Abstract
Background
The transoral thyroidectomy vestibular approach has been utilized via both robotic (TORTVA) and endoscopic (TOETVA) techniques to perform thyroidectomy. However, there have been no studies evaluating outcomes between these approaches. Here we describe our outcomes for thyroid lobectomy with TORTVA and TOETVA.
Methods
All cases of transoral vestibular approach thyroid lobectomy at Johns Hopkins Hospital were reviewed. Primary outcomes and demographic data were then compared between TORTVA and TOETVA.
Results
Twenty-seven cases were identified, 7 using the robotic approach and 20 using the endoscopic approach. The procedural success rate for the robotic and endoscopic cohorts was 5 of 7 (71%) and 19 of 20 (95%), respectively (P = .15). There were no persistent nerve injuries, mental, or recurrent in either cohort. Median operative time for TOETVA was 188 minutes versus 322 minutes for TORTVA (P = .001).
Conclusion
Thyroid lobectomy can be safely performed via both techniques, although performed more quickly endoscopically, which is likely due in part to differences in the learning curves.
Keywords: remote access thyroidectomy, robotic thyroid surgery, transoral endoscopic thyroidectomy vestibular approach, transoral robotic thyroidectomy vestibular approach, transoral thyroidectomy
1 | INTRODUCTION
The transcervical incision has long been the primary approach to the thyroid gland and central neck since its description by Kocher in the late 1880s.1 Although it provides direct access to the central compartment, it can lead to unsightly neck scarring, which may negatively impact a patient’s quality of life.2–4 This consequence has become of particular interest given the growing volume of thyroid pathology in young women in conjunction with increasing societal emphasis on aesthetics.
Over the last 2 decades, there has been increasing motivation to avoid these consequences, encouraging the development of aesthetically favorable remote access and minimally invasive alternatives.5,6 These include the transaxillary approach, bilateral axillo-breast approach, and facelift approach, among others.7–9 Although these procedures succeed in avoiding a central neck scar, they require compromise between exposure and aesthetics, necessitating either a small but visible scar10 or extensive tissue dissection with a remote, concealed scar.11–18 The transoral vestibular approach first described with the use of the DaVinci robot (Intuitive Surgical, Sunnyvale, CA)19 and then successfully adapted for an endoscopic approach,20–24 most successfully navigates these competing interests. The incision in the mandibular gingivobuccal sulcus provides a midline approach with access to both thyroid lobes and paratracheal basins without a cutaneous scar.20–27 To date, this transoral vestibular approach has been utilized to successfully perform thyroid lobectomy, total thyroidectomy, parathyroidectomy, and central neck dissection via both robotic and endoscopic techniques.20–24,28 However, to our knowledge, there is no literature comparing the robotic and endoscopic techniques in regard to outcomes or learning curves within a single institution.
In this article, we report our early outcomes for thyroid lobectomy via both the transoral endoscopic thyroidectomy vestibular approach (TOETVA) and the transoral robotic thyroidectomy vestibular approach (TORTVA). We aim to further define the safety and efficacy of both techniques, while evaluating for any differences in the learning curves or outcomes with either approach.
2 | MATERIALS AND METHODS
2.1 | Data collection and analysis
After institutional review board approval, all cases of TOETVA and TORTVA at the Johns Hopkins Hospital were retrospectively reviewed between April 2016 and September 2017. Two fellowship-trained surgeons, one a high-volume endocrine surgeon and the other a high volume transoral robotic head and neck surgeon, completed all cases. The TORTVA cases were completed with the aid of an assistant at the head of the bed, similar to transoral robotic surgery cases, whereas an assistant operated the endoscope during TOETVA cases. Both surgeons were right-handed. Demographic data including, age, sex, body mass index, nodule size, and pathology were recorded. The primary outcomes of interest included completion of the intended procedure, presence of persistent (symptoms present for 3 months or longer), recurrent laryngeal nerve (RLN) or mental nerve injury, operative time (incision to closure), inadvertent presence of parathyroid glands within the specimen, and use of a surgical drain. Differences in means of parametric demographic data between the endoscopic and robotic groups were compared and analyzed using unpaired t tests. Operative times and largest specimen dimension were assumed to be nonparametric and, as such, differences in medians between the cohorts were compared using a Mann-Whitney U test. The proportion of nerve injuries, permanent hypoparathyroidism, presence of parathyroid tissue in the final specimen, need for a surgical drain, and completion of the intended approach in each cohort were compared using a Fisher’s exact test. A subgroup analysis of the first 7 endoscopic cases versus the 7 robotic cases was performed to capture differences in outcomes that may be attributable to experience with the procedure, as there were less total robotic cases. The entire endoscopic cohort was then additionally compared to the robotic cohort. All analyses were done in R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
2.2 | Surgical procedure
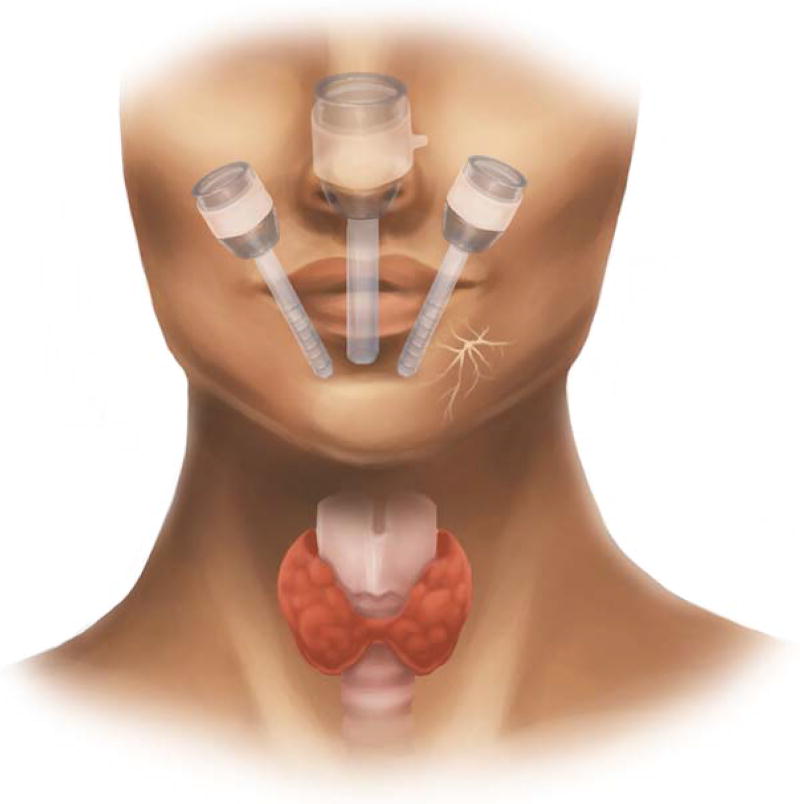
Our technique for TOETVA and TORTVA are based on those by Richmon et al19 and Anuwong et al20 and have been previously described in detail.22,29 In brief, patients are positioned supine and intubated orotracheally with a neural integrity monitor electromyogram endotracheal tube. Three incisions are then made in the oral vestibule for placement of the robotic or endoscopic trocars. The central incision is placed 5 to 10 mm cranial to the buccal-mandibular frenulum and is 15 mm in length. The lateral stab incisions are placed at the mucosal border of the oral commissure bilaterally. We have found that placement of trocars in this manner helps to minimize instrument collision and avoid mental nerve injury (see Figure 1). The subplatysmal space is then entered via the central incision through blunt dissection along the mandible and the use of mechanical dilators to facilitate placement of the central trocar. With the trocars in place, the subplatysmal flap is elevated endoscopically for both techniques to the level of the sternal notch inferiorly and the sternocleidomastoid muscles laterally, with CO2 insufflation used to maintain this working space. At this point, if TORTVA is planned, the DaVinci SI Surgical System (Intuitive Surgical, Sunnyvale, CA), is docked and the remainder of the case is performed robotically, otherwise it continues via endoscopic instrumentation, which most frequently consists of two of the following: Maryland dissector, hook cautery, suction, and an ultrasonic energy device. The midline raphe is identified and divided and the strap muscles are elevated off the thyroid capsule. The isthmus is then divided and lobectomy is performed in a superior to inferior fashion. The anterior trachea is utilized as a landmark for the RLN, which is often found at 2 o’clock and 3 o’clock on the right side, and between 9 o’clock and 10 o’clock on the left. Of note, the ability to visualize the RLNs at their insertion sites and the favorable angle of dissection along the nerves are distinct advantages provided by both TORTVA and TOETVA in comparison with other remote-access approaches. Once the lobe has been freed it is placed in an endocatch bag and removed through the central incision. Oral incisions are closed in a single layer with absorbable suture.
FIGURE 1.
Schematic illustration of transoral vestibular trocar placement for both robotic and endoscopic techniques. Care is taken with lateral port placement to avoid injury to the mental nerve, which is depicted on the patient’s left [Color figure can be viewed at wileyonlinelibrary.com]
3 | RESULTS
Twenty-seven thyroid lobectomies were attempted via a transoral vestibular approach between April 2016 and September 2017, with 7 being robotic and 20 endoscopic. Selection criteria and demographic/characteristic data for the robotic and endoscopic cohorts can be found in Tables 1 and 2. Of note, there was no statistically significant difference between cohorts or on subgroup analysis in demographic or characteristic data (Table 2).
TABLE 1.
Patient selection
| Case number |
Technique | Preoperative index nodule size, cm |
Preoperative pathology |
Final pathology |
|---|---|---|---|---|
| 1a | Robotic | 4.3 | Benign | Benign |
| 2 | Robotic | 4.3 | Benign | Benign |
| 3 | Robotic | 3.2 | AUS | Benign |
| 4 | Robotic | 3.4 | AUS | NIFT-P |
| 5 | Robotic | 2.8 | AUS | Benign |
| 6 | Endoscopic | 3.6 | Hürthle cell neoplasm | Benign |
| 7 | Endoscopic | 3.8 | Benign | Benign |
| 8 | Endoscopic | 4.2 | AUS | Benign |
| 9 | Endoscopic | 1.9 | Benign | Benign |
| 10 | Endoscopic | 1.7 | SFN | Benign |
| 11b | Robotic | 4.1 | Benign | Benign |
| 12 | Robotic | 1.5 | PTC | PTC |
| 13 | Endoscopic | 4.0 | Benign | Benign |
| 14 | Endoscopic | 4.5 | Benign | Benign |
| 15 | Endoscopic | 1.3 | SFN | PTC |
| 16 | Endoscopic | 5.4 | Benign | Benign |
| 17 | Endoscopic | 5.3 | Benign | Benign |
| 18 | Endoscopic | 2.2 | AUS | NIFT-P |
| 19 | Endoscopic | 5.1 | AUS | Benign |
| 20 | Endoscopic | 1.2 | AUS | Benign |
| 21 | Endoscopic | 1.7 | AUS | Benign |
| 22 | Endoscopic | 2.5 | Hürthle cell neoplasm | Hürthle cell carcinoma |
| 23 | Endoscopic | NAc | Hürthle cell carcinoma | Hürthle cell carcinoma |
| 24a | Endoscopic | 7.0 | AUS | Benign |
| 25 | Endoscopic | 3.5 | Benign | Benign |
| 26 | Endoscopic | 1.2 | AUS | PTC |
| 27 | Endoscopic | 5.0 | AUS | Benign |
Abbreviations: AUS, atypia of unknown significance; NIFT-P, noninvasive thyroid neoplasm with papillary-like nuclear features; PTC, papillary thyroid carcinoma; SFN, suspicious for neoplasm.
Case converted to open.
Case converted to endoscopic.
There was no nodule present in this lobe, lobectomy was performed due to the finding of Hürthle cell carcinoma in the contralateral lobe.
TABLE 2.
Cohort characteristics
| Robotic approach |
Endoscopic approacha |
Endoscopic approacha |
P valueb | |
|---|---|---|---|---|
| No. of patients | 7 | 7 | 20 | |
| Age, mean, years | 41.3 +/− 15.8 | 39.7 +/− 14.5 | 41.3 +/− 12.2 | .42/.41 |
| Female, % | 100, 7/7 | 100, 7/7 | 80, 16/20 | NA/.5 |
| BMI, mean, kg/m2 | 32.8 +/− 7.2 | 28.3 +/− 8.1 | 27.6 +/− 7.5 | .15/.06 |
| Largest specimen dimension, median, cm | 5.1 (4.4–6.8) | 4.5 (3.0–5.3) | 5.0 (3.0–7.8) | .09/.20 |
Abbreviations: BMI, body mass index; NA, not applicable.
The 2 endoscopic columns represent a division between the first 7 endoscopic cases followed by data on the entire endoscopic cohort (20 cases). As such, all patients in the endoscopic N = 7 column are included in the N = 20 column as well.
The first P value represents column 1 versus column 2, with the second representing 1 versus 3.
The first attempted robotic case was converted to open due to the presence of a substernal component of a goiter not identified on preoperative ultrasound, whereas the 17th attempted endoscopic case was converted to open due to bleeding encountered at the superior pole. Both cases were completed without complication or mental or recurrent nerve injury after converting. One of the robotic cases included both a thyroid lobectomy and intentional parathyroidectomy. Another case in the robotic cohort was attempted robotically but the surgery was ultimately converted to an endoscopic procedure due to difficulty manipulating the larger gland with the robotic platform. One patient in the endoscopic cohort underwent a thyroid lobectomy followed by a completion thyroidectomy by the same approach after final pathology from the initial procedure revealed Hürthle cell carcinoma with angioinvasion.
In the robotic cohort, there were 5 completed left lobectomies and 1 right lobectomy, compared with 12 right lobectomies and 7 left lobectomies completed in the endoscopic cohort. The intended procedure was completed successfully in 5 of 7 cases (71%) for the robotic cohort compared to 19 of 20 cases (95%) for the endoscopic cohort (P = .15). The final pathology in the robotic cohort was benign in 5 cases, with 1 case each of papillary thyroid carcinoma (PTC) and noninvasive follicular thyroid neoplasm with papillary-like nuclear features. In the endoscopic cohort, 15 cases had benign pathology, with 1 case of noninvasive follicular thyroid neoplasm with papillary-like nuclear features, 2 cases of PTC, and the aforementioned case of Hürthle cell carcinoma; that patient underwent completion thyroidectomy 2 weeks after the initial procedure (Table 1).
There was 1 case of temporary RLN paresis, which resolved spontaneously within 2 months in the endoscopic cohort. There were no cases of permanent RLN or mental nerve injury in either cohort. No extrathyroidal parathyroid tissue was identified in the specimens on final pathology in either cohort, save for the case with planned parathyroidectomy. One parathyroid gland was autotransplanted from the endoscopic group by first mincing the gland and then injecting with a large-gauge needle under direct visualization into the ipsilateral sternohyoid. A drain was placed in the axilla in 3 of 7 cases (43%) and 2 of 20 cases (10%) in the robotic and endoscopic cohorts, respectively (P = .09). Median maximum specimen dimension was 5.1 cm (range 4.4–6.8 cm) for the robotic cohort and 5.0 cm (range 3.0–7.8 cm) for the endoscopic cohort (P = .20). In subgroup analysis, the first 7 endoscopic cases had a median maximum dimension of 4.5 cm (range 3.0–5.3 cm), again with no statistically significant difference in comparison to the robotic cohort (P = .09; Table 2).
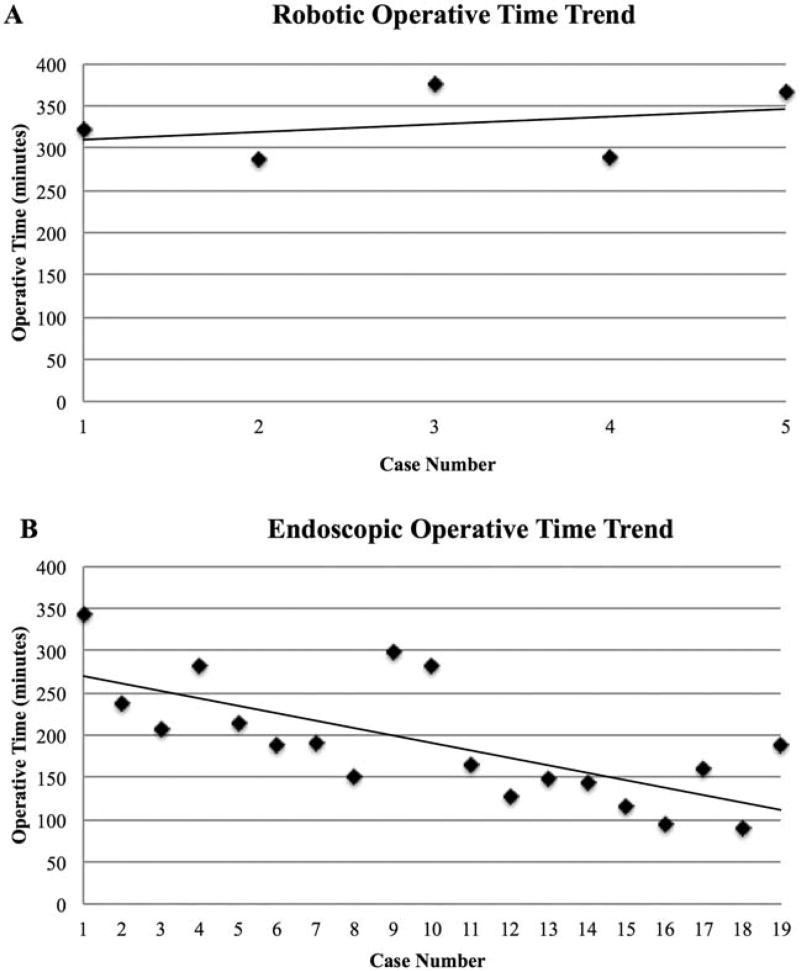
Median operative time was 322 minutes (range 287–377 minutes) in the robotic cohort compared to 188 minutes (range 89–343 minutes) in the endoscopic cohort (P = .001). On subgroup analysis, there was a median operative time of 213 minutes (range 189–343 minutes) for the first 7 endoscopic cases. This was again significantly different when compared to the robotic cohort (P = .01). Of note, cases not completed via the intended approach were not included in any cohort’s median operative time. These include the 2 cases converted to open, and the single robotic case that was converted to endoscopic (Table 3). There was no significant difference in median operative time when comparing right-sided (176 minutes) and left-sided (238 minutes) endoscopic lobectomies (P = .19). The trend in completed case operative time versus case number for both cohorts is plotted in Figure 2.
TABLE 3.
Operative outcomes
| Robotic approach |
Endoscopic approach |
Endoscopic approach |
P valuea | |
|---|---|---|---|---|
| No. of patients | 7 | 7 | 20 | |
| Operative time (median, range, minutes)b | 322 (287–377) | 213 (189–343) | 188 (89–343) | .01/.001 |
| Permanent RLN injury, % | 0 | 0 | 0 | NA |
| Permanent MN injury, % | 0 | 0 | 0 | NA |
| Extrathyroidal parathyroids within specimen, %c | 0 | 0 | 0 | NA |
| Placement of drain, % | 43 (3/7) | 14 (1/7) | 10 (2/20) | 0.56//0.09 |
| Completion of intended approach, % | 71 (5/7) | 100 (7/7) | 95 (19/20) | 0.46//0.15 |
Abbreviations: MN, mental nerve; NA, not applicable; RLN, recurrent laryngeal nerve.
The first P value represents column 1 versus column 2, with the second representing 1 versus 3.
Converted cases were not included in median operative time for any cohort.
Excluding planned parathyroidectomy.
FIGURE 2.
Comparison of A, robotic and B, endoscopic operative times for transoral lobectomy as a function of case number. Linear regression trend lines are illustrated for each technique. Cases converted to another approach than initially intended were not included in this figure
4 | DISCUSSION
Transoral vestibular approach thyroidectomy was successfully performed in 25 of 27 cases without incidence of permanent RLN or mental nerve injury. The converted cases were also completed without complications. There were also no inadvertently removed parathyroid glands and only one parathyroid gland required reimplantation. This may suggest the enhanced visualization and magnification of both techniques allowed for identification and preservation of viable parathyroid glands. These outcomes are consistent with recently reported international literature for both techniques20,22–24,28 further validating the safety and efficacy of these procedures.
Although thyroid lobectomy was safely performed via both techniques, there was a statistically significant difference in median operative time between the cohorts when comparing the initial 7 endoscopic cases to the 7 cases in the robotic cohort (213 minutes vs 322 minutes, respectively). This difference in operative time was magnified when including the subsequent 13 cases in the endoscopic cohort, as the median operative time dropped to 188 minutes. These differences were statistically significant in both cases with P values of .01 and .001, respectively. Moreover, there was no statistically significant difference between the cohorts or in subgroup analysis in demographic data or median specimen size, and, as such, the difference in operative time is not attributable to these potential confounders.
One explanation for the long median operative time for TORTVA in our series is that the bulk of the cohort was subject to a very steep institutional learning curve as they were Johns Hopkins’ first experience with transoral surgery. As the initial dissection is done endoscopically even in the robotic approach, these skills were developed and more finely honed at the start of the endoscopic case series. The sequential roll-out of the vestibular approach at our institution, therefore, does introduce a bias that may artificially inflate the operative time difference between the 2 approaches. Nonetheless, we believe that this difference is real, as the endoscopic approach does not require the additional robotic instrumentation and conversion from robotic to endoscopic and vice versa, which is required for the robotic approach. This is further supported by the largest published robotic transoral vestibular series to date, which reports a median operative time of 232 minutes for their 24 cases. This study went on to perform a subgroup analysis of their first 12 cases versus their second 12 and found no statistically significant difference in median operative time between the 2 time periods (234 minutes vs 230 minutes, respectively; P = .10) suggesting the learning curve had not been reached after 24 cases.23 This is in contrast to our TOETVA series, which had a median operative time of 188 minutes after 20 cases with a statistically significant difference in median operative times between our first 10 and last 9 completed cases (225.5 minutes vs 143 minutes; P = .007). A more objective assessment of surgical duration would require comparison of various endoscopic and robotic surgeons whom have all submitted their learning curves.
In our experience, we found that the ability to quickly remove/replace instrumentation with TOETVA led to extensive timesaving. When performing TOETVA/TORTVA, the fixed and limited working space often leads to obstruction of the camera with smoke, requiring removal and cleaning to reestablish visualization. This is most frequently encountered when utilizing energy-based instruments, which create surgical plumes that condense on the camera lens if it is in close proximity to the active blade of the device. We combat this in TOETVA by having the assistant withdraw the endoscope slightly when energy devices are active. In this way, the assistant in TOETVA works much in the same fashion as in the open approach, aiding the primary surgeon in maintaining adequate visualization. This process is much less streamlined in TORTVA, where a single operator must control all variables. More importantly, when the camera does become obstructed the process of disengaging, removing, cleaning, and reengaging the camera is significantly more time-consuming with TORTVA. In a similar fashion, any replacement of instrumentation, such as alternating between classes of energy devices, is much more time-consuming with the robotic technique. We do not believe, however, that difference in instrument availability between techniques was a significant affecter of operative time, as frequently used energy devices (monopolar cautery and ultrasonic scalpel) and available dissecting instruments are largely the same between techniques. Although available robotic instrumentation was not designed for use in the head and neck, the same is true for instrumentation utilized with TOETVA, which was initially designed for laparoscopic abdominal surgery. In this way, both techniques would benefit from the design and development of dedicated instrumentation.
In our experience, we did not find that the lack of haptic feedback with TORTVA altered our ability to manipulate and extract the specimen, and, as such, do not believe this to have affected our operative time. However, it did lead to uncertainty in the magnitude of force being applied to the trocars and surrounding tissue outside the field of view. Although we did not have any such complications in our series, injuries to the maxilla and tears of the oral commissure have been previously reported with TORTVA, both of which may be attributed to the lack of haptic feedback.23
Although left-sided laterality of disease was initially perceived as subjectively more difficult for the right-handed surgeon with TOETVA, this was quickly overcome, as is evidenced by the lack of a significant difference in operative time between right-sided and left-sided lobectomies in the TOETVA cohort (176 minutes vs 238 minutes; P = .19). Our experience with TORTVA was subjectively similar; however, the relatively limited sample size made it difficult to draw definitive conclusions.
The difference in operative time trends between approaches suggests a difference in the learning curves between the robotic and endoscopic techniques. In reports of other robotic thyroidectomy techniques, it has been noted that the learning curve is on the order of 40 to 50 cases.14,30,31 If the learning curve is assumed to be similar for TORTVA, it is not surprising that there was not a significant downtrend in operative time after 7 cases (see Figure 2), especially given the flat time trend seen in the study by Kim et al23 with their experience after 24 cases. The fact that there was a negative slope in operative time in the endoscopic cohort, a significant difference in median operative time in comparison to the robotic cohort, and a significant difference in operative time between our first 10 and last 9 completed endoscopic cases suggest that the learning curve may be substantially <40 cases for TOETVA. This finding in conjunction with the known cost burden32 with the commercially available robotic system suggests the barriers to introducing a transoral vestibular approach to thyroid and parathyroid pathology at a new institution may be more significant for the robotic approach. Further studies are needed to better elucidate this.
5 | CONCLUSION
The TORTVA and TOETVA techniques can both be safely performed without a cervical incision, although more quickly endoscopically, likely due to differences in the learning curves between techniques and ease of instrument exchange with TOETVA. The advantages in visualization and wristed instrumentation may not justify the loss of haptic feedback and increased operative time with the robotic technique for institutions wishing to adopt the transoral vestibular approach. Further studies are needed to better delineate the learning curves of TOETVA and TORTVA and determine the true cost burden of each approach in comparison to the traditional transcervical approach.
Acknowledgments
The authors acknowledge Halley Darrach for her aid in the figure illustrations.
References
- 1.Adam MA, Speicher P, Pura J, et al. Robotic thyroidectomy for cancer in the US: patterns of use and short-term outcomes. Ann Surg Oncol. 2014;21:3859–3864. doi: 10.1245/s10434-014-3838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora A, Swords C, Garas G, et al. The perception of scar cosmesis following thyroid and parathyroid surgery: a prospective cohort study. Int J Surg. 2016;25:38–43. doi: 10.1016/j.ijsu.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Arora A, Garas G, Sharma S, et al. Comparing transaxillary robotic thyroidectomy with conventional surgery in a UK population: a case control study. Int J Surg. 2016;27:110–117. doi: 10.1016/j.ijsu.2016.01.071. [DOI] [PubMed] [Google Scholar]
- 4.Choi Y, Lee JH, Kim YH, et al. Impact of postthyroidectomy scar on the quality of life of thyroid cancer patients. Ann Dermatol. 2014;26(6):693–699. doi: 10.5021/ad.2014.26.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berber E, Bernet V, Fahey TJ, III, et al. American Thyroid Association Statement on Remote-Access Thyroid Surgery. Thyroid. 2016;26(3):331–337. doi: 10.1089/thy.2015.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 7.Yoon JH, Park CH, Chung WY. Gasless endoscopic thyroidectomy via an axillary approach: experience of 30 cases. Surg Laparosc Endosc Percutan Tech. 2006;16(4):226–231. doi: 10.1097/00129689-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Choe JH, Kim SW, Chung KW, et al. Endoscopic thyroidectomy using a new bilateral axillo-breast approach. World J Surg. 2007;31(3):601–606. doi: 10.1007/s00268-006-0481-y. [DOI] [PubMed] [Google Scholar]
- 9.Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: patient selection and technical considerations. Surg Laparosc Endosc Percutan Tech. 2011;21(4):237–242. doi: 10.1097/SLE.0b013e3182266dd6. [DOI] [PubMed] [Google Scholar]
- 10.Miccoli P. Minimally invasive surgery for thyroid and parathyroid diseases. Surg Endosc. 2002;16(1):3–6. doi: 10.1007/s00464-001-8140-8. [DOI] [PubMed] [Google Scholar]
- 11.Bärlehner E, Benhidjeb T. Cervical scarless endoscopic thyroidectomy: axillo-bilateral-breast approach (ABBA) Surg Endosc. 2008;22(1):154–157. doi: 10.1007/s00464-007-9393-7. [DOI] [PubMed] [Google Scholar]
- 12.Jackson NR, Yao L, Tufano RP, Kandil EH. Safety of robotic thyroidectomy approaches: meta-analysis and systematic review. Head Neck. 2014;36(1):137–143. doi: 10.1002/hed.23223. [DOI] [PubMed] [Google Scholar]
- 13.Kandil EH, Noureldine SI, Yao L, Slakey DP. Robotic transaxillary thyroidectomy: an examination of the first one hundred cases. J Am Coll Surg. 2012;214(4):558–564. doi: 10.1016/j.jamcollsurg.2012.01.002. discussion 564–566. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Yun JH, Nam KH, Soh EY, Chung WY. The learning curve for robotic thyroidectomy: a multicenter study. Ann Surg Oncol. 2011;18(1):226–232. doi: 10.1245/s10434-010-1220-z. [DOI] [PubMed] [Google Scholar]
- 15.Ohgami M, Ishii S, Arisawa Y, et al. Scarless endoscopic thyroidectomy: breast approach for better cosmesis. Surg Laparosc Endosc. 2000;10(1):1–4. [PubMed] [Google Scholar]
- 16.Shimazu K, Shiba E, Tamaki Y, et al. Endoscopic thyroid surgery through the axillo-bilateral-breast approach. Surg Laparosc Endosc Percutan Tech. 2003;13(3):196–201. doi: 10.1097/00129689-200306000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Terris DJ, Singer MC, Seybt MW. Robotic facelift thyroidectomy: II. Clinical feasibility and safety. Laryngoscope. 2011;121(8):1636–1641. doi: 10.1002/lary.21832. [DOI] [PubMed] [Google Scholar]
- 18.Kim HY, d’Ajello F, Woo SU, Son GS, Lee JB, Bae JW. Robotic thyroid surgery using bilateral axillo-breast approach: personal initial experience over two years. Minerva Chir. 2012;67(1):39–48. [PubMed] [Google Scholar]
- 19.Richmon JD, Holsinger FC, Kandil E, Moore MW, Garcia JA, Tufano RP. Transoral robotic-assisted thyroidectomy with central neck dissection: preclinical cadaver feasibility study and proposed surgical technique. J Robot Surg. 2011;5(4):279–282. doi: 10.1007/s11701-011-0287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anuwong A. Transoral endoscopic thyroidectomy vestibular approach: a series of the first 60 human cases. World J Surg. 2016;40(3):491–497. doi: 10.1007/s00268-015-3320-1. [DOI] [PubMed] [Google Scholar]
- 21.Russell JO, Clark J, Noureldine SI, et al. Transoral thyroidectomy and parathyroidectomy – a North American series of robotic and endoscopic transoral approaches to the central neck. Oral Oncol. 2017;71:75–80. doi: 10.1016/j.oraloncology.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Richmon JD, Kim HY. Transoral robotic thyroidectomy (TORT): procedures and outcomes. Gland Surg. 2017;6(3):285–289. doi: 10.21037/gs.2017.05.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HY, Chai YJ, Dionigi G, Anuwong A, Richmon JD. Transoral robotic thyroidectomy: lessons learned from an initial consecutive series of 24 patients. Surg Endosc. 2017;32(2):688–694. doi: 10.1007/s00464-017-5724-5. [DOI] [PubMed] [Google Scholar]
- 24.Anuwong A, Ketwong K, Jitpratoom P, Sasanakietkul T, Duh QY. Safety and outcomes of the transoral endoscopic thyroidectomy vestibular approach. JAMA Surg. 2018;153(1):21–27. doi: 10.1001/jamasurg.2017.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell JO, Noureldine SI, Al Khadem MG, et al. Transoral robotic thyroidectomy: a preclinical feasibility study using the da Vinci Xi platform. J Robot Surg. 2017;11(3):341–346. doi: 10.1007/s11701-016-0661-1. [DOI] [PubMed] [Google Scholar]
- 26.Dionigi G, Tufano RP, Russell J, Kim HY, Piantanida E, Anuwong A. Transoral thyroidectomy: advantages and limitations. J Endocrinol Invest. 2017;40(11):1259–1263. doi: 10.1007/s40618-017-0676-0. [DOI] [PubMed] [Google Scholar]
- 27.Dionigi G, Lavazza M, Wu CW, et al. Transoral thyroidectomy: why is it needed? Gland Surg. 2017;6(3):272–276. doi: 10.21037/gs.2017.03.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jitpratoom P, Ketwong K, Sasanakietkul T, Anuwong A. Transoral endoscopic thyroidectomy vestibular approach (TOETVA) for Graves’ disease: a comparison of surgical results with open thyroidectomy. Gland Surg. 2016;5(6):546–552. doi: 10.21037/gs.2016.11.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark JH, Kim HY, Richmon JD. Transoral robotic thyroid surgery. Gland Surg. 2015;4(5):429–434. doi: 10.3978/j.issn.2227-684X.2015.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang SW, Lee SC, Lee SH, et al. Robotic thyroid surgery using a gasless, transaxillary approach and the da Vinci S system: the operative outcomes of 338 consecutive patients. Surgery. 2009;146(6):1048–1055. doi: 10.1016/j.surg.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Kim WW, Jung JH, Park HY. The learning curve for robotic thyroidectomy using a bilateral axillo-breast approach from the 100 cases. Surg Laparosc Endosc Percutan Tech. 2015;25(5):412–416. doi: 10.1097/SLE.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 32.Barbash GI, Glied SA. New technology and health care costs --- the case of robot-assisted surgery. N Engl J. Med. 2010;363(8):701–704. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]