to provide local catecholamine within WAT. In addition, groups of type 2 innate lymphoid cells (ILC2s) and eosinophils are the major source of type 2 cytokines IL-33, IL-4, and IL-13 that are necessary for proliferation and commitment of beige precursor cells into beige adipocytes [41, 42]. Despite numerous indications, a missing link remains as to whether fish oil supplementation (or GPR120 activation itself) amends the immunological makeup of WAT into a favorable state for proliferation of beige progenitor cells.
New evidence suggests FGF21 is an important mediator for type 2 innate immune responses in WAT. Huang et al. reported that FGF21 acts on adipocytes in an autocrine manner to promote the production of CCL11, which subsequently promotes eosinophil recruitment, IL-4 release, M2 macrophage polarization, and proliferation and differentiation of beige progenitor cells into thermogenic beige fat cells [43]. Consistently, Quesada-Lopez et al. have demonstrated that GPR120 activation failed to induce beige fat induction in FGF21 null mice and vice versa [21], suggesting that a GPR120/FGF21 axis is essential for WAT browning. Given the indispensable function of GPR120 on FGF21 production, it is likely that fish oil-induced beige fat may trigger type 2 innate immune responses and eosinophil recruitment in WAT. To validate this notion, it is necessary to evaluate the signaling relay of FGF21/CCL11/type 2 lymphoid cells proposed by Huang et al. [43] in GPR120 null mice.
In summary, the activation of GPR120 in inguinal adipose tissue mediates the production of FGF21, resulting in immunological remodeling of adipose tissue such as recruitment of type 2 innate immune responses, recruitment of eosinophils, ILC2s and M2 macrophages, and proliferation of beige precursor cells. (Figure 1B).
2.2. Thermogenic activation by fish oil through GPR120-independent mechanisms
A. Thermogenic activation by n-3 PUFA through TRPV1
N-3 PUFA supplementation triggers multiple signaling pathways for thermogenic reprogramming, which GPR120 activation alone cannot explain. Activation of the sympathetic nervous system (SNS) plays a critical role in non-shivering thermogenesis by innervating BAT and, to a lesser extent, WAT. Detected in the skin, cold is a strong afferent signal to stimulate the SNS in the hypothalamus. Stimulation of the SNS induces efferent signaling acting upon BAT and WAT to produce catecholamines, which activate β3-adrenergic receptors (ADRB3), produce cAMP, and thereby triggers the thermogenic program [44]. Though the sympathetic outflow signaling to BAT/WAT could be identical, the afferent signal to SNS by food-borne molecules originates from temperature-sensing mechanism in the gut [45, 46]. An example of a dietary component is capsaicin, the major pungent component in hot red peppers. Dr. Saito’s group has extensively investigated the ability of capsaicin (or capsinoids) to bind to transient receptor potential vanilloid 1 (TRPV1), a nonselective calcium channel located on peripheral sensory neurons in the gut, sending out a thermogenic stimulus to the SNS [4, 47, 48].
Similar to capsaicin, fish oil induces brown/beige fat through activation of the SNS. Kim et al. demonstrated that fish oil supplementation for 10 weeks increased oxygen consumption and core body temperature in mice [20], which was abolished by propranolol, a potent β-blocker. Consistently, elevated levels of levels of cAMP in serum and peripheral tissue after fish oil consumption was reported [19, 20]. The gut-brain-adipose tissue axis seems to be essential, as evidenced by browning effects were abolished in mice with the removal of the vagal nerve, vagotomy, or genetic deletion of TRPV1 [20]. In agreement with this notion, n-3 PUFAs are well-known ligands for TRPV1 [49]. Ohyama et al. proposed new evidence that capsaicin-mediated TRPV1/SNS axis activation may stimulate β2-adrenoceptors (ADRB2) in white fat rather than the β3-adrenoceptor. Furthermore, this study demonstrated that synergistic SNS activation by sub-ambient temperature (17°C) and TRPV1 activation in the gut by capsaicin strongly enhanced WAT browning, resulting in > 2-fold weight loss relative to that caused by cold or capsaicin alone [50].
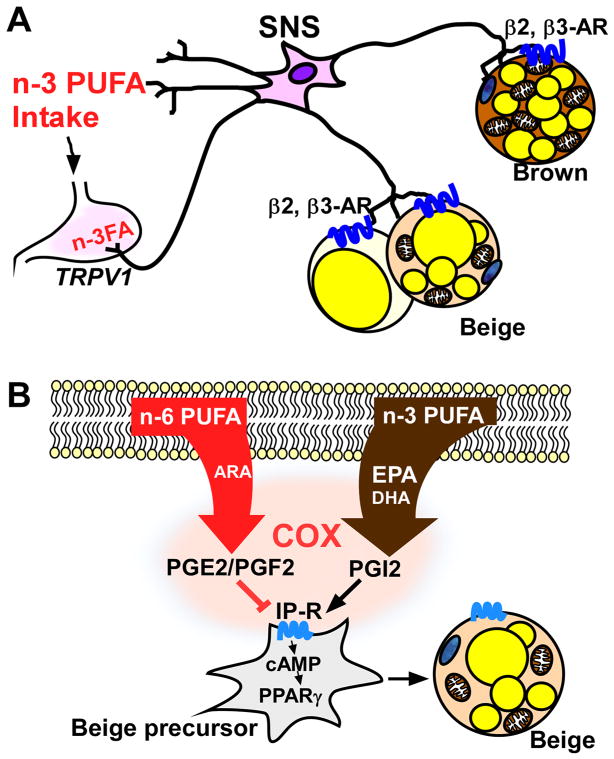
Collectively, these studies suggest that n-3 PUFA, independent of GPR120-mediated signaling, serve as a stimulator for TRPV1 in the gut, activating the SNS to trigger ADRB3-mediated signaling cascades in both BAT and WAT (Figure 2A). It is worth testing whether the combined strategy of chronic sub-ambient temperature and dietary regimen with fish oil will constitute a practical approach to promote brown/beige thermogenesis in humans.
Figure 2. Activation of brown and beige thermogenesis by n-3 PUFA by GPR120-independent mechanisms.
A. n-3 PUFA serve as ligands of the TRPV1 receptor in the gut, activating the sympathetic nervous system (SNS), which in turn triggers the ADRB2- or ADRB3-mediated signaling cascade for thermogenic activation in both brown and beige adipocytes. B.A high n-6/n-3 ratio is linked with ARA mediated anti-adipogenic oxylipin production of PGE2 or PGF2 inhibiting beige fat differentiation. In contrast, a low n-6/n-3 ratio in adipocytes increases thermogenic oxylipin PGI2 production. PGI2 triggers cAMP/PPARγ signaling through its cognate receptor IP-R in beige precursor cells, leading to beige fat induction.
B. Impact of n-3 PUFA-driven oxylipins on thermogenic activation of beige adipocytes
The Western diet is deficient in n-3 fatty acids (n-6/n-3=15~17), and a high ratio of dietary n-6/n-3 is associated with increased risk of various metabolic diseases including obesity [51]. Pisani et al. first addressed the impact of n-6/n-3 ratio on beige fat conversion [52]. By using hADS, it has been shown that n-6 PUFA, i.e., linoleic acid (LA, C18:2n-6) and arachidonic acid (ARA, C20:4n-6), strongly inhibit adipocyte browning. This inhibitory effect was associated with ARA-derived eicosanoids of PGE2 and PGF2 via cyclooxygenase (COX) activity [52]. Ghandour et al. have compared the effective thermogenic potential of an n-6 enriched diet (n-6/n-3=30) composed of linoleic acid (LA, C18:2n-6) and oleic acid (OA, C18:1n-6) with an n-3 supplemented diet (n-6/n-3=3.7) mostly composed of alpha-linolenic acid (ALA, C18:n-3) [53]. This study revealed that n-2 prostaglandin series derived from n-6 PUFA, i.e., PGF2 and PGE2, were inhibitors of adaptive thermogenesis. More importantly, it was proposed that a high intake of n-3 PUFA and its oxygenated lipid mediator prostacyclin (PGI2) promotes beige fat formation by suppressing PGE2 and PGF2 production in adipocytes, implicating the competition between n-3 vs. n-6 FA for COX activities [53]. In accord with this idea, PGI2 or a stable analog of prostacyclin promotes browning of hADS through mechanisms associated with cognate receptor IP-R/cAMP signaling and upregulation of PPARγ [54]. Taken together, these studies suggest that the n-6/n-3 ratio per se is an important modulator for white-to-beige thermogenic conversion through oxygenated lipid mediators (oxylipins) independent of GPR120-mediated signaling cascades (Figure 2B).
2.3. Current knowledge, controversies, and directions for future research
We summarized recent studies that investigated the role of n-3 PUFA in influencing thermogenic function in animals and relevant cell models of brown and beige adipocytes (Table 1). n-3 PUFA possess unique properties to promote brown/beige thermogenesis compared to other long-chain saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), or n-6 PUFA [19, 38]. This agrees with literature suggesting that n-3 PUFA are better ligands for GPR120 than n-6 PUFA [22]. Moreover, these data are consistent with recent results that metabolites of n-3 PUFA are much more potent agonists for the TRPV1 receptor than metabolites of ARA [55]. N-3 PUFA seemingly exhibit distinctive mechanisms on brown vs. beige precursor cells, although GPR120 is involved in both brown and beige fat development. Based on literature, we suggest a molecular framework that n-3 PUFA act on brown precursor cells at the transcriptional level during brown adipogenesis (Figure 1A), whereas n-3 PUFA alter the microenvironment of WAT at the level of recruitment and proliferation of beige precursors (Figure 1B). In agreement with this current hypothesis, trans-differentiation of white to beige adipocytes was not triggered by n-3 PUFA [37]. Herein, we categorized ADRB3 activation by n-3 PUFA as a GPR120-independent pathway for convenience’s sake. Given that adaptive thermogenesis is an integrative response linking the central nervous system to peripheral adipose tissues, the activation of sympathetic neurons by n-3 PUFA can be regarded as a concurrent and synergistic signal parallel to the GPR120-dependent peripheral responses rather than exclusive to each other.
Table 1.
Thermogenic effects of n-3 PUFA in animals and brown and beige adipocyte models
| Test model | Treatment/Dose | Duration | Metabolic Responses | Mechanism | References |
|---|---|---|---|---|---|
| iBAT primary mSV | EPA/100μM | 5 days | ↑ Brown specific gene profile (Ucp1,Cidea, Prdm16, Pparγ) ↑brown specific miRNAs miR-30b, miR193/365, and miR-378 |
GPR120 ↑ cAMP, | Kim et al. 2016 (19) |
| C57BL/6 mice | DHA-enriched fish oil (DHA 25%, EPA 8%) EPA-enriched fish oil (EPA 28%, DHA 12%) |
10 Weeks | ↑ Thermogenesis ↑ Oxygen consumption ↑ Glucose tolerance ↑ Insulin sensitive ↓ Fat mass ↓ body weight. |
TRVP1 | Kim et.al 2015 (20) |
| WT GPR120-KO mice FGF21 KO mice |
Cold treatment (4 °C) GPR120 agonist GW9508 (50ug/g BW/day) |
Cold 24 hours/GW9508 1wk | GW9508 ↑ brown and beige activity ↑ FGF21 release, which is missing in GPR120 KO mice |
GPR120 FGF21secretion | Quesada-López, et al. 2016 (21) |
| C57BL/6 mice | HF 50% supplementation with 12.5% from EPA and DHA | 8 weeks | ↓BW ↑glucose tolerance, ↑insulin sensitive, ↑thermogenic markers (beta3-AR, Pgc1α and Ucp1), ↑PPAR (the three isoforms) | beta3-AR | Bargut et al. 2016 (26) |
| HIB1B cell (murine brown cell line) | EPA100μM | 48 hours | ↑mRNA expression of Pgc1α and Sirt2 in cell ↑maximum oxidative and peak glycolytic metabolism | ↑Glycolysis ↑mitochondrial respiration |
Pahlavani et al. 2017 (27) |
| hADS from overweight females | EPA100–200μM | 24 hours | ↑ Pgc1α, Sirt1, AMPK | ↑ mitochondrial function | Laiglesia et al. 2016 (29) |
| Primary SV From 129 mice | Cold treatment | 10 days | cold treatment ↑ GPR120 expression ~2.5 fold intrinsically |
beta3-AR | Rosell et al. 2014 (30) |
| mSV cells C57BL/6 mice | EPA 200 μM | 24 hours | ↑ UCP1, CIDEA and VEGFα ↑mitochondrial biogenesis and mtDNA content |
↑Mitochondrial biogenesis | Zhao et.al 2014 (37) |
| hADS from lean women | EPA, AA 20 μM | 12 days | EPA ↑ UCP1 and ↑ mitochondrial function. AA ↑ lipid droplet size and ↓ mitochondrial respiratory function. |
↑Mitochondrial function | Fleckenstein-Elsen et al. 2016 (38) |
| C57BL6 mice GPR120 KO mice |
GPR120 agonist TUG-891 35mg/kg BW |
daily for 2.5 weeks | ↓ BW ↓ Fat mass ↑ Fatty acid uptake ↑ Fat oxidation ↓ Lilpid content in BAT and WAT |
GPR120 | Schilperoor t et. al 2018(39) |
| iBAT and sWAT Primary mSV Immortalized GPR120 KO brown adipocytes | Vehicle TUG891 with/without GPR120 antagonist AH7614/ |
30 min | ↑ Oxygen consumption ↑ Browning of white adipocytes ↑ Intracellular calcium release |
GPR120 | Schilperoor t et. al 2018(39) |
| hADS cell | ARA 10 μM | 3 days | ↓UCP1, PPARg-targets ↓ mitochondria activity ↓ Oxygen consumption rate ↑ secretion of PGE2, PGF2α |
Oxylipins (PGE2, PGF2α) COX activities | Pisani et. al 2014 (52) |
| C57BL6 mice | ARA or OA Stimulation with CL316,214 (1 mg/kg/day) | 4 weeks | ↑PGF2a and PGE2 in sWAT ↓ recruitment of beige adipocytes |
n-6/n-3 ratio COX activities | Pisani et. al 2014 (52) |
| C57BL/6 mice | n6- or n3-supplemented diets (12% energy) CL316,214 stimulation | 12 weeks | n3-PUFA ↓ n-6 derived oxylipin production |
n-6/n-3 ratio | Ghandour et.al 2018 (53) |
| hADS | EPA (molar ARA/EPA=3) 10 μM ARA with or w/o 3.3 μM EPA | 3 days | EPA ↓ PGF2α and ↑Oxygen consumption |
n-6/n-3 ratio | Ghandour et.al 2018(53) |
| hADS | Carbaprostacyclin (cPGI2) | 4 days | cPGI2 ↑ UCP1 mRNA ↑ PPAR signaling |
IP-R/cAMP signaling by PGI2 | Ghandour et. al 2016 (54) |
Abbreviation used: iBAT: interscapular brown adipose tissue, mSV: mice stromal vascular cells, EPA: Eicosapentaenoic acid, UCP1: Uncoupling Protein 1, CIDEA: cell death-inducing DNA fragmentation factor alpha-like effector A, PRDM16: PR domain-containing 16, PPARγ: Peroxisome proliferator-activated receptor gamma, GPR120: G-protein coupled receptor 120, beta3-AR: beta-adrenergic receptor, Sirt2: Sirtuin 2, AMPK: AMP-activated protein kinase, FGF21: Fibroblast growth factor 21, Zic1: Zinc finger of the cerebellum-1, AA: Arachidonic acid, DPA: Docosapentaenoic acid, TRVP1: transient receptor potential vanilloid receptor1, cpdA: compound A (GPR120 agonist), 20-HETE, x-hydroxy ARA: hADS: Human adipose-derived stem cells, PGI2: prostacyclin, cPGI2: carbaprostacyclin (analog of PGI2), COX: cyclooxygenase, PGF2α: Prostaglandin F2α
One important but unanswered question is the thermogenic effectiveness among the different dietary n-3 PUFA, i.e., DHA, EPA, ALA or docosapentaenoic acid (DPA, C22:5n-3). To date, most studies tested the thermogenic function of n-3 PUFA by using fish oil that contains mixtures of EPA and DHA, and no study was conducted to evaluate the thermogenic function of ALA or DPA alone. Despite some controversies, EPA seems to be a stronger thermogenic stimulus than DHA in general, based on studies on murine brown adipocytes or hADS [19]. One reasonable answer is found in studies conducted by Dr. Pisani’s group. A fascinating hypothesis is proposed that oxylipins derived from different PUFA are downstream effectors to modulate thermogenic remodeling of beige fat. In particular, Pisani et al. claimed that PGI2 is a pro-thermogenic eicosanoid wherein EPA is a better substrate of COX for PGI2 production rather than DHA [56]. Conversely, it is suggested that DHA is insufficient to compete with ARA and less effective in suppressing anti-adipogneic oxylipin production of PGF2 and PGE2, although DHA features a stronger effect on inhibiting COX2 activity. This notion seems to align with the several cohort studies demonstrating EPA, but not DHA, is effective in reducing triglyceride levels in humans [57, 58]. However, the likelihood of the impact of n-6/n-3 ratio on beige fat development warrants additional support from other researchers, as all studies were published from a single group [56–58]. Also, it is uncertain how competition between n-3 vs. n-6 PUFA toward COX activity affects recruitment of beige precursor or the immunological setting of WAT. Further research is required to test these hypotheses in a relevant system. For example, use of the transgenic fat-1 mouse that converts n-6 PUFA into n-3 endogenously would be a useful model system to investigate the function of a low n-6/n-3 ratio in regulating adaptive thermogenesis probably without the influence of GPR120 signaling. Moreover, future studies to distinguish the thermogenic function of plant-based n-3 PUFA, e.g., flaxseed oil or nut oil (rich in ALA), from marine-based fish oil (rich in DHA and EPA) would be essential to establish a solid role of a low n-6/n-3 ratio in regulating adaptive thermogenesis.
There are some caveats since most cell-based studies used approximately 100–200 μM of EPA, which may not be attainable by oral intake of a fish oil-containing diet or pills. Nonetheless, these studies suggest a new research avenue that n-3 PUFA-enriched diets possess the potential to alter the fate of adipogenic stem cells into mitochondria loaded, fat-burning beige adipocytes rather than fat-depositing white adipocytes.
3. Human clinical studies supporting the thermogenic function of n-3 PUFA
The physiological relevance of brown/beige fat in obesity has been well established in humans [59]. It is estimated that healthy adults contain upward of ~60 g of brown/beige fat (<0.1% of body weight), which could be responsible for >20 % of daily energy expenditure [60]. Unfortunately, induction of thermogenic fat in response to cold exposure is severely compromised in obese individuals [61, 62]. Conversely, loss of thermogenic activity, whether classical brown or beige fat, contributes to obesity. There are numerous studies supporting that n-3 PUFA decreased inflammation and improved insulin sensitivity in metabolically unhealthy humans [63–69]. However, the implication of thermogenic function of n-3 PUFA is less evident in human clinical trials, despite the clear indication in the aforementioned animal studies. This is likely due to technical difficulties in measuring thermogenic energy expenditure in humans; thus, few studies were designed to assess thermogenic heat release or energy expenditure. In this section, we revisit some of the previous human clinical trials showing that n-3 PUFA affect metabolic rates and adiposity, which may help us provide insight into the thermogenic function of n-3 PUFA in humans.
3.1. Revisiting the clinical evidence on the thermogenic function of n-3 PUFA
One of the most noticeable effects of n-3 FA supplementation is reduced fat mass. Based on the meta-analysis involving 11 randomized clinical trials, Zhang et al. revealed that n-3 PUFA intake significantly reduced serum levels of triglyceride and waist circumference without affecting body mass index (BMI) [70]. Results reported from this study confirm the potential function of n-3 PUFA on reducing visceral fat. However, conclusions from this report are uncertain due to limitations, such as small-scale randomization and poor quality control. The fat loss effect by fish oil was also noted in a study of insulin-resistant adults conducted by Ramel et al [71]. A total of 324 participants aged 20–40 years with BMI 27.5–32.5 were randomly assigned 0–2.1g/day of n-3 PUFA for 8 weeks. This study revealed that 2.1g/day n-3 PUFA was linked to a significant decrease in body weight, plasma levels of fasting insulin, glucose and triglyceride, and improved insulin sensitivity [71]. However, these studies were not designed to understand the underlying mechanisms, and thus it is difficult to infer a thermogenic function of fish oil solely due to reduced adiposity.
The very first crossover-study to determine the effects of n-3 PUFA on resting metabolic rate (RMR), basal energy expenditure, and body composition was conducted by Couet el al. Supplementation of 6g/day of fish oil for three weeks resulted in reduced body fat mass. It also decreased respiratory exchange ratio (RER), indicating increased fuel usage from fat, and increased basal lipid oxidation without altering resting metabolic rate [72]. This study posed several limitations such as small size (only six participants), gender imbalance (5 males and one female), and seasonal differences between two cohorts. Noreen et al. conducted another study to determine the oxidative potential after fish oil intake [73]. In this study, 6 weeks of fish oil supplementation (4g/day) significantly increased lean body mass and reduced fat mass in healthy adults (total of 44 men and 34 women), although significant differences in basal metabolic rate (BMR) or RER were not observed [73]. In parallel with these results, the inclusion of fish or fish oil in randomized trials of weight-loss-diets offered increased weight loss in healthy humans [74, 75]. Most recently, Jannas-Vela et al. reported an interesting study [76] that determined the BMR and substrate oxidation in young healthy males subjects after 12 weeks of fish oil supplementation (2g/d EPA, 1g/d DHA) in comparison to olive oil intake. The authors identified that fish oil intake increased fatty acid and carbohydrate oxidation in the winter season, but not in summer, regardless of RMR. These results imply that fish oil-mediated thermogenic activation may require additional environmental stimuli, such as cold temperature [76]. In the same context, the effects of n-3 PUFA were augmented with exercise [77], another signaling factor to promote adaptive thermogenesis [78]. Another important aspect to consider is gender differences in WAT browning. In contrast to the study performed by Jannas-Vela et al. [76], Logan et al. reported that 12 weeks of fish oil supplementation (3g/day) significantly increased resting BMR as well as exercise-induced fat oxidation in females [79]. This result is also consistent with the finding that women contain a detectably higher amount of brown fat mass than males [80].
3.2. Limitations of studies and directions for future research
In Table 2, we summarized the clinical trials discussed in this review. The thermogenic effects of n-3 PUFA in human clinical trials, mostly fish oil supplementation, remain inconclusive, despite a strong correlation between fish oil intake and reduced visceral adiposity. The inconsistency between animal studies and human clinical trials seems to originate from the confounding factors of study design and technical difficulties in brown fat identification in humans. Unlike experimental design for rodents, direct stimulation of ADRB3 via pharmacological agonists or chronic exposure to suboptimal low temperature is not appropriate for human clinical trials. Hence, better study designs are required to address the thermogenic function of n-3 PUFA intake in adaptive thermogenesis in humans. In agreement with this notion, the reduced adiposity by fish oil supplementation seems to become increasingly evident with additional signaling cues for thermogenesis such as calorie restriction, cold temperature, and exercise [74–76]. Therefore, fish oil supplementation in combination with other lifestyle modifications could be a better strategy to promote adaptive thermogenesis. This hypothesis needs further research with well-controlled and large-scale human trials in both genders. In addition, direct evidence such as 18F-2-deoxy-glucose (FDG) uptake using positron emission tomography (PET)-scans should be provided to establish stronger links with adaptive thermogenesis in humans [81].
Table 2.
Human clinical studies showing that n-3 PUFA affect metabolic rates and adiposity
| Subject | Treatment | Dose / Duration | Results | References | ||
|---|---|---|---|---|---|---|
| ΔBW | ΔFat | Metabolic Responses | ||||
| 74 psychiatric patients | EPA and placebo plus optional EPA supplementation | Ethyl-EPA 2 g/day 12 weeks double blinded following 40 weeks | - | - | EPA 2 g/day is generally safe when used in patients with schizophrenia for up to 1 year | Emsley et al. 2008 (63) |
| Insulin-resistant, nondiabetic 34 participants | n-3 EPA Ethyl Esters, placebo | 4g/day for 12 weeks | - | ↓ | n-3 PUFA ↓adipose macrophages, ↓MCP-1 expression ↑n-3 PUFA in adipose tissue and muscle |
Spencer et al. 2013 (64) |
| 31 insulin-resistant adults | EPA+DHA or placebo | 3.9 g/day / 6 months | - | - | EPA+DHA ↑ hepatic insulin sensitivity | Lalia et al. 2015 (65) |
| 54 T2DM Mexican adults | DHA + EPA-enriched fish-oil, placebo | 520 mg / 24 weeks | ↓ | ↓ | ↓waist circumference, ↑glucose, HbA1c, leptin, leptin/adiponectin ratio, and lipid profile | Jacobo-Cejudo et al. 2017 (66) |
| 60 overweight (BMI > 25), healthy adults, aged 40–60 years | n-3 fatty acid (capsule) probiotic VSL#3 both omega-3 and probiotic | 180 mg EPA and 120 mg DHA daily / 6 weeks | - | - | Omega-3 fatty acid ↓ IR, ↓ hsCRP |
Rajkumar et al. 2014 (67) |
| 29 subjects (Avg 44 years with MetS) | n-3 PUFA, placebo | 2 g/day of omega-3 PUFAs 12 weeks | - | - | ↓ IL-6, TG, Cholesterol ↑PAI-1 levels |
Tousoulis et al. 2014 (68) |
| 37 patients (50.6±9.8 y) with well-controlled diabetes | Mixture EPA and DHA Corn oil Placebo EPA 2160 mg DHA 1440 mg |
48 weeks | - | - | Improved inflammation compared with placebo | Dasarathy et al. 2015 (69) |
| 324 participants (20–40 years, BMI 27.5–32.5, from Iceland, Spain and Ireland) | (30% of total energy) -no seafood (control; 6×500 mg sunflower oil capsules/day); -lean fish (150 g cod, three times/week); -fatty fish (150 g salmon, three times/week); -fish oil capsules (6×500 mg capsules/day) | 8 weeks | ↓ | ↓ | ↓ fasting insulin, glucose, triacylglycerol and adiponectin levels and HOMA-IR ↑ LC n-3 PUFA content in membrane lipids significantly increased (all p<0.001) |
Ramel et al. 2008 (71) |
| Six volunteers, 23±2 years, BMI 21.9±1.6 | Isocaloric diet (32% fat) Control diet and fish oil diet |
6 g/day, 15 weeks | ↓ | ↓ | ↑ Lipid oxidation | Couet et al. 1997 (72) |
| A total of 44 participants, 34 ± 13years | 4 g/d of Safflower Oil (SO) 4 g/d of Fish oil (FO) |
1600 mg/d EPA 800 mg/d DHA for 6 weeks |
- | ↓ | ↓ fat mass, ↑ lean mass ↓ salivary cortisol |
Noreen et al. 2010 (73) |
| 324 men and women, (20–40 years) BMI 27.5–32.5 | (1) control (sunflower oil) (2) cod diet (3x 150 g/week) (3) salmon diet (3x 150 g/week) (4) fish oil (DHA/EPA capsules) |
Cod 272 mg n-3 PUFA/d Salmon 3004 mg n-3 PUFA/d Fish oil 1418 mg n-3 PUFA/d / 8 weeks |
↓ | ↓ | ↓ TG, LDL, TC, greatest in salmon group | Gunnarsdottir et al. 2008 (74) Thorsdottir et.al 2007 (75) |
| 26 healthy males (22.8 ± 2.6 years) | Olive oil capsule Fish oil capsule |
3g Olive oil/day 2g EPA, 1g DHA/d / 12 weeks |
- | - | ↑ Resting FA oxidation no change in RMR | Jannas-Vela et al. 2017 (76) |
| 81 adult volunteers (25–65 years old) | Fish oil (FO), FO and exercise (FOX), Sunflower oil (SO; control), SO and exercise (SOX). | 6 g tuna FO/d (1.9 g n-3 PU FA) 6 g SO/d 12 weeks |
↓ | ↓ | fish oil intake ↓ TG ↑ HDL cholesterol |
Hill et al. 2007 (77) |
| 24 female (66 ± 1 years) | EPA + DHA Placebo | 3g/day, 12 weeks | - | ↓ | FO significantly ↑ RMR ↑EE ↑ Fat oxidation ↑Lean mass ↓TG |
Logan et al. 2015 (79) |
Abbreviation used: EPA: Eicosapentaenoic acid, DHA: Docosahexaenoic acid, EE: Energy expenditure, n-3 PUFA: Omega-3 polyunsaturated fatty acid, HbA1c: Hemoglobin A1c , T2DM: Type 2 Diabetes Mellitus, HOMA-IR: Homeostatic model assessment - insulin resistance, RMR: Resting metabolic rate, SO: Sunflower oil, FO: Fish oil, MCP-1: Monocyte chemoattractant protein-1, IR: Insulin resistance, hsCRP: High sensitivity C-reactive protein, IL-6: Interleukin 6, TG: Triglyceride, PAI-1: Plasminogen activator inhibitor-1, BMI: Body mass index, MetS: Metabolic syndrome, LC n-3 PUFA: Long chain omega-3 polyunsaturated fatty acid, LDL: Low-density lipoprotein, TC: Total cholesterol, FA: Fatty acid, HDL: High-density lipoprotein
4. Perspective of n-3 PUFA supplementation on thermogenic activity in different life stages
In this section, we would like to discuss the physiological perspective of n-3 PUFA supplementation on brown/beige fat activity at different life stages of pregnancy and senescence.
4.1. Prenatal exposure of n-3 PUFA, BAT development, and childhood obesity
Pregnancy, especially in the late gestation period, is the critical window of time for fetal growth including classical BAT development. Augmentation of classical BAT development at the time of birth and increased retention in childhood could be a promising intervention strategy to counteract obesity and metabolic syndrome. Until now, several non-nutritional factors have been shown to increase the development of BAT formation in the fetus. These include thyroid hormone, catecholamines [82, 83] or maternal cold exposure [84], suggesting that activation of maternal sympathetic innervation promotes ADRB3 signaling in the fetus, thereby enhancing the development of BAT. Currently, there is growing information that maternal or infant nutritional status alters BAT mass/activity in newborns and its maintenance in adulthood. Malnutrition at late gestational phases, such as low protein intake [85–87] or HF diet [88], results in reduced BAT development in animal models.
Maternal supplementation of n-3 PUFA increases the n-3 PUFA concentration in the placenta and uterus cord blood, suggesting that n-3 FA are effectively transferred to the fetus in utero [89]. Pregnant women are recommended to take 600~800 mg of n-3 PUFA daily during pregnancy, and no adverse effects were found with up to 2.8 g/day of n-3 PUFA at the late gestation period until delivery [90]. Several human studies report a negative correlation between maternal n-3 PUFA intake and prevalence of childhood obesity [91–94]. In a large population-based cohort study with 4830 mothers, Vidakovic et al. showed that higher maternal n-6/n-3 ratio was correlated with higher risk of childhood obesity [91]. In another prospective cohort study, Moon et al. identified that maternal n-3 FA intake was associated with offspring lean body mass in 12,583 participants [93]. More interestingly, a long-term follow-up study showed that fish oil supplementation during pregnancy in obese mothers had long-lasting effects on reduced adiposity in their offspring [94]. Intriguingly, a recent study by Rudolph et al. supports this model demonstrating that low perinatal n-6/n-3 ratio serves as a metabolic cue to attenuate the susceptibility against diet-induced obesity in adult offspring [95].
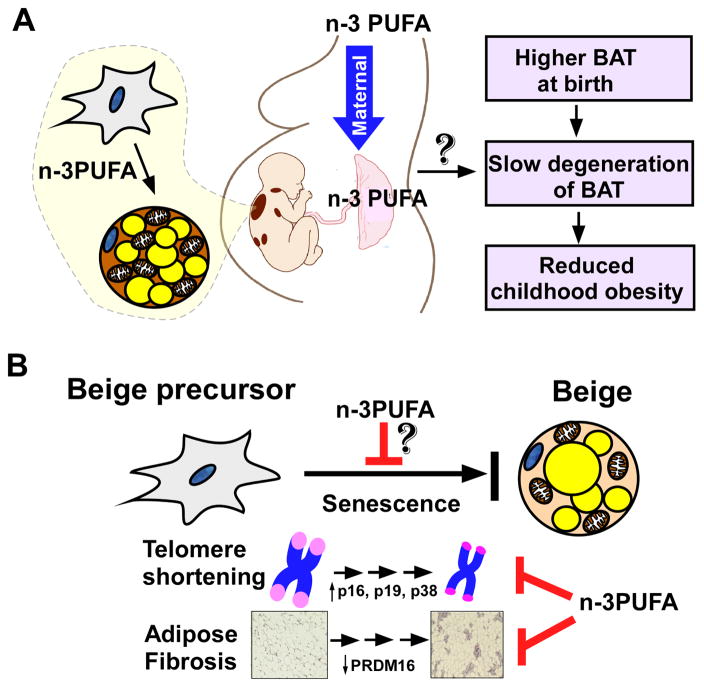
Based on these results, it is conceivable that maternal fish oil intake may promote prenatal BAT reprogramming via the aforementioned mechanisms found in section 2.1 or may delay the postnatal degeneration of BAT in early life (Figure 3A). Our group is currently undertaking a pilot study to address the metabolic benefit of maternal n-3 PUFA nutrition on fetal BAT development in animals. While our results are promising, this possibility is still to be reported in the primary literature. In addition, the broader implications of maternal n-3 PUFA supplementation on gestational diabetes, obesity outcomes, and BAT activity in mothers in addition to infants remains to be determined in the context of thermogenic activation.
Figure 3. Nutritional perspectives regarding n-3 PUFA intake on thermogenic activity during pregnancy and senescence.
A. Maternal intake of n-3 PUFA would be effective in promoting prenatal BAT development across the placenta. The benefits of maternal n-3 PUFA nutrition remain to be determined regarding BAT activity at birth, obesity outcome in childhood and later life. B. The decline of beige thermogenic potential with aging is associated with telomere shortening via increased cell cycle inhibitors/oxidative stressors (i.e., p16, p19, p38). Recently, it has also been proposed that adipose tissue fibrosis is inversely linked to adipocyte browning. There are open possibilities that n-3 PUFA supplementation can rejuvenate thermogenesis by interfering with cellular senescence of beige precursors.
4.2. n-3 PUFA supplementation and aging-mediated reduction in thermogenesis
The probability to detect brown/beige adipose tissue is inversely correlated with age, implying that thermogenic potential declines with senescence [80]. Understanding the exact cause and identifying an intervention strategy to revert this thermogenic reduction have strong clinical implications for improving metabolic health. Berry et al. have found that old mice (1-year-old) failed to activate cold-induced beige thermogenesis compared to young mice (2-month-old) [96]. They identified that 1) cellular aging of beige progenitors is driven by cell cycle inhibitors (i.e., p21cip and p16Ink4a, and p19Arf) and stress-activated kinases p38, and 2) senescent beige precursors are unable to proliferate and differentiate upon cold stimulus. Blockade of cellular senescence by genetic deletion of Ink4a/Arf or a small molecule inhibitor of p38 reversed the aging-mediated decrease in thermogenesis [96].
Based on the proposed mechanism above, dietary molecules that possess the ability to suppress cellular senescence of beige precursor cells could be effective in rejuvenating WAT-browning with aging. Compared to other health benefits, the anti-aging function of n-3 PUFA is less clear and poorly understood [97]. Although the exact mechanism was not presented, several clinical trials have reported anti-aging effects of n-3 PUFA; marine n-3 PUFA intake (DHA+EPA) was associated with significant attenuation of telomere shortening in patients with coronary artery disease [98] and elderly individuals [99]. Regarding this mechanism, Chen et al. revealed that fish oil supplementation suppressed aging-mediated reduced-telomere activity by maintaining redox homeostasis; DHA intake was effective in suppressing overexpression of p16 and p53, which are metabolic culprits to promote cellular senescence [100]. Supporting this concept, fish oil supplementation improved energy expenditure and promoted RMR in elderly females [79]. It remains to be determined whether the proposed axis activation of ‘redox-telomere-cell cycle inhibition’ will be relevant to beige precursor cells (Figure 3B).
4.3. Limitations and directions for future research
We have discussed the basic promise of n-3 PUFA supplementation as a lifetime stimulator for brown/beige fat. This offers the possibility that n-3 PUFA intake exerts inter-generational metabolic benefits through fetal BAT development, and reverses age-related metabolic slowdown by suppressing telomere shortening in beige precursor cells. In fact, there is a paucity of evidence to support these hypotheses to date. Hence, the impact of prenatal n-3 PUFA exposure on fetal BAT development, and its metabolic ramifications in diet-induced obesity should be assessed before implementing maternal n-3 PUFA as a realistic and long-lasting therapeutic target to mitigate childhood obesity. To achieve this aim, randomized clinical trials are required with careful consideration of several factors such as supplementation periods, dose, the source of n-3 PUFA, and the ratio of DHA to EPA. Similarly, innovative study designs are required to distinguish metabolic improvement due to adipose tissue browning from other n-3 PUFA related metabolic benefits in elderly. In addition, rejuvenating WAT browning in the elderly poses a challenge since it demands two separate signaling cues, suppression of cellular senescence and activation of ADRB3 signaling. It will be of great interest to determine whether n-3 PUFA supplementation in the elderly can exert a coordinated role in inhibiting cellular senescence as well as stimulating ADRB3 for WAT browning.
For future research, the emerging role of n-3 PUFA as an epigenetic modulator [19, 101] needs to be counted as a contributing mechanism for maternal nutrition. Intriguingly, there is a challenging hypothesis that requires our attention. Hasegawa et al. demonstrated PRDM16-mediated adipocyte browning is inversely associated with adipocyte fibrosis [102]. It will be exciting to identify whether n-3 PUFA promotes beige fat activation by attenuating aging-mediated fibrosis. Lastly, the role of n-3 PUFA in UCP1-independent thermogenesis in beige adipocytes would be another fascinating aspect to consider. Recently Ikeda et al. reported that calcium cycling in beige adipocytes elevates energy expenditure in the absence of UCP1 [103]. Supported by work showing GPR120 agonism promotes thermogenesis via calcium influx-mediated mitochondrial fission independent of UCP1 activity [39], exploration of calcium signaling-dependent thermogenesis by n-3 PUFA will be of interest, especially in older adults whose UCP1 activation is compromised.
5. Conclusion
The metabolic role of brown/beige fat activity in humans has gained great attention during the last decade. The current review highlights the molecular networks that n-3 PUFA can serve as a safe source of dietary molecules to promote adaptive thermogenesis via GPR-120 dependent or independent mechanisms (Figure 1 and 2). To date, our current understanding regarding the thermogenic function of n-3 PUFA in humans is inconsistent, despite numerous indications in experimental animals. The existing discrepancy between animal studies and human clinical trials is expected to reconcile in the near future provided that FDG-PET becomes accessible to human clinical trials with improved resolution of PET imaging.
Supported by literature, it is conceivable that n-3 PUFA supplementation at targeted-lifetime stages such as pregnancy or senescence could promote brown and beige fat activity, counteracting childhood obesity or aging-associated metabolic slowdown, respectively (Figure 3). It also reinforces the importance of future work to determine other factors that regulate the collective efficacy of thermogenic function of n-3 PUFA such as supplementation dose, period, and EPA/DHA ratio in human clinical trials.
Highlights.
GPR120 is a signaling platform for adaptive thermogenesis by n-3 PUFA
n-3 PUFA induce endocrine-immune interactions for beige fat differentiation
n-3 PUFA activate TRPV1 to trigger ADRB3 signaling for brown/beige fat formation
A low n-6/n-3 ratio in adipocytes promote white to beige adipocyte conversion.
n-3 PUFA intake in pregnancy and senescence may promote adaptive thermogenesis
Acknowledgments
We would like to thank Ashley Mulcahy for reviewing the manuscript.
Abbreviations used
- ADRB3
beta 3-adrenergic receptor
- ARA
arachidonic acid
- ALA
alpha-linolenic acid
- BAT
brown adipose tissue
- BMR
basal metabolic rate
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FGF21
fibroblast growth factor 21
- GPR120
G-protein-coupled receptor 120
- hADS
human adipose-derived stem cells
- HF
high fat
- iBAT
interscapular BAT
- miRNAs
microRNAs
- MUFA
monounsaturated fatty acids
- PUFA
polyunsaturated fatty acids
- RER
respiratory exchange ratio
- SFA
saturated fatty acids
- SV
stromal vascular
- SNS
sympathetic nervous system
- TRPV1
transient receptor potential vanilloid 1
- UCP1
uncoupling protein 1
- WAT
white adipose tissue)
Footnotes
Conflict of Interest: Fan, Koehler, and Chung, no conflicts of interest.
Funding Disclosure: This work was supported by NIH-1P20GM104320 (Project 5 to S.C.). It was also supported by a Nebraska EPSCoR Food for Health Initiative grant awarded to S.C.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Okla M, Kim J, Koehler K, Chung S. Dietary Factors Promoting Brown and Beige Fat Development and Thermogenesis. Adv Nutr. 2017;8:473–483. doi: 10.3945/an.116.014332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santulli G, Iaccarino G. Adrenergic signaling in heart failure and cardiovascular aging. Maturitas. 2016 doi: 10.1016/j.maturitas.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mele L, Bidault G, Mena P, Crozier A, Brighenti F, Vidal-Puig A, Del Rio D. Dietary (Poly)phenols, Brown Adipose Tissue Activation, and Energy Expenditure: A Narrative Review. Adv Nutr. 2017;8:694–704. doi: 10.3945/an.117.015792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saito M. Capsaicin and Related Food Ingredients Reducing Body Fat Through the Activation of TRP and Brown Fat Thermogenesis. Adv Food Nutr Res. 2015;76:1–28. doi: 10.1016/bs.afnr.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Chen LH, Chien YW, Liang CT, Chan CH, Fan MH, Huang HY. Green tea extract induces genes related to browning of white adipose tissue and limits weight-gain in high energy diet-fed rat. Food Nutr Res. 2017;61:1347480. doi: 10.1080/16546628.2017.1347480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee MS, Shin Y, Jung S, Kim Y. Effects of epigallocatechin-3-gallate on thermogenesis and mitochondrial biogenesis in brown adipose tissues of diet-induced obese mice. Food Nutr Res. 2017;61:1325307. doi: 10.1080/16546628.2017.1325307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neyrinck AM, Bindels LB, Geurts L, Van Hul M, Cani PD, Delzenne NM. A polyphenolic extract from green tea leaves activates fat browning in high-fat-diet-induced obese mice. J Nutr Biochem. 2017;49:15–21. doi: 10.1016/j.jnutbio.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Alberdi G, Rodriguez VM, Miranda J, Macarulla MT, Churruca I, Portillo MP. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 2013;141:1530–1535. doi: 10.1016/j.foodchem.2013.03.085. [DOI] [PubMed] [Google Scholar]
- 9.Andrade JM, Frade AC, Guimaraes JB, Freitas KM, Lopes MT, Guimaraes AL, de Paula AM, Coimbra CC, Santos SH. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr. 2014;53:1503–1510. doi: 10.1007/s00394-014-0655-6. [DOI] [PubMed] [Google Scholar]
- 10.Arias N, Pico C, Teresa Macarulla M, Oliver P, Miranda J, Palou A, Portillo MP. A combination of resveratrol and quercetin induces browning in white adipose tissue of rats fed an obesogenic diet. Obesity (Silver Spring) 2017;25:111–121. doi: 10.1002/oby.21706. [DOI] [PubMed] [Google Scholar]
- 11.Lee SG, Parks JS, Kang HW. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J Nutr Biochem. 2017;42:62–71. doi: 10.1016/j.jnutbio.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Mele L, Carobbio S, Brindani N, Curti C, Rodriguez-Cuenca S, Bidault G, Mena P, Zanotti I, Vacca M, Vidal-Puig A, Del Rio D. Phenyl-gamma-valerolactones, flavan-3-ol colonic metabolites, protect brown adipocytes from oxidative stress without affecting their differentiation or function. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201700074. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Zhang H, Li B, Meng X, Wang J, Zhang Y, Yao S, Ma Q, Jin L, Yang J, Wang W, Ning G. Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun. 2014;5:5493. doi: 10.1038/ncomms6493. [DOI] [PubMed] [Google Scholar]
- 14.Bang HO, Dyerberg J, Hjoorne N. The composition of food consumed by Greenland Eskimos. Acta medica Scandinavica. 1976;200:69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- 15.Jeromson S, Gallagher IJ, Galloway SD, Hamilton DL. Omega-3 fatty acids and skeletal muscle health. Marine drugs. 2015;13:6977–7004. doi: 10.3390/md13116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochemical Society transactions. 2017 doi: 10.1042/BST20160474. [DOI] [PubMed] [Google Scholar]
- 17.Bhaswant M, Poudyal H, Brown L. Mechanisms of enhanced insulin secretion and sensitivity with n-3 unsaturated fatty acids. The Journal of nutritional biochemistry. 2015;26:571–584. doi: 10.1016/j.jnutbio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Wang DD, Hu FB. Dietary Fat and Risk of Cardiovascular Disease: Recent Controversies and Advances. Annu Rev Nutr. 2017;37:423–446. doi: 10.1146/annurev-nutr-071816-064614. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Okla M, Erickson A, Carr T, Natarajan SK, Chung S. Eicosapentaenoic Acid Potentiates Brown Thermogenesis through FFAR4-dependent Up-regulation of miR-30b and miR-378. J Biol Chem. 2016;291:20551–20562. doi: 10.1074/jbc.M116.721480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim M, Goto T, Yu R, Uchida K, Tominaga M, Kano Y, Takahashi N, Kawada T. Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system. Sci Rep. 2015;5:18013. doi: 10.1038/srep18013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quesada-Lopez T, Cereijo R, Turatsinze JV, Planavila A, Cairo M, Gavalda-Navarro A, Peyrou M, Moure R, Iglesias R, Giralt M, Eizirik DL, Villarroya F. The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Commun. 2016;7:13479. doi: 10.1038/ncomms13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song T, Yang Y, Zhou Y, Wei H, Peng J. GPR120: a critical role in adipogenesis, inflammation, and energy metabolism in adipose tissue. Cell Mol Life Sci. 2017;74:2723–2733. doi: 10.1007/s00018-017-2492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonet ML, Mercader J, Palou A. A nutritional perspective on UCP1-dependent thermogenesis. Biochimie. 2017 doi: 10.1016/j.biochi.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. Faseb j. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 26.Bargut TC, Silva ESAC, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. Eur J Nutr. 2016;55:159–169. doi: 10.1007/s00394-015-0834-0. [DOI] [PubMed] [Google Scholar]
- 27.Pahlavani M, Razafimanjato F, Ramalingam L, Kalupahana NS, Moussa H, Scoggin S, Moustaid-Moussa N. Eicosapentaenoic acid regulates brown adipose tissue metabolism in high-fat-fed mice and in clonal brown adipocytes. J Nutr Biochem. 2016;39:101–109. doi: 10.1016/j.jnutbio.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Kalupahana NS, Claycombe K, Newman SJ, Stewart T, Siriwardhana N, Matthan N, Lichtenstein AH, Moustaid-Moussa N. Eicosapentaenoic acid prevents and reverses insulin resistance in high-fat diet-induced obese mice via modulation of adipose tissue inflammation. The Journal of nutrition. 2010;140:1915–1922. doi: 10.3945/jn.110.125732. [DOI] [PubMed] [Google Scholar]
- 29.Laiglesia LM, Lorente-Cebrian S, Prieto-Hontoria PL, Fernandez-Galilea M, Ribeiro SM, Sainz N, Martinez JA, Moreno-Aliaga MJ. Eicosapentaenoic acid promotes mitochondrial biogenesis and beige-like features in subcutaneous adipocytes from overweight subjects. J Nutr Biochem. 2016;37:76–82. doi: 10.1016/j.jnutbio.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 30.Rosell M, Kaforou M, Frontini A, Okolo A, Chan YW, Nikolopoulou E, Millership S, Fenech ME, MacIntyre D, Turner JO, Moore JD, Blackburn E, Gullick WJ, Cinti S, Montana G, Parker MG, Christian M. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab. 2014;306:E945–964. doi: 10.1152/ajpendo.00473.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin X, Park HG, Zhang JY, Lawrence P, Liu G, Subramanian N, Kothapalli KS, Brenna JT. Brown but not white adipose cells synthesize omega-3 docosahexaenoic acid in culture. Prostaglandins Leukot Essent Fatty Acids. 2016;104:19–24. doi: 10.1016/j.plefa.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopes PA, Bandarra NM, Martins SV, Madeira MS, Ferreira J, Guil-Guerrero JL, Prates JAM. Docosahexaenoic acid (DHA) at the sn-2 position of triacylglycerols increases DHA incorporation in brown, but not in white adipose tissue, of hamsters. Int J Food Sci Nutr. 2017:1–14. doi: 10.1080/09637486.2017.1372390. [DOI] [PubMed] [Google Scholar]
- 33.Fumagalli M, Moltke I, Grarup N, Racimo F, Bjerregaard P, Jorgensen ME, Korneliussen TS, Gerbault P, Skotte L, Linneberg A, Christensen C, Brandslund I, Jorgensen T, Huerta-Sanchez E, Schmidt EB, Pedersen O, Hansen T, Albrechtsen A, Nielsen R. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science. 2015;349:1343–1347. doi: 10.1126/science.aab2319. [DOI] [PubMed] [Google Scholar]
- 34.Oh DY, Walenta E, Akiyama TE, Lagakos WS, Lackey D, Pessentheiner AR, Sasik R, Hah N, Chi TJ, Cox JM, Powels MA, Di Salvo J, Sinz C, Watkins SM, Armando AM, Chung H, Evans RM, Quehenberger O, McNelis J, Bogner-Strauss JG, Olefsky JM. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med. 2014;20:942–947. doi: 10.1038/nm.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. 2012 doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- 36.Bargut TC, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB. Fish oil diet modulates epididymal and inguinal adipocyte metabolism in mice. Food Funct. 2016;7:1468–1476. doi: 10.1039/c5fo00909j. [DOI] [PubMed] [Google Scholar]
- 37.Zhao M, Chen X. Eicosapentaenoic acid promotes thermogenic and fatty acid storage capacity in mouse subcutaneous adipocytes. Biochem Biophys Res Commun. 2014;450:1446–1451. doi: 10.1016/j.bbrc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Fleckenstein-Elsen M, Dinnies D, Jelenik T, Roden M, Romacho T, Eckel J. Eicosapentaenoic acid and arachidonic acid differentially regulate adipogenesis, acquisition of a brite phenotype and mitochondrial function in primary human adipocytes. Mol Nutr Food Res. 2016;60:2065–2075. doi: 10.1002/mnfr.201500892. [DOI] [PubMed] [Google Scholar]
- 39.Schilperoort M, van Dam AD, Hoeke G, Shabalina IG, Okolo A, Hanyaloglu AC, Dib LH, Mol IM, Caengprasath N, Chan YW, Damak S, Miller AR, Coskun T, Shimpukade B, Ulven T, Kooijman S, Rensen PC, Christian M. The GPR120 agonist TUG-891 promotes metabolic health by stimulating mitochondrial respiration in brown fat. EMBO molecular medicine. 2018 doi: 10.15252/emmm.201708047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexaki VI, Chavakis T. The role of innate immunity in the regulation of brown and beige adipogenesis. Reviews in endocrine & metabolic disorders. 2016;17:41–49. doi: 10.1007/s11154-016-9342-7. [DOI] [PubMed] [Google Scholar]
- 41.Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Z, Zhong L, Lee JTH, Zhang J, Wu D, Geng L, Wang Y, Wong CM, Xu A. The FGF21-CCL11 Axis Mediates Beiging of White Adipose Tissues by Coupling Sympathetic Nervous System to Type 2 Immunity. Cell Metab. 2017;26:493–508.e494. doi: 10.1016/j.cmet.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Messina G, Valenzano A, Moscatelli F, Salerno M, Lonigro A, Esposito T, Monda V, Corso G, Messina A, Viggiano A, Triggiani AI, Chieffi S, Guglielmi G, Monda M, Cibelli G. Role of Autonomic Nervous System and Orexinergic System on Adipose Tissue. Front Physiol. 2017;8:137. doi: 10.3389/fphys.2017.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ono K, Tsukamoto-Yasui M, Hara-Kimura Y, Inoue N, Nogusa Y, Okabe Y, Nagashima K, Kato F. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J Appl Physiol (1985) 2011;110:789–798. doi: 10.1152/japplphysiol.00128.2010. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe T, Kawada T, Kurosawa M, Sato A, Iwai K. Adrenal sympathetic efferent nerve and catecholamine secretion excitation caused by capsaicin in rats. Am J Physiol. 1988;255:E23–27. doi: 10.1152/ajpendo.1988.255.1.E23. [DOI] [PubMed] [Google Scholar]
- 47.Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. 2016;173:2369–2389. doi: 10.1111/bph.13514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawabata F, Inoue N, Masamoto Y, Matsumura S, Kimura W, Kadowaki M, Higashi T, Tominaga M, Inoue K, Fushiki T. Non-pungent capsaicin analogs (capsinoids) increase metabolic rate and enhance thermogenesis via gastrointestinal TRPV1 in mice. Biosci Biotechnol Biochem. 2009;73:2690–2697. doi: 10.1271/bbb.90555. [DOI] [PubMed] [Google Scholar]
- 49.Matta JA, Miyares RL, Ahern GP. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J Physiol. 2007;578:397–411. doi: 10.1113/jphysiol.2006.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohyama K, Nogusa Y, Shinoda K, Suzuki K, Bannai M, Kajimura S. A Synergistic Antiobesity Effect by a Combination of Capsinoids and Cold Temperature Through Promoting Beige Adipocyte Biogenesis. Diabetes. 2016;65:1410–1423. doi: 10.2337/db15-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2002;56:365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 52.Pisani DF, Ghandour RA, Beranger GE, Le Faouder P, Chambard JC, Giroud M, Vegiopoulos A, Djedaini M, Bertrand-Michel J, Tauc M, Herzig S, Langin D, Ailhaud G, Duranton C, Amri EZ. The omega6-fatty acid, arachidonic acid, regulates the conversion of white to brite adipocyte through a prostaglandin/calcium mediated pathway. Molecular metabolism. 2014;3:834–847. doi: 10.1016/j.molmet.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghandour RA, Colson C, Giroud M, Maurer S, Rekima S, Ailhaud GP, Klingenspor M, Amri EZ, Pisani DF. Impact of dietary omega3 polyunsaturated fatty acid supplementation on brown and brite adipocyte function. J Lipid Res. 2018 doi: 10.1194/jlr.M081091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghandour RA, Giroud M, Vegiopoulos A, Herzig S, Ailhaud G, Amri EZ, Pisani DF. IP-receptor and PPARs trigger the conversion of human white to brite adipocyte induced by carbaprostacyclin. Biochimica et biophysica acta. 2016;1861:285–293. doi: 10.1016/j.bbalip.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Hwang SH, Wagner K, Xu J, Yang J, Li X, Cao Z, Morisseau C, Lee KS, Hammock BD. Chemical synthesis and biological evaluation of omega-hydroxy polyunsaturated fatty acids. Bioorganic & medicinal chemistry letters. 2017;27:620–625. doi: 10.1016/j.bmcl.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saito J, Terano T, Hirai A, Shiina T, Tamura Y, Saito Y. Mechanisms of enhanced production of PGI2 in cultured rat vascular smooth muscle cells enriched with eicosapentaenoic acid. Atherosclerosis. 1997;131:219–228. doi: 10.1016/s0021-9150(97)00048-8. [DOI] [PubMed] [Google Scholar]
- 57.Bhatt DL, Steg PG, Brinton EA, Jacobson TA, Miller M, Tardif JC, Ketchum SB, Doyle RT, Jr, Murphy SA, Soni PN, Braeckman RA, Juliano RA, Ballantyne CM. Rationale and design of REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial. Clinical cardiology. 2017;40:138–148. doi: 10.1002/clc.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohnishi H, Saito Y. Eicosapentaenoic acid (EPA) reduces cardiovascular events: relationship with the EPA/arachidonic acid ratio. Journal of atherosclerosis and thrombosis. 2013;20:861–877. doi: 10.5551/jat.18002. [DOI] [PubMed] [Google Scholar]
- 59.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. The Journal of clinical investigation. 2015;125:478. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. The Journal of clinical investigation. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Orava J, Nuutila P, Noponen T, Parkkola R, Viljanen T, Enerback S, Rissanen A, Pietilainen KH, Virtanen KA. Blunted metabolic responses to cold, insulin stimulation in brown adipose tissue of obese humans. Obesity (Silver Spring Md) 2013;21:2279–2287. doi: 10.1002/oby.20456. [DOI] [PubMed] [Google Scholar]
- 62.Vosselman MJ, van der Lans AA, Brans B, Wierts R, van Baak MA, Schrauwen P, van Marken Lichtenbelt WD. Systemic beta-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes. 2012;61:3106–3113. doi: 10.2337/db12-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Emsley R, Niehaus DJ, Oosthuizen PP, Koen L, Ascott-Evans B, Chiliza B, van Rensburg SJ, Smit RM. Safety of the omega-3 fatty acid, eicosapentaenoic acid (EPA) in psychiatric patients: results from a randomized, placebo-controlled trial. Psychiatry research. 2008;161:284–291. doi: 10.1016/j.psychres.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 64.Spencer M, Finlin BS, Unal R, Zhu B, Morris AJ, Shipp LR, Lee J, Walton RG, Adu A, Erfani R. Omega-3 fatty acids reduce adipose tissue macrophages in human subjects with insulin resistance. Diabetes. 2013;62:1709–1717. doi: 10.2337/db12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lalia AZ, Johnson ML, Jensen MD, Hames KC, Port JD, Lanza IR. Effects of dietary n-3 fatty acids on hepatic and peripheral insulin sensitivity in insulin-resistant humans. Diabetes care. 2015;38:1228–1237. doi: 10.2337/dc14-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacobo-Cejudo MG, Valdés-Ramos R, Guadarrama-López AL, Pardo-Morales R-V, Martínez-Carrillo BE, Harbige LS. Effect of n-3 Polyunsaturated Fatty Acid Supplementation on Metabolic and Inflammatory Biomarkers in Type 2 Diabetes Mellitus Patients. Nutrients. 2017;9:573. doi: 10.3390/nu9060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajkumar H, Mahmood N, Kumar M, Varikuti SR, Challa HR, Myakala SP. Effect of probiotic (VSL# 3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediators of inflammation. 2014;2014 doi: 10.1155/2014/348959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tousoulis D, Plastiras A, Siasos G, Oikonomou E, Verveniotis A, Kokkou E, Maniatis K, Gouliopoulos N, Miliou A, Paraskevopoulos T. Omega-3 PUFAs improved endothelial function and arterial stiffness with a parallel antiinflammatory effect in adults with metabolic syndrome. Atherosclerosis. 2014;232:10–16. doi: 10.1016/j.atherosclerosis.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 69.Dasarathy S, Dasarathy J, Khiyami A, Yerian L, Hawkins C, Sargent R, McCullough AJ. Double blind randomized placebo controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. Journal of clinical gastroenterology. 2015;49:137. doi: 10.1097/MCG.0000000000000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang YY, Liu W, Zhao TY, Tian HM. Efficacy of Omega-3 Polyunsaturated Fatty Acids Supplementation in Managing Overweight and Obesity: A Meta-Analysis of Randomized Clinical Trials. J Nutr Health Aging. 2017;21:187–192. doi: 10.1007/s12603-016-0755-5. [DOI] [PubMed] [Google Scholar]
- 71.Ramel A, Martinez A, Kiely M, Morais G, Bandarra NM, Thorsdottir I. Beneficial effects of long-chain n-3 fatty acids included in an energy-restricted diet on insulin resistance in overweight and obese European young adults. Diabetologia. 2008;51:1261–1268. doi: 10.1007/s00125-008-1035-7. [DOI] [PubMed] [Google Scholar]
- 72.Couet C, Delarue J, Ritz P, Antoine JM, Lamisse F. Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int J Obes Relat Metab Disord. 1997;21:637–643. doi: 10.1038/sj.ijo.0800451. [DOI] [PubMed] [Google Scholar]
- 73.Noreen EE, Sass MJ, Crowe ML, Pabon VA, Brandauer J, Averill LK. Effects of supplemental fish oil on resting metabolic rate, body composition, and salivary cortisol in healthy adults. J Int Soc Sports Nutr. 2010;7:31. doi: 10.1186/1550-2783-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gunnarsdottir I, Tomasson H, Kiely M, Martinez JA, Bandarra NM, Morais MG, Thorsdottir I. Inclusion of fish or fish oil in weight-loss diets for young adults: effects on blood lipids. Int J Obes (Lond) 2008;32:1105–1112. doi: 10.1038/ijo.2008.64. [DOI] [PubMed] [Google Scholar]
- 75.Thorsdottir I, Tomasson H, Gunnarsdottir I, Gisladottir E, Kiely M, Parra MD, Bandarra NM, Schaafsma G, Martinez JA. Randomized trial of weight-loss-diets for young adults varying in fish and fish oil content. Int J Obes (Lond) 2007;31:1560–1566. doi: 10.1038/sj.ijo.0803643. [DOI] [PubMed] [Google Scholar]
- 76.Jannas-Vela S, Roke K, Boville S, Mutch DM, Spriet LL. Lack of effects of fish oil supplementation for 12 weeks on resting metabolic rate and substrate oxidation in healthy young men: A randomized controlled trial. PLoS One. 2017;12:e0172576. doi: 10.1371/journal.pone.0172576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hill AM, Buckley JD, Murphy KJ, Howe PR. Combining fish-oil supplements with regular aerobic exercise improves body composition and cardiovascular disease risk factors. Am J Clin Nutr. 2007;85:1267–1274. doi: 10.1093/ajcn/85.5.1267. [DOI] [PubMed] [Google Scholar]
- 78.Flouris AD, Dinas PC, Valente A, Andrade CMB, Kawashita NH, Sakellariou P. Exercise-induced effects on UCP1 expression in classical brown adipose tissue: a systematic review. Horm Mol Biol Clin Investig. 2017;31 doi: 10.1515/hmbci-2016-0048. [DOI] [PubMed] [Google Scholar]
- 79.Logan SL, Spriet LL. Omega-3 Fatty Acid Supplementation for 12 Weeks Increases Resting and Exercise Metabolic Rate in Healthy Community-Dwelling Older Females. PLoS One. 2015;10:e0144828. doi: 10.1371/journal.pone.0144828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borga M, Virtanen KA, Romu T, Leinhard OD, Persson A, Nuutila P, Enerback S. Brown adipose tissue in humans: detection and functional analysis using PET (positron emission tomography), MRI (magnetic resonance imaging), and DECT (dual energy computed tomography) Methods Enzymol. 2014;537:141–159. doi: 10.1016/B978-0-12-411619-1.00008-2. [DOI] [PubMed] [Google Scholar]
- 82.Symonds ME, Mostyn A, Pearce S, Budge H, Stephenson T. Endocrine and nutritional regulation of fetal adipose tissue development. The Journal of endocrinology. 2003;179:293–299. doi: 10.1677/joe.0.1790293. [DOI] [PubMed] [Google Scholar]
- 83.Mostyn A, Pearce S, Stephenson T, Symonds ME. Hormonal, nutritional regulation of adipose tissue mitochondrial development, function in the newbornm, Experimental and clinical endocrinology & diabetes : official journal. German Society of Endocrinology [and] German Diabetes Association. 2004;112:2–9. doi: 10.1055/s-2004-815719. [DOI] [PubMed] [Google Scholar]
- 84.Symonds ME, Bryant MJ, Clarke L, Darby CJ, Lomax MA. Effect of maternal cold exposure on brown adipose tissue and thermogenesis in the neonatal lamb. The Journal of physiology. 1992;455:487–502. doi: 10.1113/jphysiol.1992.sp019313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ojha S, Robinson L, Yazdani M, Symonds ME, Budge H. Brown adipose tissue genes in pericardial adipose tissue of newborn sheep are downregulated by maternal nutrient restriction in late gestation. Pediatric research. 2013;74:246–251. doi: 10.1038/pr.2013.107. [DOI] [PubMed] [Google Scholar]
- 86.Budge H, Edwards LJ, McMillen IC, Bryce A, Warnes K, Pearce S, Stephenson T, Symonds ME. Nutritional manipulation of fetal adipose tissue deposition and uncoupling protein 1 messenger RNA abundance in the sheep: differential effects of timing and duration. Biology of reproduction. 2004;71:359–365. doi: 10.1095/biolreprod.103.018986. [DOI] [PubMed] [Google Scholar]
- 87.Dumortier O, Roger E, Pisani DF, Casamento V, Gautier N, Lebrun P, Johnston H, Lopez P, Amri EZ, Jousse C, Fafournoux P, Prentki M, Hinault C, Van Obberghen E. Age-Dependent Control Of Energy Homeostasis by Brown Adipose Tissue in Progeny Subjected to Maternal Diet-Induced Fetal Programming. Diabetes. 2016 doi: 10.2337/db16-0956. [DOI] [PubMed] [Google Scholar]
- 88.Liang X, Yang Q, Zhang L, Maricelli JW, Rodgers BD, Zhu MJ, Du M. Maternal high-fat diet during lactation impairs thermogenic function of brown adipose tissue in offspring mice. Sci Rep. 2016;6:34345. doi: 10.1038/srep34345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Larque E, Gil-Sanchez A, Prieto-Sanchez MT, Koletzko B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br J Nutr. 2012;107(Suppl 2):S77–84. doi: 10.1017/S0007114512001481. [DOI] [PubMed] [Google Scholar]
- 90.Knudsen VK, Hansen HS, Osterdal ML, Mikkelsen TB, Mu H, Olsen SF. Fish oil in various doses or flax oil in pregnancy and timing of spontaneous delivery: a randomised controlled trial. Bjog. 2006;113:536–543. doi: 10.1111/j.1471-0528.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- 91.Vidakovic AJ, Gishti O, Voortman T, Felix JF, Williams MA, Hofman A, Demmelmair H, Koletzko B, Tiemeier H, Jaddoe VW, Gaillard R. Maternal plasma PUFA concentrations during pregnancy and childhood adiposity: the Generation R Study. Am J Clin Nutr. 2016;103:1017–1025. doi: 10.3945/ajcn.115.112847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Donahue SM, Rifas-Shiman SL, Gold DR, Jouni ZE, Gillman MW, Oken E. Prenatal fatty acid status and child adiposity at age 3 y: results from a US pregnancy cohort. Am J Clin Nutr. 2011;93:780–788. doi: 10.3945/ajcn.110.005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moon RJ, Harvey NC, Robinson SM, Ntani G, Davies JH, Inskip HM, Godfrey KM, Dennison EM, Calder PC, Cooper C. Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J Clin Endocrinol Metab. 2013;98:299–307. doi: 10.1210/jc.2012-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Foster BA, Escaname E, Powell TL, Larsen B, Siddiqui SK, Menchaca J, Aquino C, Ramamurthy R, Hale DE. Randomized Controlled Trial of DHA Supplementation during Pregnancy: Child Adiposity Outcomes. Nutrients. 2017;9 doi: 10.3390/nu9060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rudolph MC, Jackman MR, Presby DM, Houck JA, Webb PG, Johnson GC, Soderborg TK, de la Houssaye BA, Yang IV, Friedman JE, MacLean PS. Low Neonatal Plasma N-6/N-3 Pufa Ratios Regulate Offspring Adipogenic Potential and Condition Adult Obesity Resistance. Diabetes. 2017 doi: 10.2337/db17-0890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berry DC, Jiang Y, Arpke RW, Close EL, Uchida A, Reading D, Berglund ED, Kyba M, Graff JM. Cellular Aging Contributes to Failure of Cold-Induced Beige Adipocyte Formation in Old Mice and Humans. Cell Metab. 2017;25:166–181. doi: 10.1016/j.cmet.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Magalhaes JP, Muller M, Rainger GE, Steegenga W. Fish oil supplements, longevity and aging. Aging (Albany NY) 2016;8:1578–1582. doi: 10.18632/aging.101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. Jama. 2010;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O'Callaghan N, Parletta N, Milte CM, Benassi-Evans B, Fenech M, Howe PR. Telomere shortening in elderly individuals with mild cognitive impairment may be attenuated with omega-3 fatty acid supplementation: a randomized controlled pilot study. Nutrition (Burbank, Los Angeles County Calif) 2014;30:489–491. doi: 10.1016/j.nut.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 100.Chen J, Wei Y, Chen X, Jiao J, Zhang Y. Polyunsaturated fatty acids ameliorate aging via redox-telomere-antioncogene axis. Oncotarget. 2017;8:7301–7314. doi: 10.18632/oncotarget.14236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Dijk SJ, Zhou J, Peters TJ, Buckley M, Sutcliffe B, Oytam Y, Gibson RA, McPhee A, Yelland LN, Makrides M, Molloy PL, Muhlhausler BS. Effect of prenatal DHA supplementation on the infant epigenome: results from a randomized controlled trial. Clinical epigenetics. 2016;8:114. doi: 10.1186/s13148-016-0281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hasegawa Y, Ikeda K, Chen Y, Alba DL, Stifler D, Shinoda K, Hosono T, Maretich P, Yang Y, Ishigaki Y, Chi J, Cohen P, Koliwad SK, Kajimura S. Repression of Adipose Tissue Fibrosis through a PRDM16-GTF2IRD1 Complex Improves Systemic Glucose Homeostasis. Cell Metab. 2018;27:180–194.e186. doi: 10.1016/j.cmet.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, Shinoda K, Chen Y, Lu X, Maretich P, Tajima K, Ajuwon KM, Soga T, Kajimura S. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med. 23:201. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]