Abstract
Background
Nonresected, nonmetastatic (NR‐M0) pancreatic cancer involves both locally advanced pancreatic cancer and patients who did not undergo resection due to poor health status or patient preference. This study investigates nationwide trends of characteristics, treatment, and survival of patients with NR‐M0 pancreatic cancer.
Methods
From the Netherlands Cancer Registry, all patients diagnosed with pancreatic cancer between 2006 and 2014 were selected. Chemotherapy and overall survival (OS) of NR‐M0 patients were evaluated for 3‐year time periods and 2 age groups using chi‐square tests for trend and Cox proportional hazard regression analysis.
Results
Of 18 234 patients, 33% had NR‐M0 pancreatic cancer, which decreased over time (in consecutive 3‐year periods: 38%‐33%‐28%, P < 0.001). Of 5964 NR‐M0 patients, 52% was over 75 years of age, 16% received chemotherapy, and median OS was 5.1 months. Chemotherapy use increased over time in younger patients (<75 years: from 23 to 36%, P‐trend < 0.001, ≥75 years: 3% to 4%, P‐trend = 0.053). In multivariable survival analysis, elderly age, low SES, nonconfirmed cancer, stage II‐III disease, and earlier years of diagnosis were independently associated with a worse OS. Age of patients who received chemotherapy increased over time (median 62‐66 years) and median OS was 10.4 months without significant differences between time periods (P = 0.177) or age groups (P = 0.207).
Conclusions
Overall survival of NR‐M0 pancreatic cancer remains poor which is partly related to advanced age of many patients. Despite an increase, chemotherapy is infrequently used. Future research should investigate to what extent the more widespread use of chemotherapy could improve survival in relation to age‐related morbidity.
Keywords: chemotherapy, nonresected, pancreatic adenocarcinoma, survival
1. INTRODUCTION
Pancreatic cancer remains one of the most lethal cancers with a 5‐year survival rate of 5‐7%.1, 2 Since symptoms usually emerge late, about 50%‐60% of patients are diagnosed with metastatic disease.3, 4 Only 10%‐20% of patients have resectable disease. The intermediate group of 30%‐40% generally is referred to as locally advanced pancreatic cancer (LAPC).5, 6 Nonresected patients in cancer registries have metastatic or unresectable disease diagnosed at imaging or at time of surgical exploration or are ineligible for surgery due to a poor health status or patient preference. As a result of increased resection rates for pancreatic cancer,7, 8 characteristics of the patient group with nonresected, nonmetastatic (NR‐M0) disease may have changed.
For patients with pancreatic cancer not undergoing resection, chemotherapy is the main treatment modality. In patients with metastatic disease, population‐based studies have shown that the administration of palliative chemotherapy steeply increased in the past decades.3, 9, 10 Notably, this increased use of chemotherapy was found in the gemcitabine era, and thus, before the studies on FOLFIRINOX (5‐fluorouracil, leucovorin, irinotecan, and oxaliplatin) and nab‐paclitaxel plus gemcitabine reported favorable results compared with gemcitabine alone.11, 12 No randomized controlled trials have yet been published on these chemotherapy schemes in patients with LAPC. Despite a lack of randomized studies, an increased use of chemotherapy may also be found in patients with NR‐M0 disease.
Population‐based data on treatment and survival of patients with LAPC or NR‐M0 disease are scarce.13 In addition, little is known about survival of elderly patients with NR‐M0 disease, with or without chemotherapy.
Therefore, the aim of this nationwide study was to investigate time trends in characteristics, treatment, and survival of patients with NR‐M0 pancreatic cancer.
2. METHODS
2.1. Data collection
The Netherlands Cancer Registry (NCR) records data on all patients with newly diagnosed cancer in the Netherlands, a country with 17 million inhabitants. Since 1989, newly diagnosed malignancies are notified to the NCR by the automated pathological archive (PALGA), supplemented with data from the National Registry of Hospital Discharge Diagnoses. Completeness is estimated to be at least 95%. Trained registrars in all Dutch hospitals routinely extract data on patient, tumor, and treatment characteristics. Tumor location and histology are registered according to the International Classification of Diseases for Oncology (ICD‐O‐3).14 The tumour‐node‐metastasis (TNM) staging classification is used (6th edition in 2003‐2009,15 7th edition in 2010‐201616) for pathologically confirmed malignancies, while in other cases, a 1‐digit extend of disease (EoD) is recorded. From 2012 onwards, TNM was recorded for all patients. Actual vital status (dead or alive, date of death or emigration) is obtained by periodically linking the NCR to the Municipal Personal Records Database which keeps record on the vital status of all Dutch inhabitants.
2.2. Patients
From the NCR, all patients were selected who were diagnosed with pancreatic (ductal) adenocarcinoma between 2006 and 2014 (ICD‐O‐3 C25, morphology codes 8010, 8012, 8020, 8140,8141, 8260, 8310, 8440, 8480, 8481, 8490, 8500, 8560, or a nonconfirmed supposed adenocarcinoma). Patients diagnosed at autopsy, younger than 18 years or residing abroad, were excluded. The total population was divided into three groups: resected, nonresected nonmetastatic (NR‐M0), and metastatic pancreatic cancers. Since this division was based on findings of imaging and surgical exploration, a number of patients with nonresected disease underwent a laparotomy or laparoscopy (11% of NR‐M0 patients in 2012‐2014). The intermediate group of NR‐M0 patients was the focus of the present study.
The study period was evenly divided into three 3‐year periods: 2006‐2008, 2009‐2011, and 2012‐2014. Patients were divided into two age groups: younger patients <75 years and elderly patients ≥75 years at diagnosis. Comorbidity was recorded regionwide in 2 out of 9 Dutch cancer regions (16% of all patients) according to a slightly modified version of the Charlson classification. Serious comorbid conditions included chronic obstructive pulmonary diseases, cardiovascular diseases, cerebrovascular diseases, digestive tract diseases, diabetes mellitus, and other serious diseases. The number of comorbidities was categorized into three groups (0, 1, and ≥2). In addition, due to the nature of the NCR, information on previous malignancies was available in all patients. Furthermore, socioeconomic status (SES)17 was based on reference data from The Netherlands Institute for Social Research. Social deprivation scores were derived from data on income, education, and occupation per 4‐digit postal code and were broken into three SES categories (high: 1st‐3rd, intermediate: 4th‐7th, and low: 8th‐10th deciles). Both types of information on tumor stage (TNM and EoD) were combined into one summary stage: (a) “localized”: tumor confined to the pancreas (TNM I); (b) “nonlocalized”: “tumor extension into adjacent organs or tissues and/or into regional lymph nodes” (TNM II‐III); (c) “metastatic”: distant metastasis (TNM IV); and (d) unknown stage. In the period 2012‐2014, a distinction between stage II (T3/N1M0) and stage III (T4M0) could be made. Registered treatments comprise tumor resection, chemotherapy, and/or local treatment such as radiotherapy applied for stage at diagnosis. No information was available about type of chemotherapy treatment. Survival time was calculated from the date of diagnosis to the date of death or emigration. Patients who were alive on 1 February 2017 were censored (1.6%). To investigate early mortality after diagnosis, 30‐ and 90‐day mortality of any cause after date of diagnosis were calculated.
2.3. Statistical analysis
Chi‐square tests for trend were used to analyze characteristics and treatment of the NR‐M0 patients in consecutive 3‐year periods. A two‐sided P‐value <0.05 was considered statistically significant. To evaluate overall survival of NR‐M0 patients, Kaplan‐Meier analyses and log‐rank tests were used, as well as univariable and multivariable Cox proportional hazard analyses. In multivariable models, a backward stepwise selection was used with a P > 0.10 for removal of variables in likelihood ratio tests. Characteristics that were included (if applicable) were time periods, age, sex, history of cancer, SES, pathological confirmation of cancer, tumor location, summary tumor stage, chemotherapy, and local treatment. Sensitivity analyses were performed using regionwide data to investigate associations of the number and type of comorbid conditions (adjusted for predictors derived from the multivariable model in all patients). STATA/SE (version 14.0; STATA Corp., College Station, TX, USA) was used in all analyses.
3. RESULTS
3.1. All patients
Median age of 18 234 patients diagnosed with pancreatic cancer in 2006‐2014 was 71 years and 37% was 75 years or older. Pathology confirmation of pancreatic cancer occurred less frequently in patients with nonmetastatic disease (62% vs 69% in metastatic disease, P < 0.001). Metastatic disease was present in 9934 (54%) of patients, and 2336 (13%) of patients underwent tumor resection. The remaining 5964 (33%) patients had nonresected, nonmetastatic (NR‐M0) pancreatic cancer. Compared with patients with resected and metastatic cancer, patients with NR‐M0 pancreatic cancer were older (median 75 years vs 67 and 69 years, respectively) and had an intermediate overall survival (median 5.1 months [95% confidence interval: 4.9‐5.2 months] vs 17.5 and 2.3 months, respectively).
Both tumor resection and diagnosis of metastatic disease increased over time. As a result, NR‐M0 pancreatic cancer decreased from 2052 (38%) patients in 2006‐2008 to 2026 (33%) in 2009‐2011 and 1886 (28%) in 2012‐2014 (P‐trend < 0.001). This time trend was found within younger and elderly age groups alike, as was shown in Figure 1.
Figure 1.
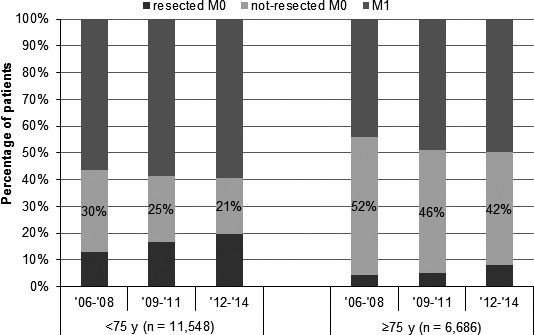
Distribution of resected, nonresected nonmetastatic (NR‐M0), and metastatic (M1) pancreatic cancer, by periods within younger and elderly age categories (both P < 0.001)
3.2. Patients with NR‐M0 pancreatic cancer
Overall, 52% of 5964 patients with NR‐M0 pancreatic cancer was aged 75 years and older, with a significant increase over time (50%, 52%, and 54%, P‐trend = 0.008; Table 1). Pathological confirmation of cancer occurred in 47% of NR‐M0 patients and increased over time in younger patients only. Arterial involvement was found in 39% of NR‐M0 patients (TNM stage III, 2012‐2014).
Table 1.
Characteristics of patients with nonresected, nonmetastatic (NR‐M0) pancreatic carcinoma, by time periods within younger and elderly age groups
| All patients | Patients younger than 75 y | Patients 75 y and older | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | 2006‐2008 | 2009‐2011 | 2012‐2014 | Chi‐square P‐trend | All | 2006‐2008 | 2009‐2011 | 2012‐2014 | Chi‐square P‐trend | All | 2006‐2008 | 2009‐2011 | 2012‐2014 | Chi‐square P‐trend | |
| N | 5964 (%) | 2052% | 2026% | 1886% | 2872 (%) | 1035% | 965% | 872% | 3092 (%) | 1017% | 1061% | 1014% | |||
| Pathological confirmation | |||||||||||||||
| Confirmed | 2790 (47) | 45 | 46 | 49 | 0.022 | 1874 (65) | 63 | 63 | 71 | <0.001 | 916 (30) | 28 | 30 | 30 | 0.208 |
| Not confirmed | 3174 (53) | 55 | 54 | 51 | 998 (35) | 37 | 37 | 29 | 2176 (70) | 72 | 70 | 70 | |||
| Primary tumor | |||||||||||||||
| Head of pancreas | 4499 (75) | 77 | 76 | 74 | 0.098 | 2057 (72) | 73 | 73 | 68 | 0.014 | 2442 (79) | 80 | 79 | 79 | 0.860 |
| Body or tail | 784 (13) | 12 | 12 | 16 | 464 (16) | 15 | 15 | 18 | 320 (10) | 9.1 | 8.9 | 13 | |||
| Overlapping/NOS | 681 (11) | 11 | 12 | 11 | 351 (12) | 11 | 12 | 14 | 330 (11) | 11 | 12 | 8.3 | |||
| Summary stage | |||||||||||||||
| Localized | 1467 (25) | 26 | 26 | 22 | 0.595 | 352 (12) | 15 | 12 | 9.5 | 0.260 | 1115 (36) | 37 | 39 | 32 | 0.631 |
| Nonlocalized | 3789 (64) | 59 | 64 | 67 | 2287 (80) | 75 | 80 | 84 | 1502 (49) | 43 | 49 | 53 | |||
| Unknown | 708 (12) | 15 | 10 | 11 | 233 (8) | 9.8 | 7.8 | 6.5 | 475 (15) | 20 | 12 | 15 | |||
| TNM stage I‐II‐X | 61 | 43 | 77 | ||||||||||||
| TNM stage III | 39 | 57 | 23 | ||||||||||||
| Chemotherapy (%yes) | 967 (16) | 13 | 17 | 19 | <0.001 | 858 (30) | 23 | 31.5 | 36 | <0.001 | 109 (3.5) | 2.8 | 3.5 | 4.3 | 0.053 |
| Local therapy (%yes)a | 324 (5.4) | 5.5 | 4.2 | 6.7 | 0.121 | 288 (10) | 10 | 7.8 | 13 | 0.074 | 36 (1.2) | 1.0 | 0.9 | 1.6 | 0.212 |
| Deceased within 30 d of diagnosis (%yes) | 739 (12) | 12 | 12 | 13 | 0.275 | 191 (6.7) | 6.5 | 6.3 | 7.2 | 0.529 | 548 (18) | 17 | 18 | 18 | 0.704 |
| Deceased within 90 d of diagnosis (%yes) | 1978 (33) | 33.2 | 32.8 | 33.51 | 0.838 | 697 (24) | 25 | 24 | 24 | 0.938 | 1281 (41) | 42 | 41 | 41 | 0.761 |
| Median OS (95% CI) in mo | 5.1 (4.9‐5.2) | 4.9 (4.6‐5.2) | 5.1 (4.8‐5.5) | 5.1 (4.8‐5.5) | 0.088 b | 6.3 (6.0‐6.6) | 5.9 (5.5‐6.4) | 6.7 (6.0‐7.1) | 6.4 (6.1‐6.7) | 0.052 b | 3.9 (3.7‐4.1) | 3.8 (3.5‐4.2) | 3.9 (3.6‐4.4) | 4.0 (3.6‐4.3) | 0.322 b |
CI, confidence interval; NOS, not otherwise specified; OS, overall survival.
For example, conventional radiotherapy, SBRT, RFA, and IRE.
Log‐rank test.
Only 16% (967/5964) of patients with NR‐M0 disease received chemotherapy, with an increase in consecutive 3‐year periods from 13%, 17%, to 19% (P‐trend < 0.001), particularly in younger patients (<75 years: from 23% to 36%, P‐trend < 0.001, Table 1). Of patients over 75 years, only 3.5% were treated with chemotherapy (2.8% to 4.3%, P‐trend = 0.053). In addition, 5.4% of patients received local therapy such as radiotherapy or (sporadic) ablative treatments (in consecutive periods: 5.5%‐4.2%‐6.7%, respectively, P‐trend = 0.121).
At time of 90‐days after diagnosis, 33% (1978/5964) of patients had died, particularly elderly patients (<75 years: 24%, ≥75 years: 41%, P < 0.001). No time trends were found in early mortality (Table 1). One‐ and 2‐year overall survival (OS) of patients with NR‐M0 pancreatic cancer were 18% and 5%, respectively (data not shown). In consecutive 3‐year periods, median OS was 4.9, 5.1, and 5.1 months, respectively (P = 0.088, Table 1). For patients aged <75 years and ≥75 years, median OS was 6.3 and 3.9 months, respectively (P < 0.001). In the younger age group, a very small improvement of OS was found in the study period (from 5.9 to 6.4 months, P = 0.052; ≥75 years: 3.8 to 4.0 months, P = 0.322). Furthermore, median OS was 10.4 months in patients who received chemotherapy vs 4.2 months in untreated patients (P < 0.001). In the multivariable Cox proportional hazard model, elderly age, low SES, nonconfirmed cancer, nonlocalized disease, and diagnosis in earlier years of the study period were independently associated with a worse OS (Table 2). In a second model including treatment, the increased use of chemotherapy could not completely remove differences between time periods. Among cases with available comorbidity data, only the presence of pulmonary disease was additionally associated with a worse OS (n = 864, HR = 1.29, 95% CI 1.06‐1.59).
Table 2.
Univariable and multivariable Cox proportional hazards analyses predicting overall survival of patients with nonresected, nonmetastatic (NR‐M0) pancreatic cancer
| N | MS (mo) | Univariable analysis | Multivariable analysis | Multivariable analysis including treatment | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95%CI) | P‐value | HR (95%CI) | P‐value | |||
| Overall | 5964 | 5.1 | ||||||
| Period of diagnosis | ||||||||
| 2005‐2008 | 2052 | 4.9 | Ref | 0.090 | Ref | Ref | ||
| 2009‐2011 | 2026 | 5.1 | 0.93 (088‐0.99) | 0.93 (0.87‐0.99) | 0.020 | 0.94 (0.88‐1.00) | 0.043 | |
| 2012‐2014 | 1886 | 5.1 | 0.97 (0.89‐1.02) | 0.94 (0.88‐1.00) | 0.067 | 0.99 (0.93‐1.05) | 0.664 | |
| Age (y) | ||||||||
| <75 | 2870 | 6.3 | Ref | <0.001 | Ref | Ref | ||
| ≥75 | 3092 | 3.9 | 1.36 (1.29‐1.43) | 1.39 (1.31‐1.47) | <0.001 | 1.21 (1.14‐1.28) | <0.001 | |
| Sex | ||||||||
| Male | 2745 | 5.1 | Ref | 0.249 | ||||
| Female | 3219 | 5.1 | 1.03 (0.98‐1.08) | |||||
| History of cancer | ||||||||
| No | 4956 | 5.1 | Ref | 0.008 | ||||
| Yes | 1008 | 4.5 | 1.10 (1.03‐1.18) | |||||
| Socioeconomic status | ||||||||
| High | 1718 | 5.4 | Ref | 0.001 | Ref | Ref | ||
| Intermediate | 2405 | 4.9 | 1.03 (0.96‐1.09) | 1.03 (0.96‐1.09) | 0.416 | 1.01 (0.95‐1.08) | 0.762 | |
| Low | 1841 | 4.7 | 1.12 (1.05‐1.20) | 1.12 (1.05‐1.20) | 0.001 | 1.11 (1.04‐1.19) | 0.002 | |
| Pathological confirmation | ||||||||
| Confirmed | 2790 | 6.0 | Ref | <0.001 | Ref | Ref | ||
| Not confirmed | 3174 | 4.1 | 1.18 (1.12‐1.24) | 1.09 (1.03‐1.16) | 0.004 | 0.99 (0.94‐1.05) | 0.827 | |
| Primary tumor location | ||||||||
| Head of pancreas | 4499 | 5.1 | Ref | 0.108 | ||||
| Body or tail | 784 | 5.2 | 0.93 (0.86‐1.00) | |||||
| Overlapping/NOS | 681 | 4.7 | 1.02 (0.94‐1.10) | |||||
| Summary stage | ||||||||
| Localized | 1467 | 4.9 | Ref | <0.001 | Ref | Ref | ||
| Nonlocalized | 3789 | 5.5 | 1.04 (0.98‐1.11) | 1.27 (1.18‐1.36) | <0.001 | 1.34 (1.25‐1.44) | <0.001 | |
| Unknown | 708 | 3.5 | 1.25 (1.14.1.37) | 1.31 (1.19‐1.43) | <0.001 | 1.27 (1.16‐1.39) | <0.001 | |
| Chemotherapy | ||||||||
| No | 4997 | 4.2 | Ref | <0.001 | X | Ref | ||
| Yes | 967 | 10.4 | 0.53 (0.49‐0.56) | 0.56 (0.52‐0.61) | <0.001 | |||
| Local therapy | ||||||||
| No | 5640 | 4.7 | Ref | <0.001 | X | Ref | ||
| Yes | 324 | 11.3 | 0.55 (0.49‐0.62) | 0.77 (0.68‐0.87) | <0.001 | |||
MS, median survival.
3.3. Patients with NR‐M0 disease who received chemotherapy
Median age of 967 patients with NR‐M0 pancreatic cancer who received chemotherapy was 64 years (range 34‐85 years) and increased in consecutive 3‐year periods (median age 62, 63, 66 years, respectively, P = 0.007). Of these treated patients, as many as 17% did not undergo pathological confirmation of cancer (Table 3), which decreased over time (19%, 20%, 12% of treated patients in consecutive time periods, P = 0.015). Most patients receiving chemotherapy had locally advanced disease (stage II‐III: 87%, 91%, and 94% in consecutive 3‐year periods, P = 0.013; stage III: 67% of 357 treated patients diagnosed in 2012‐2014). One‐ and 2‐year survival were 41% and 11%, respectively. Median OS of treated patients was 10.5, 9.6, and 10.8 months in consecutive time periods (P = 0.177; Table 3) and did not differ significantly between age groups (<75 years: 10.6 and ≥75 years: 9.2 months, P = 0.207; data not shown).
Table 3.
Characteristics of patients with nonresected, nonmetastatic (NR‐M0) pancreatic carcinoma receiving chemo(radio)therapy, by time periods
| All patients | 2006‐2008 | 2009‐2011 | 2012‐2014 | Chi‐square | |
|---|---|---|---|---|---|
| N = 967% | N = 269% | N = 341% | N = 357% | P‐trend | |
| Median age (range) | 64 (34‐85) | 62 (34‐83) | 64 (36‐84) | 66 (38‐85) | 0.007 |
| Pathological confirmation | |||||
| Confirmed | 807 (83) | 81 | 80 | 88 | 0.015 |
| Not confirmed | 160 (17) | 19 | 20 | 12 | |
| Primary tumor | |||||
| Head of pancreas | 645 (67) | 68 | 68 | 64 | 0.102 |
| Body or tail | 206 (21) | 22 | 17 | 24 | |
| Overlapping/NOS | 116 (12) | 9.7 | 14 | 11 | |
| Summary stage | |||||
| Localized | 62 (6.4) | 9.7 | 5.3 | 5.0 | 0.013 |
| Nonlocalized | 878 (91) | 87 | 91 | 94 | |
| Unknown | 27 (2.8) | 3.7 | 3.8 | 1.1 | |
| TNM stage I‐II‐X | 117 (33) | 33 | |||
| TNM stage III | 240 (67) | 67 | |||
| Local therapy (%yes)a | 247 (26) | 36 | 19 | 24 | <0.001 |
| Deceased within 90 days of start chemotherapy (%yes) | 101 (14) | 11 | 16 | 13 | 0.342 |
| Median OS (95%CI) in mo | 10.4 (9.9‐10.9) | 10.5 (9.4‐11.8) | 9.6 (8.7‐10.7) | 10.8 (10.2‐11.5) | 0.177b |
CI, confidence interval; NOS, not otherwise specified; OS, overall survival.
For example, conventional radiotherapy, SBRT, RFA, and IRE.
Log‐rank test.
4. DISCUSSION
One‐third of patients with pancreatic cancer in the Netherlands (2006‐2014) had nonresected, nonmetastatic (NR‐M0) disease. At least half of these nearly 6.000 NR‐M0 patients were over 75 years of age and two‐fifth of patients had stage III disease. The median overall survival of NR‐M0 pancreatic cancer was 5.1 months. Only 16% of NR‐M0 patients received chemotherapy with a median survival of 10.4 months. In the course of our study, a 50% increase in chemotherapy use was found within the younger age group (<75 years), though without significant improvement of survival.
In the past decade, the resection rate for pancreatic cancer in the Netherlands has increased,7, 8 whereas detection of metastatic disease also increased (stage migration).3 Consequently, the proportion of patients in the remaining group with NR‐M0 pancreatic cancer decreased until less than one‐third in 2012‐2014, while age of patients with NR‐M0 disease increased. In addition, only 40% of the NR‐M0 patient group in 2014‐2016 had stage III disease. Particularly in the remaining 60% of NR‐M0 pancreatic cancer patient stage I‐II, elderly patients were overrepresented (≥75 years: 68%). Several retrospective studies suggested underutilization of surgical treatment in elderly patients with localized pancreatic cancer.18, 19, 20 However, many NR‐M0 patients die soon after diagnosis; in our study, 41% of patients over 75 years died within 90 days. Though in a previous study comorbidity of (elderly) patients was not associated with the application of pancreatic surgery,18 a poor general health status at time of diagnosis may have precluded surgical treatment. Accurate identification of a poor health status of patients is of utmost importance for optimal treatment decision making.21
Most patients not eligible for pancreatic surgery due to a poor performance status are also not candidates for chemotherapy. In the current study period in the Netherlands, the administration of chemotherapy to NR‐M0 patients was very limited (16%), which can largely be attributed to the high number of elderly patients with early‐stage disease (77% of stage I‐II and 43% of stage III patients were aged ≥75 years). In addition, a restraint of medical oncologists to give chemotherapy to elderly patients and patient preferences could have added to limited chemotherapy use. Also in the subgroup of patients with stage III disease, chemotherapy use in our study (34% in 2012‐2014) was limited compared with population‐based studies in the United States (>50%).13, 22 Similar data on chemotherapy use were found in a previous study of our group in patients with metastatic pancreatic cancer.10
Despite a major increase in chemotherapy use in NR‐M0 patients under 75 years in the current study, overall survival hardly improved. However, the study period mainly covers the gemcitabine era, chemotherapy use and response rates may simply be too low to show a survival improvement in all NR‐M0 pancreatic cancer patients. Possibly, increasing prescription of more effective chemotherapy schemes such as FOLFIRINOX and nab‐paclitaxel with gemcitabine may affect overall survival in years following the current study period. In addition, the age of chemotherapy‐treated patients in our study has substantially risen from median 62 to 66 years, though still few elderly patients received chemotherapy (≥75 years: 11%). A careful selection and better support of elderly patients for chemotherapy treatment is therefore relevant and can be facilitated by the use of geriatric assessment tools.23
Strikingly, pathological confirmation of cancer in our study was lacking in one in six NR‐M0 patients who received chemotherapy. Because a misdiagnosis cannot be ruled out,24 pathological confirmation before chemotherapy is highly recommended.25 The absence of pathological confirmation in treated patients is worrisome and requires further attention in multidisciplinary team discussions in the Netherlands.
In recent systematic reviews of nonrandomized studies with LAPC patients only, approximately one quarter of patients could undergo resection after neoadjuvant chemo(radio)therapy26, 27 and overall survival of patients receiving FOLFIRINOX28 with or without resection was 24 months,26 which was comparable with survival of patients with initially resectable pancreatic cancer. In the current nationwide study, chemotherapy was combined with radiotherapy in only a minority of chemotherapy‐treated NR‐M0 patients.25, 29 Re‐evaluation of NR‐M0 patients after several months of chemo(radio)therapy may be worthwhile to identify patients for possible resection or eligibility for other treatments directed at local tumor control. An experienced multidisciplinary team or expert panel can provide in this need.
This study has several limitations that are related to the retrospective data that were used. Firstly, due to the available notification sources, the NCR is at risk of incompleteness of pancreatic cancer in elderly patients.30 Therefore, chemotherapy use and survival of elderly NR‐M0 patients may be slightly overestimated in our study, while early mortality may be underestimated. Survival may also be slightly overestimated because some patients of the large group without histological confirmation of pancreatic cancer were incorrectly diagnosed.24 Despite these limitations, the available unselected data of an often neglected group of pancreatic cancer patients revealed important findings about trends in everyday clinical practice. Secondly, survival trends of NR‐M0 patients in the course of the study period must be interpreted with caution as a result of changing characteristics of this subgroup and possible residual confounding of unmeasured characteristics. Thirdly, although the proportion of patients with stage III disease in our study (12% of all stage I‐IV in 2012‐2014) was comparable with other population‐based studies (7%‐13%),13, 19, 31 staging may be suboptimal in patients who were staged based on imaging only. Locally advanced and metastatic disease was found in a substantial proportion of patients who preoperatively were thought to have resectable disease.32, 33 Furthermore, TNM staging information cannot discriminate between the currently used categories of resectable, borderline resectable, and irresectable pancreatic cancer, based on the extent of arterial and venous involvement.34 Finally, data on comorbid conditions were available in only a subgroup of patients, and no information was available about performance status and quality of life of patients. In the future, the Dutch nationwide PAncreatic CAncer Project (PACAP) will provide more detailed information.35
In conclusion, our study showed that the group of NR‐M0 pancreatic cancer patients is heterogeneous, consisting of patients with irresectable tumors due to arterial involvement (stage III) and many patients with advanced age and (supposed) stage I‐II tumors. Despite an increase in the use of chemotherapy in younger patients, overall survival of all patients hardly improved over the described time period.
ACKNOWLEDGMENTS
The authors would like to thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for collecting data for the NCR in all hospitals in the Netherlands.
van der Geest LGM, van Eijck CHJ, Bas GK, et al.; for the Dutch Pancreatic Cancer Group. Trends in treatment and survival of patients with nonresected, nonmetastatic pancreatic cancer: A population‐based study. Cancer Med. 2018;7:4943–4951. 10.1002/cam4.1750
REFERENCES
- 1. Netherlands Cancer Registry (NCR) . Dutch Cancer Figures. http://www.cijfersoverkanker.nl/. Accessed April 01, 2018.
- 2. Lepage C, Capocaccia R, Hackl M, et al. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999‐2007: results of EUROCARE‐5. Eur J Cancer. 2015;51(15):2169‐2178. [DOI] [PubMed] [Google Scholar]
- 3. Bernards N, Haj Mohammad N, Creemers GJ, de Hingh IH, van Laarhoven HW, Lemmens VE. Ten weeks to live: a population‐based study on treatment and survival of patients with metastatic pancreatic cancer in the south of the Netherlands. Acta Oncol. 2015;54(3):403‐410. [DOI] [PubMed] [Google Scholar]
- 4. Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol. 2007;14(4):1320‐1326. [DOI] [PubMed] [Google Scholar]
- 5. Heestand GM, Murphy JD, Lowy AM. Approach to patients with pancreatic cancer without detectable metastases. J Clin Oncol. 2015;33(16):1770‐1778. [DOI] [PubMed] [Google Scholar]
- 6. Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta‐analysis of response and resection percentages. PLoS Med. 2010;7(4):e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gooiker GA, Lemmens VE, Besselink MG, et al. Impact of centralization of pancreatic cancer surgery on resection rates and survival. Br J Surg. 2014;101(8):1000‐1005. [DOI] [PubMed] [Google Scholar]
- 8. van der Geest LG, Besselink MG, van Gestel YR, et al. Pancreatic cancer surgery in elderly patients: balancing between short‐term harm and long‐term benefit. A population‐based study in the Netherlands. Acta Oncol. 2016;55(3):278‐285. [DOI] [PubMed] [Google Scholar]
- 9. David M, Lepage C, Jouve JL, et al. Management and prognosis of pancreatic cancer over a 30‐year period. Br J Cancer. 2009;101(2):215‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Geest LGM, Haj Mohammad N, Besselink MGH, et al. Nationwide trends in chemotherapy use and survival of elderly patients with metastatic pancreatic cancer. Cancer Med. 2017;6:2840‐2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817‐1825. [DOI] [PubMed] [Google Scholar]
- 12. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Enewold L, Harlan LC, Tucker T, McKenzie S. Pancreatic cancer in the USA: persistence of undertreatment and poor outcome. J Gastrointest Cancer. 2015;46(1):9‐20. [DOI] [PubMed] [Google Scholar]
- 14. Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology (ICD‐O). Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 15. Sobin LH, Wittekind C. TNM Classifications of Malignant Tumours, 6th edn New York, NY: Wiley; 2002. [Google Scholar]
- 16. Sobin LH, Gospodarowitz MK, Wittekind C. TNM Classifications of Malignant Tumours, 7th edn Hoboken, NJ: Wiley‐Blackwell; 2009. [Google Scholar]
- 17. Louwman WJ, Aarts MJ, Houterman S, van Lenthe FJ, Coebergh JW, Janssen‐Heijnen ML. A 50% higher prevalence of life‐shortening chronic conditions among cancer patients with low socioeconomic status. Br J Cancer. 2010;103(11):1742‐1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. King JC, Zenati M, Steve J, et al. Deviations from expected treatment of pancreatic cancer in octogenarians: analysis of patient and surgeon factors. Ann Surg Oncol. 2016;23(13):4149‐4155. [DOI] [PubMed] [Google Scholar]
- 19. Visser BC, Ma Y, Zak Y, Poultsides GA, Norton JA, Rhoads KF. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. HPB (Oxford). 2012;14(8):539‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He W, Zhao H, Chan W, Lopez D, Shroff RT, Giordano SH. Underuse of surgical resection among elderly patients with early‐stage pancreatic cancer. Surgery. 2015;158(5):1226‐1234. [DOI] [PubMed] [Google Scholar]
- 21. Chow WB, Rosenthal RA, Merkow RP, et al. Optimal preoperative assessment of the geriatric surgical patient: a best practices guideline from the American College of Surgeons National Surgical Quality Improvement Program and the American Geriatrics Society. J Am Coll Surg. 2012;215(4):453‐466. [DOI] [PubMed] [Google Scholar]
- 22. Abraham A, Al‐Refaie WB, Parsons HM, Dudeja V, Vickers SM, Habermann EB. Disparities in pancreas cancer care. Ann Surg Oncol. 2013;20(6):2078‐2087. [DOI] [PubMed] [Google Scholar]
- 23. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;14:442‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zijlstra M, Bernards N, de Hingh IH, et al. Does long‐term survival exist in pancreatic adenocarcinoma? Acta Oncol. 2016;55(3):259‐264. [DOI] [PubMed] [Google Scholar]
- 25. Netherlands Comprehensive Cancer Organisation (IKNL) . National evidence‐based guideline for pancreatic carcinoma. 2011. http://www.oncoline.nl. Accessed March 01, 2016.
- 26. Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient‐level meta‐analysis. Lancet Oncol. 2016;17(6):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rombouts SJ, Walma MS, Vogel JA, et al. Systematic review of resection rates and clinical outcomes after FOLFIRINOX‐based treatment in patients with locally advanced pancreatic cancer. Ann Surg Oncol. 2016;23(13):4352‐4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vogel JA, Rombouts SJ, de Rooij T, et al. Induction chemotherapy followed by resection or irreversible electroporation in Locally Advanced Pancreatic Cancer (IMPALA): a prospective cohort study. Ann Surg Oncol. 2017;24:2734‐2743. [DOI] [PubMed] [Google Scholar]
- 29. Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2015;26(Suppl 5):v56‐v68. [DOI] [PubMed] [Google Scholar]
- 30. Fest J, Ruiter R, van Rooij FJ, et al. Underestimation of pancreatic cancer in the national cancer registry – Reconsidering the incidence and survival rates. Eur J Cancer. 2016;72:186‐191. [DOI] [PubMed] [Google Scholar]
- 31. Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246(2):173‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Balzano G, Capretti G, Callea G, Cantu E, Carle F, Pezzilli R. Overuse of surgery in patients with pancreatic cancer. A nationwide analysis in Italy. HPB (Oxford). 2016;18(5):470‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van der Geest LGM, Lemmens V, de Hingh I, et al. Nationwide outcomes in patients undergoing surgical exploration without resection for pancreatic cancer. Br J Surg. 2017;104:1568‐1577. [DOI] [PubMed] [Google Scholar]
- 34. Al‐Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the American pancreatic association. Gastroenterology. 2014;146(1):291‐304.e1. [DOI] [PubMed] [Google Scholar]
- 35. PAncreatic CAncer Project (PACAP) . https://pacap.nl/ and http://www.dpcg.nl/projecten/pacap.html. Accessed January 17, 2017.


