Abstract
Previously, we detected circulating tumor DNA that contained two EGFR mutations (p.L858R and exon19 del) in plasma of patients with late-stage non–small-cell lung carcinoma (NSCLC) using the electric field–induced release and measurement (EFIRM) platform. Our aim was to determine whether EFIRM technology can detect these mutations in patients with early-stage NSCLC. Prospectively, 248 patients with radiographically determined pulmonary nodules were recruited. Plasma was collected before biopsy and histologic examination of the nodule. Inclusion criteria were histologic diagnosis of benign nodule (control) and stage I or II adenocarcinoma harboring either p.L858R or exon19 delEGFR mutations. Plasma samples were available from 44 patients: 23 with biopsy-proven benign pulmonary nodules and 21 with stage I or II adenocarcinoma (12 p.L858R and 9 exon19 delEGFR variants). Samples were analyzed for the EGFR mutations using the EFIRM platform. Assay sensitivity was 92% for p.L858R (11 of 12 samples positive) and 77% for exon19 del (7 of 9 samples positive). Specificity was 91% with two false-positive results in 23 patients with EGFR-positive nodules and 95% for the entire 44-patient series. Concordance was 100% with identical mutations discovered in plasma and nodule biopsy. The EFIRM platform is able to noninvasively detect two EGFR mutations in individuals with early-stage NSCLC.
Lung cancer is the leading cause of cancer mortality globally, with only 15% to 20% of all patients being surgical candidates.1 If detected at an early stage (stage I and II), 5-year survival increases to 70% to 80% if the lesion is resectable.2 The National Lung Screening Trial reported that low-dose computed tomography (CT) screening significantly reduced mortality from lung cancer in a high-risk population by detecting early-stage disease.3 The promise of CT is tempered by the high number of false-positive findings, which generate costs and unnecessarily expose patients to ionizing radiation.3 The knowledge of genetic alterations in the EGFR gene, a pharmacologically relevant target for tyrosine kinase inhibitors,4 is valuable because it informs chemotherapy options. A significant percentage of non–small-cell lung carcinoma (NSCLC) tumors (27%) will harbor either the p.L858R mutation or exon19 del variants.5 Traditionally, EGFR mutation analysis is performed at the time of initial diagnosis or recurrence on tissue DNA extracted from surgery. Performing biopsies to detect and monitor EGFR mutation status is costly, invasive, and may be technically difficult or impossible to achieve because of small samples size or other technical factors.
Variants in circulating tumor DNA (ctDNA) fragments can be detected in the biofluids of patients in early stages of lung cancer.6 An increasing body of literature has demonstrated the ability to noninvasively screen for actionable variants in a process described as liquid biopsy (LB).
Plasma-based EGFR variant detection can be performed on plasma samples from patients with late-stage NSCLC using a technology known as electric field–induced release and measurement (EFIRM), which uses an electric field to enhance hybridization efficiency and detect perfect duplexes. EFIRM is plate-based and automatable and analyzes native plasma or direct saliva. With the speed and simplicity of the method, EFIRM has the potential to be a suitable tool for mutation monitoring in a clinical setting. EFIRM can detect two actionable EGFR mutations (p.L858R and exon19 del) in samples from patients with late-stage lung cancer samples with >90% concordance with tissue biopsy genotype of the tumor.7, 8 We investigated the ability of an EFIRM LB (eLB) assay to detect p.L858R and exon19 del EGFR variants in direct plasma samples from patients with early-stage NSCLC and correlated the results with those from the biopsy of the tumor itself.
Materials and Methods
Patients and Clinical Specimens
The clinical trial described in this article was approved and performed under the supervision of the University of California, Los Angeles (UCLA) Institutional Review Board protocol 11-000592 and National Cheng Kung University Hospital Institutional Review Board protocol BR-100-034. From December 2014 through March 2016, patients presenting to the National Cheng Kung University Hospital for percutaneous CT-guided fine-needle aspiration biopsies and video-assisted thoracoscopic surgery resections to evaluate a pulmonary nodule were offered participation in the study. After consent, plasma and saliva were obtained before the scheduled procedure. Biopsy specimens were examined histologically, and the presence of EGFR mutations was determined using EGFR PCR kits as previously described (Qiagen, Hilden, Germany).7 Patients with the final histologic diagnosis of a benign nodule (normal controls) or adenocarcinoma of the lung were enrolled for further analysis. Patients without a final histologic diagnosis, those diagnosed as stage III or IV disease, or those diagnosed as nonadenocarcinoma were excluded from further study. The staging criteria used were those from the American Joint Committee on Cancer TNM system (https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/staging.html, last accessed March 6, 2018).
Specimen Handling
Ten milliliters of blood was drawn into EDTA collection tubes and centrifuged immediately at 2500 × g for 10 minutes at 4°C, and the upper plasma layer was collected and immediately flash-frozen and stored at −80°C. The specimens were shipped frozen to the laboratory at the UCLA School of Dentistry and stored at −80°C. At the time of analysis, plasma was thawed for the EFIRM assay as described below.
eLB Assay
The basic eLB assay has been described previously.7 Of note is that each measurement is performed in duplicate as part of the standard operating procedure. The published method has been modified to increase signal intensity. Briefly, eLB is an open platform signal amplification technology based on a microtiter plate of 96 gold electrodes obtained from EZLife Bio (Guangzhou, China). Initially, capture probes (100 nmol/L) are copolymerized with conduction gel and pyrrole onto the gold electrodes so that each well contains a single capture probe specific to a single variant. In this assay, either of two well-characterized tyrosine kinase inhibitor–sensitizing EGFR variants, the exon19 del or p.L858R point mutation, were measured.
Paired probes (capture and detector; Integrated DNA Technologies, San Diego, CA) specific for the two tyrosine kinase inhibitor–sensitizing mutations were designed for EFIRM as follows: a capture probe for the exon 19 deletion, 5′-TGTTGCTTCCTTG-3′; a detector probe for the exon 19 deletion, 5′-ATAGCGACGGGAATTTTAACTTTCTCACCT-3′; a capture probe for the L858R point mutation: 5′-TGGCCCGCCC-3′; and a detector probe for the L858R mutation: 5′-AAAATCTGTGATCTTGACATGCTGCGGTGTTTTGTGCAG-3′. The detector probes were biotinylated on the 3′ end. The capture probes (100 nmol/L) were first co-polymerized with pyrrole onto the bare gold electrodes by applying a cyclic square wave electric field at 300 mV for 1 second and 1100 mV for 1 second. In total, polymerization proceeded for four cycles of 2 seconds each.
Synthetic 50-bp oligonucleotides corresponding to the mutant alleles were used for controls in the EFIRM assay. The synthetic oligonucleotides were diluted in Ultrahyb Ultra-Sensitive Hybridization Buffer (Thermo Fisher Scientific, Waltham, MA) at a 1:99 ratio, and plasma was thawed and diluted 1:2 with the same buffer. The resulting diluted buffer was divided into two wells. Hybridization was performed at −300 mV for 1 second and 500 mV for 1 second for a total of 150 cycles of 2 seconds each followed by a 30-minute incubation period in the EFIRM instrument at room temperature. After washing, detector probes were mixed with casein-phosphate–buffered saline (Invitrogen, Carlsbad, CA) at a 1:100 dilution and transferred onto the electrodes. Hybridization was performed at 300 mV for 1 second and 500 mV for 1 second for a 150-second cycle of 2 seconds each for 30 minutes in the EFIRM instrument at room temperature. Subsequently, streptavidin poly–horseradish peroxidase (poly-HRP) (Thermo Fisher Scientific) was mixed with casein-phosphate–buffered saline (Invitrogen) at a 1:1000 ratio and incubated for 30 minutes. In the published method, signal generation was accomplished by the addition of HRP, tetramethylbenzidine substrate, and measurement of the amperometric signal.
In the modified technique, after the addition of HRP, anti-HRP antibody (ab195239: Abcam, Cambridge, UK) at a concentration of 1/6 μg/mL with casein-phosphate–buffered saline (Invitrogen) was added followed by a 30-minute incubation at room temperature. Subsequently, streptavidin poly-HRP80 conjugate (Fitzgerald Industries, Acton, MA) mixed with casein-phosphate–buffered saline (Invitrogen) at a 1:1000 ratio was added and incubated for 30 minutes to increase the amount of available HRP molecules. Signal was measured in nanoamperes by current generation through the gold electrodes. The EFIRM instrument is capable of measuring current in picoamperes.
Statistical Analysis
To evaluate the performance of EFIRM in detecting EGFR mutations, the receiver operating characteristic curve for each probe was plotted, and the 95% CIs were calculated. All analyses were performed using SAS statistical software version 9.3 TS level 1M1.9 The G* power program was used to estimate the sample size needed for validation in a blinded (F.W., C.M.S., J.C., C.-C.L., C.-Y.H., G.W.S.H., D.C., Y.K., F.L., D.E., T.G., M.T., W.L., W.-C.S., and D.T.W.W.) group using one-way analysis of variance with a power of 0.95 at a = 0.05.
Results
Patient Recruitment
Plasma was collected from 248 patients with suspected lung cancer. A total of 152 patients were excluded because of the diagnosis of advanced NSCLC (stages III and IV), and six patients were excluded because of the diagnosis of other malignant tumors.
Of the 90 remaining patients, 46 were diagnosed with early-stage NSCLC. Of these 46 patients, 12 patients had a p.L858R EGFR mutation, and nine had an exon19 del EGFR mutation. Twenty-five patients with early-stage NSCLC were excluded from the study for the following reasons: 12 patients had no detectable EGFR mutations, seven patients had no EGFR mutation results available, two patients had two EGFR mutations, and four patients had uncommon EGFR mutations.
Demographic Characteristics
There were 23 patients with benign nodules (12 males and 11 females). There were 12 samples from patients with biopsy samples that contained p.L858R variants (five males and seven females). Eleven of these 12 patients with p.L858R variants had stage I disease, and a single patient had stage II disease. There were nine samples from patients with a biopsy-proven exon19 del (four males and five females). Seven of the nine patients had stage I disease, and two patients had stage II disease.
eLB
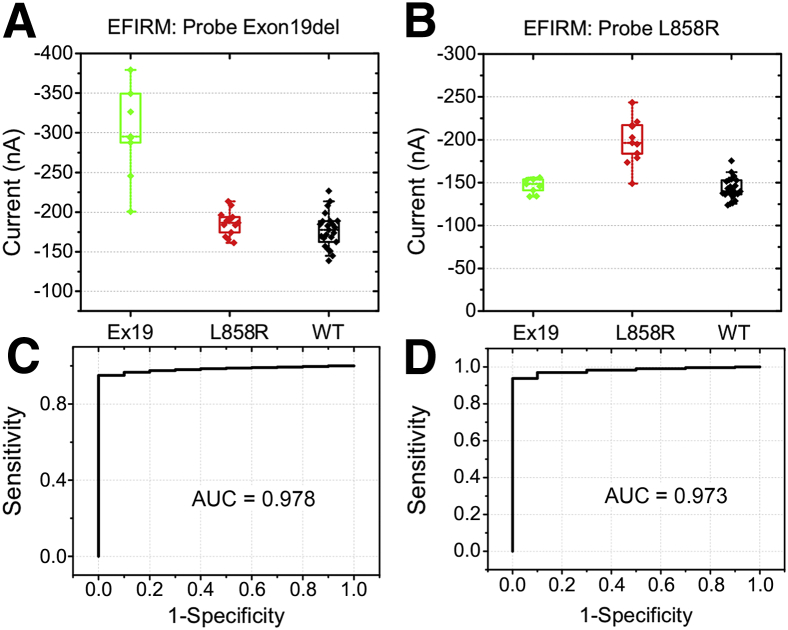
Plasma samples were blinded (F.W., C.M.S., J.C., C.-C.L., C.-Y.H., G.W.S.H., D.C., Y.K., F.L., D.E., T.G., M.T., W.L., W.-C.S., and D.T.W.W.) and analyzed on the eLB platform, and results returned to the statistician. All samples were analyzed in duplicate, and the mean value used. The mean variance for the two replicate samples was 8.7% for the p.L858R assay and 9.2% for the exon19 del assay. Scatterplots of the results are shown in Figure 1. The results for the exon19 del (Figure 1A) and p.L858R (Figure 1B) capture probe are shown. Visual inspection demonstrates excellent discrimination between the individuals with tumors harboring the exon19 del variant and both the patients with benign nodule (wild type) and those with the p.L858R variant. Results with the exon19 del capture probe demonstrated that none of the patients with benign nodules or those with the p.L858R mutation had electrical current values of <−230 nA, whereas all but one sample from patients with biopsy-proven exon19 del had values of <−240 nA. The 0.978 area under the AUC curve reinforces this observation (Figure 1C).
Figure 1.
Performance of electric field–induced release and measurement (EFIRM) on p.l858r and exon19 del (EX19) mutation detection in early-stage samples. A and B: EX19 samples (green), L858R samples (red), and wild-type (WT) samples (black). C and D: Area under curves (AUCs). n = 9 EX19d samples; n = 13 L858R samples; n = 22 WT samples.
Although there is more overlap using the p.L858R probe than with the exon19 del probe, there is still good discrimination between patients with tumors carrying the p.L858R mutation and those with benign nodules or the exon19 del variant (Figure 1, C and D) with the area under the receiver operating characteristic curve of 0.973.
Using statistically derived cutoffs to optimize sensitivity and specificity, there were two false-positive results (both for p.L858R) in the controls, yielding an overall specificity of 91% for the two single-nucleotide polymorphism eLB assay in the 23 patients with benign nodules and therefore 95% for the entire 44-patient series. The sensitivity was 92% for p.L858R detection, with only one negative result in 12 samples in a patient with stage 1 disease and 77% for exon19 del with two false-negative results in nine samples in a single patient each for stage 1 and stage II disease. It is impossible to determine whether the negative results in patients with nodules that contain EGFR mutations are false-negative results because of the platform measurement or whether these patients do not have ctDNA in sufficient quantity for detection.
In this small series, there is an apparent difference in sensitivity between the p.l858r and exon19 del assays. This observation may be caused by biological variation in the amount of circulating cell-free DNA in those particular patients or could be the result of a true technical difference between the ability of the capture and signal probes to detect small amounts of circulating DNA. Because each mutation requires different capture and signal probes, it would not be surprising for the assays to vary in sensitivity. Experiments with synthetic oligonucleotides have shown that the two assays have similar abilities to detect their respective targets (data not shown).
Discussion
LB is an exciting development in cancer biology. It has been asserted that LB could be useful in screening for disease,10, 11 monitoring treatment response,10, 12 and guiding treatment when biopsy material is not available.12 This study demonstrates that EFIRM, a novel platform for LB, is capable of accurately detecting mutations in cell-free DNA in patients with early-stage lung cancer. The clinical utility for eLB has yet to be established in any of these areas and will require further study. If necessary, the assay could be modified as needed for a particular clinical indication. As in any assay development, there is an inverse association between sensitivity and specificity. Reference ranges can be adjusted to optimize sensitivity or specificity. In addition, the content of the assay could be altered to the clinical indication. For example, if the application were screening for NSCLC, additional mutations or biomarkers, such as miRNA, could be added to the EFIRM panel.
Although it is tempting to speculate that detecting EGFR mutations in the plasma is predictive of that patient having cancer, there is no direct evidence to support this claim. Further investigation will be necessary to determine what the positive predictive value of detecting an EGFR mutation in the circulation of an individual with, for example, an indeterminate lung nodule or a high-risk smoking history.
Guiding treatment selection in patients for whom biopsy material is not available is the most immediate potential use for eLB. There is ample evidence that LB is valuable in this setting.10, 11, 12 For other applications, clinical utility will need to be established by convincing clinical studies. Other currently available platforms will require the same clinical validation.
Three reports regarding the validity of LB for early-stage lung cancer have been published.13, 14, 15 For all three groups, their approach combines optimized library preparation for DNA from patient samples, use of next-generation sequencing technology to sequence key regions of oncogenic genes for driver mutations, and bioinformatics analysis to derive a conclusion from their sequenced data.
The results of these studies reveal that their concordance (defined as their platform's ability to successfully predict biopsy or tissue malignant tumors versus noncancer) is 50% and 100%,10 45% and 72%,14 and 37% and 75%12 for stage I and stage II disease, respectively. In addition, two of these studies have small patient numbers, with only five and 16 patients with early-stage lung cancer enrolled, respectively.
In this report, eLB demonstrated superior performance to those previously reported studies in its ability to noninvasively detect p.L858R and exon19 del in ctDNA from patients with stages 1 and II NSCLC.7, 8 Set at 91% to 95% specificity, the sensitivity for L858R and exon19 del detection was determined to be 92% and 77%, respectively, in stage I and II disease.
There are several potential explanations for the improved performance of eLB compared with next-generation sequencing technologies. The eLB is performed directly on unprocessed samples without the need for DNA isolation, adaptor ligation, bead purification, and other sample manipulation. Each manipulation or process will inevitably result in the loss of material because of physical phenomena and the fact that enzymatic reactions are not 100% efficient. Because the amount of ctDNA is low to begin with, these accumulated losses of DNA can have significant effects on the downstream ability to detect genomic variants. In addition, because no amplification or sequencing is performed in eLB, there is no need for the addition of unique identifiers or complex bioinformatics manipulations to eliminate false-positive results attributable to potential amplification and sequencing errors.
Currently, the clinical sensitivity of eLB to detect patients with NSCLC is limited by the percentage of tumors that contain either or both of the two variants in the eLB assay. We are currently developing a 10-variant panel that detects mutations expressed in half of all lung malignant tumors. Once designed and validated, this assay could be used in clinical trials to establish the clinical utility for screening patients with indeterminate pulmonary nodules. Individuals with ctDNA that contains known pathologic variants could be at increased risk for malignant tumors, and the eLB could help stratify patients into early biopsy versus watchful waiting cohorts.
More studies will be necessary to optimize the technical and clinical performance of the eLB assay. If the specificity of the assay can be improved, clinical studies could be designed to assess the performance of eLB in a screening setting. The observation that eLB can detect pathologic EGFR variants with high sensitivity and specificity in patients with stage I NSCLC despite their low tumor burdens and at a time when resection for cure is possible may facilitate more effective surveillance programs for high risk individuals currently followed by spiral CT.
The eLB assay is plate-based, robust, and automatable and can be performed in 4 hours, making it an attractive platform for high-throughput testing. Once validated for clinical use, the assay could be useful in many applications.
Footnotes
Supported by NIH grants UH2 CA206126 (D.T.W.W.) and UH3 TR000923 (D.T.W.W.) and by the Center of Applied Nanomedicine, National Cheng Kung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
F.W., C.M.S., J.C., and C.-C.L. contributed equally to this work.
Disclosures: D.T.W.W. is a co-founder and scientific adviser of RNAmeTRIX Inc., a molecular diagnostic company, and holds equity in the company; the University of California holds equity in RNAmeTRIX; intellectual property invented by D.T.W.W. and patented by the University of California has been licensed to RNAmeTRIX.
Supplemental material for this article can be found at https://doi.org/10.1016/j.jmoldx.2018.06.008.
Contributor Information
Charles M. Strom, Email: cstrom@mednet.ucla.edu.
Wu-Chou Su, Email: sunnysu@mail.ncku.edu.tw.
Supplemental Data
References
- 1.Wong M.C.S., Lao X.Q., Ho K.-F., Goggins W.B., Tse S.L.A. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci Rep. 2017;7:14300. doi: 10.1038/s41598-017-14513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre L.A., Siegel R.L., Jemal A. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. doi: 10.1007/978-3-319-24223-1_1. [DOI] [PubMed] [Google Scholar]
- 3.National Lung Screening Trial Research Team. Aberle D.R., Adams A.M., Berg C.D., Black W.C., Clapp J.D., Fagerstrom R.M., Gareen I.F., Gatsonis C., Marcus P.M., Sicks J.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mok T.S., Wu Y.-L., Thongprasert S., Yang C.-H., Chu D.-T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y., Nishiwaki Y., Ohe Y., Yang J.-J., Chewaskulyong B., Jiang H., Duffield E.L., Watkins C.L., Armour A.A., Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 6.Aravanis A.M., Lee M., Klausner R.D. Next-generation sequencing of circulating tumor DNA for early cancer detection. Cell. 2017;168:571–574. doi: 10.1016/j.cell.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Wei F., Lin C., Joon A., Feng Z., Troche G., Lira M.E., Chia D., Mao M., Ho C., Su W.-C., Wong D.T.W. Noninvasive saliva-based EGFR gene mutation detection in patients with lung cancer. Am J Respir Crit Care Med. 2014;190:1117–1126. doi: 10.1164/rccm.201406-1003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pu D., Liang H., Wei F., Akin D., Feng Z., Yan Q., Li Y., Zhen Y., Xu L., Dong G., Wan H., Dong J., Qiu X., Qin C., Zhu D., Wang X., Sun T., Zhang W., Li C., Tang X., Qiao Y., Zhou Q., Wong D.T.W. Evaluation of a novel saliva–based epidermal growth factor receptor mutation detection for lung cancer: a pilot study. Thorac Cancer. 2016;7:428–436. doi: 10.1111/1759-7714.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 10.Brock G., Castellanos-Rizaldos E., Hu L., Coticchia C., Skog J. Liquid biopsy for cancer screening, patient stratification and monitoring. Transl Cancer Res. 2015;4:280–290. [Google Scholar]
- 11.Alix-Panabieres C., Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;10:479–491. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- 12.Stewart C.M., Tsui D.W.Y. Circulating cell-free DNA for non-invasive cancer management. Cancer Genet. 2018 doi: 10.1016/j.cancergen.2018.02.005. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman A., Bratman S., To J., Wynne J., Eclov N.C.W., Modlin L.A., Liu C.L., Neal J.W., Wakelee H.A., Merritt R.E., Shrager J.B., Loo B.W., Alizadeh A.A., Diehn M. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phallen J., Sausen M., Adleff V., Leal A., Hruban C., White J., Anagnostou V., Fiksel J., Cristiano S., Papp E., Speir S., Reinert T., Orntoft M., Woodward B.D., Murphy D., Parpart-Li S., Riley D., Nesselbush M., Sengamalay N., Georgiadis A., Li Q.K., Madsen M.R., Mortensen F.V., Husikens J., Punt C., van Grieken N., Fijneman R., Meijer G., Husain H., Scharpf R.B., Diaz L.A., Jones S., Angiuoli S., Ørntoft T., Nielsen H.J. Direct detection of early-stage cancers using circulating tumor DNA. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aan2415. pii: eaan2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen J.D., Li L., Wang Y., Thoburn C., Afsari B., Danilova L. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359:926–930. doi: 10.1126/science.aar3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.