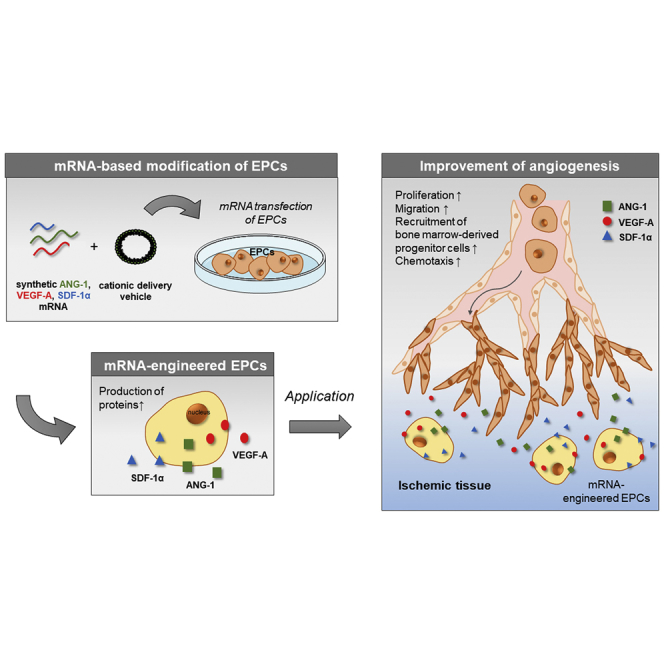
Abstract
The application of endothelial progenitor cells (EPCs) for the revascularization of ischemic tissues, such as after myocardial infarction, stroke, and acute limb ischemia, has a huge clinical potential. However, the low retention and engraftment of EPCs as well as the poor survival of migrated stem cells in ischemic tissues still hamper the successful clinical application. Thus, in this study, we engineered, for the first time, murine EPCs with synthetic mRNAs to transiently produce proangiogenic factors vascular endothelial growth factor-A (VEGF-A), stromal cell-derived factor-1α (SDF-1α), and angiopoietin-1 (ANG-1). After the transfection of cells with synthetic mRNAs, significantly increased VEGF-A, SDF-1α, and ANG-1 protein levels were detected compared to untreated EPCs. Thereby, mRNA-engineered EPCs showed significantly increased chemotactic activity versus untreated EPCs and resulted in significantly improved attraction of EPCs. Furthermore, ANG-1 mRNA-transfected EPCs displayed a strong wound-healing capacity. Already after 12 hr, 94% of the created wound area in the scratch assay was closed compared to approximately 45% by untreated EPCs. Moreover, the transfection of EPCs with ANG-1 or SDF-1α mRNA also significantly improved the in vitro tube formation capacity; however, the strongest effect could be detected with EPCs simultaneously transfected with VEGF-A, SDF-1α, and ANG-1 mRNA. In the in vivo chicken chorioallantoic membrane (CAM) assay, EPCs transfected with ANG-1 mRNA revealed the strongest angiogenetic potential with significantly elevated vessel density and total vessel network length. In conclusion, this study demonstrated that EPCs can be successfully engineered with synthetic mRNAs encoding proangiogenic factors to improve their therapeutic angiogenetic potential in patients experiencing chronic or acute ischemic disease.
Keywords: angiogenesis, EPCs, ischemia, proangiogenic factors, synthetic mRNA
Graphical Abstract
Introduction
The insufficient perfusion of tissues or organs with blood results in ischemia and often leads to conditions such as stroke, myocardial infarction, or acute limb ischemia. During ischemia, the affected tissues are damaged due to hypoxia and the lack of nutrient supply and waste removal. Thus, the formation of new blood vessels by angiogenesis and vasculogenesis is required. During angiogenesis, new blood vessels are formed from pre-existing vessels and, during vasculogenesis, de novo blood vessel formation occurs through bone marrow-derived endothelial progenitor cells (EPCs).1 Thereby, the blood supply can be restored to ischemic tissues, the tissue damage can be reduced, and, as a result, the function of affected tissues or organs can be improved.
EPCs are circulating stem cells in the bloodstream, and they are recruited by chemokines, which are secreted by injured and activated cells at the injury site to induce revascularization.2 EPCs settle and differentiate at sites of vascular lesions, and they appear to participate in vascular repair and homeostasis.3 Previous studies even identified EPCs as biomarkers in cardiovascular diseases, and a decline of EPC numbers and dysfunction were related to ischemic diseases.4, 5 Several preclinical studies have demonstrated the vasculogenic and angiogenic potential,6, 7 as well as beneficial paracrine effects, of transplanted EPCs in the treatment of ischemic diseases.8, 9 However, the success of clinical applications is limited due to low retention and engraftment of EPCs as well as the poor survival of migrated EPCs in ischemic tissues.10, 11 Furthermore, the low quantity and quality of isolated autologous EPCs is challenging for clinical applications.12 Thus, multiple animal and clinical studies with EPCs demonstrated different results in their effectiveness to treat ischemic diseases.9, 13 Consequently, novel strategies are needed to enhance the number and function of EPCs and to obtain a successful autologous EPC therapy for a more efficient tissue regeneration.
In previous studies, various growth factors, such as vascular endothelial growth factor (VEGF)14 or fibroblast growth factor (FGF),15 were applied to improve the revascularization of tissues.16 Local levels of proangiogenic proteins were upregulated by delivering recombinant proteins17, 18 or genes19, 20 using nano- or microparticles,17, 18 direct injection into the target tissue,19 ultrasound-targeted microbubble destruction (UTMD),20, 21 or sustained release from implants.22 The use of recombinant proteins is costly and it is difficult to maintain adequate protein levels in the ischemic regions due to their relatively short half-lives.23 Therefore, gene therapy with viral and non-viral vectors was used as an alternative strategy to express the desired proangiogenic proteins, and it has shown to be promising, for example, for the treatment of myocardial ischemia.13, 23
In recent years, the expression of exogenous proteins by the delivery of synthetic mRNAs has gained great importance as an alternative strategy to the viral vector or plasmid-based gene delivery methods.24 In contrast to genome-integrating gene delivery methods, the application of synthetic mRNA is a non-integrating method with no risk of insertional mutagenesis. Since the mRNA does not need to enter the nucleus, the synthetic mRNA can be efficiently delivered in dividing as well as non-dividing cells. The transfection of synthetic mRNA leads to the transient production of exogenous proteins in the cells, and, after the natural degradation of mRNA, no footprint is left in the cells.
The most intensively studied growth factor for the induction of vascularization and angiogenesis is the vascular endothelial growth factor-A (VEGF-A). It is involved in the chemotaxis, migration, and differentiation of progenitor cells; endothelial cell (EC) survival and proliferation; as well as the sprouting of vessels and vessel permeability.25 Among alternative splice variants of VEGF-A, VEGF-A165 is the quantitatively and qualitatively most important variant for angiogenesis.26 VEGF-A binds and activates VEGF receptors 1 and 2 (VEGFR-1 and VEGFR-2) expressed on vascular ECs and EPCs. VEGFR-2 has approximately 10-fold higher kinase activity than VEGFR-1, and the major proangiogenic signal is generated from the ligand-activated VEGFR-2.26 Angiopoietin-1 (ANG-1) is produced by peri-ECs and platelets.27 It binds to Tie2 receptors on ECs and maintains endothelial integrity and reduces the effects of inflammation,28 which prevents vascular leakage and stabilizes vessels. ANG-1 is further involved in EC migration and the reorganization of ECs.29 Stromal cell-derived factor-1α (SDF-1α, also known as CXCL12) is a chemokine that mediates the mobilization and recruitment of bone marrow-derived progenitor cells that express CXCR4 receptor on the cell surface, such as EPCs. Additionally, SDF-1α attenuates EC apoptosis and stimulates new vessel capillary tube formation, and the expression of SDF-1α is regulated by hypoxia.25, 30, 31
In this study, for the first time, murine EPCs were engineered by the exogenous delivery of synthetic mRNAs to increasingly express the proangiogenic proteins VEGF-A, SDF-1α, and ANG-1. The ability of EPCs to produce the desired proteins was analyzed after the transfection with synthetic mRNAs using ELISA. Next, the migration behavior of mRNA-engineered EPCs was investigated by chemotactic and wound-healing migration assay and compared with non-engineered EPCs in vitro. Then, the in vitro and in vivo angiogenic potential of mRNA-engineered EPCs was analyzed using tube formation and chick chorioallantoic membrane (CAM) assay.
Results
Generation of Synthetic mRNA Encoding ANG-1, SDF-1α, and VEGF-A
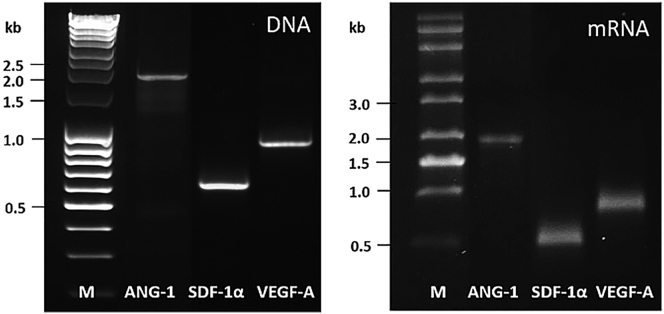
The specific length and purity of the generated PCR products and the in vitro-transcribed modified mRNAs were analyzed by agarose gel electrophoresis. After the gel electrophoresis, single bands without misamplifications and expected lengths were detected for the PCR products ANG-1 (1,794 bp), SDF-1α (567 bp), and VEGF-A (873 bp) and the resulting in vitro-transcribed mRNA containing the coding sequence, 3′ and 5′ UTR regions, and PolyA120 tail (Figure 1).
Figure 1.
Quality Control of the Generated PCR Products and In Vitro-Transcribed mRNAs
ANG-1-, SDF-1α-, and VEGF-A-encoding mRNAs were synthesized and modified with 3′-PolyA120 tail, 5′-ARCA, 100% m5CTP, 100% Ψ-UTP, and post-transcriptional phosphatase treatment. The specific lengths of the amplified DNA and the synthetic mRNA were detected using 1% agarose gel electrophoresis and GelRed staining at approximately 1.8 kb for ANG-1, 0.6 kb for SDF-1α, and 0.9 kb for VEGF-A. The 0.08- to 10-kb range mix DNA ladder and the 0.5- to 10-kb RNA ladder were used as length markers (Ms).
Expression of ANG-1, SDF-1α, and VEGF-A after the Transfection of EPCs with Synthetic Modified mRNAs
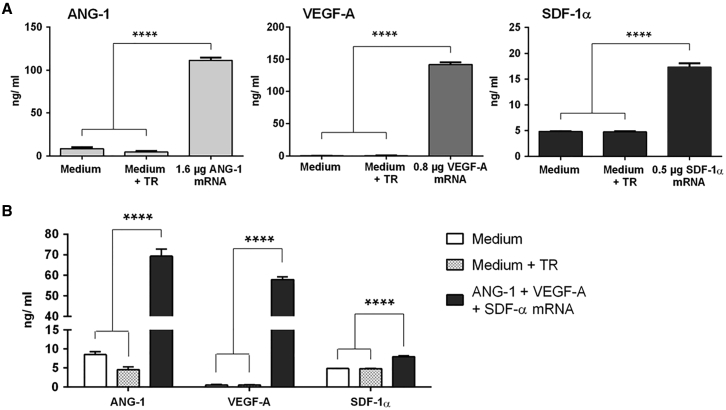
After the successful production of synthetic mRNAs, the expression of specific proteins was determined. Therefore, single-mRNA transfections with 1.6 μg ANG-1, 0.8 μg VEGF-A, or 0.5 μg SDF-1α or transfections with an mRNA cocktail consisting of 1.6 μg ANG-1, 0.8 μg VEGF-A, and 0.5 μg SDF-1α were performed. The concentrations of produced proteins were measured in supernatants 24 hr after the transfections using ELISA (Figure 2). The transfection of EPCs with ANG-1, VEGF-A, or SDF-1α mRNA resulted in significantly increased production of ANG-1 (112 ± 6.1 ng/mL), VEGF-A (142 ± 6.7 ng/mL), and SDF-1α (17 ± 1.4 ng/mL) compared to the cells incubated with medium or medium containing the transfection reagent (Figure 2A). The transfection of EPCs with the mRNA cocktail also resulted in significantly increased protein synthesis (Figure 2B), and approximately 70 ± 7.1 ng/mL ANG-1, 58 ± 2.7 ng/mL VEGF-A, and 8 ± 0.4 ng/mL SDF-1α were detected. Here, the amount of produced ANG-1 was 1.6-fold, VEGF-A 2.4-fold, and SDF-1α 2.1-fold less than after the transfection of EPCs with single mRNAs. In the used mRNA cocktail, the total amount of mRNAs was 2.9 μg.
Figure 2.
Expression of ANG-1, VEGF-A, and SDF-1α after the Transfection of Synthetic mRNA into Murine EPCs
1 × 105 EPCs were seeded and transfected the next day with (A) 1.6 μg ANG-1, 0.8 μg VEGF-A, or 0.5 μg SDF-1α mRNA or with (B) an mRNA cocktail containing 1.6 μg ANG-1, 0.8 μg VEGF-A, and 0.5 μg SDF-1α mRNA. The protein expression was analyzed in supernatants 24 hr after the transfection using ELISA. Cells treated with only medium or medium and transfection reagent (TR) served as negative controls. Results are shown as mean + SEM (n = 4). Statistical differences were determined using one-way ANOVA followed by Bonferroni multiple comparison test (****p < 0.0001).
To test the influence of higher mRNA amounts on protein synthesis, the cells were transfected with each single mRNA and EGFP mRNA, which was used as a filler mRNA, to obtain a total mRNA amount of 2.9 μg. Especially, after the co-transfection of ANG-1 mRNA with EGFP mRNA, a significant reduction of ANG-1 protein amount could be detected (Figure S1). In the case of VEGF-A mRNA, no influence could be detected. A slightly higher amount of SDF-1α was detected after the simultaneous transfection of cells with SDF-1α and EGFP mRNA. Furthermore, double combinations of ANG-1, VEGF-A, and SDF-1α mRNA transfections were tested (Figure S2). Here, the simultaneous transfection of cells with ANG-1 and VEGF-A or SDF-1α mRNA also resulted in a reduction in ANG-1 protein expression. These results indicate that the higher mRNA amount has an influence on ANG-1 production.
Influence of mRNA Engineering on the Viability of EPCs
The influence of mRNA transfections on the viability of EPCs was analyzed using PrestoBlue cell viability assay. 1 × 105 EPCs were cultivated overnight and transfected with 1.6 μg ANG-1, 0.8 μg VEGF-A, or 0.5 μg SDF-1α mRNA or with a triple mRNA cocktail. As a control, cells were treated with Opti-MEM containing the maximal amount of Lipofectamine 2000 (4 μL), which was used for the generation of transfection complexes. The viability of cells incubated with Opti-MEM (medium) was set to 100%, and the viability of samples was displayed relative to these cells. As shown in Figure 3, 24 hr post-transfection, the transfection of cells with synthetic mRNAs resulted in no significant differences in cell viability compared to the controls, cells incubated with medium or medium containing transfection reagent.
Figure 3.
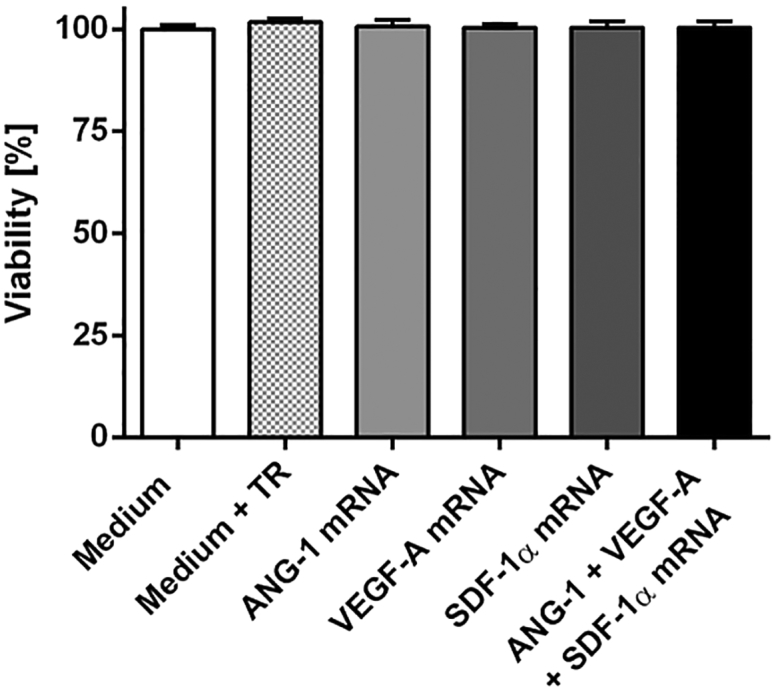
Influence of mRNA Transfection on the Viability of Murine EPCs
1 × 105 EPCs were transfected with 1.6 μg ANG-1, 0.8 μg VEGF-A, or 0.5 μg SDF-1α mRNA or with an mRNA cocktail containing 1.6 μg ANG-1, 0.8 μg VEGF-A, and 0.5 μg SDF-1α mRNA. Viability was determined 24 hr post-transfection using PrestoBlue assay. The viability of cells incubated with Opti-MEM (medium) was set to 100%, and the viability of samples was expressed relative to these cells. The data are shown as mean + SEM (n = 3). No statistically significant differences were determined using one-way ANOVA followed by Bonferroni multiple comparison test.
Chemotactic Migration of EPCs toward mRNA-Engineered EPCs
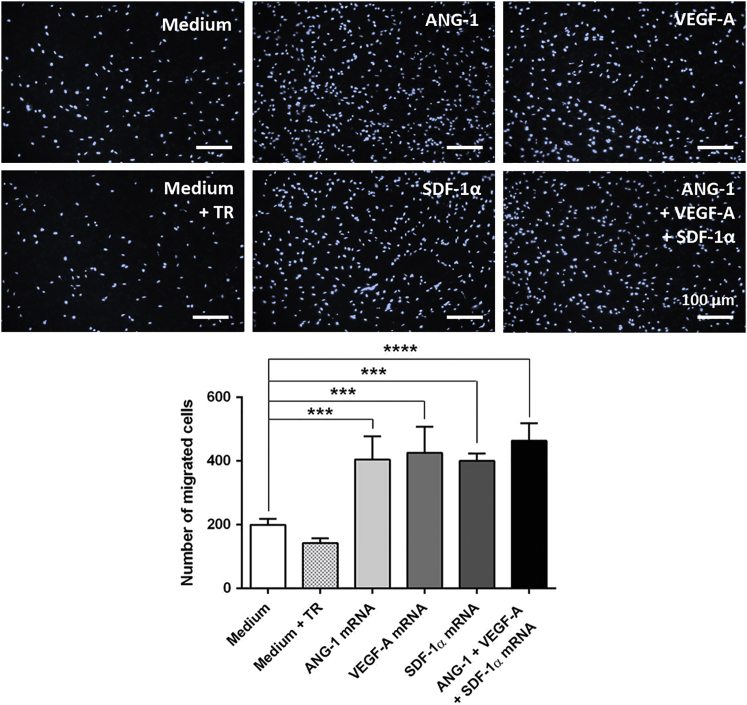
The chemotactic activity of mRNA-engineered EPCs was determined using a migration assay. Transwell inserts seeded with 5 × 104 untreated EPCs were placed in wells containing 1 × 105 mRNA-transfected or untransfected EPCs. After 6 hr, the migrated cells were stained with 1 μg/mL DAPI and counted. The mRNA-transfected EPCs were able to attract significantly more EPCs compared to EPCs without mRNA transfection (ANG-1 mRNA, 405 ± 36 cells; VEGF-A mRNA, 426 ± 41 cells; SDF-1α mRNA, 400 ± 12 cells; and mRNA cocktail, 464 ± 27 cells versus EPCs without mRNA transfection [medium], 200 ± 10 cells; Figure 4). Compared to single-mRNA-transfected EPCs, the transfection of EPCs with the mRNA cocktail resulted in a slight increase of migrated cell numbers; however, this increase was not statistically significant. The treatment of cells with the transfection reagent also had no statistically significant influence on the migration behavior compared to the cells treated with medium. Interestingly, all EPCs transfected with a single mRNA or with the mRNA cocktail showed comparable migration activity. Overall, these results demonstrated an improvement of EPC migration toward EPCs transfected with proangiogenic mRNAs.
Figure 4.
Chemotactic Migration of EPCs toward mRNA-Engineered EPCs
1 × 105 murine EPCs were cultivated overnight, and then they were transfected with 1.6 μg ANG-1, 0.8 μg VEGF-A, or 0.5 μg SDF-1α mRNA or with an mRNA cocktail containing 1.6 μg ANG-1, 0.8 μg VEGF-A, and 0.5 μg SDF-1α mRNA. Migration behavior of untreated EPCs (5 × 104) seeded on transwell inserts toward mRNA-transfected EPCs was analyzed using a chemotactic migration assay. As a control, EPCs incubated with medium or medium containing transfection reagent (TR) were used. After 6 hr, migrated EPCs through 8-μm transwell inserts were stained with DAPI, and cell numbers were calculated using ImageJ software. Scale bars represent 100 μm. Results are shown as mean + SEM (n = 4). Statistical differences were determined using one-way ANOVA followed by Bonferroni multiple comparison test (***p < 0.001 and ****p < 0.0001).
Migration Capacity of mRNA-Engineered EPCs in Wound Scratch Migration Assay
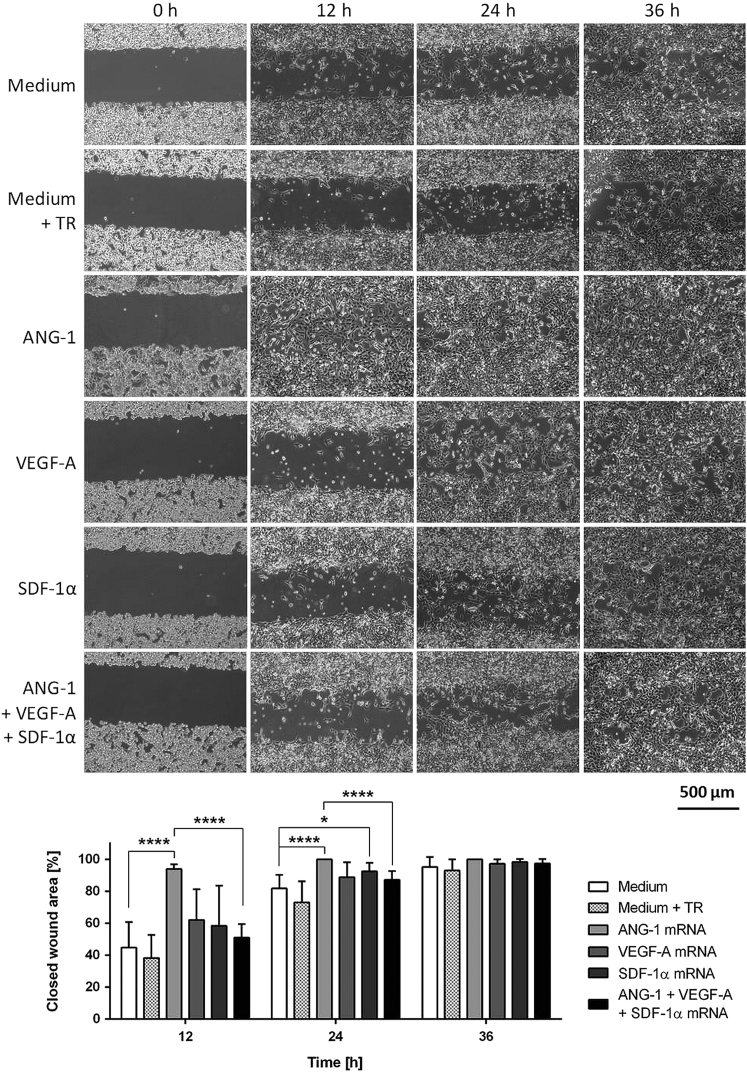
The in vitro wound-healing assay, which mimics the in vivo wound-healing process, was used to analyze the migration behavior of mRNA-engineered EPCs growing in a monolayer culture. Therefore, 28,000 EPCs with or without mRNA transfection were seeded in each chamber of Culture-Insert 3 wells. After reaching confluence, the culture inserts were removed from the dish to generate an open wound field. To quantify the closed wound area, pictures were taken immediately after removing the insert (0 hr) and after 12, 24, and 36 hr. After 36 hr, the wound areas were closed for each sample (Figure 5). EPCs transfected with ANG-1 mRNA showed fastest closure of the wound area, with 94% already after 12 hr. In comparison, a wound closure area of about 45% was detected with EPCs treated only with medium and 38% with EPCs treated with the medium containing transfection reagent. After 24 hr, 100% of the wound area was closed in ANG-1 mRNA-transfected EPCs and 93% wound area was closed in SDF-1α mRNA-transfected EPCs. The closure of the wound area was significantly higher in these EPCs compared to the medium control. Especially, the transfection of EPCs with ANG-1 mRNA accelerated the migration process so that the wound area was completely closed already after 12 hr. In comparison, EPCs transfected with the mRNA cocktail showed significantly less wound closure area. Furthermore, the treatment of the cells with the transfection reagent had no significant influence on the migration and the speed of wound closure compared to untreated cells (medium control).
Figure 5.
Analysis of Wound-Healing Capacity of mRNA-Engineered EPCs via Wound Scratch Migration Assay
1 × 105 murine EPCs were cultivated overnight, and then they were transfected with 1.6 μg ANG-1, 0.8 μg VEGF-A, or 0.5 μg SDF-1α mRNA or with an mRNA cocktail containing 1.6 μg ANG-1, 0.8 μg VEGF-A, and 0.5 μg SDF-1α mRNA. The next day, cells were detached, and 28,000 EPCs with or without mRNA transfection were seeded in each chamber of Culture-Insert 3 wells in μ-dishes. After 5 hr, when the cells completely attached and covered the surface, an open wound field was generated. Immediately after the generation of wound areas (0 hr) and after 12, 24, and 36 hr, phase-contrast images were taken and closed wound areas were calculated using Tscratch software. Scale bar represents 500 μm. Results are shown as mean + SD (n = 8). Statistical differences were determined using one-way ANOVA followed by Bonferroni multiple comparison test (*p < 0.05 and ****p < 0.0001).
In Vitro Angiogenic Potential of mRNA-Engineered EPCs
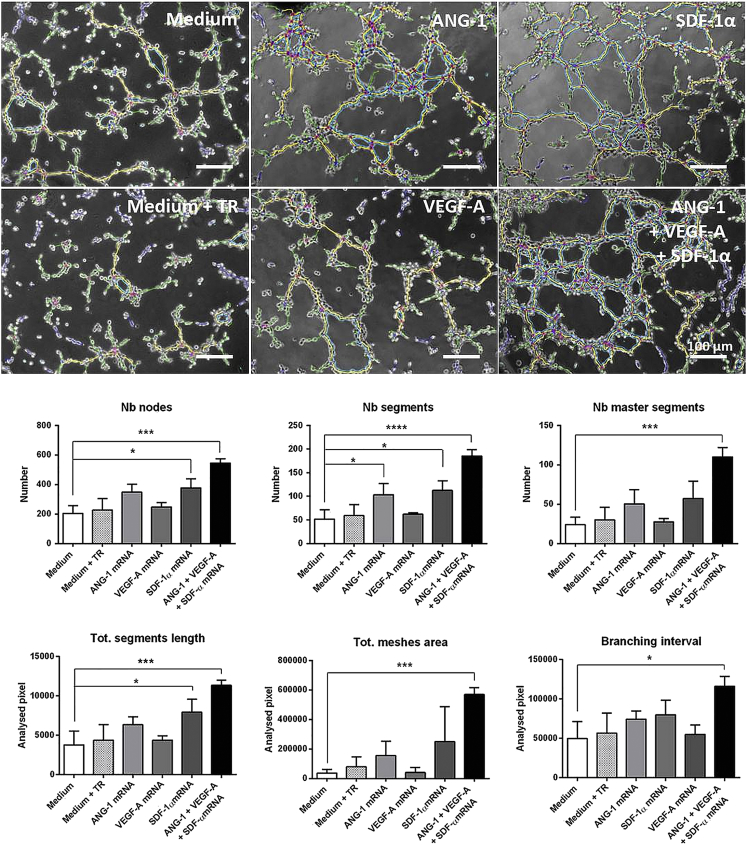
The tube formation assay was performed to assess the angiogenic potential of mRNA-engineered EPCs in vitro. Therefore, 1 × 104 EPCs transfected with 1.6 μg ANG-1, 0.8 μg VEGF-A, or 0.5 μg SDF-1α mRNA or with a triple mRNA cocktail were seeded on Matrigel. Already within 4 hr, EPCs spontaneously initiated vascular morphogenesis and formed multicellular tubular networks (Figure 6). Using Angiogenesis Analyzer ImageJ plugin, the number of segments (elements, which are delimited by two junctions), number of master segments (segments, which are none exclusively implicated with one branch), number of nodes (pixels with at least 3 neighboring elements, corresponding to a bifurcation), total segment length (sum of lengths of the segments in the analyzed area), the total mesh areas (sum of areas enclosed by the segments or master segments), and the branching interval (the mean distance separating two branches [total segment length/number of branches]) were obtained. The unit of area and length is the pixel (px).
Figure 6.
Analysis of Angiogenic Potential of mRNA-Engineered EPCs by Tube Formation Assay
1 × 105 murine EPCs were cultivated overnight, and then they were transfected with 1.6 μg ANG-1, 0.8 μg VEGF-A, or 0.5 μg SDF-1α mRNA or with an mRNA cocktail containing 1.6 μg ANG-1, 0.8 μg VEGF-A, and 0.5 μg SDF-1α mRNA. After 24 hr, EPCs were detached and 1 × 104 EPCs were seeded on Matrigel-coated angiogenesis slides. After 4 hr of incubation at 37°C, the formation of tubes was examined by phase-contrast microscopy. Microscopic images were analyzed using NIH ImageJ software with Angiogenesis Analyzer plugin, and segments are shown in magenta, master segments in orange, branches in green, and meshes in blue. The numbers (Nb) of nodes, segments, and master segments and the total (Tot.) segment length, total mesh area, and branching interval were quantified and compared to the medium control. The unit of area and length is pixel (px). Scale bars represent 100 μm. Results are shown as mean + SD (n = 3). Statistical differences were determined using one-way ANOVA followed by Bonferroni multiple comparison test (*p < 0.05, ***p < 0.001, and ****p < 0.0001).
EPCs engineered to simultaneously produce ANG-1, VEGF-A, and SDF-1α demonstrated highly increased network formation, compared to the untreated EPCs (medium). Thereby, the networks formed by EPCs transfected with the mRNA cocktail displayed higher numbers of nodes (545 ± 29 versus 205 ± 52), segments (185 ± 14 versus 52 ± 20), and master segments (110 ± 12 versus 24 ± 10), with an increase in total segment length (11,343 ± 660 px versus 3,786 ± 1750 px), total mesh area (569,094 ± 47,325 px versus 36,107 ± 24,228 px), as well as branching interval (116,306 ± 12,322 px versus 49,573 ± 21,438 px). The single transfection of EPCs either with ANG-1 or SDF-1α mRNA also led to an increase of parameters related to network formation. However, the increase was in the case of SDF-1α mRNA-transfected EPCs, statistically significant for number of nodes, segments, and total segment length, and in the case of ANG-1 mRNA-transfected EPCs, statistically significant for number of segments. In the case of VEGF-A, no increase could be detected compared to untreated EPCs. The overall results demonstrated an improvement of network formation characteristics in triple-mRNA-transfected EPCs, which led to a denser, highly branched tubular network with increased numbers of tubes, suggesting an improved induction of angiogenesis.
In Vivo Angiogenic Potential of mRNA-Engineered EPCs
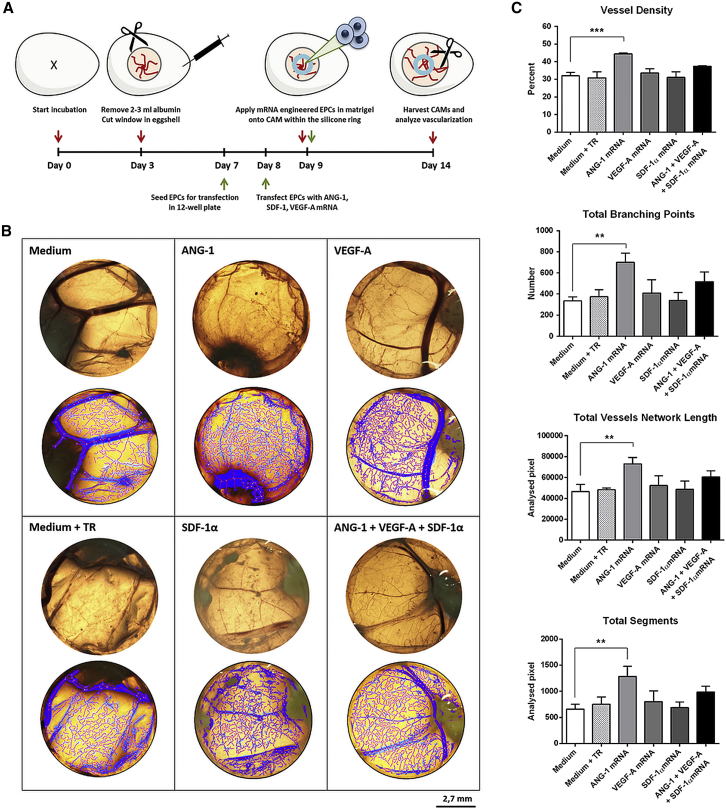
The in vivo angiogenic potential of mRNA-engineered EPCs was analyzed using the CAM assay. Therefore, 1 × 105 EPCs were seeded and transfected the next day with 1.6 μg ANG-1, 0.8 μg VEGF-A, or 0.5 μg SDF-1α mRNA or with a triple mRNA cocktail. After 24 hr, cells were detached, and 4 × 105 cells were mixed in Matrigel and placed within a silicone ring on the CAM. As controls, EPCs incubated with medium or medium containing transfection reagent were also applied to the CAMs. For evaluation and quantification of angiogenesis, the area of sample application (within the Ø 0.8-mm silicone ring) of the fixed CAMs was photographed and analyzed using Wimasis WimCAM web-based service (Figure 7). Compared to the mRNA untreated cells (medium), EPCs transfected with the ANG-1 mRNA resulted in an augmented angiogenesis/vascularization determined by the significant increase in numbers of total branching points (701 ± 85 versus 337 ± 37), a higher vessel density (44.5% ± 0.6% versus 32% ± 2%), and increases in total segments (1,285 ± 195 px versus 659 ± 96 px) and total vessel network length (73,101 ± 5,986 px versus 46,551 ± 6908 px) in the analyzed area. Although EPCs transfected with the mRNA cocktail also showed an improvement of parameters regarding network formation on CAMs, the differences were not significantly different from untransfected EPCs. EPCs treated with only SDF-1α mRNA or VEGF-A showed no improved effect on the formation of new vasculature compared to the controls.
Figure 7.
Analysis of In Vivo Angiogenetic Potential of mRNA-Engineered EPCs in Chick Embryo Chorioallantoic Membrane Assay
(A) Schematic representation of the chorioallantoic membrane (CAM) assay. ANG-1, SDF-1α, and VEGF-A mRNA-transfected and untreated cells (medium or medium containing transfection reagent [TR]) were applied in silicone rings (8-mm inner diameter) onto the CAMs at the ninth day of incubation at 37°C and 60% humidity. At the fourth day of incubation, the CAMs were fixed and excised. (B) Analysis of the region of the inner ring circle with Wimasis WimCAM image software to quantify angiogenesis. Scale bar of photographs (upper) and analyzed pictures (lower) represents 2.7 mm. (C) Quantification of angiogenesis using Wimasis WimCAM web-based service. The vessel density, total branching points, total vessel network length, and total segments were quantified and compared to the medium control. Results are shown as mean + SD (n = 3). Statistical differences were determined using one-way ANOVA followed by Bonferroni multiple comparison test (**p < 0.01 and ***p < 0.001).
Discussion
The improvement of angiogenic potential of EPCs by the use of synthetic modified mRNA represents a promising strategy for the revascularization of ischemic tissues. In this study, we transiently modified murine EPCs with synthetic mRNAs encoding ANG-1, VEGF-A, and SDF-1α to augment the proangiogenic potential. Thereby, tissue repair and regeneration can be improved in the affected regions, first by the local production of growth factors improving the proliferation and maintenance of implanted EPCs, and second by the local production of chemokines attracting other EPCs to the required location.
The transfection of EPCs with synthetic mRNAs showed no influence on cell viability, and, after the transfection of EPCs with single synthetic mRNAs, the expression of ANG-1, VEGF-A, and SDF-1α was demonstrated. However, the transfection of EPCs with a triple mRNA cocktail resulted in a reduced production of proteins compared to single-mRNA transfections. Especially, the production of ANG-1 protein was significantly reduced due to the increased mRNA amount in the triple mRNA cocktail-transfected cells. In eukaryotic cells, most secreted and membrane proteins undergo maturation steps in the lumen of the endoplasmic reticulum (ER).32 There, after the translation, nascent polypeptides are chemically modified and folded into mature proteins, and an accumulation of unfolded proteins in the ER lumen can lead to an unfolded protein response (UPR) stress. Thus, cells can selectively degrade mRNAs encoding secreted proteins.32, 33 Presumably, the higher total amount of synthetic mRNA per cell compared to single-mRNA transfection could lead to an overload and to a translational repression. Using EGFP mRNA as a filler mRNA, no negative influence of mRNA amount on the production of VEGF-A and SDF-1α was detected. However, in triple-mRNA-transfected cells, feedback mechanisms due to produced protein combinations could lead to the translational repression or degradation of synthetic mRNA and, thereby, result in a reduction of produced protein amounts.
In the chemotactic migration assay, all of the mRNA-engineered EPCs were able to attract non-modified EPCs. Contrary to the expectation that EPCs transfected with the mRNA cocktail could have an augmented chemoattractant activity, this could be not demonstrated. The reason, therefore, could be the production of reduced protein amounts in the mRNA cocktail-transfected EPCs compared to the single-mRNA-transfected EPCs. However, the tube formation assay demonstrated significantly improved angiogenic potential of EPCs transfected with the mRNA cocktail, which could be caused by the synergistic effects of simultaneously produced ANG-1, VEGF-A, and SDF-1α. EPCs transfected with SDF-1α or ANG-1 mRNA, or simultaneously with SDF-1α and VEGF-A mRNA (Figure S3), also showed an improved effect on tube formation.
In the wound scratch migration assay, EPCs transfected with ANG-1 mRNA showed the fastest closure of the wound area with 94% already after 12 hr. After 24 hr, the ANG-1 mRNA-transfected EPCs resulted in 100% closure of the wound area, which was significantly higher than that in EPCs transfected with the mRNA cocktail that also contained the ANG-1 mRNA. An improved wound closure compared to the untreated cells was also detected after 24 hr with SDF-1α mRNA-transfected EPCs. In the in vivo CAM assay, the highest angiogenic potential was obtained by the ANG-1 mRNA engineering of EPCs. Here, EPCs transfected with the mRNA cocktail also showed an improvement of angiogenesis-related parameters, however, this increase was not statistically significant.
VEGF is the most investigated growth factor for the treatment of cardiovascular diseases and ischemic conditions, since it is a key regulator of blood vessel formation. However, studies demonstrated that newly formed blood vessels induced by VEGF are immature and leaky.34, 35 Su and colleagues35 demonstrated that the simultaneous expression of ANG-1 and VEGF delivered by an adeno-associated virus (AAV)-mediated gene delivery approach has positive effects on the therapy of infarcted mouse hearts, with more capillaries, a smaller infarct size, and better cardiac function. Moreover, the neovasculature was less leaky compared to VEGF alone treatment.
The results of this study proved that the modification of EPCs only with ANG-1 mRNA could be sufficient for the improvement of angiogenesis. SDF-1α mRNA transfection also showed an improvement of wound closure and in vitro tube formation. In contrast, after the single transfection of EPCs with VEGF mRNA, an improvement in angiogenic potential could not be detected. In their study, Zangi et al.36 injected 100 μg VEGF mRNA into cardiac muscle of mice and demonstrated increased formation of systemically perfused vessels in the area of injection. Thus, the production of higher VEGF amounts by the mRNA-modified EPCs could be required to improve the angiogenesis.
Compared to the in vivo injection of synthetic mRNA into the affected tissue, using the approach described in this study, increased numbers of EPCs can be obtained by the isolation and/or expansion of EPCs. The ex vivo modification of these cells by synthetic mRNA to produce their own proangiogenic proteins, after the in vivo injection for a limited time, could further increase the angiogenic potential compared to the injection of high amounts of VEGF mRNA into the affected tissue. In this study, due to high transfection efficiency and cell compatibility, Lipofectamine 2000 was used as a transfection reagent; however, the quick delivery of synthetic mRNA into the cells, for example, by using methods such as electroporation, could further significantly improve the in vivo results. Thereby, the cells could be immediately injected into the tissue after the mRNA transfection. However, care should be taken that the selected transfection method does not harm the cells.
The overexpression of paracrine factors in ischemic patients could be especially beneficial to increase the mobilization and recruitment of autologous stem cells to the damaged area to support healing and regeneration by improving revascularization. In previous studies, AAV or lentiviral vectors were applied to produce ANG-1 in rat mesenchymal stem cells (MSCs),37 VEGF-A in rat skeletal muscles,38 and SDF-1α in human MSCs.39 Furthermore, EPCs were genetically modified with adenoviral vectors encoding hypoxia-induced factor-1α (HIF-1α),40 FGF-1,41 and VEGF;42 lentiviral vectors encoding ANG-1;43 and retroviral vectors encoding SDF-1α and VEGF-A44 to improve angiogenesis in animal models of ischemia. Using these vectors, the expression of proteins is maintained for an extended period of time. However, the prolonged presence of exogenously expressed proteins can have adverse effects. For example, the prolonged exposure of vessels to VEGF-A due to DNA-mediated gene transfer can result in edema due to an increased vessel permeability.36 The synthetic mRNA-mediated protein expression in the cells ceases after the natural degradation of delivered synthetic mRNA, which is approximately after 2–3 days,45 and no footprint is left. We suggest that this time frame could be sufficient for further homing of endogenous EPCs to the ischemic tissue and the rapid connection of implanted EPCs to the blood vessels of the surrounding intact tissue.
Conclusions
In this study, for the first time, we demonstrated that the angiogenic potential of EPCs can be improved by the transfection of cells with synthetic mRNAs encoding proangiogenic factors. The strongest in vivo angiogenic potential could be detected with ANG-1 mRNA-transfected cells. In vitro, EPCs transfected with the mRNA cocktail showed significantly improved tube formation. Thus, this promising synthetic mRNA-based treatment method could be beneficial in conditions associated with insufficient formation of new blood vessels in damaged tissues, e.g., after myocardial infarction, stroke, or limb ischemia, or in the field of tissue engineering to improve the vascularization of scaffolds and to promote vessel anastomosis with the host vasculature. Furthermore, this method could be applied to the treatment of various diseases by transfecting the desired cell type with one or multiple mRNAs to modify cell activity and cell fate, in order to improve cellular functions for sustained clinical outcomes.
Materials and Methods
In Vitro mRNA Synthesis
The pcDNA 3.3 plasmid containing the coding sequences for SDF-1α or VEGF-A165 (referred to as VEGF-A) was produced by Aldevron (Fargo, ND, USA), and pEX-K4 plasmid containing ANG-1 was produced by Eurofins Genomics (Ebersberg, Germany). To synthesize the mRNA, first of all, DNA templates were generated by PCR using 50–100 ng plasmid DNA, 0.7 μM forward primer (5′-TTGGACCCTCGTACAGAAGCTAATACG-3′) and 0.7 μM reverse primer (5′-T120CTTCCTACTCAGGCTTTATTCAAAGACCA-3′) (Ella Biotech, Martinsried, Germany), and HotStar HiFidelity Polymerase Kit (QIAGEN, Hilden, Germany), according to the manufacturer’s instructions. PCR products were generated using the following amplification protocol: initial denaturation step at 94°C for 3 min, followed by 25 cycles of denaturation at 94°C for 45 s, annealing at 57°C for 1 min, extension at 72°C for 1 min, and final extension at 72°C for 5 min. Afterward, PCR products were purified using MinElute PCR purification kit (QIAGEN).
Next, the mRNA was produced by in vitro transcription (IVT) using MEGAscript T7 Kit (Ambion, Glasgow, Scotland), according to the manufacturer’s instructions. The IVT reaction contained 1.5 μg template DNA, 7.5 mM ATP, 1.875 mM guanosine triphosphate (GTP), 7.5 mM pseudoruridine-5′-triphosphate (Ψ-UTP), and 7.5 mM 5-methylcytidine-5′-triphosphate (m5CTP) (TriLink Biotech, San Diego, CA, USA). Furthermore, 2.5 mM 3′-0-Me-m7G(5′)ppp(5′)G RNA Cap Structure Analog (New England Biolabs, Frankfurt, Germany) was used for 5′ end capping, and the mRNA was dephosphorylated using 5 U/mL Antarctic phosphatase (New England Biolabs, Frankfurt am Main, Germany). Additionally, the IVT reaction mixture contained 40 U RNase inhibitor (Thermo Fisher Scientific, Waltham, MA, USA) to prevent mRNA degradation. After each reaction step, the mRNA was purified using RNeasy kit (QIAGEN), and the mRNA concentration was adjusted to 100 ng/μL in nuclease-free water. The purity and specific length of generated DNA templates and mRNA products were analyzed using 1% agarose gel electrophoresis at 100 V for 45 min and staining with 1× GelRed (Biotium, Fremont, CA, USA) in 1× Tris-borate-EDTA (TBE) buffer.
Cultivation of Murine EPCs
In this study, murine embryonal EPCs (T17b cells), which were previously isolated and characterized by Hatzopoulos et al.,46 were used. These cells can differentiate into mature ECs and form vascular structures in vitro and in vivo.47 EPCs were cultivated in DMEM with high glucose and L-glutamine supplemented with 20% fetal bovine serum (FBS), 1× minimum essential medium (MEM) non-essential amino acids (NEAA), 100 μM 2-mercaptoethanol, and 1% penicillin and streptomycin. All these cell culture reagents were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Cells were cultivated at 37°C with 5% CO2 and medium was changed every 2–3 days. Cells were detached at about 70% confluency using 0.04% trypsin/0.03% EDTA, and trypsin was neutralized using trypsin-neutralizing solution (TNS, 0.05% trypsin inhibitor in 0.1% BSA; PromoCell, Heidelberg, Germany). Afterward, cells were centrifuged for 5 min at 300 × g and seeded on 0.1% gelatin- (Sigma-Aldrich Chemie, Steinheim, Germany) coated tissue flasks or cell culture plates.
Transfection of EPCs with Synthetic mRNAs
To perform the transfection of EPCs with synthetic mRNAs, 1 × 105 EPCs were seeded per well of a 12-well plate coated with 0.1% gelatin and incubated overnight at 37°C. The next day, lipoplexes were generated by incubation of mRNAs with Lipofectamine 2000 (Thermo Fisher Scientific) in 0.5 mL Opti-MEM I reduced serum medium (Opti-MEM, Thermo Fisher Scientific). The transfections were performed with equimolar amounts of mRNAs. Thus, EPCs were transfected with 1.6 μg ANG-1, 0.8 μg VEGF-A, or 0.5 μg SDF-1α mRNA or with all mRNAs together (called triple-mRNA cocktail). To perform single-mRNA transfections, transfection complexes were generated by using 2 μL Lipofectamine 2000 for VEGF-A165 and SDF-1α mRNA and 4 μL for ANG-1 mRNA. To generate transfection complexes with the mRNA cocktail, 4 μL Lipofectamine 2000 was used. The mRNAs were incubated in Opti-MEM for 15 min at room temperature (RT) with the transfection reagent, and then Opti-MEM containing lipoplexes were added to the cells. After 4 hr of incubation, transfection medium was discarded and 1 mL fresh cell culture medium was added for further overnight incubation at 37°C. As controls, cells were incubated with only Opti-MEM or Opti-MEM containing transfection reagent.
Detection of Protein Expression Using ELISA
After the transfection of EPCs with synthetic mRNAs, the expression of proteins was analyzed using ELISA. Therefore, supernatants of transfected cells were collected and diluted 1:100 in Dulbecco’s PBS (DPBS)/1% BSA. The concentrations of ANG-1, VEGF-A, and SDF-1α were determined as duplicate in 100 μL using human ANG-1, CXCL-12/SDF-1α, and VEGF-A DuoSet ELISA (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions. The absorbance was measured using a microplate reader (Eon Synergy 2, BioTek Instruments) at 450 nm, with the correction wavelength set at 540 nm.
Influence of mRNA Engineering on EPC Viability
The influence of mRNA treatment on the viability of EPCs was assessed using PrestoBlue assay (Invitrogen, Carlsbad, CA, USA). Therefore, 1 × 105 EPCs were transfected with 1.6 μg ANG-1, 0.8 μg VEGF-A, or 0.5 μg SDF-1α mRNA or with a triple-mRNA cocktail containing 1.6 μg ANG-1, 0.8 μg VEGF-A, and 0.5 μg SDF-1α mRNA for 4 hr at 37°C. Afterward, transfection complexes were discarded and 1 mL cell culture medium was added per well of a 12-well plate. After 24 hr, 110 μL PrestoBlue cell viability reagent was added per well and incubated for 1.5 hr at 37°C. Fluorescence intensity of 50 μL supernatant was measured in triplicates at an excitation wavelength of 530 nm and an emission wavelength of 600 nm, using a multimode microplate reader (Mithras LB 940, Berthold Technologies).
Chemotactic Migration Assay
Migration of unmodified EPCs toward mRNA-engineered EPCs was analyzed using chemotactic migration assay. After the transfection of 1 × 105 EPCs with mRNAs, lipoplexes were removed and serum-reduced cell culture medium containing 1% FBS was added for overnight cultivation to the cells. The next day, 5 × 104 untreated EPCs were seeded onto transwell inserts with 8-μm pores coated with 0.1% gelatin. Subsequently, transwell inserts were transferred into wells of a 12-well plate containing the mRNA-transfected or untransfected EPCs and incubated for 6 hr at 37°C. Afterward, the transwell inserts were rinsed with DPBS containing Ca2+/Mg2+ (DPBS+) and fixed for 10 min with ice-cold methanol (AnalaR NORMAPUR, VWR, Darmstadt, Germany). After an additional washing of transwell inserts with DPBS+, the cells at the bottom of the transwell were stained for 10 min at RT with 1 μg/mL DAPI (Sigma-Aldrich) in DPBS+. The migrated cells were detected using fluorescence microscopy (Axiovert135, Carl Zeiss, Oberkochen, Germany), and the numbers of cells that migrated through the transwell inserts were determined at 3 different regions of each insert membrane using ImageJ 1.51 software.48
Wound Scratch Migration Assay
1 × 105 EPCs were transfected in 12-well plates with synthetic mRNAs and cultivated at 37°C and 5% CO2 overnight. Then, cells were detached, and 28,000 cells were seeded in 70 μL cell culture medium into each chamber of the 0.1% gelatin-coated Culture-Insert 3 wells in μ-Dish35mm, high (ibidi, Martinsried, Germany). Cells were cultivated at 37°C and 5% CO2 for 5 hr. After allowing the cells to form a confluent monolayer, a 500-μm open wound field between the cells was generated by removing the culture insert from the dish. Subsequently, 1.5 mL fresh medium was added to each μ-dish. After 0, 12, 24, and 36 hr, the wound fields were documented using a phase-contrast microscope (Axiovert 135). The percentage of closed wound area was calculated using Tscratch software.49
Tube Formation Assay
Tube formation capacity of mRNA-engineered EPCs was analyzed after the transfection with synthetic mRNAs. Therefore, 1 × 105 EPCs were transfected in 12-well plates with synthetic mRNAs and cultivated at 37°C and 5% CO2 overnight in serum-reduced culture medium containing 1% FBS. Each well of the μ-slides for angiogenesis (ibidi) was coated with 10 μL Matrigel (hESC qualified, Corning, Corning, NY, USA) solution (1:5 diluted in DMEM) for 1 hr at 37°C. Then, transfected and untransfected EPCs were detached, and 50 μL cell suspension containing 10,000 EPCs was added to the Matrigel-coated wells. Tube formation was assessed after 4 hr of incubation at 37°C using a light microscope (Axiovert 135) equipped with a digital camera AxioCam MRm (Carl Zeiss). Pictures were analyzed using Angiogenesis Analyzer plugin of ImageJ 1.51 software.48
Chicken Embryo CAM Assay
Fresh fertilized chicken eggs of the Lohmann White × White Rock breed chicken variety were purchased from the breeding facility Matthias Sittig (Buchholz, Germany). The eggs were incubated at 37°C and 60% relative humidity in an egg incubator (Heka-Brutgeräte, Rietberg-Varensell, Germany) and completely rotated twice a day. At day 3 of incubation, 2–3 mL albumen was aspirated by inserting an 18G needle at the tip of the egg without harming the yolk. Next, a semi-permeable adhesive tape Suprasorb F (Lohmann & Rauscher, Rengsdorf, Germany) was stuck to the eggshell, and a circular window (Ø 1–1.5 cm) was cut into the shell. Unfertilized eggs showing no vasculature or heart beating were removed. Then, the window was sealed using the adhesive tape to prevent dehydration and to minimize the risk of infection. The eggs were then incubated without rotation.
At day 9 of incubation, EPCs transfected with 1.6 μg ANG-1, 0.8 μg VEGF-A, or 0.5 μg SDF-1α mRNA or with a triple mRNA cocktail were applied to the CAM. Per egg, 4 × 105 EPCs were resuspended in 50 μL cell culture medium and mixed with 50 μL Matrigel (hESC qualified, Corning). A silicone ring (inner diameter: 0.8 cm) (neoLab, Leonberg, Germany) was carefully placed onto the CAM, and 100 μL matrigel and cell suspension was applied into the inner circle of the ring. The eggs were sealed and further incubated. At day 14, eggs were kept at RT for 3 hr and CAMs were then excised and fixed with 4% paraformaldehyde (Merck, Darmstadt, Germany) for 24 hr at 4°C. After washing with DPBS, color photographs of the circular application area were taken using a photomacroscope (Wild Heerbrugg M400) and a digital camera (Canon EOS 550D). The analysis of angiogenesis-associated parameters, such as vessel density, branching points, and segment length, was performed using Wimasis image analysis web-based system.
Statistical Analysis
Data are shown as mean + SD or SEM. One-way ANOVA for repeated measurements followed by Bonferroni multiple comparison test was performed to compare the means. All statistical analyses were performed double-tailed using GraphPad Prism version 6.01. Differences of p < 0.05 were considered significant.
Author Contributions
H.S. and M.A.-A. conceived and designed the experiments. H.S. performed the experiments with support from S.G., A.B., M.A.-A., and H.P.W. and analyzed the data. H.P.W. and C.S. contributed reagents, materials, and analysis tools. H.S. and M.A.-A. wrote the paper. M.A.-A. supervised the project.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This study was funded by the European Social Funds in Baden-Wuerttemberg, Germany and the Ministry of Science, Research, and the Arts of the State of Baden-Wuerttemberg (MWK-BW). We thank Prof. Antonis K. Hatzopoulos for generously providing the embryonal murine T17b endothelial progenitor cells.
Footnotes
Supplemental Information includes three figures and can be found with this article online at https://doi.org/10.1016/j.omtn.2018.09.005.
Supplemental Information
References
- 1.Takahashi T., Kalka C., Masuda H., Chen D., Silver M., Kearney M., Magner M., Isner J.M., Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T., Masuda H., Takahashi T., Kalka C., Pastore C., Silver M., Kearne M., Magner M., Isner J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T., Kawamoto A., Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29:1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 4.Sen S., McDonald S.P., Coates P.T., Bonder C.S. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovascular disease. Clin. Sci. (Lond.) 2011;120:263–283. doi: 10.1042/CS20100429. [DOI] [PubMed] [Google Scholar]
- 5.Kachamakova-Trojanowska N., Bukowska-Strakova K., Zukowska M., Dulak J., Jozkowicz A. The real face of endothelial progenitor cells - Circulating angiogenic cells as endothelial prognostic marker? Pharmacol. Rep. 2015;67:793–802. doi: 10.1016/j.pharep.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Kawamoto A., Gwon H.C., Iwaguro H., Yamaguchi J.I., Uchida S., Masuda H., Silver M., Ma H., Kearney M., Isner J.M., Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103:634–637. doi: 10.1161/01.cir.103.5.634. [DOI] [PubMed] [Google Scholar]
- 7.Minami Y., Nakajima T., Ikutomi M., Morita T., Komuro I., Sata M., Sahara M. Angiogenic potential of early and late outgrowth endothelial progenitor cells is dependent on the time of emergence. Int. J. Cardiol. 2015;186:305–314. doi: 10.1016/j.ijcard.2015.03.166. [DOI] [PubMed] [Google Scholar]
- 8.Fazel S., Cimini M., Chen L., Li S., Angoulvant D., Fedak P., Verma S., Weisel R.D., Keating A., Li R.K. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J. Clin. Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong M.S.K., Ng W.K., Chan J.K.Y. Concise Review: Endothelial Progenitor Cells in Regenerative Medicine: Applications and Challenges. Stem Cells Transl. Med. 2016;5:530–538. doi: 10.5966/sctm.2015-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terrovitis J.V., Smith R.R., Marbán E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ. Res. 2010;106:479–494. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipiec P., Krzemińska-Pakuła M., Plewka M., Kuśmierek J., Płachcińska A., Szumiński R., Robak T., Korycka A., Kasprzak J.D. Impact of intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction on left ventricular perfusion and function: a 6-month follow-up gated 99mTc-MIBI single-photon emission computed tomography study. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:587–593. doi: 10.1007/s00259-008-0988-6. [DOI] [PubMed] [Google Scholar]
- 12.Sukmawati D., Tanaka R. Introduction to next generation of endothelial progenitor cell therapy: a promise in vascular medicine. Am. J. Transl. Res. 2015;7:411–421. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H., van Olden C., Sweeney D., Martin-Rendon E. Blood vessel repair and regeneration in the ischaemic heart. Open Heart. 2014;1:e000016. doi: 10.1136/openhrt-2013-000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeshita S., Zheng L.P., Brogi E., Kearney M., Pu L.Q., Bunting S., Ferrara N., Symes J.F., Isner J.M. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J. Clin. Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baffour R., Berman J., Garb J.L., Rhee S.W., Kaufman J., Friedmann P. Enhanced angiogenesis and growth of collaterals by in vivo administration of recombinant basic fibroblast growth factor in a rabbit model of acute lower limb ischemia: dose-response effect of basic fibroblast growth factor. J. Vasc. Surg. 1992;16:181–191. [PubMed] [Google Scholar]
- 16.Sun X.-T., Ding Y.-T., Yan X.-G., Wu L.-Y., Li Q., Cheng N., Qiu Y.D., Zhang M.Y. Angiogenic synergistic effect of basic fibroblast growth factor and vascular endothelial growth factor in an in vitro quantitative microcarrier-based three-dimensional fibrin angiogenesis system. World J. Gastroenterol. 2004;10:2524–2528. doi: 10.3748/wjg.v10.i17.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J.H., Xu Y.M., Fu Q., Song L.J., Li C., Zhang Q., Xie M.K. Continued sustained release of VEGF by PLGA nanospheres modified BAMG stent for the anterior urethral reconstruction of rabbit. Asian Pac. J. Trop. Med. 2013;6:481–484. doi: 10.1016/S1995-7645(13)60078-4. [DOI] [PubMed] [Google Scholar]
- 18.Chappell J.C., Song J., Burke C.W., Klibanov A.L., Price R.J. Targeted delivery of nanoparticles bearing fibroblast growth factor-2 by ultrasonic microbubble destruction for therapeutic arteriogenesis. Small. 2008;4:1769–1777. doi: 10.1002/smll.200800806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgartner I., Pieczek A., Manor O., Blair R., Kearney M., Walsh K., Isner J.M. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- 20.Kuliszewski M.A., Kobulnik J., Lindner J.R., Stewart D.J., Leong-Poi H. Vascular gene transfer of SDF-1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle. Mol. Ther. 2011;19:895–902. doi: 10.1038/mt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong-Poi H., Kuliszewski M.A., Lekas M., Sibbald M., Teichert-Kuliszewska K., Klibanov A.L., Stewart D.J., Lindner J.R. Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle. Circ. Res. 2007;101:295–303. doi: 10.1161/CIRCRESAHA.107.148676. [DOI] [PubMed] [Google Scholar]
- 22.Swanson N., Hogrefe K., Javed Q., Malik N., Gershlick A.H. Vascular endothelial growth factor (VEGF)-eluting stents: in vivo effects on thrombosis, endothelialization and intimal hyperplasia. J. Invasive Cardiol. 2003;15:688–692. [PubMed] [Google Scholar]
- 23.Gupta R., Tongers J., Losordo D.W. Human studies of angiogenic gene therapy. Circ. Res. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 25.Adams R.H., Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 26.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer. 2011;2:1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milam K.E., Parikh S.M. The angiopoietin-Tie2 signaling axis in the vascular leakage of systemic inflammation. Tissue Barriers. 2015;3:e957508. doi: 10.4161/21688362.2014.957508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brouwers J., Noviyanti R., Fijnheer R., de Groot P.G., Trianty L., Mudaliana S., Roest M., Syafruddin D., van der Ven A., de Mast Q. Platelet activation determines angiopoietin-1 and VEGF levels in malaria: implications for their use as biomarkers. PLoS ONE. 2013;8:e64850. doi: 10.1371/journal.pone.0064850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brindle N.P.J., Saharinen P., Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ. Res. 2006;98:1014–1023. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho T.K., Tsui J., Xu S., Leoni P., Abraham D.J., Baker D.M. Angiogenic effects of stromal cell-derived factor-1 (SDF-1/CXCL12) variants in vitro and the in vivo expressions of CXCL12 variants and CXCR4 in human critical leg ischemia. J. Vasc. Surg. 2010;51:689–699. doi: 10.1016/j.jvs.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 31.Ceradini D.J., Kulkarni A.R., Callaghan M.J., Tepper O.M., Bastidas N., Kleinman M.E., Capla J.M., Galiano R.D., Levine J.P., Gurtner G.C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 32.Ron D. Cell biology. Stressed cells cope with protein overload. Science. 2006;313:52–53. doi: 10.1126/science.1130469. [DOI] [PubMed] [Google Scholar]
- 33.Hollien J., Weissman J.S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 34.Dvorak H.F., Nagy J.A., Feng D., Brown L.F., Dvorak A.M. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr. Top. Microbiol. Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- 35.Su H., Takagawa J., Huang Y., Arakawa-Hoyt J., Pons J., Grossman W., Kan Y.W. Additive effect of AAV-mediated angiopoietin-1 and VEGF expression on the therapy of infarcted heart. Int. J. Cardiol. 2009;133:191–197. doi: 10.1016/j.ijcard.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zangi L., Lui K.O., von Gise A., Ma Q., Ebina W., Ptaszek L.M., Später D., Xu H., Tabebordbar M., Gorbatov R. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L., Cui M., Wang Z., Feng X., Mao J., Chen P., Kangtao M., Chen F., Zhou C. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem. Biophys. Res. Commun. 2007;357:779–784. doi: 10.1016/j.bbrc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Shimpo M., Ikeda U., Maeda Y., Takahashi M., Miyashita H., Mizukami H., Urabe M., Kume A., Takizawa T., Shibuya M. AAV-mediated VEGF gene transfer into skeletal muscle stimulates angiogenesis and improves blood flow in a rat hindlimb ischemia model. Cardiovasc. Res. 2002;53:993–1001. doi: 10.1016/s0008-6363(01)00546-6. [DOI] [PubMed] [Google Scholar]
- 39.Liang X., Su Y.-P., Kong P.-Y., Zeng D.-F., Chen X.-H., Peng X.-G., Zou Z.M., Xu H. Human bone marrow mesenchymal stem cells expressing SDF-1 promote hematopoietic stem cell function of human mobilised peripheral blood CD34+ cells in vivo and in vitro. Int. J. Radiat. Biol. 2010;86:230–237. doi: 10.3109/09553000903422555. [DOI] [PubMed] [Google Scholar]
- 40.Jiang M., Wang B., Wang C., He B., Fan H., Shao Q., Gao L., Liu Y., Yan G., Pu J. In vivo enhancement of angiogenesis by adenoviral transfer of HIF-1α-modified endothelial progenitor cells (Ad-HIF-1α-modified EPC for angiogenesis) Int. J. Biochem. Cell Biol. 2008;40:2284–2295. doi: 10.1016/j.biocel.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Chen S.Y., Wang F., Yan X.Y., Zhou Q., Ling Q., Ling J.X., Rong Y.Z., Li Y.G. Autologous transplantation of EPCs encoding FGF1 gene promotes neovascularization in a porcine model of chronic myocardial ischemia. Int. J. Cardiol. 2009;135:223–232. doi: 10.1016/j.ijcard.2008.12.193. [DOI] [PubMed] [Google Scholar]
- 42.Iwaguro H., Yamaguchi J., Kalka C., Murasawa S., Masuda H., Hayashi S., Silver M., Li T., Isner J.M., Asahara T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y.Q., Song J.J., Han X., Liu Y.Y., Wang X.H., Li Z.M., Tzeng C.M. Effects of angiopoietin-1 on inflammatory injury in endothelial progenitor cells and blood vessels. Curr. Gene Ther. 2014;14:128–135. doi: 10.2174/1566523214666140307111138. [DOI] [PubMed] [Google Scholar]
- 44.Yu J.X., Huang X.F., Lv W.M., Ye C.S., Peng X.Z., Zhang H., Xiao L.B., Wang S.M. Combination of stromal-derived factor-1alpha and vascular endothelial growth factor gene-modified endothelial progenitor cells is more effective for ischemic neovascularization. J. Vasc. Surg. 2009;50:608–616. doi: 10.1016/j.jvs.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 45.Warren L., Manos P.D., Ahfeldt T., Loh Y.H., Li H., Lau F., Ebina W., Mandal P.K., Smith Z.D., Meissner A. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hatzopoulos A.K., Folkman J., Vasile E., Eiselen G.K., Rosenberg R.D. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development. 1998;125:1457–1468. doi: 10.1242/dev.125.8.1457. [DOI] [PubMed] [Google Scholar]
- 47.Bleiziffer O., Horch R.E., Hammon M., Arkudas A., Naschberger E., Rath S., Pryymachuk G., Beier J.P., Hatzopoulos A.K., Stürzl M., Kneser U. T17b murine embryonal endothelial progenitor cells can be induced towards both proliferation and differentiation in a fibrin matrix. J. Cell. Mol. Med. 2009;13:926–935. doi: 10.1111/j.1582-4934.2008.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gebäck T., Schulz M.M., Koumoutsakos P., Detmar M. TScratch: a novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques. 2009;46:265–274. doi: 10.2144/000113083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.