Abstract
Adequate maternal vascular adaptations and blood supply to the uterus and placenta are crucial for optimal oxygen and nutrient transport to growing fetuses of eutherian mammals, including humans. Multiple factors contribute to hemodynamics and structuring of placental vasculature essential for term pregnancy with minimal complications. In women, failure to achieve or sustain favorable pregnancy progression is, not surprisingly, associated with high incidence of antenatal complications, including preeclampsia, a hypertensive disorder of pregnancy. While the pathogenesis of preeclampsia in women remains unknown, a role for androgens is emerging. The relationship between androgens and maternal cardiovascular and placental function deserves particular consideration because testosterone levels in the circulation of preeclamptic women are elevated approximately two- to three-fold and are positively correlated with vascular dysfunction. Preeclampsia is also associated with elevated placental androgen receptor (AR) gene expression. Studies in animal models mimicking the pattern and level of increase of adult female testosterone levels to those found in preeclamptic pregnancies, replicate key features of preeclampsia, including gestational hypertension, endothelial dysfunction, exaggerated vasoconstriction to angiotensin II, reduced spiral artery remodeling, placental hypoxia, decreased nutrient transport and fetal growth restriction. Taken together, these data strongly implicate AR-mediated testosterone action as an important pathway contributing to clinical manifestation of preeclampsia. This review critically addresses this hypothesis, taking into consideration both clinical and preclinical data.
Introduction
Pregnancy is characterized by major cardiovascular adaptations, including marked decreases in systemic vascular resistance and mean arterial pressure, along with increases in maternal cardiac output and total blood volume (Magness 1998, Thornburg et al. 2000, Chinnathambi et al. 2013a ). Studies suggest that pregnancy-enhanced vasodilatory actions allow peripheral vessels to accommodate increases in blood flow and volume (Conrad et al. 1993). Consistently, maternal vascular adaptations are accompanied by blunted vascular contractility (Naden & Rosenfeld 1981, Magness & Rosenfeld 1986) and enhanced release of endothelium-derived vasodilatory factors (Kawano & Mori 1983, Magness et al. 1990, 1996, 2000, Conrad et al. 1993, Sladek et al. 1997, Williams et al. 1997, Gillham et al. 2003, Gokina et al. 2010). Failure of these vascular adaptations during pregnancy are directly related to several maternal/fetal pathologies, such as increased systemic vascular resistance, hypertension, proteinuria, poor placental growth, decreased nutrient transport and low birth weight, all characteristics associated with a diagnosis of preeclampsia (Powe et al. 2011) outlined in Table 1. Despite being a leading contributor of maternal and perinatal morbidity and death worldwide, the etiology and pathogenesis of preeclampsia remain unclear (Roberts et al. 1991, Palei et al. 2013, Salam et al. 2015).
Table 1.
Diagnostic criteria for preeclampsia in women (ACOG Guidelines 2013).
| Parameter | Diagnostic criteria |
|---|---|
| Blood pressure | Greater than or equal to 140 mmHg systolic or greater than or equal to 90 mmHg diastolic on two occasions at least 4 h apart after 20 weeks of gestation in a woman with a previously normal blood pressure |
| OR | |
| Greater than or equal to 160 mmHg systolic or greater than or equal to 110 mmHg diastolic, hypertension can be confirmed within a short interval (minutes) to facilitate timely antihypertensive therapy | |
| Proteinuria | Greater than or equal to 300 mg per 24 h urine collection (or this amount extrapolated from a timed collection) |
| OR | |
| Protein/creatinine ratio greater than or equal to 0.3* | |
| Dipstick reading of 1+ (used only if other quantitative methods not available) | |
| OR in the absence of proteinuria, new-onset hypertension plus new onset of any of the following: | |
| Thrombocytopenia | Platelet count less than 100,000/microliter |
| Renal insufficiency | Serum creatinine concentrations greater than 1.1 mg/dL or a doubling of the serum creatinine concentration in the absence of other renal disease |
| Impaired liver function | Elevated blood concentrations of liver transaminases to twice normal concentration |
| Pulmonary edema | |
| Cerebral or visual symptoms | |
*Each measured as mg/mL.
Treatment options for preeclampsia are limited to management of high blood pressure using antihypertensives, such as methyldopa, hydralazine, labetalol and nifedipine (ACOG 2013), as well as magnesium sulfate for prevention of eclamptic seizures (Al Khaja et al. 2014); however, these treatments have limited efficacy, and the only cure is the delivery of the placenta with baby, a totally undesired outcome before late preterm (≥34 weeks gestation). While the exact causes of preeclampsia remain unknown, a large body of evidence, supported by preclinical models of preeclampsia, indicates that abnormal placentation early in pregnancy is an important initial event in the onset of preeclampsia (Roberts & Redman 1993, Myatt 2002). Such preeclamptic abnormal placentation stimulates the production of anti-angiogenic factors and cytokines, resulting in generalized vascular dysfunction and the clinical manifestation of preeclampsia. In the past several years, dysregulation of steroid hormones, specifically increases in maternal testosterone levels, has emerged as an important endocrinopathy repeatedly associated with clinical manifestations of preeclampsia. In order to develop more effective therapeutic interventions for preeclampsia, it is important to fully understand the role of testosterone in maternal vascular and placental function, as well as blood pressure control, during normal pregnancy and in preeclampsia. This review critically addresses the hypothesis of testosterone-mediated pathogenesis of preeclampsia, taking into consideration data from both clinical (human) and preclinical (animal) studies.
Testosterone levels in clinical preeclampsia
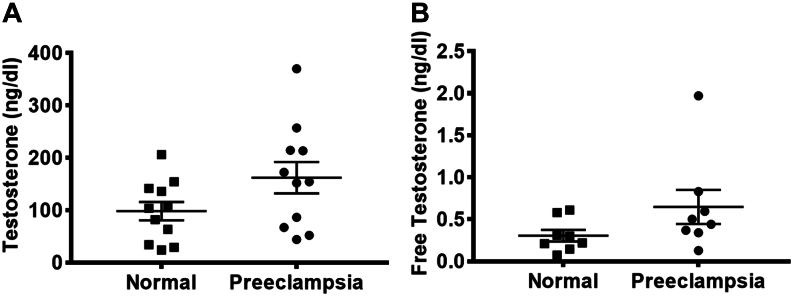
Most studies have investigated the beneficial role of sex steroid hormones, especially estradiol and progesterone, on cardiovascular function during pregnancy in women (Magness 1998). The relationship between testosterone and maternal cardiovascular function, however, is relatively understudied. PubMed search with keywords, ‘testosterone, preeclampsia and women’, generated 40 publications that were manually screened to identify 14 full-length papers reporting testosterone levels in both preeclampsia and control groups. Twelve of these 14 studies reported elevated plasma levels of testosterone during preeclamptic compared to normotensive (control) pregnancies (Table 2) (Acromite et al. 1999, Serin et al. 2001, Steier et al. 2002, Ficicioglu & Kutlu 2003, Miller et al. 2003, Troisi et al. 2003, Atamer et al. 2004, Baksu et al. 2004, Carlsen et al. 2005, Gerulewicz-Vannini et al. 2006, Salamalekis et al. 2006, Ghorashi & Sheikhvatan 2008, Hsu et al. 2009, Sharifzadeh et al. 2012). These studies report that during late pregnancy, plasma testosterone concentrations range between 100 and 150 ng/dL and these are 1.5- to 2.4-fold higher in preeclamptic compared to normotensive pregnant women (Fig. 1A). The reported mean unbound or ‘free’ testosterone level circulating in preeclamptic women is also 1.4- to 3.4-fold higher compared to normotensive pregnancies (Fig. 1B). Some studies also indicate that circulating testosterone levels correlate with the severity of preeclampsia, although this is not a universal finding (Ficicioglu & Kutlu 2003, Atamer et al. 2004). While there are many androgens, including the relatively bio-ineffective testosterone precursors of dehydroepiandrosterone (DHEA) and androstenedione (A4), only circulating levels of testosterone are increased during preeclampsia (Table 2). Preeclamptic hyperandrogenic measures include elevated total testosterone, free testosterone, free androgen index (FAI, total testosterone × 100/sex hormone binding globulin) and the testosterone-to-estradiol ratio. Hyperandrogenic pregnant women with polycystic ovary syndrome (PCOS) are at increased risk for preeclampsia (de Vries et al. 1998, Kjerulff et al. 2011, Kamalanathan et al. 2013), and it has been proposed that overproduction of testosterone by the polycystic ovary is the causal factor engaging preeclampsia in PCOS women (Diamant et al. 1982, Sir-Petermann et al. 2002, Codner & Escobar-Morreale 2007). Obesity, and accompanying insulin resistance-induced compensatory hyperinsulinemia, is predictive of preeclampsia (Seely & Solomon 2003). Insulin stimulates androgen release, including testosterone, from theca cells of normal ovaries (Franks et al. 1999), and thus, the exaggerated hyperinsulinemia of obesity during preeclamptic gestation (Kaaja et al. 1995, Lorentzen et al. 1998) likely contributes to increased maternal testosterone levels (Andersen et al. 1995, Pasquali et al. 2000, Sutton-Tyrrell et al. 2010). Since both obesity, hyperinsulinemia and preeclampsia are more prevalent among hyperandrogenic pregnant women with PCOS than in pregnant women without PCOS (Lonnebotn et al. 2018), obesity-enhanced maternal testosterone levels may contribute to PCOS-associated preeclampsia.
Table 2.
Plasma androgen levels in healthy and preeclamptic pregnant women.
| Reference/Androgen | Normal vs preeclampsia | P | % Increase in preeclampsia# | Gestational age (weeks) |
|---|---|---|---|---|
| Acromite et al. (1999) | 36–38 | |||
| Total TS Free TS DHEA-S Estradiol | 154.5 vs 213.6 ng/dL0.3 vs 0.5 ng/dL175.5 vs 171.0 µg/dL33.8 vs 36.4 g/mL | <0.01<0.05NSNS | 3867 | |
| Salamalekis et al. (2006) | 30–31 | |||
| Total TS Free TS DHEA-S Androstenedione | 106.3 vs 154.4 ng/dL0.21 vs 0.34 ng/dL76.15 vs 57.62 µg/dL110.5 vs 107.1 ng/dL | <0.05<0.05>0.05>0.05 | 4562 | |
| Ghorashi and Sheikhvatan (2008) | 28–39 | |||
| Free TS | 0.58 vs 1.97 ng/dL | 0.001 | 240 | |
| Serin et al. (2001) | 34–39 | |||
| Total TS Free TS DHEA-S Androstenedione Estradiol | 24.3 vs 44.1 ng/dL0.22 vs 0.44 ng/dL90.5 vs 162.5 µg/dL210 vs 220 ng/dL92.2 vs 73.5 pg/mL | <0.05<0.05NSNSNS | 81100 | |
| Carlsen et al. (2005) | 33 | |||
| Total TS Free TS index DHEA-S Androstenedione | 63.4 vs 86.5 ng/dL0.61 vs 0.83102.3 vs 121.5 μg/dL280 vs 337 ng/dL | 0.0010.012NSNS | 3636 | |
| Baksu et al. (2004) | 34 | |||
| Total TS Free TS index DHEA-S Estradiol | 136 vs 257 ng/dL0.31 vs 0.37109.1 vs 104.3 µg/dL5830.1 vs 6164.2 pg/mL | 0.0010.01NSNS | 8919 | |
| Steier et al. (2002) | 30–38 | |||
| Total TS | 82.1 vs 172.4 ng/dL | <0.01 | 110 | |
| Hsu et al. (2009) | 37 | |||
| Total TS | 34 vs 52 ng/dL | <0.01 | 53 | |
| Gerulewicz-Vannini et al. (2006) | 37 | |||
| Total TS Free TS DHEA-S | 103.7 vs 152.2 ng/dL0.144 vs 0.594 ng/dL70.0 vs 51.1 µg/dL | 0.020.002NS | 47312 | |
| Atamer et al. (2004) | 34–35 | |||
| Total TS DHEA-S Androstenedione Estradiol | 29 vs 67 ng/dL108 vs 112 μg/dL189 vs 158 ng/dL2927 vs 3572 pg/mL | <0.001NSNSNS | 131 | |
| Troisi et al. (2003) | 37 | |||
| Total TS Androstenedione | 141.9 vs 214.5 ng/dL316.0 vs 506.3 ng/dL | 0.00070.004 | 5160 | |
| Sharifzadeh et al. (2012) | 32–33 | |||
| Total TS Free TS DHEA-S | 206 vs 370 ng/dL0.074 vs 0.12851 vs 75 μg/dL | <0.01<0.01NS | 8073 | |
| Miller et al. (2003) | 35 | |||
| Total TS Free TS index DHEA-S Estradiol | 206 vs 147 ng/dL2.03 vs 1.5075 vs 75 μg/dL18,536 vs 9619 pg/mL | NSNSNSNS | ||
| Ficicioglu and Kutlu (2003) | 34–35 | |||
| Total TS Free TS index DHEA-S Estradiol | 218 vs 209 ng/dL4.16 vs 5.24104 vs 77 μg/dL21,880 vs 21,370 pg/mL | NSNS<0.05NS |
All these studies used immunoassays (ELISA and RIA) to measure TS levels. This raises concern regarding assay sensitivity and the specificity because of risk of cross-reactivity between steroids and their multiple placental metabolites. Recently, liquid chromatography tandem mass spectrometry (LC-MS/MS) has been suggested as the new ‘gold standard’ method for measurement of TS levels. This recommendation is more geared towards situations in which measurements of TS are below detectable levels (such as in hypogonadal men, women, children etc.) or in species for which no specific antibodies are available (such as sheep). Recent studies that compared the predictive values of TS levels measured by LC-MS/MS and immunoassay showed no significant difference between the two analytical methods (Czeloth et al. 2017, Mitchell 2012). The TS levels reported in the studies cited here may be appropriate for two reasons. First, the TS levels in pregnant women are within detectable range, and second, the objective is to detect relative change in preeclamptic group compared to controls.
# % increase is calculated as 100 x (preeclampsia - normal)/normal.
TS, testosterone; NS, not significant.
Figure 1.
Mean higher total (A) and free testosterone (B) levels reported in preeclamptic patients compared normotensive controls in published studies cited in Table 1. Each point represents a single published study.
Ethnicity has also been implicated in contributing hyperandrogenism-related preeclampsia. Pregnant African-American women exhibit high maternal testosterone levels (120–130%), including elevated fetal cord blood testosterone levels at term (Henderson et al. 1988, Potischman et al. 2005, Rohrmann et al. 2009, Agurs-Collins et al. 2012) and are at increased risk for developing preeclampsia (Samadi et al. 2001, Iavazzo & Vitoratos 2010). In addition, plasma testosterone levels are increased during pregnancy in a variety of situations (Fig. 2), including classical congenital adrenal hyperplasia (Warmann et al. 2000, Mains et al. 2007), high caffeine intake (Ferrini & Barrett-Connor 1998, Svartberg et al. 2003) and stress (Sarkar et al. 2007, 2008), all of which are known risk factors for preeclampsia. Furthermore, pregnant women are inadvertently exposed to elevated testosterone levels via environmental pollutants and anabolic steroids (endocrine disruptors). High androgenic activity is reported in water from craft pulp and paper mills, as well as concentrated animal feed operations in the United States and Europe (Parks et al. 2001, Orlando et al. 2004). Reports have shown that an androgenic growth promoter used in beef cattle, trenbolone, has a half-life of greater than 260 days in animal by-products (Schiffer et al. 2001, Hotchkiss & Nelson 2007).
Figure 2.
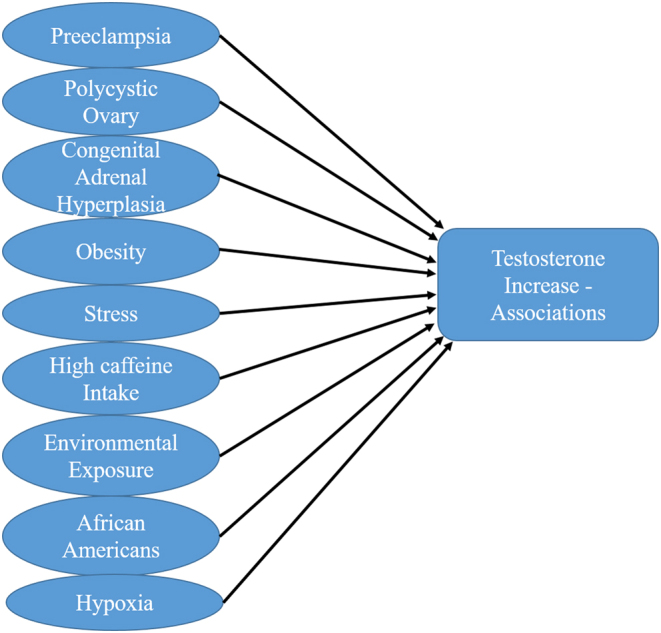
Possible associations for testosterone increase in females and pregnancy.
The degree of hyperandrogenism in preeclamptic women varies depending on the sex of their fetus. Preeclamptic women bearing sons exhibit higher testosterone levels than preeclamptic women bearing daughters (Steier et al. 2002), contributing to the notion that a male fetus and its placenta contribute additional amounts of testosterone to the maternal circulation (Sathishkumar et al. 2012). Such pregnant women bearing male fetuses are at increased risk for developing preeclampsia and placental dysfunction (Stark et al. 2006, Murji et al. 2012, Sykes et al. 2014, Li et al. 2018). Furthermore, daughters experiencing a preeclamptic gestation demonstrate higher circulating testosterone levels when they reach puberty (Alsnes et al. 2016). Such female offspring are at increased risk of developing hypertension and cardiovascular disease as adults (King et al. 2007, Sathishkumar et al. 2011c , Chinnathambi et al. 2012, 2013b , Vyas et al. 2016), and possibly preeclampsia and other pregnancy-related complications. High testosterone levels persist for at least 17 years in women with a documented history of preeclampsia (Laivuori et al. 1998). These studies thus provide consistent circumstantial evidence linking increased testosterone levels with preeclampsia.
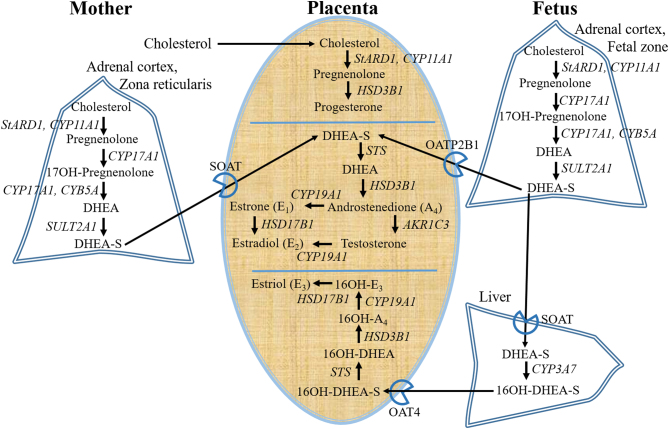
The origin of the increased testosterone levels during preeclampsia remain uncertain. Studies suggest a placental contribution (Steier et al. 2002, Dokras et al. 2003). The human placenta, however, lacks the key androgen biosynthetic enzymes, 17β-hydroxylase and 17,20-desmolase (Christensen 1974). It nevertheless expresses 3β-hydroxysteroid dehydrogenase type 1 (HSD3B1) (Mason et al. 1993), endowing a ready ability to convert DHEA into A4, as well as the estrogen-preferring 17β-hydroxysteroid dehydrogenase type 1 (HSD17B1) (Takeyama et al. 1998), endowing a weak ability to synthesize testosterone from A4. After mid-gestation, both maternal and fetal adrenals equally contribute as the major sources of C19 steroids for placental androgen biosynthesis (Turnipseed et al. 1976, Kowalczyk et al. 1998). The human fetal adrenal cortex includes a fetal zone, expressing StARD1, CYP11A1, CYP17A1 and SULT2A1, essential for production of DHEA and DHEAS sulfate (DHEA-S), analogous to the maternal zona reticularis, the innermost zone of the maternal adrenal cortex (Fig. 3). After membrane uptake carrier transport into placental syncytiotrophoblast cells, sulfonated testosterone precursors (i.e. DHEA-S) are desulfonated by the enzyme sulfatase (STS) to yield DHEA. Placental steroidogenic enzymes (Fig. 3) then convert DHEA to A4 (HSD3B1), and A4 to testosterone (ARK1C3). Accompanying high levels of placental aromatase expression (Mason et al. 1993) ensure ready conversion of placental androgens, including testosterone, into non-androgenic, estrogenic metabolites (Gant et al. 1971, Buster et al. 1979, Dokras et al. 2003), including estrone, estradiol and their catechol and methoxy metabolites, some of which display placental bioactivity rivaling that of E2 (Jobe et al. 2011, Landeros et al. 2018).
Figure 3.
Pathway of biosynthesis and metabolism of testosterone, primary estrogens and progesterone during pregnancy. StARD1, steroidogenic acute regulatory protein; CYP11A1, cholesterol side-chain cleavage enzyme; HSD3B1, 3beta-hydroxysteroid dehydrogenase; CYP17A1, 17α-hydroxylase/17,20-lyase; CYB5A, cytochrome b5; SULT2A1, sulfotransferase; STS, steroid sulfatase; AKR1C3, aldo-keto reductase type 1C3; HSD17B1, hydroxysteroid 17-beta dehydrogenase 1; CYP19A1, aromatase – cell membrane-located uptake carriers of DHEAS; SOAT, sodium dependent organic anion transporter; OATP2B1 and OAT4, organic anion transporters.
The finding that there are no statistically significant differences regarding circulating maternal levels of DHEA-S and A4 between control and preeclamptic pregnancies implies that there is no contribution of adrenal steroids to the hyperandrogenism of preeclampsia. Placental aromatase mRNA and protein expression, however, is decreased in the preeclamptic placenta, diminishing metabolism of A4 and testosterone into estrogenic metabolites, and tipping the equilibrium between estrogens and androgens in favor of androgens (Sathishkumar et al. 2012, Perez-Sepulveda et al. 2015). Hepatic conjugation, and thus inactivation, of estrogens also appears diminished during preeclamptic gestations. Maternal circulating levels of unconjugated estrogens, however, remain unchanged from those in normotensive pregnant women (Rosing & Carlstrom 1984). Studies show that testosterone, alone, diminishes aromatase mRNA expression in human trophoblast cells through a miR-22-mediated mechanism (Shao et al. 2017). Other factors, tumor necrosis factor alpha (Lau et al. 2013) and lipid radicals (Mori et al. 2014), which are increased during preeclampsia downregulate aromatase (Milczarek et al. 2008, Diaz et al. 2009). In addition, hypoxia (which mirrors the actual conditions of the placenta in the context of preeclampsia) also downregulates placental aromatase (Jiang et al. 2000, Perez-Sepulveda et al. 2015, Yu et al. 2015). It would be interesting to assess whether compromised expression of aromatase also exists in tissues and organs other than placenta during preeclamptic gestation. A recent study also indicated that overexpression of CYP11A1 mRNA (commonly referred as cholesterol side-chain cleavage enzyme that catalyzes the first steps of steroidogenesis) in human trophoblast cells induces increased testosterone production and preeclampsia-like placental dysfunction that could be reversed with flutamide, an androgen receptor antagonist (Pan et al. 2017). Taken together, these findings support the notion that increased testosterone during preeclamptic pregnancies may be of placental origin, although other sources cannot be excluded.
Testosterone and maternal blood pressure and uterine artery blood flow
In healthy women experiencing no complications during pregnancy, arterial pressure is stable during the early part of first trimester and then gradually decreases, reaching a nadir during the second trimester (Magness 1998, Bosio et al. 1999). Lack of this pregnancy-related decrease in blood pressure indicates a failure in normal cardiovascular adaptation and is considered to be a cardinal feature of preeclampsia (Ishikuro et al. 2012). Several independent investigators have demonstrated, through human and animal studies, the association of androgens, especially testosterone, with hypertension (Nakao et al. 1981, Reckelhoff et al. 1998, Gonzales et al. 2004, 2005, Park et al. 2004, Chen et al. 2007, Yanes et al. 2009, Makinen et al. 2011). Testosterone levels correlate positively with systolic blood pressure and diastolic blood pressure during and after preeclampsia (Laivuori et al. 1998, Serin et al. 2001, Carlsen & Heimstad 2012). Experimentally induced increases in maternal testosterone levels during pregnancy in rats, at concentrations that mimic testosterone levels found in human preeclamptic pregnancies, induce increases in systemic arterial pressure (Chinnathambi et al. 2013a , 2014b , Fornes et al. 2016), implying a causal role for testosterone in raising blood pressure during gestation. The exact mechanism by which testosterone mediates an increase in maternal blood pressure during pregnancy is not clear, but accumulating evidence indicates that testosterone increases vascular reactivity, activating the renin-angiotensin system and altering eicosanoid metabolism, thus favoring an increase in the thromboxane A2 to prostacyclin (PGI2) ratio and causing platelet aggregation in ways that are strikingly similar to those reported in preeclampsia (Acromite et al. 1999). Our unpublished observations also show that elevating rat maternal testosterone levels during pregnancy induce renal hypertrophy and proteinuria, a hallmark feature of preeclampsia (Sathishkumar et al. 2011a ). Treatment with a selective angiotensin type 1 receptor (AT1R) antagonist, losartan, markedly attenuated the hypertension induced by testosterone in the pregnant rats (Chinnathambi et al. 2014b ). These findings suggest that AT1R activation contributes, at least in part, to the testosterone-induced increase in blood pressure in rat pregnancies.
In addition to adjustments in systemic vasculature, the uteroplacental circulation normally adapts to maintain a low vascular tone to accommodate a more than 20-fold increase in uterine blood flow near-term (Rosenfeld et al. 1974, Magness 1998, Osol & Mandala 2009). Studies in hyperandrogenic women with PCOS have shown that their high maternal testosterone levels are associated with increased uterine artery resistance index and reduced blood flow (Palomba et al. 2010, 2012). Experimentally induced increase in maternal testosterone levels in pregnant rats show significantly reduced uterine arterial blood flow by 40% (measured using transcutaneous micro ultrasound) (Gopalakrishnan et al. 2016). In addition, elevated testosterone decreases uterine arterial diameter and increases resistance and pulsatile index (Gopalakrishnan et al. 2016). These findings suggest that the mechanisms controlling blood pressure and uterine artery hemodynamics during pregnancy are perturbed by elevated maternal testosterone levels. Primary estrogens, estrone, estradiol-17β and estriol play an important role in maintaining uterine blood flow and blunting vascular responses during pregnancy (Albrecht & Pepe 1990, Magness 1998). Jobe et al. (2013) elegantly demonstrated that these primary estrogens, and the majority of their catechol and methoxy metabolites, including those with demonstrable placental bioactivity (Jobe et al. 2011, Landeros et al. 2018), are reduced in preeclampsia (Jobe et al. 2013). The lower levels of primary estrogens, together with the reduced expression of placental aromatase could induce precursor steroid hormone accumulation, causing C19 steroids, especially testosterone, to be elevated. It is unclear if elevated testosterone acts independently or if it synergies with reduced downstream C18 estrogens to cause preeclampsia progression. Progesterone levels, however, are reported to be normal (Rosing & Carlstrom 1984, Bussen et al. 1998, Hertig et al. 2010), decreased (Acikgoz et al. 2013, Wan et al. 2018) or increased (Tamimi et al. 2003, Metz et al. 2014) in preeclampsia. Further studies will be needed to clarify whether there is hitherto unrecognized relationship between progesterone and testosterone, and if they work in concert in preeclampsia pathogenesis.
Testosterone-induced mechanisms of vascular dysfunction during pregnancy
Effects on endothelium-dependent relaxation
In humans, normal maternal vascular adaptations are accompanied by enhanced release of three major endothelium-derived vasodilatory factors including nitric oxide (NO) (Conrad et al. 1993, Sladek et al. 1997, Williams et al. 1997), PGI2 (Kawano & Mori 1983, Magness et al. 1990, 1996, 2000) and endothelium-derived hyperpolarizing factor (EDHF) (Gillham et al. 2003, Gokina et al. 2010). This is accompanied with concomitant pregnancy-induced increases in mRNA and protein expression of endothelial NO synthase (eNOS) (Sladek et al. 1997, Williams et al. 1997, Nelson et al. 2000, Magness et al. 2001), endothelial prostaglandin-I synthase (PGIS) (Bird et al. 2000, Magness et al. 2000) and EDHF activity (Gokina et al. 2010). In the systemic circulation, the principal endothelium-dependent vasodilators are NO and EDHF (Chinnathambi et al. 2013a ). In the uterine arteries, in addition to NO and EDHF, PGI2 also plays a role in mediating vascular relaxation (Cooke & Davidge 2003). Elevated testosterone is shown to inhibit acetylcholine-induced relaxation of rat mesenteric and uterine arteries suggesting that elevated testosterone impairs endothelium-dependent relaxation. Specifically, the NO-mediated vasodilation was significantly decreased in mesenteric and uterine arteries in a pregnant rat model of elevated maternal testosterone (Chinnathambi et al. 2013a , 2014a ). This testosterone-induced decrease in NO-mediated arterial relaxation was found not related to decreased vascular smooth muscle sensitivity to NO, as relaxation of arterial rings to sodium nitroprusside, an exogenous NO donor, was not affected (Chinnathambi et al. 2013a , 2014a ). These findings indicate that testosterone likely alters synthesis/release of NO. Consistently, studies in rats have shown that testosterone decreases plasma levels of NO x (marker of NO production) with decreases in eNOS protein expression in uterine arteries (Chinnathambi et al. 2014a ) and eNOS activity (decreased phosphorylation at excitatory Ser1177 site and increased phosphorylation at inhibitory Thr495 site) in mesenteric arteries (Chinnathambi et al. 2013a ). The effect of testosterone in rat uterine arteries appears to be more profound than that in mesenteric arteries as in addition to decreasing NO pathway, it also compromises the EDHF- and PGI2-mediated relaxation by decreasing expression of small conductance calcium-activated channel-3 and PGI2 receptor, respectively (Chinnathambi et al. 2014a ). Taken together, these results suggest that elevated testosterone during pregnancy may specifically impair the NO-mediated relaxation in systemic (mesenteric) vessels, while it compromises all three major vasodilatory pathways in reproductive (uterine) vessels.
Effects on vascular smooth muscle contractile response
Systemic and uterine vasculature are refractory to vasoconstrictions during pregnancy. In contrast, enhanced contractile responses to vasoconstrictors is a characteristic feature of preeclampsia (Naden & Rosenfeld 1981, Magness & Rosenfeld 1986, Benoit et al. 2007, Stanhewicz et al. 2017). Elevated testosterone during pregnancy is shown to enhance contractile responses to many vasoconstrictors in endothelium-intact vessels, but in endothelium-denuded vessels, there is enhanced contractile response specific to angiotensin II in rat mesenteric (Chinnathambi et al. 2014b ) and uterine arteries (Chinnathambi et al. 2014a ). These enhanced responses observed in rat endothelium-denuded vessels indicate that enhanced arterial sensitivity is primarily because of increased angiotensin II-induced contractions, per se, rather than the loss of the endothelium-mediated relaxation component (Chinnathambi et al. 2014a ). Since elevated testosterone does not alter the vasomotor response to other potent constrictors, such as K+ depolarization, thromboxane agonist U46619 and phenylephrine in endothelium-denuded vessels (Chinnathambi et al. 2014a , b ), it appears that testosterone has a selective effect in enhancing vascular smooth muscle response to angiotensin II. It is possible that testosterone-mediated vascular smooth muscle dysfunction occurs at the agonist-specific receptor level rather than at common intracellular signaling pathways. Consistently, studies show that gestational elevation in testosterone levels causes selective upregulation of vasocontractile AT1 receptor and downregulation of vasodilatory AT2 receptor in mesenteric and uterine arteries implying that increased AT1/AT2 receptor ratio may play an underlying role in testosterone-induced exaggerated vasoconstriction to angiotensin II (Chinnathambi et al. 2014a , b ).
Testosterone on placental development and function
The progenitor cytotrophoblast cell is the stem cell of the placenta. These cells proliferate throughout gestation, differentiating along two pathways to form either villous cytotrophoblast, which ultimately can become syncytiotrophoblasts (outer cellular layer) or extravillous cytotrophoblasts (inner cellular layer). Syncytiotrophoblast is a specialized epithelium that has several functions, including transport of gases, nutrients and waste products and synthesis of peptide and steroid hormones that regulate placental, fetal and maternal systems. Extravillous trophoblasts have a proliferative component and an invasive component. There is also a migratory extravillous trophoblast, which is neither invasive nor proliferative. AR is present in syncytiotrophoblasts and in the decidua during the first trimester of human gestation (Horie et al. 1992). The expression of AR in human preeclamptic placentae is considerably higher than its expression in healthy placentae from uncomplicated pregnancies (Hsu et al. 2009, Sathishkumar et al. 2012). Also, genetic polymorphisms in the AR gene are associated with increased risk of preeclampsia (Lim et al. 2011). Rat models show that experimentally elevated maternal testosterone levels during pregnancy induce a reduction in placental size and weight (Sathishkumar et al. 2011b , Sun et al. 2012). The reason for smaller placenta in testosterone-exposed dams is not known, but may involve increased apoptosis or decreased proliferation (Ling et al. 2002). Pan et al. (2017) revealed a critical role for testosterone in human trophoblast invasion and demonstrated that flutamide, an AR antagonist, could rescue testosterone-induced reduction in invasion (Pan et al. 2017). It is possible that testosterone-induced autophagy (human) (Pan et al. 2017), reduced invasion (human) (Pan et al. 2017) or advanced placental differentiation (sheep) (Veiga-Lopez et al. 2011), may all contribute to such alterations in placental weight/morphology.
Vasculogenesis and angiogenesis are critical processes that lead to the formation of the placental vascular network necessary for optimal uteroplacental circulation (Huppertz & Peeters 2005, Arroyo & Winn 2008). Testosterone, however, downregulates the expression of genes related to vascular development and angiogenesis (Ccr3, Stra6, Dhcr7, Arid1a, Ptprj, Col1a2, Lef1, Col1a1 and Mmp2) in the rat placenta (Gopalakrishnan et al. 2016). Along with this antivasculogenic gene expression profile, testosterone also decreases the radial and spiral artery diameters and inhibits branching angiogenesis (Gopalakrishnan et al. 2016). One of the important functions of the placenta is to promote nutrient transport to the fetus. Elevated testosterone is shown to decrease placental amino acid transport to rat fetuses (Sathishkumar et al. 2011b ). This reduction in amino acid transport is related to reduced expression of the system A amino acid transporters (slc38a2/Snat 2) in the rat placenta (Sathishkumar et al. 2011b ). Testosterone also decreases placental oxygenation with associated increase in hypoxia-inducible factor 1α and hypoxia responsive genes, presumable due to compromised placental vascularization (Gopalakrishnan et al. 2016). In addition to placental compromise, the fetuses of testosterone -exposed pregnant rats also receive less oxygen and are hypoxic (Gopalakrishnan et al. 2016). Elevated testosterone, however, does not alter glucose transport across the rat placenta (Sathishkumar et al. 2011b ). Thus, testosterone increases during pregnancy alter placental structure and function leading to decreases in amino acid and oxygen availability to the fetus.
Testosterone effects on fetal growth
Studies have shown that elevated maternal testosterone levels are associated with reduced birth weights in certain human populations (Sir-Petermann et al. 2005, Carlsen et al. 2006, Mehrabian & Kelishadi 2012), rats (Sun et al. 2012, Fornes et al. 2016), sheep (Manikkam et al. 2004, Steckler et al. 2005, Recabarren et al. 2008, Beckett et al. 2014) and marmoset monkeys (Smith et al. 2010), but not in rhesus monkeys (Abbott et al. 2010) and not in human populations of non-Spanish descent (Abbott et al. 2016). In rhesus monkeys, testosterone was experimentally increased during early-to-mid-gestation, 2 months prior to parturition, hence, it is possible that initial fetal growth restriction is masked by subsequent in utero catchup growth. Female infant monkeys, rats and sheep exposed to such gestational testosterone excess, however, exhibit accelerated body weight gain 2 months following parturition (Manikkam et al. 2004, Abbott et al. 2010, Sathishkumar et al. 2011c ) and demonstrate increased abdominal adiposity and onset of type 2 diabetes and hypertension in adulthood (Chinnathambi et al. 2012, 2013b , Abbott et al. 2016). Testosterone is a lipophilic hormone and can diffuse across tissues, including placenta (Dell’Acqua et al. 1966, Meulenberg & Hofman 1991, Wang et al. 2005); however, whether fetal growth restriction induced by testosterone is the result of a direct effect on the fetus, or is secondary to decreased uterine blood flow or compromised placental function, remains to be resolved.
Conclusions
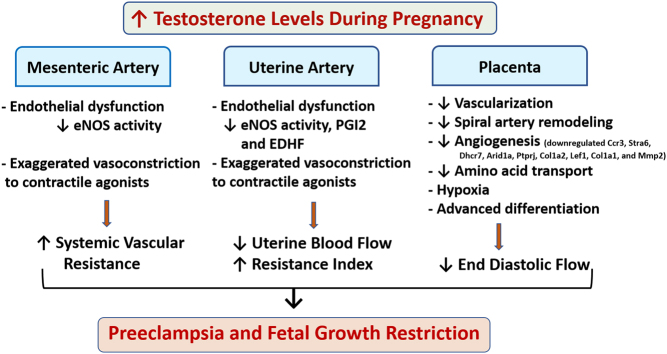
Several studies show that circulating levels of testosterone are two- to three-fold higher in preeclamptic pregnancies compared to those of healthy women experiencing uncomplicated pregnancies. Elevated testosterone in pregnant rats results in significantly increased arterial pressure and decreased uterine arterial hemodynamics. Testosterone, in pregnant rats, also causes endothelial dysfunction and exaggerated vasoconstriction to contractile agonists and dysregulates renin-angiotensin system with exaggerated vascular smooth muscle sensitivity to angiotensin II. In addition, testosterone compromises rat placenta vascularization and nutrient transport leading to placental hypoxia and fetal growth restriction. It is therefore possible that some of the vascular and placental effects observed during preeclampsia may indeed be testosterone mediated (Fig. 4). Therefore, strategies that (1) diminish excessive testosterone action in the cardiovascular and placental system and (2) identify the cause(s) of testosterone elevations during pregnancy, could have important therapeutic potential in treatment of pregnancies complicated by vascular dysfunction and fetal growth restriction.
Figure 4.
Unifying model depicting the central role of testosterone in preeclampsia. Increased testosterone level causes systemic, uterine and placental vascular dysfunction leading to increased blood pressure, decreased uterine artery blood flow and placental insufficiency, which may contribute to fetal growth restriction.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
Financial support from the National Institute of Health (NIH) through grants HD069750, HL119869 and HL134779 (PI: S K), as well as HD044405 (PI: A Dunaif) and HD028934 (PI: J Marshall) supporting DHA, is greatly appreciated.
References
- Abbott DH, Bruns CR, Barnett DK, Dunaif A, Goodfriend TL, Dumesic DA, Tarantal AF. 2010. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. American Journal of Physiology: Endocrinology and Metabolism 299 E741–E751. ( 10.1152/ajpendo.00058.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott DH, Levine JE, Dumesic DA. 2016. Translational insight into polycystic ovary syndrome (PCOS) from female monkeys with PCOS-like traits. Current Pharmaceutical Design 22 5625–5633. ( 10.2174/1381612822666160715133437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acikgoz S, Bayar UO, Can M, Guven B, Mungan G, Dogan S, Sumbuloglu V. 2013. Levels of oxidized LDL, estrogens, and progesterone in placenta tissues and serum paraoxonase activity in preeclampsia. Mediators of Inflammation 2013 862982. ( 10.1155/2013/862982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACOG, Task Force on Hypertension in Pregnancy 2013. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstetrics and Gynecology 122 1122–1131. ( 10.1097/01.AOG.0000437382.03963.88) [DOI] [PubMed] [Google Scholar]
- Acromite MT, Mantzoros CS, Leach RE, Hurwitz J, Dorey LG. 1999. Androgens in preeclampsia. American Journal of Obstetrics and Gynecology 180 60–63. ( 10.1016/S0002-9378(99)70150-X) [DOI] [PubMed] [Google Scholar]
- Agurs-Collins T, Rohrmann S, Sutcliffe C, Bienstock JL, Monsegue D, Akereyeni F, Bradwin G, Rifai N, Pollak MN, Platz EA. 2012. Racial variation in umbilical cord blood sex steroid hormones and the insulin-like growth factor axis in African-American and white female neonates. Cancer Causes and Control 23 445–454. ( 10.1007/s10552-011-9893-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Khaja KA, Sequeira RP, Alkhaja AK, Damanhori AH. 2014. Drug treatment of hypertension in pregnancy: a critical review of adult guideline recommendations. Journal of Hypertension 32 454–463. ( 10.1097/HJH.0000000000000069) [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Pepe GJ. 1990. Placental steroid hormone biosynthesis in primate pregnancy. Endocrine Reviews 11 124–150. ( 10.1210/edrv-11-1-124) [DOI] [PubMed] [Google Scholar]
- Alsnes IV, Janszky I, Asvold BO, Okland I, Forman MR, Vatten LJ. 2016. Maternal preeclampsia and androgens in the offspring around puberty: a follow-up study. PLoS ONE 11 e0167714. ( 10.1371/journal.pone.0167714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Seljeflot I, Abdelnoor M, Arnesen H, Dale PO, Lovik A, Birkeland K. 1995. Increased insulin sensitivity and fibrinolytic capacity after dietary intervention in obese women with polycystic ovary syndrome. Metabolism: Clinical and Experimental 44 611–616. ( 10.1016/0026-0495(95)90118-3) [DOI] [PubMed] [Google Scholar]
- Arroyo JA, Winn VD. 2008. Vasculogenesis and angiogenesis in the IUGR placenta. Seminars in Perinatology 32 172–177. ( 10.1053/j.semperi.2008.02.006) [DOI] [PubMed] [Google Scholar]
- Atamer Y, Erden AC, Demir B, Kocyigit Y, Atamer A. 2004. The relationship between plasma levels of leptin and androgen in healthy and preeclamptic pregnant women. Acta Obstetricia et Gynecologica Scandinavica 83 425–430. ( 10.1111/j.0001-6349.2004.00276.x) [DOI] [PubMed] [Google Scholar]
- Baksu A, Gurarslan H, Goker N. 2004. Androgen levels in pre-eclamptic pregnant women. International Journal of Gynecology and Obstetrics 84 247–248. ( 10.1016/S0020-7292(03)00318-7) [DOI] [PubMed] [Google Scholar]
- Beckett EM, Astapova O, Steckler TL, Veiga-Lopez A, Padmanabhan V. 2014. Developmental programing: impact of testosterone on placental differentiation. Reproduction 148 199–209. ( 10.1530/REP-14-0055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit C, Zavecz J, Wang Y. 2007. Vasoreactivity of chorionic plate arteries in response to vasoconstrictors produced by preeclamptic placentas. Placenta 28 498–504. ( 10.1016/j.placenta.2006.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird IM, Sullivan JA, Di T, Cale JM, Zhang L, Zheng J, Magness RR. 2000. Pregnancy-dependent changes in cell signaling underlie changes in differential control of vasodilator production in uterine artery endothelial cells. Endocrinology 141 1107–1117. ( 10.1210/endo.141.3.7367) [DOI] [PubMed] [Google Scholar]
- Bosio PM, McKenna PJ, Conroy R, O’Herlihy C. 1999. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstetrics and Gynecology 94 978–984. ( 10.1016/s0029-7844(99)00430-5) [DOI] [PubMed] [Google Scholar]
- Bussen SS, Sutterlin MW, Steck T. 1998. Plasma renin activity and aldosterone serum concentration are decreased in severe preeclampsia but not in the HELLP-syndrome. Acta Obstetricia et Gynecologica Scandinavica 77 609–613. ( 10.1034/j.1600-0412.1998.770606.x) [DOI] [PubMed] [Google Scholar]
- Buster JE, Chang RJ, Preston DL, Elashoff RM, Cousins LM, Abraham GE, Hobel CJ, Marshall JR. 1979. Interrelationships of circulating maternal steroid concentrations in third trimester pregnancies. II. C18 and C19 steroids: estradiol, estriol, dehydroepiandrosterone, dehydroepiandrosterone sulfate, delta 5-androstenediol, delta 4-androstenedione, testosterone, and dihydrotestosterone. Journal of Clinical Endocrinology and Metabolism 48 139–142. ( 10.1210/jcem-48-1-139) [DOI] [PubMed] [Google Scholar]
- Carlsen SM, Heimstad R. 2012. Androgen levels are associated with blood pressure in pregnant women after term. Acta Obstetricia et Gynecologica Scandinavica 91 232–236. ( 10.1111/j.1600-0412.2011.01280.x) [DOI] [PubMed] [Google Scholar]
- Carlsen SM, Romundstad P, Jacobsen G. 2005. Early second-trimester maternal hyperandrogenemia and subsequent preeclampsia: a prospective study. Acta Obstetricia et Gynecologica Scandinavica 84 117–121. ( 10.1111/j.0001-6349.2005.00493.x) [DOI] [PubMed] [Google Scholar]
- Carlsen SM, Jacobsen G, Romundstad P. 2006. Maternal testosterone levels during pregnancy are associated with offspring size at birth. European Journal of Endocrinology 155 365–370. ( 10.1530/eje.1.02200) [DOI] [PubMed] [Google Scholar]
- Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. 2007. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension 49 1442–1447. ( 10.1161/HYPERTENSIONAHA.106.083972) [DOI] [PubMed] [Google Scholar]
- Chinnathambi V, Balakrishnan M, Yallampalli C, Sathishkumar K. 2012. Prenatal testosterone exposure leads to hypertension that is gonadal hormone-dependent in adult rat male and female offspring. Biology of Reproduction 86 131–137. ( 10.1095/biolreprod.111.097550) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnathambi V, Balakrishnan M, Ramadoss J, Yallampalli C, Sathishkumar K. 2013a. Testosterone alters maternal vascular adaptations: role of the endothelial NO system. Hypertension 61 647–654. ( 10.1161/HYPERTENSIONAHA.111.00486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnathambi V, Yallampalli C, Sathishkumar K. 2013b. Prenatal testosterone induces sex-specific dysfunction in endothelium-dependent relaxation pathways in adult male and female rats. Biology of Reproduction 89 97. ( 10.1095/biolreprod.113.111542) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnathambi V, Blesson CS, Vincent KL, Saade GR, Hankins GD, Yallampalli C, Sathishkumar K. 2014a. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension 64 405–414. ( 10.1161/HYPERTENSIONAHA.114.03283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnathambi V, More AS, Hankins GD, Yallampalli C, Sathishkumar K. 2014b. Gestational exposure to elevated testosterone levels induces hypertension via heightened vascular angiotensin II type 1 receptor signaling in rats. Biology of Reproduction 91 6. ( 10.1095/biolreprod.114.118968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A. 1974. Hormone and enzyme assays in pregnancy. I. Studies on the placental and the tissue cystine-aminopeptidase activity in peripheral plasma from non-pregnant and pregnant women, and in plasma from the umbilical cord. Acta Endocrinology 76 189–200. [PubMed] [Google Scholar]
- Codner E, Escobar-Morreale HF. 2007. Clinical review: hyperandrogenism and polycystic ovary syndrome in women with type 1 diabetes mellitus. Journal of Clinical Endocrinology and Metabolism 92 1209–1216. ( 10.1210/jc.2006-2641) [DOI] [PubMed] [Google Scholar]
- Conrad KP, Joffe GM, Kruszyna H, Kruszyna R, Rochelle LG, Smith RP, Chavez JE, Mosher MD. 1993. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB Journal 7 566–571. ( 10.1096/fasebj.7.6.7682524) [DOI] [PubMed] [Google Scholar]
- Cooke CL, Davidge ST. 2003. Pregnancy-induced alterations of vascular function in mouse mesenteric and uterine arteries. Biology of Reproduction 68 1072–1077. ( 10.1095/biolreprod.102.009886) [DOI] [PubMed] [Google Scholar]
- Czeloth K, Bobjer J, Bronton K, Christofferson O, Frederiksen H, Holmboe S, Anderson A, Juul A, Giwercman A. 2017. Comparison of testosterone measurement by the new ‘gold standard’ method liquid chromatography tandem mass spectrometry versus the traditionally in clinical routine used immunoassays in the prediction of symptoms of male hypogonadism. European Urology Supplements 16 e3018. [Google Scholar]
- de Vries MJ, Dekker GA, Schoemaker J. 1998. Higher risk of preeclampsia in the polycystic ovary syndrome. A case control study. European Journal of Obstetrics, Gynecology and Reproductive Biology 76 91–95. ( 10.1016/S0301-2115(97)00164-4) [DOI] [PubMed] [Google Scholar]
- Dell’Acqua S, Mancuso S, Eriksson G, Diczfalusy E. 1966. Metabolism of retrotestosterone and testosterone by midterm human placentas perfused in situ. Biochimica et Biophysica Acta/General Subjects 130 241–248. ( 10.1016/0304-4165(66)90028-6) [DOI] [Google Scholar]
- Diamant YZ, Rimon E, Evron S. 1982. High incidence of preeclamptic toxemia in patients with polycystic ovarian disease. European Journal of Obstetrics, Gynecology and Reproductive Biology 14 199–204. ( 10.1016/0028-2243(82)90097-1) [DOI] [PubMed] [Google Scholar]
- Diaz L, Noyola-Martinez N, Barrera D, Hernandez G, Avila E, Halhali A, Larrea F. 2009. Calcitriol inhibits TNF-alpha-induced inflammatory cytokines in human trophoblasts. Journal of Reproductive Immunology 81 17–24. ( 10.1016/j.jri.2009.02.005) [DOI] [PubMed] [Google Scholar]
- Dokras A, Spaczynski RZ, Behrman HR, Duleba AJ. 2003. Testosterone levels in pregnant women correlate with the insulin response during the glucose tolerance test. Fertility and Sterility 79 492–497. ( 10.1016/S0015-0282(02)04764-7) [DOI] [PubMed] [Google Scholar]
- Ferrini RL, Barrett-Connor E. 1998. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. American Journal of Epidemiology 147 750–754. ( 10.1093/oxfordjournals.aje.a009519) [DOI] [PubMed] [Google Scholar]
- Ficicioglu C, Kutlu T. 2003. The role of androgens in the aetiology and pathology of pre-eclampsia. Journal of Obstetrics & Gynaecology 23 134–137. [DOI] [PubMed] [Google Scholar]
- Fornes R, Hu M, Maliqueo M, Kokosar M, Benrick A, Carr D, Billig H, Jansson T, Manni L, Stener-Victorin E. 2016. Maternal testosterone and placental function: effect of electroacupuncture on placental expression of angiogenic markers and fetal growth. Molecular and Cellular Endocrinology 433 1–11. ( 10.1016/j.mce.2016.05.014) [DOI] [PubMed] [Google Scholar]
- Franks S, Gilling-Smith C, Watson H, Willis D. 1999. Insulin action in the normal and polycystic ovary. Endocrinology and Metabolism Clinics of North America 28 361–378. ( 10.1016/S0889-8529(05)70074-8) [DOI] [PubMed] [Google Scholar]
- Gant NF, Hutchinson HT, Siiteri PK, MacDonald PC. 1971. Study of the metabolic clearance rate of dehydroisoandrosterone sulfate in pregnancy. American Journal of Obstetrics and Gynecology 111 555–563. ( 10.1016/0002-9378(71)90472-8) [DOI] [PubMed] [Google Scholar]
- Gerulewicz-Vannini D, Camero Y, Salas J, Hernandez-Andrade E. 2006. High plasmatic androgen levels in women affected with pregnancy-induced hypertension. Revista de Investigacion Clinica 58 228–233. [PubMed] [Google Scholar]
- Ghorashi V, Sheikhvatan M. 2008. The relationship between serum concentration of free testosterone and pre-eclampsia. Endokrynologia Polska 59 390–392. [PubMed] [Google Scholar]
- Gillham JC, Kenny LC, Baker PN. 2003. An overview of endothelium-derived hyperpolarising factor (EDHF) in normal and compromised pregnancies. European Journal of Obstetrics, Gynecology and Reproductive Biology 109 2–7. ( 10.1016/S0301-2115(03)00044-7) [DOI] [PubMed] [Google Scholar]
- Gokina NI, Kuzina OY, Vance AM. 2010. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. American Journal of Physiology: Heart and Circulatory Physiology 299 H1642–H1652. ( 10.1152/ajpheart.00227.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RJ, Krause DN, Duckles SP. 2004. Testosterone suppresses endothelium-dependent dilation of rat middle cerebral arteries. American Journal of Physiology: Heart and Circulatory Physiology 286 H552–560. ( 10.1152/ajpheart.00663.2003) [DOI] [PubMed] [Google Scholar]
- Gonzales RJ, Ghaffari AA, Duckles SP, Krause DN. 2005. Testosterone treatment increases thromboxane function in rat cerebral arteries. American Journal of Physiology: Heart and Circulatory Physiology 289 H578–585. ( 10.1152/ajpheart.00958.2004) [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan K, Mishra JS, Chinnathambi V, Vincent KL, Patrikeev I, Motamedi M, Saade GR, Hankins GD, Sathishkumar K. 2016. Elevated testosterone reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant rats. Hypertension 67 630–639. ( 10.1161/HYPERTENSIONAHA.115.06946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BE, Bernstein L, Ross RK, Depue RH, Judd HL. 1988. The early in utero oestrogen and testosterone environment of blacks and whites: potential effects on male offspring. British Journal of Cancer 57 216–218. ( 10.1038/bjc.1988.46) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertig A, Liere P, Chabbert-Buffet N, Fort J, Pianos A, Eychenne B, Cambourg A, Schumacher M, Berkane N, Lefevre G. et al. 2010. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. American Journal of Obstetrics and Gynecology 203 477.e471–477.e479. ( 10.1016/j.ajog.2010.06.011) [DOI] [PubMed] [Google Scholar]
- Horie K, Takakura K, Imai K, Liao S, Mori T. 1992. Immunohistochemical localization of androgen receptor in the human endometrium, decidua, placenta and pathological conditions of the endometrium. Human Reproduction 7 1461–1466. ( 10.1093/oxfordjournals.humrep.a137595) [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Nelson RJ. 2007. An environmental androgen, 17beta-trenbolone, affects delayed-type hypersensitivity and reproductive tissues in male mice. Journal of Toxicology and Environmental Health: Part A 70 138–140. ( 10.1080/15287390600755091) [DOI] [PubMed] [Google Scholar]
- Hsu TY, Lan KC, Tsai CC, Ou CY, Cheng BH, Tsai MY, Kang HY, Tung YH, Wong YH, Huang KE. 2009. Expression of androgen receptor in human placentas from normal and preeclamptic pregnancies. Taiwanese Journal of Obstetrics and Gynecology 48 262–267. ( 10.1016/S1028-4559(09)60301-6) [DOI] [PubMed] [Google Scholar]
- Huppertz B, Peeters LL. 2005. Vascular biology in implantation and placentation. Angiogenesis 8 157–167. ( 10.1007/s10456-005-9007-8) [DOI] [PubMed] [Google Scholar]
- Iavazzo C, Vitoratos N. 2010. Polycystic ovarian syndrome and pregnancy outcome. Archives of Gynecology and Obstetrics 282 235–239. ( 10.1007/s00404-010-1495-0) [DOI] [PubMed] [Google Scholar]
- Ishikuro M, Obara T, Metoki H, Ohkubo T, Yaegashi N, Kuriyama S, Imai Y. 2012. Blood pressure changes during pregnancy. Hypertension Research. ( 10.1038/hr.2012.33) [DOI] [PubMed] [Google Scholar]
- Jiang B, Kamat A, Mendelson CR. 2000. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor Mash-2 (mammalian achaete-scute homologous protein-2). Molecular Endocrinology 14 1661–1673. ( 10.1210/mend.14.10.0539) [DOI] [PubMed] [Google Scholar]
- Jobe SO, Fling SN, Ramadoss J, Magness RR. 2011. A novel role for an endothelial adrenergic receptor system in mediating catecholestradiol-induced proliferation of uterine artery endothelial cells. Hypertension 58 874–881. ( 10.1161/HYPERTENSIONAHA.111.178046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe SO, Tyler CT, Magness RR. 2013. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension 61 480–487. ( 10.1161/HYPERTENSIONAHA.111.201624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaja R, Tikkanen MJ, Viinikka L, Ylikorkala O. 1995. Serum lipoproteins, insulin, and urinary prostanoid metabolites in normal and hypertensive pregnant women. Obstetrics and Gynecology 85 353–356. ( 10.1016/0029-7844(94)00380-V) [DOI] [PubMed] [Google Scholar]
- Kamalanathan S, Sahoo JP, Sathyapalan T. 2013. Pregnancy in polycystic ovary syndrome. Indian Journal of Endocrinology and Metabolism 17 37–43. ( 10.4103/2230-8210.107830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M, Mori N. 1983. Prostacyclin producing activity of human umbilical, placental and uterine vessels. Prostaglandins 26 645–662. ( 10.1016/0090-6980(83)90201-0) [DOI] [PubMed] [Google Scholar]
- King AJ, Olivier NB, Mohankumar PS, Lee JS, Padmanabhan V, Fink GD. 2007. Hypertension caused by prenatal testosterone excess in female sheep. American Journal of Physiology: Endocrinology and Metabolism 292 E1837–E1841. ( 10.1152/ajpendo.00668.2006) [DOI] [PubMed] [Google Scholar]
- Kjerulff LE, Sanchez-Ramos L, Duffy D. 2011. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. American Journal of Obstetrics and Gynecology 204 558.e551–558.e556. ( 10.1016/j.ajog.2011.03.021) [DOI] [PubMed] [Google Scholar]
- Kowalczyk TD, Cabaniss ML, Cusmano L. 1998. Association of low unconjugated estriol in the second trimester and adverse pregnancy outcome. Obstetrics and Gynecology 91 396–400. ( 10.1016/S0029-7844(97)00677-7) [DOI] [PubMed] [Google Scholar]
- Laivuori H, Kaaja R, Rutanen EM, Viinikka L, Ylikorkala O. 1998. Evidence of high circulating testosterone in women with prior preeclampsia. Journal of Clinical Endocrinology and Metabolism 83 344–347. ( 10.1210/jcem.83.2.4543) [DOI] [PubMed] [Google Scholar]
- Landeros RV, Pastore MB, Magness RR. 2018. Effects of the catechol and methoxy metabolites of 17beta-estradiol on nitric oxide production by ovine uterine artery endothelial cells. Reproductive Sciences Epub. ( 10.1177/1933719118783265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, Chamley LW. 2013. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. American Journal of Reproductive Immunology 70 412–427. ( 10.1111/aji.12138) [DOI] [PubMed] [Google Scholar]
- Li X, Zhang W, Lin J, Liu H, Yang Z, Teng Y, Duan S, Lin X, Xie Y, Li Y. et al. 2018. Risk factors for adverse maternal and perinatal outcomes in women with preeclampsia: analysis of 1396 cases. Journal of Clinical Hypertension 20 1049–1057. ( 10.1111/jch.13302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Kim S, Lee SW, Park SY, Han JY, Chung JH, Kim MY, Yang JH, Ryu HM. 2011. Association between genetic polymorphisms in androgen receptor gene and the risk of preeclampsia in Korean women. Journal of Assisted Reproduction and Genetics 28 85–90. ( 10.1007/s10815-010-9485-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S, Dai A, Williams MR, Myles K, Dilley RJ, Komesaroff PA, Sudhir K. 2002. Testosterone (T) enhances apoptosis-related damage in human vascular endothelial cells. Endocrinology 143 1119–1125. ( 10.1210/endo.143.3.8679) [DOI] [PubMed] [Google Scholar]
- Lonnebotn M, Natvig GK, Benediktsdottir B, Burgess JA, Holm M, Jogi R, Lindberg E, Macsali F, Schlunssen V, Skulstad SM. et al. 2018. Polycystic ovary syndrome, body mass index and hypertensive disorders in pregnancy. Pregnancy Hypertension 11 32–37. ( 10.1016/j.preghy.2017.12.006) [DOI] [PubMed] [Google Scholar]
- Lorentzen B, Birkeland KI, Endresen MJ, Henriksen T. 1998. Glucose intolerance in women with preeclampsia. Acta Obstetricia et Gynecologica Scandinavica 77 22–27. ( 10.1080/00016349808565805) [DOI] [PubMed] [Google Scholar]
- Magness RR. 1998. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy. In The Endocrinology of Pregnancy (Contemporary Endocrinology), pp 507–540. Ed Bazer FW. Totowa, NJ: Humana Press Inc. ( 10.1007/978-1-4612-1804-3_18) [DOI] [Google Scholar]
- Magness RR, Rosenfeld CR. 1986. Systemic and uterine responses to alpha-adrenergic stimulation in pregnant and nonpregnant ewes. American Journal of Obstetrics and Gynecology 155 897–904. ( 10.1016/S0002-9378(86)80047-3) [DOI] [PubMed] [Google Scholar]
- Magness RR, Mitchell MD, Rosenfeld CR. 1990. Uteroplacental production of eicosanoids in ovine pregnancy. Prostaglandins 39 75–88. ( 10.1016/0090-6980(90)90096-E) [DOI] [PubMed] [Google Scholar]
- Magness RR, Rosenfeld CR, Hassan A, Shaul PW. 1996. Endothelial vasodilator production by uterine and systemic arteries. I. Effects of ANG II on PGI2 and NO in pregnancy. American Journal of Physiology 270 H1914–H1923. ( 10.1152/ajpheart.1996.270.6.H1914) [DOI] [PubMed] [Google Scholar]
- Magness RR, Shideman CR, Habermehl DA, Sullivan JA, Bird IM. 2000. Endothelial vasodilator production by uterine and systemic arteries. V. Effects of ovariectomy, the ovarian cycle, and pregnancy on prostacyclin synthase expression. Prostaglandins and Other Lipid Mediators 60 103–118. ( 10.1016/S0090-6980(99)00055-6) [DOI] [PubMed] [Google Scholar]
- Magness RR, Sullivan JA, Li Y, Phernetton TM, Bird IM. 2001. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x). American Journal of Physiology: Heart and Circulatory Physiology 280 H1692–H1698. ( 10.1152/ajpheart.2001.280.4.H1692) [DOI] [PubMed] [Google Scholar]
- Mains LM, Lathi RB, Burney RO, Dahan MH. 2007. Serum total testosterone levels in a patient with late onset 21-hydroxylase deficiency and a twin gestation. Fertility and Sterility 87 1212.e1215–1212.e1218. ( 10.1016/j.fertnstert.2006.07.1545) [DOI] [PubMed] [Google Scholar]
- Makinen JI, Perheentupa A, Irjala K, Pollanen P, Makinen J, Huhtaniemi I, Raitakari OT. 2011. Endogenous testosterone and brachial artery endothelial function in middle-aged men with symptoms of late-onset hypogonadism. Aging Male 14 237–242. ( 10.3109/13685538.2011.593655) [DOI] [PubMed] [Google Scholar]
- Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. 2004. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 145 790–798. ( 10.1210/en.2003-0478) [DOI] [PubMed] [Google Scholar]
- Mason JI, Ushijima K, Doody KM, Nagai K, Naville D, Head JR, Milewich L, Rainey WE, Ralph MM. 1993. Regulation of expression of the 3 beta-hydroxysteroid dehydrogenases of human placenta and fetal adrenal. Journal of Steroid Biochemistry and Molecular Biology 47 151–159. ( 10.1016/0960-0760(93)90069-9) [DOI] [PubMed] [Google Scholar]
- Mehrabian F, Kelishadi R. 2012. Comparison of the metabolic parameters and androgen level of umbilical cord blood in newborns of mothers with polycystic ovary syndrome and controls. Journal of Research in Medical Sciences 17 207–211. [PMC free article] [PubMed] [Google Scholar]
- Metz TD, Allshouse AA, Euser AG, Heyborne KD. 2014. Preeclampsia in high risk women is characterized by risk group-specific abnormalities in serum biomarkers. American Journal of Obstetrics and Gynecology 211 512.e511–512.e516. ( 10.1016/j.ajog.2014.04.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg PM, Hofman JA. 1991. Maternal testosterone and fetal sex. Journal of Steroid Biochemistry and Molecular Biology 39 51–54. ( 10.1016/0960-0760(91)90012-T) [DOI] [PubMed] [Google Scholar]
- Milczarek R, Sokolowska E, Hallmann A, Kaletha K, Klimek J. 2008. NADPH- and iron-dependent lipid peroxidation inhibit aromatase activity in human placental microsomes. Journal of Steroid Biochemistry and Molecular Biology 110 230–235. ( 10.1016/j.jsbmb.2007.11.004) [DOI] [PubMed] [Google Scholar]
- Miller NR, Garry D, Cohen HW, Figueroa R. 2003. Serum androgen markers in preeclampsia. Journal of Reproductive Medicine 48 225–229. [PubMed] [Google Scholar]
- Mitchell F. 2012. Reproductive endocrinology: Mass spectrometry 'gold standard' for measuring steroid sex hormones? Nature Reviews in Endocrinology 8 320. [DOI] [PubMed] [Google Scholar]
- Mori T, Watanabe K, Iwasaki A, Kimura C, Matsushita H, Shinohara K, Wakatsuki A. 2014. Differences in vascular reactivity between pregnant women with chronic hypertension and preeclampsia. Hypertension Research 37 145–150. ( 10.1038/hr.2013.131) [DOI] [PubMed] [Google Scholar]
- Murji A, Proctor LK, Paterson AD, Chitayat D, Weksberg R, Kingdom J. 2012. Male sex bias in placental dysfunction. American Journal of Medical Genetics: Part A 158A 779–783. ( 10.1002/ajmg.a.35250) [DOI] [PubMed] [Google Scholar]
- Myatt L. 2002. Role of placenta in preeclampsia. Endocrine 19 103–111. ( 10.1385/ENDO:19:1:103) [DOI] [PubMed] [Google Scholar]
- Naden RP, Rosenfeld CR. 1981. Effect of angiotensin II on uterine and systemic vasculature in pregnant sheep. Journal of Clinical Investigation 68 468–474. ( 10.1172/JCI110277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao J, Change WC, Murota SI, Orimo H. 1981. Testosterone inhibits prostacyclin production by rat aortic smooth muscle cells in culture. Atherosclerosis 39 203–209. ( 10.1016/0021-9150(81)90070-8) [DOI] [PubMed] [Google Scholar]
- Nelson SH, Steinsland OS, Wang Y, Yallampalli C, Dong YL, Sanchez JM. 2000. Increased nitric oxide synthase activity and expression in the human uterine artery during pregnancy. Circulation Research 87 406–411. ( 10.1161/01.RES.87.5.406) [DOI] [PubMed] [Google Scholar]
- Orlando EF, Kolok AS, Binzcik GA, Gates JL, Horton MK, Lambright CS, Gray LE, Jr, Soto AM, Guillette LJ., Jr 2004. Endocrine-disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environmental Health Perspectives 112 353–358. ( 10.1289/ehp.6591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osol G, Mandala M. 2009. Maternal uterine vascular remodeling during pregnancy. Physiology 24 58–71. ( 10.1152/physiol.00033.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. 2013. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiologica 208 224–233. ( 10.1111/apha.12106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomba S, Falbo A, Russo T, Battista L, Tolino A, Orio F, Zullo F. 2010. Uterine blood flow in pregnant patients with polycystic ovary syndrome: relationships with clinical outcomes. British Journal of Obstetrics and Gynaecology 117 711–721. ( 10.1111/j.1471-0528.2010.02525.x) [DOI] [PubMed] [Google Scholar]
- Palomba S, Russo T, Falbo A, Di CA, Amendola G, Mazza R, Tolino A, Zullo F, Tucci L, La Sala GB. 2012. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: an experimental case-control study. Journal of Clinical Endocrinology and Metabolism 97 2441–2449. ( 10.1210/jc.2012-1100) [DOI] [PubMed] [Google Scholar]
- Pan T, He G, Chen M, Bao C, Chen Y, Liu G, Zhou M, Li S, Xu W, Liu X. 2017. Abnormal CYP11A1 gene expression induces excessive autophagy, contributing to the pathogenesis of preeclampsia. Oncotarget 8 89824–89836. ( 10.18632/oncotarget.21158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. 2004. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. Journal of Biological Chemistry 279 52282–52292. ( 10.1074/jbc.M407629200) [DOI] [PubMed] [Google Scholar]
- Parks LG, Lambright CS, Orlando EF, Guillette LJ, Jr, Ankley GT, Gray LE., Jr 2001. Masculinization of female mosquitofish in Kraft mill effluent-contaminated Fenholloway River water is associated with androgen receptor agonist activity. Toxicological Sciences 62 257–267. ( 10.1093/toxsci/62.2.257) [DOI] [PubMed] [Google Scholar]
- Pasquali R, Gambineri A, Biscotti D, Vicennati V, Gagliardi L, Colitta D, Fiorini S, Cognigni GE, Filicori M, Morselli-Labate AM. 2000. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism 85 2767–2774. ( 10.1210/jcem.85.8.6738) [DOI] [PubMed] [Google Scholar]
- Perez-Sepulveda A, Monteiro LJ, Dobierzewska A, Espana-Perrot PP, Venegas-Araneda P, Guzman-Rojas AM, Gonzalez MI, Palominos-Rivera M, Irarrazabal CE, Figueroa-Diesel H. et al. 2015. Placental aromatase is deficient in placental ischemia and preeclampsia. PLoS ONE 10 e0139682. ( 10.1371/journal.pone.0139682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potischman N, Troisi R, Thadhani R, Hoover RN, Dodd K, Davis WW, Sluss PM, Hsieh CC, Ballard-Barbash R. 2005. Pregnancy hormone concentrations across ethnic groups: implications for later cancer risk. Cancer Epidemiology, Biomarkers and Prevention 14 1514–1520. ( 10.1158/1055-9965.EPI-04-0869) [DOI] [PubMed] [Google Scholar]
- Powe CE, Levine RJ, Karumanchi SA. 2011. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 123 2856–2869. ( 10.1161/CIRCULATIONAHA.109.853127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recabarren SE, Rojas-Garcia PP, Recabarren MP, Alfaro VH, Smith R, Padmanabhan V, Sir-Petermann T. 2008. Prenatal testosterone excess reduces sperm count and motility. Endocrinology 149 6444–6448. ( 10.1210/en.2008-0785) [DOI] [PubMed] [Google Scholar]
- Reckelhoff JF, Zhang H, Granger JP. 1998. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 31 435–439. ( 10.1161/01.HYP.31.1.435) [DOI] [PubMed] [Google Scholar]
- Roberts JM, Redman CW. 1993. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet 341 1447–1451. ( 10.1016/0140-6736(93)90889-O) [DOI] [PubMed] [Google Scholar]
- Roberts JM, Taylor RN, Goldfien A. 1991. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. American Journal of Hypertension 4 700–708. ( 10.1093/ajh/4.8.700) [DOI] [PubMed] [Google Scholar]
- Rohrmann S, Sutcliffe CG, Bienstock JL, Monsegue D, Akereyeni F, Bradwin G, Rifai N, Pollak MN, Agurs-Collins T, Platz EA. 2009. Racial variation in sex steroid hormones and the insulin-like growth factor axis in umbilical cord blood of male neonates. Cancer Epidemiology, Biomarkers and Prevention 18 1484–1491. ( 10.1158/1055-9965.EPI-08-0817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CR, Morriss FH, Jr, Makowski EL, Meschia G, Battaglia FC. 1974. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecologic Investigation 5 252–268. ( 10.1159/000301658) [DOI] [PubMed] [Google Scholar]
- Rosing U, Carlstrom K. 1984. Serum levels of unconjugated and total oestrogens and dehydroepiandrosterone, progesterone and urinary oestriol excretion in pre-eclampsia. Gynecologic and Obstetric Investigation 18 199–205. ( 10.1159/000299081) [DOI] [PubMed] [Google Scholar]
- Salam RA, Das JK, Ali A, Bhaumik S, Lassi ZS. 2015. Diagnosis and management of preeclampsia in community settings in low and middle-income countries. Journal of Family Medicine and Primary Care 4 501–506. ( 10.4103/2249-4863.174265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamalekis E, Bakas P, Vitoratos N, Eleptheriadis M, Creatsas G. 2006. Androgen levels in the third trimester of pregnancy in patients with preeclampsia. European Journal of Obstetrics, Gynecology and Reproductive Biology 126 16–19. ( 10.1016/j.ejogrb.2005.07.007) [DOI] [PubMed] [Google Scholar]
- Samadi AR, Mayberry RM, Reed JW. 2001. Preeclampsia associated with chronic hypertension among African-American and White women. Ethnicity and Disease 11 192–200. [PubMed] [Google Scholar]
- Sarkar P, Bergman K, Fisk NM, O’Connor TG, Glover V. 2007. Amniotic fluid testosterone: relationship with cortisol and gestational age. Clinical Endocrinology 67 743–747. ( 10.1111/j.1365-2265.2007.02955.x) [DOI] [PubMed] [Google Scholar]
- Sarkar P, Bergman K, O’Connor TG, Glover V. 2008. Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: possible implications for foetal programming. Journal of Neuroendocrinology 20 489–496. ( 10.1111/j.1365-2826.2008.01659.x) [DOI] [PubMed] [Google Scholar]
- Sathishkumar K, Balakrishnan M, Gao HJ, Yallampalli C. 2011a. Elevated testosterone levels during pregnancy induce preeclampsia-like syndrome in pregnant rats. Reproductive Sciences 18 359a.20959645 [Google Scholar]
- Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GD, Yallampalli C. 2011b. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reproductive Biology and Endocrinology 9 110. ( 10.1186/1477-7827-9-110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar K, Elkins R, Yallampalli U, Balakrishnan M, Yallampalli C. 2011c. Fetal programming of adult hypertension in female rat offspring exposed to androgens in utero. Early Human Development 87 407–414. ( 10.1016/j.earlhumdev.2011.03.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar K, Balakrishnan M, Chinnathambi V, Chauhan M, Hankins GD, Yallampalli C. 2012. Fetal sex-related dysregulation in testosterone production and their receptor expression in the human placenta with preeclampsia. Journal of Perinatology 32 328–335. ( 10.1038/jp.2011.101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer B, Daxenberger A, Meyer K, Meyer HH. 2001. The fate of trenbolone acetate and melengestrol acetate after application as growth promoters in cattle: environmental studies. Environmental Health Perspectives 109 1145–1151. ( 10.1289/ehp.011091145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely EW, Solomon CG. 2003. Insulin resistance and its potential role in pregnancy-induced hypertension. Journal of Clinical Endocrinology and Metabolism 88 2393–2398. ( 10.1210/jc.2003-030241) [DOI] [PubMed] [Google Scholar]
- Serin IS, Kula M, Basbug M, Unluhizarci K, Gucer S, Tayyar M. 2001. Androgen levels of preeclamptic patients in the third trimester of pregnancy and six weeks after delivery. Acta Obstetricia et Gynecologica Scandinavica 80 1009–1013. ( 10.1034/j.1600-0412.2001.801107.x) [DOI] [PubMed] [Google Scholar]
- Shao X, Liu Y, Liu M, Wang Y, Yan L, Wang H, Ma L, Li YX, Zhao Y, Wang YL. 2017. Testosterone represses estrogen signaling by upregulating miR-22: a mechanism for imbalanced steroid hormone production in preeclampsia. Hypertension 69 721–730. ( 10.1161/HYPERTENSIONAHA.116.08468) [DOI] [PubMed] [Google Scholar]
- Sharifzadeh F, Kashanian M, Fatemi F. 2012. A comparison of serum androgens in pre-eclamptic and normotensive pregnant women during the third trimester of pregnancy. Gynecological Endocrinology 28 834–836. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. 2002. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Human Reproduction 17 2573–2579. ( 10.1093/humrep/17.10.2573) [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Hitchsfeld C, Maliqueo M, Codner E, Echiburu B, Gazitua R, Recabarren S, Cassorla F. 2005. Birth weight in offspring of mothers with polycystic ovarian syndrome. Human Reproduction 20 2122–2126. ( 10.1093/humrep/debib9) [DOI] [PubMed] [Google Scholar]
- Sladek SM, Magness RR, Conrad KP. 1997. Nitric oxide and pregnancy. American Journal of Physiology 272 R441–R463. ( 10.1152/ajpregu.1997.272.2.R441) [DOI] [PubMed] [Google Scholar]
- Smith AS, Birnie AK, French JA. 2010. Maternal androgen levels during pregnancy are associated with early-life growth in Geoffroy’s marmosets, Callithrix geoffroyi. General and Comparative Endocrinology 166 307–313. ( 10.1016/j.ygcen.2009.10.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhewicz AE, Jandu S, Santhanam L, Alexander LM. 2017. Alterations in endothelin type B receptor contribute to microvascular dysfunction in women who have had preeclampsia. Clinical Science 131 2777–2789. ( 10.1042/CS20171292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark MJ, Dierkx L, Clifton VL, Wright IM. 2006. Alterations in the maternal peripheral microvascular response in pregnancies complicated by preeclampsia and the impact of fetal sex. Journal of the Society for Gynecologic Investigation 13 573–578. ( 10.1016/j.jsgi.2006.06.006) [DOI] [PubMed] [Google Scholar]
- Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. 2005. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology 146 3185–3193. ( 10.1210/en.2004-1444) [DOI] [PubMed] [Google Scholar]
- Steier JA, Ulstein M, Myking OL. 2002. Human chorionic gonadotropin and testosterone in normal and preeclamptic pregnancies in relation to fetal sex. Obstetrics and Gynecology 100 552–556. ( 10.1097/00006250-200209000-00024) [DOI] [PubMed] [Google Scholar]
- Sun M, Maliqueo M, Benrick A, Johansson J, Shao R, Hou L, Jansson T, Wu X, Stener-Victorin E. 2012. Maternal androgen excess reduces placental and fetal weights, increases placental steroidogenesis, and leads to long-term health effects in their female offspring. American Journal of Physiology: Endocrinology and Metabolism 303 E1373–E1385. ( 10.1152/ajpendo.00421.2012) [DOI] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Zhao X, Santoro N, Lasley B, Sowers M, Johnston J, Mackey R, Matthews K. 2010. Reproductive hormones and obesity: 9 years of observation from the Study of Women’s Health across the Nation. American Journal of Epidemiology 171 1203–1213. ( 10.1093/aje/kwq049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svartberg J, Midtby M, Bonaa KH, Sundsfjord J, Joakimsen RM, Jorde R. 2003. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromso Study. European Journal of Endocrinology 149 145–152. ( 10.1530/eje.0.1490145) [DOI] [PubMed] [Google Scholar]
- Sykes SD, Pringle KG, Zhou A, Dekker GA, Roberts CT, Lumbers ER. & SCOPE Consortium 2014. Fetal sex and the circulating renin-angiotensin system during early gestation in women who later develop preeclampsia or gestational hypertension. Journal of Human Hypertension 28 133–139. ( 10.1038/jhh.2013.51) [DOI] [PubMed] [Google Scholar]
- Takeyama J, Sasano H, Suzuki T, Iinuma K, Nagura H, Andersson S. 1998. 17Beta-hydroxysteroid dehydrogenase types 1 and 2 in human placenta: an immunohistochemical study with correlation to placental development. Journal of Clinical Endocrinology and Metabolism 83 3710–3715. ( 10.1210/jcem.83.10.5212) [DOI] [PubMed] [Google Scholar]
- Tamimi R, Lagiou P, Vatten LJ, Mucci L, Trichopoulos D, Hellerstein S, Ekbom A, Adami HO, Hsieh CC. 2003. Pregnancy hormones, pre-eclampsia, and implications for breast cancer risk in the offspring. Cancer Epidemiology, Biomarkers and Prevention 12 647–650. [PubMed] [Google Scholar]
- Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. 2000. Hemodynamic changes in pregnancy. Seminars in Perinatology 24 11–14. ( 10.1016/S0146-0005(00)80047-6) [DOI] [PubMed] [Google Scholar]
- Troisi R, Potischman N, Roberts JM, Ness R, Crombleholme W, Lykins D, Siiteri P, Hoover RN. 2003. Maternal serum oestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. International Journal of Epidemiology 32 455–460. ( 10.1093/ije/dyg094) [DOI] [PubMed] [Google Scholar]
- Turnipseed MR, Bentley K, Reynolds JW. 1976. Serum dehydroepiandrosterone sulfate in premature infants and infants with intrauterine growth retardation. Journal of Clinical Endocrinology and Metabolism 43 1219–1225. ( 10.1210/jcem-43-6-1219) [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. 2011. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biology of Reproduction 84 87–96. ( 10.1095/biolreprod.110.086686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas AK, Hoang V, Padmanabhan V, Gilbreath E, Mietelka KA. 2016. Prenatal programming: adverse cardiac programming by gestational testosterone excess. Scientific Reports 6 28335. ( 10.1038/srep28335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Hu Z, Zeng K, Yin Y, Zhao M, Chen M, Chen Q. 2018. The reduction in circulating levels of estrogen and progesterone in women with preeclampsia. Pregnancy Hypertension 11 18–25. ( 10.1016/j.preghy.2017.12.003) [DOI] [PubMed] [Google Scholar]
- Wang YC, Su HY, Liu JY, Chang FW, Chen CH. 2005. Maternal and female fetal virilization caused by pregnancy luteomas. Fertility and Sterility 84 509. ( 10.1016/j.fertnstert.2005.02.029) [DOI] [PubMed] [Google Scholar]
- Warmann S, Roth C, Gluer S, Fuchs J. 2000. Congenital adrenal hyperplasia associated with maternal pregnancy luteoma and the Antley-Bixler syndrome. Journal of Pediatric Surgery 35 528–530. ( 10.1016/S0022-3468(00)90232-X) [DOI] [PubMed] [Google Scholar]
- Williams DJ, Vallance PJ, Neild GH, Spencer JA, Imms FJ. 1997. Nitric oxide-mediated vasodilation in human pregnancy. American Journal of Physiology 272 H748–H752. ( 10.1152/ajpheart.1997.272.2.H748) [DOI] [PubMed] [Google Scholar]
- Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. 2009. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. American Journal of Physiology: Renal Physiology 296 F771–F779. ( 10.1152/ajprenal.90389.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu RM, Chaturvedi G, Tong SK, Nusrin S, Giesy JP, Wu RS, Kong RY. 2015. Evidence for microRNA-mediated regulation of steroidogenesis by hypoxia. Environmental Science and Technology 49 1138–1147. ( 10.1021/es504676s) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a