Abstract
Esophageal cancer is one of the leading causes of cancer-related death worldwide. Surgery plays an important role in the treatment strategies for esophageal cancer. Recent advances in surgical techniques and perioperative management have dramatically improved the mortality rate; however, esophagectomy remains a highly invasive procedure that can lead to severe postoperative complications. Future advances in thoracoscopic surgery with the development of surgical endoscopy systems such as three-dimensional (3D) imaging systems with a 4K ultra high-definition camera or two-dimensional (2D) imaging systems with an 8K camera, which is expected to provide 3D-like visual sensation, will enable us to further understand the microscopic anatomy of the thoracic cavity and mediastinum, and to perform delicate surgical procedures that enable minimally invasive esophagectomy with mediastinal lymphadenectomy. A robot-assisted thoracoscopic esophagectomy is attractive for surgeons and may be beneficial to esophageal cancer patients. Preoperative simulation and intraoperative real-time navigation are expected to further help surgeons safely perform esophagectomy with lymphadenectomy. Reduction of the lymphadenectomy field and setting of lymphadenectomy areas with highest priority may be feasible when sentinel node (SN) navigation is appropriately performed in cN0 early-stage esophageal cancer. These technical advances are expected to decrease the morbidity and mortality rate of surgery for esophageal cancer and hopefully improve oncological outcomes.
Keywords: esophagectomy, minimally invasive surgery, simulation, navigation, sentinel node
Esophageal cancer is one of the leading causes of cancer-related death worldwide. In the Asia-Pacific region, including Japan, the vast majority of esophageal cancers are squamous cell carcinomas (SCC), for which alcohol drinking and cigarette smoking are well-known risk factors.1) In contrast, in western countries, esophageal SCC is rare and most esophageal cancers are adenocarcinoma in the lower portion of the thoracic esophagus, the major risk factors for which are cigarette smoking and Barrett’s esophagus, which develops in approximately 5%–8% of patients with gastroesophageal reflux disease.2) Although pathological findings and biologic behavior differ between SCC and adenocarcinoma, surgery plays an important role in achieving locoregional control in the treatment strategies for both types of esophageal carcinoma. Surgical treatment of esophageal cancer consists of esophagectomy, two- or three-field lymphadenectomy and reconstruction using certain organs such as the stomach. Recent advances in surgical techniques and perioperative management have enabled us to perform one-stage esophagectomy with two- or three-field lymphadenectomy followed by reconstruction for patients with esophageal cancer, which dramatically improved the mortality rate; however, esophagectomy remains a highly invasive procedure that can lead to severe postoperative complications.3)
Esophagectomy was originally performed by thoracotomy and open laparotomy, and those approaches are still selected as a standard operative procedure for some cases or in some institutions. Thoracoscopic surgery with or without the laparoscopic approach has been developed as a minimally invasive surgery for esophageal cancer; however, its advantages with regard to short-term outcomes and the oncological feasibility of minimally invasive esophagectomy have not been adequately established.4) Although the non-inferiority of thoracoscopic esophagectomy to open esophagectomy in terms of overall survival for clinical Stage I–III esophageal cancer is currently being investigated in a multi-institutional randomized Phase III trial (JCOG1409) in Japan,5) thoracoscopic surgery is becoming a major method for esophagectomy with lymphadenectomy for patients with esophageal cancer in developed countries including Japan.
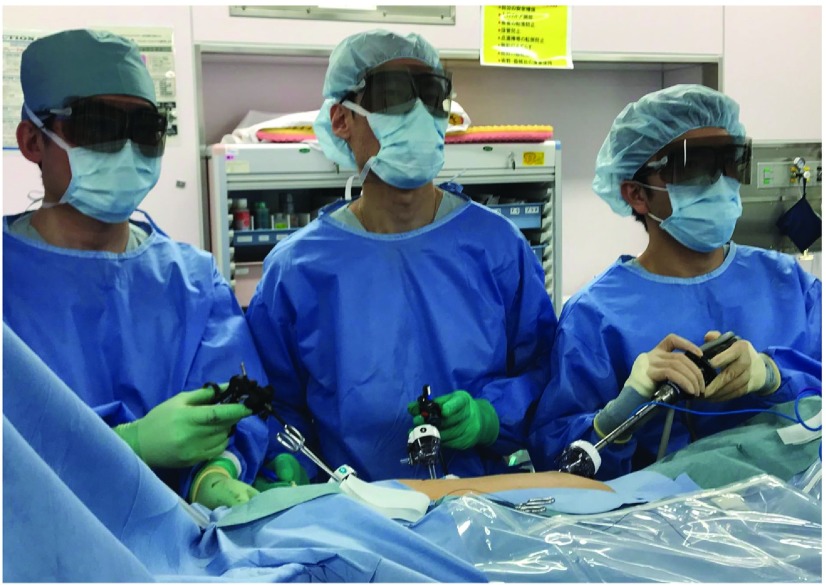
Recent advances in thoracoscopic surgery with the development of surgical endoscopy systems have helped us to understand the microscopic anatomy of the thoracic cavity and mediastinum, and perform fine operations with mediastinal lymphadenectomy. Currently, three-dimensional (3D) imaging systems with a digital 3D full high-definition (HD) camera and 2D imaging systems with a 4K ultra HD (UHD) camera are available for thoracoscopic surgery, which provide real images in surgical fields and allow surgeons to observe fine patterns and structures in high precision. In future, 3D imaging systems with a 4K UHD camera will be developed, which will enable us to further understand the microscopic anatomy and to perform fine and safe operations. In the vast majority of current 3D imaging systems, surgeons need to wear 3D glasses to see a slightly different picture on screen by each eye, which decreases light and causes somewhat dim images (Fig. 1). Because 4K UHD generates a wider color gamut by adopting the 4K color format, which enables rich color reproducibility and light images on screen, future 3D imaging systems with 4K UHD may overcome the limitations of current 3D imaging systems. Furthermore, glasses-free 3D display systems have been developed and will be widely available in the near futre. In addition, downsizing of the 8K camera has been exploited and future surgical endosopy may be equipped with an 8K camera, which is expected to provide 3D-like visual sensation even with 2D imaging systems. A robot-assisted thoracoscopic esophagectomy using the Da Vinci surgical system has also been performed in many institutions and is reportedly feasible for performing an effective lymphadenectomy.6–8) The 3D effect of the field of view and articulated forceps of the Da Vinc surgical system are thought to enable delicate surgical procedures. Although a robot-assisted thoracoscopic esophagectomy is attractive for surgeons and may be beneficial to esophageacl cancer patients, its cost is a matter of great concern, and special training is necessary for the use of the Da Vinc surgical system. As several campanies are developing robot-assisted surgical systems other than the Da Vinc, further technical development of surgical systems and decreased costs are to be expected in the future.
Fig. 1. Intraperative image of surgeons wearing 3D glasses during throracoscopic esophagectomy on the 3D imaging system.
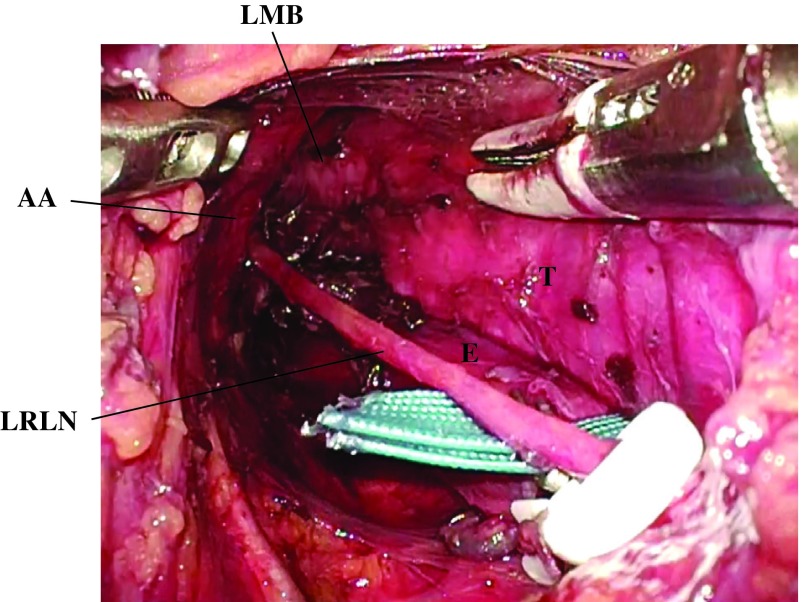
Recently, single-port mediastinoscope-assisted transhiatal esophagectomy with lymphadenectomy has been developed as a minimally invasive surgery for thoracic esophageal cancer that can reportedly be applied to patients with low pulmonary function (Fig. 2).9,10) Although special skills are needed to safely perform this surgical procedure as a curative operation without complications such as recurrent laryngeal nerve paralysis, future development of surgical instruments and intraoperative recurrent laryngeal nerve monitoring may enable us to more safely perform this surgical procedure. Robot-assisted surgical systems will also help perform delicate and safe single-port mediastinoscope-assisted transhiatal esopagectomy with lymphadenectomy. However, oncological outcomes of single-port mediastinoscopeassisted transhiatal esophagectomy need to be investigated in future studies.
Fig. 2. Intraoperative findings in the upper mediastinum during the single-port mediastinoscope-assisted transhiatal esophagectomy with lymphadenectomy. AA: aortic arch; E: esophagus; LMB: left main bronchus; LRLN: left recurrent laryngeal nerve; T: trachea.
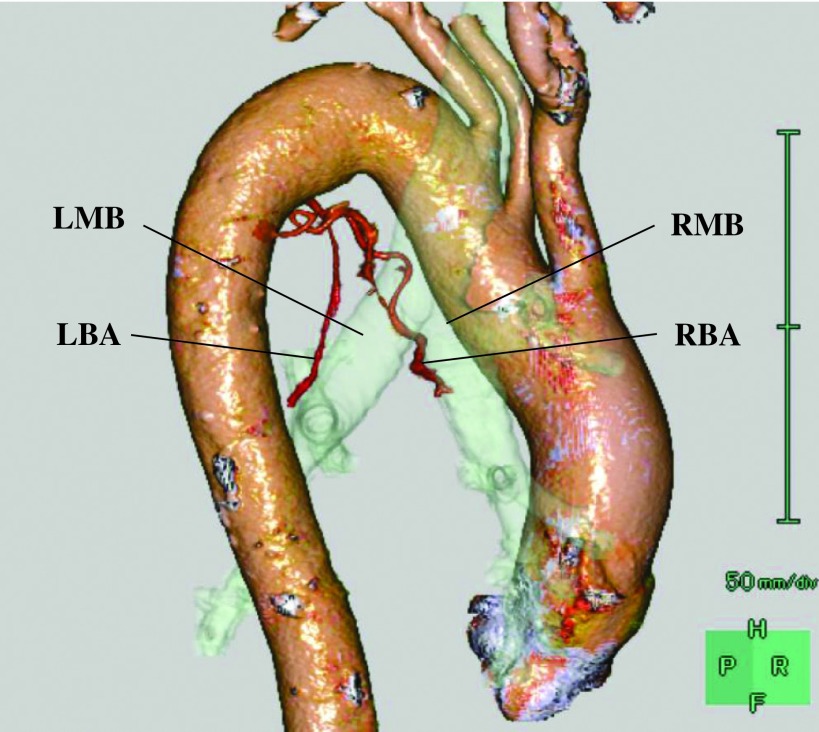
Preoperative simulation for vessels such as bronchial arteries using three-dimensional computed tomographic angiography (3D-CTA) is useful to understand vessel anatomy, which we need to take care during esophagectomy to avoid tracheobronchial ischemia and unexpected massive bleeding (Fig. 3).11) Furthermore, intraoperative real-time navigation is expected to further help surgeons safely perform esophagectomy with lymphadenectomy. A magnetic resonance imaging (MRI)-guided robotassisted interventional surgical system as well as an MRI-compatible endoscope are reported to allow the performance of precise image-guided interventional therapy and endoscopic surgery.12) To identify esophageal lesion localization, the usefulness of laparoscopic ultrasonography using endoscopically placed marking clips (LUEMC) in esophageal cancer patients undergoing minimally invasive esophagectomy was reported.13) Although intraoperative real-time navigation systems are investigated in some clinical studies, those are preliminary models and further studies are needed to develop clinically available real-time navigation systems. In addition to preoperative anatomical simulation and intraoperative navigation for each patient, virtual reality (VR) simulators present a new paradigm in surgical education and may provide an alternative means of improving performance in thoracoscopic surgery.14)
Fig. 3. Three-dimensional computed tomography angiography image of the right and left bronchial arteries. LBA: left bronchial artery; LMB: left main bronchus; RBA: right bronchial artery; RMB: right main bronchus.
Because esophagectomy with radical lymphadenectomy is a highly invasive operation with a relatively high morbidity rate, reduction of lymphadenectomy area may be indicated for some patients with limited stage of esophageal cancer. Recently, the sentinel node (SN) concept was successfully validated for early gastric cancer through a multicenter prospective trial that mapped SNs using a dualtracer method with a radioactive colloid and blue dye.15) In gastric cancer treatment, mapping of the distribution of sentinel lymphatic basins with their pathological status could be useful in determining whether minimal degree of gastric resection is feasible.16) In contrast, SNs in thoracic esophageal cancer are widely distributed from cervical to abdominal areas.17–20) Because esophageal cancer is a very aggressive disease and advanced esophageal cancer frequently shows muitiple lymph node metastases, SN navigation may be indicated for cT1N0 esophageal cancers. A previous study showed that approximately 85% of pT1 patients had no lymph node metastasis or had lymph node metastasis only in SNs, and that radio-guided SN mapping is useful not only as an accurate diagnostic tool for detecting lymph node metastasis but also as a tool for prognostic stratification in patients with cN0 early-stage esophageal cancer.19) Because of the wide distribution of SNs and the unpredictable pattern of metastasis, esophagectomy with extended lymphadenectomy appears to be a reasonable procedure for thoracic esophageal cancer; however, optimized and individualized prophylactic nodal irradiation targeting SNs might be effective in controlling superficial cN0 esophageal SCC by chemoradiation therapy (CRT) for patients who refused esophagectomy.19) Although further studies are needed, reduction of the lymphadenectomy field and setting of lymphadenectomy areas with highest priority may be feasible when SN navigation is appropriately performed in cN0 early-stage esophageal cancer.
In conclusion, future development of thoracoscope and robotic surgical systems will help us further understand microscopic anatomy and perform delicate surgical procedures that enable minimally invasive esophagectomy. Preoperative simulation and intraoperaative navigation may also help surgeons safely perform esophagectomy with lymphadenectomy. SN mapping and SN navigation surgery would be a promising strategy for a less invasive individualized surgery for early-stage esophageal cancer. These technical advances are expected to decrease the morbidity and mortality rate of surgery for esophageal cancer and hopefully improve oncological outcomes.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1).Morita M, Saeki H, Mori M, et al. Risk factors for esophageal cancer and the multiple occurrence of carcinoma in the upper aerodigestive tract. Surgery 2002; 131: S1-6. [DOI] [PubMed] [Google Scholar]
- 2).Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349: 2241-52. [DOI] [PubMed] [Google Scholar]
- 3).Takeuchi H, Miyata H, Gotoh M, et al. A risk model for esophagectomy using data of 5354 patients included in a Japanese nationwide web-based database. Ann Surg 2014; 260: 259-66. [DOI] [PubMed] [Google Scholar]
- 4).Takeuchi H, Kawakubo H, Kitagawa Y. Current status of minimally invasive esophagectomy for patients with esophageal cancer. Gen Thorac Cardiovasc Surg 2013; 61: 513-21. [DOI] [PubMed] [Google Scholar]
- 5).Kataoka K, Takeuchi H, Mizusawa J, et al. A randomized phase III trial of thoracoscopic versus open esophagectomy for thoracic esophageal cancer: Japan clinical oncology group study JCOG1409. Jpn J Clin Oncol 2016; 46: 174-7. [DOI] [PubMed] [Google Scholar]
- 6).Bodner JC, Zitt M, Ott H, et al. Robotic-assisted thoracoscopic surgery (RATS) for benign and malignant esophageal tumors. Ann Thorac Surg 2005; 80: 1202-6. [DOI] [PubMed] [Google Scholar]
- 7).van Hillegersberg R, Boone J, Draaisma WA, et al. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc 2006; 20: 1435-9. [DOI] [PubMed] [Google Scholar]
- 8).Suda K, Ishida Y, Kawamura Y, et al. Robot-assisted thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve for esophageal squamous cell carcinoma in the prone position: technical report and short-term outcomes. World J Surg 2012; 36: 1608-16. [DOI] [PubMed] [Google Scholar]
- 9).Fujiwara H, Shiozaki A, Konishi H, et al. Single-port mediastinoscopic lymphadenectomy along the left recurrent laryngeal nerve. Ann Thorac Surg 2015; 100: 1115-7. [DOI] [PubMed] [Google Scholar]
- 10).Fujiwara H, Shiozaki A, Konishi H, et al. Perioperative outcomes of single-port mediastinoscope-assisted transhiatal esophagectomy for thoracic esophageal cancer. Dis Esophagus 2017; 30: 1-8. [DOI] [PubMed] [Google Scholar]
- 11).Wada T, Takeuchi H, Kawakubo H, et al. Clinical utility of preoperative evaluation of bronchial arteries by three-dimensional computed tomographic angiography for esophageal cancer surgery. Dis Esophagus 2013; 26: 616-22. [DOI] [PubMed] [Google Scholar]
- 12).Hashizume M. MRI-guided laparoscopic and robotic surgery for malignancies. Int J Clin Oncol 2007; 12: 94-8. [DOI] [PubMed] [Google Scholar]
- 13).Miyazaki T, Honjyo H, Sohda M, et al. Successful tumor navigation technique during intrathoracoscopic esophagectomy: laparoscopic ultrasonography using endoscopically placed marking clips. J Am Coll Surg 2015; 221: e125-8. [DOI] [PubMed] [Google Scholar]
- 14).Yiannakopoulou E, Nikiteas N, Perrea D, et al. Virtual reality simulators and training in laparoscopic surgery. Int J Surg 2015; 13: 60-4. [DOI] [PubMed] [Google Scholar]
- 15).Kitagawa Y, Takeuchi H, Takagi Y, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol 2013; 31: 3704-10. [DOI] [PubMed] [Google Scholar]
- 16).Takeuchi H, Kitagawa Y. New sentinel node mapping technologies for early gastric cancer. Ann Surg Oncol 2013; 20: 522-32. [DOI] [PubMed] [Google Scholar]
- 17).Takeuchi H, Fujii H, Ando N, et al. Validation study of radio-guided sentinel lymph node navigation in esophageal cancer. Ann Surg 2009; 249: 757-63. [DOI] [PubMed] [Google Scholar]
- 18).Uenosono Y, Arigami T, Yanagita S, et al. Sentinel node navigation surgery is acceptable for clinical T1 and N0 esophageal cancer. Ann Surg Oncol 2011; 18: 2003-9. [DOI] [PubMed] [Google Scholar]
- 19).Takeuchi H, Kawakubo H, Nakamura R, et al. Clinical significance of sentinel node positivity in patients with superficial esophageal cancer. World J Surg 2015; 39: 2941-7. [DOI] [PubMed] [Google Scholar]
- 20).Akutsu Y, Kato K, Igaki H, et al. The prevalence of overall and initial lymph node metastases in clinical T1N0 thoracic esophageal cancer: from the results of JCOG0502, a prospective multicenter study. Ann Surg 2016; 264: 1009-15. [DOI] [PubMed] [Google Scholar]