Abstract
Objective
Women diagnosed with breast cancer younger than 45 years (young breast cancer survivors—YBCS) and their biological relatives face significant stressors. Although family support is an important coping resource, little is known about YBCS’ and relatives’ support and whether it is interdependent. The study described family support in YBCS and their biological relatives; identified demographic, clinical, and psychosocial predictors of support; and determined the interdependence of support in YBCS‐relatives family units.
Methods
Data were collected from a random sample of YBCS and their first‐ or second‐degree female relatives. Actor‐partner interdependence models (APIM) explored predictors and interdependence of YBCS’ and relatives’ family support in dyads (YBCS and relative) and triads (YBCS and two relatives).
Results
Among n = 310 YBCS and n = 431 first‐ or second‐degree relatives, family support was higher in triads compared to dyads. APIMs identified actor effects in dyads, and actor and partner effects in triads. Across all family units, YBCS’ higher self‐efficacy was associated with higher YBCS support (actor effect) and relative support (partner effect); YBCS’ prior diagnosis of depression was associated with lower YBCS and relative support (actor and partner effect); cost‐related lack of access to care was associated with lower support among YBCS (actor effect) and relatives (actor and partner effect).
Conclusions
Family support was interdependent and was affected by self‐efficacy, depression, and access to care. Interventions should include YBCS and relatives, enhance self‐efficacy and access to care.
Keywords: Actor‐Partner Interdependence Model, dyads, principal component analysis, random sample, triads, young breast cancer survivors
1. BACKGROUND
Breast cancer is the most prevalent female cancer worldwide, with 1.38 million new cases annually.1 About 25% of all breast cancer cases are diagnosed in women under 50 years old, constituting a growing clinical population of younger women with breast cancer.2 Early onset breast cancer presents several challenges, including tumors that are more aggressive, higher recurrence rates, and increased mortality, and is associated with genetic predisposition. First‐ and second‐degree relatives of young breast cancer survivors (YBCS) have a 2.3 and 1.5 increased relative risk for breast cancer, respectively.3
YBCS often report poorer outcomes compared to their older counterparts due to different stressors and social roles.4, 5 YBCS caring for young children may face additional difficulties communicating concerns and may feel responsible for transmitting an increased cancer risk to their offspring.4, 5 Caring for children and older parents, combined with the challenges of the disease, can cause additional distress, anxiety, depression, fear of recurrence, and difficulties returning to work.4, 6, 7 Loss of income due to inability to work can lead to additional financial stressors and lack of access to care.5 Thus, YBCS may need significantly more support to overcome these challenges compared to older breast cancer patients.8
Although biological female relatives have an elevated risk for breast cancer, they may not always cope with this risk and manage it effectively. Young women with a strong family history may have heightened perceptions of breast cancer risk, chronic depression, anxiety, and increased breast cancer worry.9, 10, 11 Family members are an important source of information about risk factors, genetics, and available screening and risk‐reducing strategies, especially for women from medically underserved communities.12 Multiple family members are likely to be involved, directly or indirectly, in appraisals regarding the magnitude of the health threat and the availability of coping resources. However, family members may perceive different levels of vulnerability and stigmatization associated with hereditary breast cancer, experience different levels of distress, and disagree about the extent of family involvement needed to reduce these stressors.13, 14, 15 Input from different family members affects support they are willing to give and receive to each other.
There is a need to promote long‐term coping in YBCS and relatives and to mitigate the burden of early onset breast cancer.4, 5 Family support is essential to successful coping of breast cancer patients16 and can decrease cancer‐related distress.17 Individuals involved in reciprocal relationships usually influence each other's thoughts, emotions, and coping behaviors.18 Yet, little is known about a possible interdependence of family support in YBCS and biological relatives, who also face an increased breast cancer risk due to heredity. The study addressed this gap in the literature. Specific aims were to describe family support in YBCS and their relatives; identify demographic, clinical, and psychosocial characteristics as predictors of family support; and determine the interdependence of support in YBCS‐relatives family units.
1.1. Theoretical framework
The study was guided by the integration of the theory of stress and coping19 with the theory of family systems in genetic illness20 applied to families with hereditary breast cancer risk.21 Stress occurs when primary appraisals of a health problem threaten one's well‐being.19 Primary appraisals include YBCS’ and relatives’ assessment of stressors associated with early onset breast cancer, for example, cost of health care. Primary appraisals may interfere with the ability to withstand stress because they can exacerbate YBCS’ depression and fear of cancer recurrence, and increase relatives’ perceived breast cancer risk.22, 23 Initial appraisals are followed by appraisals about the availability of personal (eg, self‐efficacy for managing breast cancer23) and social coping resources (eg, family support) that can help manage the health threat. Family support is the primary outcome of the study in both YBCS and their relatives (Figure 1).
Figure 1.
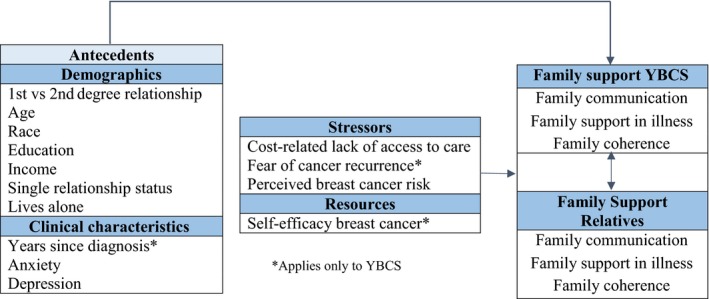
Theoretical framework
2. METHODS
2.1. Design, setting, and sample
The study used baseline data obtained with a self‐administered survey for an efficacy trial designed to increase surveillance and use of cancer genetic services in YBCS and their first‐ and second‐degree relatives (ClinicalTrial.gov ID:NCT01612338).24 All Institutional Review Boards involved in the identification, recruitment, and consent of participants approved the study protocol. Methodological and recruitment details have been reported.24, 25 A random sample of 3000 YBCS was selected from the Michigan Cancer Surveillance Program (MCSP). Age criteria for YBCS vary among studies from 40 to 50 years old and under; we were conservative in our sample selection and we chose a cutoff of 45 years or younger at the time of diagnosis. The sample was stratified by race (1500 Black vs 1500 White/Other YBCS) to ensure an adequate representation of Black YBCS. The “Other” category includes about 7% of Michigan YBCS not recorded in the registry as Black or White (eg, Arab American). Due to their small numbers, YBCS of other racial/ethnic backgrounds could not form a separate stratum. YBCS were eligible to participate if they were diagnosed with invasive breast cancer or ductal carcinoma in situ (DCIS); were younger than 45 years old at the time of diagnosis and younger than 65 years old at the time of the study; and were willing to invite one or two relatives in the study. Relatives had to be female and in first‐ or second‐degree biological relationship with the YBCS. They had to be younger than 65 years old and cancer‐free at the time of the study.
Prior to contacting the YBCS, the director of the MCSP inquired with the reporting facility and physician of record whether there was any reason that the YBCS could not participate in the study. If MCSP did not receive a negative response within 30 days, an invitation letter explaining the study, a consent form, a self‐administered baseline survey, and a stamped return envelope were mailed to YBCS. Eligible YBCS received up to three mailed invitations to participate in the study. In order to have family units with comparable size, the study invited up to two relatives per YBCS. There were 58 YBCS carrying a mutation associated with hereditary breast cancer who were excluded from this paper; their relatives were not invited in the efficacy trial since the focus was to increase use of genetic services among untested families.
2.2. Instruments
The study outcome was family support in YBCS and relatives. According to the theoretical framework, predictors included stressors for YBCS (lack of access to care due to cost, anxiety, depression, and fear of cancer recurrence) and for relatives (perceived breast cancer risk), resources for YBCS (self‐efficacy dealing with breast cancer), and demographic characteristics for both YBCS and relatives.
2.2.1. Family support
Family support was conceptualized as open communication, support in times of illness, and coherence, and was measured with three well‐established scales. All items were rated on a seven‐point Likert scale, ranging from one “Strongly Disagree” to seven “Strongly Agree.” Family communication was assessed with the Lewis Mutuality and Interpersonal Sensitivity Scale (MIS),26 validated with breast cancer survivors and their family members.27, 28 MIS includes 15 items (eg, “The people in my family change the topic when I discuss my concerns”); internal consistency in this study was 0.94. Family support in times of illness was assessed with the Family Support in Illness scale, originally developed for women pursuing breast cancer screening.29 The scale includes 10 items (eg, “In our family, when I have a health problem, there is someone helping me get the care that I need”); internal consistency in this study was 0.91. Family coherence is the ability of the family to cope with adverse events and was assessed with the Family Hardiness Index (FHI),30 validated with cancer and noncancer patients.31, 32 FHI includes 20 items (eg, “In our family we have a sense of being strong even when we face big problems”); internal consistency was 0.90.
A family support index was created from these three scales. Principal component analysis (PCA) examined the correlations of items (n = 45). The Kaiser‐Meyer‐Olkin measure of sampling adequacy and Bartlett's test of sphericity indicated that PCA was possible. PCA identified a primary component of family support. Pearson correlation coefficients in the component matrices ranged between 0.40 and 0.80. Four items did not correlate adequately with the principal component and were not used. An overall family support index was created by calculating a mean score from the three scales as the dependent variable.
2.2.2. Stressors
Cost‐Related Lack of Access to Care was assessed with one item asking YBCS and relatives “Has there been a time within the past 12 months that you needed to see a doctor or have a medical test but you could not because of high out‐of‐pocket cost?” yes/no; yes indicates cost‐related lack of access to care.
Anxiety and Depression were assessed with two items asking YBCS and relatives “Have you ever been told by a healthcare provider that you have anxiety?” yes/no and “Have you ever been told by a health care provider that you have depression?” yes/no. These variables were assessed because they interfere with support and communication,33, 34 and better family functioning mitigates depressive symptoms among cancer patients.35
Fear of Cancer Recurrence (YBCS only) was assessed with four items from the Concerns About Recurrence Scale (CARS) (eg, “How much time do you spend thinking about your breast cancer coming back”) using a seven‐point Likert scale from one “Not at all” to seven “All the time”.36 Internal consistency was 0.91.
Perceived Breast Cancer Risk was assessed with one item asking YBCS and relatives to rate their chances of (another) getting breast cancer on a 10‐point Likert scale with verbal anchors “Definitely will not” to “Definitely will”.37
2.2.3. Resources
Self‐efficacy managing breast cancer (YBCS only) was assessed with 14 items (eg, “Since my breast cancer diagnosis, I am able to do the things that are important for me”) scored on a seven‐point Likert scale, ranging from one “Strongly Disagree” to seven “Strongly Agree”.38 Internal consistency was 0.95.
Demographic and Clinical Characteristics such as age, education, income, living alone, relative being first vs. second degree, years since diagnosis, number of cancer diagnoses were assessed in YBCS and relatives with items from the Behavioral Risk Factors Surveillance System Survey39 and items developed by the team.40
2.3. Statistical analyses
Analyses were performed with SPSS® version 22.041 and MPlus version 7.0.42 Sample characteristics, stressors, and resources were described with means, standard deviations (SD), frequencies (n), or percentages (%), depending on scaling and data distribution. A P value ˂0.05 was considered statistically significant in all analyses. Demographics and clinical characteristics were included in all models as covariates.
Data from dyads and triads often violate the fundamental assumption of many data analyses methods that data are collected from independent subjects. YBCS and relatives have an existing interpersonal relationship; thus, correlations between YBCS’ and relatives’ data need to be taken into account. The Actor‐Partner Interdependence Model (APIM) has been used to study complex dynamics in families and close relationships.43 It assesses interdependence and bidirectional effects within interpersonal relationships.44 Observation interdependence necessitates examining the dyad (ie, the pair) as a single unit of analyses, rather than two units (ie, as single individuals). Interdependence means that observations from two or more individuals are linked. Knowledge of one's characteristics (actor) can provide information about the other person's (partner) attitudes, etc. Assessing bidirectionality involves examining each person's influence on the other person's outcomes.
We identified three types of family units for this study: dyads consisting of one YBCS and one relative; triads consisting of one YBCS and two relatives; and YBCS with no eligible relatives or whose relatives did not accept participation. The latter group was excluded from APIM analyses and this paper. APIM examined predictors of family support in dyads and triads. A dyadic model captured the interdependence of family support between YBCS and one relative (Figure 2A), and a triadic model among YBCS and two relatives (Figure 2B). Actor effects are observed when characteristics of one person (eg, their own resources) are significant predictors of their own outcome (ie, family support), regardless of whether this is an YBCS or a relative. Partner effects refer to cross‐dyadic or cross‐triadic associations and are observed when characteristics of one person in the family influence family support reported by another member.
Figure 2.
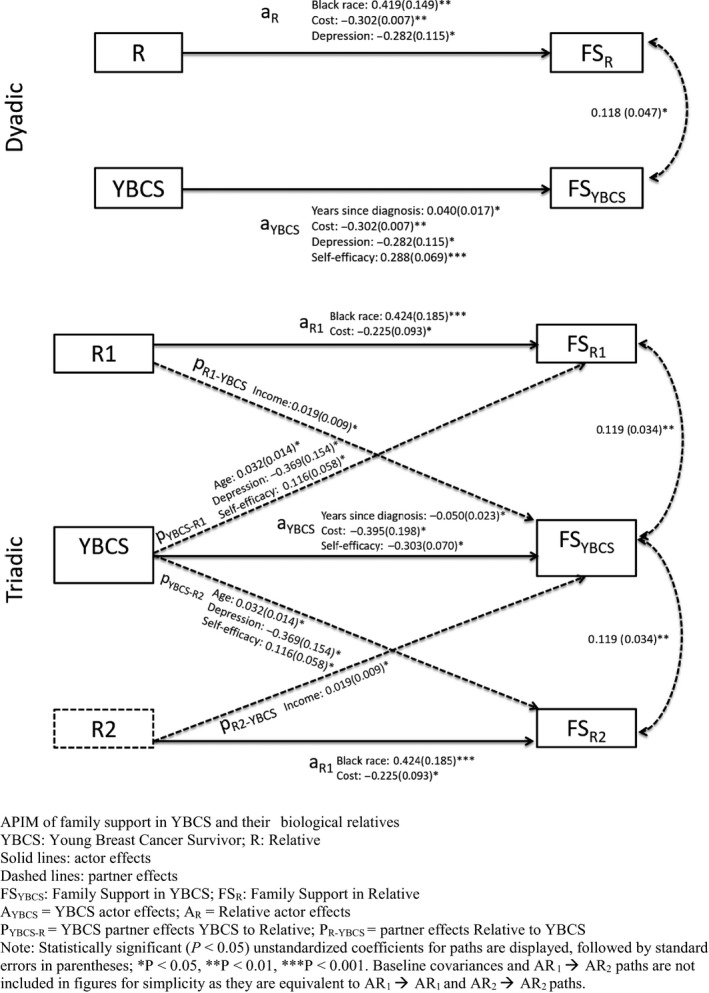
Results of actor‐partner interdependence models (APIM) of family support
We examined evidence of nonindependence in the outcome variable (family support index) by assessing the level of intraclass correlations.18, 43 Intraclass correlations in dyads and triads were statistically significant (0.31, 95% CI 0.17‐0.43, ANOVA F = 1.88, P < 0.001; and 0.37, 95% CI 0.23‐0.50, ANOVA F = 2.16, P < 0.001, respectively), suggesting interdependence of support in family units and that analyses should follow a dyadic and triadic format. Data were restructured in dyads including one YBCS and one relative and triads including one YBCS and two relatives. Mixed predictor variables (variables that exhibit both between‐ and within‐dyad/triad variability) were used to estimate the interdependence effects of family support.18, 43, 44 Full information on maximum likelihood estimation (FIML) was used in path analyses. FIML produces unbiased estimates based on all available information from variables included in an analysis, when data are missing at random or missing completely at random.
A fundamental tenet of dyadic analyses is determining distinguishability, meaning, whether or not there is a way to treat each individual as different.18 Distinguishable dyads are, for example, heterosexual couples; indistinguishable dyads are, for example, same‐sex roommates. We could distinguish members of dyads (YBCS and relatives), but we were unable to distinguish the two female relatives included in triads. The two relatives in triads were treated as indistinguishable, because there were no specific criteria that could designate one relative as a primary participant and the other relative as a secondary participant. Consequently, we used a novel approach for triadic analyses based on analyses methods of indistinguishable dyads. In indistinguishable dyads, data from the two individuals are pooled in order to produce parameter estimates.18, 45, 46 Following this approach, in the triadic APIM we pooled the estimates from the two relatives into one estimate by imposing equality constraints. Pooling the estimates from two indistinguishable relatives allowed us to estimate the effects from the two relatives as a single “relative” effect.
Indicators of adequate model fit in triadic APIM were a comparative fit index (CFI) above 0.90, a nonsignificant chi‐square statistic or a ratio of chi‐square statistic to degrees of freedom (df) less than two, a root mean squared error of approximation (RMSEA) value of 0.08 or less, and a standardized root mean square residual (SRMR) value of 0.08 or less.47, 48 We tested both an unconstrained dyadic model (fully saturated) and a constrained dyadic model (with actor = actor and partner = partner) constraints.49 A significant difference between the unconstrained and constrained model suggests that the actor effects and partner effects were equal for YBCS and relatives.
3. RESULTS
3.1. Sample characteristics
From the 3000 identified YBCS, n = 883 accepted participation (response rate 33.2% after excluding deceased, missing current address etc.). From these 883 YBCS, n = 573 participated in the study alone, either because they had no eligible relatives or because their relatives did not accept study participation.25 To address APIM analyses, in this paper we include only those YBCS, n = 310, who participated in the study with one or two relatives.
The 310 YBCS and their 431 blood female relatives constituted 189 dyads (one YBCS and one relative) and 121 triads (one YBCS and two relatives). Most family units (n = 249) included only first‐degree relatives (n = 164 dyads; n = 85 triads); 32 family units included only second‐degree relatives (n = 25 dyads; n = 7 triads); 29 triads were mixed, with both first‐ and second‐degree relatives. YBCS were on average 51 years old at the time of the study and 11 years postdiagnosis; approximately one in five (19.7%) had more than one cancer diagnoses. Most YBCS were White/Other, had an annual household income less than $80 000, had some college‐level education or above, and were married. Relatives were on average 43 years old; most were White/Other, had an annual household income less than $60 000, had some college‐level education or above, and were married (Table 1).
Table 1.
Sample characteristics
| YBCS (n = 310) | Relatives (n = 431) | P value | |
|---|---|---|---|
| Demographics | |||
| Age (mean ± SDa) | 51.4 ± 5.8 | 43.4 ± 11.9 | <0.001 |
| Race (N, %b) | |||
| White/other | 242 (78.1%) | 344 (79.8%) | |
| Black | 68 (21.9%) | 87 (20.2%) | 0.629 |
| Education (N, %b) | |||
| Elementary school only | 0 (0%) | 2 (0.5%) | |
| High school (grades 9‐11) | 2 (0.7%) | 9 (2.1%) | |
| Compulsory education (K‐12) | 56 (18.3%) | 61 (14.4%) | |
| Some college | 113 (36.9%) | 157 (37.0%) | |
| Completed college | 74 (24.2%) | 126 (29.7%) | |
| Postgraduate degree | 61 (19.9%) | 69 (16.3%) | 0.109 |
| Employment (N, %b) | |||
| Full time | 157 (50.7%) | 237 (57%) | |
| Part time | 42 (13.5%) | 64 (15.4%) | |
| Unemployed/otherc | 111 (35.8%) | 115 (27.6%) | 0.357 |
| Income (N, %b) | |||
| <$20 000 | 24 (8.7%) | 51 (13.2%) | |
| $20 000‐$39 999 | 42 (15.2%) | 84 (21.8%) | |
| $40 000‐$59 999 | 50 (18.1%) | 68 (17.6%) | |
| $60 000‐$79 999 | 51 (18.4%) | 53 (13.7%) | |
| $80 000‐$99 999 | 30 (10.8%) | 36 (9.3%) | |
| $100 000‐$119 999 | 29 (10.4%) | 33 (8.5%) | |
| >$120 000 | 51 (18.4%) | 61 (15.8%) | 0.103 |
| Marital status (N, %b) | |||
| Married/life partner | 212 (68.4%) | 257 (59.8%) | |
| Single/divorced/widowed | 98 (31.6%) | 173 (40.2%) | 0.020 |
| Lives alone | 44 (14.2%) | 60 (13.9%) | 0.998 |
| Clinical characteristics | |||
| Years since 1st diagnosis (mean ± SDa) | 11.6 ± 4.0 | N/Ad | |
| Anxiety (N, %b) | 89 (29.1%) | 115 (27.1%) | 0.604 |
| Depression (N, %b) | 84 (27.5%) | 111 (26.1%) | 0.731 |
| Stressors | |||
| Cost‐related lack of access to care (N, %b) | 56 (18.1%) | 82 (19.2%) | 0.779 |
| Fear of cancer recurrence (mean ± SDa) | 3.4 ± 1.6 | N/Ad | |
| Perceived breast cancer risk (mean ± SDa) | N/Ad | 4.7 ± 2.0 | |
| Resources | |||
| Self‐efficacy (mean ± SDa) | 5.9 ± 1.1 | N/Ad | |
Standard deviation.
Valid percentages.
Student, retired, housewife.
N/A: not applicable.
Participants reported high levels of family support on the three family support scales (ie, communication, support in illness, and coherence) (observed range: 5.4‐6.1) and the family support index (YBCS: 5.6 ± 0.9; relatives: 5.7 ± 0.9) (Table 2). Relatives reported significantly higher family communication compared to YBCS. White/Other participants (YBCS and relatives combined) reported a higher score on the Family Support in Illness scale compared to Black participants (6.0 ± 1.1 vs. 5.8 ± 1.2, P = 0.001); this difference was not observed in the other two scales. Family support in illness was also higher in triads than dyads (Table 3).
Table 2.
Family support in YBCS and relatives
| YBCS (N = 310) mean ± SDa | Relatives (N = 431) mean ± SDa | P value | |
|---|---|---|---|
| Family communication | 5.4 ± 1.1 | 5.6 ± 1.1 | 0.040 |
| Family support in illness | 6.0 ± 1.1 | 6.1 ± 1.0 | 0.087 |
| Family coherence | 5.4 ± 0.8 | 5.5 ± 0.8 | 0.688 |
| Family Support Index | 5.6 ± 0.9 | 5.7 ± 0.9 | 0.112 |
Standard deviation.
Table 3.
Mean family support in family units
| Dyads (n = 189) mean ± SDa | Triads (n = 121) mean ± SDa | P value | |
|---|---|---|---|
| Family communication | 5.4 ± 1.2 | 5.6 ± 1.0 | 0.437 |
| Family support in illness | 6.0 ± 1.1 | 6.2 ± 1.0 | 0.038 |
| Family coherence | 5.4 ± 0.8 | 5.5 ± 0.7 | 0.287 |
| Family Support Index | 5.6 ± 0.9 | 5.7 ± 0.8 | 0.134 |
Standard deviation.
3.2. Predictors of family support in dyads and triads—APIM
3.2.1. Dyadic findings
APIM with 189 dyads (YBCS and one relative) identified significant actor effects in YBCS and relatives, but no partner effects (Table 4). First, we tested an unconstrained dyadic model, which allowed actor and partner effects to differ between YBCS and relatives and found significant actor effects. As this dyadic model was a saturated model without fit indices, we tested an additional model where we constrained the actor effects to be equal to each other and the partner effects also to be equal to each other. No constraints were placed on dyad‐level predictors, that is, years since diagnosis, race, and degree type; on variables measured only in YBCS, that is, fear of recurrence and self‐efficacy. Chi‐square difference tests revealed that the effects did not differ across YBCS and relatives in the dyadic model (Χ2 diff = 15.390, df = 18, P = 0.6350). Thus, we report the constrained model results in Table 4. Similar to the unconstrained model, only actor effects were significant; a prior diagnosis of depression and cost‐related lack of access to care were associated with lower family support for YBCS and relatives. The strength of the actor effects between depression and cost‐related lack of access to care did not differ between YBCS and relatives. More years since diagnosis and higher self‐efficacy were associated with higher family support for YBCS, whereas Black race was associated with higher family support in relatives.
Table 4.
Actor‐partner interdependence models^
| Actor effects | Dyads | Triads | ||
|---|---|---|---|---|
| Estimate | P value | Estimate | P value | |
| YBCS | ||||
| First‐degree relationship* | −0.166 | 0.364 | 0.191 | 0.226 |
| Age | 0.001 | 0.788 | 0.011 | 0.502 |
| Black race* | 0.285 | 0.068 | 0.014 | 0.949 |
| Education | 0.082 | 0.053 | −0.073 | 0.329 |
| Income | −0.010 | 0.308 | 0.008 | 0.573 |
| Single relationship status | −0.159 | 0.175 | −0.082 | 0.724 |
| Lives alone | −0.103 | 0.491 | 0.101 | 0.714 |
| Years since diagnosis* | 0.040 | 0.015 | −0.050 | 0.031 |
| Anxiety | −0.198 | 0.075 | −0.212 | 0.250 |
| Depression | −0.282 | 0.014 | −0.308 | 0.098 |
| Cost‐related lack of access to care | −0.302 | 0.007 | −0.395 | 0.046 |
| Fear of recurrence* | 0.048 | 0.286 | 0.025 | 0.649 |
| Self‐efficacy* | 0.288 | <0.001 | 0.303 | <0.001 |
| Perceived risk | −0.004 | 0.857 | −0.004 | 0.911 |
| Relatives | ||||
| First‐degree relationship* | 0.098 | 0.572 | 0.219 | 0.092 |
| Age | 0.001 | 0.788 | 0.001 | 0.697 |
| Black race* | 0.419 | 0.005 | 0.424 | 0.022 |
| Education | 0.082 | 0.053 | 0.057 | 0.171 |
| Income | −0.010 | 0.308 | 0.002 | 0.807 |
| Single relationship status | −0.159 | 0.175 | −0.087 | 0.340 |
| Lives alone | −0.103 | 0.491 | 0.113 | 0.408 |
| Years since diagnosis* | −0.002 | 0.883 | −0.023 | 0.224 |
| Anxiety | −0.198 | 0.075 | 0.119 | 0.265 |
| Depression | −0.282 | 0.014 | −0.025 | 0.819 |
| Cost‐related lack of access to care | −0.302 | 0.007 | −0.225 | 0.016 |
| Perceived risk | 0.004 | 0.857 | −0.026 | 0.198 |
| Partner effects† | ||||
| YBCS → relative | ||||
| Age | 0.007 | 0.202 | 0.032 | 0.017 |
| Depression | −0.168 | 0.148 | −0.369 | 0.017 |
| Self‐efficacy* | 0.067 | 0.309 | 0.116 | 0.047 |
| Relative → YBCS | ||||
| Income | 0.010 | 0.298 | 0.019 | 0.034 |
Unstandardized estimates (B) and standard errors (SE) reported.
^The dependent variable of the APIM analyses is the Family Support Index (PCA of the three scales); boldface indicates P < 0.05.
†Only significant (P < 0.05) partner effects in the triad data are reported along with the complementary nonsignificant results in the dyadic analysis; no significant (P < 0.05) partner effects were observed in dyadic data.
*Dyad‐level predictors were degree type, race, and years since diagnosis; fear of recurrence and self‐efficacy were reported only by YBCS.
3.2.2. Triadic findings
With the exception of the CFI (0.616), model fit indices were acceptable in the APIM triad analyses (nonsignificant chi‐square, P = 0.0552; X2/df ratio = 1.18; RMSEA = 0.038; SRMR = 0.057). APIM with 121 triads (YBCS and two relatives) identified significant actor and partner effects (Table 4). YBCS’ self‐efficacy was associated with higher YBCS family support. YBCS having cost‐related problems to accessing care and more years since diagnosis were associated with lower YBCS family support. Black race in relatives was associated with higher relative family support, while relatives’ cost‐related lack of access to care was associated with relatives’ lower family support. Four partner effects were identified in triads. YBCS’ prior diagnosis of depression was associated with relatives’ lower family support; YBCS’ older age and higher self‐efficacy were associated with relatives’ higher support; and relatives’ higher income was associated with YBCS’ higher support.
4. DISCUSSION
Early onset breast cancer can have a profound impact on cancer patients and their families. YBCS are a special group who have to manage both their own disease and their family roles. Their biological relatives also have to realize, accept, and manage a higher breast cancer risk. Family support is a valuable resource that can help address these challenges.
4.1. Family support in YBCS and relatives
Relatives reported higher family communication compared to YBCS, possibly due to YBCS’ unmet communication needs, especially for illness‐related issues.50, 51 White/Other participants (YBCS and relatives combined) reported higher family support at times of illness compared to Black participants. This could be partly because Black YBCS were significantly more likely to invite relatives living further than 50 miles away to participate in the study with them (data shown elsewhere),25 which could affect tangible support offered and received at times of illness. Triads reported higher family support at times of illness compared to dyads, presumably because it can be more difficult to find support from others at that time. Participating in the study with two vs one relative may indicate a stronger and larger support network.
4.2. Predictors and interdependence of family support
APIM examined both actor and partner effects, with the former being primary predictors of family support. Partner effects were observed only in triads, possibly due to greater chances of one person affecting the other person's responses.
4.2.1. Self‐efficacy
Consistent with other studies, YBCS’ breast cancer self‐efficacy was an important predictor of their own family support (actor effect).52, 53 In dyads and triads, YBCS with more confidence in their ability to manage demands associated with breast cancer reported higher family support. Self‐efficacy is a key resource for cancer survivors associated with important outcomes, such as better mental health54 and higher quality of life.55 A novel finding of our study was that YBCS’ higher self‐efficacy had a significant partner effect on relatives’ perceived family support in triads. Relatives may find it easier to help YBCS who have higher self‐efficacy and fewer needs, as it may be less burdensome. YBCS who are better able to manage disease‐related stressors by themselves may feel more self‐reliant, creating less strains and demands on their family. YBCS with lower self‐efficacy is a group at risk for adverse outcomes and warrants further assessment and early intervention. In contrast, YBCS with higher self‐efficacy could be a resource for their relatives, who may also benefit from support in managing their own anxiety about cancer risk. This reciprocal relationship merits more investigation.
4.2.2. Cost and access to care
About one in five YBCS and one in five relatives reported that there was a time during the past 12 months that they needed medical care but could not get it due to high out‐of‐pocket costs. Higher income is usually associated with availability of expendable resources to address illness‐related expenses.7, 56 Cancer may cause significant financial burdens and high out‐of‐pocket costs for YBCS; lack of support and available resources at times of need may further deter YBCS from accessing care. Relatives with higher income had a positive partner effect on YBCS’ family support in triads, possibly because they are considered an actual or potential resource to YBCS, accounting for YBCS perceiving higher support from them.
4.2.3. Depression
YBCS with a prior diagnosis of depression reported lower family support (actor effect). Depression during diagnosis and treatment worsens for some breast cancer patients, especially for those lacking a partner or other forms of support.57, 58 Relatives who reported lower family support were also more likely to report a prior diagnosis of depression (actor effect in dyadic relationships) or to be associated with an YBCS with depression (partner effect in triadic relationships). The partner effects of YBCS’ prior diagnosis of depression indicate that YBCS’ depressive symptoms may influence relatives’ perceived family support. Relatives are expected to provide support to cancer survivors, although they also experience stressors and need support59; when relatives are depressed, they may perceive receiving less support, possibly as appreciation for their efforts.60 In a prior APIM analyses with a different sample of cancer survivors and their family caregivers, we also identified significant longitudinal partner effects between cancer patients’ depression and their family caregivers.61 Helping YBCS and relatives identify and manage depression is an important intervention area. However, we acknowledge that our findings may be influenced by participants’ recall bias, since we measured anxiety and depression with single‐item questions asking participants to recall what was told to them.
4.2.4. Race
The activation of family support, which often increases with the burden of illness, can strengthen family relationships but can also strain network ties. In the face of a cancer diagnosis, family resources may be more readily available for some YBCS, or conversely not available for others, bringing up any differences in perceived family support that may have existed prediagnosis. Relatives of Black YBCS may hold strong beliefs about their familial obligations, due to strong familial and community orientations.62 Expressions of familialism and collectivism are more evident in Blacks than other racial groups, due to a traditional caregiving ideology, related to collectivism in social relationships.63 However, this finding should be interpreted with caution, since there were a smaller number of Black relatives in the study and the other group included White YBCS and a small proportion of YBCS of other ethnic/racial background.
4.2.5. Length of survivorship
Finally, being a longer‐term cancer survivor was associated with less family support reported by YBCS in triadic relationships. Since YBCS in the study were diagnosed on average 11 years prior, family members may assume that YBCS need less support over time. This may not necessarily be accurate, as some YBCS may have to cope with late effects of cancer treatment or pervasive fear of cancer recurrence.64, 65 YBCS’ age had a positive partner effect on relatives’ family support, presumably because some longer‐term cancer survivors focus less on their own needs and more on the needs of their families.66
4.3. Strengths and limitations
Limitations include self‐reporting and recruitment preferences. Relatives were invited directly from YBCS, which assumes that relatives in good relationship with the YBCS were prioritized; this may explain the small range of scores in the family support index (5.3‐6.1; possible range 1‐7). The study invited up to two relatives per YBCS for comparable family units, although some YBCS may be receiving support from larger networks. Stressors, that is anxiety and depression, were assessed with single‐item measures instead of lengthier instruments to reduce overall burden, but responses may be influenced by recall bias. YBCS of “other” racial/ethnic backgrounds were combined with White YBCS, thus findings related to race may not be generalized. Finally, we did not assess whether YBCS were receiving treatment, which may have implications for family support they needed at the time of the survey.
Future research with YBCS and relative triads may consider a conceptually meaningful way to distinguish relatives as part of the study design and in line with the study aims. For example, the study could have required YBCS to designate one relative who provides more support. However, prior work of the research team demonstrated that cancer patients have difficulty and may feel uncomfortable about making a choice and indicating who provides more support. Thus, we consider that extending the term “family” to include more than two people and attending to the challenges associated with these complex analyses are significant strengths of our study.
We examined self‐efficacy as a predictor of family support, which is not the way it has been traditionally examined in prior literature. Our findings indicate a strong correlation between family support and patients’ self‐efficacy (ie, patients’ confidence in their own ability to manage the disease). Traditionally, family support has been examined as a predictor of self‐efficacy. An alternative hypothesis suggests that the ability of people to provide support is associated with the characteristics of the person needing support. It may be more difficult to support cancer patients who have low self‐efficacy (ie, low self‐confidence) in their ability to manage the disease. It is possible that the support person may need to spend more time bolstering or encouraging the patient with low self‐efficacy, which may become burdensome over time. Further research is needed in this area. Due to the cross‐sectional nature of the data, we can only report the significant correlation between self‐efficacy and support rather than confirm causation from one variable to the other variable.
4.4. Implications
Family support enhances the long‐term physical and mental well‐being of cancer patients and their family members.6, 16, 67, 68, 69 It also provides greater cohesion and strengthens the interpersonal contacts among breast cancer patients and their relatives.67 Our findings demonstrate the interdependence of family support between YBCS and their close biological relatives. APIM provided valuable insights into complex family relationships taking also into account relatives’ increased breast cancer risk due to possible hereditary susceptibility. Consistent with our theoretical framework, self‐efficacy and access to care were important resources that influence family support. Perceived stressors associated with early onset breast cancer and availability of resources may affect the level of support family members are willing to give and receive to each other. Due to the interdependence among YBCS and their relatives, supportive programs need to focus on the YBCS‐relative as the unit of care and include family‐based interventions that enhance each person's self‐efficacy and access to high‐quality services.70, 71, 72 Existing evidence‐based interventions for cancer patients and their family caregivers72, 73, 74, 75 could be adapted to address the needs of YBCS and their relatives. It is also important for researchers and clinicians to work together and develop technology‐based dyadic interventions that are cost‐effective and accessible to a large number of families.76, 77, 78
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
Centers for Disease Control and Prevention, 5U48DP001901‐03, PI: M.C. Katapodi; Robert Wood Johnson Foundation, Nurse Faculty Scholars Award 68039, PI: M.C. Katapodi. Debra Duquette, CGC, Beth Anderson, MPH, Jenna McLosky, CGC, Cancer Genomics Program ‐ Michigan Department of Health and Human Services for YBCS and relative identification, recruitment, assessment of eligibility, and development of survey. Glenn Copeland, MBA, Director, Michigan Cancer Surveillance Program for YBCS identification and recruitment.
Katapodi MC, Ellis KR, Schmidt F, Nikolaidis C, Northouse LL. Predictors and interdependence of family support in a random sample of long‐term young breast cancer survivors and their biological relatives. Cancer Med. 2018;7:4980–4992. 10.1002/cam4.1766
REFERENCES
- 1. Eccles SA, Aboagye EO, Ali S, et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013;15(5):R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenberg S, Newman L, Partridge A. Breast cancer in young women: rare disease or public health problem? JAMA Oncol. 2015;1(7):877‐878. [DOI] [PubMed] [Google Scholar]
- 3. King M‐C, Levy‐Lahad E, Lahad A. Population‐based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312(11):1091‐1092. [DOI] [PubMed] [Google Scholar]
- 4. Arès I, Lebel S, Bielajew C. The impact of motherhood on perceived stress, illness intrusiveness and fear of cancer recurrence in young breast cancer survivors over time. Psychol Health. 2014;29(6):651‐670. [DOI] [PubMed] [Google Scholar]
- 5. Takahashi M. Psychosocial distress among young breast cancer survivors: implications for healthcare providers. Breast Cancer. 2014;21(6):664‐669. [DOI] [PubMed] [Google Scholar]
- 6. Thewes B, Bell ML, Butow P, et al. Psychological morbidity and stress but not social factors influence level of fear of cancer recurrence in young women with early breast cancer: results of a cross‐sectional study. Psychooncology. 2013;22(12):2797‐2806. [DOI] [PubMed] [Google Scholar]
- 7. Ziner KW, Sledge GW, Bell CJ, Johns S, Miller KD, Champion VL. Predicting fear of breast cancer recurrence and self‐efficacy in survivors by age at diagnosis. Oncol Nurs Forum. 2012;39(3):287‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sammarco A. Quality of life of breast cancer survivors: a comparative study of age cohorts. Cancer Nurs. 2009;32(5):347‐356; quiz 57‐8. [DOI] [PubMed] [Google Scholar]
- 9. Garner MJ, McGregor BA, Murphy KM, Koenig AL, Dolan ED, Albano D. Optimism and depression: a new look at social support as a mediator among women at risk for breast cancer. Psychooncology. 2015;24(12):1708‐1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glassey R, O'Connor M, Ives A, Saunders C, O'Sullivan S, Hardcastle SJ. Heightened perception of breast cancer risk in young women at risk of familial breast cancer. Fam Cancer. 2017;17(1):15‐22. [DOI] [PubMed] [Google Scholar]
- 11. Underhill ML, Habin KR, Shannon KM. Perceptions of cancer risk, cause, and needs in participants from low socioeconomic background at risk for hereditary cancer. Behav Med. 2017;43(4):259‐267. [DOI] [PubMed] [Google Scholar]
- 12. Underhill ML, Crotser CB. Seeking balance: decision support needs of women without cancer and a deleterious BRCA1 or BRCA2 mutation. J Genet Couns. 2014;23(3):350‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. den Heijer M, Vos J, Seynaeve C, et al. The impact of social and personal resources on psychological distress in women at risk for hereditary breast cancer. Psychooncology. 2012;21(2):153‐160. [DOI] [PubMed] [Google Scholar]
- 14. Mellon S, Berry‐Bobovski L, Gold R, Levin N, Tainsky MA. Communication and decision‐making about seeking inherited cancer risk information: findings from female survivor‐relative focus groups. Psychooncology. 2006;15(3):193‐208. [DOI] [PubMed] [Google Scholar]
- 15. Nycum G, Avard D, Knoppers BM. Factors influencing intrafamilial communication of hereditary breast and ovarian cancer genetic information. Eur J Hum Genet. 2009;17(7):872‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kvillemo P, Branstrom R. Coping with breast cancer: a meta‐analysis. PLoS ONE. 2014;9(11):e112733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baider L, Ever‐Hadani P, Goldzweig G, Wygoda MR, Peretz T. Is perceived family support a relevant variable in psychological distress?. A sample of prostate and breast cancer couples. J Psychosom Res. 2003;55(5):453‐460. [DOI] [PubMed] [Google Scholar]
- 18. Kenny D, Kashy D, Cook W. The Analysis of Dyadic Data. New York: Guilford; 2006. [Google Scholar]
- 19. Folkman S, Lazarus RS, Gruen RJ, DeLongis A. Appraisal, coping, health status, and psychological symptoms. J Pers Soc Psychol. 1986;50(3):571. [DOI] [PubMed] [Google Scholar]
- 20. Rolland JS, Williams JK. Toward a biopsychosocial model for 21st‐century genetics. Fam Process. 2005;44(1):3‐24. [DOI] [PubMed] [Google Scholar]
- 21. Katapodi MC, Northouse LL, Milliron KJ, Liu G, Merajver SD. Individual and family characteristics associated with BRCA1/2 genetic testing in high‐risk families. Psychooncology. 2013;22(6):1336‐1343. [DOI] [PubMed] [Google Scholar]
- 22. Freeman‐Gibb LA, Janz NK, Katapodi MC, Zikmund‐Fisher BJ, Northouse L. The relationship between illness representations, risk perception and fear of cancer recurrence in breast cancer survivors. Psychooncology. 2016;26(9):1270‐1277. [DOI] [PubMed] [Google Scholar]
- 23. Maly RC, Liu Y, Liang LJ, Ganz PA. Quality of life over 5 years after a breast cancer diagnosis among low‐income women: effects of race/ethnicity and patient‐physician communication. Cancer. 2015;121(6):916‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katapodi MC, Northouse LL, Schafenacker AM, et al. Using a state cancer registry to recruit young breast cancer survivors and high‐risk relatives: protocol of a randomized trial testing the efficacy of a targeted versus a tailored intervention to increase breast cancer screening. BMC Cancer. 2013;13:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katapodi MC, Duquette D, Yang JJ, et al. Recruiting families at risk for hereditary breast and ovarian cancer from a statewide cancer registry: a methodological study. Cancer Causes Control. 2017;28(3):191‐201. [DOI] [PubMed] [Google Scholar]
- 26. Lewis FM, Hammond MA, Woods NF. The family's functioning with newly diagnosed breast cancer in the mother: the development of an explanatory model. J Behav Med. 1993;16(4):351‐370. [DOI] [PubMed] [Google Scholar]
- 27. Harden JK, Sanda MG, Wei JT, et al. Partners’ long‐term appraisal of their caregiving experience, marital satisfaction, sexual satisfaction, and quality of life 2 years after prostate cancer treatment. Cancer Nurs. 2013;36(2):104‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song L, Northouse LL, Zhang L, et al. Study of dyadic communication in couples managing prostate cancer: a longitudinal perspective. Psychooncology. 2012;21(1):72‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Katapodi MC, Facione NC, Miaskowski C, Dodd MJ, Waters C. The influence of social support on breast cancer screening in a multicultural community sample. Oncol Nurs Forum. 2002;29(5):845‐852. [DOI] [PubMed] [Google Scholar]
- 30. McCubbin MA. Family Hardiness Index. Madison, Wisconsin: University of Wisconsin; 1987. [Google Scholar]
- 31. Carey PJ, Oberst MT, McCubbin MA, Hughes SH. Appraisal and caregiving burden in family members caring for patients receiving chemotherapy. Oncol Nurs Forum. 1991;18(8):1341‐1348. [PubMed] [Google Scholar]
- 32. Munkres A, Oberst M, Hughes S, editors. Appraisal of illness, symptom distress, self‐care burden, and mood states in patients receiving chemotherapy for initial and recurrent cancer. Oncol Nurs Forum; 1992. [PubMed]
- 33. Gonzalez‐Saenz de Tejada M, Bilbao A, Baré M, et al. Association between social support, functional status, and change in health‐related quality of life and changes in anxiety and depression in colorectal cancer patients. Psycho‐oncology. 2017;26(9):1263‐1269. [DOI] [PubMed] [Google Scholar]
- 34. Jeong A, Shin DW, Kim SY, Yang HK, Park JH. Avoidance of cancer communication, perceived social support, and anxiety and depression among patients with cancer. Psychooncology. 2016;25(11):1301‐1307. [DOI] [PubMed] [Google Scholar]
- 35. Hann D, Baker F, Denniston M, et al. The influence of social support on depressive symptoms in cancer patients: age and gender differences. J Psychosom Res. 2002;52(5):279‐283. [DOI] [PubMed] [Google Scholar]
- 36. Vickberg SM. The Concerns About Recurrence Scale (CARS): a systematic measure of women's fears about the possibility of breast cancer recurrence. Ann Behav Med. 2003;25(1):16‐24. [DOI] [PubMed] [Google Scholar]
- 37. Katapodi MC, Dodd MJ, Lee KA, Facione NC. Underestimation of breast cancer risk: influence on screening behavior. Oncol Nurs Forum. 2009;36(3):306‐314. [DOI] [PubMed] [Google Scholar]
- 38. Champion VL, Ziner KW, Monahan PO, et al. Development and psychometric testing of a breast cancer survivor self‐efficacy scale. Oncol Nurs Forum. 2013;40(6):E403‐E410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. CDC . Behavorial risk factor surveillance system (BRFSS) 2016. [Available from: https://www.cdc.gov/brfss/.
- 40. Anderson B, McLosky J, Wasilevich E, Lyon‐Callo S, Duquette D, Copeland G. Barriers and facilitators for utilization of genetic counseling and risk assessment services in young female breast cancer survivors. J Cancer Epidemiol. 2012;2012:298745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. IBM Corp . Released 2013. IBM SPSS Statistics for Windows, Version 22.0. 22.0 ed. Armonk, NY 2013.
- 42. M Plus Statistical Analysis with Latent Variables [Computer program] Version 7. 7 ed. Los Angeles, CA; 2012.
- 43. Cook WL, Kenny DA. The actor–partner interdependence model: a model of bidirectional effects in developmental studies. Int J Behav Dev. 2005;29(2):101‐109. [Google Scholar]
- 44. Rayens MK, Svavarsdottir EK. A new methodological approach in nursing research: an actor, partner, and interaction effect model for family outcomes. Res Nurs Health. 2003;26(5):409‐419. [DOI] [PubMed] [Google Scholar]
- 45. Kashy DA, Kenny DA. The Aanalysis of Data from Dyads and Groups. In: H.T. Reis. & C.M. Judd. (Eds). Handbook of Research Methods in Social and Personality Psychology, vol 38 New York, NY, US: Cambridge University Press; 2000:451‐477. [Google Scholar]
- 46. Olsen JA, Kenny DA. Structural equation modeling with interchangeable dyads. Psychol Methods. 2006;11(2):127. [DOI] [PubMed] [Google Scholar]
- 47. Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1‐55. [Google Scholar]
- 48. Little TD, Card NA. Longitudinal Structural Equation Modeling. New York: Guilford Press; 2013. [Google Scholar]
- 49. Peugh JL, DiLillo D, Panuzio J. Analyzing mixed‐dyadic data using structural equation models. Struct Equ Modeling. 2013;20(2):314‐337. [Google Scholar]
- 50. Jones SMW, Walker R, Fujii M, Nekhlyudov L, Rabin BA, Chubak J. Financial difficulty, worry about affording care, and benefit finding in long‐term survivors of cancer. Psychooncology. 2018;27:1320‐1326. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 51. Lambert S, Girgis A, Descallar J, Levesque JV, Jone B. Trajectories of mental and physical functioning among spouse caregivers of cancer survivors over the first five years following the diagnosis. Patient Educ Couns. 2017;100(6):1213‐1221. [DOI] [PubMed] [Google Scholar]
- 52. Ahern T, Gardner A, Courtney M. Exploring patient support by breast care nurses and geographical residence as moderators of the unmet needs and self‐efficacy of Australian women with breast cancer: results from a cross‐sectional, nationwide survey. Eur J Oncol Nurs. 2016;23:72‐80. [DOI] [PubMed] [Google Scholar]
- 53. Dockham B, Schafenacker A, Yoon H, et al. Implementation of a psychoeducational program for cancer survivors and family caregivers at a cancer support community affiliate: a pilot effectiveness study. Cancer Nurs. 2016;39(3):169‐180. [DOI] [PubMed] [Google Scholar]
- 54. Ellis KR, Janevic MR, Kershaw T, Caldwell CH, Janz NK, Northouse L. The influence of dyadic symptom distress on threat appraisals and self‐efficacy in advanced cancer and caregiving. Support Care Cancer. 2017;25(1):185‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Northouse LL, Mood D, Kershaw T, et al. Quality of life of women with recurrent breast cancer and their family members. J Clin Oncol. 2002;20(19):4050‐4064. [DOI] [PubMed] [Google Scholar]
- 56. Rottmann N, Dalton SO, Christensen J, Frederiksen K, Johansen C. Self‐efficacy, adjustment style and well‐being in breast cancer patients: a longitudinal study. Qual Life Res. 2010;19(6):827‐836. [DOI] [PubMed] [Google Scholar]
- 57. Borstelmann NA, Rosenberg SM, Ruddy KJ, et al. Partner support and anxiety in young women with breast cancer. Psychooncology. 2015;24(12):1679‐1685. [DOI] [PubMed] [Google Scholar]
- 58. Deckx L, van Abbema DL, van den Akker M, et al. A cohort study on the evolution of psychosocial problems in older patients with breast or colorectal cancer: comparison with younger cancer patients and older primary care patients without cancer. BMC Geriatr. 2015;15:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Ryn M, Sanders S, Kahn K, et al. Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue? Psychooncology. 2011;20(1):44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Litzelman K, Kent EE, Mollica M, Rowland JH. How does caregiver well‐being relate to perceived quality of care in patients with cancer? Exploring associations and pathways. J Clin Oncol. 2016;34(29):3554‐3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kershaw T, Ellis KR, Yoon H, Schafenacker A, Katapodi M, Northouse L. The interdependence of advanced cancer patients’ and their family caregivers’ mental health, physical health, and self‐efficacy over time. Ann Behav Med. 2015;49(6):901‐911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Knight BG, Sayegh P. Cultural values and caregiving: the updated sociocultural stress and coping model. J Gerontol B Psychol Sci Soc Sci. 2010;65B(1):5‐13. [DOI] [PubMed] [Google Scholar]
- 63. Pinquart M, Sorensen S. Ethnic differences in stressors, resources, and psychological outcomes of family caregiving: a meta‐analysis. Gerontologist. 2005;45(1):90‐106. [DOI] [PubMed] [Google Scholar]
- 64. Dumalaon‐Canaria JA, Prichard I, Hutchinson AD, Wilson C. Fear of cancer recurrence and psychological well‐being in women with breast cancer: the role of causal cancer attributions and optimism. Eur J Cancer Care (Engl). 2018;27:e12579. [DOI] [PubMed] [Google Scholar]
- 65. Jones SM, Ziebell R, Walker R, et al. Association of worry about cancer to benefit finding and functioning in long‐term cancer survivors. Support Care Cancer. 2017;25(5):1417‐1422. [DOI] [PubMed] [Google Scholar]
- 66. Lebel S, Beattie S, Ares I, Bielajew C. Young and worried: age and fear of recurrence in breast cancer survivors. Health Psychol. 2013;32(6):695‐705. [DOI] [PubMed] [Google Scholar]
- 67. Casellas‐Grau A, Vives J, Font A, Ochoa C. Positive psychological functioning in breast cancer: an integrative review. Breast. 2016;27:136‐168. [DOI] [PubMed] [Google Scholar]
- 68. Coyne E, Wollin J, Creedy DK. Exploration of the family's role and strengths after a young woman is diagnosed with breast cancer: views of women and their families. Eur J Oncol Nurs. 2012;16(2):124‐130. [DOI] [PubMed] [Google Scholar]
- 69. Snyder KA, Pearse W. Crisis, social support, and the family response: exploring the narratives of young breast cancer survivors. J Psychosoc Oncol. 2010;28(4):413‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Badr H, Carmack CL, Kashy DA, Cristofanilli M, Revenson TA. Dyadic coping in metastatic breast cancer. Health Psychol. 2010;29(2):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brandão T, Schulz MS, Matos PM. Psychological intervention with couples coping with breast cancer: a systematic review. Psychol Health. 2014;29(5):491‐516. [DOI] [PubMed] [Google Scholar]
- 72. Northouse L, Kershaw T, Mood D, Schafenacker A. Effects of a family intervention on the quality of life of women with recurrent breast cancer and their family caregivers. Psychooncology. 2005;14(6):478‐491. [DOI] [PubMed] [Google Scholar]
- 73. Keefe FJ, Ahles TA, Sutton L, et al. Partner‐guided cancer pain management at the end of life: a preliminary study. J Pain Symptom Manage. 2005;29(3):263‐272. [DOI] [PubMed] [Google Scholar]
- 74. Manne S, Babb J, Pinover W, Horwitz E, Ebbert J. Psychoeducational group intervention for wives of men with prostate cancer. Psychooncology. 2004;13:37‐46. [DOI] [PubMed] [Google Scholar]
- 75. Northouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer. 2007;110(12):2809‐2818. [DOI] [PubMed] [Google Scholar]
- 76. Katapodi MC, Jung M, Schafenacker AM, et al. Development of a Web‐based family intervention for BRCA carriers and their biological relatives: acceptability, feasibility, and usability study. JMIR Cancer. 2018;4(1):e7 10.2196/cancer.9210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Northouse L, Schafenacker A, Barr K, et al. A tailored web‐based psycho‐educational intervention for cancer patients and family caregivers. Cancer Nursing Int J Cancer Care. 2014;37(5):321‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Strecher V. Internet methods for delivering behavioral and health‐related interventions (eHealth). Annu Rev Clin Psychol. 2007;3:53‐76. [DOI] [PubMed] [Google Scholar]


