Abstract
This study evaluated the possible protective effect of royal jelly (RJ) on sperm parameters and sperm malondialdehyde (MDA) concentration and in vitro fertilizing potential in nicotine (NIC) exposed male mice. Thrtiy-six male BALB/c mice were randomly divided into six groups (n = 6). Group 1 received normal saline, group 2 received 100 mg kg-1 per day RJ, groups 3 and 4 received NIC at doses of 0.50 and 1.00 mg kg-1 per day, respectively and groups 5 and 6 received NIC at doses of 0.50 and 1.00 mg kg-1 per day, respectively plus RJ. Caudal epididymal sperm characteristics, lipid peroxidation and in vitro fertilizing capacity and embryo development were evaluated after 35 days. The NIC treatment caused a significant decrease in sperm motility and viability and fertilization rate along with poor blastocyst formation and increased sperm DNA damage and MDA levels. Moreover, the incidences of chromatin abnormality in spermatozoa were significantly higher in NIC-exposed mice than those of control. Nevertheless, RJ treatment improved sperm parameters and in vitro fertilization outcome as well as sperm lipid peroxidation level. Data from the current study suggest that RJ has a potential repro-protective action against NIC-induced sperm abnormalities and embryotoxicity in mice.
Key Words: Malondialdehyde, Mice, Nicotine, Royal jelly, Sperm
Introduction
Infertility, as one of the health problems, causes detrimental effects in personal, social and economic domains and is observed in 10.00 to 15.00% of the couples. For many years, it was imagined that most reproductive failures could be attributed to the female partner but numerous reports have demonstrated that 30.00 to 50.00% of infertilities are caused by a male factor.1
Cigarette smoke, one of the largest public health problems, contains many toxic chemical compounds causing reduced sperm quality and reproductive failure.2 Indeed, nicotine (NIC) is an active substance in tobacco and many researchers have focused on its adverse effects on male reproductive function. It was found that NIC administration in experimental animals affects spermatogenesis and epididymal sperms count, motility and fertilizing capacity, decreases testosterone level, impairs Leydig cells function and leads to various histopathological changes in testicular tissue.2,3-6
Accordingly, NIC has been attributed to increased oxidative stress level in seminal plasma.7 Moreover, NIC not only increases sperm abnormalities, but also causes negative effects on sperm plasma membrane integrity and DNA reducing the capacity of fusion with oocytes.8
Royal jelly (RJ) as the most important beehive product is secreted from the salivary glands of worker honeybees and serves as a primary super food for the queen and larvae during their first three days of life.9 The RJ includes several important compounds such as sugars, free amino acids, fatty acids, minerals (e.g., calcium) and vitamins with biological activities.10 It has been shown to have anti-inflammatory, immunomodulatory and anti-tumor functions as well as antioxidant properties.10 Although previous studies have indicated that RJ has significant positive effects on reproductive system and fertility, less attention has been directed towards disclosing its potential in protection against drug-induced sperm lipid peroxidation and abnormalities and embryotoxicities.11
In vitro fertilization (IVF) was developed primarily for treatment of female infertility.12 However, with improved methods; it became apparent that lower concentrations of motile spermatozoa were required to achieve fertilization than originally expected. With this in mind, the idea was put forward that as long as suitable numbers of motile spermatozoa were obtained, IVF could be used for treatment of infertility due to subfertile semen.13
Based on this concept, the present study was carried out to examine the possible protective role of RJ in preventing NIC-induced sperm impairment and lipid peroxidation and IVF outcome disturbances in mice.
Materials and Methods
Preparation of NIC and RJ. The NIC solution (C10H14N2, CAS No. 54-11-5) was purchased from Merck Company (Merck, Germany). The doses and administration route were selected according to Oyeyipo et al.2 Fresh RJ was obtained from a local beekeeping association (Urmia, Iran) and stored at –20 ˚C until use.
Animals. For this study, 36 male BALB/c mice with weight range of 25-30 g were purchased from authorized laboratory animal breeding center (Laboratory Animal House, Urmia University, Urmia, Iran). They were housed in a specific pathogen-free environment under standard conditions of temperature (22.00 ± 2.00 ˚C), relative humidity (50.00 ± 10.00%) and light (12 hr light/dark), fed with a standard pellet diet and had free access to water. Clinical and behavioral observations were recorded throughout the study. Animal work was conducted in compliance with Guidelines for the Humane Care and Use of Laboratory Animals using protocols approved by the Urmia University (No. 2.PAD.159, 2018.01.29). Following 15 days of acclimatization to the new environment, the male mice were randomly divided into six experimental groups, each comprises of six animals. Group 1 was provided as a control group receiving 0.20 mL normal saline orally throughout the experiment. Group 2 was provided as a RJ control group receiving 100 mg kg-1 per day RJ dissolved in 0.20 mL normal saline orally.14 Groups 3 and 4 were received NIC dissolved in 0.20 mL normal saline at doses of 0.50 and 1.00 mg kg-1 per day; orally, respectively. Groups 5 and 6 were received NIC at doses of 0.50 and 1.00 mg kg-1 per day; orally, dissolved in 0.20 mL normal saline, respectively plus 100 mg kg-1 per day RJ; orally. The experiment period was 35 days.
Caudal epididymal sperm sampling. All animals were euthanized following anesthesia with ketamine (75 mg kg-1; IP) (Alfasan International, Woerden, Holland) 24 hr after the experiment period.15 A vertical midline lower abdominal incision was made and epididymides were carefully dissected out and cleaned of adhering connective tissue under a 20× magnification provided by a stereo zoom microscope (Model TL2; Olympus, Tokyo, Japan). Epididymal tails were cut into 2-3 pieces, transferred to 1 mL of human tubal fluid (HTF; Sigma, St. Louis, USA) medium and incubated for 10 min at 37 ˚C in an atmosphere of 5.00% CO2 incubator to allow sperms to swim out of the epididymal tubules.14 After that, samples were evaluated for sperm characteristics and in vitro fertilizing potential as well as lipid peroxidation levels.
Evaluation of sperm parameters. For counting sperm density, after dilution of epididymal sperm to 1:20 in HTF medium, approximately 10 μL of diluted specimen was transferred to each of the counting chambers of the hemocytometer, which was allowed to stand for five min in a humid chamber to prevent drying. The cells were sedimented during this time and counted with a light microscope at 400×. The sperm density was expressed as the number of sperm per milliliter.14 The percentage of sperm motility was evaluated by a light microscope (Olympus) at 400×. For this analysis, one drop of sperm suspension was placed on a hot (37 ˚C) glass slide which was then covered with a lamella. After that, the numbers of sperms with rapid progressive forward movement (RPFM), slow progressive forward movement (SPFM), circumferential motion (CM) and those which remained motionless (ML) were recorded in ten different microscopic fields.16 In order to sperm viability evaluation, 20 µL of 0.50% eosin Y and nigrosin were added into an equal volume of sperm suspension. After 2 min of incubation at room temperature, slides were examined by light microscope at 400×. Dead sperms were appeared to be pink and live sperms were not stained. In each sample, 400 sperms were counted and viability percentages were calculated.17 To determine typical form percentages, sperm smears were prepared on clean and grease free slides, allowed to be air-dried overnight, stained with 1.00% eosin-Y/5.00% nigrosin and examined at 400× (Fig. 1). The teratozoospermia index (TZI) was defined as the number of abnormalities present per abnormal spermatozoon. Each abnormal spermatozoon can have one to four abnormalities including head, neck/mid piece and tail defects or presence of cytoplasmic residues. The spermatozoa were recorded as normal or abnormal and distributed into specific groups (head, neck/mid piece and tail defects or cytoplasmic residues groups). The total number of abnormalities was then added together and divided by the number of abnormal spermatozoa.18 The sperm deformity index (SDI) was calculated through dividing the total number of deformities observed by the number of sperms that were randomly selected and evaluated irrespective of their morphological normality.19
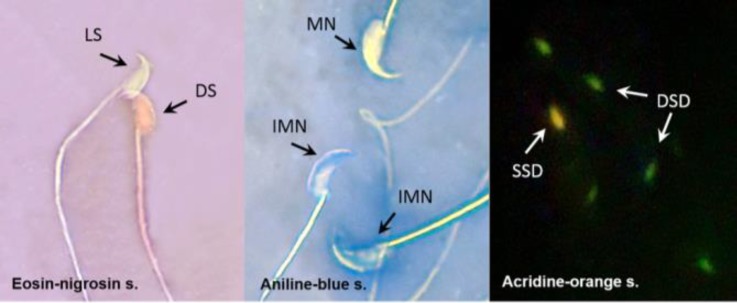
Fig. 1.
Photomicrograph of mice epididyimal spermatozoa. DS: Dead sperm, LS: Live sperm, MN: Mature nucleus, IMN: Immature nuclei, SSD: Single-strand DNA, DSD: Double-strand DNA (1000×).
Assessment of sperm chromatin quality. Aniline blue (AB), a cytochemically based dye, is used for detection of excessive histones in process of sperm chromatin condensation.20 A drop of spermatozoa suspension was spread on glass slides and allowed to be air-dried. All smears were fixed in 3.00% glutaraldehyde in phosphate buffered saline. The slides were then stained with 5.00% aqueous AB and mixed with 4.00% acetic acid (pH=3.50) for 5 min. Sperm heads containing immature nuclear chromatin were stained blue, whereas those with mature nuclei did not stain (Fig. 1). The percentage of spermatozoa that stained with AB was determined by counting 400 spermatozoa.21
Assessment of sperm DNA damage. Sperm DNA integrity was determined by acridine-orange (AO) staining. A drop of sperm suspension was spread on the glass slides and allowed to be air-dried. All smears were fixed in methanol acetic acid at 1:3 v/v for 2 hr. The slides were then stained with AO solution in phosphate citrate for 5 min and rinsed with deionized water. The sperms were evaluated by a fluorescence microscope (Zeiss, Oberkochen, Germany) and two types of staining patterns were identified including green (double-stranded DNA) and yellow (single-stranded DNA) sperms (Fig. 1).22
Assessment of sperm lipid peroxidation. Sperm lipid peroxidation was assessed by a specific spectro-photometric method.23 Briefly, 50 µL of 0.20% butylated hydroxytoluene (dissolved in ethanol) and 1.00 mL of 15.00% aqueous trichloroacetic acid were added to 50 µL of sperm. The mixture was then centrifuged at 4,000 g for 15 min at 4 ˚C. An aliquot of 500 μL of deproteinized supernatant was added to 1.00 mL thiobarbituric acid (0.375% in 0.25 M HCl) and the mixture was heated at 100 ˚C for 20 min. After cooling, the solution was analyzed by a spectrophotometer at 532 nm.
Oocyte pickup. To induce superovulation, 108 female BALB/c mice were injected intraperitoneally with 10 IU of pregnant mare’s serum gonadotropin (PMSG; Folligon, Boxmeer, Netherlands) and 10.00 IU of human chorionic gonadotropin (hCG; Folligon) 48 hr later.24 Fourteen hr after hCG administration, females were sacrificed by cervical dislocation following anesthesia with ketamine (75 mg kg-1; IP) and their oviducts were immediately excised and placed in Petri dishes containing HTF medium. Using the TL2 stereo zoom microscope, the ampullary portion was found and cumulus–oocyte complex was picked up and moved to the fertilization droplets under mineral oil containing HTF medium.
The IVF process and microscopic evaluation. The IVF was performed as previously described.25 Following capacitation step, sperms (1×106 mL-1 HTF) were added to the medium. Fertilization rate was determined after 4 to 6 hr through two pronuclei observations. Then, granulosa cells were denuded and washed and zygotes were transferred into the fresh pre-equilibrated medium and cultured for five days. Evaluation of two-cell embryos was done 24 hr after fertilization. The percentages of blastocysts and hatched embryos were estimated 4 and 5 days after IVF, respectively (Fig. 2).26
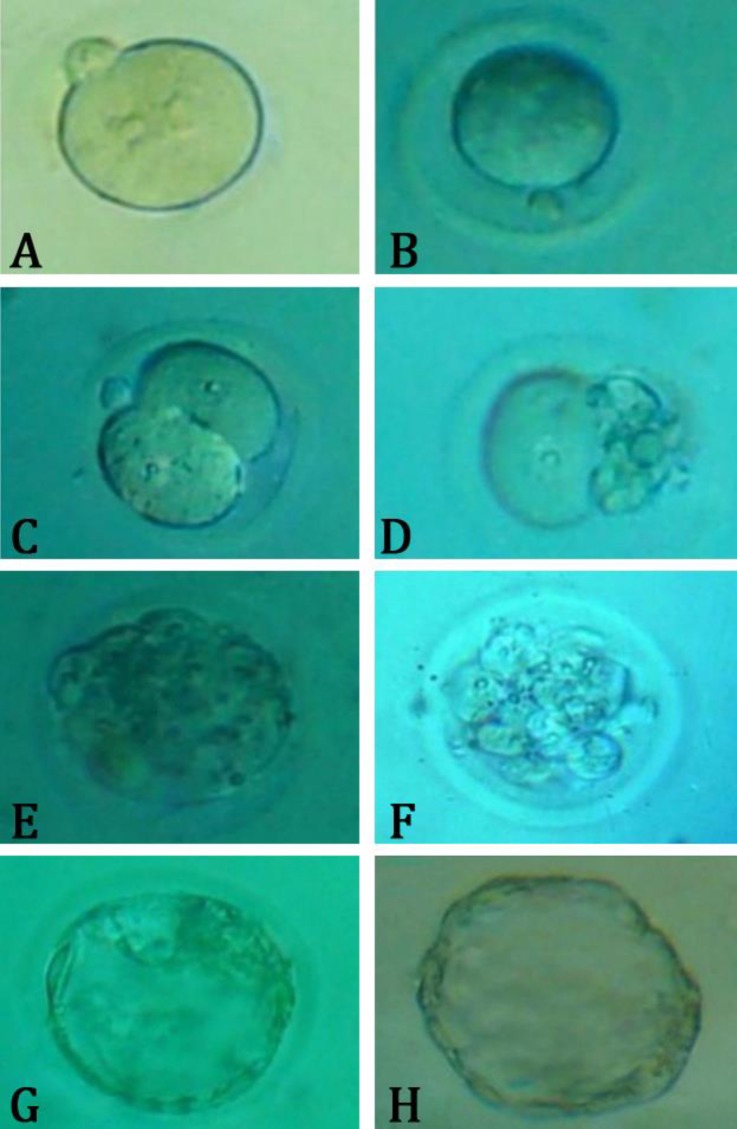
Fig. 2.
Photomicrograph of pre-implantation embryo development. A) Fertilized oocyte, B) Unfertilized oocyte, C) 2-cell embryo, D) Arrested 2-cell embryo, E) Compact morula, F) Arrested morula, G) Blastocyst, H) Hatched embryo.
Statistical analysis. The data were expressed as mean ± SD. The variables were analyzed by one-way analysis of variance followed by Tukey’s test for post hoc comparisons using Statistical Package for the Social Sciences, (version 18.0, SPSS Inc., Chicago, USA). The statistical significance level was set at p < 0.05.
Results
Sperm characteristics. Observations revealed a significant reduction in sperm density of NIC-received animals compared to control groups, meanwhile, co-administration of RJ led to increases in sperm density compared to NIC groups. In the groups which received low and high doses of NIC compared to controls, significant reductions in RPFM were also observed (p < 0.05). Furthermore, SPFM and ML were increased in the NIC groups. Co-administration of RJ led to increase in RPFM compared to NIC groups. Moreover, there were significant (p < 0.05) lower percentages of sperm viability in NIC groups in comparison with control and RJ groups, meanwhile co-administration of RJ led to significant (p < 0.05) increase in sperm viability compared to NIC groups. The SDI and TZI significantly (p < 0.05) increased in NIC-treated groups compared to control and RJ groups. Co-administration of RJ caused a significant (p < 0.05) decrease in the SDI and TZI. Additionally, a significant (p < 0.05) lower percentage of normal morphology was seen in NIC-treated animals. However, RJ co-treatment caused a significant (p < 0.05) increase in this parameter (Table 1).
Table 1.
Effect of nicotine and royal jelly on epididymal sperm parameters. Data are presented as mean ± SD.
| parameters | CON | RJ | NIC 0.50 | NIC 1.00 | NIC 0.50+ RJ | NIC 1.00+ RJ |
|---|---|---|---|---|---|---|
| Sperm density (10 6 mL -1 ) | 47.04 ± 3.01 a | 47.35 ± 1.55a | 34.38 ± 2.60b | 26.03 ± 1.87c | 46.24 ± 2.65 a | 42.90 ± 2.35 a |
| Sperm viability (%) | 86.79 ± 4.21 a | 89.55 ± 3.10 a | 67.94 ± 3.00b | 54.04 ± 2.77 c | 85.04 ± 4.62 a | 81.78 ± 2.37 a |
| Rapid progressive forward movement (%) | 65.38 ± 2.54 a | 64.75 ± 2.41 a | 53.58 ± 2.50b | 48.93 ± 3.31 c | 63.80 ± 2.40 a | 61.95 ± 1.89 a |
| Slow progressive forward movement (%) | 16.30 ± 1.14 a | 17.16 ± 1.09 a | 20.50 ± 2.02 b | 24.64 ± 2.41 c | 16.55 ± 0.91 a | 16.93 ± 1.06 a |
| Circumferential motion (%) | 11.89 ± 0.55 a | 12.13 ± 0.83 a | 9.72 ± 0.63 b | 9.75 ± 0.59 b | 11.49 ± 0.37 a | 11.10 ± 0.89 a |
| Motionless (%) | 6.71 ± 0.73 a | 8.22 ± 0.54 b | 9.88 ± 0.54c | 10.93 ± 0.96d | 8.06 ± 0.60ab | 6.90 ± 0.43 a |
| Typical form (%) | 90.68 ± 1.29 a | 91.19 ± 1.05 a | 68.34 ± 2.78b | 57.22 ± 2.81 c | 86.62 ± 2.27 a | 82.32 ± 2.71 d |
| Teratozoospermia index | 1.08 ± 0.49 a | 1.05 ± 0.03 a | 1.33 ± 0.03b | 1.59 ± 0.07c | 1.14 ± 0.04 a | 1.25 ± 0.06b |
| Sperm deformity index | 0.36 ± 0.02 a | 0.33 ± 0.02 a | 0.52 ± 0.03 b | 0.72 ± 0.03 c | 0.37 ± 0.03 a | 0.39 ± 0.02 a d |
CON: Control, RJ: Royal jelly, NIC 0.50: Nicotine 0.50 mg kg-1 per day, NIC 1.00: Nicotine 1.00 mg kg-1 per day.
Different superscripts in the same row show significant differences between groups (p < 0.05).
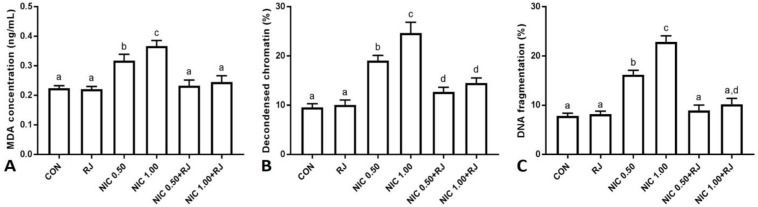
Sperm MDA level. The NIC administration resulted in significant (p < 0.05) sperm MDA level elevation in comparison with control and RJ groups, meanwhile co-administration of RJ significantly (p < 0.05) improved MDA concentration compared to NIC groups (Fig. 3A).
Fig. 3.
Effect of nicotine and royal jelly on A) sperm malondialdehyde concentration, B) percentage of decondensed chromatin and C) percentage of sperm DNA fragmentation. The different superscripts are representative of significant differences. CON: Control, RJ: Royal jelly, NIC 0.50: Nicotine 0.50 mg kg-1 per day, NIC 1.00: Nicotine 1.00 mg kg-1 per day.
Sperm chromatin condensation. Significant (p < 0.05) increases in the percentage of spermatozoa with chromatin abnormalities were observed in low and high doses of NIC groups compared to control and RJ groups. The RJ co-administration resulted in a significant (p < 0.05) decrease in the percentage of spermatozoa with chromatin abnormality compared to NIC groups (Fig. 3B).
Sperm DNA integrity. Administration of low and high doses of the NIC significantly (p < 0.05) increased the number of spermatozoa with DNA damage, whereas co-administration of RJ led to significant (p < 0.05) reductions in this parameter compared to NIC groups (Fig. 3C).
The IVF outcome. In the groups which received low and high doses of NIC compared the controls, significant (p < 0.05) reductions in the numbers of zygote and two-cell, blastocyst stage and hatched embryos along with significant (p < 0.05) increases in the percentages of arrested embryos were observed. Co-administration of RJ led to increases in the numbers of zygote and two-cell, blastocyst stage and hatched embryos as well as reductions in arrested embryos percentages compared to NIC groups (Table 2).
Table 2.
Effect of nicotine and royal jelly on in vitro fertilization outcome. Data are presented as mean ± SD.
| Groups | Oocyte (n) | Number of Zygote | Zygote (%) | Two cell (%) | Blastocyst (%) | Hatched (%) | Arrest (%) |
|---|---|---|---|---|---|---|---|
| CON | 57 | 51 | 90.89 ± 1.71a | 88.01 ± 1.89 a | 77.26 ± 3.00 a | 69.51 ± 2.70 a | 10.09 ± 0.82 a |
| RJ | 60 | 54 | 91.25 ± 2.83 a | 89.55 ± 1.97 a | 78.45 ± 2.87 a | 70.41 ± 2.44 a | 9.43 ± 1.37 a |
| NIC 0.50 | 53 | 40 | 77.34 ± 2.79b | 74.81 ± 1.83 b | 63.97 ± 3.64 b | 52.13 ± 1.85 b | 21.85 ± 1.25 b |
| NIC 1.00 | 57 | 66 | 66.53 ± 2.97c | 67.38 ± 3.88c | 49.11 ± 4.06 c | 53.61 ± 2.44c | 28.11 ± 1.84c |
| NIC 0.50 +RJ | 63 | 55 | 87.44 ± 2.12 a | 84.29 ± 4.53 a | 73.03 ± 3.44 a | 66.49 ± 3.51 a | 12.04 ± 1.43 a d |
| NIC 1.00 + RJ | 53 | 45 | 85.46 ± 3.32 a | 83.69 ± 2.92 a | 70.32 ± 1.77 a b | 63.89 ± 3.86 a | 13.96 ± 1.37d |
CON: Control, RJ: Royal jelly, NIC 0.50: Nicotine 0.50 mg kg-1 per day, NIC 1.00: Nicotine 1.00 mg kg-1 per day.
Different superscripts in the same row show significant differences between groups (p < 0.05).
Discussion
The present study demonstrated that RJ co-treatment attenuates NIC-induced reproductive failure in mice. According to pharmaceutical and nutritive features, RJ is a highly effective antioxidant and protects cells against reactive oxygen species (ROS)-induced oxidative stress, lipid peroxidation and DNA damages.27 In vivo studies have shown that RJ leads to increases in the production of hormones such as testosterone, progesterone and luteinizing hormone.28 In addition, administration of RJ to the heat-stressed male rabbits has resulted in remarkable reproductive performance increases and physiological status improvements.29 It is well known that smoking increases the ROS production that may defeat the antioxidants defense system of the body resulting in oxidative damages to proteins, lipids and DNA.30 Moreover, previous reports have revealed that cigarette smoke exposure can lead to sperm concentration, motility and normal morphology reductions.31 Further, it has been shown that NIC as a pharmacologically active and addictive alkaloid component of the cigarette smoke can adversely affect male reproductive system and fertility.6
In the present study, confirming a previous report, NIC at both doses of 0.50 and 1.00 mg kg-1 per day caused reductions in epididymal sperm quality and density.2 Previously, in vitro studies have showed that short-term consumption of cigarettes cannot affect the sperm motility. However, higher doses of NIC, which is used chronically, can significantly reduce sperm motility. Furthermore, it has been demonstrated that serum level of NIC increases with smoking leading to spermatogenesis and sperm motility reductions.32 In fertile individuals, sperm motility levels have a direct relation to fertilization ability.33 Sperm motility is an important factor in natural fertility and low sperm motility is the cause of most of infertilities.34 Sperm motility examinations showed that in NIC-exposed animals, spermatozoa had low progressive movements, which might have negatively affect acrosomal reaction.4 Moreover, following NIC administration, significant reductions in sperm count and motility along with remarkable increases of dead and abnormal sperms were recorded. Oxidative damage of unsaturated fatty acids in cell membranes of sperm cells and disruption of membrane permeability can lead to spermatogenesis impairment and sperm damages.35 Spermatological findings in this study may also be due to the effects of NIC on the epididymis via acting as a spermatotoxic agent on maturing and/or matured spermatozoa.36
Cotinine, a NIC metabolite, similar to NIC, produces free radicals and ROS in different tissues and ROS in turn induce oxidative stress and lipid peroxidation leading to cellular damages.37 In addition, it is well-established that ROS are involved in etiology of male infertility.34 Sperm functions are extremely dependent on ROS due to high content of polyunsaturated fatty acids and limited ability of DNA repair.38 Recently, it has been revealed that ROS over-generations not only affect the fertilization process negatively but also cause spermatozoa damages through sperm dysfunctions induction such as motility, acrosomal reaction and/or DNA integrity disruptions.39 Our findings showed that NIC at both doses of 0.50 and 1.00 mg kg-1 per day caused increase in sperm MDA concentration. Previous studies have shown that abnormal sperms and seminal leukocytes are the main sources of ROS production.40
Moreover, it has been indicated that 8.00% of infertile men with normal sperm parameters show high degrees of defective sperm chromatin and DNA.41
The findings of the present study are in accordance with previous reports in which the administration of NIC led to high degrees of DNA and chromatin disorders in a dose-dependent manner.15 Accordingly, it has been shown that significant increases in ROS and lipid peroxidation products concentrations in spermatozoa of cigarette smoke-exposed mice lead to remarkable increases in DNA single- and double-strand breaks.42 Normal sperm chromatin structure and integrity are essential for successful fertilization, embryo development and also appropriate function of sperm in the fertilization process.43 It has been shown that protamine deficiency and increased histone remnants in sperms result in premature chromatin condensation as a noticeable cause of fertilization and embryo development failures.44 In addition, sperm DNA is an important target of ROS attacks which lead to the formation of adducts between nitrogen bases, DNA molecule destabilization and eventually DNA-strand breaks. A growing body of evidence confirms negative associations between frequency of sperm DNA fragmentation and fertilization rate and embryo development.45
Our finding also exhibited that NIC causes fertilization, blastulation and hatching rates reductions and increases arrested embryos percentages in a dose-dependent manner. The NIC-induced sperm motility suppression may result in reduction of spermatozoa number reaching the ampulla of oviduct and reduce the chances of fertilization. Previously, it has been demonstrated that cigarette smoking has detrimental effects on sperm fertilizing potentials.46 Further, NIC and cotinine, two important ingredients of cigarette smoke, were found to induce trophoblastic apoptosis in several cell lines through triggering caspase activation.47
In this study, RJ co-treatment in mice improved epididymal sperms quantity, quality and in vitro fertilizing capacity as well as sperm lipid peroxidation level compared to NIC-only groups. It has been reported that RJ reinforces antioxidant defense system and ameliorates histological changes in diabetic rats.48 It has also been found that RJ improves sperm kinematic characteristics and DNA fragmentation and lipid peroxidation in oxymetholone-treated mice and provides protection against Stanozolol-induced spermatotoxicity and early embryonic development arrest.14
The RJ contains vitamins such as vitamin E and vitamin C which have been reported to reduce oxidative stress in diabetic patients.49 Moreover, it has been indicated that RJ has protective activity during sperm freezing.50
In conclusion, taken together, the protection offered by RJ against NIC reprotoxicity in mice is likely thanks to its ability to inhibit oxidative stress by ROS neutralization as well as the chemical composition and physiological functions of its proteins and vitamins.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.McLachlan RI, de Kretser DM. Male infertility: The case for continued research. Med J Aust. 2001;174(3):116–117. doi: 10.5694/j.1326-5377.2001.tb143180.x. [DOI] [PubMed] [Google Scholar]
- 2.Oyeyipo IP, Raji Y, Emikpe BO, et al. Effects of nicotine on sperm characteristics and fertility profile in adult male rats: A possible role of cessation. J Reprod Infertil. 2011;12(3):201–207. [PMC free article] [PubMed] [Google Scholar]
- 3.Jana K, Samanta PK, De DK. Nicotine diminishes testicular gametogenesis, steroidogenesis, and steroidogenic acute regulatory protein expression in adult albino rats: Possible influence on pituitary gonadotropins and alteration of testicular antioxidant status. Toxicol Sci. 2010;116(2):647–659. doi: 10.1093/toxsci/kfq149. [DOI] [PubMed] [Google Scholar]
- 4.Nesseim W, Haroun H, Mostafa E, et al. Effect of nicotine on spermatogenesis in adult albino rats. Andrologia. 2011;43(6):398–404. doi: 10.1111/j.1439-0272.2010.01086.x. [DOI] [PubMed] [Google Scholar]
- 5.Kavitharaj N, Vijayammal P. Nicotine administration induced changes in the gonadal functions in male rats. Pharmacology. 1999;58(1):2–7. doi: 10.1159/000028262. [DOI] [PubMed] [Google Scholar]
- 6.Aydos K, Güven M, Can B, et al. Nicotine toxicity to the ultrastructure of the testis in rats. BJU Int. 2001;88(6):622–626. doi: 10.1046/j.1464-4096.2001.02384.x. [DOI] [PubMed] [Google Scholar]
- 7.Yildiz D, Ercal N, Armstrong DW. Nicotine enantiomers and oxidative stress. Toxicology. 1998;130(2):155–165. doi: 10.1016/s0300-483x(98)00105-x. [DOI] [PubMed] [Google Scholar]
- 8.Arabi M. Nicotinic infertility: Assessing DNA and plasma membrane integrity of human spermatozoa. Andrologia. 2004;36(5):305–310. doi: 10.1111/j.1439-0272.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- 9.Han B, Li C, Zhang L, et al. Novel royal jelly proteins identified by gel-based and gel-free proteomics. J Agric Food Chem. 2011;59(18):10346–10355. doi: 10.1021/jf202355n. [DOI] [PubMed] [Google Scholar]
- 10.Hattori N, Nomoto H, Fukumitsu H, et al. Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed Res. 2007;28(5):261–266. doi: 10.2220/biomedres.28.261. [DOI] [PubMed] [Google Scholar]
- 11.Abdelhafiz AT, Muhamad JA. Midcycle pericoital intravaginal bee honey and royal jelly for male factor infertility. Int J Gynecol Obstet. 2008;101(2):146–149. doi: 10.1016/j.ijgo.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Edwards R, Steptoe P, Purdy J. Fertilization and cleavage in vitro of preovulator human oocytes. Nature. 1970;227(5265):1307–1309. doi: 10.1038/2271307a0. [DOI] [PubMed] [Google Scholar]
- 13.Trounson A. Current perspectives of in vitro fertilization and embryo transfer. Clin Reprod Fertil. 1982;1(1):55–65. [PubMed] [Google Scholar]
- 14.Shalizar Jalali A, Najafi G, Hosseinchi M, et al. Royal jelly alleviates sperm toxicity and improves in vitro fertilization outcome in Stanozolol-treated mice. Iran J Reprod Med. 2015;13(1):15–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Khosh AH, Hasanzadeh S, Shalizar Jalali A. Ameliorative effects of Achillea millefolium inflorescences alcoholic extract on nicotine-induced reproductive toxicity in male rat: Apoptotic and biochemical evidences. Vet Res Forum. 2017;8(2):97–104. [PMC free article] [PubMed] [Google Scholar]
- 16.Jin L, Xue HY, Jin LJ, et al. Antioxidant and pancreas-protective effect of aucubin on rats with streptozotocin-induced diabetes. Eur J Pharmacol. 2008;582(1):162–167. doi: 10.1016/j.ejphar.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Wyrobek AJ, Gordon LA, Burkhart JG, et al. An evaluation of the mouse sperm morphology test and other sperm tests in nonhuman mammals: A report of the US environmental protection agency gene-tox program. Mutat Res. 1983;115(1):1–72. doi: 10.1016/0165-1110(83)90014-3. [DOI] [PubMed] [Google Scholar]
- 18.Jalali AS, Najafi G, Rahimzadeh P. Summer savory (Satureja hortensis) can reduce spermatotoxic effects of doxorubicin in rats. Caspian J Reprod Med. 2015;1(1):2–7. [Google Scholar]
- 19.Aziz N, Said T, Paasch U, et al. The relationship between human sperm apoptosis, morphology and the sperm deformity index. Hum Reprod. 2007;22(5):1413–1419. doi: 10.1093/humrep/dem016. [DOI] [PubMed] [Google Scholar]
- 20.Talebi AR, Ghasemzadeh J, Khalili MA, et al. Sperm chromatin quality and DNA integrity in partial versus total globozoospermia. Andrologia. 2018;50(1) doi: 10.1111/and.12823. doi: 10.1111/and.12823. Epub 2017 May 18. [DOI] [PubMed] [Google Scholar]
- 21.Kazerooni T, Asadi N, Jadid L. Evaluation of sperm's chromatin quality with acridin orange test, Chromomycine A3 and aniline blue staining in couple with recurrent abortion. Fertil Steril. 2009;92(3):S206. doi: 10.1007/s10815-009-9361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erenpreiss J, Bars J, Lipatnikova V, et al. Comprative study of cytochemical tests for sperm chromatin integrity. J Androl. 2001;22: 45–53. [PubMed] [Google Scholar]
- 23.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9(6):515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 24.Byers SL, Payson SJ, Taft RA. Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs) Theriogenology. 2006;65(9):1716–1726. doi: 10.1016/j.theriogenology.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 25.González R, Ruiz-León Y, Gomendio M, et al. The effect of glucocorticoids on mouse oocyte in vitro maturation and subsequent fertilization and embryo development. Toxicol In Vitro. 2010;24(1):108–115. doi: 10.1016/j.tiv.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Falcone T, Attaran M, et al. Vitamin C and vitamin E supplementation reduce oxidative stress- induced embryo toxicity and improve the blastocyst development rate. Fertil Steril. 2002;78(6):1272–1277. doi: 10.1016/s0015-0282(02)04236-x. [DOI] [PubMed] [Google Scholar]
- 27.Silici S, Ekmekcioglu O, Eraslan G, et al. Antioxidative effect of royal jelly in cisplatin-induced testes damage. Urology. 2009;74(3):545–551. doi: 10.1016/j.urology.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 28.Guo H, Ekusa A, Iwai K, et al. Royal jelly peptides inhibit lipid peroxidation in vitro and in vivo. J Nutr Sci Vitaminol (Tokyo) 2008;54(3):191–195. doi: 10.3177/jnsv.54.191. [DOI] [PubMed] [Google Scholar]
- 29.Elnagar SA. Royal jelly counteracts bucks’ “summer infertility”. Anim Reprod Sci. 2010;121(1):174–180. doi: 10.1016/j.anireprosci.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Winrow V, Winyard P, Morris C, et al. Free radicals in inflammation: Second messengers and mediators of tissue destruction. Br Med Bull. 1993;49(3):506–522. doi: 10.1093/oxfordjournals.bmb.a072627. [DOI] [PubMed] [Google Scholar]
- 31.Sankako MK, Garcia PC, Piffer RC, et al. Semen and reproductive parameters during some abstinence periods after cigarette smoke exposure in male rats. Braz Arch Biol Technol. 2013;56(1):93–100. [Google Scholar]
- 32.Jorsaraei SGA, Shibahara H. The in-vitro effects of nicotine, cotinine and leptin on sperm parameters analyzed by CASA system. Iran J Reprod Med. 2008;6(3):157–165. [Google Scholar]
- 33.Barrios F, Filipponi D, Pellegrini M, et al. Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci. 2010;123(6):871–880. doi: 10.1242/jcs.057968. [DOI] [PubMed] [Google Scholar]
- 34.Aitken RJ. Free radicals, lipid peroxidation and sperm function. Reprod Fertil Dev. 1995;7(4):659–668. doi: 10.1071/rd9950659. [DOI] [PubMed] [Google Scholar]
- 35.Sikka SC. Andrology lab corner: Role of oxidative stress and antioxidants in andrology and assisted reproductive technology. J androl. 2004;25(1):5–18. doi: 10.1002/j.1939-4640.2004.tb02751.x. [DOI] [PubMed] [Google Scholar]
- 36.Pacifici R, Altieri I, Gandini L, et al. Environmental tobacco-smoke-nicotine and cotinine concentration in semen. Environ Res. 1995;68(1):69–72. doi: 10.1006/enrs.1995.1009. [DOI] [PubMed] [Google Scholar]
- 37.Goss D, Oyeyipo IP, Skosana BT, et al. Ameliorative potentials of quercetin against cotinine-induced toxic effects on human spermatozoa. Asian Pac J Reprod. 2016;5(3):193–197. [Google Scholar]
- 38.Shen HM, Ong CN. Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med. 2000;28(4):529–536. doi: 10.1016/s0891-5849(99)00234-8. [DOI] [PubMed] [Google Scholar]
- 39.Moazamian R, Polhemus A, Connaughton H, et al. Oxidative stress and human spermatozoa: Diagnostic and functional significance of aldehydes generated as a result of lipid peroxidation. Mol Hum Reprod. 2015;21(6):502–515. doi: 10.1093/molehr/gav014. [DOI] [PubMed] [Google Scholar]
- 40.Saleh RA, Agarwal A, Sharma RK, et al. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: A prospective study. Fertil Steril. 2002;78(3):491–499. doi: 10.1016/s0015-0282(02)03294-6. [DOI] [PubMed] [Google Scholar]
- 41.Schulte RT, Ohl DA, Sigman M, et al. Sperm DNA damage in male infertility: Etiologies, assays, and outcomes. J Assist Reprod Genet. 2010;27(1):3–12. doi: 10.1007/s10815-009-9359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.La Maestra S, de Flora S, Micale RT. Effect of cigarette smoke on DNA damage, oxidative stress, and morphological alterations in mouse testis and spermatozoa. Int J Hyg Environ Health. 2015;218(1):117–122. doi: 10.1016/j.ijheh.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Sadeghi MR, Hodjat M, Lakpour N, et al. Effects of sperm chromatin integrity on fertilization rate and embryo quality following intracytoplasmic sperm injection. Avicenna J Med Biotechnol. 2009;1(3):173–180. [PMC free article] [PubMed] [Google Scholar]
- 44.Cho C, Jung-Ha H, Willis WD, et al. Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biol Reprod. 2003;69(1):211–217. doi: 10.1095/biolreprod.102.015115. [DOI] [PubMed] [Google Scholar]
- 45.Sharma R, Said T, Agarwal A. Sperm DNA damage and its clinical relevance in assessing reproductive outcome. Asian J Androl. 2004;6(2):139–148. [PubMed] [Google Scholar]
- 46.Yamamoto Y, Isoyama E, Sofikitis N, et al. Effects of smoking on testicular function and fertilizing potential in rats. Urol Res. 1998;26(1):45–48. doi: 10.1007/s002400050022. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Wilcken DE, Wang XL. Cigarette smoke activates caspase-3 to induce apoptosis of human umbilical venous endothelial cells. Mol Genet Metab. 2001;72(1):82–88. doi: 10.1006/mgme.2000.3115. [DOI] [PubMed] [Google Scholar]
- 48.Ghanbari E, Nejati V, Khazaei M. Antioxidant and protective effects of royal jelly on histopathological changes in testis of diabetic rats. Int J Reprod Biomed. 2016;14(8):519–526. [PMC free article] [PubMed] [Google Scholar]
- 49.Pourmoradian S, Mahdavi R, Mobasseri M, et al. Effects of royal jelly supplementation on glycemic control and oxidative stress factors in type 2 diabetic female: A randomized clinical trial. Chin J Integr Med. 2014;20(5):347–352. doi: 10.1007/s11655-014-1804-8. [DOI] [PubMed] [Google Scholar]
- 50.Shahzad Q, Mehmood MU, Khan H, et al. Royal jelly supplementation in semen extender enhances post-thaw quality and fertility of Nili-Ravi buffalo bull sperm. Anim Reprod Sci. 2016;167:83–88. doi: 10.1016/j.anireprosci.2016.02.010. [DOI] [PubMed] [Google Scholar]