Abstract
This set of raw and analyzed data are complement to the research article that is titled “Mechanical, structural and physical aspects of chitosan-based films as antimicrobial dressings” (Escárcega-Galaz et al., 2018) [1]. The mechanical, structural and biological properties of the chitosan-based films determine their potential application in biomedicine. The films were prepared from pure chitosan and in combination with honey or glycerol. Afterwards, the characterization data related to thermal analysis, elementary composition, tensile strength and degree crystallinity was collected. The data of the antimicrobial activity of the films correspond to Klebsiella pneumoniae and Pseudomonas aeruginosa, both isolated from cutaneous ulcers. This set of data indicate that the chitosan-based films possess biological and physicochemical characteristics for their application as antimicrobial dressings for their action when are used by direct contact during the treatment of cutaneous ulcers.
Keywords: Chitosan, Thermal analysis, Honey-glycerol, Characterization data, Films, Degree crystallinity, Carbon-oxygen, Klebsiella pneumoniae, Pseudomonas aeruginosa, Differential scanning calorimetry
Specifications table
| Subject area | Carbohydrates, natural polyacids and lignins |
| More specific subject area | Polymers, biomedical, |
| Type of data | Tables, Figures, Text. |
| How data was acquired | Scanning electron microscope (SEM EVO LS15), texture analyzer (TA-XT2®), calorimeter (TA Instruments DSC Q100), X-ray Diffractometer (Phillips PW1710). |
| Data format | Analyzed |
| Experimental factors | Samples were only cut to an appropriate size to perform the tests. |
| Experimental features | The films of pure chitosan and others in mixture with honey-glycerol were characterized in relation to their antimicrobial activity, thermal analysis, elementary composition, tensile strength and degree crystallinity. All this to confirm its potential use as antimicrobial dressings during the healing of infected and chronic ulcers. |
| Data source location | Instituto Tecnológico de Sonora, Ciudad Obregón, Sonora, MX-85000, México. |
| Centro de Investigación en Alimentos y Desarrollo, Hermosillo, Sonora, MX-83304, México. | |
| Universidad Santiago de Compostela, Santiago de Compostela 15782, La Coruña, Spain. | |
| Data accessibility | The data are supplied with this article. |
| Related research article | Ana A. Escárcega-Galaz, Dalia I. Sánchez-Machado, Jaime López-Cervantes, Ana Sanches-Silva, Tomás J. Madera-Santana, Perfecto Paseiro-Losada (2018). Mechanical, structural and physical aspects of chitosan-based films as antimicrobial dressings. International Journal of Biological Macromolecules. |
| doi:10.1016/j.ijbiomac.2018.04.149 |
Value of the data
-
•
Data set for the characterization of six films based on chitosan and mixed with honey or glycerol.
-
•
The characterization data of the chitosan films are related to the structural, mechanical and physical properties that favor its availability as an antimicrobial dressing.
-
•
Between honey and chitosan there is a synergistic effect that improves their ability to eliminate infections and heal skin ulcers.
-
•
This set of data of the essential properties of a biomaterial tends to strengthen the applications of chitosan as a film former for medical use, in addition to its biocompatibility and biodegradability.
1. Data
1.1. Antimicrobial activity in vitro
The composition of honey and its origin plays a very important role in its biological properties, specifically in the antimicrobial activity [2]. The honey is characterized for its sugars such as fructose, glucose, maltose and sucrose [3].
Table 1 shows the antimicrobial activity data of the chitosan-based films against Klebsiella pneumoniae and Pseudomonas aeruginosa, both are clinical origin. For the two microorganisms an area increment was observed. In the pure chitosan films, a similar behavior was observed between them and the addition of glycerol did not affect the increase in area values to a great extent. However, when honey was added the films increased their diameter considerably. Honey has high sugar content and low pH, these help prevent the growth of microorganisms [1].
Table 1.
Area increase of chitosan films against Klebsiella pneumonia and Pseudomonas aeruginosa.
| Formulation |
Klebsiella pneumoniae |
Pseudomonas aeruginosa |
||
|---|---|---|---|---|
| Increase in area| (mm)a | % Increase in area | Increase in area (mm)a | % Increase in area | |
| Ch 1% | 254.58 ± 2.08 | 9.13 ± 0.89 | 253.13 ± 1.46 | 8.51 ± 0.62 |
| Ch 2% | 255.98 ± 2.32 | 9.57 ± 0.99 | 252.14 ± 2.08 | 8.08 ± 0.89 |
| Ch 3% | 263.88 ± 1.55 | 13.08 ± 0.69 | 250.90 ± 1.23 | 7.55 ± 0.52 |
| Ch 2% / Gly | 255.03 ± 2.05 | 9.10 ± 0.87 | 251.93 ± 1.34 | 7.99 ± 0.57 |
| Ch 2% / Honey | 317.13 ± 8.02 | 36.73 ± 3.22 | 339.66 ± 11.22 | 45.60 ± 4.81 |
| Ch 2% / Honey / Gly | 336.68 ± 6.99 | 44.32 ± 2.99 | 338.24 ± 2.70 | 44.99 ± 1.16 |
233.27 mm2 initial area
1.2. Thermal analysis
The data of differential scanning calorimetry (DSC) measurements are shown in Table 2. The enthalpy variation shows with film formulation. By thermal analysis was possible found the temperature that resist the chitosan when is used pure or combined with other agents. These assays are indispensable for the process of manufacturing the biomaterial, and thus confirm that the chemical structure of chitosan remains intact, due it is not degraded during processing.
Table 2.
Thermal events of chitosan films.
| Formulation | Tg (°C) | Cp (W/g) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|
| Ch 1% | 63.938 | 0.022764 | 169.384 | 77.03 |
| Ch 2% | 63.754 | 0.221945 | 170.563 | 121.9 |
| Ch 3% | 62.362 | 0.011058 | 134.968 | 107.6 |
| Ch 2% / Gly | 63.307 | 0.0175212 | 152.051 | 119.2 |
| Ch 2% / Honey | 62.755 | 0.0061252 | 144.266 | 164.8 |
| Ch 2% / Honey / Gly | 63.513 | 0.0077765 | 140.498 | 185.6 |
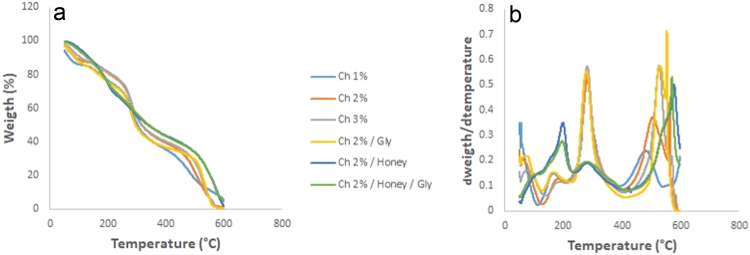
The thermogravimetric analysis (TGA) data revealed valuable information that confirms weight loss as the temperature increases. Fig. 1 shows the thermogram of the original data and the derivative.
Fig. 1.
TGA thermograms (a) and derivative (b).
1.3. Elementary composition
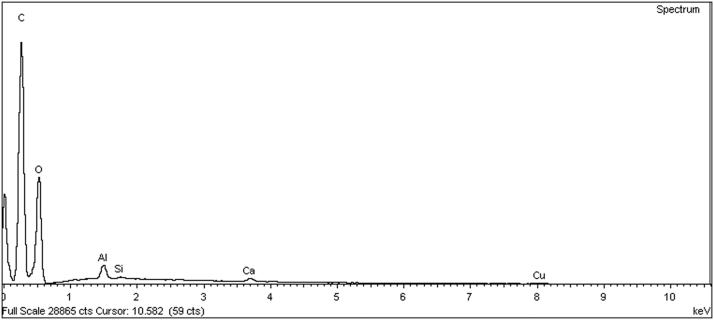
Table 3 shows the carbon (C) and oxygen (O) content data of all the chitosan-based films formulations. Both elements are major components in all samples, around to 99%. Fig. 2 shows a characteristics energy-dispersive spectrum (EDS) of a 1% pure chitosan films.
Table 3.
Carbon and oxygen data in chitosan-based films.
| Sample |
Weight% |
Atomic% |
||
|---|---|---|---|---|
| C | O | C | O | |
| Ch 1% | 52.27 ± 0.18 | 47 ± 0.13 | 59.54 ± 0.17 | 40.19 ± 0.13 |
| Ch 2% | 53.31 ± 0.23 | 45.44 ± 0.62 | 60.66 ± 0.32 | 38.81 ± 0.49 |
| Ch 3% | 50.43 ± 0.05 | 48.64 ± 0.22 | 57.80 ± 0.04 | 41.86 ± 0.14 |
| Ch 2% / Gly | 51.75 ± 0.14 | 47.70 ± 0.18 | 58.98 ± 0.14 | 40.81 ± 0.17 |
| Ch 2% / Honey | 43.88 ± 4.47 | 54.52 ± 3.93 | 51.29 ± 4.47 | 47.94 ± 4.23 |
| Ch 2% / Honey / Gly | 38.31 ± 0.69 | 58.37 ± 0.76 | 45.94 ± 0.71 | 52.55 ± 0.71 |
Fig. 2.
EDS spectrum of a 2% chitosan film.
In the films of pure chitosan and glycerol the behavior is similar, the variation was found in the films combined with honey where the carbon decreases and the oxygen increases, due to sugar being made up of carbon, hydrogen and oxygen.
The data in Table 4 show the trace elements found in the films such as Al, Ca, Si, Cu and Na. However, in all the formulations were found Al, Ca y Cu. Particularly in the films combined with honey, the aluminum content increased abundantly.
Table 4.
Trace elements in chitosan-based films.
| Muestra |
Weight % |
|||||
|---|---|---|---|---|---|---|
| Al | Si | Ca | Cu | K | Na | |
| Ch 1% | 0.21 ± 0.04 | 0.11 ± 0.01 | 0.27 ± 0.01 | 0.19 ± 0.03 | ND | ND |
| Ch 2% | 0.53 ± 0.37 | 0.08 ± 0.01 | 0.3 ± 0.01 | 0.22 ± 0.08 | ND | 0.16 ± 0.01 |
| Ch 3% | 0.29 ± 0.15 | ND | 0.26 ± 0.01 | 0.26 ± 0.08 | ND | ND |
| Ch 2% / Gly | 0.05 ± 0.03 | 0.07 ± 0.01 | 0.24 ± 0.03 | 0.16 ± 0.02 | ND | 0.12 ± 0.01 |
| Ch 2% / Honey | 1.32 ± 0.29 | ND | 0.15 ± 0.01 | 0.25 ± 0.07 | 0.15 ± 0.01 | ND |
| Ch 2% / Honey / Gly | 2.12 ± 0.13 | 0.11 ± 0.01 | 0.31 ± 0.08 | 0.54 ± 0.26 | 0.20 ± 0.07 | ND |
| Atomic % | ||||||
| Muestra | Al | Si | Ca | Cu | K | Na |
| Ch 1% | 0.11 ± 0.02 | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.04 ± 0.01 | ND | ND |
| Ch 2% | 0.27 ± 0.18 | 0.04 ± 0.01 | 0.10 ± 0.01 | 0.05 ± 0.01 | ND | 0.09 ± 0.01 |
| Ch 3% | 0.15 ± 0.07 | ND | 0.09 ± 0.01 | 0.05 ± 0.02 | ND | ND |
| Ch 2% / Gly | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.08 ± 0.01 | 0.03 ± 0.01 | ND | 0.07 0.01 |
| Ch 2% / Honey | 0.69 ± 0.16 | ND | 0.05 ± 0.01 | 0.05 ± 0.02 | 0.05 ± 0.01 | ND |
| Ch 2% / Honey / Gly | 1.13 ± 0.07 | 0.06 ± 0.01 | 0.11 ± 0.02 | 0.12 ± 0.05 | 0.07 ± 0.02 | ND |
1.4. Tensile strength
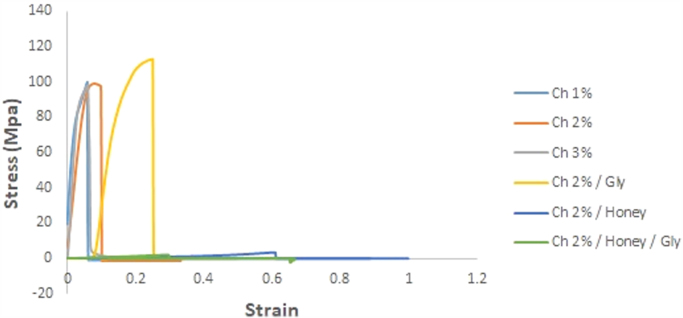
With the data of the tensile strength the mechanical behavior of the chitosan-based films is complemented. All the formulations of the films showed a similar behavior. However, the force required to reach the rupture varied from one sample to another, see Fig. 3.
Fig. 3.
Tensile strength of the chitosan-based films.
1.5. Degree of crystallinity
Table 5 shows the data of crystallinity of the pure chitosan-based films and their formulations, indicating that the grade of crystallinity decreases when the chitosan is combined with other agents. Consequently, all chitosan films were mainly in an amorphous state.
Table 5.
Data of degree crystallinity of chitosan films.
| Sample | Crystal intensity | Intensity amorphous part | Total intensity | % Crystallinity |
|---|---|---|---|---|
| Ch 1% | 10567.25 | 119700.10 | 130267.40 | 8.1 |
| Ch 2% | 16054.56 | 158195.9 | 174250.4 | 9.2 |
| Ch 3% | 16933.84 | 168150.50 | 185084.30 | 9.1 |
| Ch 2% / Gly | 11323.5 | 129193.9 | 140517.3 | 8.0 |
| Ch 2% / Honey | 20228.23 | 337796.90 | 358025.1 | 5.6 |
| Ch 2% / Honey / Gly | 16521.16 | 315417.50 | 331938.60 | 4.9 |
2. Experimental design, materials, and methods
The chitosan-based films were prepared by the solvent evaporation technique. All the experiments were performed in triplicate minimum and the data analysis was carried out with the STATGRAPHICS Plus 5.1 software.
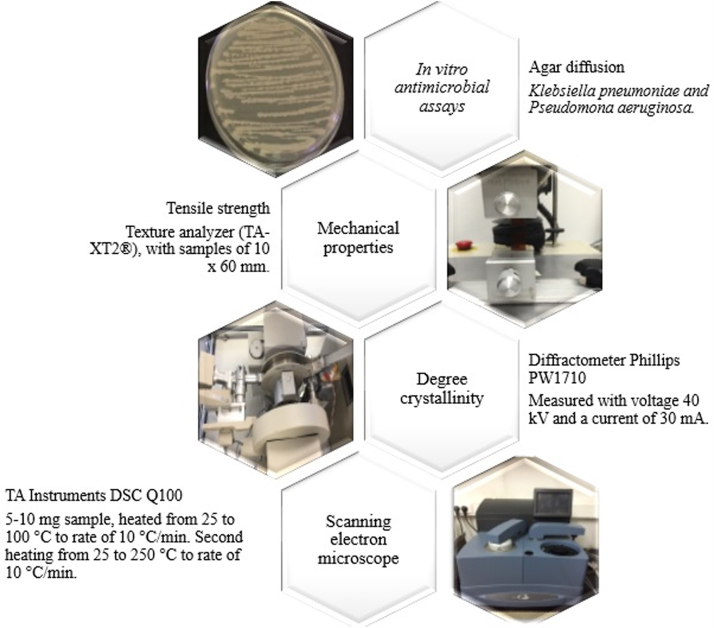
Fig. 4 shows a flow chart of the methodologies for the characterization of chitosan-based films. Specifically, the details are described to evaluate the antimicrobial activity, thermal analysis by DSC, tensile strength and degree crystallinity.
Fig. 4.
Methodology for the characterization of chitosan-based films.
Additionally, to evaluate the elementary composition was used a scanning electron microscope SEM EVO LS15 ZEISS brand (Oberkochen, Baden-Württemberg, Alemania) with an EDX detector. The sample was placed on a scanning support and the measurements were carried out with variable pressure vacuum (VP, 6.05 × 10-6 mbar), the voltage acceleration was 20 kV and the working distance (WD) was 8.5 mm.
Likewise, the thermogravimetric analysis (TGA) was carried out in TA Instruments Inc. Discovery (New Castle, DE, USA). The test was carried out with 20 mg of sample, which was heated from 50 to 600 °C at heating rate of 10 °C/min, under a nitrogen atmosphere with a flow of 60 ml/min.
Acknowledgments
A. A. Escárcega-Galaz gratefully acknowledges the Consejo Nacional de Ciencia y Tecnología (CONACYT) by Ph.D. scholarship: 417707. This research was funded by the Instituto Tecnológico de Sonora with the project PROFAPI (2018-0010) and by the Consejo Nacional de Ciencia y Tecnología (PDCPN2014:248160).
Footnotes
Transparency document associated with this article can be found in the online version at 10.1016/j.dib.2018.09.121.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.Escárcega-Galaz A.A., Sánchez-Machado D.I., López-Cervantes J., Sanches-Silva A., Madera-Santana T.J., Paseiro-Losada P. Mechanical, structural and physical aspects of chitosan-based films as antimicrobial dressings. Int. J. Biol. Macromol. 2018 doi: 10.1016/j.ijbiomac.2018.04.149. [DOI] [PubMed] [Google Scholar]
- 2.Meo S.A., Al-Asiri S.A., Mahesar A.L., Ansari M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017;24:975–978. doi: 10.1016/j.sjbs.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang T., Zhu X.K., Xue X.T., Wu D.Y. Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohyd. Polym. 2012;88:75–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material