Abstract
Gaucher disease (GD) is an autosomal recessive condition that results from a deficiency of the enzyme β-glucocerebrosidase. The increased risk of primary parkinsonism symptoms among individuals affected with GD and carriers for the disorder is well-documented in the literature. However, these risks and case reports often reflect patients with classical Parkinson's disease (PD) symptoms. We report a patient with GD type 1 who was diagnosed with corticobasal syndrome (CBS), a clinical atypical parkinsonism diagnosis, in his sixth decade of life. Our case highlights the need to consider forms of atypical parkinsonism such as CBS in addition to PD in the differential diagnosis of cognitive and motor changes in patients with GD type 1. We also recommend careful assessment and routine monitoring of cognition, mood, behavior, sleep patterns, olfaction, and memory in patients with GD type 1 to identify early symptoms indicative of neurological involvement.
Abbreviations: GD, Gaucher disease; PD, Parkinson's disease; DLB, dementia with Lewy bodies; CBS, corticobasal syndrome; ERT, enzyme replacement therapy; CHITO, chitotriosidase; TRAP, tartrate-resistant acid phosphatase; ACE, angiotensin-converting enzyme; RBD, rapid eye movement (REM) sleep behavior disorder
Keywords: Gaucher disease, Atypical parkinsonism, Corticobasal syndrome, Apraxia, Stereoagnosia, Astereognosis
1. Introduction
Gaucher disease (GD) is an autosomal recessive condition that results from a deficiency of the enzyme β-glucocerebrosidase, causing pathogenic glucocerebroside accumulation within the lysosomes of reticuloendothelial cells [1]. Individuals with GD, one of the most common lysosomal storage disorders, often present with hepatosplenomegaly, pancytopenia, and skeletal abnormalities [2,3]. Classically GD is divided into three clinical subtypes: a non-neuronopathic form (type 1) and two neuronopathic forms (types 2 and 3). Types 2 and 3 disease typically include neurological features such as seizures, saccadic eye movement abnormalities, and spasticity. These clinical subtype classifications are largely determined by the specific disease-causing genetic variants present in the GBA1 gene (OMIM #606463) [1,4]. One of the most common disease-causing variants, N409S (formerly reported as N370S), is most often associated with type 1 disease in the homozygous or compound heterozygous states and traditionally has been thought to provide a protective effect against the neuronopathic manifestations of GD [5].
Although GD type 1 (GD1) is traditionally considered non-neuronopathic in that it does not include the neurological features typically seen in types 2 and 3 disease, peripheral neuropathy and Parkinson's disease (PD) are known to occur in some patients with type 1 disease. Biegstraaten and colleagues [6] estimated the prevalence of peripheral polyneuropathy in the general population to be between 0.09% and 1.3%. In their prospective, longitudinal, observational cohort study of patients with GD1 (n = 103), 10.7% of the patients were diagnosed with sensory motor axonal polyneuropathy at baseline. An additional 5.8% of the patients were diagnosed with polyneuropathy during a two-year follow-up period. These results led to the conclusion that there is a significantly higher prevalence of peripheral polyneuropathy among the GD1 patient population relative to the general population [6].
Similarly, mutations in the GBA1 gene appear to be one of the most significant genetic risk factors for synucleinopathies, including idiopathic PD and dementia with Lewy bodies (DLB) [7,8]. Idiopathic PD affects approximately 0.3% of the entire general population and 1% of the general population over the age of 60 years [9]. Among the GD1 patient population, PD is estimated to be present in 4% of all patients [10]. Patients with GD1 also have a lower mean age of onset of parkinsonian symptoms relative to the general population: 48 years of age in comparison to 71 years, respectively [11]. In addition to cardinal motor symptoms of PD, there are several reports of atypical motor symptoms among GD1 patients including myoclonus and supranuclear gaze palsy [[12], [13], [14], [15], [16], [17]]. In their multicenter study, Nalls and colleagues report a significant association between carrier GBA1 mutations and DLB, a form of atypical parkinsonism, with a higher odds ratio than that between these mutations and idiopathic PD [18]. Their work highlights the importance of considering atypical parkinsonism during the treatment of patients with GD and those who are carriers for GBA1 mutations.
We present a patient with GD type 1 who was diagnosed with corticobasal syndrome (CBS), a clinical atypical parkinsonism diagnosis, in his sixth decade of life, adding to the literature on the spectrum of parkinsonian syndromes seen in GD type 1.
2. Case
The patient is a 62-year-old Caucasian male born to non-consanguineous parents. At age 12 years, he was hospitalized for a fracture of the vertebra resulting from a football injury. Upon examination, hepatosplenomegaly was observed. The patient reported experiencing frequent nose bleeds and easy bruising as a child. He was subsequently diagnosed with GD via bone marrow aspirate at age 14 years. A common genetic variant panel revealed compound heterozygosity for the N409S (formerly reported as N370S) variant and the R120W variant in the GBA1 gene, ultimately leading to a diagnosis of type 1 disease. At age 15 years, he was splenectomized as a means of reducing the hematologic complications of hypersplenism, primarily pancytopenia. The patient reported bone crises during the remainder of his teenage years; in particular, he reported bone pain in his right knee at approximately 16 years of age. He has undergone three hip replacements: the right hip at age 37 years, revised right hip at age 49, and the left hip at age 50. At age 60 years, a dual-energy X-ray absorptiometry (DXA) scan yielded bone mineral density T-score values between −1 and − 2.5, fulfilling the World Health Organization criteria for a diagnosis of osteopenia/osteoporosis.
The patient began treatment with intravenous enzyme replacement therapy (ERT), imiglucerase, at age 49 years and, with the exception of a national imiglucerase shortage, responded well with improvement to his overall disease state. At age 55 years, the patient presented at the Duke Metabolic Clinic. With regard to family history, the patient reported having an older sister also diagnosed with GD who exhibits anemia and thrombocytopenia; he has two other siblings, who are unaffected. The patient's father had Parkinson's disease and died in his seventies.
At the time of presentation, the patient's platelet count (363 × 109/L) and hemoglobin (13.9 g/dL) were within normal ranges. The patient had high chitotriosidase (CHITO) at 701 nmol/h/mL (normal range: 4–120 nmol/h/mL) as well as high tartrate-resistant acid phosphatase (TRAP) at 11.0 IU/L (normal range: 3–10 IU/L). Angiotensin-converting enzyme (ACE) was not evaluable as the patient was on an ACE inhibitor, which would affect the testing results. An MRI of the abdomen revealed increased hepatic iron storage. Blood testing revealed a high level of ferritin at 1163 ng/mL (normal range: 11–204 ng/mL). A workup for hemochromatosis was performed, which was negative. The patient enrolled in the ENCORE trial (NCT00943111, Sanofi Genzyme) at age 55 years and began receiving oral eliglustat, which stabilized his systemic Gaucher symptoms. After three years of eliglustat treatment, his TRAP was within normal range and his CHITO remained elevated but stable at approximately 489 nmol/h/mL.
At age 58 years, cognitive decline was reported by the patient and his spouse characterized by long-term memory impairment, slurring of speech, and word-finding difficulty. Vitamin B12 (411 pg/mL) and folate (6.9 ng/mL) were within normal ranges as were his results for serum protein electrophoresis (7.0 g/dL) and thyroid function (thyroid stimulating hormone: 1.67 uIU/mL; free thyroxine: 0.65 ng/dL), which ruled out causes for mental lapses associated with vitamin deficiency and hormonal imbalance. Additionally, cerebrospinal fluid analysis demonstrated normal levels of glucose, protein, and cell count and was free of oligoclonal bands, making autoimmune causes of neurological symptoms less likely. Brain magnetic resonance imaging (MRI) at this time displayed evidence of ventriculomegaly, significant diffuse T2 hyperintensities, and cortical atrophy considered advanced for his age. Ventriculomegaly raised concern for normal pressure hydrocephalus, but there was no significant improvement in motor and cognitive symptoms after a large-volume lumbar puncture.
Neuropsychological testing at age 58 years revealed non-amnestic mild cognitive impairment with deficits in executive function and in lexical and semantic retrieval. Movement disorders examination revealed masked facies and reduced left arm rapid alternating movements. Given that eliglustat does not cross the blood-brain barrier, the likelihood of the drug affecting cognitive functioning was deemed very low, and the patient expressed a desire to continue on oral treatment. At age 59 years, at the conclusion of the ENCORE trial, he transitioned from study eliglustat to clinical eliglustat. Additionally, based upon a reported episode of parasomnia in which the patient acted out his dream, a sleep study at age 59 years noted sleep maintenance insomnia and possible REM sleep behavior disorder (RBD).
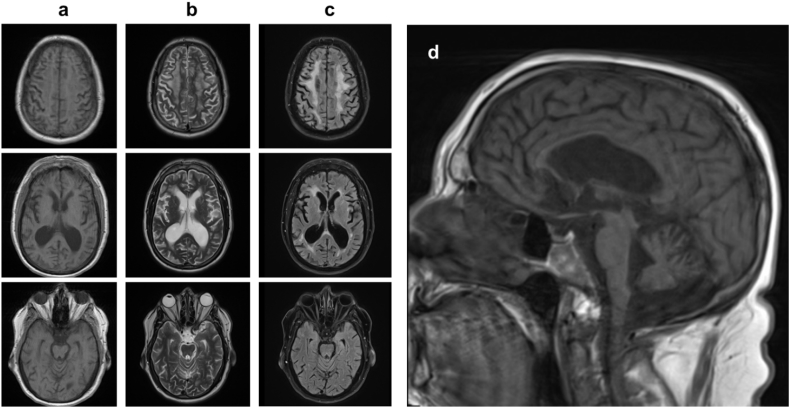
At age 60 years, the patient was seen by specialists at the National Institutes of Health (NIH). Exam demonstrated asymmetric rigidity (which was associated with muscle pain and cramping), left hand tremor, and asymmetric bradykinesia, with all symptoms notably worse on the left. Micrographia was noted. He had difficulty rising from a chair without assistance, and he was unsteady with shuffling of gait. In addition, exam demonstrated dementia, left hand apraxia, and vertical gaze palsy, which are not typically seen with classical PD. A brain MRI obtained at the NIH at age 60 years exhibited generalized cerebral and cerebellar volume loss along with associated ventriculomegaly (Fig. 1). It also showed diffuse confluent T2/FLAIR signal hyperintensity within the periventricular white matter, deep white matter, and subcortical white matter structures of the cerebral hemispheres bilaterally. Electroencephalogram displayed mild to moderate diffuse background slowing with focal slowing in the right frontal region. Sleep study demonstrated severe obstructive sleep apnea. He was overweight with a body mass index of 35.51 kg/m2. No parasomnias were noted during the sleep study. A diagnosis of atypical parkinsonism was determined by the NIH as the patient exhibited apraxia, dementia, and vertical gaze palsy, which are not typical features of idiopathic PD. At age 60.5 years, he was started on a trial of carbidopa/levodopa 25/100 mg three times daily but without any clear impact on his motor symptoms; however, he did start to experience visual hallucinations of human figures.
Fig. 1.
Brain MRI axial T1 (column a), T2 (column b), and FLAIR (column c) as well as sagittal midline T1 (d) at age 60 years. Imaging was notable for generalized atrophy with associated ex-vacuo ventriculomegaly as well as diffuse, confluent T2/FLAIR signal hyperintensity within the subcortical, periventricular, and deep white matter of bilateral cerebral hemispheres.
At age 61 years, he was evaluated at the Duke Movement Disorders Center, and his neurological exam was notable for highly left lateralized rigidity and bradykinesia as well as apraxia and stereoagnosia in the left upper limb. He also exhibited gait impairment with shuffling steps, restriction of extra-ocular movements in both the vertical and lateral planes, progressive dementia without fluctuations, irritability, and visual hallucinations. No autonomic abnormalities were noted. His hallucinations initially resolved after starting quetiapine but then returned after carbidopa/levodopa 25/100 mg was increased to 1.5 and then 2 tablets, three times daily. There was no improvement in his motor symptoms on 600 mg/day of levodopa, so it was slowly weaned without worsening of motor symptoms. He was diagnosed with clinically probable corticobasal syndrome based upon his strongly lateralized motor symptoms, signs and symptoms of cortical dysfunction including apraxia and stereoagnosia, and lack of response to levodopa, following consensus criteria established by Armstrong et al. [19].
3. Discussion
The increased risk of primary parkinsonism symptoms among individuals affected with Gaucher disease and carriers for the disorder is well-documented in the literature. Our case highlights the need to consider forms of atypical parkinsonism such as CBS in addition to PD in the differential diagnosis of cognitive and motor changes in patients with GD type 1. At least 60 cases of patients with GD type 1 who have exhibited symptoms consistent with atypical parkinsonism have been reported in the literature [[12], [13], [14], [15], [16], [17],20]. These patients have been reported to exhibit a range of neurological manifestations including mild cognitive impairment, dementia, horizontal gaze abnormalities, and myoclonus. Of these patients, there appears to be only one other case of CBS explicitly reported [20]. Several parallels exist between this case and ours. Both cases exhibited cortical atrophy, progressive dementia, apraxia, supranuclear gaze abnormality in the vertical plane, resting tremor, abnormal gait, difficulty sleeping, and changes in mood and personality. Additionally, both individuals are male and compound heterozygous for the N409S variant and began exhibiting neurological manifestations at similar ages: age 58 years for our patient and age 60 years in the case of Alonso-Canovas et al.
GD type 1 is traditionally characterized as non-neuronopathic, and literature to date suggests that the presence of certain disease-causing variants, particularly N409S, confers a protection against the neurological symptoms associated with types 2 and 3 disease. These neurological symptoms include seizures, spasticity, and saccadic eye movements [1,5]. However, as many reports in the literature have demonstrated, a diagnosis of GD type 1 does not preclude a patient from exhibiting an array of other neuronopathic manifestations including peripheral neuropathy, PD, and forms of atypical parkinsonism later in life [6,20,21]. Our case illustrates a patient compound heterozygous for the N490S variant who developed neurological manifestations in the form of atypical parkinsonism, namely CBS, later in life. While the traditional clinical subtype classifications for GD provide a useful framework to conceptualize the most common hematological, visceral, skeletal, and neurological manifestations of GD, strict adherence to this framework can result in less common, but important, manifestations to be overlooked.
The mean age of onset of parkinsonian symptoms is much earlier in patients with GD1 (48 years of age) relative to the general population (71 years of age), a difference of more than 20 years [11]. Careful assessment and routine monitoring of cognition, mood, and behavior in patients with GD type 1 should be completed to identify early symptoms indicative of neurological involvement. Additionally, given that insomnia and RBD are among some of the non-motor symptoms reported in the literature that often precede the onset of more classic motor manifestations of PD and other parkinsonian syndromes, sleep patterns in these patients should also be monitored closely [22]. Anosmia has also been reported as a precursory PD non-motor manifestation; while not observed in the case presented in this report, monitoring olfaction in patients with GD1 could prove to be beneficial [23]. Finally, while currently not a part of management guidelines for patients with GD1, memory testing and neuropathy questionnaires could be utilized to screen for neurological manifestations, similar to how serum protein electrophoresis is used to screen for malignancies and monoclonal gammopathies in patients with GD1 [24].
This report does not suggest a definite causal relationship between GBA1 mutations, the underlying cause of GD, and the signs and symptoms of CBS in this patient. Whole exome sequencing was not performed, so other possible genetic causes of CBS cannot be excluded. Corticobasal syndrome is a non-specific clinical diagnosis associated with a number of pathologically-proven conditions including corticobasal degeneration (CBD), dementia with Lewy bodies (DLB), progressive supranuclear palsy (PSP), and frontotemporal lobar degeneration (FTLD) [25,26]. This case was not positively diagnosed with a synucleinopathy, for which GBA1 mutations are a major risk factor. It is possible that the underlying pathology of this patient's CBS presentation is a tauopathy such as CBD, PSP, or FTLD. While the vertical gaze palsy noted in this case is suggestive of PSP, the white matter changes are not typical of PSP or DLB; without brain pathology, a definitive diagnosis cannot be made.
4. Conclusions
Our case adds to the literature on patients with GD1 later diagnosed with atypical parkinsonism, specifically CBS. To reach this rare diagnosis, careful assessment and monitoring of our patient's neurological symptoms was crucial, achieved through collaboration with neurologists who are part of the multidisciplinary team in the management of patients with Gaucher disease. While it is well known that patients with GD1 and GD1 carriers are at higher risk for developing PD, this report augments the value of considering CBS and other forms of atypical parkinsonism in discussions with patients with GD1 and their families.
Acknowledgments
Acknowledgements
We would like to thank Dr. Grisel Lopez at the National Institutes of Health for contributing to this patient's care and collaborating with our institution.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
Priya S. Kishnani has received research/grant support and honoraria from Sanofi Genzyme and Shire. She is a member of the Pompe and Gaucher Disease Registry Advisory Board for Genzyme Corporation. Roy N. Alcalay has received consultation fees from Sanofi Genzyme, Biogen and Denali. He is a member of the Gaucher Disease Registry Advisory Board for Genzyme Corporation. Lauren B. Flueckinger has received honoraria from Sanofi Genzyme and Shire.
References
- 1.Jmoudiak M., Futerman A.H. Gaucher disease: pathological mechanisms and modern management. Br. J. Haematol. 2005;129(2):178–188. doi: 10.1111/j.1365-2141.2004.05351.x. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira C.R., Gahl W.A. Lysosomal storage diseases. Translational Science of Rare Diseases. 2017;2(1–2):1–71. doi: 10.3233/TRD-160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceravolo F., Grisolia M., Sestito S. Combination therapy in a patient with chronic neuronopathic Gaucher disease: a case report. J. Med. Case Rep. 2017;11(19):1–5. doi: 10.1186/s13256-016-1147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pastores G.M., Hughes D.A. 2000. Gaucher Disease. GeneReviews. updated 2018. (Internet) [Google Scholar]
- 5.Neudorfer O., Giladi N., Elstein D. Occurrence of Parkinson's syndrome in type I Gaucher disease. QJM. 1996;89(9):691–694. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- 6.Biegstraaten M.K., Mengel E., Marodi L. Peripheral neuropathy in adult type 1 Gaucher disease: a 2-year prospective observational study. Brain. 2010;133(10):2909–2919. doi: 10.1093/brain/awq198. [DOI] [PubMed] [Google Scholar]
- 7.Schapira A.H. Glucocerebrosidase and Parkinson disease: recent advances. Mol. Cell. Neurosci. 2015;66(Pt A):37–42. doi: 10.1016/j.mcn.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sardi S.P., Cheng S.H., Shihabuddin L.S. Gaucher-related synucleinopathies: the examination of sporadic neurodegeneration from a rare (disease) angle. Prog. Neurobiol. 2015;125(Feb 2015):47–62. doi: 10.1016/j.pneurobio.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 9.McLean G., Hindle J.V., Guthrie B. Co-morbidity and polypharmacy in Parkinson's disease: insights from a large Scottish primary care database. BMC Neurol. 2017;17(126):1–8. doi: 10.1186/s12883-017-0904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Porcel F., Espay A., Carecchio M. Parkinson disease in Gaucher disease. Journal of Clinical Movement Disorders. 2017;4(7):1–4. doi: 10.1186/s40734-017-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherin P., Rose C., de Roux-Serratrice C. The neurological manifestations of Gaucher disease type 1: the French Observatoire on Gaucher disease (FROG) J. Inherit. Metab. Dis. 2010;33(4):331–338. doi: 10.1007/s10545-010-9095-5. [DOI] [PubMed] [Google Scholar]
- 12.Lwin A., Orvisky E., Goker-Alpan O. Glucocerebrosidase mutations in subjects with parkinsonism. Mol. Genet. Metab. 2004;81(1):70–73. doi: 10.1016/j.ymgme.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Wong K., Sidransky E., Verma A. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol. Genet. Metab. 2004;82(3):192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Tayebi N., Walker J., Stubblefield B. Gaucher disease with parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol. Genet. Metab. 2003;79(2):104–109. doi: 10.1016/s1096-7192(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 15.Capablo J.L., Saenz De Cabezon A., Fraile J. Neurological evaluation of patients with Gaucher disease diagnosed as type 1. J. Neurol. Neurosurg. Psychiatry. 2008;79(2):219–222. doi: 10.1136/jnnp.2006.111518. [DOI] [PubMed] [Google Scholar]
- 16.Biegstraaten M., van Schaik I.N., Aerts J.M. ‘Non-neuronopathic’ Gaucher disease reconsidered. Prevalence of neurological manifestations in a Dutch cohort of type I Gaucher disease patients and a systematic review of the literature. J. Inherit. Metab. Dis. 2008;31(3):337–349. doi: 10.1007/s10545-008-0832-y. [DOI] [PubMed] [Google Scholar]
- 17.Goker-Alpan O., Lopez G., Vithayathil J. The spectrum of parkinsonian manifestations associated with glucocerebroside mutations. Arch. Neurol. 2008;65(10):1353–1357. doi: 10.1001/archneur.65.10.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nalls M.A., Duran R., Lopez G. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurology. 2013;70(6):727–735. doi: 10.1001/jamaneurol.2013.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong M.J., Litvan I., Lang A.E. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80(5):496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alonso-Canovas A., Katschnig P., Tucci A. Atypical Parkinsonism with Apraxia and Supranuclear Gaze Abnormalities in Type 1 Gaucher Disease. Expanding the Spectrum: Case Report and Literature Review. Mov. Disord. 2010;25(10):1506–1508. doi: 10.1002/mds.23109. [DOI] [PubMed] [Google Scholar]
- 21.Bultron G., Kacena K., Pearson D. The risk of Parkinson's disease in type 1 Gaucher disease. J. Inherit. Metab. Dis. 2010;33(2):167–173. doi: 10.1007/s10545-010-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loddo G., Calandra-Buonaura G., Sambati L. The Treatment of sleep Disorders in Parkinson's Disease: from Research to Clinical Practice. Front. Neurol. 2017;8(42):1–15. doi: 10.3389/fneur.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morley J.F., Cohen A., Silveira-Moriyama L. Optimizing olfactory testing for the diagnosis of Parkinson's disease: item analysis of the University of Pennsylvania smell identification test. Nature Partner Journals: Parkinson's Disease. 2018;4(2):1–7. doi: 10.1038/s41531-017-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes D., Cappellini M.D., Berger M. Recommendations for the management of the haematological and onco-haematological aspects of Gaucher disease. Br. J. Haematol. 2007;138(6):676–686. doi: 10.1111/j.1365-2141.2007.06701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S.E., Rabinovici G.D., Mayo M.C. Clinicopathological correlations in corticobasal degeneration. Ann. Neurol. 2011;70(2):327–340. doi: 10.1002/ana.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasanuki K., Josephs K.A., Ferman T.J. Diffuse Lewy body disease manifesting as corticobasal syndrome: a rare form of Lewy body disease. Neurology. 2018;91(3):e268–e279. doi: 10.1212/WNL.0000000000005828. [DOI] [PMC free article] [PubMed] [Google Scholar]