Abstract
This paper offers a review of the implementation of current wearable sensing technologies in monitoring the movement and activity of patients suffering from movement disorders. Recent literature has focused on incorporating simple and reliable wearable technologies for the continuous and objective monitoring of patient movement during normal daily activities. However, the use of such wearable sensing technologies has yet to find its way to clinical practice. In the following, the basic elements of such monitoring systems and their applications are introduced, and a discussion regarding current clinical applications is presented.
Keywords: Wearable sensors, Movement disorders, Activity monitoring
Wearable sensing technology is a category of technology devices worn by subjects that allow continuous physiological monitoring with reduced manual intervention and at low cost. Wearable sensors concerned with quantification of movement have been the focus of research efforts to further enhance clinical assessment of motor dysfunction. The aim of these efforts is to shift clinical assessment of motor dysfunction from the current subjective methods applied in some rating scales to quantifiable and accurate measures and to provide long-term quantified measures that monitor the patient's condition and overall motor progression [1], [2], [3], [4]. In the past couple of decades, there have been significant advances in the miniaturization, proliferation, accessibility and sophistication of sensor technology. These advances have fueled the exploration and implementation of wearable sensors and feedback devices for the mass population with the main objective of improving healthcare. Monitoring a patient's health status remotely and continuously for a long duration without the need for hospitalization not only provides the opportunity for better management of the patient's condition, but also reduces the consequent healthcare costs [5], [6], [7].
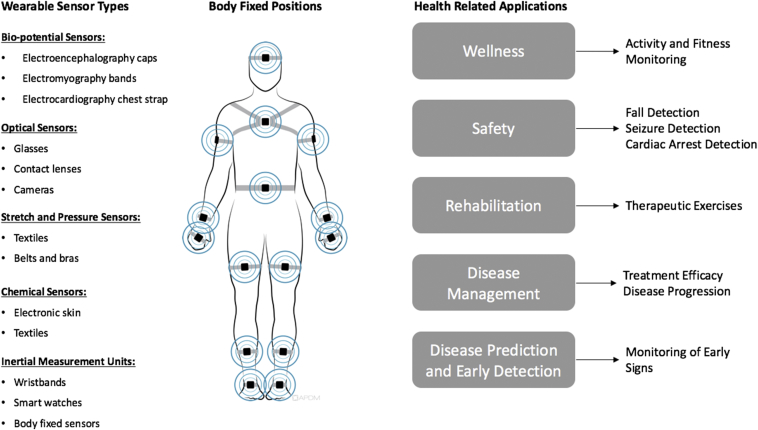
Wearable sensors in healthcare applications can be categorized based on the measured entity into the following subtypes: biopotential sensors, optical sensors, stretch and pressure sensors, chemical sensors, and inertial measurement units [Fig. 1].
Fig. 1.
Diagram showing the different categories of wearable sensors, common body fixed positions based on sensor type, and a general summary of health related applications [16].
Commercially available devices of the different sensor types have been gradually increasing. For example, existing biopotential sensors, include a wearable EEG system that is designed for brain monitoring and cognitive assessment and has been used in different studies for brain computer interface development, emotion recognition and assessment of auditory events [8], [9]. Other examples include a chest strap [10], which acts as a portable ECG device designed for heart rate monitoring commonly used in fitness domains. Stretch sensors have been used for human body motion sensing [11] and monitoring of chronic diseases [12].
The incorporation of wearable sensors in rehabilitation and disease management applications has also seen its fair share of growth over the past few decades [13], [14], [15]. In the most part, wearable sensors have been used to facilitate the implementation of home-based rehabilitation and therapeutic programs. The importance of such systems in monitoring patients suffering from movement disorders lies in their ability to provide objective and continuous quantification of patients' movements. This has been a main concern for physicians, seeing as common evaluation of movement disorders is normally conducted through clinical visits that include subjective methods and are spread out over long periods of time: an issue which hinders the physician's ability to properly evaluate the patient's progression. The following sections include the basic elements of wearable sensor-based monitoring systems used for the evaluation of movement disorders, a review of some of the applications of such systems, and finally a discussion of their clinical application.
Wearable monitoring systems for the quantification of movement
The quantification of human movement is based on key technologies that can be summed up into three parts: the sensors and data collection hardware, the communication hardware and software to transfer the collected data, and finally the processing and analysis methods used to extract meaningful information from the collected data. The current methods for physical activity monitoring and motion analysis are mostly based on inertial parameters such as acceleration, angular velocity, and in some cases by measuring the magnetic field surrounding the subject. These inertial parameters are well established as suitable measures for quantifying and analyzing states of rigid body kinematics [17]. Because of the segmental decomposition of the human body defined in biomechanics, these parameters have shown great relevance in quantifying movement, detecting pathological aspects and movement dysfunction, and assessing performance. As the understanding of the complexity of simple human movements and gestures grew along with the advances in electronics technology, micro-electromechanical systems (MEMS) emerged, allowing for the combination of multiple sensors into miniaturized devices along with processors and data storage capabilities [18]. As a result, major leaps in the field of inertial sensing followed, leading to the development of IMUs [19], which are devices that house a number of sensor components and aim to better quantify different aspects of complex movements [20], [21], [22].
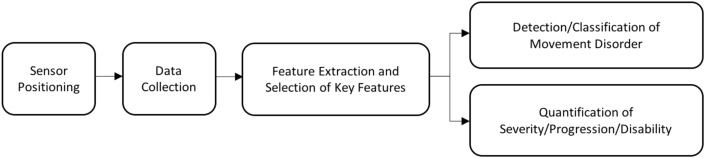
Generally, quantification of patient movement for the evaluation and detection of disorders follows the scheme in Fig. 2. While several advances have been made in this domain, certain design issues have been addressed in recent years. Some of these issues include choice of sensors, optimal sensor positions, data collection protocols, as well as attribute selection [23]. However, the large scope of sensors, methods and applications considered, has made it very difficult to properly identify optimal conditions for quantification of movement disorders for implementation in clinical assessment.
Fig. 2.
General scheme of development of a monitoring system for the quantification of movement.
Clinical applications of wearable sensors
One of the most common clinical applications of wearable sensors is in the field of movement disorders, where patient movement is monitored in an objective and continuous manner during day-to-day activities or through clinical assessments during patient visits. Table 1 introduces some of the recent literature dedicated to this subject, showing the quantified movement disorder, sensor choice and placement, and the protocol of activities for data acquisition.
Table 1.
Recent literature on the implementation of wearable sensors for evaluating and quantifying movement disorders.
| Reference | Movement disorder | Sensor | Placement | Protocol |
|---|---|---|---|---|
| Rigas 2012 [24] | Tremor | Accelerometers | Right and left wrist, right and left leg, chest and waist | Postural activities |
| Mariani 2013 [25] | Gait | Physilog module | On-shoe fixation | Standard 3-m TUG and gait tests |
| Cole 2014 [26] | Tremor & Dyskinesia | Triaxial accelerometers and sEMG | Wrist | unconstrained activities of daily living in home environment |
| Bernard 2015 [27] | Motor response fluctuations | Triaxial accelerometer | Lower back | Walking and being inactive (i.e. lying, sitting) |
| Lennon 2015 [28] | Dyskineisa | Accelerometer & gyroscopes | Wrists and ankles | Daily life activities |
| Jalloul 2015 [29], [30] | Dyskinesia | Shimmer IMUs | Ankle, hip, thigh, neck, wrist and arm | Simple daily life activities |
| Howcroft 2016 [31] | Gait | Pressure-sensing insoles and Triaxial accelerometers | Head, pelvis, and Left and right shanks | Dual-Task gait exercises |
| Bourke 2016 [32] | Fall detection | Triaxial accelerometer and triaxial gyroscope | L5 lumbar position | Activities of daily living |
| Kobsar 2017 [33] | Knee Osteoarthritis | Accelerometers | Back, thigh and shank | Hip-strengthening exercise intervention |
| Denault 2017 [34] | Bradykinesia | Triaxial accelerometers | Left & right upper arm, left & right forearm, left & right thigh, left & right hank | Clinical evaluation |
Specific movement disorders such as essential tremor and Parkinson's disease (PD) motor symptoms, including tremor, bradykinesia, and dyskinesia, are some of the most explored for objective evaluation through wearable sensors. For example, the authors were able to successfully quantify tremor severity and distinguish between resting and postural tremors [24]. The ability to replace standard gait and TUG tests with an objective system was also validated [25]. The authors, on the other hand, introduced a dynamic method for tracking presence and intensity of tremor and dyskinesia in PD patients with an error rate below 10% [26]. Another method for the detection of dyskinesia in PD patients is introduced in where activity classification in done prior to the detection of dyskinesia in order to enhance the accuracy of the system [29], [30]. While accelerometer data was used to estimate clinical scores of bradykinesia and showed good correlation with limb-specific scores [34].
Wearable sensors have also been used to facilitate the implementation of home-based rehabilitation therapeutic programs post trauma or surgeries. Evaluation of rehabilitation programs for patients with Knee Osteoarthritis was done [33], where accelerometers were used to determine post-intervention response for patients following a six week rehabilitation program. Another area which has been extensively researched includes monitoring activities of the elderly and individuals with disease and/or movement dysfunction, who are prone to falls and show a high risk of injury [31], where the use of wearable sensors helped to identify gait changes in the elderly and distinguish between fallers and non-fallers. While the authors were able to distinguish between real-world falls and normal activity using lumbar-positioned sensors with an accuracy of 88% [32].
It is worth noting that identifying the context in which the movement disorder/disability is occurring offers significant insight into the patient's condition. For example, in the case of fall risk assessment for elderly patients or patients who are prone to falls, the rate of occurrence can determine the patient's overall condition and progress. While in the case of patients suffering from movement disorders brought on by neurological diseases such as PD, then features relative to the quality of movement and overall physical activity offers information about the patient's disease progression and response to therapeutic plans.
Conflicts of interest
There are no conflicts of interest to disclose.
Discussion and conclusion
The ability to capture movement data and employ it to optimize treatment strategies relies on the understanding of behaviors that occur over long periods of time. In the absence of continuous visual observation, providing context to the measured quantities is essential in identifying the incidents occurring. Many of the already published literature has been reviewed [35], [36], [37], [38] and it has been shown clear that the results displayed in these studies are highly dependent on the variable sensing modules, protocols and processing techniques.
The transition from subjective towards objective and automated assessment methods of movement disorders is a process that requires the integration of the several aspects of movement analysis. Furthermore, monitoring activities and providing descriptive measures of certain parameters, such as gait or posture analysis, gives physicians and nurses the ability to better assess a patient's condition. A better understanding of the progression and/or deterioration of physical activities could play a major role in early diagnosis, allowing for more efficient treatment and consequently lower healthcare costs.
While it seems that the interest in applying wear-able sensors for the evaluation of patients suffering from movement disorders is only increasing, a general consensus on standards, such as sensor placement and evaluation techniques, is imperative for their eventual implementation in clinical practice.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Johansson D., Malmgren K., Alt Murphy M. Wearable sensors for clinical applications in epilepsy, Parkinson's disease, and stroke: a mixed-methods systematic review. J Neurol. 2018:1432–1459. doi: 10.1007/s00415-018-8786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulliam C.L., Heldman D.A., Brokaw E.B., Mera T.O., Mari Z.K., Burack M.A. Continuous assessment of levodopa response in Parkinson's disease using wearable motion sensors. IEEE Trans Biomed Eng. 2018;65:159–164. doi: 10.1109/TBME.2017.2697764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ameli S., Naghdy F., Stirling D., Naghdy G., Aghmesheh M. Objective clinical gait analysis using inertial sensors and six minute walking test. Pattern Recogn. 2017;63:246–257. [Google Scholar]
- 4.Gilmore G., Jog M. Movement disorders rehabilitation. Springer; Cham: 2017. Future perspectives: assessment tools and rehabilitation in the new age; pp. 155–182. [Google Scholar]
- 5.Muennig P.A., Glied S.A. What changes in survival rates tell us about US health care. Health Aff. 2010;29:2105–2113. doi: 10.1377/hlthaff.2010.0073. [DOI] [PubMed] [Google Scholar]
- 6.Gulley S.P., Rasch E.K., Chan L. If we build it, who will come?: Working-age adults with chronic health care needs and the medical home. Med Care. 2011;49:149–155. doi: 10.1097/MLR.0b013e3182028380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gulley S.P., Rasch E.K., Chan L. Ongoing coverage for ongoing care: access, utilization, and out-of-pocket spending among uninsured working-aged adults with chronic health care needs. Am J Public Health. 2011;101:368–375. doi: 10.2105/AJPH.2010.191569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y., Jiang X., Cao T., Wan F., Mak P.U., Mak P.I. Virtual environments human-computer interfaces and measurement systems (VECIMS) IEEE International Conference; 2012. Implementation of SSVEP based BCI with Emotiv EPOC; pp. 34–37. [Google Scholar]
- 9.Vokorokos L., Mados B., Ádám N., Baláz A. Data acquisition in non-invasive brain-computer interface using emotiv epoc neuroheadset. Acta Electrotechnica et Informatica. 2012;12:5–8. [Google Scholar]
- 10.Mateu-Mateus M., Guede-Fernández F., García-González M.A. 6th European conference of the International Federation for Medical and Biological Engineering. Springer; Cham: 2015. RR time series comparison obtained by H7 polar sensors or by photoplethysmography using smartphones: breathing and devices influences; pp. 264–267. [Google Scholar]
- 11.O'Brien B., Gisby T., Anderson I.A. Electroactive polymer actuators and devices (EAPAD) International Society for Optics and Photonics; 2014. Stretch sensors for human body motion; p. 9056. 905618. [Google Scholar]
- 12.OQuigley C., Sabourin M., Coyle S., Connolly J., Condall J., Curran K. Wearable and Implantable body sensor networks workshops (BSN workshops) IEEE; 2014. Characteristics of a piezo-resistive fabric stretch sensor glove for home-monitoring of rheumatoid arthritis; pp. 23–26. [Google Scholar]
- 13.Daneault J.F., Kanzler C., Lee S., Golabchi F., Vergara-Diaz G., Carvalho G.F. Exploring the use of wearable sensors to monitor drug response of patients with Parkinson’s disease in the home setting. Neurology. 2017;88:P4.002. [Google Scholar]
- 14.Eskofier B.M., Lee S.I., Baron M., Simon A., Martindale C.F., Garner H. An overview of smart shoes in the internet of health things: gait and mobility assessment in health promotion and disease monitoring. Appl Sci. 2017;7:986. [Google Scholar]
- 15.Vogel J., Auinger A., Riedl R., Kindermann H., Helfert M., Ocenasek H. Digitally enhanced recovery: Investigating the use of digital self-tracking for monitoring leisure time physical activity of cardiovascular disease (CVD) patients undergoing cardiac rehabilitation. PloS one. 2017;12:e0186261. doi: 10.1371/journal.pone.0186261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel S., Park H., Bonato P., Chan L., Rodgers M. A review of wearable sensors and systems with application in rehabilitation. J Neuroeng Rehabil. 2012;9:21. doi: 10.1186/1743-0003-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luinge H.J. Twente University Press; 2002. Inertial sensing of human movement. [Google Scholar]
- 18.Ghodssi R., Lin P., editors. MEMS materials and processes handbook. Springer Science & Business Media; 2011. [Google Scholar]
- 19.Woodman O.J. University of Cambridge, Computer Laboratory; 2007. An introduction to inertial navigation. [Google Scholar]
- 20.Luinge H.J., Veltink P.H. Measuring orientation of human body segments using miniature gyroscopes and accelerometers. Med Biol Eng Comput. 2005;43:273–282. doi: 10.1007/BF02345966. [DOI] [PubMed] [Google Scholar]
- 21.Bulling A., Blanke U., Schiele B. A tutorial on human activity recognition using body-worn inertial sensors. ACM Comput Surv (CSUR) 2014;46:33. [Google Scholar]
- 22.Lambrecht S., del-Ama A.J. Springer. Emerging Therapies in Neurorehabilitation; 2014. Human movement analysis with inertial sensors; pp. 305–328. [Google Scholar]
- 23.Aggarwal J.K., Ryoo M.S. Human activity analysis: A review. ACM Comput Surv (CSUR) 2011;43:16. [Google Scholar]
- 24.Rigas G., Tzallas A.T., Tsipouras M.G., Bougia P., Tripoliti E.E., Baga D. Assessment of tremor activity in the Parkinson’s disease using a set of wearable sensors. IEEE Trans Inf Technol Biomed. 2012;16:478–487. doi: 10.1109/TITB.2011.2182616. [DOI] [PubMed] [Google Scholar]
- 25.Mariani B., Jiménez M.C., Vingerhoets F.J., Aminian K. On-shoe wearable sensors for gait and turning assessment of patients with Parkinson's disease. IEEE Trans Biomed Eng. 2013;60:155–158. doi: 10.1109/TBME.2012.2227317. [DOI] [PubMed] [Google Scholar]
- 26.Cole B.T., Roy S.H., De Luca C.J., Nawab S.H. Dynamical learning and tracking of tremor and dyskinesia from wearable sensors. IEEE Trans Neural Syst Rehabil Eng. 2014;22:982–991. doi: 10.1109/TNSRE.2014.2310904. [DOI] [PubMed] [Google Scholar]
- 27.Bernad-Elazari H., Weiss A., Oren S., Cohen Y., Mirelman A., Giladi N. Using a wearable sensor to evaluate activity and motor response fluctuations in patients with Parkinson's disease (pd): Preliminary findings: 675. Mov Disord. 2015;30:S265. [Google Scholar]
- 28.Lennon T., Bernier T., Tamayo D., Goldberg C., Mankodiya K. IEEE; 2015. Multi-sensory system for monitoring dyskinesia in movement disorders. Biomedical engineering conference (NEBEC) pp. 1–2. [Google Scholar]
- 29.Jalloul N., Porée F., Viardot G., L'Hostis P., Carrault G. Engineering in Medicine and Biology Society (EMBC), IEEE; 2015. Detection of Levodopa induced Dyskinesia in Parkinson's DIsease patients based on activity classification; pp. 5134–5137. [DOI] [PubMed] [Google Scholar]
- 30.Jalloul N., Porée F., Viardot G., L'Hostis P., Carrault G. Advances in Biomedical Engineering (ICABME), IEEE; 2015. Feature selection for activity classification and Dyskinesia detection in Parkinson's disease patients; pp. 146–149. [DOI] [PubMed] [Google Scholar]
- 31.Howcroft J., Kofman J., Lemaire E.D., McIlroy W.E. Analysis of dual-task elderly gait in fallers and non-fallers using wearable sensors. J Biomech. 2016;49:992–1001. doi: 10.1016/j.jbiomech.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Bourke A.K., Klenk J., Schwickert L., Aminian K., Ihlen E.A., Mellone S. Engineering in Medicine and Biology Society (EMBC), IEEE; 2016. Fall detection algorithms for real-world falls harvested from lumbar sensors in the elderly population: a machine learning approach; pp. 3712–3715. [DOI] [PubMed] [Google Scholar]
- 33.Kobsar D., Osis S.T., Boyd J.E., Hettinga B.A., Ferber R. Wearable sensors to predict improvement following an exercise intervention in patients with knee osteoarthritis. J Neuroeng Rehabil. 2017;14:94. doi: 10.1186/s12984-017-0309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daneault J.F., Lee S.I., Golabchi F.N., Patel S., Shih L.C., Paganoni S. Proceedings of the second IEEE/ACM international conference on connected health: applications, systems and engineering technologies. 2017. Estimating bradykinesia in Parkinson's disease with a minimum number of wearable sensors; pp. 264–265. [Google Scholar]
- 35.Thilarajah S., Clark R.A., Williams G. Wearable sensors and Mobile Health (mHealth) technologies to assess and promote physical activity in stroke: a narrative review. Brain Impair. 2016;17:34–42. [Google Scholar]
- 36.Chen S., Lach J., Lo B., Yang G.Z. Toward Pervasive Gait Analysis With Wearable Sensors: A Systematic Review. IEEE J Biomed Health Inf. 2016;20:1521–1537. doi: 10.1109/JBHI.2016.2608720. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez-Ferro Á., Elshehabi M., Godinho C., Salkovic D., Hobert M.A., Domingos J. New methods for the assessment of Parkinson's disease (2005 to 2015): A systematic review. Mov Disord. 2016;31:1283–1292. doi: 10.1002/mds.26723. [DOI] [PubMed] [Google Scholar]
- 38.de Lima A.L., Evers L.J., Hahn T., Bataille L., Hamilton J.L., Little M.A. Freezing of gait and fall detection in Parkinson’s disease using wearable sensors: a systematic review. J Neurol. 2017;264:1642–1654. doi: 10.1007/s00415-017-8424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]