Abstract
Introduction
People who inject drugs (PWID) experience high HIV incidence and face significant barriers to engagement in HIV care and substance use treatment. Strategies for HIV treatment as prevention and substance use treatment present unique challenges in PWID that may vary regionally. Understanding differences in the risk structure for HIV transmission and disease progression among PWID is essential in developing and effectively targeting intervention strategies of HIV treatment as prevention.
Methods
We present a baseline analysis of HIV Prevention Trials Network (HPTN) 074, a two‐arm, randomized controlled trial among PWID in Indonesia (n = 258), Ukraine (n = 457) and Vietnam (n = 439). HPTN 074 was designed to determine the feasibility, barriers and uptake of an integrated intervention combining health systems navigation and psychosocial counselling for the early engagement of antiretroviral therapy (ART) and substance use treatment for PWID living with HIV. Discordant PWID networks were enrolled, consisting of an HIV‐positive index and their HIV‐negative network injection partner(s). Among the enrolled cohort of 1154 participants (502 index participants and 652 network partners), we examine regional differences in the baseline risk structure, including sociodemographics, HIV and substance use treatment history, and injection and sexual risk behaviours.
Results
The majority of participants were male (87%), with 82% of the enrolled females coming from Ukraine. The overall mean age was 34 (IQR: 30, 38). Most commonly injected substances included illegally manufactured methadone in Ukraine (84.2%), and heroin in Indonesia (81.8%) and Vietnam (99.5%). Injection network sizes varied by region: median number of people with whom participants self‐reported injecting drugs was 3 (IQR: 2, 5) in Indonesia, 5 (IQR: 3, 10) in Ukraine and 3 (IQR: 2, 4) in Vietnam. Hazardous alcohol use, assessed using the Alcohol Use Disorders Identification Test – Alcohol Consumption Questions (AUDIT‐C), was prominent in Ukraine (54.7%) and Vietnam (26.4%). Reported sexual risk behaviours in the past month, including having two or more sex partners and giving/receiving money or drugs in exchange for sex, were uncommon among all participants and regions.
Conclusions
While regional differences in risk structure exist, PWID particularly in Ukraine need immediate attention for risk reduction strategies. Substantial regional differences in risk structure will require flexible, tailored treatment as prevention interventions for distinct PWID populations.
Keywords: injection drug use, PWID, HIV, substance use treatment, ART, treatment as prevention
1. Introduction
HIV epidemics in eastern Europe, central Asia and many parts of South East Asia are concentrated among people who inject drugs (PWID) 1. Serial use and sharing of drug preparation and injection equipment create heightened risks for acquiring and transmitting HIV 2, 3. The persistently high incidence of HIV infection among PWID in many locations with concentrated epidemics necessitates aggressive efforts to prevent HIV transmission 1.
Given the ethical complexity of mitigating stigma and legal risks and providing effective harm reduction services, PWID have been largely excluded from HIV prevention trials 4, 5, 6. Although HIV Prevention Trials Network (HPTN) 052 demonstrated the benefit of early antiretroviral therapy (ART) among people living with HIV to prevent sexual HIV transmission 7, the trial excluded active PWID. Consequently, the concept of treatment as prevention, in which individuals are treated with ART to prevent transmission of HIV to others, has not been validated in PWID living with HIV. Parenteral exposure to HIV during injection drug use typically has a higher infectious dose, leading to a higher transmission probability 8. Thus, the effectiveness of ART to reduce HIV transmission may be lower among PWID than the 96% reduction in HIV found among HIV discordant sexual partnerships in HPTN 052 7.
Testing HIV treatment as prevention strategies among PWID presents unique challenges. HIV transmission in PWID occurs in the context of risk networks, typically with the involvement of multiple injection partners, varied injection practices and sexual risk behaviours 9, 10. Injection behaviours among PWID after initiating ART may change due to alterations in risk behaviours or increased attention to health concerns leading to a reduction in injection related risks or an increased use of sterile injection equipment 11, 12, 13. Continued substance use may also lead to poor ART adherence and retention, treatment failure and transmission of resistant strains 14, 15, 16. To maximize the potential for treatment success, novel approaches for offering ART in conjunction with substance use treatment modalities to PWID must be explored, particularly across multiple regions 1, 17, 18, 19.
PWID in need of HIV care across varying regions likely experience different risk structures for HIV transmission and disease progression. In Indonesia, Ukraine and Vietnam, the HIV epidemic is primarily concentrated among PWID 20, 21, 22, 23. Yet, differences in sociodemographics and injection substances and practices that increase risk may highlight the need for region‐specific strategies to prevent HIV transmission 3, 24. An enhanced understanding of risk structure among PWID within these countries will directly inform treatment as prevention interventions that are flexible enough to address potential regional differences. HPTN 074 was designed to determine the feasibility, barriers and uptake of a multisite, integrated intervention combining supported referrals and brief psychosocial counselling for the early engagement of ART and substance use treatment for PWID living with HIV. HPTN 074 enrolled HIV discordant PWID networks of HIV‐positive PWID with unsuppressed viraemia and their HIV‐negative PWID injection partners in three geographically and culturally distinct regions: Indonesia, Ukraine and Vietnam.
Here, we examine regional differences in the baseline risk structure, including sociodemographics, HIV and substance use treatment history, and injection and sexual risk behaviours, among the HPTN 074 study cohort.
2. Methods
2.1. Study settings
This study was conducted among PWID in three distinct locations with documented HIV epidemics among PWID: Jakarta, Indonesia; Kyiv, Ukraine; and Thai Nguyen, Vietnam. These study sites were chosen based on HIV prevalence and incidence among PWID. In Jakarta, the estimated prevalence among PWID is 54%. In Kyiv, HIV prevalence among the estimated 31,300 PWID was 20% in 2013; incidence observed in a prospective study was 4.5 (95% CI: 2.3, 7.9) per 100 person‐years (PY) 20, 21, 22. Among PWID in Thai Nguyen 2005 to 2007 trial, the calculated HIV prevalence was 35% and HIV seroconversion incidence rate was 5.2 (95% CI: 3.5, 7.6) per 100 PY 23.
2.2. Study population
The study population included PWID networks with two participants’ types: index participants living with HIV with unsuppressed viraemia (≥1000 copies/mL) and their HIV‐negative network injection partners 25. The inclusion criteria for both types of participants included: male or female gender, age between 18 and 60 years (the upper age limit was increased from 45 years to 60 years in September 2015 26); active injection drug use (defined initially as self‐report of (i) injecting drugs approximately two or more times per week for the past three months and (ii) ability to identify the anatomical location of the most recent injection site that was confirmed by site research staff; updated in September 2015 to (i) injecting 12 times or more in the past three months and at least six times in the past month and (ii) a PWID in the opinion of site research staff); having no plans to move outside the study area for at least one year after study enrolment; and ability to provide written informed consent. Inclusion criteria specific for index participants also included: HIV infection based on local standard of care testing; viral load ≥1000 copies/mL and CD4 cell count >50 cells/μL at screening; willingness and ability to identify, recruit and enrol at least one HIV‐negative network injection partner who was eligible for study participation; and willingness to participate in intervention activities including regular phone contact 25.
2.3. Parent study design
This multisite, two‐arm, randomized study was designed to: (1) determine the feasibility of a future randomized controlled trial by estimating HIV incidence among network partners and assessing the potential for enrolment and retention of PWID living with HIV and their HIV‐network partners; and (2) assess the feasibility, barriers and uptake of the integrated interventions 25.
Index participants in the intervention arm received a standard harm reduction package and an integrated intervention that included: (1) systems navigators to facilitate engagement, retention and adherence in substance use treatment and HIV care; (2) psychosocial counselling to facilitate substance use treatment and HIV care and medication adherence; and (3) referral for ART at any CD4 count. Index participants in the standard of care arm received the World Health Organization (WHO) package of care for PWID, including HIV testing and counselling and referrals for ART, diagnosis and treatment of sexually transmitted infections, hepatitis B and C virus, and tuberculosis, as appropriate. All network partners received a standardized harm reduction package with referral for syringe service programmes and substance use treatment, consistent with national guidelines.
2.4. Recruitment procedures for index participants
Index participants were recruited using a variety of methods, including referral from HIV‐testing sites, community outreach and injection‐network referrals. Trained outreach workers who were knowledgeable about community dynamics, including geographic areas, settings and organizations frequented by PWID, were selected from the community and harm reduction programmes. Outreach workers were trained on basic methods of rapid assessment procedures to target areas of high drug use. These workers disseminated information about the study, provided oral and written descriptions of the study to prospective participants and encouraged index participants to participate in screening activities at the local study site. Potential index participants were asked to share the information to other PWID in their networks.
2.5. Identification of network partners
After confirmation of positive HIV status via local standard of care testing, index participants were asked to identify members of their injection network with whom they engage in HIV‐related injection exposures, such as sharing injection equipment. Index participants were provided with referral identification cards, which did not contain identifying information, and encouraged to accompany network partners to the study site. Index participants were asked to provide descriptions, such as name, age and gender, of members of their injection network with whom they may provide referral identification cards. Up to five HIV‐negative network partners per index participant were allowed to enrol. Network partners had to match the description provided by the index to participate in the screening process and were then asked to provide study informed consent for enrolment and to be tested for HIV infection. Index participants received compensation for successful enrolment of network partners. Network partners were compensated for their time and participation. The amount and form of compensation was approved by the local institutional review boards (IRB) and varied by site.
2.6. Cohort enrolment
Enrolment began in April 2015 in Vietnam, June 2015 in Ukraine and July 2015 in Indonesia (Figure 1). The study enrolled 504 network units (504 index participants, 656 baseline network partners). Of the 504 enrolled index participants, one had a viral load <1000 copies/mL at screening and was excluded from analyses. Of the 656 enrolled network partners, four participants were excluded; the network partner of the excluded index and three other network partners who were found to be HIV positive at a screening based on testing performed retrospectively at the HPTN LC. One of the excluded network partners was the sole partner of an index and that index participant was removed from the analyses. The final baseline analysis sample included 1154 participants (502 network units: 502 index participants and 652 network partners).
Figure 1. Cumulative enrolment by site in HPTN 074 (N = 1154).
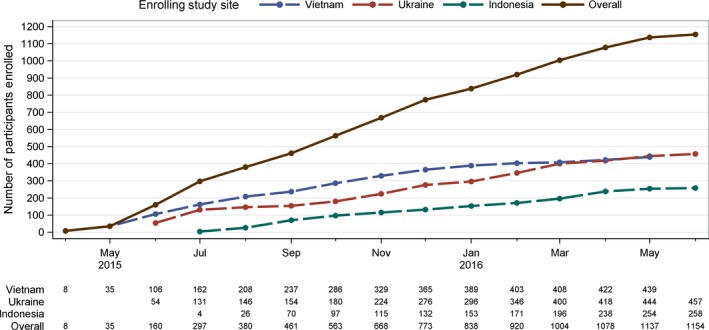
2.7. Data collection
At screening, index participants provided blood samples for HIV viral load testing and CD4 cell counts. After enrolment consent was obtained, index participants and network partners completed face‐to‐face interviews with study staff who had extensive experience with non‐judgemental interviewing techniques.
2.8. Measures
All participants provided information on sociodemographics characteristics: sex, age, marital status, highest level of education completed and employment. Participants self‐reported HIV testing and treatment history, including ART use (currently on ART, previously on ART or ART naïve) and date of their HIV diagnosis. Years since HIV diagnosis was calculated based on the difference of dates between HIV diagnosis and survey completion. Baseline substance use measures were collected for the prior three months and included: alcohol use, non‐injection and injection drug use, and number of people used drug with. The measure used to define whether participants displayed hazardous alcohol use was the Alcohol Use Disorders Identification Test – Alcohol Consumption Questions (AUDIT‐C) score 27. Males with AUDIT‐C scores ≥4 and females with scores ≥3 were classified as displaying hazardous alcohol use behaviours 28. Injection practices were collected for last injection, which included shared rinse water, shared cooker/container, shared filter cotton, used a new needle, cleaned needle before injection, used pre‐filled syringe and injected drugs that were frontloaded or backloaded into the syringe. Participants reported whether they had ever participated methadone maintenance or any other medication‐assisted treatment (MAT) programme. Sexual behaviour measures, including number of reported sexual partners and number of times giving and receiving sex for money, were collected for the prior month. Additional testing was performed at the HPTN Laboratory Center (LC), Johns Hopkins University, Baltimore, MD, using FDA‐cleared assays; baseline testing included confirmation of HIV status for index and network partners and viral load testing.
2.9. Statistical analysis
Frequency distributions and descriptive statistics were used to summarize enrolment, sociodemographic characteristics, HIV testing and treatment history, self‐reported substance use and treatment history, and injection and sexual behaviours of the enrolled study cohort at baseline. Pearson's chi‐square test was used to evaluate differences in baseline categorical variables by region, with Fisher's exact test used when any expected cell counts were less than 5. Continuous measures were compared using one‐way ANOVA. Analyses were conducted in Linux SAS version 9.4(SAS/STAT 14.2, Cary, NC).
2.10. Ethics approval
The study protocol, which is available at clinicaltrials.gov (NCT02935296), was approved by at least one local IRB affiliated with each site: University of Indonesia, Ukrainian Institute on Public Health Policy, Thai Nguyen Center for Preventive Medicine, University of North Carolina‐Chapel Hill and Faculty of Medicine. All study participants provided written informed consent in their local languages, or English, if preferred.
3. Results
3.1. Sociodemographic characteristics of the enrolled cohort
The baseline cohort, including index and network partners, was predominantly male (87.3%), with most females enrolled at the Ukraine site (Table 1). The median age at enrolment was 34 years (IQR: 30, 38). Unlike Indonesia and Vietnam, most Ukrainian participants were married or living with a sexual partner (57.7%). Overall, 54.7% of participants in Indonesia and 41.8% in Ukraine completed college or technical college, compared to only 4.8% in Vietnam. Most Ukrainian participants were unemployed (65.0%), while most participants in Indonesia (73.6%) and Vietnam (77.5%) were employed either full‐ or part‐time.
Table 1.
Baseline sociodemographic characteristics by site in HPTN 074
| Indonesian = 258 | Ukrainen = 457 | Vietnam n = 439 | Total n = 1154 | p‐valuea | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Sex | |||||||||
| Male | 238 | (92.2) | 337 | (73.7) | 432 | (98.4) | 1007 | (87.3) | <0.01 |
| Female | 20 | (7.8) | 120 | (26.3) | 7 | (1.6) | 147 | (12.7) | |
| Age (years) | |||||||||
| 18 to 19 | 5 | (1.9) | 0 | (0.0) | 1 | (0.2) | 6 | (0.5) | <0.01 |
| 20 to 29 | 88 | (34.1) | 83 | (18.2) | 98 | (22.3) | 269 | (23.3) | |
| 30 to 39 | 137 | (53.1) | 287 | (62.8) | 225 | (51.3) | 649 | (56.2) | |
| 40 to 45 | 23 | (8.9) | 80 | (17.5) | 101 | (23.0) | 204 | (17.7) | |
| >45 | 5 | (1.9) | 7 | (1.5) | 14 | (3.2) | 26 | (2.3) | |
| Marital status | |||||||||
| Married | 96 | (37.2) | 102 | (22.3) | 203 | (46.2) | 401 | (34.7) | <0.01 |
| Living with sexual partner but not married | 7 | (2.7) | 162 | (35.4) | 2 | (0.5) | 171 | (14.8) | |
| Separated, divorced, widowed | 61 | (23.6) | 64 | (14.0) | 96 | (21.9) | 221 | (19.1) | |
| Single | 94 | (36.4) | 129 | (28.2) | 138 | (31.4) | 361 | (31.3) | |
| Education | |||||||||
| No schooling or some primary school | 14 | (5.4) | 0 | (0.0) | 36 | (8.2) | 50 | (4.3) | <0.01 |
| Completed primary school or some secondary school | 32 | (12.4) | 35 | (7.7) | 279 | (63.6) | 346 | (30.0) | |
| Completed secondary school or some technical training/college/university | 71 | (27.5) | 231 | (50.5) | 103 | (23.5) | 405 | (35.1) | |
| Completed technical training or college/university | 141 | (54.7) | 191 | (41.8) | 21 | (4.8) | 353 | (30.6) | |
| Employmentb | |||||||||
| Employed full‐time | 79 | (30.6) | 66 | (14.4) | 222 | (50.6) | 367 | (31.8) | <0.01 |
| Employed part‐time | 111 | (43.0) | 89 | (19.5) | 118 | (26.9) | 318 | (27.6) | |
| Unemployed but seeking work | 57 | (22.1) | 214 | (46.8) | 64 | (14.6) | 335 | (29.0) | |
| Unemployed – not seeking work | 11 | (4.3) | 83 | (18.2) | 34 | (7.7) | 128 | (11.1) | |
| Retired | 0 | (0.0) | 4 | (0.9) | 1 | (0.2) | 5 | (0.4) | |
Pearson's chi‐square test was used to test association between each row variable and site; Fisher's exact test was used when any of the expected cell counts were less than 5 or when 0 counts were present.
Refused to answer, n = 1 (Ukraine).
3.2. HIV treatment history of index participants
Among HIV‐positive index participants, the median time since their self‐reported HIV diagnosis was longer in Ukraine (4.16 years, IQR: 1.87, 9.37; Table 2), compared to Indonesia (1.50 years, IQR: 0.02, 6.91) and Vietnam (0.09 years, IQR: 0.06, 0.45). Across all three sites, most index participants (~70% of in Indonesia and >80% in Ukraine and Vietnam) reported that they were ART naïve at enrolment. The median viral load of all index participants at screening was 4.56 log10 copies/mL (IQR: 3.99, 4.99), with a narrow range of medians among sites (4.53, 4.61). The median baseline CD4 count for index participants across all sites at screening was 293 cells/μL overall (IQR: 166, 463). The median baseline CD4 count was lowest in Indonesia (271 cells/μL; IQR: 147, 418) and highest in Vietnam (314 cells/μL; IQR: 187, 492).
Table 2.
Baseline antiretroviral drug use, CD4 cell count and HIV viral load among index participants in HPTN 074 (n = 502)
| Indonesian = 121 | Ukrainen = 187 | Vietnam n = 194 | Total n = 502 | p‐valuea | |||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||||||
| ART useb | |||||||||
| Currently on ART | 23 (19.0) | 3 (1.6) | 28 (14.4) | 54 (10.8) | <0.01 | ||||
| Previously on ART | 14 (11.6) | 28 (15.0) | 4 (2.1) | 46 (9.2) | |||||
| ART naïve | 84 (69.4) | 156 (83.4) | 162 (83.5) | 402 (80.1) | |||||
| Median | (IQR) | Median | (IQR) | Median | (IQR) | Median | (IQR) | ||
| Years since HIV diagnosis | 1.50 | (0.02,6.91) | 4.16 | (1.87,9.37) | 0.09 | (0.06,0.45) | 1.41 | (0.07,6.41) | <0.01 |
| HIV‐1 viral load (log10 copies/mL) | 4.53 | (4.18, 4.94) | 4.54 | (3.83, 5.07) | 4.61 | (4.05, 4.96) | 4.56 | (3.99, 4.99) | 0.99 |
| CD4 cell count (cells/μL) | 271 | (147, 418) | 310 | (178, 465) | 314 | (187, 492) | 293 | (166, 463) | 0.09 |
IQR: interquartile range; ART: antiretroviral therapy.
Pearson's chi‐square test was performed for ART use and one‐way ANOVA was used for continuous measures.
Based on self‐report.
3.3. Risk structure of enrolled cohort
Among all index participants and network partners at all three sites, recent substance use was common (Table 3). In addition to injection drug use, hazardous alcohol use was uncommon in Indonesia (9.3%), but prominent in Ukraine (54.7%) and Vietnam (26.4%). Marijuana use in the past three months was common among Indonesian (41.5%) and Ukrainian (64.8%) participants, but substantially less common in Vietnam (0.9%). Three‐quarters (75.6%) of Indonesian participants reported recent (prior three months) non‐injectable stimulant use, as compared to only 28.4% in Ukraine and 18.0% in Vietnam. The most commonly injected drugs were heroin (81.8%) and buprenorphine (37.6%) in Indonesia; illegally manufactured methadone (84.2%), home‐made opioids (75.7%) and amphetamines (35.7%) in Ukraine; and heroin (99.5%) in Vietnam.
Table 3.
Baseline substance use behaviours by site in HPTN 074 (n = 1154)
| Indonesian = 258 | Ukrainen = 457 | Vietnamn = 439 | Totaln = 1154 | p‐valuea | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Hazardous alcohol useb | 24 (9.3) | 250 (54.7) | 116 (26.4) | 390 (33.8) | <0.01 |
| Non‐injection drug use, past three monthsc | |||||
| Marijuana | 107 (41.5) | 296 (64.8) | 4 (0.9) | 407 (35.3) | <0.01 |
| Stimulants (cocaine, methamphetamines) | 195 (75.6) | 130 (28.4) | 79 (18.0) | 404 (35.0) | |
| Opiates | 30 (11.6) | 21 (4.6) | 55 (12.5) | 106 (9.2) | |
| Benzodiazepine | 136 (52.7) | 2 (0.4) | 0 (0.0) | 138 (12.0) | |
| Methadone (illegally manufactured) | 0 (0.0) | 4 (0.9) | 0 (0.0) | 4 (0.3) | |
| Injection drug use, past three monthsc | |||||
| Amphetamines | 2 (0.8) | 163 (35.7) | 1 (0.2) | 166 (14.4) | <0.01 |
| Heroin | 211 (81.8) | 40 (8.8) | 437 (99.5) | 688 (59.6) | |
| Opium | 3 (1.2) | 58 (12.7) | 0 (0.0) | 61 (5.3) | |
| Buprenorphine | 97 (37.6) | 65 (14.2) | 0 (0.0) | 162 (14.0) | |
| Methadone (illegally manufactured) | 4 (1.6) | 385 (84.2) | 1 (0.2) | 390 (33.8) | |
| Home‐made opioids | 0 (0.0) | 346 (75.7) | 0 (0.0) | 346 (30.0) | |
| Shared rinse water, last injection | |||||
| Yes | 149 (57.8) | 88 (19.3) | 15 (3.4) | 252 (21.8) | <0.01 |
| No | 109 (42.2) | 369 (80.7) | 424 (96.6) | 902 (78.2) | |
| Shared cooker/container, last injectiond | |||||
| Yes | 149 (57.8) | 301 (65.9) | 116 (26.4) | 566 (49.0) | <0.01 |
| No | 109 (42.2) | 155 (33.9) | 323 (73.6) | 587 (50.9) | |
| Shared filter cotton, last injectiond | |||||
| Yes | 3 (1.2) | 254 (55.6) | 1 (0.2) | 258 (22.4) | <0.01 |
| No | 255 (98.8) | 202 (44.2) | 437 (99.5) | 894 (77.5) | |
| Used a new needle, last injection | |||||
| Yes | 208 (80.6) | 398 (87.1) | 393 (89.5) | 999 (86.6) | <0.01 |
| No | 50 (19.4) | 59 (12.9) | 46 (10.5) | 155 (13.4) | |
| Cleaned needle before injection, last injectiond | |||||
| Yes | 106 (41.1) | 52 (11.4) | 71 (16.2) | 229 (19.8) | <0.01 |
| No | 152 (58.9) | 403 (88.2) | 367 (83.6) | 992 (86.0) | |
| Used a pre‐filled syringe, last injection | |||||
| Yes | 140 (54.3) | 90 (19.7) | 26 (5.9) | 256 (22.2) | <0.01 |
| No | 118 (45.7) | 367 (80.3) | 413 (94.1) | 898 (77.8) | |
| Injected drugs that were frontloaded or backloaded into the syringe or needle, last injectiond | |||||
| Yes | 254 (98.4) | 372 (81.4) | 102 (23.2) | 728 (63.1) | <0.01 |
| No | 4 (1.6) | 84 (18.4) | 337 (76.8) | 425 (36.8) | |
| Number of people used drugs with, past three months | |||||
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <0.01 |
| 1 | 22 (8.5) | 17 (3.7) | 69 (15.7) | 108 (9.4) | |
| 2 to 4 | 170 (65.9) | 166 (36.3) | 321 (73.1) | 657 (56.9) | |
| ≥5 | 66 (25.6) | 274 (60.0) | 49 (11.2) | 389 (33.7) | |
| Ever participated in methadone maintenance or any other medication‐assisted treatment programme | |||||
| Yes | 138 (53.5) | 214 (46.8) | 99 (22.6) | 451 (39.1) | <0.01 |
| No | 120 (46.5) | 243 (53.2) | 340 (77.4) | 703 (60.9) | |
Pearson's chi‐square test was used to test association between each row variable and site; Fisher's exact test was used when any of the expected cell counts were less than 5 or when 0 counts were present.
Hazardous alcohol use was determined as an Alcohol Use Disorders Identification Test – Alcohol Consumption Questions (AUDIT‐C) score of ≥4 among males and ≥3 among females.
Participants may report more than one substance type.
Missing data due to not knowing or refused to answer: shared cooker/container, n = 1; shared filter cotton, n = 2; cleaned needle before injection, n = 3; injected drugs that were frontloaded or backloaded into the syringe or needle, n = 1.
Injection risk behaviours were common among index participants and network partners across all three sites. In Indonesia, 57.8% reported sharing rinse water and sharing a cooker/container at last injection. Sharing of filter cotton at last injection was more common in Ukraine (55.6%) than in Indonesia (1.2%) and Vietnam (0.2%). Most participants at all three sites (80.6% in Indonesia, 87.1% in Ukraine and 89.5% in Vietnam) reported using a new needle at last injection.
Many of the participants in Ukraine (60.0%) reported using injection drugs with five or more different people in the prior three months. Participants in Indonesia and Vietnam reported using drugs with a median of three people (IQR: 2, 5 in Indonesia; IQR: 2, 4 in Vietnam). Participants in Ukraine reported using drugs with a median number of five people (IQR: 3, 10).
Almost half (46.5%) of participants in Indonesia, over half in Ukraine (53.2%) and over three‐quarters in Vietnam (77.4%) reported never having participated in methadone maintenance or any other MAT programme prior to enrolment.
Reported sexual risk behaviours were uncommon among all participants (Table 4). Nearly all male participants (91.5%) reported one or no female sexual partners in the past month. Similarly, 94.6% of female participants reported one or no male sexual partners in the past month. Overall, 3.1% of male participants and zero female participants reported giving money or drugs in exchange for sex, and 0.8% of male participants and 2.0% of female participants reported receiving money or drugs in exchange for sex in the past month.
Table 4.
Baseline sexual risk behaviours in past month by site in HPTN 074 (N = 1154)
| Indonesia | Ukraine | Vietnam | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Malea n = 238 | Female n = 20 | Male n = 337 | Female n = 120 | Male n = 432 | Female n = 7 | Malea n = 1007 | Female n = 147 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Number of different female sex partners | ||||||||
| 0 | 95 (39.9) | 20 (100.0) | 60 (17.8) | 117 (97.5) | 215 (49.8) | 7 (100.0) | 370 (36.7) | 144 (98.0) |
| 1 | 130 (54.6) | 0 (0.0) | 234 (69.4) | 3 (2.5) | 187 (43.3) | 0 (0.0) | 551 (54.8) | 3 (2.0) |
| ≥2 | 12 (5.0) | 0 (0.0) | 43 (12.8) | 0 (0.0) | 30 (6.9) | 0 (0.0) | 85 (8.4) | 0 (0.0) |
| Number of different male sex partners | ||||||||
| 0 | 237 (99.6) | 3 (15.0) | 337 (100.0) | 35 (29.2) | 432 (100.0) | 2 (28.6) | 1006 (99.9) | 40 (27.2) |
| 1 | 0 (0.0) | 13 (65.0) | 0 (0.0) | 81 (67.5) | 0 (0.0) | 5 (71.4) | 0 (0.0) | 99 (67.4) |
| ≥2 | 0 (0.0) | 4 (20.0) | 0 (0.0) | 4 (3.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (5.4) |
| Number of times giving sex partners money or drugs in exchange for sex | ||||||||
| 0 | 224 (94.1) | 20 (100.0) | 330 (97.9) | 120 (100.0) | 422 (97.7) | 7 (100.0) | 976 (96.9) | 147 (100.0) |
| 1 | 2 (0.8) | 0 (0.0) | 2 (0.6) | 0 (0.0) | 4 (0.9) | 0 (0.0) | 8 (0.8) | 0 (0.0) |
| ≥2 | 12 (5.0) | 0 (0.0) | 5 (1.5) | 0 (0.0) | 6 (1.4) | 0 (0.0) | 23 (2.3) | 0 (0.0) |
| Number of times receiving money or drugs in exchange for sex | ||||||||
| 0 | 229 (96.2) | 19 (95.0) | 337 (100.0) | 118 (98.4) | 432 (100.0) | 7 (100.0) | 998 (99.1) | 144 (98.0) |
| 1 | 2 (0.8) | 0 (0.0) | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 2 (0.2) | 1 (0.7) |
| ≥2 | 6 (2.5) | 1 (5.0) | 0 (0.0) | 1 (0.8) | 0 (0.0) | 0 (0.0) | 6 (0.6) | 2 (1.3) |
Missing data due to not knowing or refused to answer, n = 1.
4. Discussion
To our knowledge, this study represents one of the largest multisite cohorts of PWID living with HIV and their HIV‐negative injection partners in an HIV prevention trial. Several significant regional differences in PWID risk structure exist across Indonesia, Ukraine and Vietnam. Notably, PWID in Ukraine may be uniquely vulnerable to a continued HIV epidemic given the proportion of female PWID, time since HIV diagnosis and need for HIV care for viral suppression, substance use variation and density of drug use networks. These findings have important implications for HIV and substance use treatment strategies among PWID worldwide.
Although the sociodemographic characteristics of participants were largely similar in each country, PWID in Vietnam reported lower education and Ukraine had a significantly larger proportion of female PWID. For PWID in Vietnam, low levels of education may serve as a barrier for HIV and substance use treatment 29. We believe the limited number of females in Indonesia and Vietnam accurately reflects the population of PWID in these countries based on culture and historic precedence 30, 31, 32. The gender distribution in Ukraine is similar to the distribution in Russia and the Baltic States 27, 33. Female PWID often face more stigma and discrimination than their male PWID, which can be an additional barrier for engaging in HIV and substance use treatment 34. Treatment as prevention interventions, as well as substance use treatment, should address vary education levels and integrate female tailored approaches, where appropriate.
Variations in HIV status awareness highlight unique regional needs for routine HIV testing and counselling. Index participants in Ukraine reported that they knew their HIV status for a median of over four years, compared to one and a half years in Indonesia and less than one year in Vietnam. This difference was assumably due to barriers in receiving routine HIV testing for PWID, especially in Vietnam 35, 36, 37. In Ukraine, the primary recruitment location was staffed by an organization that fostered long‐term relationships with PWID and provided routine HIV testing and counselling and needle exchange. Additional efforts should be made to leverage relationships and enhance engagement and adherence to HIV treatment across all regions.
The significant variability in both injectable and non‐injectable substance likely indicates availability and affordability of drugs at the time of enrolment. Heroin in Indonesia and Vietnam is typically cheap and available, given the proximity to the Golden Triangle where the borders of Thailand, Laos and Myanmar meet and opium trafficking is prominent 38. In contrast, in Ukraine, heroin is expensive and less available than other substances, such as home‐made opiates 39, 40. Over half of Ukrainian participants reported hazardous alcohol use, while two‐thirds reported using marijuana. Additionally, methadone, home‐made opioids and amphetamines were the predominant injected substances. The extensive use of varying types of substances, may delay engagement and hinder benefits of HIV and substance use treatment among PWID 41, 42, 43.
The density of injection drug networks in Ukraine depicts potential HIV transmission dynamics in need of appropriate prevention interventions. The size of the injection drug networks appears to be larger in Ukraine than in Indonesia or Vietnam. Injection network size likely reflects the social norms related to injection behaviour in each area 44. Large, dense networks are associated with injection practices that increase the risk for HIV transmission 45, 46, 47. The larger networks in Ukraine may arise because of the uncertainty of the drug sources and the culture of home‐made drug preparation 39, 40, 48. Further examination of contact patterns within these networks will identify target for HIV treatment as prevention interventions 49.
Within our study population, injection risk behaviours were more commonly reported than sexual risk behaviours across all three sites. However, this does not necessarily suggest injection as the primary mode of potential HIV transmission. Given recruitment and enrolment targeted PWID, participants may have felt comfortable reporting injection behaviours. Additionally, sexual risk behaviours are likely under‐reported due to the stigmatization, particularly among sexual relationships of the same sex or outside of marriage 50, 51, 52.
The high prevalence of injection risk behaviours across all sites emphasizes the importance of integrating effective harm reduction with innovative strategies to prevent ongoing transmission. Sharing injection equipment significantly increases the risk of HIV acquisition among PWID 53, 54, 55. Harm reduction approaches, such as needle and syringe exchange programmes and MAT, can significantly reduce the number of new HIV infections, even when coverage is high 56, 57, 58. Access and use of MAT appears to be less in Vietnam where almost four out of five PWID had never participated in a substance use programme, compared to approximately half in Indonesia and Ukraine. In all three countries, the number of available MAT clinics is increasing due to changes in health policy 59, 60, 61. Consequently, uptake of MAT services among the enrolled cohort may have increased throughout study follow‐up.
We characterized a cohort of HIV‐infected PWID and their HIV‐uninfected network partners in three distinct settings, providing insights on the risk structure of the study population. Enrolled PWID may not be generalizable to PWID who were not reached or consented within each region. Recruitment efforts may have varied, particularly in terms of access to PWID populations, despite the structured and coordinated recruitment strategies across each site. This may have resulted in differences in the risk structure across the sites. However, there were clear sociodemographic differences that likely contributed to the varying risk structures. Furthermore, all injection and sexual behaviours measures were self‐reported and collected by an interviewer and thus estimates may be over‐ or under‐reported and susceptible to social desirability bias. Self‐administered questionnaires or audio computer‐assisted self‐interview were not feasible and likely inappropriate given the limited education and poor literacy rates of the study population 62, 63. Questionnaires were administered face‐to‐face by trained interviewers with extensive experience with non‐judgemental interviewing techniques, thus reducing the potential for social desirability bias.
5. Conclusions
HIV treatment of PWID, whether for the prevention of HIV transmission or to increase the health and wellbeing of PWID living with HIV, presents unique, region‐specific challenges. While notable regional differences in the risk structure exist among our cohort, PWID within Ukraine have a unique vulnerability for a continued HIV epidemic that will require urgent efforts to address HIV transmission and acquisition. The regional differences in the risk structure among this cohort highlight the need for treatment as prevention interventions that are sufficiently flexible to address the needs of distinct PWID populations they are serving.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KEL, IFH, WCM and the HPTN Study Team had overall responsibility for implementing the study, and conceived and designed the study, analysed the data and led the manuscript writing. KEL, IFH, BH, TVH, KD, HS, SR, VFG, SRM, MGH, EMP‐M, PR, SD, ZD, TK, OZ, SD, CL, DM, DNB, JS, SAS, SHE, W, DD, LE, LES, LM, NS, ELH, JPL, BDD, NVV, RS, WCM and the HPTN Study Team contributed to developing the study concept and design. BH, TVH, KD, HS, SR, VFG, EMP‐M, PR, SD, ZD, TK, OZ, SD, CVA, SHE, WC, DD, LE, LES, LM, ELH and RS contributed to data collection. KEL, IFH, BH, SAR, KRM, MGH and WCM assisted with data analysis and results interpretation. KEL, IFH, WCM and the HPTN Study Team contributed to drafting the manuscript. All authors reviewed the manuscript critically for intellectual content. All authors read and approved the final draft of the submitted manuscript.
Acknowledgements
We thank the members of the HPTN 074 study site teams for their hard work and dedication to this project, including: the HPTN 074 Indonesia Team; the 074 Ukraine Team (Olena Makarenko, MD; Irina Zaviryukha, MD; Mariya Liulchuk, MD; Svitlana Antonyak, MD; Volodymyr Yaryy, MD; Sergey Parkhomenko; Natalia Saichuk; Velta Parchomenko; Natalya Kasyanenko; Vita Omelyanchik; Igor Kichuk; Roman Timchenko; Maksim Shelengivsky); the HPTN 074 Vietnam Team (Ngo Kim Hoa, MD; Tran Thi Mo, Nguyen Vu Tuyet Mai; Teerada Sripaipan; Nguyen Duc Vuong, MD; Le Thi Thuc Anh); and all the outreach community workers from each of the three sites. Also, we are grateful for the support from the Vietnam Administration of HIV/AIDS Control, Thai Nguyen Department of Health, Pho Yen Health District Center, NGO “Club Eney” and Gromashevsky Institute of Epidemiology and Infectious Diseases. We also thank the HPTN Laboratory Center staff (Barbara Debevec, Stephanie Veater, Tamara Walsky and Michelle Xing) for their assistance with the laboratory testing and the SCHARP staff (Sue Tracy‐Waisanen and Amber Guo). Lastly, we thank the participants for their invaluable contributions to this research.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (NIH); award numbers UM1AI068619 [HPTN Leadership and Operations Center], UM1AI068617 [HPTN Statistical and Data Management Center], UM1AI068613 [HPTN Laboratory Center] and the University of North Carolina at Chapel Hill Center for AIDS Research (P30 AI50410). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Lancaster, K. E. , Hoffman, I. F. , Hanscom B., Ha, T. V. , Dumchev, K. , Susami, H. , Rose, S. , Go, V. F. , Reifeis, S. A. , Mollan K. R., Hudgens, M. G. , Piwowar‐Manning, E. M. , Richardson, P. , Dvoriak, S. , Djoerban, Z. , Kiriazova, T. , Zeziulin, O. , Djauzi, S. , Ahn, C. V. , Latkin, C. , Metzger, D. , Burns, D. N. , Sugarman, J. , Strathdee, S. A. , Eshleman, S. H. , Clarke, W. , Donnell, D. , Emel, L. , Sunner, L. E. , McKinstry, L. , Sista, N. , Hamilton, E. L. , Lucas, J. P. , Duong, B. D. , Van Vuong, N. , Sarasvita, R. and Miller, W. C. Regional differences between people who inject drugs in an HIV prevention trial integrating treatment and prevention (HPTN 074): a baseline analysis. J Int AIDS Soc. 2018;21(10):e25195
Partial results were reported at the 9th IAS Conference on HIV Science (IAS 2017); July 23‐26, 2017; Paris, France.
Contributor Information
Kathryn E Lancaster, Email: lancaster.111@osu.edu.
Irving F Hoffman, Email: irving_hoffman@med.unc.edu.
Brett Hanscom, Email: bhanscom@fredhutch.org.
Tran Viet Ha, Email: vietha@live.unc.edu.
Kostyantyn Dumchev, Email: k.dumchev@gmail.com.
Hepa Susami, Email: eva.susami@gmail.com.
Scott Rose, Email: SRose@fhi360.org.
Vivian F Go, Email: vgo@unc.edu.
Sarah A Reifeis, sreifeis@email.unc.edu.
Katie R Mollan, kmollan@email.unc.edu.
Michael G Hudgens, mhudgens@email.unc.edu.
Estelle M Piwowar‐Manning, Email: epiwowa@jhmi.edu.
Paul Richardson, Email: pricha18@jhmi.edu.
Sergii Dvoriak, Email: dvoryak@uiphp.org.ua.
Zubairi Djoerban, Email: zubairi_djoerban@yahoo.com.
Tetiana Kiriazova, Email: Kiriazova@uiphp.org.ua.
Oleksandr Zeziulin, Email: zeziulin@uiphp.org.ua.
Samsuridjal Djauzi, Email: samsuridjal@yahoo.com.
Chu Viet Ahn, Email: cva@live.unc.edu.
Carl Latkin, Email: carl.latkin@jhu.edu.
David Metzger, Email: dsm@pennmedicine.upenn.edu.
David N Burns, Email: burnsda@niaid.nih.gov.
Jeremy Sugarman, Email: jsugarman@jhu.edu.
Steffanie A Strathdee, Email: sstrathdee@ucsd.edu.
Susan H Eshleman, Email: seshlem@jhmi.edu.
William Clarke, Email: wclarke@jhmi.edu.
Deborah Donnell, Email: deborah@scharp.org.
Lynda Emel, Email: lemel@scharp.org.
Lisa E Sunner, Email: lsunner@iavi.org.
Laura McKinstry, Email: lmckinst@scharp.org.
Nirupama Sista, Email: NSista@fhi360.org.
Erica L Hamilton, Email: EHamilton@fhi360.org.
Jonathan P Lucas, Email: JLucas@fhi360.org.
Bui D Duong, Email: bdduong06@gmail.com.
Nguyen Van Vuong, Email: vuongttytpy@gmail.com.
Riza Sarasvita, Email: rizapram@yahoo.com.
William C Miller, Email: miller.8332@osu.edu.
References
- 1. Mathers BM, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick RP, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375(9719):1014–28. [DOI] [PubMed] [Google Scholar]
- 2. Stimson G. Drug injecting and HIV infection. London, UK: Routledge; 1998. [Google Scholar]
- 3. Booth RE, Kwiatkowski CF, Brewster JT, Sinitsyna L, Dvoryak S. Predictors of HIV sero‐status among drug injectors at three Ukraine sites. AIDS. 2006;20(17):2217–23. [DOI] [PubMed] [Google Scholar]
- 4. Strathdee SA, Shoptaw S, Dyer TP, Quan VM, Aramrattana A, the Substance Use Scientific Committee of the HIVPTN . Towards combination HIV prevention for injection drug users: addressing addictophobia, apathy and inattention. Curr Opin HIV AIDS. 2012;7(4):320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sugarman J, Rose SM, Metzger D. Ethical issues in HIV prevention research with people who inject drugs. Clin Trials. 2014;11(2):239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dawson L, Strathdee SA, London AJ, Lancaster KE, Klitzman R, Hoffman I, et al. Addressing ethical challenges in HIV prevention research with people who inject drugs. J Med Ethics. 2018;44(3):149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV‐1 Infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hudgens MG, Longini IM Jr, Vanichseni S, Hu DJ, Kitayaporn D, Mock PA, et al. Subtype‐specific transmission probabilities for human immunodeficiency virus type 1 among injecting drug users in Bangkok, Thailand. Am J Epidemiol. 2002;155(2):159–68. [DOI] [PubMed] [Google Scholar]
- 9. Gyarmathy VA, Li N, Tobin KE, Hoffman IF, Sokolov N, Levchenko J, et al. Correlates of unsafe equipment sharing among injecting drug users in St. Petersburg, Russia. Eur Addict Res. 2009;15(3):163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gyarmathy VA, Li N, Tobin KE, Hoffman IF, Sokolov N, Levchenko J, et al. Injecting equipment sharing in Russian drug injecting dyads. AIDS Behav. 2010;14:141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu T‐C, Westergaard RP, Lau B, Celentano DD, Vlahov D, Mehta SH, et al. Changes in sexual and drug‐related risk behavior following antiretroviral therapy initiation among HIV‐infected injection drug users. AIDS. 2012;26(18):2383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuyper L, Milloy MJ, Marshall BDL, Zhang R, Kerr T, Montaner JSG, et al. Does initiation of HIV antiretroviral therapy influence patterns of syringe lending among injection drug users? Addict Behav. 2011;36(5):560–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vlahov D, Safaien M, Lai S, Strathdee SA, Johnson L, Sterling T, et al. Sexual and drug risk‐related behaviours after initiating highly active antiretroviral therapy among injection drug users. AIDS. 2001;15(17):2311–6. [DOI] [PubMed] [Google Scholar]
- 14. Nolan S, Milloy MJ, Zhang R, Kerr T, Hogg RS, Montaner JS, et al. Adherence and plasma HIV RNA response to antiretroviral therapy among HIV‐seropositive injection drug users in a Canadian setting. AIDS Care. 2011;23(8):980–7. [DOI] [PubMed] [Google Scholar]
- 15. Roux P, Carrieri MP, Villes V, Dellamonica P, Poizot‐Martin I, Ravaux I, et al. The impact of methadone or buprenorphine treatment and ongoing injection on highly active antiretroviral therapy (HAART) adherence: evidence from the MANIF2000 cohort study. Addiction. 2008;103(11):1828–36. [DOI] [PubMed] [Google Scholar]
- 16. Roux P, Carrieri MP, Cohen J, Ravaux I, Poizot‐Martin I, Dellamonica P, et al. Retention in opioid substitution treatment: a major predictor of long‐term virological success for HIV‐infected injection drug users receiving antiretroviral treatment. Clin Infect Dis. 2009;49(9):1433–40. [DOI] [PubMed] [Google Scholar]
- 17. Volkow ND, Montaner J. The urgency of providing comprehensive and integrated treatment for substance abusers with HIV. Health Aff. 2011;30(8):1411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Volkow ND, Baler RD, Normand JL. The unrealized potential of addiction science in curbing the HIV epidemic. Curr HIV Res. 2011;9(6):393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. CampBinford M, Kahana SY, Altice FL. A systematic review of antiretroviral adherence interventions for HIV‐infected people who use drugs. Curr HIV/AIDS Rep. 2012;9(4):287–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berleva G, Dumchev K, Kasianchuk M, Nikolko M, Saliuk T, Shvab I, et al. Estimation of the Size of Populations Most‐at‐Risk for HIV Infection in Ukraine. Technical Working Group on Monitoring and Evaluation of HIV/AIDS Response in Ukraine; 2012.
- 21. Balakireva OM, Bondar TV, Loktieva I, Sazonova YO, Sereda YV. Summary of the analytical report: monitoring the behaviour and HIV‐infection prevalence among people who inject drugs as a component of HIV second generation surveillance. Results of the 2013 survey. 2014. [Cited 2018 Jan 14]. Available from: http://www.aidsalliance.org.ua/ru/library/our/2014/arep14/zvit%20IDU_obl_eng.pdf
- 22. Dumchev K, Samko M, Barska J, Salyuk T. HIV testing and ART are associated with lower HIV incidence among PWID in Ukraine. 21st International AIDS Conference; Durban, South Africa, Poster #LBPE015; 2016. [Google Scholar]
- 23. Quan VM, Minh NL, Ha TV, Ngoc NP, Vu PT, Celentano DD, et al. Mortality and HIV transmission among male Vietnamese injection drug users. Addiction. 2011;106(3):583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tavitian‐Exley I, Vickerman P, Bastos FI, Boily MC. Influence of different drugs on HIV risk in people who inject: systematic review and meta‐analysis. Addiction. 2015;110(4):572–84. [DOI] [PubMed] [Google Scholar]
- 25. Miller WC, Hoffman IF, Hanscom B, Ha TV, Dumchev K, Djoerban Z, et al. A scalable intervention to increase antiretroviral therapy and medication‐assisted treatment and reduce mortality and HIV transmission among people who inject drugs: a randomized, controlled vanguard trial (HPTN 074). Lancet. 2018;392(10149):P747–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2013;381(9883):2083–90. [DOI] [PubMed] [Google Scholar]
- 27. Gyarmathy VA, Li N, Tobin KE, Hoffman IF, Sokolov N, Levchenko J, et al. Unprotected sex in heterosexual partnerships of injecting drug users in St. Petersburg, Russia. AIDS Behav. 2011;15(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Babor T, Higgins‐Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: guidelines for use in primary care. Geneva, Switzerland: World Health Organization, Department of Mental Health and Substance Dependence; 2001. [Google Scholar]
- 29. Zelenev A, Shea P, Mazhnaya A, Rozanova J, Madden L, Marcus R, et al. Assessment of barrier severity and willingness to enter opioid agonist treatment among people who inject drugs in Ukraine. Drug Alcohol Depend. 2018;190:82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hammett TM, Phan S, Nguyen P, Kieu B, Dang S, Nguyen D, et al. Female sexual partners of male people who inject drugs in Vietnam have poor knowledge of their male partners’ HIV status. J Acquir Immune Defic Syndr. 2015;68(5):562–7. [DOI] [PubMed] [Google Scholar]
- 31. Kato M, Long NH, Duong BD, Nhan DT, Nguyen TTV, Hai NH, et al. Enhancing the benefits of antiretroviral therapy in Vietnam: towards ending AIDS. Current HIV/AIDS Reports. 2014;11:487–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jarlais DCD, Thi HD, Hai OKT, Minh KP, Thi GH, Tuyet TNT, et al. Prospects for ending the HIV epidemic among persons who inject drugs in Haiphong, Vietnam. Int J Drug Policy. 2016;32:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shaboltas AV, Toussova OV, Hoffman IF, Heimer R, Verevochkin SV, Ryder RW, et al. HIV prevalence, sociodemographic, and behavioral correlates and recruitment methods among injection drug users in St. Petersburg, Russia. J Acquir Immune Defic Syndr. 2006;41(5):657–63. [DOI] [PubMed] [Google Scholar]
- 34. Azim T, Bontell I, Strathdee SA. Women, drugs and HIV. Int J Drug Policy. 2015;26(0 1):S16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rangarajan S, Tram HN, Todd CS, Thinh T, Hung V, Hieu PT, et al. Risk factors for delayed entrance into care after diagnosis among patients with late‐stage HIV disease in southern Vietnam. PLoS ONE. 2014;9(10):e108939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maher L, Coupland H, Musson R. Scaling up HIV treatment, care and support for injecting drug users in Vietnam. Int J Drug Policy. 2007;18(4):296–305. [DOI] [PubMed] [Google Scholar]
- 37. Ford K, Wirawan DN, Sumantera GM, Sawitri AAS, Stahre M. Voluntary HIV testing, disclosure, and stigma among injection drug users in Bali, Indonesia. AIDS Educ Prev. 2004;16(6):487–98. [DOI] [PubMed] [Google Scholar]
- 38. Opium cultivation rising in Golden Triangle. Addiction. 2014;109(7):1217. [PubMed] [Google Scholar]
- 39. Booth RE, Kennedy J, Brewster T, Semerik O. Drug injectors and dealers in Odessa, Ukraine. J Psychoactive Drugs. 2003;35(4):419–26. [DOI] [PubMed] [Google Scholar]
- 40. Abdala N, Grund JPC, Tolstov Y, Kozlov AP, Heimer R. Can home‐made injectable opiates contribute to the HIV epidemic among injection drug users in the countries of the former Soviet Union? Addiction. 2006;101(5):731–7. [DOI] [PubMed] [Google Scholar]
- 41. Sacamano PL, Farley JE. Behavioral and other characteristics associated with HIV viral load in an outpatient clinic. PLoS ONE. 2016;11(11):e0166016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carter A, Roth EA, Ding E, Milloy MJ, Kestler M, Jabbari S, et al. Substance use, violence, and antiretroviral adherence: a latent class analysis of women living with HIV in Canada. AIDS Behav. 2018;22(3):971–85. [DOI] [PubMed] [Google Scholar]
- 43. Fairbairn N, Hayashi K, Kaplan K, Suwannawong P, Qi J, Wood E, et al. Factors associated with methadone treatment among injection drug users in Bangkok, Thailand. J Subst Abuse Treat. 2012;43(1):108–13. [DOI] [PubMed] [Google Scholar]
- 44. Latkin C, Donnell D, Celentano DD, Aramrattna A, Liu TY, Vongchak T, et al. Relationships between social norms, social network characteristics, and HIV risk behaviors in Thailand and the United States. Health Psychol. 2009;28(3):323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Latkin C, Mandell W, Vlahov D, Oziemkowska M, Celentano D. People and places: behavioral settings and personal network characteristics as correlates of needle sharing. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(3):273–80. [DOI] [PubMed] [Google Scholar]
- 46. Suh T, Mandell W, Latkin C, Kim J. Social network characteristics and injecting HIV‐risk behaviors among street injection drug users. Drug Alcohol Depend. 1997;47(2):137–43. [DOI] [PubMed] [Google Scholar]
- 47. Latkin C, Kuramoto S, Davey‐Rothwell M, Tobin K. Social norms, social networks, and HIV risk behavior among injection drug users. AIDS Behav. 2010;14(5):1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Booth RE, Davis JM, Dvoryak S, Brewster JT, Lisovska O, Strathdee SA, et al. HIV incidence among people who inject drugs (PWIDs) in Ukraine: results from a clustered randomised trial. Lancet HIV. 2016;3(10):e482–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith MK, Graham M, Latkin CA, Go VL. Using contact patterns to inform HIV Interventions in persons who inject drugs in Northern Vietnam. J Acquir Immune Defic Syndr. 2018;78(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kaljee LM, Green M, Riel R, Lerdboon P, Tho LH, Thoa LTK, et al. Sexual stigma, sexual behaviors, and abstinence among Vietnamese adolescents: implications for risk and protective behaviors for HIV, STIs, and unwanted pregnancy. J Assoc Nurses AIDS Care. 2007;18(2):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cakalo JI, Bozicevic I, Vitek C, Mandel JS, Salyuk T, Rutherford GW. Misclassification of men with reported HIV infection in Ukraine. AIDS Behav. 2015;19(10):1938–40. [DOI] [PubMed] [Google Scholar]
- 52. Morineau G, Nugrahini N, Riono P, Nurhayati DE, Girault P, Mustikawati DE, et al. Sexual risk taking, STI and HIV prevalence among men who have sex with men in six Indonesian cities. AIDS Behav. 2011;15(5):1033–44. [DOI] [PubMed] [Google Scholar]
- 53. Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV‐1 transmission for parenteral exposure and blood transfusion: a systematic review and meta‐analysis. AIDS. 2006;20(6):805–12. [DOI] [PubMed] [Google Scholar]
- 54. Thiede H, Hagan H, Campbell JV, Strathdee SA, Bailey SL, Hudson SM, et al. Prevalence and correlates of indirect sharing practices among young adult injection drug users in five U.S. cities. Drug Alcohol Depend. 2007;91 Suppl 1:S39–47. [DOI] [PubMed] [Google Scholar]
- 55. Armstrong G, Nuken A, Medhi GK, Mahanta J, Humtsoe C, Lalmuanpuaii M, et al. Injecting drug use in Manipur and Nagaland, Northeast India: injecting and sexual risk behaviours across age groups. Harm Red J. 2014;11(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Aspinall EJ, Nambiar D, Goldberg DJ, Hickman M, Weir A, Van Velzen E, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta‐analysis. Int J Epidemiol. 2013;43(1):235–48. [DOI] [PubMed] [Google Scholar]
- 57. MacArthur GJ, van Velzen E, Palmateer N, Kimber J, Pharris A, Hope V, et al. Interventions to prevent HIV and hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int J Drug Policy. 2014;25(1):34–52. [DOI] [PubMed] [Google Scholar]
- 58. Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2011; 10(8):CD004145. [DOI] [PubMed] [Google Scholar]
- 59. Dumchev K, Dvoryak S, Chernova O, Morozova O, Altice FL. Retention in medication‐assisted treatment programs in Ukraine – identifying factors contributing to a continuing HIV epidemic. Int J Drug Policy. 2017;48:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nguyen TT, Nguyen LT, Pham MD, Vu HH, Mulvey KP. Methadone maintenance therapy in Vietnam: an overview and scaling‐up plan. Adv Prev Med. 2012;2012:732484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mesquita F, Winarso I, Atmosukarto II, Eka B, Nevendorff L, Rahmah A, et al. Public health the leading force of the Indonesian response to the HIV/AIDS crisis among people who inject drugs. Harm Reduct J. 2007;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Edwards EM, Cheng DM, Levenson S, Bridden C, Meli S, Egorova VY, et al. Behavioral assessments in Russian addiction treatment inpatients: a comparison of audio computer‐assisted self‐interviewing and interviewer‐administered questionnaires. HIV Clin Trials. 2008;9(4):247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Potdar R, Koenig MA. Does Audio‐CASI improve reports of risky behavior? Evidence from a randomized field trial among young urban men in India. Stud Fam Plann. 2005;36(2):107–16. [DOI] [PubMed] [Google Scholar]


