Abstract
Aim
To evaluate the clinical efficacy and safety of an intra-articular injection of bone marrow aspirate concentrate (BMAC) as a treatment option for osteoarthritis (OA) of the knee.
Materials and methods
Between June 2014 and February 2017, data from 233 patients with knee osteoarthritis treated with BMAC injection at a single center, were retrospectively evaluated. Only patients with idiopathic osteoarthritis were included. Exclusion criteria were post-traumatic osteoarthritis, previous knee surgery, age less than 50 years old or more than 85 years old, active infection, uncontrolled diabetes mellitus, rheumatological or other systemic disease, malignancy, or treatment with immunosuppressive drugs. Bone marrow from the iliac crest was aspirated/concentrated with a standardized technique using a single-spin manual method. Patients were evaluated before and after the procedure, using the numeric pain scale (NPS) and Oxford knee score (OKS). Mean follow-up period was 11 months, range (6–30 months).
Results
A total of 121 of 233 patients had completed data as previously defined and were included in the statistical analysis. There were 85 females and 36 males, with mean age 70 years (range 50–85). Compared to baseline, the mean NPS decreased from 8.33 to 4.49 (p < 0.001) and the mean OKS increased from 20.20 to 32.29 (P < 0.001) at final follow-up. There were no complications.
Conclusion
A single intra-articular injection of BMAC is a safe and reliable procedure that results in clinical improvement of knee OA.
Keywords: Evidence-based medicine, Surgery
1. Introduction
Osteoarthritis (OA) is a chronic progressive degenerative disorder consisting of degeneration and loss of articular cartilage with accompanying synovitis, subchondral bone remodelling, and osteophyte formation [1, 2]. It constitutes a major cause of disability with pain, stiffness, resulting in severe functional limitations [3]. OA is the most common joint disorder in the United States, affecting 1 in 5 Americans over 60 years of age [4] and with projected 67 million patients in the United States alone by 2030 [5]. This has huge socio-economic implications [6, 7] and a substantial financial burden on the economy [8], and it is estimated that it accounts between 1% and 2.5% of the gross national product of Western world [9].
Although OA is a process of articular cartilage “wear and tear”, its changes are biochemically mediated [10], through an imbalance between intra-articular anabolic and catabolic cytokines [11]. This results in cartilage loss, synovial inflammation and eventually leads to mechanical and biological dysfunction of the joint [12].
The articular cartilage due to its avascular nature and the limited self-renewal [13] capacity of chondrocytes has remarkably poorer regenerative ability than other tissues [14, 15].
Current treatments for early phase of degenerative arthritis focus on relieving inflammation and pain [16], but have no effect on the natural progression of the disease [17] because it does not improve the biochemical environment (homeostasis) of the joint.
Conservative treatments including medications such as non-steroidal anti-inflammatory drugs (NSAIDs) and steroids as well as supplements including glucosamine, chondroitin sulphate, omega-3 fatty acids and intra-articular use of viscosupplementation [16, 18], cannot alter the natural history of the disease. Specifically, viscosupplementation is efficient in the early stage of osteoarthritis but pain relief is limited to a few months, offering only a temporary benefit [19, 20], whereas injection of corticosteroids provides short-term improvement of symptoms while posing the risk of aggravating cartilage damage and producing tissue atrophy [21].
Costly total knee arthroplasty (TKA) then follows when other treatment options have been exhausted [22], however patients experience a higher risk of death from mental and inflammatory musculoskeletal diseases, with a serious adverse event rate of 5.6% and a 0.2% mortality rate [23].
With recent increase of interest in field of regenerative medicine, research has been directed towards the development of treatment strategies to provide a symptomatic improvement by influencing joint homeostasis [24]. Recently, extraction of the mesenchymal stem cells (MSCs) obtained from autologous bone marrow (BMAC) followed by concentration was introduced represents the next generation of injectable intra-articular orthobiologic therapy for patients with cartilage disease [25, 26].
MSCs are multipotent cells that exhibit strong self-renewal abilities, combined with a differentiation capacity to form chondrocytes, adipocytes, and osteocytes [27]. These cells also have very important local paracrine affects to alter their local microenvironment to conditions favourable for regeneration and repair [22, 28].
BMAC represents the safest and most feasible source of MSCs. Intra-articular application has resulted in pain reduction, functional improvement and/or tissue regeneration [29]. BMAC is obtained through density gradient centrifugation of bone marrow aspirate (BMA) typically aspirated from the iliac crest [30]. BMAC has been shown to provide elevated levels of hematopoietic stem cells (HSCs), MSCs, platelets, chemokines and cytokines including PDGF and TGF-β [31]. These growth factors (GFs) are not only contained within the alpha granules of platelets, but they are also secreted by MSCs [32, 33] and can induce chondrogenesis of MSCs [32, 33, 34]. GFs also initiate stem cell migration to the injury site and provide adhesion sites for the migrating stem cells [35]. Moreover BMAC possesses in general, anti-inflammatory, angiogenic trophic and immunomodulatory properties that can potentially have anabolic and anti-inflammatory effects enhancing cartilage repair [32, 33, 36].
To date, there have been several studies that have looked at BMAC for the treatment of osteoarthritis, with conflicting results secondary to the differences and/or inconsistencies in methodologies used throughout the studies [37]. Therefore, the role of BMAC in osteoarthritis is not yet been established.
Encouraged by the positive preliminary results of BMAC-induced bone regeneration [22, 38, 39, 40, 41, 42], the authors initiated a retrospective clinical trial to evaluate the results of a single, intra-articular injection of BMAC with knee OA. This study is one of the largest cohorts in the literature.
2. Materials & methods
Between June 2014 and February 2017, data from 233 patients with knee osteoarthritis, treated with BMAC injection, were retrospectively evaluated. All procedures were performed at one Institution by the Authors. The Mediterraneo Hospital Scientific Committee approved the study protocol and informed consents were obtained from each participant.
Inclusion criteria were a longstanding knee pain from idiopathic osteoarthritis unresponsive to activity modification, weight loss, physical therapy, bracing, analgesics, nonsteroidal anti-inflammatory drugs, injection therapy or arthroscopy for at least 6 weeks with a Kellgren–Lawrence [43] grade III or higher radiographic OA.
Exclusion criteria included post-traumatic osteoarthritis, previous knee surgery, age less than 50 years old or more than 85 years old, active infection, uncontrolled diabetes mellitus, rheumatological or other systemic disease, malignancy, treatment with immunosuppressive drugs. Although in cases of bilateral osteoarthritis both knees were treated, the worst knee was taken into account for comparison analysis. Patients who elected to participate in the study and had a follow-up time of less than 60 days were also excluded. Additionally, patients who elected to proceed with total knee arthroplasty before their post-procedure evaluation, were also excluded.
As BMAC treatment works by stimulating the normal inflammatory healing mechanisms, medications such as NSAIDs [44] or corticosteroids [45] that can impair soft tissue healing and also reduce MSC proliferation, were discontinued at least 10 days and 4–6 weeks prior to the procedure respectively. In addition, no patient received pre-procedural antibiotics. If the patient was under anticoagulants, routine bridging was performed with subcutaneous enoxaparin until the day before the procedure.
2.1. Procedure
With the patient in a supine position on the operating table, the iliac crest was surgically prepped and draped in the usual fashion. A combination of conscious sedation and local anesthesia (1% lidocaine) was used. Prior to aspiration, an 11-Gauge Bone Access Needle (Medtronic, Inc) and eight 10ml-syringes are flushed with heparin (5000 U/20ml) and then filled with 1 ml heparin solution.
Using a stab incision, the bone access needle is inserted and advanced through the periosteum of the Anterior Superior Iliac Spine. After the periosteum is pierced, the driver and stylet are removed and a 10ml syringe containing 1ml of heparin dilution is used to aspirate 8 ml of bone marrow [Fig. 1]. There was extra care to hold the needle still throughout the procedure. The needle was not advanced nor rotated after each successive 10 mL aspiration, in contrast to other authors who recommend this practice in order to reduce peripheral blood contamination (hemodilution) [46]. All aspirations were performed by the same surgeon (IMK). The total amount of bone marrow harvested (BMA) was 80 ml for the treatment of both knees and 60 ml for one knee. Following bone marrow aspiration, the bone access needle is withdrawn, pressure is applied to the skin entry site, followed by dressing application.
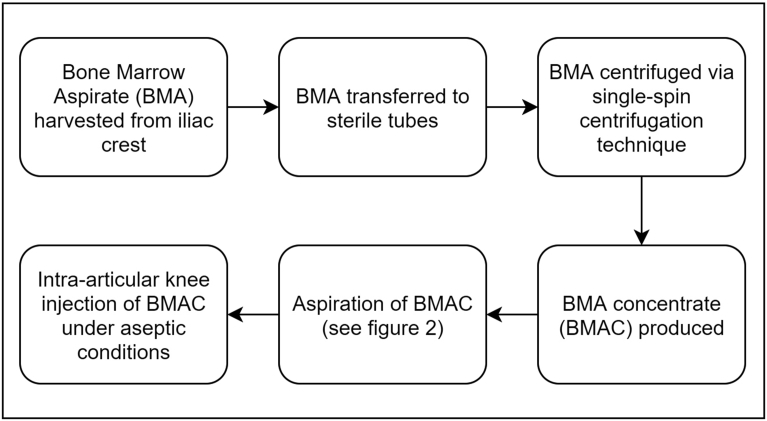
Fig. 1.
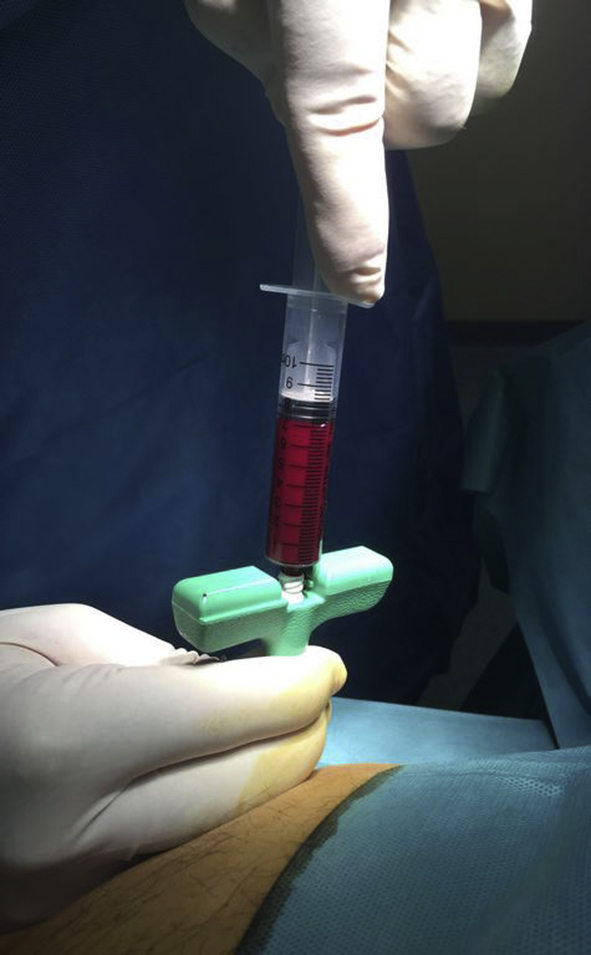
Bone marrow aspiration.
Following extraction, the aspirate is transferred to sterile tubes and is carefully processed by hand in a separate room under sterile conditions to isolate the buffy coat through centrifugation [Fig. 2]. A single-spin centrifugation technique (Hettich® Rotofix 32A centrifuge, 15 min at 2800 RPMs) yielded approximately 20ml of BMAC for every 80 ml of BMA which was then transported back to the operating room and under sterile conditions, each knee joint was injected with 10 ml of BMAC. The supernatant part of the 80 ml aspirate was preserved and additionally used as prolotherapy at the level of the joint line. All injections were performed using an anterolateral approach to the knee joint by the same physician (G.S.T.) without anaesthesia in order to prevent any interaction with the BMAC. Immediately following injection, the knee joint was passively moved throughout its range of motion to disseminate the fluid throughout the joint. A summary flow diagram of the procedure is shown in Fig. 3. Mean duration of the procedure was approximately 1 hour, while the time between extraction and injection was approximately 35 minutes.
Fig. 2.
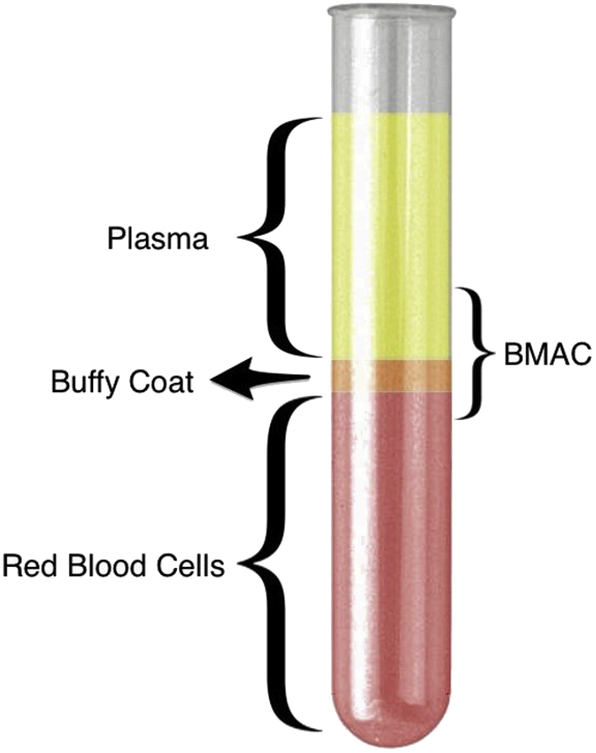
Cell separation layers starting from the top: Plasma mainly containing platelets, buffy coat with mononuclear HSCs and MSCs and bottom fraction with red blood cells (RBCs). BMAC consists mainly from buffy coat, a supernatant with platelets and small size MSCs and the very top of the RBC layer containing the largest size MSCs.
Fig. 3.
Flow diagram showing a summary of the procedure.
The patients were allowed full weight bearing and instructed to return to light activity as tolerated avoiding oral NSAID's and corticosteroids for at least four weeks post procedure. Within six weeks' patients were allowed to return to full activities. There was no other therapeutic intervention (bracing, physical therapy, etc). There were no adverse events or complications and all patients recovered completely.
2.2. Outcome measurements
Outcome was assessed using a numeric Pain Scale (NPS) (0–10) for pain intensity, (NPS has eleven levels of pain ranging from 0 for no pain, to 10, indicating worst possible pain), and a validated Oxford knee score (OKS) questionnaire for functional assessment [47]. OKS is a self-administered questionnaire, which is designed specifically for the knee joint. The questionnaire includes 12 items with a maximum total score of 48 indicating maximum function.
Evaluations were performed prior to the administration of treatment and post-procedure in varying time periods (mean follow-up time: 11 months, range, 6–30 months) via phone calls and direct patient evaluations. At final follow-up, the patients were asked if they were satisfied with the procedure and if they would suggest the treatment to someone else.
2.3. Statistical analysis
The pre- and post-treatment scores were compared using the Paired t test. Probability (p) values less than 0.05 were considered statistically significant. All statistical analyses were carried out using SPSS software (SPSS 17.0, SPSS, Chicago, IL, USA).
3. Results
According to the inclusion and exclusion criteria, 121 patients (121 knees) were included in this study. Mean age was 70 years, ranging from 50 to 85 years, and there were 36 (29.7%) males and 85 (70.3%) females, 76 right knees and 45 left knees. The degree of the degenerative arthritis was evaluated by K–L grade (Kellgren–Lawrence grading scale) on standing anteroposterior (AP) view: there were 46 (38%) cases of grade III, and 75 (62%) cases of grade IV. At final follow-up, the mean NPS decreased from 8.33 preoperatively to 4.49 postoperatively (p < 0.001). Also the mean OKS increased from 20.20 pre-operatively to 32.92 postoperatively (p < 0.001). A total of 6 patients (5%) elected to proceed to total knee arthroplasty, 89 patients (73.5%) indicated that they would repeat the procedure, and 105 patients (86.7%) indicated that they would recommend the procedure to a friend. The results are summarized in Table 1. In the group of the current study, knee pain recurrence was not further recorded beyond the follow-up time and this would be a subject for a future follow-up investigation. There was no correlation between age, grade of OA and decreased scores. There were no complications, including pain on the site of the harvest, hematoma or paresthesias.
Table 1.
Summary of results, 121 patients (121 knees).
| Outcome Instrument | Pre-treatment | Post-treatment | p value |
|---|---|---|---|
| NPS | 8.33 (range 5–10) | 4.49 (range 1–10) | <0.001 |
| OKS | 20.20 (range 7–39) | 32.29 (range 16–48) | <0.001 |
NPS: Numeric Pain Scale; OKS: Oxford Knee Score.
4. Discussion
Orthobiologics has emerged as a therapeutic option with special emphasis on regenerating damaged or diseased tissue, correcting the systems biology and delaying or preventing disease progression [36]. The potential of MSCs to differentiate into the cell lineage of interest to form chondrocytes, adipocytes, and osteocytes [27], and the capacity of self-renewal has created huge interest in trauma and orthopedic surgery [28, 48]. Additionally, MSCs may favourably alter the microenvironment conditions for regeneration and repair [49], due to down-regulation of inflammatory cytokines, including interleukin 1 (IL-1), interleukin 6 (IL-6), interferon-γ, and tumor necrosis factor alpha (TNF-α) [50, 51]. Apart from bone marrow, MSCs are present in numerous tissues in the body including adipose, synovium and blood [52], however their chondrogenic potential is lower than that of bone MSCs [36]. BMAC may represent the safest and most feasible source of MSCs for bone tissue regeneration [53], the procedure is technically easy and fast, enabling the harvesting and intraoperative transplantation in one sitting [54]. Additionally, BMAC contains hematopoietic stem cells, platelets, growth factors (GFs), cytokines and chemokines [55]. The GFs are released from platelet alpha granules [32, 33], and mainly include TGF- β, PDGF, VEGF, FGF, BMP, and IGF [56], which additionally help to initiate stem cell migration to the injury site and provide adhesion sites for the migrating stem cells [35].
Currently there are many published studies supporting the feasibility, safety, and efficacy of bone marrow derived MSC therapy in knee OA [22, 36, 57, 58]. Specifically human trials showed improvement in range of motion, pain scores, functional status of the knee, and walking distance even with patients with grade 4 arthritis [22, 59, 60, 61, 62], shortened hospital stay [63], production of cartilage and bone regeneration [58, 60, 64], increase in cartilage thickness, decrease in the size of subchondral edema [15, 58, 61, 62, 65], treatment of patello-femoral cartilage defects [66], complete filling of cartilage defects (MRI-confirmed) [59, 67], combination of BMAC with ACL reconstruction [68], and increase in meniscus volume [62]. In this study we report the clinical results on 134 patients with osteoarthritis who were retrospectively followed after receiving a single, intra-articular injection of BMAC.
The procedure was well tolerated and improved pain and function at both short and long-term follow-up among the vast majority of the patients.
Potential complications of bone marrow aspiration from the iliac crest are rare, (0.05%) with the most common being hemorrhage, while others include, infection, donor site morbidity with persistent pain [69]. No adverse effects were recorded and none of the patients reported worsening of symptoms following the BMAC procedure.
In addition, 86.7% of patients would recommend the procedure to a friend. A number of studies using BMAC for cartilage regeneration and repair have focused on preparations in which MSCs are pre-cultured, assuming that number of delivered MSCs is critical [60, 62, 68]. However, excellent clinical results have been demonstrated with low cell numbers compared to culture-expanded techniques [22, 65, 70, 71]. Therefore, it has been suggested that the mechanism of action of MSCs is modulation of the joint homeostasis via paracrine signalling [57], instead of being an actual building block of cartilage [72]. In an effort to obtain the maximum possible number of MSCs, several authors strive to minimize blood “contamination” of the sample; however this minimizes a number of platelets and therefore their growth factors obtained in the final aspirate. Therefore, in this study there has been no effort to increase the number of MSCs and minimize blood contamination as suggested by several authors [73], including multiple sampling sites, multi-hole needles as well as successive needle displacement and/or rotation following each 10 mL aspiration.
Our previous experience (Themistocleous et al., unpublished data) consisted of administration of isolates of large numbers of cultured MSCs that had relatively poor results compared to our current protocol. Therefore, we believe that the administration of MSCs with platelets and therefore growth factors acting similarly to platelet rich plasma would be desirable. Further research is needed in order to establish the optimal fractions of each of the elements. This simple and fast technique applied herein uses a thin (11 gauge) needle to acquire a maximum number of MSCs by a single puncture in order to minimize procedural time and patient discomfort. In addition, the authors did a single spin with low centrifuge settings (580G) in order to maximize stem cell viability and achieve optimal separation between the bone marrow layers. There have been very few studies in which BMAC was injected intra-articularly without any additional processing. A recent study by Centeno et al. [42], in which 424 knees of 373 patients with knee osteoarthritis were injected with BMAC, supported that the final MSC number plays a critical role for optimal outcomes in contrast to our protocol. Sampson et al. [40], performed a single intra-articular injection of BMAC in 73 knee osteoarthritis patients (73 knees) with an average follow-up of 148 days followed by a single platelet rich plasma injection at eight weeks and concluded that this combination is beneficial in the short-term in moderate to severe osteoarthritis. Shapiro et al. [41], conducted a double blinded randomized control trial in 25 patients with bilateral knee osteoarthritis. One side was injected with BMAC whereas the contralateral side was injected with normal saline 0.9%. There was no statistically significant difference in pain relief and function at 6 months. However, given the fact that MSCs administered to any site have the ability to travel to sites of inflammation, it cannot be concluded with certainty that the MSCs injected may have not played a role in improving the contralateral knee symptoms as well [41]. Kim et al. [22], injected with BMAC 75 knees of 41 patients with osteoarthritis degrees ranging from 1 to 4 and showed improved quality of life in all osteoarthritis grades, however even in grade 4 patients the difference was less significant. Our study represents one of the largest cohorts of patients with knee osteoarthritis treated exclusively with a single injection of concentrated BMAC which demonstrates the efficacy of this treatment in the absence of any additional procedure. However, several questions remain yet to be addressed:
-
1.
What is the ideal/optimal proportion of MSCs and platelet derived growth factors to be injected? More is not always necessarily better.
-
2.
Should treatment with BMAC be combined with and/or followed by other modalities, for example PRP, hyaluronic acid, prolotherapy?
-
3.
Is there any added benefit in repeating treatment with BMAC at regular intervals for maintenance and if so, what is the optimal frequency/timing?
This study has several limitations. First, the results should be interpreted in the light of absence of a control group. Second, the retrospective nature of the study did not allow for data collection at the designated regular intervals and therefore follow up was variable on a case-by-case basis. Third, patients enrolled in this study have advanced (grades 3 and 4) knee osteoarthritis, and therefore the effects of treatment in patients with milder disease were not evaluated. This is greatly attributed to the fact that in our country's culture, a typical patient will seek several orthopedic opinions and undergo several nonoperative treatments and only visit our practice when they desperately try to avoid the total joint arthroplasty surgery that has been proposed to them as final solution. Fourth, another confounding factor may be that many of our “word-of-mouth” patients visiting our practice may be biased in favour of this nonoperative modality, at the expense of other possible operative or nonoperative management options. Lastly, although there was consistency throughout the sampling and injection process, there was no laboratory analysis of the aspirate and BMAC to measure cell numbers, presence of growth factors or other constituents.
5. Conclusion
MSCs are a promising option for the treatment of knee OA. Given the aforementioned limitations, this study showed that a single intra-articular injection of BMAC appears to provide long-term benefits. The procedure is simple, fast, well-tolerated, avoids the need for hospitalization and generated no complications or adverse effects. Further research is needed to better understand the role of BMAC therapy, determine the optimal dose, the best way of separation, delivery and frequency of treatment.
Declarations
Author contribution statement
George S. Themistocleous: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
George D. Chloros: Analyzed and interpreted the data; Wrote the paper.
Ioannis M. Kyrantzoulis: Performed the experiments.
Ioannis A. Georgokostas: Conceived and designed the experiments; Performed the experiments.
Marios S. Themistocleous, Olga D. Savvidou: Analyzed and interpreted the data; Wrote the paper.
Panayiotis J. Papagelopoulos: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank Maria Koutsa, RN for her valuable contribution in data collection.
References
- 1.Goldring M.B., Goldring S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 2.Ishiguro N., Kojima T., Poole A.R. Mechanism of cartilage destruction in osteoarthritis. Nagoya J. Med. Sci. 2002;65:73–84. [PubMed] [Google Scholar]
- 3.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21:1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hootman J.M., Helmick C.G., Barbour K.E., Theis K.A., Boring M.A. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable Activity limitation among US adults, 2015-2040. Arthritis Rheumatol. (Hoboken, N.J.) 2016;68:1582–1587. doi: 10.1002/art.39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hootman J.M., Helmick C.G. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 6.Guccione A.A., Felson D.T., Anderson J.J. The effects of specific medical conditions on the functional limitations of elders in the Framingham study. Am. J. Public Health. 1994;84:351–358. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson V.L., Hunter D.J. The epidemiology of osteoarthritis. Best Prac. Res. Clin. Rheumatol. 2014;28:5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Bitton R. The economic burden of osteoarthritis. Am. J. Manag. Care. 2009;15:S230–S235. [PubMed] [Google Scholar]
- 9.March L.M., Bachmeier C.J. Economics of osteoarthritis: a global perspective. Bailliere’s Clin. Rheumatol. 1997;11:817–834. doi: 10.1016/s0950-3579(97)80011-8. [DOI] [PubMed] [Google Scholar]
- 10.Lee A.S., Ellman M.B., Yan D. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527:440–447. doi: 10.1016/j.gene.2013.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapoor M., Martel-Pelletier J., Lajeunesse D., Pelletier J.P., Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 12.Baltzer A.W., Ostapczuk M.S., Stosch D., Seidel F., Granrath M. A new treatment for hip osteoarthritis: clinical evidence for the efficacy of autologous conditioned serum. Orthop. Rev. 2013;5:59–64. doi: 10.4081/or.2013.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng D., Dan Y., Yang S.H. Controlled chondrogenesis from adipose-derived stem cells by recombinant transforming growth factor-beta3 fusion protein in peptide scaffolds. Acta Biomater. 2015;11:191–203. doi: 10.1016/j.actbio.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 14.Mazor M., Lespessailles E., Coursier R., Daniellou R., Best T.M., Toumi H. Mesenchymal stem-cell potential in cartilage repair: an update. J. Cell Mol. Med. 2014;18:2340–2350. doi: 10.1111/jcmm.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gigante A., Cecconi S., Calcagno S., Busilacchi A., Enea D. Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthrosc. Techniq. 2012;1:e175–e180. doi: 10.1016/j.eats.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kon E., Filardo G., Roffi A., Andriolo L., Marcacci M. New trends for knee cartilage regeneration: from cell-free scaffolds to mesenchymal stem cells. Curr. Rev. Musculoskel. Med. 2012;5:236–243. doi: 10.1007/s12178-012-9135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon L.S. Osteoarthritis. Curr. Rheumatol. Rep. 1999;1:45–47. doi: 10.1007/s11926-999-0024-2. [DOI] [PubMed] [Google Scholar]
- 18.McAlindon T.E., Bannuru R.R., Sullivan M.C. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Benke M., Shaffer B. Viscosupplementation treatment of arthritis pain. Curr. Pain Headache Rep. 2009;13:440–446. doi: 10.1007/s11916-009-0072-3. [DOI] [PubMed] [Google Scholar]
- 20.Jevsevar D., Donnelly P., Brown G.A., Cummins D.S. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J. Bone Joint Surg. Am. Vol. 2015;97:2047–2060. doi: 10.2106/JBJS.N.00743. [DOI] [PubMed] [Google Scholar]
- 21.Bellamy N., Campbell J., Robinson V., Gee T., Bourne R., Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev. 2006 doi: 10.1002/14651858.CD005328.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Kim J.D., Lee G.W., Jung G.H. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2014;24:1505–1511. doi: 10.1007/s00590-013-1393-9. [DOI] [PubMed] [Google Scholar]
- 23.Belmont P.J., Jr., Goodman G.P., Waterman B.R., Bader J.O., Schoenfeld A.J. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J. Bone Joint Surg. Am. Vol. 2014;96:20–26. doi: 10.2106/JBJS.M.00018. [DOI] [PubMed] [Google Scholar]
- 24.Anz A.W., Hackel J.G., Nilssen E.C., Andrews J.R. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J. Am. Acad. Orthop. Surg. 2014;22:68–79. doi: 10.5435/JAAOS-22-02-68. [DOI] [PubMed] [Google Scholar]
- 25.Koelling S., Miosge N. Stem cell therapy for cartilage regeneration in osteoarthritis. Expet Opin. Biol. Ther. 2009;9:1399–1405. doi: 10.1517/14712590903246370. [DOI] [PubMed] [Google Scholar]
- 26.Szychlinska M.A., Stoddart M.J., D'Amora U., Ambrosio L., Alini M., Musumeci G. Mesenchymal stem cell-based cartilage regeneration approach and cell senescence: can we manipulate cell aging and function? Tissue Eng. B Rev. 2017;23:529–539. doi: 10.1089/ten.TEB.2017.0083. [DOI] [PubMed] [Google Scholar]
- 27.Pittenger M.F., Mackay A.M., Beck S.C. Multilineage potential of adult human mesenchymal stem cells. Science (New York, N.Y.). 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 28.Lee E.H., Hui J.H. The potential of stem cells in orthopaedic surgery. J. Bone Joint Surg. Br. Vol. 2006;88:841–851. doi: 10.1302/0301-620X.88B7.17305. [DOI] [PubMed] [Google Scholar]
- 29.Turner L.G. Federal regulatory oversight of US clinics marketing adipose-derived autologous stem cell interventions: insights from 3 new FDA draft guidance documents. Mayo Clin. Proc. 2015;90:567–571. doi: 10.1016/j.mayocp.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Jager M., Hernigou P., Zilkens C., Herten M., Fischer J., Krauspe R. [Cell therapy in bone-healing disorders] Orthopä. 2010;39:449–462. doi: 10.1007/s00132-009-1583-7. quiz 463. [DOI] [PubMed] [Google Scholar]
- 31.McCarrel T., Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J. Orthop. Res. Off. Publ Orthop. Res. Soc. 2009;27:1033–1042. doi: 10.1002/jor.20853. [DOI] [PubMed] [Google Scholar]
- 32.Huang A.H., Motlekar N.A., Stein A., Diamond S.L., Shore E.M., Mauck R.L. High-throughput screening for modulators of mesenchymal stem cell chondrogenesis. Ann. Biomed. Eng. 2008;36:1909–1921. doi: 10.1007/s10439-008-9562-4. [DOI] [PubMed] [Google Scholar]
- 33.Indrawattana N., Chen G., Tadokoro M. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem. Biophys. Res. Commun. 2004;320:914–919. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 34.Chen F.H., Tuan R.S. Mesenchymal stem cells in arthritic diseases. Arthritis Res. Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinert A.F., Rackwitz L., Gilbert F., Noth U., Tuan R.S. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl. Med. 2012;1:237–247. doi: 10.5966/sctm.2011-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sampson S., Botto-van Bemden A., Aufiero D. Autologous bone marrow concentrate: review and application of a novel intra-articular orthobiologic for cartilage disease. Physician Sportsmed. 2013;41:7–18. doi: 10.3810/psm.2013.09.2022. [DOI] [PubMed] [Google Scholar]
- 37.Jevotovsky D.S., Alfonso A.R., Einhorn T.A., Chiu E.S. Osteoarthritis and stem cell therapy in humans: a systematic review. Osteoarthritis Cartilage. 2018;26:711–729. doi: 10.1016/j.joca.2018.02.906. [DOI] [PubMed] [Google Scholar]
- 38.Hendrich C., Franz E., Waertel G., Krebs R., Jager M. Safety of autologous bone marrow aspiration concentrate transplantation: initial experiences in 101 patients. Orthop. Rev. 2009;1:e32. doi: 10.4081/or.2009.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jager M., Jelinek E.M., Wess K.M. Bone marrow concentrate: a novel strategy for bone defect treatment. Curr. Stem Cell Res. Ther. 2009;4:34–43. doi: 10.2174/157488809787169039. [DOI] [PubMed] [Google Scholar]
- 40.Sampson S., Smith J., Vincent H., Aufiero D., Zall M., Botto-van-Bemden A. Intra-articular bone marrow concentrate injection protocol: short-term efficacy in osteoarthritis. Regen. Med. 2016;11:511–520. doi: 10.2217/rme-2016-0081. [DOI] [PubMed] [Google Scholar]
- 41.Shapiro S.A., Kazmerchak S.E., Heckman M.G., Zubair A.C., O'Connor M.I. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am. J. Sports Med. 2016 doi: 10.1177/0363546516662455. [DOI] [PubMed] [Google Scholar]
- 42.Centeno C.J., Al-Sayegh H., Bashir J., Goodyear S., Freeman M.D. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Muscoskel. Disord. 2015;16:258. doi: 10.1186/s12891-015-0714-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schippinger G., Pruller F., Divjak M. Autologous platelet-rich plasma preparations: influence of nonsteroidal anti-inflammatory drugs on platelet function. Orthop. J. Sports Med. 2015;3 doi: 10.1177/2325967115588896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyles C.C., Houdek M.T., Wyles S.P., Wagner E.R., Behfar A., Sierra R.J. Differential cytotoxicity of corticosteroids on human mesenchymal stem cells. Clin. Orthop. Relat. Res. 2015;473:1155–1164. doi: 10.1007/s11999-014-3925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters A.E., Watts A.E. Biopsy needle advancement during bone marrow aspiration increases mesenchymal stem cell concentration. Front. Vet. Sci. 2016;3:23. doi: 10.3389/fvets.2016.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strimpakos N., Dapka F., Papachristou A., Kapreli E. The 12-item oxford knee score: cross-cultural adaptation into Greek and assessment of its psychometric properties. Physiotherapy. 2015;101(Supplement 1):e1445–e1446. [Google Scholar]
- 48.O'Driscoll S.W. The healing and regeneration of articular cartilage. J. Bone Joint Surg. Am. Vol. 1998;80:1795–1812. [PubMed] [Google Scholar]
- 49.Bianco P., Riminucci M., Gronthos S., Robey P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells (Dayton, Ohio) 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 50.Maxson S., Lopez E.A., Yoo D., Danilkovitch-Miagkova A., Leroux M.A. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl. Med. 2012;1:142–149. doi: 10.5966/sctm.2011-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li H., Fu X. Mechanisms of action of mesenchymal stem cells in cutaneous wound repair and regeneration. Cell Tissue Res. 2012;348:371–377. doi: 10.1007/s00441-012-1393-9. [DOI] [PubMed] [Google Scholar]
- 52.Abumaree M., Al Jumah M., Pace R.A., Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012;8:375–392. doi: 10.1007/s12015-011-9312-0. [DOI] [PubMed] [Google Scholar]
- 53.Quarto R., Mastrogiacomo M., Cancedda R. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 54.Sauerbier S., Rickert D., Gutwald R. Bone marrow concentrate and bovine bone mineral for sinus floor augmentation: a controlled, randomized, single-blinded clinical and histological trial--per-protocol analysis. Tissue Eng. 2011;17:2187–2197. doi: 10.1089/ten.TEA.2010.0516. [DOI] [PubMed] [Google Scholar]
- 55.Gupta P.K., Das A.K., Chullikana A., Majumdar A.S. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res. Ther. 2012;3:25. doi: 10.1186/scrt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oh J.H., Kim W., Park K.U., Roh Y.H. Comparison of the cellular composition and cytokine-release kinetics of various platelet-rich plasma preparations. Am. J. Sports Med. 2015;43:3062–3070. doi: 10.1177/0363546515608481. [DOI] [PubMed] [Google Scholar]
- 57.Centeno C., Pitts J., Al-Sayegh H., Freeman M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. BioMed Res. Int. 2014;2014:370621. doi: 10.1155/2014/370621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giannini S., Buda R., Cavallo M. Cartilage repair evolution in post-traumatic osteochondral lesions of the talus: from open field autologous chondrocyte to bone-marrow-derived cells transplantation. Injury. 2010;41:1196–1203. doi: 10.1016/j.injury.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 59.Kim Y.S., Choi Y.J., Lee S.W. Assessment of clinical and MRI outcomes after mesenchymal stem cell implantation in patients with knee osteoarthritis: a prospective study. Osteoarthritis Cartilage. 2016;24:237–245. doi: 10.1016/j.joca.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 60.Davatchi F., Abdollahi B.S., Mohyeddin M., Shahram F., Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 2011;14:211–215. doi: 10.1111/j.1756-185X.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 61.Emadedin M., Aghdami N., Taghiyar L. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch. Iran. Med. 2012;15:422–428. [PubMed] [Google Scholar]
- 62.Centeno C.J., Busse D., Kisiday J., Keohan C., Freeman M., Karli D. Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells. Med. Hypotheses. 2008;71:900–908. doi: 10.1016/j.mehy.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 63.Varma H.S., Dadarya B., Vidyarthi A. The new avenues in the management of osteo-arthritis of knee--stem cells. J. Indian Med. Assoc. 2010;108:583–585. [PubMed] [Google Scholar]
- 64.Gigante A., Calcagno S., Cecconi S., Ramazzotti D., Manzotti S., Enea D. Use of collagen scaffold and autologous bone marrow concentrate as a one-step cartilage repair in the knee: histological results of second-look biopsies at 1 year follow-up. Int. J. Immunopathol. Pharmacol. 2011;24:69–72. doi: 10.1177/03946320110241S213. [DOI] [PubMed] [Google Scholar]
- 65.Buda R., Vannini F., Cavallo M., Grigolo B., Cenacchi A., Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J. Bone Joint Surg. Am. Vol. 2010;92(Suppl 2):2–11. doi: 10.2106/JBJS.J.00813. [DOI] [PubMed] [Google Scholar]
- 66.Wakitani S., Nawata M., Tensho K., Okabe T., Machida H., Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J. Tissue Eng. Regen. Med. 2007;1:74–79. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 67.Haleem A.M., Singergy A.A., Sabry D. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1:253–261. doi: 10.1177/1947603510366027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nejadnik H., Hui J.H., Feng Choong E.P., Tai B.C., Lee E.H. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am. J. Sports Med. 2010;38:1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 69.Bain B.J. Bone marrow biopsy morbidity and mortality. Br. J. Haematol. 2003;121:949–951. doi: 10.1046/j.1365-2141.2003.04329.x. [DOI] [PubMed] [Google Scholar]
- 70.Sampson S., Botto-van Bemden A., Aufiero D. Stem cell therapies for treatment of cartilage and bone disorders: osteoarthritis, avascular necrosis, and non-union fractures. PM R J. Inj. Funct. Rehabil. 2015;7:S26–S32. doi: 10.1016/j.pmrj.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 71.Veronesi F., Giavaresi G., Tschon M., Borsari V., Nicoli Aldini N., Fini M. Clinical use of bone marrow, bone marrow concentrate, and expanded bone marrow mesenchymal stem cells in cartilage disease. Stem Cell. Dev. 2013;22:181–192. doi: 10.1089/scd.2012.0373. [DOI] [PubMed] [Google Scholar]
- 72.Ankrum J., Karp J.M. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol. Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hernigou P., Poignard A., Beaujean F., Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J. Bone Joint Surg. Am. Vol. 2005;87:1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]