Abstract
The present study aimed to assess the clinical impact of BCR‐ABL1 transcript levels determined at an earlier time point than the 3‐month early molecular response (EMR) in chronic‐phase chronic myeloid leukemia (CML‐CP) patients. BCR‐ABL1 transcript levels of CML‐CP patients (n = 258; median age, 43 [range, 18‐81] years) treated with various tyrosine kinase inhibitors (TKIs) were determined at 4 weeks (28 ± 3 days) and at every 3 months of treatment initiation. At 4 weeks, receiver operating characteristic curves revealed that cutoff values of BCR‐ABL1 transcripts for achieving major molecular responses (MMRs) by 12 and 60 months were 40.89% and 39.16%, respectively (95% CI, 0.658‐0.772 and 95% CI, 0.643‐0.758; P < 0.0001). With 40% of BCR‐ABL1 transcripts at 4 weeks (very early MR; VEMR), patients with VEMR achieved higher 3‐month EMR and 4‐week VEMR significantly associated with higher cumulative incidences of 5‐year MMR (89.1% vs 72.3%; P < 0.001) and 5‐year deep molecular response (DMR) (56.5% vs 29.4%; P = 0.001). Furthermore, event‐free survival (EFS)‐a (93.0% vs 84.8%; P = 0.068) and EFS‐b (71.1% vs 57.9%; P = 0.061) by 5 years were also marginally significant. VEMR and 3‐month EMR were achieved in 89 patients, with significantly superior outcomes. In multivariate analyses, lower leukocyte count (P = 0.008) and frontline second‐generation TKI therapy size (P < 0.001) were significantly associated with VEMR achievement, but not baseline BCR‐ABL1 level and CML duration. In conclusion, the 4‐week BCR‐ABL1 transcript levels including VEMR could be important to predict long‐term outcomes and may provide additional information about innate intrinsic sensitivity to CML among individuals.
Keywords: chronic myeloid leukemia, early molecular response, molecular response, predictor, tyrosine kinase inhibitor
1. INTRODUCTION
Delayed cytogenetic and molecular responses are associated with increased tumor progression and poor outcome in imatinib‐treated patients with newly diagnosed chronic‐phase chronic myeloid leukemia (CML‐CP).1, 2, 3 Early molecular response (EMR; BCR‐ABL1 ≤10% at 3 months, ≤1% at 6 months) predicts long‐term response and survival.4, 5, 6 Therefore, EMR achievement at an early time point of treatment has been reported as an important parameter for further treatment and monitoring.7, 8, 9
Hughes et al4 reported that patients achieving EMR at 6 months had superior event‐free survival (EFS) and decreased tumor progression. Moreover, numerous studies including those on second‐generation (2G) tyrosine kinase inhibitors (TKIs) revealed that patients achieving EMR had significantly lower overall survival (OS), progression‐free survival (PFS), complete cytogenetic response (CCyR), major molecular response (MMR), and/or complete molecular response (CMR).5, 10, 11, 12, 13, 14 The prognostic information from 3‐month vs 6‐month EMR achievement has been analyzed, and earlier achievement of EMR is reported to have superior prognostic value.6, 15, 16 In addition, as various factors such as baseline risk score, spleen size, baseline leukocyte count, TKI dose intensity, and blood TKI levels can be associated with EMR achievement, more cautious analyses are warranted.13, 17
To investigate prognostic significance using molecular data at earlier time points, recent studies highlighted that 3‐month EMR depends on the baseline leukemia burden and a decline in BCR‐ABL1 transcripts.10, 18, 19 Hanfstein et al10 found that absolute BCR‐ABL1 transcript level at diagnosis was not predictive, and an individual reduction to the half‐log of the baseline level (0.35‐fold; 0.46 log) at 3 months significantly discriminated for 5‐year OS and PFS. Moreover, the rate of BCR‐ABL1 decline as assessed by halving time was a critical predictor for very poor outcomes among non‐EMR patients at 3 months.18 Additional studies have reported that the halving time was an important predictor for the achievement of molecular responses and was shorter in patients administered frontline 2G TKIs.19, 20, 21 Recently, El Missiry et al22 found that a fold change in BCR‐ABL1 transcript after 1 month could distinguish poor responders, suggesting a possibility of earlier assessment.
In addition, White et al23 reported a 36% probability of achieving a 2‐log reduction in low in vitro baseline inhibitory concentration 50% for imatinib (IC50 ≤0.6 μmol/L) by 3 months, suggesting the importance of intrinsic sensitivity to TKIs. Theoretically, BCR‐ABL1 transcript levels at baseline and at specific time points within 3 months can precisely reflect the in vivo intrinsic sensitivity irrespective of external contributing factors.
Therefore, the present study aimed to identify the prognostic significance of an earlier molecular cutoff and kinetics of BCR‐ABL1 transcript levels.
2. MATERIALS AND METHODS
2.1. Patients
The study objectives were (a) to identify the optimal cutoff of BCR‐ABL1 transcript levels at 4 weeks (very early molecular response; VEMR), (b) to identify predictive factors for the achievement of VEMR, (c) to evaluate the prognostic significance of VEMR, and (d) to evaluate the clinical implication of initial change in BCR‐ABL1 transcript level within 3 months. In total, 258 patients with CML‐CP, treated with various frontline TKIs (130 treated with imatinib; 128, 2G TKIs) and for whom molecular data were available at 4 weeks (28 ± 3 days), were included in this study. This study included patients from a clinical trial (n = 150) and routine clinical practice (n = 108). To evaluate the optimal VEMR cutoff of BCR‐ABL1 transcript level and clinical implication of VEMR, data from all 258 patients were prospectively collected. Among them, 183 patients diagnosed at our center had available baseline BCR‐ABL1 data through retrospective review of clinical records. To evaluate early molecular dynamics and the associated clinical implication, data from 183 patients were analyzed for 4‐week fold change, followed by 4‐week and 3‐month halving time analyses in 156 and 146 patients with no increasing BCR‐ABL1 transcript level, respectively. The number of patients tested at each time point is presented in Table S1. Patients having minor and atypical BCR‐ABL1 transcript levels were excluded. Informed consent was obtained from the patients in accordance with the tenets of the Declaration of Helsinki, and all human samples were obtained from the Korea Leukemia Bank, with approval from the institutional review board of the participating institutes.
2.2. Cytogenetic and molecular monitoring
Routine cytogenetic analyses were performed using the standard G‐banding method in bone marrow (BM) aspirates, and all cytogenetic responses were estimated on the basis of analyses of more than 20 metaphase cells in a single institution. Cytogenetic responses were monitored at 3‐month intervals until a CCyR was achieved. Molecular responses were monitored using quantitative real‐time polymerase chain reaction (qRT‐PCR) analysis at 3‐month intervals and then at 6‐month intervals after MMRs were achieved. All qRT‐PCR analyses were performed with at least 4.5‐log sensitivity in the central laboratory, and only those qRT‐PCR results with ABL1 copy numbers greater than 50 000 were analyzed. MMR was defined as a BCR‐ABL1 transcript level ≤0.1%IS. Deep molecular response (DMR) was defined as a reduction in BCR‐ABL1 transcript levels to 0.0032% or lower on the international scale.
2.3. Statistical analysis
OS was measured from the first day of initiation of TKI treatment to any death regardless of cause, and PFS was measured considering progression to the accelerated phase (AP) or blast crisis (BC) as well as death from any cause. OS and PFS were also determined for patients treated with other TKIs after the first‐line TKI administration was discontinued. EFS was divided into (a) EFS‐a, calculated from TKI initiation until any death, progression, treatment failure, and treatment warning (in accordance with the 2013 ELN criteria), and (b) EFS‐b, including EFS‐a, treatment failure with/without frontline TKI discontinuation (except treatment‐free remission), whichever was observed first. Survival curves for OS, PFS, and EFS were plotted using the Kaplan–Meier method and were compared using the log‐rank test. Receiver operating characteristic (ROC) analyses were performed to identify optimal cutoff values of BCR‐ABL1 transcript at 4 weeks for predicting landmark responses and survival. Potential predictive factors for achieving VEMR were assessed using logistic regression analysis and included age, sex, transcript type, Sokal risk scores, leukocyte count, platelet count, blast %, basophil %, spleen size, hydroxyurea use, type of TKI, CML duration, transcript type, and baseline BCR‐ABL1 level as a continuous variable. Covariates with a P‐value of less than 0.1 in the univariate analyses were added to the multivariate analysis model. Time to events was compared using the log‐rank test with SPSS software (SPSS, Inc.). ROC analyses of fold change and halving time were also performed.
3. RESULTS
3.1. Patients
Of 258 CML‐CP patients, 130 (50.4%) received imatinib and 128 received 2G TKIs (80 dasatinib, 33 nilotinib, 13 radotinib, and 2 bosutinib). The median age was 43 years (range, 18‐81 years), and 50.8% (n = 131) were female. All patients had major BCR‐ABL1 transcripts (101 e13a2, 156 e14a2, and 1 e13a2 + e14a2), and the median baseline BCR‐ABL1 transcript level in 183 patients was 74.6% (range, 4.63‐601.5). Other baseline characteristics including Sokal, Hasford, EUTOS, and ELTS scores are shown in Table 1. Median follow‐up duration was 24 months (range, 1‐182 months), and five patients died (three due to disease progression and two due to non‐CML causes). An additional patient progressed to AP and survived.
Table 1.
Patient characteristics
| Parameters | Total (n = 258) |
|---|---|
| Age, y | |
| Median (range) | 43 (18‐81) |
| Sex, number (%) | |
| Male/female | 153 (59.3)/105 (40.7) |
| Leukocyte count (× 109/L) (NA = 16) | |
| Median (range) | 95.9 (2.82‐532.8) |
| Platelet (× 109/L) (NA = 6) | |
| Median (range) | 465 (82‐3660) |
| Blasts (%) (NA = 10) | |
| Median (range) | 1 (0‐14) |
| Basophils (%) (NA = 13) | |
| Median (range) | 5 (0‐18) |
| Spleen size, cm (NA = 7) | |
| Median (range) | 3.5 (0‐20) |
| Sokal risk, number (%) | |
| Low | 88 (34.1) |
| Intermediate | 109 (42.2) |
| High | 59 (22.9) |
| Unknown | 2 (0.8) |
| Hasford, number (%) | |
| Low | 104 (40.3) |
| Intermediate | 107 (41.5) |
| High | 29 (11.2) |
| Unknown | 18 (7.0) |
| EUTOS, number (%) | |
| Low | 193 (74.8) |
| High | 45 (17.4) |
| Unknown | 20 (7.8) |
| ELTS, number (%) | |
| Low | 173 (67.0) |
| Intermediate | 66 (25.6) |
| High | 17 (6.6) |
| Unknown | 2 (0.8) |
| Transcript type, number (%) | |
| e13a2 | 101 (39.1) |
| e14a2 | 156 (60.5) |
| e13a2 + e14a2 | 1 (0.4) |
| Baseline BCR‐ABL1 IS (%) in 183 patients | |
| Median (range) | 74.6 (4.63‐601.5) |
| Frontline therapy, number (%) | |
| Imatinib | 130 (50.4) |
| Nilotinib | 33 (12.8) |
| Dasatinib | 80 (31.0) |
| Bosutinib | 2 (0.8) |
| Radotinib | 13 (5.0) |
| From Dx to TKI treatment in 258 patients, mo | |
| Median (range) | 0.6 (0‐6) |
| Follow‐up duration, mo | |
| Median (range) | 24 (1‐182) |
| Outcome | |
| Alive/death | 253/5a |
| Progression | 4b |
Three disease progression and two non‐CML death.
Three BC (death) and one AP (alive).
3.2. Optimal cutoffs for BCR‐ABL1 transcript levels at 4 weeks
ROC analysis to identify the optimal cutoff with 4‐week transcript levels allowed us to classify patients as low risk and high risk, with maximal sensitivity and specificity for outcomes, except OS and PFS at specific time points (Table 2). At 4 weeks, patients with transcript levels <41.69% and <40.89% had significantly higher CCyR and MMR rates at 1 year, respectively. The optimal cutoffs for 1‐year MMR achievement were 42.57% and 38.41% in patients treated with frontline imatinib and 2G TKIs, respectively (Tables S2 and S3).
Table 2.
Relative risk for CCyR, MMR, DMR, and survivals according to the BCR‐ABL1 transcript level at 4 wk
| Outcome | Cutoff (%) | No. of patients at risk | RR for transcript level (log) | |
|---|---|---|---|---|
| RR (95% CI) | P‐value | |||
| BCR‐ABL1 transcript level at 1 mo | ||||
| CCyR_1yr | ||||
| Low risk | ≤41.69 | 131 | 1 | |
| High risk | >41.69 | 127 | 0.58 (0.44‐0.76) | <0.0001 |
| MMR_1yr | ||||
| Low risk | ≤40.89 | 124 | 1 | |
| High risk | >40.89 | 134 | 0.26 (0.17‐0.40) | <0.0001 |
| MMR_5yr | ||||
| Low risk | ≤39.16 | 120 | 1 | |
| High risk | >39.16 | 138 | 0.36 (0.25‐0.50) | <0.0001 |
| DMR_1yr | ||||
| Low risk | ≤30.45 | 84 | 1 | |
| High risk | >30.45 | 174 | 0.12 (0.04‐0.36) | <0.0001 |
| DMR_5yr | ||||
| Low risk | ≤33.50 | 95 | 1 | |
| High risk | >33.50 | 163 | 0.29 (0.16‐0.55) | <0.0001 |
| OS_5yr | ||||
| Low risk | ≤26.58 | 59 | 1 | |
| High risk | >26.58 | 199 | 0.21 (0.03‐1.23) | 0.083 |
| PFS_5yr | ||||
| Low risk | ≤26.58 | 59 | 1 | |
| High risk | >26.58 | 199 | 0.31 (0.06‐1.53) | 0.151 |
| EFS_5yr‐aa | ||||
| Low risk | ≤42.75 | 136 | 1 | |
| High risk | >42.75 | 122 | 2.77 (1.20‐6.38) | 0.017 |
| EFS_5yr‐bb | ||||
| Low risk | ≤42.75 | 136 | 1 | |
| High risk | >42.75 | 122 | 2.17 (1.30‐3.62) | 0.003 |
DMR, deep molecular response; EFS, event‐free survival; MMR, molecular response; OS, overall survival; PFS, progression‐free survival; RR, relative risk; yr, year.
EFS‐a: PFS + ELN treatment failure + ELN warning.
EFS‐b: PFS + ELN treatment failure + ELN warning + frontline TKI discontinuation (except treatment free remission).
Furthermore, other cutoffs significantly predicted 5‐year MMR (P < 0.0001), 1‐year DMR (P < 0.0001), 5‐year DMR (P < 0.0001), 5‐year EFS‐a (P = 0.017), and 5‐year EFS‐b (P = 0.003). Interestingly, the optimal cutoffs for confidence intervals of 1‐year CCyR (41.69%), 1‐year MMR (40.89%), 5‐year MMR (39.16%), and 5‐year EFS‐a/‐b (42.75%) were similar. However, the cutoffs for CIs of 1‐year DMR (30.45%), 5‐year DMR (33.50%), 5‐year OS (26.58%), and 5‐year PFS (26.58%) were lower.
3.3. Rate of VEMR and early molecular responses
Based on the results of ROC analysis, we established a universal cutoff of 40% as VEMR and classified the patients as low risk (BCR‐ABL1 ≤40%) or high risk (BCR‐ABL1 >40%) based on VEMR achievement at 4 weeks. The proportion of the 258 patients achieving VEMR was 47% (121 patients), and VEMR rates between imatinib and 2G TKIs were similar at 45% (58 patients) and 49% (63 patients), respectively (Figure 1).
Figure 1.
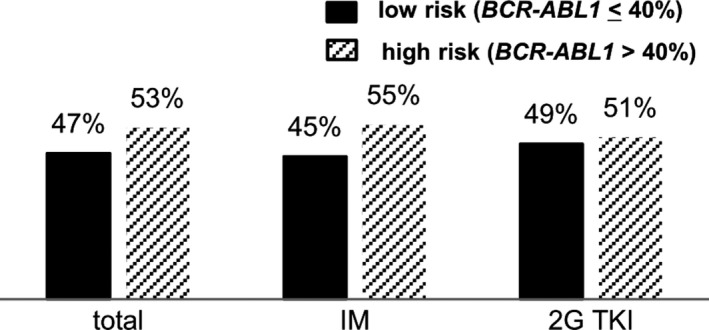
The proportion of low‐risk (BCR‐ABL1 ≤40%) and high‐risk (BCR‐ABL1 >40%) patients according to VEMR achievement at 4 wk
Results of molecular analyses were available for 213 patients at 3 months, and 83% (176 patients) achieved EMR. EMR was achieved in 74% of imatinib‐treated patients and 91% of 2G TKI‐treated patients. At 6 months, molecular analyses were conducted for 185 patients, and EMR was achieved in 71% (131) patients. The EMR rates were 59% and 83% for imatinib‐ and 2G TKI‐treated patients, respectively (Figure 2A‐C).
Figure 2.
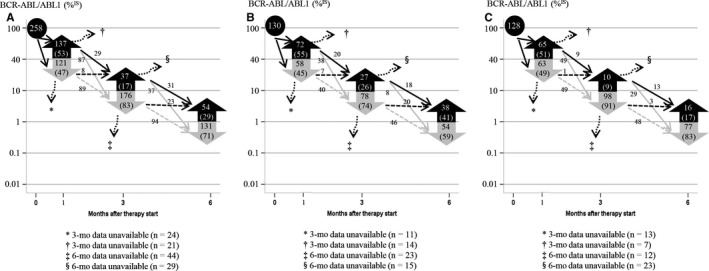
Subsequent change in molecular response. A, total patients; B, imatinib‐treated patients; C, 2G TKI‐treated patients; N (%)
3.4. Subsequent change in molecular response by VEMR
Among 121 VEMR patients at 4 weeks, results of molecular analyses were available for 97 patients at 3 months and 89 (92%) patients who achieved EMR. Results of molecular analyses were available for 116 of 137 non‐VEMR patients at 3 months, and 75% (87 patients) achieved 3‐month EMR (92% vs 75%; P < 0.0001). Of 130 imatinib‐treated patients, results of qRT‐PCR analysis for 3‐month EMR assessment were available for 47 of 58 VEMR patients, and 40 patients (85%) achieved 3‐month EMR. Data were available for 58 of 72 non‐VEMR patients, and 38 patients (66%) achieved 3‐month EMR (85% vs 66%; P = 0.026). Of 128 2G TKI‐treated patients, results of molecular analysis for 3‐month EMR assessment were available for 50 of 63 VEMR patients, and 49 patients (98%) achieved 3‐month EMR. Results of molecular analysis were available for 58 of 65 non‐VEMR patients, and 49 patients (84%) achieved 3‐month EMR (Figure 2A‐C) (98% vs 84%; P = 0.041). Of 121 VEMR patients, 73 (60.3%) achieved better 12‐month MMR than 21.9% (P < 0.001) of 137 non‐VEMR patients (Figure S1). The VEMR cutoff also significantly predicted 5‐year MMR (P < 0.001) and 5‐year DMR (P = 0.001), and it marginally predicted 5‐year EFS‐a (P = 0.068) and 5‐year EFS‐b (P = 0.061), but not OS and PFS owing to a small number of events and switching to an alternative TKI (Figure 3).
Figure 3.
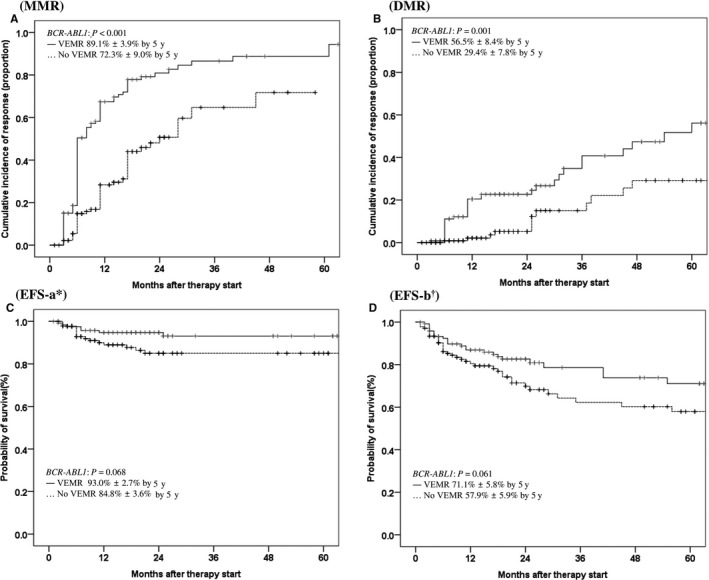
Cumulative incidences of MMR, DMR, and EFS according to VEMR (≤40% and >40%) achievement at 4 wk. VEMR, very early molecular response; MMR, molecular response; DMR, deep molecular response; EFS, event‐free survival; y, year. *EFS‐a: PFS + ELN treatment failure + ELN warning. † EFS‐b: PFS + ELN treatment failure + ELN warning + frontline TKI discontinuation (except treatment‐free remission)
3.5. Outcomes based on 4‐week VEMR and 3‐month EMR
To evaluate the combined clinical significance of BCR‐ABL1 transcript levels at 4 weeks and 3 months, 213 patients, for whom data for molecular analysis were available at both time points, were classified on the basis of VEMR at 4 weeks (≤ or >40% of BCR‐ABL1 transcript) and EMR at 3 months (≤ or >10% of BCR‐ABL1 transcript; Table 3). VEMR + EMR were achieved in 89 patients (group 1); these patients had MMR by 1 year (62.9%) and 5 years (91.7%), and a 5‐year DMR of 62.1%. Five‐year EFS‐a and EFS‐b were achieved in 92.3% and 73.6%, respectively. Twenty‐nine patients had no optimal MRs on both occasions (group 4); these patients had significantly poorer outcomes in terms of 1‐year MMR (6.9%), 5‐year MMR (74.1%), 5‐year DMR (20.0%), 5‐year EFS‐a (70.0%), and EFS‐b (48.2%) than those in group 1. However, there were no differences in 5‐year OS and PFS. Eight patients had VEMR but no EMR (group 2), and 87 patients had EMR but no VEMR (group 3). These patients with discordant BCR‐ABL1 transcript levels between 4 weeks and 3 months (groups 2 and 3) displayed significant differences in 1‐year CCyR (93.3% vs 77.9%, P = 0.006), 1‐year MMR (62.9% vs 23.2%, P < 0.0001), 5‐year MMR (91.7% vs 64.8%, P < 0.0001), 5‐year EFS‐a (92.3% vs 84.0%, P = 0.013), and 5‐year EFS‐b (73.6% vs 56.7%, P = 0.016), but no differences in OS (98.8% vs 97.5%, P = 0.603) and PFS (98.8% vs 96.9%, P = 0.389) compared with those in group 1.
Table 3.
Distribution of patients and outcomes combining BCR‐ABL1 transcript levels at 4‐wk and 3‐mo
| Group | VEMR | 3 mos EMR | Number of patients | CCyR by 1 y | MMR by 1 y | CI of MMR by 5 y | CI of DMR by 5 y | OS by 5 y | PFS by 5 y | EFS‐aa by 5 y | EFS‐bb by 5 y |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Yes | Yes | 89 | 83/89 (93.3%) | 56/89 (62.9%) | 91.7% ± 4.1 | 62.1% ± 10.1 | 98.8% ± 1.2 | 98.8% ± 1.2 | 92.3% ± 4.5 | 73.6% ± 7.1 |
| Group 2 | No | 8 | 74/95 (77.9%) | 22/95 (23.2%) | 64.8% ± 9.7 | 33.8% ± 10.0 | 97.5% ± 1.7 | 96.9% ± 1.8 | 84.0% ± 4.1 | 56.7% ± 7.3 | |
| Group 3 | No | Yes | 87 | ||||||||
| Group 4 | No | 29 | 14/29 (48.3%) | 2/29 (6.9%) | 74.1% ± 21.5 | 20.0% ± 17.9 | 100% | 100% | 70.0% ± 11.8 | 48.2% ± 12.8 | |
| Log‐rank P | <0.0001 | <0.0001 | <0.0001 | 0.006 | 0.723 | 0.512 | 0.005 | 0.007 | |||
| Log‐rank P between subgroups | Group 1 vs 2 and 3 | 0.006 | 0.001 | <0.0001 | 0.063 | 0.603 | 0.389 | 0.013 | 0.016 | ||
| Group 1 vs 4 | <0.0001 | <0.0001 | <0.0001 | 0.015 | 0.992 | 0.991 | 0.005 | 0.005 | |||
| Group 2 and 3 vs 4 | 0.002 | 0.121 | 0.169 | 0.342 | 0.971 | 0.967 | 0.385 | 0.285 |
2G TKI, second‐generation tyrosine kinase inhibition; CI, confidence interval; DMR, deep molecular response; EFS, event‐free survival; MMR, molecular response; OS, overall survival; PFS, progression‐free survival.
EFS‐a: PFS + ELN treatment failure + ELN warning.
EFS‐b: PFS + ELN treatment failure + ELN warning + frontline TKI discontinuation (except treatment free remission).
3.6. Predictive factors for VEMR and long‐term clinical significance of early molecular dynamics
In univariate analyses of factors affecting VEMR achievement, a P‐value of less than 0.1 presented in sex, leukocyte count, platelet count, percentage of blast, percentage of basophil, spleen size, hydroxyurea use, frontline TKI therapy, CML duration, and baseline BCR‐ABL1 transcript. After adjusting for potential predictive factors, multivariate analyses revealed that low leukocyte count (P = 0.008) and frontline 2G TKI therapy (P < 0.001) but not baseline BCR‐ABL1 transcript level (P = 0.305) were significantly associated with VEMR achievement (Table S4). However, hydroxyurea use and CML duration were not significantly associated with VEMR achievement and long‐term outcome (Table S5).
As the BCR‐ABL1 transcript level change within 3 months is a key component in prediction of long‐term outcome, we performed further analyses using baseline transcript level (n = 183), 4‐week transcript level (n = 258), VEMR (n = 258), fold change (n = 183), 4‐week halving time (n = 156), 3‐month halving time (n = 146), and 3‐month EMR (n = 213) in our cohorts. Interestingly, the 4‐week transcript level (≤41.57%), VEMR (≤40%), and 3‐month EMR (≤10%) significantly predicted 12‐month MMR, 5‐year MMR, and 5‐year DMR. The halving time assessed with data from baseline to 3 months more significantly predicted MMR and survival (OS, P = 0.031; PFS, P = 0.035). However, baseline transcript level, 4‐week fold change, and 4‐week halving time were not predictive for long‐term outcome owing to the direct influence of the baseline BCR‐ABL1 value calculated with the ABL1 control gene (Table 4). When the consistency of the clinical significance of the 4‐week BCR‐ABL1 transcript level in imatinib and 2G TKI cohorts was investigated, as expected, 4‐week BCR‐ABL1 value significantly predicted MMR in both cohorts, with higher significance in the 2G TKI cohort (Table S6).
Table 4.
Outcomes according to various BCR‐ABL1 values between baseline and 3 mo
| Parameters | Value | MMR by 12 mo | P‐value | MMR by 5 y | P‐value | DMR by 5 y | P‐value | OS by 5 y | P‐value | PFS by 5 y | P‐value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline BCR‐ABL1 | Continuous variable (n = 183) | 1.00 (0.99‐1.00) | 0.326 | 1.00 (1.00‐1.00) | 0.555 | 1.00 (0.99‐1.00) | 0.394 | 1.00 (0.99‐1.01) | 0.922 | 1.00 (0.99‐1.01) | 0.923 |
| 4‐wk BCR‐ABL1 | Continuous variable (n = 258) | 0.98 (0.97‐0.99) | <0.001 | 0.98 (0.97‐0.99) | <0.001 | 0.97 (0.95‐0.98) | <0.001 | 0.99 (0.95‐1.03) | 0.596 | 0.99 (0.97‐1.03) | 0.901 |
| 4‐wk BCR‐ABL1, median | ≤41.57% (n = 129) | 65.7% ± 4.5 | <0.001 | 88.6% ± 3.9 | <0.001 | 54.7% ± 8.0 | 0.002 | 97.5% ± 1.4 | 0.679 | 97.6% ± 1.4 | 0.996 |
| >41.57% (n = 129) | 29.1% ± 4.8 | 71.4% ± 9.3 | 28.7% ± 8.1 | 97.1% ± 2.2 | 96.3% ± 2.3 | ||||||
| VEMR | ≤40% (n = 121) | 67.9% ± 4.6 | <0.001 | 89.1% ± 3.9 | <0.001 | 56.5% ± 8.4 | 0.001 | 97.3% ± 1.5 | 0.581 | 97.4% ± 1.5 | 0.873 |
| >40% (n = 137) | 29.0% ± 4.6 | 72.3% ± 9.0 | 29.4% ± 7.8 | 97.2% ± 2.1 | 96.5% ± 2.2 | ||||||
| 3‐mo EMR | ≤10% (n = 176) | 52.5% ± 4.2 | <0.001 | 85.2% ± 5.1 | <0.001 | 50.6% ± 7.5 | 0.041 | 98.8% ± 0.9 | 0.478 | 98.2% ± 1.0 | 0.692 |
| >10% (n = 37) | 8.5% ± 5.9 | 47.6% ± 18.8 | 16.7% ± 15.2 | 96.8% ± 3.2 | 97.1% ± 2.8 | ||||||
| 4‐wk FC | FC≤1 (n = 156) | 47.3% ± 4.3 | 0.899 | 82.7% ± 5.1 | 0.485 | 43.9% ± 7.3 | 0.433 | 97.9% ± 1.2 | 0.464 | 98.0% ± 1.1 | 0.466 |
| FC>1 (n = 27) | 44.5% ± 10.1 | 63.0% ± 13.4 | 33.4% ± 15.7 | 100% | 100% | ||||||
| 4‐wk HTa | ≤22 d (n = 53) | 39.8% ± 7.4 | 0.209 | 79.5% ± 8.8 | 0.292 | 38.6% ± 13.4 | 0.450 | 95.8% ± 2.9 | 0.231 | 96.1% ± 2.7 | 0.214 |
| >22 d (n = 103) | 50.8% ± 5.3 | 83.7% ± 6.1 | 46.1% ± 8.7 | 99.0% ± 1.0 | 99.0% ± 1.0 | ||||||
| 3‐mo HTb | ≤21 d (n = 101) | 52.4% ± 5.4 | 0.015 | 82.1% ± 6.9 | 0.039 | 46.2% ± 8.2 | 0.097 | 100% | 0.031 | 100% | 0.035 |
| >21 d (n = 45) | 35.4% ± 7.7 | 62.1% ± 9.5 | 37.7% ± 18.2 | 94.9% ± 3.5 | 95.4% ± 3.2 |
DMR, deep molecular response; FC, fold change; HT, halving time; MMR, molecular response; OS, overall survival; PFS, progression‐free survival; VEMR, very early molecular response.
Twenty‐seven patients with increasing transcript level were excluded in this analysis.
Additional 10 patients with increasing transcript level during this period were excluded in this analysis.
4. DISCUSSION
In the TKI era, early molecular response was first reported to predict short‐term outcomes such as MCyR with BCR‐ABL1 transcript levels reducing to 20% and 50% of baseline within 2 months and 4 weeks of imatinib initiation, respectively.24, 25
Thereafter, several studies involving frontline TKIs for CML‐CP patients reported that EMRs at 3 and 6 months strongly predict long‐term responses and survival.4, 15, 26
Three‐month EMR was previously predicted to be achieved in 50%‐71% of imatinib‐treated new CP patients and in 75%‐91% of frontline 2G TKI‐treated patients. In addition, 6‐month EMR (BCR‐ABL1 transcript level ≤1%) was predicted to be achieved in 49%‐58% of imatinib‐treated patients and in 69%‐82% of 2G TKI‐treated patients.14, 27, 28
In the present study, of 213 patients eligible for 3‐month molecular analysis, 83% (176 patients) achieved EMR at 3 months and 71% (results of molecular analysis available for 131 of 185 patients) achieved EMR at 6 months. Among 130 frontline imatinib‐treated patients, the EMR rates at 3 and 6 months were 74% and 59%, respectively. Of 128 patients administered frontline 2G TKIs, 91% and 83% achieved EMR at 3 and 6 months, respectively. In this study, EMR rates were comparable with those of DASISION, ENESTnd, RERISE, and BFORE studies,13, 14, 27, 28 indicating the use of homogeneous approaches in terms of molecular analysis and study population. Interestingly, the optimal cutoffs for higher CCyR, MMR, and EFS were similar (approximately 40%), which was albeit as a VEMR. However, DMR, OS, and PFS were different (26.58%‐33.50%) owing to rare events. In addition, the optimal cutoff for DMR prediction could be determined in only the 2G TKI‐treated patients (Tables S2 and S3). Hence, early assessment of BCR‐ABL1 transcript levels has a better predictive power for deeper response with more potent TKI.
With VEMR defined as 40% of BCR‐ABL1 transcript levels, the proportion of patients achieving VEMR was 47% of all 258 patients. VEMR predicted subsequent 3‐month EMR, and the 3‐month EMR rate was higher in 2G TKI‐treated patients (98% vs 85%). Of patients who failed to achieve VEMR, 84% and 66% achieved 3‐month EMR among those administered 2G TKI and imatinib, respectively. Our results suggest that more potent TKI can maintain optimal molecular response and may better rescue patients with VEMR failure. Regardless of the variety of first‐line TKI used, VEMR significantly predicted the cumulative MMR and DMR, and EFS was marginally predicted.
In our previous study, patients who achieved EMR both at 3 and 6 months had better outcomes than those achieving no EMR on both occasions. Moreover, patients with discordant BCR‐ABL1 transcript levels between 3 and 6 months also showed poor outcomes.17 Therefore, we analyzed the clinical significance of combining 4‐week VEMR and 3‐month EMR. In our study, 89 patients who achieved VEMR and 3‐month EMR showed a significant predictive power for landmark responses and survival such as CCyR, MMR, DMR, and EFS. Patients who showed discordant BCR‐ABL1 transcript levels between 4 weeks and 3 months (groups 2 and 3) displayed similar poor responses and outcomes compared with those who failed to display optimal responses at two occasions of 4 weeks and 3 months (group 4). This finding suggests that the additional molecular analysis at early time points may improve the predictive accuracy and may be a powerful predictor of subsequent long‐term outcomes.
Hanfstein et al10 and Branford et al29 reported that an initial decline in BCR‐ABL1 transcript levels was a critical predictor for poor outcomes. In addition, leukocyte count, blast count, risk score, spleen size, lactate dehydrogenase levels, achievement of CHR, TKI dose intensity, and blood level of TKI were significantly associated with 3‐month EMR achievement.13, 17, 30 Therefore, the factors influencing VEMR achievement need to be further evaluated. In the present study, low leukocyte count and frontline 2G TKI therapy were significantly associated with VEMR achievement.
Recently, El Missiry et al22 suggested that an initial decline in BCR‐ABL1 transcript level determined using the GUS control gene after 1 month (as a fold change) may distinguish early responders based on disease biology. However, analysis of our cohort without interruption or dose reduction of TKI treatment during the first 4 weeks did not reveal any associations between 4‐week fold change, halving time, and further molecular responses, suggesting that assessment of earlier molecular dynamics with baseline BCR‐ABL1 transcript level as assessed by the ABL1 control gene is considerably limited. In contrast, the 4‐week transcript level (as a continuous variable and with median value of 41.57%), VEMR (≤40%), and 3‐month EMR (≤10%) significantly predicted further molecular responses including 5‐year MMR and DMR, with higher significance in the 2G TKI cohort. Moreover, the halving time to 3 months significantly predicted outcome. Consistent with our findings, Branford et al18 found that the 3‐month halving time may provide a significant predictive power to distinguish responders. As the initial dosage of TKIs was maintained for the majority of our patients by the first qRT‐PCR assay, the 4‐week BCR‐ABL1 transcript levels of individual patients may reflect the true cellular sensitivity to TKI. In addition, identifying very‐low‐risk patients, considering baseline biological factors, may be critical for affordable treatment and further treatment‐free remission trials.
This study involved subgroup analyses of a heterogeneous population, suggesting a limitation in interpretation.
However, this study supports the possibility of earlier assessment using the 4‐week transcript levels including VEMR. To confirm the further clinical benefit of VEMR and an early switch of therapy, a randomized prospective trial in a larger homogeneous population with longer follow‐up is warranted.
In conclusion, we have determined the clinical significance of the 4‐week transcript levels including VEMR and the predictive factors associated with its achievement, which were associated with intrinsic in vivo sensitivity to TKI. Based on our results, as a powerful predictor of molecular landmark responses including DMR, a change in BCR‐ABL1 transcript within 4 weeks and VEMR assessment might be useful to identify patients potentially eligible for treatment‐free remission.
CONFLICT OF INTEREST
None declared.
Supporting information
Song H‐Y, Noh H, Choi SY, et al. BCR‐ABL1 transcript levels at 4 weeks have prognostic significance for time‐specific responses and for predicting survival in chronic‐phase chronic myeloid leukemia patients treated with various tyrosine kinase inhibitors. Cancer Med. 2018;7:5107–5117. 10.1002/cam4.1753
REFERENCES
- 1. O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low‐dose cytarabine for newly diagnosed chronic‐phase chronic myeloid leukemia. N Engl J Med. 2005;348:994‐1004. [DOI] [PubMed] [Google Scholar]
- 2. Cortes J, Talpaz M, O'Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425‐3432. [DOI] [PubMed] [Google Scholar]
- 3. Quintás‐Cardama A, Kantarjian H, Jones D, et al. Delayed achievement of cytogenetic and molecular response is associated with increased risk of progression among patients with chronic myeloid leukemia in early chronic phase receiving high‐dose or standard‐dose imatinib therapy. Blood. 2009;113:6315‐6321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hughes TP, Hochhaus A, Branford S, et al. Long‐term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood. 2010;116:3758‐3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR‐ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanfstein B, Müller MC, Hehlmann R, et al. Early molecular and cytogenetic response is predictive for long‐term progression‐free and overall survival in chronic myeloid leukemia (CML). Leukemia. 2012;26:2096‐2102. [DOI] [PubMed] [Google Scholar]
- 7. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872‐884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pallera A, Altman JK, Berman E, et al. NCCN guidelines insights: chronic myeloid leukemia, Version 1.2017. J Natl Compr Cancer Netw. 2016;14:1505‐1512. [DOI] [PubMed] [Google Scholar]
- 9. Klil‐Drori AJ, Yin H, Azoulay L, et al. Early switch to second‐line tyrosine kinase inhibitor in chronic myeloid leukemia patients failing to achieve early molecular response. Am J Hematol. 2017;92:E602‐E604. [DOI] [PubMed] [Google Scholar]
- 10. Hanfstein B, Shlyakhto V, Lauseker M, et al. Velocity of early BCR‐ABL transcript elimination as an optimized predictor of outcome in chronic myeloid leukemia (CML) patients in chronic phase on treatment with imatinib. Leukemia. 2014;28:1988‐1992. [DOI] [PubMed] [Google Scholar]
- 11. Marin D, Hedgley C, Clark RE, et al. Predictive value of early molecular response in patients with chronic myeloid leukemia treated with first‐line dasatinib. Blood. 2012;120:291‐294. [DOI] [PubMed] [Google Scholar]
- 12. Jain P, Kantarjian H, Nazha A, et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: results with four tyrosine kinase inhibitor modalities. Blood. 2013;121:4867‐4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hughes TP, Saglio G, Kantarjian HM, et al. Early molecular response predicts outcomes in patients with chronic myeloid leukemia in chronic phase treated with frontline nilotinib or imatinib. Blood. 2014;123:1353‐1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kwak JY, Kim SH, Oh SJ, et al. Phase III clinical trial (RERISE study) results of efficacy and safety of radotinib compared with imatinib in newly diagnosed chronic phase chronic myeloid leukemia. Clin Cancer Res. 2017;23:7180‐7188. [DOI] [PubMed] [Google Scholar]
- 15. Neelakantan P, Gerrard G, Lucas C, et al. Combining BCR‐ABL1 transcript levels at 3 and 6 months in chronic myeloid leukemia: implications for early intervention strategies. Blood. 2013;121:2739‐2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee SE, Choi SY, Oh YJ, et al. Distinct predictive factors influence on achievement of early molecular response by frontline imatinib in chronic phase chronic myeloid leukemia. J Leuk Res. 2015;39:411‐418. [DOI] [PubMed] [Google Scholar]
- 17. Lee SE, Choi SY, Kim SH, et al. Baseline BCR‐ABL1 transcript type of e13a2 and large spleen size are predictors of poor long‐term outcomes in chronic phase chronic myeloid leukemia patients who failed to achieve an early molecular response after 3 months of imatinib therapy. Leuk Lymphoma. 2018;59:105‐113. [DOI] [PubMed] [Google Scholar]
- 18. Branford S, Yeung DT, Parker WT, et al. Prognosis for patients with CML and >10% BCR‐ABL1 after 3 months of imatinib depends on the rate of BCR‐ABL1 decline. Blood. 2014;124:511‐518. [DOI] [PubMed] [Google Scholar]
- 19. Iriyama N, Fujisawa S, Yoshida C, et al. Shorter halving time of BCR‐ABL1 transcripts is a novel predictor for achievement of molecular responses in newly diagnosed chronic‐phase chronic myeloid leukemia treated with dasatinib: results of the D‐first study of Kanto CML study group. Am J Hematol. 2015;90:282‐287. [DOI] [PubMed] [Google Scholar]
- 20. Huet S, Cony‐Makhoul P, Heiblig M, et al. Major molecular response achievement in CML patients can be predicted by BCR‐ABL1/ABL1 or BCR‐ABL1/GUS ratio at an earlier time point of follow‐up than currently recommended. PLoS ONE. 2014;9:e106250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takaku T, Iriyama N, Mitsumori T, et al. Clinical efficacy and safety of first‐line dasatinib therapy and the relevance of velocity of BCR‐ABL1 transcript decline for achievement of molecular responses in newly diagnosed chronic‐phase chronic myeloid leukemia: report from the Juntendo Yamanashi Cooperative Study Group. Oncology. 2018;94:85‐91. [DOI] [PubMed] [Google Scholar]
- 22. El Missiry M, Hjorth‐Hansen H, Richter J, et al. Early BCR‐ABL1 transcript decline after 1 month of tyrosine kinase inhibitor therapy as an indicator for treatment response in chronic myeloid leukemia. PLoS ONE. 2017;12:e0171041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. White D, Saunders V, Lyons AB, et al. In vitro sensitivity to imatinib‐induced inhibition of ABL kinase activity is predictive of molecular response in patients with de novo CML. Blood. 2005;106:2520‐2526. [DOI] [PubMed] [Google Scholar]
- 24. Merx K, Müller MC, Kreil S, et al. Early reduction of BCR‐ABL mRNA transcript levels predicts cytogenetic response in chronic phase CML patients treated with imatinib after failure of interferon alpha. Leukemia. 2002;16:1579‐1583. [DOI] [PubMed] [Google Scholar]
- 25. Wang L, Pearson K, Ferguson JE, Clark RE. Early molecular response to imatinib predicts cytogenetic and clinical outcome in chronic myeloid leukaemia. Br J Haematol. 2003;120:990‐999. [DOI] [PubMed] [Google Scholar]
- 26. Lee SE, Choi SY, Kim SH, et al. BCR‐ABL1 transcripts (MR4.5) at post‐transplant 3 months as an early predictor for long‐term outcomes in chronic myeloid leukemia. Korean J Intern Med. 2015;31:126‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3‐year follow‐up from a randomized phase 3 trial (DASISION). Blood. 2014;123:494‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cortes JE, Gambacorti‐Passerini C, Deinninger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Branford S, Yeung DT, Ross DM, et al. The adverse effect of high Sokal risk for first line imatinib treated patients is overcome by a rapid rate of BCR‐ABL decline measured as early as 1 month of treatment. Blood. 2014;124:816. [Google Scholar]
- 30. Chikkodi SV, Malhotra P, Naseem S, et al. Factors affecting early molecular response in chronic myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2015;15:114‐119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


