Abstract
Objectives/Hypothesis:
Idiopathic subglottic stenosis (iSGS) is a rare and potentially life-threatening disease marked by recurrent and progressive airway obstruction frequently requiring repeated surgery to stabilize the airway. Unknown etiology and low disease prevalence have limited the ability to characterize the natural history of iSGS and resulted in variability in surgical management. It is uncertain how this variation relates to clinical outcomes.
Study Design:
Medical record abstraction.
Methods:
Utilizing an international, multi-institutional collaborative, we collected retrospective data on patient characteristics, treatment, and clinical outcomes. We investigated variation between and within open and endoscopic treatment approaches and assessed therapeutic outcomes; specifically, disease recurrence and need for tracheostomy at last follow-up.
Results:
Strikingly, 479 iSGS patients across 10 participating centers were nearly exclusively female (98%, 95% confidence interval [CI], 96.1–99.6), Caucasian (95%, 95% CI, 92.2–98.8), and otherwise healthy (mean age-adjusted Charlson Comorbidity Index 1.5; 95% CI, 1.44–1.69). The patients presented at a mean age of 50 years (95% CI, 48.8–51.1). A total of 80.2% were managed endoscopically, whereas 19.8% underwent open reconstruction. Endoscopic surgery had a significantly higher rate of disease recurrence than the open approach (chi2 = 4.09, P = 0.043). Tracheostomy was avoided in 97% of patients irrespective of surgical approach (95% CI, 94.5–99.8). Interestingly, there were outliers in rates of disease recurrence between centers using similar treatment approaches.
Conclusion:
Idiopathic subglottic stenosis patients are surprisingly homogeneous. The heterogeneity of treatment approaches and the observed outliers in disease recurrence rates between centers raises the potential for improved clinical outcomes through a detailed understanding of the processes of care.
Keywords: Idiopathic subglottic stenosis, cricotracheal, dilation, comparative effectiveness, tracheostomy
INTRODUCTION
Idiopathic subglottic stenosis (iSGS) is a rare1 (annual incidence of 1:400,000) and devastating extrathoracic fibroinflammatory process characterized by progressive lower laryngeal and upper tracheal airway obstruction. The disease manifests as life-threatening dyspnea of unknown etiology in otherwise healthy patients, and often requires repeated surgical interventions to prevent respiratory distress and airway obstruction. Low disease prevalence, coupled with the geographic distribution of affected patients, has limited the ability to define the natural history and clinical characteristics of iSGS, and has resulted in variability in surgical management. Additionally, the inciting event and underlying pathophysiology of iSGS remain unclear, leaving clinicians with little direction for novel treatment or focused research efforts.
The goal of treatment is to reestablish durable airway patency. Unfortunately, surgical interventions are rarely definitive, and many iSGS patients require several treatments annually.2 Current surgical management can be categorized into: 1) endoscopic approaches or 2) open neck surgery (cricotracheal resection of the narrowed tracheal segment with end-to-end anastomosis, or augmentative anterior and posterior cartilaginous grafting, i.e., laryngotracheoplasty); and 3) tracheostomy. High disease recidivism exists with all approaches, and each is associated with unique and often disabling side effects that significantly affect the patient’s quality of life. Comparative data on outcomes and treatment tradeoffs have not been systematically evaluated.
Comparative effectiveness research in rare, orphan surgical diseases is difficult; as such, these diseases have largely been marginalized in health care research in lieu of more common disorders. This trend continues despite the prioritization of rare diseases by the Institute of Medicine,3 Agency for Healthcare Research and Quality,4 and the Patient Centered Outcomes Research Institute.5 Many barriers exist to comparative effectiveness research in this domain beyond simply low population incidence (e.g., infrastructure, funding, perception of high risk, and lack of data from natural history studies). As a result, there has been little progress toward understanding the underlying pathophysiology or even the clinical epidemiology of these conditions.
Heterogeneity in treatments used, outcomes measured, and the lack of comparative studies highlight the need for data aggregation through collaboration. Recognizing this need, the North American Airway Collaborative (NoAAC) was established to characterize the population affected by iSGS and to study variations in treatment and their effectiveness. Using data compiled by the collaborative, this study aimed to 1) define population-based disease characteristics and the clinical course in patients with iSGS; 2) understand intercenter and intracenter variability in treatment modalities used; and 3) determine the comparative effectiveness of those modalities with respect to meaningful clinical endpoints.
MATERIALS AND METHODS
This study was performed in accordance with the Declaration of Helsinki, Good Clinical Practice, and was approved by the institutional review board of all participating institutions.
Participating Centers
Consortium centers were approached and volunteered to participate; inclusion criteria required participating centers to have iSGS case series greater than 25 patients between January 1, 2000, and January 1, 2014. Overall, 10 of 11 invited centers agreed to participate, including Baylor College of Medicine, (Houston, TX), Charing Cross Imperial College Healthcare (London, United Kingdom), Cleveland Clinic (Cleveland, OH), Johns Hopkins Medical Center (Baltimore MD), Mayo Clinic Rochester (Rochester MN), Mayo Clinic Arizona (Phoenix, AZ), Oregon Health Sciences University (Portland, OR), University of Texas Southwestern (Dallas, TX), University of Utah (Salt Lake City, UT), and Vanderbilt University (Nashville, TN). Idiopathic subglottic stenosis is commonly managed by multidisciplinary teams consisting of otolaryngologists, interventional pulmonologists, and thoracic surgeons. All specialties were represented within the NoAAC consortium. A total of 18 surgeons and three interventional pulmonologists were responsible for the clinical care of the 479 patients at the 10 participating centers.
Patients
Each iSGS diagnosis was confirmed using clinical and serologic criteria previously described: no history of significant laryngotracheal injury, no significant history of endotracheal intubation or tracheotomy within 2 years of presentation, no thyroid or major anterior neck surgery, no neck irradiation, no caustic or thermal injuries to the laryngotracheal complex, no history of vasculitis, negative titers antinuclear cytoplasmic antibody—and the lesion must involve the subglottis.6
Data Collected
Individual patient characteristics (age, gender, race, follow-up duration) and comorbidities were extracted from each iSGS patient’s medical record. Treatment approaches (i.e., endoscopic, open) and surgical dates were collected. Stenosis morphology data (% luminal obstruction, distance from glottis [cm], and overall length [cm]) were derived from intraoperative findings. Data on percent stenosis were derived from endoscopic measurement of tracheal diameter before and after therapy. The frequency and time to and between disease recurrence(s) (i.e., need for repeated surgery) was captured, as was the presence of a tracheostomy at last follow-up.
Procedures
Symptomatic dyspnea with endoscopic confirmation of airway stenosis was the singular indication for intervening at all centers. Broadly, therapeutic strategies for iSGS included: 1) endoscopic approaches (e.g., dilation with rigid instruments2 or inflatable balloons,7 resection, and/or endoluminal laser1); 2) open neck surgery (cricotracheal resection of the narrowed tracheal segment with end-to-end anastomosis8 or augmentative anterior and posterior cartilaginous grafting, i.e., laryngotracheoplasty); and 3) tracheostomy. Even within singular approaches there were subtle but potentially significant variations in care. Some of these factors are apparent (i.e., the use of CO2 laser vs. cold knife incision, or the use of balloon vs. rigid dilation), whereas other factors remain obscure. Each center’s treatment approach remained consistent throughout the study period.
Outcomes
Etiology of this condition is currently unknown. Success of treatment is based on reducing disease recurrence and avoidance of tracheostomy. Disease recurrence is defined as dyspnea severity significant to require a repeat surgical treatment (i.e., second surgery). Tracheostomy placement represented salvage therapy; that is, the failure of traditional surgical management (i.e., endoscopic or open) to adequately maintain airway patency. Thus, primary outcomes in this study are 1) time from first surgery to recurrence (if it occurred) and 2) the presence of tracheostomy at last follow-up.
Statistical Analysis
All data management and analyses were done using Stata/MP 12.1 software (StataCorp, College Station, TX). Univariate analyses were performed using analysis of variance, Pearson’s chi-squared tests, and Fisher’s exact tests, as appropriate. Kaplan-Meier survival analyses compared time to recurrence between institutions and treatment modalities (i.e., endoscopic vs. open approaches), respectively, and differences measured using log-rank tests. After excluding centers with less than five open cases during the study period (site 10), secondary analyses were performed to investigate whether surgical volume was correlated with recurrence after endoscopic and open surgical approaches, respectively.
RESULTS
The cohort consisted of 479 iSGS patients, with a homogenous phenotype across all participating centers: they were otherwise healthy (mean age-adjusted Charlson Comorbidity Index 1.5, 95% confidence interval [CI], 1.44–1.69), perimenopausal (mean age 50.4 years, 95% CI, 48.8–51.1), Caucasian (95%, 95% CI, 92.2–98.8), and female (98%, 95% CI, 96.1–99.6) (Table I). Overall, recurrences occurred at a mean 12.6 months after a procedure (95% CI, 11.4–13.8), with 90% of recurrences happening within 28 months. A total of 384 patients (80.2%) were exclusively managed endoscopically during the duration of the study (mean follow-up 54.2 months; 95% CI: 48.3–60.1), whereas 95 (19.8%) were managed with open reconstruction (mean follow-up 65.3 months: 95% CI, 54.47–76.13). The only difference between the therapeutic approaches was that the patients who underwent open reconstruction were younger (mean 46.7 [95% CI, 43.9–49.3] vs. 50.8 [95% CI, 49.5–52.1]; P = 0.019) (Table I).
TABLE I.
Patient Characteristics.
| Open (n = 95) | Endoscopic (n = 384) | Total (n = 479) | Significance (P) | |
|---|---|---|---|---|
| Demographics | ||||
| Follow-up (Mean months, 95% CI) | 65.3 (54.5–76.11) | 54.2 (48.3–60.1) | 56.4 (51.2–61.6) | ns |
| Age (Mean years, 95% CI) | 46.7* (43.9–49.3) | 50.8 (49.5–52.1) | 49.9 (48.8–51.1) | P = 0.019 |
| Sex (% female) | 97.9 | 98.4 | 98.3 | ns |
| Race (%) | ns | |||
| Caucasian | 98.9 | 96.9 | 97.3 | |
| African American | 0 | 1 | 0.8 | |
| Asian | 0 | 0 | 0 | |
| Hispanic | 1.1 | 2.1 | 1.9 | |
| Comorbidities | ||||
| Charlson Index (Mean, 95% CI) | 1.30 (1.02–1.55) | 1.60 (1.50–1.78) | 1.56 (1.44–1.69) | ns |
| DMII (%) | 7.4 | 3.4 | 4.2 | ns |
| MI (%) | 2.1 | 0.3 | 0.6 | ns |
| CHF (%) | 0 | 0 | 0 | ns |
| PVD (%) | 0 | 0 | 0 | ns |
| Chronic liver disease (%) | 0 | 0 | 0 | ns |
| COPD (%) | 1.1 | 1.3 | 1.3 | ns |
| Connective tissue (%) | 0 | 0 | 0 | ns |
| GERD (%) | 24.2 | 20.8 | 21.5 | ns |
| Disease Morphology | (n = 40) | (n = 214) | (n = 254) | |
| % stenosis (Mean %, 95% CI) | 68.25 (63.6–72.9) | 62.74 (60.7–64.8) | 63.61 (61.7–65.5) | ns |
| Cm below glottis (Mean cm, 95% CI) | 1.8 (1.2–2.3) | 1.59 (1.4–1.74) | 1.62 (1.5–1.8) | ns |
| Stenosis length (Mean cm, 95% CI) | 1.814 (1.2–2.4) | 1.706 (1.5–1.9) | 1.719 (1.5–1.89) | ns |
CHF = Congestive heart failure; CI = confidence interval; COPD = chronic obstructive pulmonary disease; DMII = Diabetes Mellitus Type II; GERD = gastroesophageal reflux disease; MI = Myocardial infarction; ns = not significant; PVD = Peripheral vascular disease.
Of the 254 patients with available intraoperative measurements, patients presenting with symptomatic dyspnea had obstruction of more than 60% of the tracheal lumen measured on bronchoscopy (mean 63%; 95% CI, 61.7–65.5) (Table I). Patient and disease characteristics (i.e., luminal compromise, distance from glottis, stenosis length) between those treated with endoscopic and open approaches were statistically equivalent.
Variation in Treatment Approach
Each center used an endoscopic approach for the majority of iSGS patients (mean 80.7%, 95% CI, 67.7–93.7). There was site-specific variability in endoscopic technique. For example, site 2 used graduated rigid bronchoscopes, whereas the remainder used controlled radial expansion balloon dilators. Uniquely, site 3 endoluminally excised subglottic scar without dilation. Despite identical patient characteristics, five centers treated greater than 25% of patients with open resection (Fig. 1A). Cricotracheal resection was the consistent open surgical approach across sites, with one exception (site 7), which used augmentative anterior and posterior grafting (laryngotracheoplasty) to expand the subglottic lumen. There was variation between centers in the number of endoscopic procedures undertaken prior to open reconstruction. Site 6 offered patients surgery significantly earlier than the other centers (P = 0.005) (Fig. 1B).
Fig. 1.
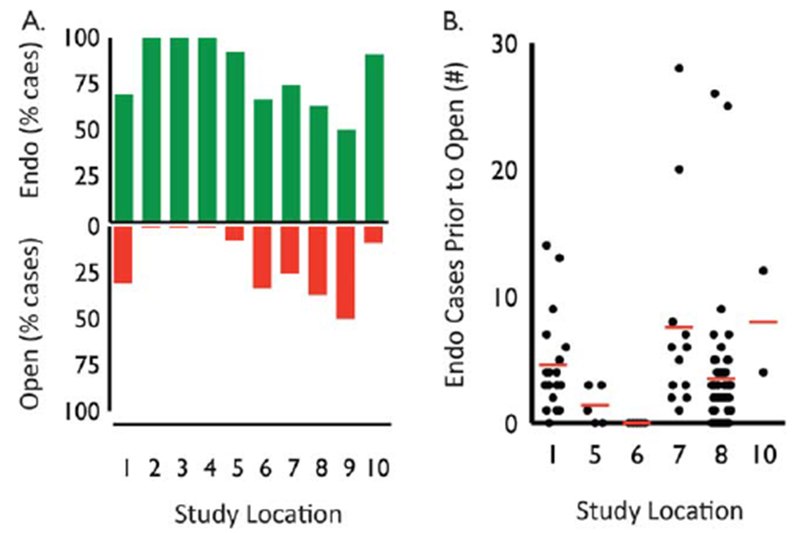
Variation of theraputic approach at each participating site. (A) Percentage of iSGS patients treated endoscopically (green bars) and open (red bars) at each participating center. (B) Number of endoscopic procedures prior to open reconstruction (red bar indicating mean) at participating centers offering open surgery. endo = endoscopic; iSGS = idiopathic subglottic stenosis.
Treatment Outcomes
Tracheostomy was avoided in 97% of patients irrespective of surgical approach (95% CI, 94.5–99.8). In general, endoscopic surgeries had a significantly higher rate of disease recurrence than open procedures (chi2 = 4.09, P = 0.043) (Fig. 2A). On average, over the course of their follow-up intervals, endoscopically managed patients underwent 3.7 surgeries (95% CI, 3.2–4.1) compared to 1.9 in the open group (95% CI, 1.5–2.3, P = 0.0001).
Fig. 2.
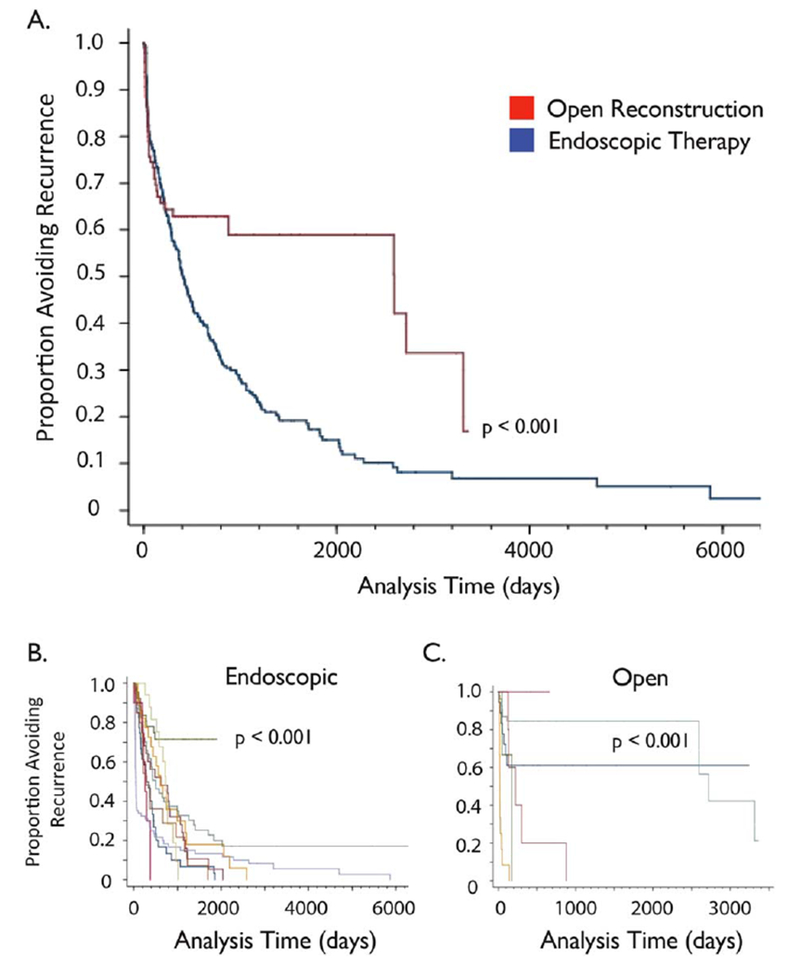
Disease recurrence in open and endoscopic therapy. (A) Kaplan Myer curve depicting the percentage of patients avoiding disease recurrence after their initial procedure at 10 pooled centers. (B) When the subgroup of patients that underwent endoscopic surgery was stratified by center, there was one significant positive outlier (site 3; P < 0.001). (C) When the subgroup of patients that underwent open surgery was stratified by center, there were two significant positive outliers (sites 1 and 8; P < 0.001).
Recurrence rate after endoscopic treatment differed significantly by location (chi2 = 58.3, P < 0.0001) (Fig. 2B). With the exception of one notable positive outlier, 80% of endoscopically treated patients at all centers recurred by 1,000 days of initial surgery. The exception was site 3, where only 25% required subsequent surgical intervention. Patients who underwent open reconstruction had a lower recurrence rate compared to patients treated with endoscopic approaches. Specifically, only 40% of the patients who had open surgery recurred by postoperative day 1,000. However, when stratified by institution, open procedures also had disparate rates of disease recurrence (chi2 = 75.32, P < 0.0001) (Fig. 2C). In particular, patients at sites 1 and 8 had higher rates of durable recurrence-free survival when compared with patients at sites 5, 6, and 7 (P = < 0.001). In contrast to disease recurrence, there was no statistical difference in the presence of tracheostomy at last follow-up between centers (chi1 = 3.886, P = 0.72). Secondary analysis showed no relationship between outcome and center endoscopic surgical volume (Spearman r = 0.16, P = 0.64). In contrast, open surgery did show a significant negative correlation between surgical volume and recurrence rate (Spearman r = −0.91, P < 0.0001); that is, higher volume was related to less recurrence (Fig. 3).
Fig. 3.
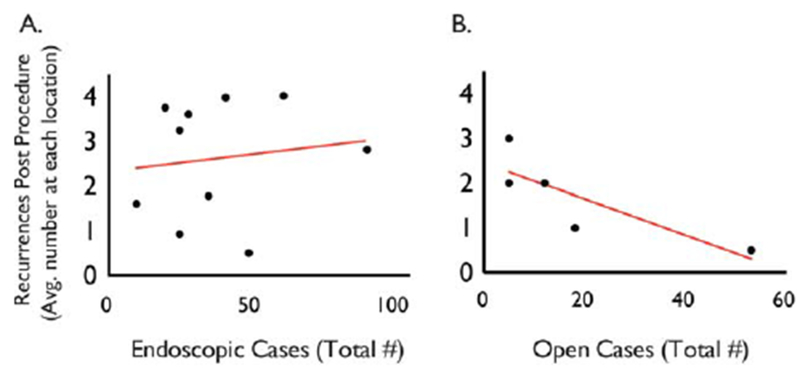
Relationship of surgical volume to outcome. There was no relationship between outcome and a center’s endoscopic surgical volume (Spearman r = 0.16, P = 0.64). In contrast, open surgery did show a significant negative correlation between surgical volume and recurrence rate (Spearman r = −0.91, P < 0.0001); that is, higher volume was related to less recurrence.
DISCUSSION
Rare diseases like idiopathic subglottic stenosis (iSGS) seem a miniscule corner of healthcare when considered in isolation. However, when the 7000 unique rare diseases recognized in the US are considered collectively, they affect over 30 million individuals or 10% of our population.9 For perspective, this is a disease magnitude similar to diabetes.10 Despite progressive and recurrent lifethreatening airway obstruction, iSGS is marginalized in healthcare research in lieu of more common diseases. Patients are an underserved minority from a research standpoint with depth of knowledge lagging significantly behind more prevalent diseases. A byproduct of this marginalization is that affected patients can face long delays in diagnosis, little decision support, and varied treatment outcomes. These effects combine to increase the psychological and physical morbidity associated with disease.
This study demonstrates a remarkable homogeneity among patients afflicted with iSGS; they are otherwise healthy, middle-aged, Caucasian, and female. The disease presentation was also nearly identical across all 10 geographically varied participating sites. Data herein represent the most complete characterization of iSGS epidemiology to date. Furthermore, it provides a window into the natural history of the disease process when treated with multiple management styles over more than a decade. Few conditions affect such a demographically similar group of patients.
The remarkable homogeneity of the iSGS population would appear to offer support for the hypothesis that a conserved and consistent biologic process is driving a singular disease. Although the clinical similarity would suggest a sex-linked genetic abnormality, the relatively mature age of presentation argues against a purely genetic etiology; additionally, there were no familial cases in our series (although they have been reported11). Alternatively, the nearly universal involvement of females, and the age of presentation (~50 years) coinciding with the hormonal alterations observed in menopause (average age 50),12 would support a hormonally mediated process; however, this also remains to be elucidated. Alternative hypotheses for disease pathogenesis have been put forward, including a subtle manifestation of collagen vascular disease, an anatomic predisposition of the smaller female subglottis, mechanical trauma,13 and a sequela of gastroesophageal reflux disease,14 as well as a fibrosing disorder in the spectrum of IgG4-related disease.15 Yet, these concepts have not proven applicable to the majority of patients or have brought tangible benefits when applied therapeutically.
Despite the homogeneity of patients and disease process across centers, there is considerable heterogeneity in the approach to treat this condition. The number of patients offered open reconstruction, and the criteria for that decision appear to vary across centers. Additionally, although broadly categorized into open and endoscopic approaches, centers all offer unique variations related to operative technique and adjunctive care (e.g., medical management pre-, intra-, or postoperatively). Some of these variations may explain intracenter differences in recurrence rates within open and endoscopic categories. The relative value of these variations is unknown and supports the need for collaborative prospective study.
Another explanation for the observed variation in outcomes between centers may relate to procedural volume. In secondary analysis, there was a moderate inverse correlation between surgical volume and recurrence rate for open approaches. Interestingly, no relationship was observed for endoscopic approaches. Volume-outcome relationships have been demonstrated for common surgical diseases,16,17 but they have not been clearly elucidated for more rare conditions. Interestingly, careful investigation into these relationships in high-risk surgical conditions has revealed that factors other than hospital volume are responsible for trends toward declining mortality.18 Similar to the present study, other reports have shown that this volume-outcome relationship is not pervasive. Some procedural outcomes showed an association to volume (e.g., pancreatectomy, esophagectomy), whereas others did not. This discrepancy may relate to adjunctive factors (i.e., process) rather than simply a volume-outcome paradigm. It can be surmised that rare disease collaborations may benefit patients by developing a process; the nature of these disease obviates high-volume treatment.
Although the present study characterized affected patients and directly compared the effectiveness of primary treatments (i.e., endoscopic dilations and open reconstruction surgery), it has highlighted the need for more definitive prospective study. Beyond the gaps in understanding of the relative effectiveness of clinical outcomes, no studies have explored health-related quality of life or functional outcomes in iSGS. These endpoints are important to patients and are arguably a primary determinant in decision making. For example, results show that endoscopic dilation is associated with a higher rate of disease recurrence and thus need for repeated surgery. Meanwhile, open reconstruction is a major surgery with significant immediate perioperative risks and has been associated with alterations in voice19,20 and swallowing.21 Open surgery appears to reduce the risk of disease recurrence, but the degree of benefit and the trade-offs associated with this invasive surgery are questions that demand prospective study.
Owing to the inherent constraints involved in rare disease research, the retrospective nature of this cohort study admittedly has several limitations, including the limited number of patients studied at each site and the lack of a priori defined treatment protocol (allowing for variation in treatment strategies among the study sites). However, as our data suggest, the population of patients with the iSGS is uniquely and strikingly homogeneous. As a group, they are a cohort of age, sex, race, and comorbidity-matched patients. Although treatment approaches varied among centers, there was little variation within centers; thus, comparing objective outcomes between centers maintains a high degree of validity. Additionally, as with any retrospective study looking at time to event data, our study has the potential for right-sided and/or left-sided censoring bias. With mean follow-up of 56 months (95% CI: 51.2–1.6), however; we believe the period of observation was sufficient to mitigate against this risk given our demonstration that 90% of recurrences occurred within 28 months.
In view of present findings, iSGS is nearly exclusively restricted to adult Caucasian females lacking comorbid disease. The homogeneity of the affected population, coupled with wide variation in treatment strategies, provides a unique opportunity to investigate the relationship between variation in therapeutic approaches and clinical outcomes in a rare surgical disease. Understanding the variables (clinical and structural) driving these relationships will require a detailed prospective investigation of the processes of care.
Acknowledgments
This was a North American Airway Collaborative (NoAAC) Study. Research in the North American Airway Collaborative is supported by Patient-Centered Outcomes Research Institute under award number 1409-22214. Additionally, David Francis is supported by the National Institutes of Health (K23DC013559). The content is solely the responsibility of the authors.
Footnotes
The authors have no financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Maldonado F, Loiselle A, Depew ZS, et al. Idiopathic subglottic stenosis: an evolving therapeutic algorithm. Laryngoscope 2014;124:498–503. [DOI] [PubMed] [Google Scholar]
- 2.Gelbard A, Francis DO, Sandulache VC, Simmons JC, Donovan DT, Ongkasuwan J. Causes and consequences of adult laryngotracheal stenosis. Laryngoscope 2014;125:1137–1143. doi: 10.1002/lary.24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Field MJ, Boat TF, eds. Institute of Medicine. Rare Diseases and Orphan Products: Accelerating Research and Development. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 4.Gliklich RE, Dreyer NA, eds. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville, MD: US Department of Health and Human Services; 2014. [PubMed] [Google Scholar]
- 5.The Patient-Centered Outcomes Research Institute (PCORI). Advisory Panel on Rare Disease Meeting Summary. Washington, DC; 2014. [Google Scholar]
- 6.Nouraei SA, Sandhu GS. Outcome of a multimodality approach to the management of idiopathic subglottic stenosis. Laryngoscope 2013;123:2474–2484. [DOI] [PubMed] [Google Scholar]
- 7.Baugnee PE, Marquette CH, Ramon P, Darras J, Wurtz A. [Endoscopic treatment of post-intubation tracheal stenosis. Apropos of 58 cases]. [Article in French]. Rev Mal Respir 1995;12:585–592. [PubMed] [Google Scholar]
- 8.Grillo HC. Primary reconstruction of airway after resection of subglottic laryngeal and upper tracheal stenosis. Ann Thorac Surg 1982;33:3–18. [DOI] [PubMed] [Google Scholar]
- 9.National Human Genome Research Institute. Frequently Asked Questions About Rare Diseases. 2015. Retrieved from http://www.genome.gov/27531963#al-2.
- 10.Grant RW, Pirraglia PA, Meigs JB, Singer DE. Trends in complexity of diabetes care in the United States from 1991 to 2000. Arch Intern Med 2004;164:1134–1139. [DOI] [PubMed] [Google Scholar]
- 11.Dumoulin E, Stather DR, Gelfand G, Maranda B, Maceachern P, Tremblay A. Idiopathic subglottic stenosis: a familial predisposition. Ann Thorac Surg 2013;95:1084–1086. [DOI] [PubMed] [Google Scholar]
- 12.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 2011;38:425–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damrose EJ. On the development of idiopathic subglottic stenosis. Med Hypotheses 2008;71:122–125. [DOI] [PubMed] [Google Scholar]
- 14.Blumin JH, Johnston N. Evidence of extraesophageal reflux in idiopathic subglottic stenosis. Laryngoscope 2011;121:1266–1273. [DOI] [PubMed] [Google Scholar]
- 15.Kobraei EM, Song TH, Mathisen DJ, Deshpande V, Mark EJ. Immunoglobulin g4-related disease presenting as an obstructing tracheal mass: consideration of surgical indications. Ann Thorac Surg 2013;96: e91–e93. [DOI] [PubMed] [Google Scholar]
- 16.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349:2117–2127. [DOI] [PubMed] [Google Scholar]
- 17.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl JMed 2002;346:1128–1137. [DOI] [PubMed] [Google Scholar]
- 18.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med 2011;364:2128–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryans L, Palmer AD, Schindler JS, Andersen PE, Cohen JI. Subjective and objective parameters of the adult female voice after cricotracheal resection and dilation. Ann Otol Rhinol Laryngol 2013;122: 707–716. [DOI] [PubMed] [Google Scholar]
- 20.Ettema SL, Tolejano CJ, Thielke RJ, Toohill RJ, Merati AL. Perceptual voice analysis of patients with subglottic stenosis. Otolaryngol Head Neck Surg 2006;135:730–735. [DOI] [PubMed] [Google Scholar]
- 21.Miller CK, Linck J, Willging JP. Duration and extent of dysphagia following pediatric airway reconstruction. Int J Pediatr Otorhinolaryngol 2009;73:573–579. [DOI] [PubMed] [Google Scholar]


