Abstract
Estradiol and progesterone rapidly induce changes in dopaminergic signaling within the dorsal striatum and nucleus accumbens of female rats. In ovariectomized females, estradiol rapidly enhances dopamine release and modulates binding of dopamine receptors. Progesterone further potentiates the effect of estradiol on dopamine release. The effects of both estradiol and progesterone are time course dependent, with increases in dopamine release immediately after acute hormone administration followed by later inhibition of dopamine release. Importantly, these changes are also seen in naturally cycling females, indicating their importance for normal physiological states and relevant reproductive behaviors. Here, we summarize the literature establishing the rapid effects of estradiol and progesterone on dopamine release and receptor expression in dorsal striatum and nucleus accumbens of both males and females. Integrating this literature with the larger body of work focusing on dopamine regulated behaviors, we propose hypotheses for adaptive reasons (i.e., ultimate causes) as to why changes in ovarian hormones modulate dopamine release. Finally, we note the importance of these studies for understanding sex differences in vulnerability to drug addiction. Research on how dopaminergic systems regulate behavior in both males and females is crucial for developing a full appreciation of dopamine’s role in both natural and drug-induced behaviors.
Introduction
There are established sex differences within the ascending mesotelencephalic dopamine (DA) system. This variation is associated with sex specific psychomotor and motivated behaviors as well as sex differences in the subjective and behavioral responses to drugs of abuse. Sex differences have been established in both the underlying organization of DA circuitry, as well as the effect of the gonadal hormones on DA activity. These sex differences are mediated by rapid effects of the steroid hormone, estradiol, acting directly within the dorsal striatum (Becker, 1990a, 1999; Becker and Beer, 1986) on a sexually dimorphic brain (Becker and Ramirez, 1981a; Perry et al., 2013a). In adult females, but not males, acute treatment with estradiol increases DA release, upregulates D2 DA receptor (DR) binding and DA transporter (DAT) activity, and enhances behavioral responses to DA agonists (Bazzett and Becker, 1994; Becker, 1990b; Calipari et al., 2017; Cummings et al., 2014; Lévesque and Di Paolo, 1988). Although work from the Becker laboratory has focused mainly on the effects of estradiol within dorsal striatum, a similar story has developed regarding the effects of estradiol on DA release within the mesolimbic DA system. Here, we describe evidence for the rapid effects of estradiol on the nigrostriatal and mesolimbic DA systems, and their implications for sex differences in motivated behaviors.
The current review takes a historical perspective on our understanding of the various roles of ovarian hormones in the dorsal striatum and nucleus accumbens and encompasses an overview of the literature in chronological order from the 1970’s until present. This perspective demonstrates how the path to a body of knowledge is not always linear and yet, findings from various research groups over the past few decades have contributed to our current understanding and working model (Figure 1) on how ovarian hormones specifically mediate DA release.
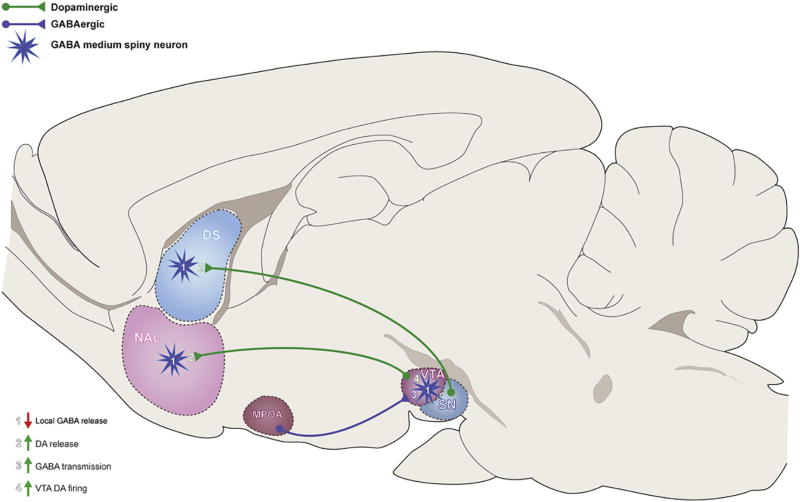
Figure 1.
Proposed model of estradiol (E2) regulation of striatal dopamine (DA) circuitry in females. E2 increases DA release with dorsal striatum by acting directly on GABAergic neurons to decrease GABA release and disinhibit DA terminals. E2 enhances DA release in NAc by acting directly on NAc GABA (1) or DA neurons (2), or by acting on GABAergic neurons in the medial preoptic area (MPOA) to increase inhibitory transmission to GABA interneurons in the VTA (3). Increased inhibition of VTA GABA interneurons leads to decreased inhibitory tone within VTA, and increased DA transmission (4).
Early studies of sex differences in striatal DA release
Much of what is known about the DA system, in general, has come from studies in male subjects. In fact, some of the first studies that identified sex differences within the striatal DA circuitry were actually investigating sex differences in DA release within the hypothalamus, and only used the striatum as a control (Becker and Ramirez, 1980). The expectation was that sex differences in DA release would exist only within the hypothalamus, a brain region that showed strong sexual dimorphisms and was known to coordinate many sexually differentiated functions and behaviors. Surprisingly, there was no sex difference in K+-stimulated DA release from medial basal hypothalamic tissue fragments (Becker and Ramirez, 1980). On the other hand, amphetamine-(AMPH) stimulated DA release from striatal tissue was significantly greater for females compared to males (Becker and Ramirez, 1981b). These studies were some of the first to have demonstrated that females showed differential regulation of neural activity outside of structures primarily implicated in reproduction.
Gonadal hormones and estrous cycle mediate sex differences in dopamine release
Understanding how DA release within the striatum is different in males and females also requires understanding how general differences in the physiology of males and females may influence differences in neural functioning. One of the most striking sex differences in animal physiology is the regulation of gonadal hormone release. While release of testosterone in males is under tonic, homeostatic control, showing slight circadian rhythms, release of female gonadal hormones is much more complex. Adult female rodents show cyclic release of estradiol and progesterone over a four or five-day ovulatory cycle (Figure 2b). During diestrus and metestrus, levels of estradiol and progesterone are low. As levels of estradiol and progesterone rise, females enter proestrus, until finally levels of ovarian hormones peak and then begin to decline during estrus.
Figure 2.
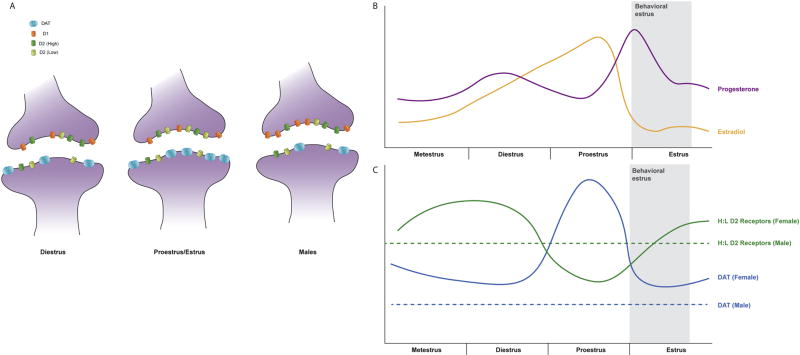
Distribution of dopamine (DA) receptors and dopamine transporter (DAT;
 ) varies by sex and reproductive state. Expression of DAT is higher in females (c; solid blue line) than males (c; dashed blue line) regardless of estrous cycle stage, and increases the morning of proestrus, when levels of estradiol (E2) are peaking and levels of progesterone (P) begin to rise (b). Males have greater expression of D1 DA receptors (
) varies by sex and reproductive state. Expression of DAT is higher in females (c; solid blue line) than males (c; dashed blue line) regardless of estrous cycle stage, and increases the morning of proestrus, when levels of estradiol (E2) are peaking and levels of progesterone (P) begin to rise (b). Males have greater expression of D1 DA receptors (
 ) in females, and D1 DA receptor expression does not change across the estrous cycle in females (a). Expression of D2 DA receptors is constant across the estrous cycle (a), but the ratio of high to low D2 DA receptors is dependent on both sex and reproductive state (c). Diestrus females have a higher level of high (
) in females, and D1 DA receptor expression does not change across the estrous cycle in females (a). Expression of D2 DA receptors is constant across the estrous cycle (a), but the ratio of high to low D2 DA receptors is dependent on both sex and reproductive state (c). Diestrus females have a higher level of high (
 ) vs low (
) vs low (
 ) D2 DA receptors than males (dashed green line), but as E2 and P rise during proestrus, the ratio of high to low D2 DA receptors in females (solid green line) decreases to lower than observed in intact males.
) D2 DA receptors than males (dashed green line), but as E2 and P rise during proestrus, the ratio of high to low D2 DA receptors in females (solid green line) decreases to lower than observed in intact males.
Sex differences in DA release from striatal tissue were only observed in estrous females, while proestrous females showed similar levels of DA release to males. Further investigation demonstrated the role of the gonadal hormones, estradiol and progesterone, in this sex difference; ovariectomized (OVX) females showed significantly reduced AMPH-stimulated DA release from striatal tissue, and treatment with estradiol benzoate (EB) for four days followed by progesterone 4 hours prior to obtaining brain tissue, to mimic endogenous hormone release on estrus, restored the sex difference seen in intact animals (Becker and Ramirez, 1981b).
Interestingly, treatment with EB for 4 days (last treatment 24 hours prior to test), or progesterone 4 hours prior to test (without EB priming), only slightly increased the release of DA from striatal tissue. This demonstrated that the pattern of release of both hormones over the course of the estrous cycle contributes to the sex difference in AMPH-stimulated DA release. Importantly, this effect was not unique to AMPH induced DA release, progesterone treatment in estradiol primed females also enhanced K+-stimulated DA release (Becker et al., 1984). These early studies not only verified that sex differences exist within the striatal DA response to synaptic activity, but also that in females, striatal DA release is modulated by circulating gonadal hormones over the course of the estrous cycle. In addition, the ability of estradiol to enhance both AMPH-and K+ -stimulated DA release indicates that rather than specifically enhancing vesicular release, as induced by K+, or non-vesicular release, as induced by AMPH, gonadal hormones induce a general enhancement of excitability of DA terminals.
Sex differences in drug-induced behaviors
One way to assess functional activity in DA systems was to use rotational behavior as an index of unilateral DA activity. Unilateral electrical stimulation of the ascending mesostriatal pathway induces turning away from the side that is stimulated. Following unilateral dorsal striatum DA denervation with a monoamine-selective neurotoxin, treatment with AMPH induces DA release only from the remaining intact DA terminals, and animals turn in circles away from the intact side and towards the side of the lesion. The intensity of the rotational behavior was related to greater DA release from the intact DA terminals, and was used as a proxy for the magnitude of DA release (Robinson et al., 1980).
Females showed greater AMPH-induced rotational behavior, compared with males, both at baseline and after unilateral lesions of dopaminergic cells within the substantia nigra (Becker and Beer, 1986; Robinson et al., 1980). Importantly, sex differences in rotational behavior were also dependent on gonadal hormones. Rotational behavior induced by unilateral electrical stimulation of the ascending mesostriatal pathway was greatest in estrous females compared to those in diestrus, and similar results were seen after AMPH administration, even when controlling for sex differences in pharmacokinetic factors (Becker et al., 1982; Robinson et al., 1982). OVX reduced rotational behavior elicited by both AMPH and electrical stimulation, and treatment with estradiol for four consecutive days increased the number of rotations made after AMPH administration to levels seen in intact females (Becker and Beer, 1986; Robinson et al., 1982). This increase in rotational behavior was seen both four hours and four days after cessation of estradiol treatment, but not 24 hours after, suggesting that multiple mechanisms contributed to the effect of estradiol on DA mediated behaviors. In fact, increases in DA release after estradiol treatment were only seen four hours after cessation of estradiol treatment, but not four days later, indicating that while increased DA release may account for changes in rotational behavior four hours following estradiol administration, later increases in rotational behavior are mediated by a different mechanism (Becker and Beer, 1986).
Rapid effects of estradiol on striatum
Further clues to one potential mechanism of estradiol enhancement of DA release came from observations that estradiol significantly altered DA activity within minutes; not on the timescale that would be expected of a typical genomic mechanism (Becker, 1990a, 1990b). Treatment with estradiol, 30 minutes prior to AMPH administration, significantly increased rotational behavior in OVX females with unilateral striatal DA lesions and this was correlated with an increase in AMPH- stimulated DA release within the intact striatum (Becker, 1990b). In addition, rapid potentiation of stimulated DA release was observed after direct application of estradiol to striatal tissue of OVX rats in vitro, indicating that estradiol acts directly on striatal tissue to rapidly enhance stimulated DA release (Becker, 1990a). In these experiments, 17β-estradiol, the most physiologically ative estrogen, and the non-steroidal estradiol analogue diethylstilbesterol both significantly increased AMPH-stimulated DA release from superfused striatal tissue from OVX female, but not castrated (CAST) male rats (Becker, 1990a). The less potent 17α-estradiol also slightly increased AMPH induced DA release in females, but this effect did not reach significance (Becker, 1990a).
Similar findings were reported when measuring K+-induced DA release. In a series of experiments, superfused striatal tissue from OVX females treated with 100 pg/ml 17β-estradiol at a rate of 100 µl/min showed increased K+-stimulated DA release, while 1000 pg/ml 17β-estradiol reduced K+-induced DA release. This rapid and direct effect of estradiol was only seen in females, and also only observed after pulsatile administration of estradiol; continuous administration of estradiol over the same period of time had no effect (Becker, 1990a). In these experiments, there was no effect of estradiol on basal DA release, indicating that estradiol selectively enhanced the excitability of DA terminals without directly inducing DA release.
Membrane receptors mediate rapid effects in striatum
At this time, it was well established that estradiol was able to modulate DA functional activity, but the specific mechanism involved was still unclear. The acute timescale by which estradiol enhanced DA release, combined with the lack of evidence for nuclear receptors within the striatum (Pfaff and Keiner, 1973), led to the development of the hypothesis that estradiol enhances DA activity by acting on membrane receptors within the dorsal striatum (Becker, 1990a).
The prediction that estradiol was acting via membrane associated receptors on dorsal striatal neurons was verified in a series of experiments using whole-cell clamp electrophysiology. These experiments demonstrated that estradiol rapidly and dose-dependently reduced Ca2+ currents via L-type Ca2+ channels in medium spiny striatal neurons (MSNs). This effect was replicated using estradiol conjugated to bovine serum albumin (BSA), a conformation that cannot penetrate the cellular membrane (Mermelstein et al., 1996). Furthermore, delivering 100 pM estradiol intracellularly, to saturate intracellular receptors, did not disrupt the ability of 1 pM estradiol, applied extracellularly, to decrease Ca2+ current, demonstrating that estradiol was acting extracellularly at the membrane. These studies also established that estradiol exerts these effects through a G-protein-coupled-receptor (GPCR), as application of GTPγS (a drug that prevents inactivation of G-protein-mediated events) prevented the reversal of estradiol attenuation of Ca2+ currents. This study was carried out on GABAergic MSNs, leading to the conclusion that estradiol inhibits Ca2+ currents on GABAergic MSNs via a membrane GPCR (Mermelstein et al., 1996). This lead to the hypothesis that estradiol enhances DA release in dorsal striatum by inhibition of GABA release.
Experiments using in vivo microdialysis went on to show that estradiol inhibited K+-stimulated GABA release within the dorsal striatum (Hu et al., 2006). Additionally, overexpression of estradiol receptor alpha (ERα) in dorsal lateral striatum enhanced the effects of estradiol to inhibit K+-stimulated GABA release (Schultz et al., 2009). In vivo experiments also verified that a membrane receptor was responsible for the effect of estradiol on AMPH-stimulated DA release, demonstrating that this mechanism likely played a role in the behavioral effects of estradiol on DA mediated behaviors (Xiao and Becker, 1998). More recent work has established that application of estradiol directly to the striatum, but not the medial prefrontal cortex (mPFC) or substantia nigra (SN) enhanced DA release induced by both AMPH administration as well as direct electrical stimulation, in OVX female rats (Shams et al., 2016; Shams et al., 2017). Taken together, these findings demonstrate that one mechanism by which estradiol enhances DA release within the dorsal striatum is through direct inhibition of GABAergic MSNs, which tonically inhibit the release of DA from nigrostriatal terminals, leading to disinhibition of DA release and elevated stimulated extracellular DA concentrations (Figure 3).
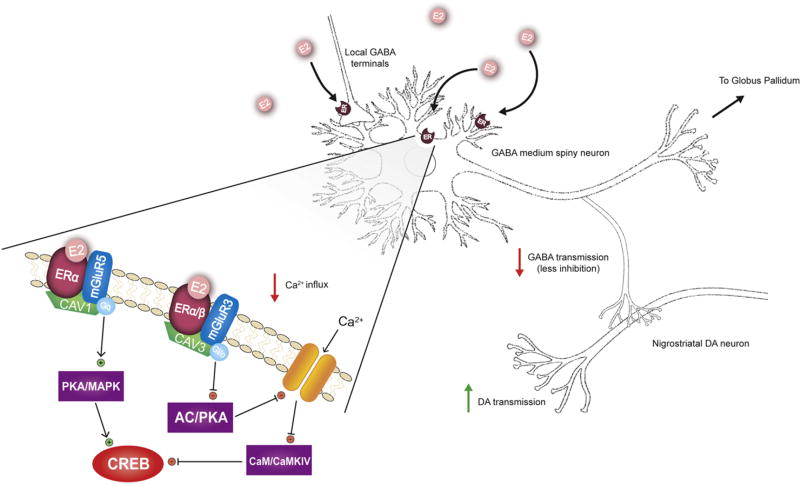
Figure 3.
Proposed mechanism for estradiol (E2) enhancement of dopamine (DA)release within dorsal striatum. Activation of estradiol receptors (ER) located on soma, dendrites, or axonal terminals of GABAergic medium spiny neurons (MSNs) leads to decreased Ca2+ release and disinhibition of DA release from nigrostriatal terminals. The expanded view of the plasma membrane depicts that ERs are anchored to the plasma membrane via caveolin proteins (CAV). This positioning allows functional coupling of ERs to metabotropic glutamate receptors (mGluR). Within striatum, ERα is coupled to both Group I (mGluR5) and Group II (mGluR3) mGluRs, whereas ERβ is coupled to only group II mGluRs. Activation of ERs coupled to Group I mGluRs results in activation of protein kinase A (PKA)/mitogen-activated protein kinase (MAPK) pathways, leading to increased activation of cAMP response binding proteins (CREB). Alternatively, activation of ERs coupled to Group II mGluRs inhibits CREB via inhibition of adenylate cyclase (AC)/PKA pathways and decreased Ca2+ influx, resulting in decreased activity of calmodulin (CaM) and calcium/calmodulin dependent kinase IV.
Effects of estradiol on dopamine receptor activity: dose and time course effects
At the same time as the effects of estradiol on DA release were being worked out, a complementary story regarding the effects of estradiol on post-synaptic DA activity was also emerging. An early study from Kumakura and colleagues (1979) found that the ability of DA to elicit adenylate cyclase activity and cylic adenosine 3’,5’ monophosphate (cAMP) generation was significantly reduced four and six weeks following ovariectomy suggesting that estradiol alters the effects of DA at its receptor(s) in addition to altering DA release.
A subsequent series of experiments investigating the effects of estradiol on apomorphine induced stereotypy found dose and time course dependent sex differences in the effects of estradiol on post-synaptic DA efficacy. Apomorphine, a non-selective DA receptor agonist with greater affinity for D2 receptors, compared to D1 receptors (Sokoloff et al., 1980), induces general locomotor activity and stereotyped rearing, sniffing, and gnawing in a dose-dependent way. OVX enhanced the effect of apomorphine on stereotyped behavior six months post-surgery, and treatment of OVX female rats with estradiol for three days reduced the efficacy of apomorphine to induce stereotyped behavior (Gordon et al., 1980). This early study indicated that in addition to enhancing DA release, estradiol may also enhance the sensitivity of DA receptors within dorsal striatum. Similar to the effect of estradiol on DA release, changes in DA receptor sensitivity were dose- and time-dependent; while short term treatment with estradiol reduced apomorphine induced stereotypy, chronic treatment over 20 days had no effect (Gordon et al., 1980). Interestingly, OVX females treated with estradiol for 14 days showed enhanced sensitivity to apomorphine six days after cessation of treatment, drawing parallels to findings that AMPH induced rotational behavior was enhanced four days following cessation of hormone treatment (Becker and Beer, 1986; Gordon et al., 1980).
Further investigation found that efficacy of apomorphine was reduced 24 hours after a three day regimen of estradiol administration, but was enhanced 2–7 days post treatment (Gordon, 1980). These changes in the behavioral response to apomorphine following estradiol treatment were associated with changes in the binding affinity of DA receptors in vitro. Repeated treatment with estradiol for three days resulted in reduced DA receptor binding affinity 24 hours later but no changes in presynaptic DA sensitivity, as measured by inhibition of tyrosine hydroxylase (TH) by apomorphine (Gordon and Perry, 1983). This indicates that while estradiol treatment reduces the sensitivity of DA receptors on the post-synaptic membrane, reduction in apomorphine efficacy 24 hours after estradiol administration are not linked to changes in the effects of presynaptic DA receptor activation. Conversely, three days following hormone treatment there was both an increase in the affinity of DA receptors post-synaptically and enhanced sensitivity to the effects of apomorphine on TH (Gordon and Perry, 1983).
These studies draw parallels to the findings of Becker and Beer (1986) which showed that treatment with estradiol enhances the effect of AMPH on rotational behavior four hours or four days after cessation of treatment, but not 24 hours later. In addition, these changes in rotational behavior were only associated with enhanced DA release at the initial four-hour time point. It is possible that initial changes in DA release then lead to secondary changes in DA receptor sensitivity: first an initial reduction in DA receptor sensitivity after 24 hours, and then a compensatory enhancement 3–4 days later.
In support of this interpretation, changes in GABAergic signaling thought to underlie the enhanced DA release seen after estradiol treatment (Figure 3) also correlate with these changes in DA receptor activity. Glutamic acid decarboxylase (GAD), the enzyme that converts glutamate to GABA, shows changes in activity within the substantia nigra (SN) concurrent to changes in DA receptor sensitivity (Gordon and Perry, 1983). GAD activity was decreased when DA receptor binding affinity was low, and increased when DA receptor binding affinity was high (Gordon and Perry, 1983). These authors interpreted their finding as a compensatory response to changes in DA activity, it is also possible that estradiol modulation of GABA activity within striatal circuitry induced homeostatic alterations in DA release and DA receptor functioning, as described previously.
Effects of estradiol on DA receptor sensitivity in males
While studies on estradiol modulation of DA release in males found limited effects of estradiol, there is an effect of estradiol on DA receptor sensitivity in males. Similar to OVX females, intact male rats given a single injection of estradiol valerate, that is metabolized slowly over 6 days in the rat, also showed enhanced sensitivity to the stereotypy inducing effects of apomorphine six days later, as well as increased duration of rotational behavior induced by AMPH (Hruska and Silbergeld, 1980a). Males, however, did not show the acute reduction in DA receptor sensitivity seen in females, or changes in DA receptor binding affinity (Hruska et al., 1980; Hruska and Silbergeld, 1980b). Changes in DA receptor sensitivity after estradiol valerate treatment in males were instead due to changes in the expression of DA receptors within striatum, where DA receptor expression (Bmax) was increased starting three days after hormone administration (Hruska et al., 1980; Hruska and Silbergeld, 1980b). Interestingly, these changes in DA receptor sensitivity in males were abolished following hypophysectomy, indicating that in males estradiol indirectly affected DA receptor expression, perhaps via prolactin (Hruska et al., 1980).
Differential effects of estradiol on dopamine receptor subtypes
Although the studies described here have been interpreted as showing changes in overall DA receptor sensitivity and expression, follow up studies have demonstrated that estradiol modulation of DA receptor binding differs with the specific DA receptor subtype. D1 DA receptors are expressed post-synaptically and activate adenylate cyclase, D2 DA receptor is expressed post-synaptically where it inhibits adenylate cyclase or has no effect, and pre-synaptically as an autoreceptor (Clark and White, 1987; Meador-Woodruff et al., 1991). As mentioned previously, apomorphine preferentially binds to D2 DA receptors, as does sulpiride, the ligand used in many studies of estradiol modulation of DA receptor binding. However, changes in ligand binding may indicate changes in binding affinity, as opposed to changes in receptor expression. D2 DA receptors exist in both high and low agonist affinity states, and the ratio of high to low affinity D2 DA receptors may explain some of the observed changes in DA receptor affinity following treatment with estradiol. In intact females, the ratio of high to low D2 DA receptor binding sites rapidly declines during the transition from diestrus to proestrus, when levels of estradiol are increasing (Figure 2); OVX increases the ratio of high vs low affinity D2 DA receptor compared to both intact females and CAST males (Bazzett and Becker, 1994; Di Paolo et al., 1988). These studies indicated that the effect of estradiol on DA receptor binding described previously may be specific to changes in D2 DA receptor, and specifically the presence of high vs low affinity D2 DA receptors. Increasing estradiol during proestrus reduced overall DA receptor binding and the efficacy of DA agonists by reducing the proportion of high to low affinity DA receptors, whereas removal of estradiol by OVX had the opposite effect.
The hyposensitivity to DAergic agonists induced 24 hours after repeated estradiol treatment was also accompanied by a reduction in the number of high affinity D2 DA receptors without any changes in overall receptor density, similar to changes in DA receptor binding seen in proestrus females (Clopton and Gordon, 1986; Di Paolo et al., 1988). Changes in D2 DA receptor binding during the hypersensitive period three days after cessation of estradiol treatment, however, was not associated with changes in D2 DA receptor affinity state, but an overall increase in the expression of D2 DA receptor within the striatum (Clopton and Gordon, 1986). These studies indicate that cessation of estradiol treatment resulted in an initial increase in the ratio of high affinity D2 DA receptor binding sites, followed by a subsequent increase in the overall expression of D2 DA receptors.
This is again consistent with a compensatory model, where changes in DA receptor binding is secondary to changes in DA release. As estradiol increases during proestrus or after exogenous hormone treatment, there is an initial decrease in the ratio of high to low affinity D2 DA receptor in response to enhanced DA release. After estradiol is removed, either by ovariectomy or cessation of treatment, the decrease in DA release onto a now hyposensitive DA synapse leads to a second, protracted compensatory response to increase overall receptor expression.
While these experiments demonstrated an effect of estradiol days after hormone administration, investigation of changes in D2 DA receptor affinity state on a shorter timescale revealed more acute and transient effects of estradiol on DA receptor binding. On the other hand, Levesque and Di Paolo (1988) found that the percentage of D2 DA receptors in the high affinity state was reduced 30 minutes following a single injection of estradiol to OVX female rats. This acute reduction, however, was not seen one or four hours after hormone treatment, and so may represent a different phenomenon than the effects seen 24 hours or three days after repeated estradiol administration. (Bazzett and Becker, 1994; Lévesque and Di Paolo, 1988).
Sex differences in the acute effects of estradiol on DA receptor subtypes
When sex differences in the acute effect of estradiol on D2 DA receptor binding are studied, males show no effect of estradiol on D2 DA receptor binding 30 minutes after treatment, but do show reductions in overall D2 DA receptor binding four hours later (Bazzett and Becker, 1994). Sex differences are also seen in the expression and binding of D1 DA receptor (Figure 2a). Expression of D1 DA receptor is higher in males than in females, and in females D1 DA receptor expression is dependent on the presence of circulating ovarian hormones (Lévesque and Di Paolo, 1990). OVX leads to an initial increase in D1 DA receptor expression, followed by a progressive decline in expression 15 days post OVX (Lévesque and Di Paolo, 1990). Interestingly, the initial increase was only observed in animals that were OVX during proestrus or estrus, and not diestrus, indicating that it was the rapid decline in levels of gonadal hormones that was responsible for the observed changes in D1DA receptor expression (Lévesque and Di Paolo, 1990). Estradiol treatment also regulated downstream elements of DA receptor signaling. Estradiol reduced D2DA receptor inhibition, while elevating D1DA receptor activation, of adenylate cyclase (Maus et al., 1989). As previously mentioned, OVX reduced DA induced adenylate cyclase and cAMP, indicating that these changes in the overall effects of estradiol on DA receptor signaling are mediated by combined regulation of D1 and D2 DA receptor activity (Kumakura et al., 1979).
Estradiol affects dopamine turnover within striatum
In addition to changes in DA receptor function, changes in levels of circulating estradiol have also been associated with changes in DA turnover, either through metabolism or reuptake by DA transporters (DAT). In experiments by DiPaolo and colleagues, acute estradiol reduced DA turnover via reuptake and DA metabolites were upregulated. It is unlikely that this is solely due to increased DA release, as the DA metabolite homovanillic acid was unchanged after estradiol treatment, indicating a selective effect on specific metabolic pathways (Di Paolo et al., 1985; Morissette and Di Paolo, 1993). Estradiol has also been found to rapidly increase concentrations of the DA metabolite dihydroxyphenylacetic acid (DOPAC), although this increase was linked to changes in DA synthesis, rather than increased release (Pasqualini et al., 1995). Experiments by Pasqualini and colleagues (1995) found that administration of physiological doses of estradiol enhanced the synthesis of DA and DOPAC without altering overall concentrations within the striatum. This was proposed to be mediated by a decrease in the inhibitory effects of DA on TH, perhaps via phosphorylation of the enzyme (Pasqualini et al., 1995).
A sex difference has been shown in DAT expression, where there was a lower DAT density in males than in intact females, and in females OVX reduced DAT binding to levels comparable to those seen in males (Morissette and Di Paolo, 1993). In intact females, DAT expression was shown to be dependent on estrous cycle phase, with the highest levels of DAT binding occurring on the morning of proestrus when estradiol is elevated (Morissette and Di Paolo, 1993). These changes in DAT activity were specifically linked to the effects of estradiol, as treating OVX females with estradiol prevented the OVX induced decrease in DAT expression (Bossé et al., 1997; Chavez et al., 2010). These changes in the activity of DAT and D2 DA receptor activity may be coordinated, as activation of D2 receptors by DA release is modulated by DAT activity (Marcott et al., 2014). As illustrated in Figure 2, when estradiol is elevated, DAT increases and the ratio of H:L D2 DA receptors decreases.
In summary, while many of the changes in DA receptor binding and DA turnover described here have been interpreted as being separate from the potentiation of DA release by estradiol, it is also possible that these changes occur as a compensatory response to changes in DA release. Changes in D2 DA receptors and DAT are not accompanied by changes in levels of mRNA, and instead may represent homeostatic responses to increased DA availability in the synapse, rather than be mediated by a separate mechanism.
Identification of estradiol receptors α and β, & GPER-1
At the time that these hypotheses were initially being developed, there was speculation that membrane estradiol receptors could modulate a number of cellular functions, but definitive evidence had not been found. Soon after, the existence of membrane estradiol receptors within the central nervous system were validated (Zheng and Ramirez, 1997). At the same time, a novel isoform of estradiol receptor was characterized (Mosselman et al., 1996). These two receptor isoforms, ERα and the newly identified, ERβ, were then cloned and transfected into Chinese hamster ovary cells, which did not normally express estradiol receptors, to allow for the characterization of membrane vs. nuclear mediated effects (Razandi et al., 1999). These transfected cells were responsive to estradiol conjugated to BSA, and activation of both ERα and ERβ by 17β-estradiol stimulated production of inositol 1,4,5-triphosphate (IP3) and cAMP, indicating that at the membrane, estradiol was acting through G-protein coupled pathways (Razandi et al., 1999).
The localization of classical ERs to the plasma membrane is dependent on their association with caveolin proteins, scaffolding proteins that mediate the association of various membrane bound signaling molecules (Evinger and Levin, 2005). Modification of ERs by palmitoylation, or the addition of covalent fatty acid groups, of specific C terminus amino acid residues, results in association of ERs with caveolin proteins, which then facilitate their trafficking to the plasma membrane (Luoma et al., 2008; Razandi et al., 2003).
Within the striatum, caveolin proteins mediated the coupling of ERα and ERβ to membrane bound metabotropic glutamate receptors (mGluRs), allowing for rapid modulation of cellular activity (Micevych and Mermelstein, 2008). Caveolin 1 (CAV1) is associated with mGluR1, while caveolin 3 (CAV3) is associated with mGluR2/3 (Luoma et al., 2008). On striatal neurons, ERα was functionally coupled to both group I and group II mGluRs, leading to activation of Gq or Gi/o subunits and activation or inhibition of CREB phosphorylation, respectively (Boulware et al., 2005; Grove-Strawser et al., 2010) . Specifically, ERα enhancement of CREB phosphorylation was dependent on mGluR5 activation of MAPK signaling, while ERα coupled to mGluR2/3 attenuates L-type Ca2+ mediated CREB phosphorylation (Figure 3; (Grove-Strawser et al., 2010). Alternatively, ERβ is coupled only to group II mGluRs, and therefore only acted to decrease CREB phosphorylation (Grove-Strawser et al., 2010).
In addition, the estradiol responsive G-protein receptor-30 (GPR-30, now known as G-protein estradiol receptor-1; GPER-1), is also found on both GABAergic MSNs as well as cholinergic interneurons in dorsal striatum (Almey et al., 2016, 2012). GPER-1 is coupled to a Gαs subunit, and activates both the protein Kinase A (PKA) and extracellular signal-regulated kinase (ERK) signaling pathways (Hadjimarkou and Vasudevan, 2017). Finally, activation of GPER-1 also rapidly induces Ca2+ influx, and interactions between GPER-1 and classical estradiol receptors are also reported (Brailoiu et al., 2007; Hadjimarkou and Vasudevan, 2017).
Multiple estradiol receptor subtypes contribute to effects of estradiol on DA activity
A full understanding of the relative contributions of each estradiol receptor subtype to the effects of estradiol on DA systems is still under investigation. Estradiol enhancement of AMPH stimulated DA release was prevented by ICI 182,780 (Xiao et al., 2003) an antagonist for ERα and ERβ. It has recently been found that ICI 182,780 is also an agonist for GPER-1, and this suggests that GPER-1 is not directly responsible for the effects of estradiol on DA release in dorsal striatum (Xiao et al., 2003). ERβ has been linked to the facilitation of AMPH-induced place preference (Boulware et al., 2005; Schultz et al., 2009; Silverman and Koenig, 2007). Selective activation of ERβ, but not ERα, also prevents the OVX induced decrease in both D2 DA receptor and DAT expression. (Le Saux et al., 2006; Morissette et al., 2008). However, estradiol facilitation of AMPH sensitization is dependent on mGluR5, a group I mGluR that only couples to ERα, and overexpression of ERα within the dorsal striatum enhances the effect of estradiol on K+ stimulated GABA release (Martinez et al., 2014; Schultz et al., 2009). These results indicate that both ERα and ERβ are involved in estradiol regulation of DA signaling, although the specific mechanisms involved have not been fully elucidated.
Estrous cycle modulation of NAc DA activity
Comparatively less work has focused on estradiol’s ability to acutely modulate DA release within the ventral striatum, although a full understanding of this system is also being developed. The ventral striatum is comprised of dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAc). Within the NAc, ERα and GPER-1 are expressed on both DA terminals and GABA MSNs, while ERβ has been found on VTA cell bodies that project to the NAc (Almey et al., 2015; Creutz and Kritzer, 2002). In the NAc, K+ stimulated DA release varies with estrous cycle in females, with the greatest release during proestrus (Thompson and Moss, 1997). Firing of VTA DA neurons is also modulated by the estrous cycle; electrophysiological recordings from DA neurons within the VTA of estrous females have shown increases in the overall firing rate as well as specific increases in burst firing, a pattern of activation that is particularly important for increases in NAc DA release (Calipari et al., 2017). Estrous females also showed increased phasic DA release at baseline and in response to cocaine when compared to males and diestrous females (Calipari et al., 2017). It is clear that estradiol regulates DA release within the NAc in additional to the dorsal striatum, although the specific mechanisms involved may show important differences.
Rapid effects of estradiol on DA release in NAc
Estrous cycle modulation of NAc DA release is mediated by estradiol. Previous studies from our lab have failed to demonstrate an acute effect of estradiol on NAc DA release using in vivo microdialysis with the probe at the core/shell interface (Cummings et al., 2014). Recent work has shown that systemic administration of estradiol benzoate or the ERβ selective agonist diarylpropionitrile (DPN) enhances the effects of cocaine on electrically stimulated DA release in the NAc shell of OVX females but not CAST males (Cummings et al., 2014; Yoest et al., 2016). The differences in results are likely due to methodological differences between the two studies. Cummings and colleagues (2014) found no effect of estradiol when measuring the average DA release using microdialysis over the course of minutes, however, measurement of phasic DA release with fast-scan cyclic voltammetry (FSCV) over the course of seconds, reveals rapid effects of acute estradiol treatment (Cummings et al., 2014; Yoest et al., 2016).
In addition to these studies, which measured changes in DA release after systemic hormone administration, there is also evidence that estradiol acts directly on ventral striatal circuitry. Local, acute administration of estradiol to the NAc enhances K+ stimulated release within 2 minutes, and these changes were accompanied by reductions in DA reuptake 15 min after estradiol administration (Thompson and Moss, 1994). However, there was no effect of estradiol on cocaine induced DA release in slices of normally cycling females (Calipari et al., 2017; Cummings et al., 2014). Again, these conflicting findings may be due to methodological differences. In the experiments performed by Calipari, Juarez, and colleagues (2017) brain slices were treated with a much higher dose of estradiol than the physiologically relevant doses applied to the NAc of anesthetized animals by Thompson and Moss (1994). As mentioned previously, there is an inverted U-shaped dose-response curve for the effects of estradiol, with high doses inhibiting DA functional activity.
Effects of estradiol on NAc DA functional activity
Similar to the dorsal striatum, estradiol dependent changes in D2 DA receptor and DAT expression have been seen within the NAc, and estradiol enhancement of AMPH induced place preference is accompanied by decreases in activity of regulator of G-protein signaling 9-2 (RGS9-2) a protein that regulates D2 DA receptor signaling (Figure 4; (Chavez et al., 2010; Morissette et al., 2008; Silverman and Koenig, 2007). Chronic treatment with either estradiol or the ERβ selective agonist DPN prevented a decrease in NAc D2 DA receptor induced by OVX, and ERβ mediate effects of estradiol on RGS9-2 (Le Saux et al., 2006; Silverman and Koenig, 2007). Overall DAT expression does not change over the course of the estrous cycle, but estrous females have shown to have significantly higher levels of phosphorylated DAT compared to both diestrous females and males; this has been associated with enhanced inhibition of DA reuptake after cocaine administration (Calipari et al., 2017).
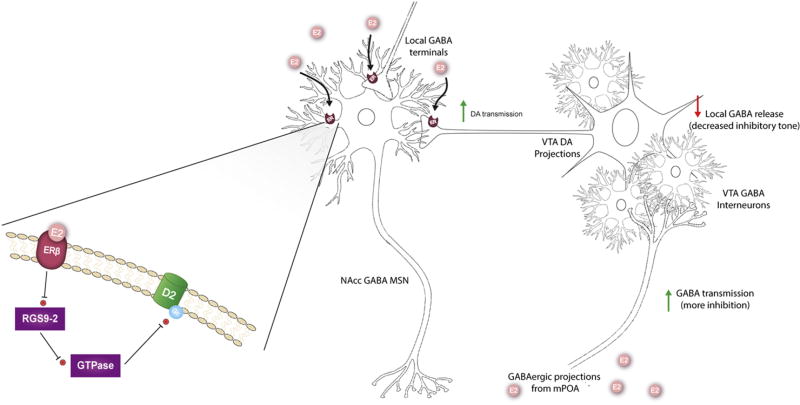
Figure 4.
Proposed mechanism for estradiol (E2) enhancement of dopamine (DA) release within nucleus accumbens (NAc). Activation of estradiol receptors (ER) located on GABAergic medium spiny neurons leads to disinhibition of DA release. ER are also expressed on pre-synaptic DA terminals within NAc. The expanded view of the plasma membrane depicts how ERβ activation decreases activity of Regulator of G-protein signaling 9-2, which leads to decreased inhibition of GTPases involved in deactivation of D2 DA receptor subunits, and ultimately increased D2 DA receptor signaling. The localization of this process to specific neuron populations within the NAcc is unknown. Alternatively, E2 can activate GABAergic projections to the ventral tegmental area (VTA) from the medial preoptic area (MPOA), which leads to decreased inhibitory tone within the VTA and increased DA transmission.
Due to the comparatively smaller body of research focusing on the ventral striatum, a conceptual model of how estradiol modulates DA release within NAc is still under investigation. The ability of estradiol to act directly on NAc to rapidly enhance DA release suggests the possibility that estradiol regulation of DA release in this brain area is similar to the mechanism seen in dorsal striatum (Figure 4). It is also possible that estradiol acts within the VTA directly to enhance cell firing, or via estradiol responsive hypothalamic projections that alter cellular activity within ventral striatal circuitry, or a combination of these mechanisms (Figure 4). With estradiol modulation of inputs from these different brain regions to the NAc, the NAc DA response may exhibit greater sensitivity to context in females, relative to dorsal striatum.
Hypothalamic contributions to estradiol effects on DA release
The medial preoptic area (MPOA) may regulate some of the effects of estradiol on ventral striatal DA activity. ER positive cells in the MPOA have been shown to project to the VTA, and inhibit DA release in the NAc (Will et al., 2016). The effect of estradiol on DA release in response to cocaine was diminished after lesions of the MPOA, and estradiol microinjections in the MPOA enhanced cocaine induced DA release in female rats (Tobiansky et al., 2015). GABAergic neurotensin neurons within the MPOA express ERα and are dynamically regulated by changes in reproductive state (McHenry et al., 2017). This circuit, which was linked to hormonal regulation of social reward, inhibited VTA GABA neurons, leading to increases in firing of DA neurons during proestrus and estrus (Figure 4; (McHenry et al., 2017). Increases in basal activity of VTA DA neurons led to increases in DAT phosphorylation and responsiveness to cocaine in estrous females (Calipari et al., 2017). Taken together, these results provide compelling evidence that the MPOA may regulate at least some of the effects of estradiol on ventral striatal DA release.
The results of previous studies that showed a direct effect of estradiol within the NAc, however, suggested that there are multiple mechanisms by which estradiol regulates DA release within females. (Figure 4). Importantly, studies that have shown a direct effect of estradiol within the NAc have emphasized the rapid effects of estradiol on DA release. On the other hand, those investigating the role of the MPOA-VTA circuit primarily have used naturally cycling estrous females or OVX females administered low doses of estradiol over extended time periods. It is therefore possible that two mechanisms may have coordinated the effects of estradiol on NAc DA signaling: a rapid effect of estradiol acting directly on NAc, coupled with more prolonged changes in DA activity mediated by estradiol regulation of the MPOA-VTA pathway.
Effect of progesterone
The majority of research has focused on the effects of estradiol on DA systems. Nevertheless, the induction of many adaptive behaviors associated with gonadal hormones in intact animals require concurrent changes in levels of circulating progesterone (Tennent et al., 1980). As mentioned previously, while treatment with estradiol alone resulted in increases in stimulated DA release, hormone priming with repeated estradiol or estradiol and progesterone resulted in even greater stimulated DA release (Becker et al., 1984; Becker and Ramirez, 1981b). Treatment of estradiol primed animals with progesterone enhanced stimulated striatal DA release above the effects of estradiol alone (Becker and Rudick, 1999; Dluzen and Ramirez, 1984). This effect was biphasic, within initial increases in DA release occurring within 30 minutes of progesterone treatment and peak DA release seen at 4 hours after treatment, followed by subsequent inhibition of DA release 24 hours after progesterone administration (Dluzen and Ramirez, 1984). Modulation of DA release by progesterone was also apparent after in vitro application of progesterone to striatal tissue from estradiol primed females, and progesterone conjugated to BSA replicates the effects of unbound progesterone, demonstrating that progesterone acts directly on membrane receptors within the striatum to enhance DA release (Dluzen and Ramirez, 1990, 1989).
Progesterone also modulates expression of DA receptors within the striatum. Animals pre-treated with estradiol showed an increase in D2 DA receptor binding four hours after progesterone administration (Fernández-Ruiz et al., 1989). Somewhat surprisingly, administration of progesterone to OVX females not treated with estradiol reduced D2 DA receptor binding, providing evidence that progesterone can modulate DA functional activity independent of the effects of estradiol (Fernández-Ruiz et al., 1989).
The timing of progesterone’s effects on DA activity may indicate its relevance for adaptive motivated behaviors. Progesterone enhancement of DA release in estradiol-primed OVX rats was greatest four hours following hormone treatment, coinciding with the maximal induction of sexual receptivity after hormone priming (Glaser et al., 1983). The inhibition of DA release 24 hours following progesterone, after estradiol priming, also corresponds with the end of behavioral estrus, and it is possible that these effects of progesterone on DA release may coordinate sexually relevant motivated behaviors across the estrous cycle.
Dopamine activity in striatum modulates motivation
Modulation of DA activity in the NAc and dorsal striatum by gonadal hormones has been proposed to mediate changes in motivation across different reproductive states. Although direct evidence testing this hypothesis is limited, integration of literature on the effects of estradiol on DA circuitry with current knowledge of DA functioning in general can be useful in understanding the functional significance estradiol/DA interactions. The dorsal striatum and NAc have both been linked to the expression of motivated behaviors and the specific contributions of each brain area have been described.
DA projections from the VTA to the NAc are implicated in approach and avoidance behaviors (Ikemoto et al., 2015), reward learning (Schultz et al., 1997), and the attribution of incentive salience to reward associated cues (Berridge and Robinson, 1998). The NAc receives DA inputs from the VTA, with glutamatergic and GABAergic afferents from the amygdala, hippocampus, cortex, and thalamus (Kalivas and Nakamura, 1999). The NAc integrates these signals in order to select and execute appropriate motivated behaviors, based on the organism’s physiological state and environmental stimuli integrated with prior learning about rewards and reward predictive cues.
With this in mind, it is possible that estradiol and progesterone act on the NAc to drive adaptive changes in motivation for rewards across various reproductive contexts. For example, changes in NAc DA release over the course of the estrous cycle may enhance sexual motivation during periods of sexually receptivity. As mentioned previously, peak enhancement of DA release after treatment with estradiol and progesterone coincides with maximal induction of sexual receptivity, and increased sexual motivation (Cummings and Becker, 2012). These increases in DA release seen in estrous females may also facilitate reward learning during estrus. Females conditioned to associate a specific context with cocaine reward during estrus showed enhanced firing of VTA DA cells when entering the drug paired context even when neural activity was measured when females are no longer in estrus (Calipari et al., 2017). Sexual activity also increased DA release within the NAc and striatum of female rats, and olfactory cues that predict sexual reward elicited neural activation of striatal DA circuitry (Coria-Avila and Pfaus, 2007; Mermelstein and Becker, 1995; Pfaus et al., 1995). From this, it seems likely that modulation of DA signaling during estrus drives increased sexual motivation and enhanced learning about sex paired cues.
It is also possible that the NAc DA system is responsive to gonadal hormones in order to guide changes in motivation during the peripartum period. Plasticity within the NAc mediates the development of pair bonds, and variation within NAc DA systems is associated with individual differences in maternal behaviors (Aragona et al., 2003; Champagne et al., 2004; Szyf et al., 2005). Interaction with pups induced DA release in postpartum dams, and the ability for pups to induce increased DA release is estradiol and progesterone dependent (Afonso et al., 2009). This underscores the importance of the MPOA in the effects of estradiol on ventral striatal DA release. The MPOA has been shown to be crucial for the regulation of maternal behavior in rats, and interactions between the MPOA and striatal DA systems are required for the onset and maintenance of maternal behavior (Numan and Stolzenberg, 2009). It is then possible that facilitation of DA activity by gonadal hormones acts to increase motivated behaviors related to offspring care, as well as allow for plasticity within motivational systems to maintain maternal behaviors in the absence of gonadal hormones during later motherhood.
Contribution of dorsal striatum to estradiol modulation of motivated behaviors
In contrast to the NAc, the dorsal striatum has largely been studied in relation to its role in the control of motor output. However, a significant body of research has also identified a role for this system in reward learning and the transition from cognitive to habitual control of behavior (Everitt and Robbins, 2016). In the context of reproductive behaviors, estradiol regulation of dorsal striatum may be important for sex specific sexual behavior. When they are able to, females will actively slow the rate of intromissions during copulation through specific patterns of approach and avoidance behaviors (Adler and McClintock, 1978). Bilateral lesions to NAc reduced sexual interaction overall and pacing behavior specifically (Jenkins and Becker, 2001). Lesions of the dorsal striatum selectively reduced avoidance of males following ejaculation (Becker et al., 2001). The striatal regulation of paced mating requires direct effects of estradiol and progesterone; when OVX female rats were sequentially administered estradiol and progesterone in the ventromedial hypothalamus to induce sexual receptivity, concurrent hormone application to the dorsal striatum or NAc facilitated active pacing of copulatory behaviors (Xiao and Becker, 1997). Estradiol enhancement of DA release within the dorsal striatum, then, is likely important for the coordination of specific motor patterns required for adaptive motivated behaviors in females.
Although the role of estradiol in modulation of DA signaling and motivated behavior is still not fully delineated, it is clear that interactions between estradiol and DA systems are important for the expression of adaptive behaviors across various reproductive states. An appreciation of these systems and the interactions between them are particularly important in understanding how estradiol may regulate disordered or maladaptive behaviors. DA circuitry is implicated in the development of a number of psychiatric disorders, and increased knowledge of how estradiol regulates this system will provide important insight into sex differences in the prevalence and trajectory of these disorders.
Role of estradiol in sex differences in addiction
Interactions between estradiol and DA systems may be particularly relevant to the understanding of sex differences in drug addiction. Although rates of drug use remain higher in men than in women, substantial evidence suggests that women who are vulnerable to addiction are also predisposed to escalate from casual drug use to pathological more rapidly than men who become addicted, and are more susceptible to relapse following periods of abstinence (Becker and Hu, 2008; Becker and Koob, 2016; Lynch et al., 2002; Robbins et al., 1999). We have hypothesized that regulation of DA activity by estradiol mediates sex differences in the motivation to take drugs of abuse. According to the National Institute on Drug Abuse, approximately 10% of adults will meet diagnostic criterion for a substance abuse disorder at one point in their lifetime. Understanding how and why gonadal hormones influence drug taking has a large clinical relevance and holds the potential for targeted therapeutic treatment for substance use disorders.
One hallmark of addiction is the advancement from occasional drug use to habitual use; in rodent models, females escalate to habitual drug taking more quickly than males do, and more females exhibit this behavior compared with males (Perry et al., 2013b). There are similar patterns in humans, where women escalated to dependence faster than men and seek treatment for dependence sooner than males did (Hernandez-Avila et al., 2004; Ridenour et al., 2005). Taken together, we understand that there is a sex difference in the acquisition of drug taking behavior as well as the progression of the disease of addiction between males and females, which we suggest is enhanced by the presence of estradiol in females.
Preclinical models find that a higher percentage of female rodents will become drug preferring compared to their male counterparts when given the choice between cocaine or a natural reward, such as palatable food (Perry et al., 2013b). Previous research also indicated that changes in levels of estradiol and progesterone during the menstrual cycle affects the subjective reinforcing effects of cocaine in females (Sofuoglu et al., 2004, 1999). In OVX females, estradiol replacement increased cocaine taking, but in males, there was no effect of this treatment (Jackson et al., 2006). These findings further support the idea that circulating gonadal hormones affect the subjective effects of drug in females, which ultimately influences their drug seeking behavior.
Increased sensitivity to drugs of abuse may be just one way in which estradiol renders females more susceptible to substance abuse disorders. The transition to addiction has been associated with long term changes in the balance of D1 and D2 DA receptor mediated DA signaling, where D1 DA receptor signaling is enhanced and D2 DA receptor signaling is diminished. Similar changes in D1 vs D2 DA receptor signaling have been observed in females after treatment with estradiol, and it may be possible that estradiol enhances drug induced plasticity in striatal circuitry. Alternatively, the inherent flexibility of the DA system in females but not males may allow for stronger or more rapid changes following drug exposure. A greater understanding of how estradiol may modulate both acute responses to drugs of abuse as well as drug induced plasticity within the striatum will allow for a more complete assessment of sex differences in motivated behaviors for both natural and drug rewards.
Future directions
Although a substantial amount of progress has been made on our understanding of the various roles of ovarian hormones, there is still progress to be made. As mentioned above, there is a gap in our understanding of the relationship between estradiol’s regulation of DA release and DA receptor binding following estradiol treatment. We propose that changes in DA receptor binding are secondary to DA release, however a direct connection between these two effects has yet to be determined and is an important next step.
As mentioned in this review, the majority of research on estradiol has been focused within dorsal striatum. Since the NAc plays a vital role in addiction, as well as other psychiatric disorders, investigation into estradiol’s role within this area of the brain is a priority. In particular, distinct subregions of the NAc are known to have differential roles in motivated behavior and addiction. A greater understanding of how estradiol specifically modulates DA release within these discrete areas of the brain will be crucial in parsing the role of estradiol in both natural and drug induced behaviors. Additionally, future research should investigate the differential roles of estrogen receptor subtypes within both the NAc and DS.
Generally, we think of estradiol as being the primary ovarian hormone involved in the effects of DA release. However, progesterone is likely playing an important role as well. Understanding how these hormones act differentially and interdependently may tell us more about how ovarian hormone fluctuations mediate natural behaviors.
Finally, estradiol is thought to be a female hormone but it also plays a vital role in male and therefore, another line of research should investigate estradiol’s role on DA release in males.
Conclusion
Sex differences exist in both the underlying organization of striatal DA circuitry, as well as the ability of estradiol to modulate DA release and DA receptor binding. Over the past few decades, it has become clear that estradiol modulates DA activity in females by acting directly on membrane receptors within the striatum. This body of work represents a shift in the conceptualization of how estradiol is able to act on neural systems and broadens our understanding of how estradiol mediates rapid changes in behavior. Understanding how estradiol interacts with DA to mediate both natural and drug induced behaviors will be particularly important as we work to understand sex differences in the prevalence and trajectory of psychiatric disorders involving DA circuitry.
Highlights.
There are sex differences in dopamine circuitry
In females, ovarian hormones induce changes in dopaminergic signaling
Estradiol and progesterone affect receptor binding within the striatum
Estradiol regulates dopamine-mediated motivated behaviors
There are sex differences in vulnerability to addiction
Abbreviations
- AC
Adenylate Cyclas
- AMPH
Amphetamine
- BSA
Bovine Serum Albumin
- CaM
Calmodulin
- CAST
Castrated
- CAV
Caveolin Protein
- cAMP
Cylic adenosine 3’,5’ monophosphate
- DPN
Diarylpropionitrile
- DOPAC
Dihydroxyphenylacetic Acid
- DA
Dopamine
- DR
Dopamine Receptor
- DAT
Dopamine Transporter
- EB
Estradiol Benzoate
- ER
Estrogen Receptor
- ERK
Extracellular Signal-Regulated Kinase
- FSCV
Fast-Scan Cyclic Voltammetry
- GPCR
G Protein Coupled Receptor
- GAD
Glutamic Acid Decarboxylase
- IP3
Inositol 1,4,5-triphosphate
- mPFC
Medial Prefrontal Cortex
- MSNs
Medium Spiny Neurons
- mGluR
Metabotropic Glutamate Receptors
- MAPK
Mitogen Activated Protein Kinase
- NAc
Nucleus Accumbens
- OVX
Ovariectomize
- PKA
Protein Kinase A
- RGS9-2
Regulator of G-protein signaling 9-2
- SN
Substantia Nigra
- TH
Tyrosine Hydroxylase
- VTA
Ventral Tegmental Area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler NT, McClintock MK. The role of the female during copulation in wild and domestic norway rats (rattus norvegicus) Behaviour. 1978;67:67–95. doi: 10.1163/156853978×00260. [DOI] [Google Scholar]
- Afonso VM, King S, Chatterjee D, Fleming AS. Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup- and food-stimuli in the female rat. Horm Behav. 2009;56:11–23. doi: 10.1016/j.yhbeh.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology. 2012;153:5373–5383. doi: 10.1210/en.2012-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Milner TA, Brake WG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav. 2015;74:125–138. doi: 10.1016/j.yhbeh.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Milner TA, Brake WG. Estrogen receptor α and G-protein coupled estrogen receptor 1 are localized to GABAergic neurons in the dorsal striatum. Neurosci Lett. 2016;622:118–123. doi: 10.1016/j.neulet.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- Becker J, Ramirez VD. Dynamics of endogenous catecholamine release from brain fragments of male and female rats. Neuroendocrinology. 1980;31:18–25. doi: 10.1159/000123045. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990a;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990b;118:169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19:27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Beer ME, Robinson TE. Striatal dopamine release stimulated by amphetamine or potassium: influence of ovarian hormones and the light-dark cycle. Brain Res. 1984;311:157–160. doi: 10.1016/0006-8993(84)91410-0. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF. Sex differences in animal models: focus on addiction. Pharmacol Rev. 2016;68:242–263. doi: 10.1124/pr.115.011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD. Experimental studies on the development of sex differences in the release of dopamine from striatal tissue fragments in vitro. Neuroendocrinology. 1981a;32:168–173. doi: 10.1159/000123151. [DOI] [PubMed] [Google Scholar]
- Becker JB, Ramirez VD. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981b;204:361–372. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- Becker JB, Robinson TE, Lorenz KA. Sex differences and estrous cycle variations in amphetamine-elicited rotational behavior. Eur J Pharmacol. 1982;80:65–72. doi: 10.1016/0014-2999(82)90178-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacol Biochem Behav. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–3241. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/S0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bossé R, Rivest R, Di Paolo T. Ovariectomy and estradiol treatment affect the dopamine transporter and its gene expression in the rat brain. Brain Res Mol Brain Res. 1997;46:343–346. doi: 10.1016/s0169-328x(97)00082-x. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C, Walker DM, Cahill ME, Ribeiro E, Roman-Ortiz C, Ramakrishnan C, Deisseroth K, Han M-H, Nestler EJ. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat Commun. 2017;8:13877. doi: 10.1038/ncomms13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez C, Hollaus M, Scarr E, Pavey G, Gogos A, van den Buuse M. The effect of estrogen on dopamine and serotonin receptor and transporter levels in the brain: an autoradiography study. Brain Res. 2010;1321:51–59. doi: 10.1016/j.brainres.2009.12.093. [DOI] [PubMed] [Google Scholar]
- Clark D, White FJ. D1 dopamine receptor--the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse. 1987;1:347–388. doi: 10.1002/syn.890010408. [DOI] [PubMed] [Google Scholar]
- Clopton J, Gordon JH. In vivo effects of estrogen and 2-hydroxyestradiol on D-2 dopamine receptor agonist affinity states in rat striatum. J Neural Transm. 1986;66:13–20. doi: 10.1007/BF01262954. [DOI] [PubMed] [Google Scholar]
- Coria-Avila GA, Pfaus JG. Neuronal activation by stimuli that predict sexual reward in female rats. Neuroscience. 2007;148:623–632. doi: 10.1016/j.neuroscience.2007.05.052. [DOI] [PubMed] [Google Scholar]
- Creutz LM, Kritzer MF. Estrogen receptor-beta immunoreactivity in the midbrain of adult rats: regional, subregional, and cellular localization in the A10, A9, and A8 dopamine cell groups. J Comp Neurol. 2002;446:288–300. doi: 10.1002/cne.10207. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Becker JB. Quantitative assessment of female sexual motivation in the rat: Hormonal control of motivation. J Neurosci Methods. 2012;204:227–233. doi: 10.1016/j.jneumeth.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Jagannathan L, Jackson LR, Becker JB. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend. 2014;135:22–28. doi: 10.1016/j.drugalcdep.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo T, Falardeau P, Morissette M. Striatal D-2 dopamine agonist binding sites fluctuate during the rat estrous cycle. Life Sci. 1988;43:665–672. doi: 10.1016/0024-3205(88)90137-3. [DOI] [PubMed] [Google Scholar]
- Di Paolo T, Rouillard C, Bédard P. 17 beta-Estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur J Pharmacol. 1985;117:197–203. doi: 10.1016/0014-2999(85)90604-1. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD. Bimodal effect of progesterone on in vitro dopamine function of the rat corpus striatum. Neuroendocrinology. 1984;39:149–155. doi: 10.1159/000123971. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD. Progesterone effects upon dopamine release from the corpus striatum of female rats. II. Evidence for a membrane site of action and the role of albumin. Brain Res. 1989;476:338–344. doi: 10.1016/0006-8993(89)91255-9. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Ramirez VD. In vitro progesterone modulation of amphetamine-stimulated dopamine release from the corpus striatum of ovariectomized estrogen-treated female rats: response characteristics. Brain Res. 1990;517:117–122. doi: 10.1016/0006-8993(90)91016-a. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Evinger AJ, Levin ER. Requirements for estrogen receptor alpha membrane localization and function. Steroids. 2005;70:361–363. doi: 10.1016/j.steroids.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Fernández-Ruiz JJ, Amor JC, Ramos JA. Time-dependent effects of estradiol and progesterone on the number of striatal dopaminergic D2-receptors. Brain Res. 1989;476:388–395. doi: 10.1016/0006-8993(89)91266-3. [DOI] [PubMed] [Google Scholar]
- Glaser JH, Rubin BS, Barfield RJ. Onset of the receptive and proceptive components of feminine sexual behavior in rats following the intravenous administration of progesterone. Horm Behav. 1983;17:18–27. doi: 10.1016/0018-506x(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Gordon JH. Modulation of apomorphine-induced stereotypy by estrogen: time course and dose response. Brain Res Bull. 1980;5:679–682. doi: 10.1016/0361-9230(80)90205-1. [DOI] [PubMed] [Google Scholar]
- Gordon JH, Gorski RA, Borison RL, Diamond BI. Postsynaptic efficacy of dopamine: possible suppression by estrogen. Pharmacol Biochem Behav. 1980;12:515–518. doi: 10.1016/0091-3057(80)90182-3. [DOI] [PubMed] [Google Scholar]
- Gordon JH, Perry KO. Pre- and postsynaptic neurochemical alterations following estrogen-induced striatal dopamine hypo- and hypersensitivity. Brain Res Bull. 1983;10:425–428. doi: 10.1016/0361-9230(83)90137-5. [DOI] [PubMed] [Google Scholar]
- Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjimarkou MM, Vasudevan N. GPER1/GPR30 in the brain: Crosstalk with classical estrogen receptors and implications for behavior. J Steroid Biochem Mol Biol. 2017 doi: 10.1016/j.jsbmb.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Hruska RE, Ludmer LM, Silbergeld EK. Characterization of the striatal dopamine receptor supersensitivity produced by estrogen treatment of male rats. Neuropharmacology. 1980;19:923–926. doi: 10.1016/0028-3908(80)90095-7. [DOI] [PubMed] [Google Scholar]
- Hruska RE, Silbergeld EK. Estrogen treatment enhances dopamine receptor sensitivity in the rat striatum. Eur J Pharmacol. 1980a;61:397–400. doi: 10.1016/0014-2999(80)90081-3. [DOI] [PubMed] [Google Scholar]
- Hruska RE, Silbergeld EK. Increased dopamine receptor sensitivity after estrogen treatment using the rat rotation model. Science. 1980b;208:1466–1468. doi: 10.1126/science.7189902. [DOI] [PubMed] [Google Scholar]
- Hu M, Watson CJ, Kennedy RT, Becker JB. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse. 2006;59:122–124. doi: 10.1002/syn.20221. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Yang C, Tan A. Basal ganglia circuit loops, dopamine and motivation: A review and enquiry. Behav Brain Res. 2015;290:17–31. doi: 10.1016/j.bbr.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Role of the striatum and nucleus accumbens in paced copulatory behavior in the female rat. Behav Brain Res. 2001;121:119–128. doi: 10.1016/s0166-4328(00)00394-6. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Current opinion in neurobiology. 1999 doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- Kumakura K, Hoffman M, Cocchi D, Trabucchi M, Spano PF, Müller EE. Long-term effect of ovariectomy on dopamine-stimulated adenylate cyclase in rat striatum and nucleus accumbens. Psychopharmacology (Berl) 1979;61:13–16. doi: 10.1007/BF00426803. [DOI] [PubMed] [Google Scholar]
- Le Saux M, Morissette M, Di Paolo T. ERbeta mediates the estradiol increase of D2 receptors in rat striatum and nucleus accumbens. Neuropharmacology. 2006;50:451–457. doi: 10.1016/j.neuropharm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Lévesque D, Di Paolo T. Rapid conversion of high into low striatal D2-dopamine receptor agonist binding states after an acute physiological dose of 17 beta-estradiol. Neurosci Lett. 1988;88:113–118. doi: 10.1016/0304-3940(88)90324-2. [DOI] [PubMed] [Google Scholar]
- Lévesque D, Di Paolo T. Effect of the rat estrous cycle at ovariectomy on striatal D-1 dopamine receptors. Brain Res Bull. 1990;24:281–284. doi: 10.1016/0361-9230(90)90216-m. [DOI] [PubMed] [Google Scholar]
- Luoma JI, Boulware MI, Mermelstein PG. Caveolin proteins and estrogen signaling in the brain. Mol Cell Endocrinol. 2008;290:8–13. doi: 10.1016/j.mce.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Marcott PF, Mamaligas AA, Ford CP. Phasic dopamine release drives rapid activation of striatal D2-receptors. Neuron. 2014;84:164–176. doi: 10.1016/j.neuron.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA, Peterson BM, Meisel RL, Mermelstein PG. Estradiol facilitation of cocaine-induced locomotor sensitization in female rats requires activation of mGluR5. Behav Brain Res. 2014;271:39–42. doi: 10.1016/j.bbr.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus M, Bertrand P, Drouva S, Rasolonjanahary R, Kordon C, Glowinski J, Premont J, Enjalbert A. Differential modulation of D1 and D2 dopamine-sensitive adenylate cyclases by 17 beta-estradiol in cultured striatal neurons and anterior pituitary cells. J Neurochem. 1989;52:410–418. doi: 10.1111/j.1471-4159.1989.tb09136.x. [DOI] [PubMed] [Google Scholar]
- McHenry JA, Otis JM, Rossi MA, Robinson JE, Kosyk O, Miller NW, McElligott ZA, Budygin EA, Rubinow DR, Stuber GD. Hormonal gain control of a medial preoptic area social reward circuit. Nat Neurosci. 2017;20:449–458. doi: 10.1038/nn.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Healy DJ, Kuehn R, Zhou QY, Bunzow JR, Akil H, Civelli O, Watson SJ. Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology. 1991;5:231–242. [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav Neurosci. 1995;109:354–365. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993;58:16–22. doi: 10.1159/000126507. [DOI] [PubMed] [Google Scholar]
- Morissette M, Le Saux M, D’Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ERβ: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-X. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Pasqualini C, Olivier V, Guibert B, Frain O, Leviel V. Acute stimulatory effect of estradiol on striatal dopamine synthesis. J Neurochem. 1995;65:1651–1657. doi: 10.1046/j.1471-4159.1995.65041651.x. [DOI] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. Impact of pubertal and adult estradiol treatments on cocaine self-administration. Horm Behav. 2013a;64:573–578. doi: 10.1016/j.yhbeh.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, Becker JB. The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS ONE. 2013b;8:e79465. doi: 10.1371/journal.pone.0079465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 1995;693:21–30. doi: 10.1016/0006-8993(95)00679-k. [DOI] [PubMed] [Google Scholar]
- Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- Ridenour TA, Maldonado-Molina M, Compton WM, Spitznagel EL, Cottler LB. Factors associated with the transition from abuse to dependence among substance abusers: implications for a measure of addictive liability. Drug Alcohol Depend. 2005;80:1–14. doi: 10.1016/j.drugalcdep.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Ramirez VD. Sex differences in amphetamine-elicited rotational behavior and the lateralization of striatal dopamine in rats. Brain Res Bull. 1980;5:539–545. doi: 10.1016/0361-9230(80)90260-9. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM, Jacknow DS, Becker JB. Sex differences and estrous cycle dependent variation in rotational behavior elicited by electrical stimulation of the mesostriatal dopamine system. Behav Brain Res. 1982;6:273–287. doi: 10.1016/0166-4328(82)90028-6. [DOI] [PubMed] [Google Scholar]
- Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29:1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shams WM, Cossette M-P, Shizgal P, Brake WG. 17β-estradiol locally increases phasic dopamine release in the dorsal striatum. Neurosci Lett. 2017;665:29–32. doi: 10.1016/j.neulet.2017.11.039. [DOI] [PubMed] [Google Scholar]
- Shams WM, Sanio C, Quinlan MG, Brake WG. 17β-Estradiol infusions into the dorsal striatum rapidly increase dorsal striatal dopamine release in vivo. Neuroscience. 2016;330:162–170. doi: 10.1016/j.neuroscience.2016.05.049. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Koenig JI. Evidence for the involvement of ERbeta and RGS9-2 in 17-beta estradiol enhancement of amphetamine-induced place preference behavior. Horm Behav. 2007;52:146–155. doi: 10.1016/j.yhbeh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacol Biochem Behav. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Martres MP, Schwartz JC. Three classes of dopamine receptor (D-2, D-3, D-4) identified by binding studies with 3H-apomorphine and 3H–domperidone. Naunyn Schmiedebergs Arch Pharmacol. 1980;315:89–102. doi: 10.1007/BF00499251. [DOI] [PubMed] [Google Scholar]
- Szyf M, Weaver ICG, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol. 2005;26:139–162. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Tennent BJ, Smith ER, Davidson JM. The effects of estrogen and progesterone on female rat proceptive behavior. Horm Behav. 1980;14:65–75. doi: 10.1016/0018-506x(80)90016-1. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neurosci Lett. 1997;229:145–148. doi: 10.1016/s0304-3940(97)00450-3. [DOI] [PubMed] [Google Scholar]
- Tobiansky DJ, Will RG, Lominac KD, Turner JM, Hattori T, Krishnan K, Martz JR, Nutsch VL, Dominguez JM. Estradiol in the Preoptic Area Regulates the Dopaminergic Response to Cocaine in the Nucleus Accumbens. Neuropsychopharmacology. 2015;41:1897–1906. doi: 10.1038/npp.2015.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will RG, Martz JR, Dominguez JM. The medial preoptic area modulates cocaine-induced locomotion in male rats. Behav Brain Res. 2016;305:218–222. doi: 10.1016/j.bbr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Hormonal activation of the striatum and the nucleus accumbens modulates paced mating behavior in the female rat. Horm Behav. 1997;32:114–124. doi: 10.1006/hbeh.1997.1412. [DOI] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Effects of estrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse. 1998;29:379–391. doi: 10.1002/(SICI)1098-2396(199808)29:4<379::AID-SYN10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Xiao L, Jackson LR, Becker JB. The Effect of Estradiol in the Striatum Is Blocked by ICI 182,780 but Not Tamoxifen: Pharmacological and Behavioral Evidence. Neuroendocrinology. 2003;77:239–245. doi: 10.1159/000070279. [DOI] [PubMed] [Google Scholar]
- Yoest KE, Cummings JA, Aragona BJ, Becker JB. 2016 Neuroscience Meeting Planner. Presented at the Society for Neuroscience, Society for Neuroscience; San Diego, CA: 2016. Contribution of estrogen receptor subtypes to the effect of estradiol on cocaine-induced dopamine release in the nucleus accumbens; p. 636.21. [Google Scholar]
- Zheng J, Ramirez VD. Demonstration of membrane estrogen binding proteins in rat brain by ligand blotting using a 17beta-estradiol-[125I]bovine serum albumin conjugate. J Steroid Biochem Mol Biol. 1997;62:327–336. doi: 10.1016/s0960-0760(97)00037-x. [DOI] [PubMed] [Google Scholar]