Abstract
Some well-established immunotherapy, radiotherapy, postoperation, anticancer drugs such as anthracyclines, antimetabolites, human epidermal growth factor receptor 2 blockers, tyrosine kinase inhibitors, alkylating agents, checkpoint inhibitors, and angiogenesis inhibitors, are significantly linked to cardiotoxicity. Cardiotoxicity is a common complication of several cancer treatments. Some studies observed complications of cardiac arrhythmia associated with the treatment of cancer, including atrial fibrillation (AF), supraventricular arrhythmias, and cardiac repolarization abnormalities. AF increases the risk of cardiovascular morbidity and mortality; it is associated with an almost doubled risk of mortality and a nearly 5-fold increase in the risk of stroke. The occurrence of AF is also usually researched in patients with advanced cancer and those undergoing active cancer treatments. During cancer treatments, the incidence rate of AF affects the prognosis of tumor treatment and challenges the treatment strategy. The present article is mainly focused on the cardiotoxicity of cancer treatments. In our review, we discuss these anticancer therapies and how they induce AF and consequently provide information on the precaution of AF during cancer treatment.
Keywords: anticancer therapies, cardiotoxicity, adverse effects, atrial fibrillation, mechanisms
Introduction
Cancer is the second leading cause of mortality in America (Siegel et al., 2016). In recent years, the mortality rate for numerous malignancies has decreased due to major progress in cancer treatment. Despite such great progress, cardiotoxicity, which can affect morbidity and mortality, is often observed in numerous therapies. Some well-established anticancer drugs, such as anthracyclines, antimetabolites, human epidermal growth factor receptor 2 (HER2) blockers, tyrosine kinase inhibitors (TKIs), alkylating agents, checkpoint inhibitors, and angiogenesis inhibitors, are significantly associated with cardiotoxicity. Cardiac arrhythmia is a common complication in the treatment of cancer patients, particularly atrial fibrillation (AF) (Tamargo et al., 2015). Cardio-oncology is an emerging academic discipline designed to resolve the complicated reciprocity between cardiovascular diseases and cancer. Monitoring, early discovery, precaution, and treatment of cardiotoxicity and well-planned cancer treatment in patients with pre-existing cardiovascular diseases protect them from the possible exacerbation/persistence of cardiotoxicity and development of heart failure (HF), respectively (Albini et al., 2010; Schwartz et al., 2013; Russell et al., 2016).
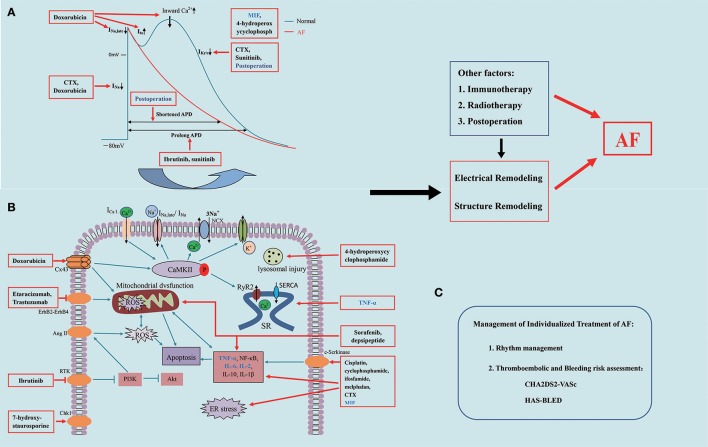
One of the key issues in cancer treatment is the occurrence of AF (Farmakis et al., 2014). AF is one of the most common persistent cardiac arrhythmias, accounting for approximately one third of all patients hospitalized owing to arrhythmia (European Heart Rhythm Association et al., 2010; Fuster et al., 2011). Further, it increases the risk of cardiovascular complications, including a 3- and 5-fold increased risk of HF and stroke, respectively, and a 2-fold increased mortality rate (Ott et al., 1997; Schmitt et al., 2009; Iwasaki et al., 2011; Camm et al., 2012; Khan et al., 2013; Guo et al., 2015). AF is usually observed in patients with advanced cancer and those undergoing active cancer treatments (O'Neal et al., 2015). Anticancer drug-induced AF is common especially in poly-medicated elderly patients. The occurrence of AF is a poor prognostic element, as well as impacts therapeutic outcomes of cancer patients (Tamargo et al., 2015). The pathophysiological etiology of cancer treatment-induced AF is complicated by various cellular and biomolecular interactions, as Figure 1 indicated in the mechanisms of cancer treatment-induced AF constant chemotherapy, the immunization therapy, and cancer surgery. In our review, we discuss anticancer therapies that induce AF and what is known about their contributing mechanisms, and offer recommendations for the management of AF during treatment of cancer.
Figure 1.
Mechanisms of anticancer therapy-induced atrial fibrillation. (A) Electrophysiological mechanism of anticancer drug-induced atrial fibrillation; (B) Signaling pathways associated with anticancer drug-induced atrial fibrillation; (C) Management of treatment of Atrial Fibrillation. AF, atrial fibrillation; ROS, reactive oxygen species; SR, sarcoplasmic reticular; CTX, cyclophosphamide; TNF-α, tumor necrosis factor -α; NF-κB, nuclear factor-κB; CaMKII, Ca2+/calmodulin dependent protein kinase II; RyR2, ryanodine receptor; ER stress, endoplasmic reticular stress; IL-2, interleukin-2; IL-6, interleukin-6; ICaL, L-type Ca2+ current; NCX, Na+/Ca2+ exchanger; INa, Na+ channels; IK, K+ currents; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; Cx43/45, connexin 43/45.
Mechanisms of anticancer drug-induced AF
With the presence of a trigger, structural and electrical remodeling occurs, which consequently initiates AF development (Nattel, 2002; European Heart Rhythm Association et al., 2010; Fuster et al., 2011; Iwasaki et al., 2011). AF induces further structural and electrophysiological changes, which can promote its persistence (Hove-Madsen et al., 2004; Vest et al., 2005; Nattel et al., 2008; Chelu et al., 2009; Neef et al., 2010; Dobrev et al., 2011; Voigt et al., 2012, 2014). The structural changes, which can also be caused by coexisting structural cardiac diseases associated with AF along with age or by some drugs, yield a steady arrhythmogenic substrate that promotes the persistence of AF. Anticancer drugs can induce AF via all kinds of mechanisms, including electrophysiology, myocardial damage, inflammation, immune responses, apoptosis, and reactive oxygen species (ROS) production (Bracci et al., 2014; Farmakis et al., 2014).
Electrophysiology
Changes in the myocardium can lead to abnormal electrophysiology, which can cause AF (Gupta et al., 2002). Chemotherapeutic drug-induced AF results in electrophysiological remodeling, which can include transient outward potassium current (Ito), K+ current (IKur), sodium channel current (INa), and L-type calcium channel current (ICa, L). These changes in currents involve shortening of the action potential (AP) and effective refractory period and thus maintenance of AF (Nattel et al., 2008). Considerably, the electrophysiological remodeling may also be associated with abnormal Ca2+ handling and the increased incidence rate of potentially pro-arrhythmic Ca2+ release events from the sarcoplasmic reticulum (SR) during diastole (Hove-Madsen et al., 2004; Vest et al., 2005; Chelu et al., 2009; Neef et al., 2010; Dobrev et al., 2011; Voigt et al., 2012, 2014; Xing et al., 2013). Ca2+/calmodulin-dependent protein kinase II (CaMKII) plays a vital part in AF by regulating cardiac-related channels and calmodulin (Neef et al., 2010; Yang et al., 2017b). Chemotherapeutic drugs can also induce CaMKII-mediated SR Ca2+ leakage and thus AF (Sag et al., 2011).
Oxidative stress
According to the principle of oxidative stress, antitumor drugs, such as doxorubicin, trastuzumab, and depsipeptide, may produce superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH−) through a series of electron transfer processes under the function of various reductases and NADH dehydrogenases (Gu, 2015; Yang et al., 2017a). These free radicals can cause mitochondrial and microsomal lipid peroxidation, which can damage a variety of cells. The production of ROS is one of the main factors of cardiotoxic side effects. For example, mtDNA damage, loss of nitrous oxide (NO), changes in gene expression, and increase or decrease in autophagy are some of the causes for cardiotoxicity that all result in elevated levels of ROS (O'Neal et al., 2015; Samman Tahhan et al., 2017).
Apoptosis
Apoptosis can eliminate aging and abnormal cells and play an important role in maintaining many cellular functions. Oxidative stress puts the body in a vulnerable state and enhances the toxic effects of pathogenic factors (Beck, 1999). It is not only related to the occurrence and development of various diseases but also has a close relationship with apoptosis (Ozaki et al., 2000). Meanwhile, the calcium ions play a major role in this process. Antitumor drugs activate the oxidative stress system of the cardiomyocytes, leading to the accumulation of ROS in the intracytoplasm. This consequently opens the ryanodine receptor on the SR of the cardiomyocytes to release a large amount of Ca2+ ions; thereafter, the intracellular Ca2+ clearance system fails, increasing the intracellular Ca2+ concentration (Keefe, 2001). A large number of Ca2+ ions causes changes in the mitochondrial membrane potential, arousing mitochondrial edema and rupture of the outer membrane, leading to the release of cytochrome c and apoptosis-induced factors, and thus promoting apoptosis of the cardiomyocytes (Gen et al., 2001).
Inflammation
Changes in inflammation are common in the tumor therapy-induced AF (Aviles et al., 2003; Siemes et al., 2006; Erichsen et al., 2012). Inflammation, determined by elevations of the concentrations of related biomarkers, is associated with the presence or development of AF (Hernández, 2006). Furthermore, cancer-related systemic inflammation promotes and maintains AF by inducing atrial structural remodeling, such as that in tumor necrosis factor (TNF)-α, nuclear factor (NF)-κB, and macrophage migration inhibitory factor (MIF) (Guzzetti et al., 2002). NF-κB is a redox-sensitive transcription factor that causes inflammation and structural remodeling by activating TNF-α, iNOS and IL-β (Wang et al., 2018). Increased density of inflammatory mediators, such as IL-6 and high-sensitivity C-reactive protein (hs-CRP), has also been recognized as a risk factor for AF (Conway et al., 2004). Inflammation plays a significant effect in the progression of cancer, and thus AF may represent an inflammatory complication in the course of cancer treatment (Ferreira et al., 2015).
Immune factors
Regulation of immune responses in patients with cancer and AF might be a potential target for cancer treatment. Cyclophosphamide (CTX) induces myocardial fibrosis and cardiac hypertrophy, as well as changes in the expressions of several cytokines, such as interleukin (IL)-2, IL-10, IL-6, and TNF-α, which can further facilitate the occurrence and development of AF (Liu et al., 2015). AF patients indicated a higher concentration of TNF-α and IL-6, lymphomonocyte infiltration, as well as the degree of myocardial fibrosis. Qu et al. (2009). In addition, inhibition of interleukin and TNF-α might be associated with attenuation of AF and even may be good for preventing the development of AF (Zhang et al., 2015).
Anticancer drugs
Anticancer drug-induced adverse effects are a serious problem, as the life expectancy in cancer treatment may be decreased by the increased mortality rate owing to a series of cardiac adverse events (CAEs). Multiple widely used anticancer drugs are associated with an increasing risk of cardiotoxicity, including anthracyclines, xuoropyrimidines, alkylating agents, interferons, IL-2, taxanes, and TKIs (Table 1) (Floyd et al., 2005; Carver et al., 2007; Curigliano et al., 2010). A single anticancer drug is often employed in combination with other anticancer drugs, immunological drugs. However, the use of anticancer drugs can increase the incidence rate of AF in patients with cancer, thereby increasing the risk of mortality.
Table 1.
AF induced by anticancer therapy.
| Classification | Drug classified | Drug | Incidence of AF | Mechanisms and actions | References |
|---|---|---|---|---|---|
| Anticancer drugs | Targeted therapies | Ibrutinib, 7-hydroxy-staurosporine, |
6.1% | PI3K–Akt pathway, the BTK and tec protein tyrosine kinase (TEC) | Honigberg et al., 2010; Herman et al., 2011; Burger et al., 2015; Byrd et al., 2015; Wang et al., 2015; Gertz, 2017; Shanafelt et al., 2017 |
| TKIs | Cetuximab, Crizotinib, Sunitinib, sorafenib |
3.3% | QT interval prolongation, decrease of nitric oxide signaling, increase of endothelin-1 production, inhibited AMPK and potassium channels, enhanced accumulation of lipid, ROS production, mitochondrial disorders, and apoptosis | Lara et al., 2005; Moslehi, 2016 | |
| Anthracycline agents | Aclacinomycin A, doxorubicin, adriamycin, 7-con-o-methylnogaril. |
6.6% | Cx43/Cx45 junction channels, CaMKII, Ca2+ ATPase, ST segment elevated, inverted T wave, long QT intervals, ROS, mitochondrial dysfunction, and apoptosis | Kluza et al., 2004; Chu et al., 2007; Lai et al., 2011; Lau et al., 2011; Xin et al., 2011; Zhang et al., 2011; Doherty et al., 2013; Kawabata et al., 2015; Varga et al., 2015 | |
| Alkylating agents | Cisplatin, Melphalan, CTX, 4-hydroperoxycyclophosphamide, cyclophosphamide, Ifosfamide. |
15.5% | cardiomyocyte contractions, mitochondrial abnormalities, ER stress and apoptosis, ROS, and inflammation, inducing cellular sodium, calcium, potassium, ATP content, the lysosome injury | Eskilsson et al., 1988; Petrella et al., 1989; Menard et al., 1991; Tomkowski et al., 2004; Pfister et al., 2006; Richards et al., 2006; Kilickap et al., 2007; Tilleman et al., 2009; Zellos et al., 2009; Liu et al., 2015 | |
| HER2/Neu receptor blockers | Etaracizumab, trastuzumab. | 19.9% | oxidative stress, apoptosis, ErbB2-ErbB4 signaling | Kupari et al., 1990; Quezado et al., 1993 | |
| Antimetabolites | 5-Fluorouracil, leucovorin. | 2.6% | the DNA synthesis, coronary spasm, myocardial ischaemia | de Forni et al., 1992; Perez-Verdia et al., 2005 | |
| Antimicrotubule agents | Paclitaxel, Docetaxel, Gemcitabine, gemcitabinevinorelbine |
9.4% | block cell division, coronary flow and left ventricular systolic pressure | Slamon et al., 1987; Keefe et al., 1993; Meydan et al., 2005 | |
| Histone deacetylase inhibitors | Depsipeptide, Belinostat. |
4.6% | No report | Bryan-Brown, 1932; Brouty-Boye et al., 1995; Alloatti et al., 1998 | |
| Antiestrogens | tamoxifen | No report | No report | Ueda et al., 1994b | |
| Proteosome inhibitors | Lenalidomide, lidomide, bortezomib. |
the cellular proliferation, apoptosis | Weber et al., 2003 | ||
| Immunotherapy | Interleukin-2, TNF-α, MIF, | 6.0% | proinflammatory cytokines, calcium homeostasis, inflammation, falling ICa, L amplitudes, and activating c-Src kinases | Thompson et al., 1994; White et al., 1994; Issac et al., 2007; Fildes et al., 2009; Rao et al., 2009; Pérez Persona et al., 2011; Guo et al., 2012a,b | |
| Radiotherapy | No report | myocardial fibrosis | Haudek et al., 2007; Lee et al., 2007 | ||
| Postoperation | 10%-20% | CRP and IL-6 increased, increased K+ outward current, and shortened action potentials | Chung et al., 2001; Craig et al., 2001; Aviles et al., 2003; Gaudino et al., 2003; Anselmi et al., 2009; Heerdt et al., 2012; Alifano et al., 2014 |
AF, atrial fibrillation; CTX, cyclophosphamide; TNF-α, tumor necrosis factor-α; ER stress, endoplasmic reticular stress; Cx43/45, connexin 43/45; BTK, bruton kinase; TEC, tec protein tyrosine kinase; HDAC, hydroxamic acid histone deacetylase; MIF, macrophage migration inhibitory factor.
Targeted therapies
Targeted cancer drugs are usually sorted as either micromolecules or monoclonal antibodies (Tamargo et al., 2015). They are aimed to disturb a specific signaling involved in the course of cancer progression.
Ibrutinib, a new kind of targeted anticancer drug, is a Bruton kinase inhibitor (Honigberg et al., 2010; Herman et al., 2011), which has been confirmed to be effective in some B-cell malignancies (Burger et al., 2015; Byrd et al., 2015; Treon et al., 2015; Wang et al., 2015; Gertz, 2017). In a recent meta-analysis of 20 studies surveying the occurrence of AF in patients treated with ibrutinib, the rate of AF in the ibrutinib-treated patients was distinctly higher than that in the non-ibrutinib-treated patients and the age-matched normal subjects (Leong et al., 2016; Yun et al., 2017). The mechanism by which ibrutinib induced cardiotoxicity likely involved the reduction of the PI3K signaling in the heart, which may increase the susceptibility to AF. McMullen et al. revealed that ibrutinib was able to suppress the PI3K-Akt signaling in an isolated rat myocardial cell (Pretorius et al., 2010; McMullen et al., 2014). In another study, ibrutinib triggered aberrant APs in isolated mouse and rabbit myocardial cells, and the defects were quickly reversed by adding PI3K to the pipette (Yang et al., 2015). These results indicate that ibrutinib causes AF by inhibiting the PI3K-Akt pathway in the heart. A previous study has shown that patients treated with ibrutinib without a history of AF had an incidence rate of AF of 6.1% (Shanafelt et al., 2017). Some patients even stopped treatment with ibrutinib owing to the occurrence of AF (Byrd et al., 2014). Thus, it is necessary to conduct further studies on the mechanism of ibrutinib-induced cardiotoxicity.
There are other drugs that can cause AF during cancer treatment. With the recent use of checkpoint inhibitors, the clinical outcomes of patients with tumors, such as metastatic melanomas and renal, lung, and bladder tumors, have dramatically improved (Ryder et al., 2014; Wolchok, 2015; Yu et al., 2015; Lee et al., 2016; Moslehi, 2016). Specifically, 7-hydroxy-staurosporine (UCN-01) is a new type of an antitumor drug. A previous phase I trial aimed to ascertain the safety and the pharmacokinetics of ascending doses of cisplatin combined with UCN-01 in patients with malignant tumors (Lara et al., 2005). Ten patients were enrolled, and treatment was halted at dose level 2 owing to dose-limiting toxicity (DLT) grade 3 AF in one patient.
TKIs
Tyrosine kinase inhibitors are significant targets for cancer treatment because they play an important role in the regulation of growth factor signaling (Guglin et al., 2009). In the chronic myelogenous leukemia, the BCR-Abl kinase is a tyrosine kinase target. Several kinds of TKIs containing nilotinib, erlotinib, dasatinib, and imatinib have targeted the kinase. These anticancer agents had been reported to induce AF, thromboembolism, and pulmonary hypertension. Some studies have reported that cetuximab, sunitinib, and alemtuzumab were linked to AF in the one case report each (Lenihan et al., 2004; Pfister et al., 2006; Mego et al., 2007). Rituximab is related with numerous reactions containing cardiac arrhythmias, such as AF and ventricular tachycardia (VT), reversible after the discontinuation of medication (Coiffier et al., 2002; Arai et al., 2005).
Sunitinib is a kind of drug which can selectively target many kinds of receptor tyrosine kinases. It works by blocking blood and nutrients needed for tumor growth. In patients with metastatic gastrointestinal stromal tumor, sunitinib treatment is reported to induce left ventricle (LV) contractile dysfunction (Chu et al., 2007). Multiple target points, such as reduced myocardial cell activity, inhibited AMPK and K+ channels, and enhanced accumulation of lipids, are also reported (Doherty et al., 2013). These underlying mechanisms of sorafenib-induced cardiotoxicity are linked with LV contractile dysfunction, ROS production, mitochondrial disorders, and apoptosis in the myocardial cell (Will et al., 2008; Duran et al., 2014; Kawabata et al., 2015).
Anthracyclines
Anthracyclines antibiotic inhibits cell growth and restrains the fleetly increasing cancer cells (Guglin et al., 2009). They are well known for the associated cardiotoxicity. There are many mechanisms underlying the cardiotoxicity associated with doxorubicin use (Gorelik et al., 2003). Dog and sheep models treated with doxorubicin showed some anomalous electrocardiogram findings, including ST segment elevation, T wave inversion, QT interval prolongation, and cardiac arrhythmia (Lau et al., 2011; Xin et al., 2011). A previous study has recognized that doxorubicin can down-regulate the expression of the Cx43/Cx45 junction, resulting in cardiac dysfunction and LV remodeling (Zhang et al., 2011). Doxorubicin-induced mitochondrial dysfunction (Varga et al., 2015), ROS production (Kluza et al., 2004), and apoptosis were observed in the cardiomyocytes (Lai et al., 2011). Doxorubicin suppresses the expression of the SR Ca2+ ATPase, impairing Ca2+ regulation and consequently cardiac function (Arai et al., 1998). Doxorubicin use can also lead to CaMKII-mediated Ca2+ leakage from the SR, which can destroy the intracell Ca2+ steady state and increase the incidence rate of AF (Bracci et al., 2014). CaMKII acts a crucial part in the occurrence and development of AF via regulating Ca2+-related proteins and cardiac ion channels, such as L-type Ca2+ currents, Na+ currents, and late Na+ currents. In addition, CaMKII inhibition can decrease the cardiotoxicity induced by doxorubicin (Bracci et al., 2014), which demonstrates the underlying CaMKII regulation regarded as a policy for alleviating anticancer drug-induced AF.
In a phase I clinical trial (Woolley et al., 1982), 22 patients with cancer were administered with the new anthracycline aclacinomycin A, and one patient developed transient AF. Conversely, 7-con-O-methylnogaril was also a novel chemotherapeutic drug used in clinical trials (Dorr et al., 1986). Twenty-four patients received this drug, and one patient developed cardiotoxicity (transient AF). A clinical study recorded paroxysmal AF in 6.9% of 393 patients during the first course of doxorubicin chemotherapy (Numico et al., 2002). Other studies have also reported similar findings (Montella et al., 2005; Kilickap et al., 2007; Lebedinsky et al., 2011). In summary, the cardiotoxicity induced by anthracyclines has been well researched, as well as association of anthracyclines with AF appears to be closely connected.
Alkylating agents
Alkylating agents (e.g., cisplatin, CTX, ifosfamide, and melphalan) can also cause AF (Eskilsson et al., 1988; Petrella et al., 1989; Menard et al., 1991; Moreau et al., 1999; Ifran et al., 2005; Pfister et al., 2006). They are normally used for the treatment of slow-growing cancers. Cisplatin has been employed extensively for locoregional perfusion in thoracic malignancies. A great number of previous studies have indicated that cisplatin-induced cardiotoxicity may result in LV dysfunction, restrained myocardial contractions (Ma et al., 2010), mitochondrial dysfunction (Pfister et al., 2006), strengthened endoplasmic reticular stress, cell apoptosis (Honigberg et al., 2010), ROS production, and inflammation (Ma et al., 2010). Cardiotoxicity is induced by cisplatin via upregulation of TNF-α and NF-κB (Albini et al., 2010). The administration of 4-hydroperoxycyclophosphamide to the cardiomyocytes stimulates cytotoxicity by inducing cellular Na+, Ca2+, and K+ activation, ATP content (Feliz et al., 2011), and lysosomal injury (Sudharsan et al., 2006). Finally, CTX use leads to myocardial hypertrophy, myocardial fibrosis, and changes in the expressions of some cytokines, such as IL-1β, TNF-α, and IL-10, which are likely to promote AF development (Liu et al., 2015).
Among patients with adenocarcinoma of the lung and pericardial tamponade who received cisplatin perfusion, 19% showed AF (Tomkowski et al., 2004; Richards et al., 2006; Tilleman et al., 2009; Zellos et al., 2009). In a recent clinic trial, carboplatin combination therapy induced AF in one of 32 patients (Illiano et al., 2000). Using high doses of CTX and ifosfamide increases the risk of paroxysmal supraventricular tachycardia and paroxysmal AF (Kupari et al., 1990; Quezado et al., 1993). In 11% of patients who underwent bone marrow transplant with high-dose melphalan treatment, AF was observed (Olivieri et al., 1998; Moreau et al., 1999; Abidi et al., 2012).
HER2 blockers
A previous experimental study has confirmed that trastuzumab-induced cardiotoxicity was associated with enhanced myocardial ROS production, apoptosis, and changes in the ultrastructure (Elzarrad et al., 2013). Another study indicated that trastuzumab use correlated with LV contractile dysfunction was regulated by the combination with the HER2 protein, accordingly interdicting the ErbB2-ErbB4 signaling channel (Jones et al., 2009). Approximately 19.9% of female patients discontinued trastuzumab treatment because of AF development. Another blocker used in patients with previously untreated metastatic melanoma was etaracizumab, an IgG1 humanized monoclonal antibody against the avb3 integrin. After treatment with etaracizumab, 9% of patients had AF (Hersey et al., 2010).
Antimetabolites
Antimetabolites are specifically bound to metabolites in the body and thus affect or antagonize metabolic functions. They have a chemical structure similar to that of nucleic acids or protein metabolites in the body. They play an antitumor role by interfering with DNA synthesis. It has been reported that the incidence rate of cardiotoxicity reached up to 2-4% in patients with cancer receiving antimetabolites, such as 5-fluorouracil (FU) or other analogs (Berliner et al., 1990; de Forni et al., 1992; Frickhofen et al., 2002; Perez-Verdia et al., 2005; Saif et al., 2009). Particularly, 5-FU is a synthetic pyrimidine antimetabolite, which acts as a cell growth inhibitor to malignant lesions; however, the cardiotoxicity associated with this has only been investigated in some clinical studies. In a previous case report, AF was found in a 60-year-old male patient within the first 24 h after receiving 5-FU treatment (Aziz et al., 1998). Meydan et al. (2005) surveyed the incidence rate of cardiotoxicity associated with high-dose leucovorin combined with 5-FU continuous infusion, and the patients underwent long-term follow-ups. They found that nine of 231 patients who were administered with high-dose leucovorin combined with 5-FU developed cardiotoxic events, revealing an overall occurrence rate of 3.9%. Myocardial ischemia appears to dominate 5-FU-induced cardiotoxicity; however, many cardiac arrhythmias appear in ischemia-reperfusion injuries as ventricular arrhythmias, AF, etc. (Slamon et al., 1987; Keefe et al., 1993; Hrovatin et al., 2006).
Antimicrotubule drugs
Tubulin, an antimicrotubule drug, plays a significant part in intracellular transportation, cell mitosis, and signal transduction. Paclitaxel is a kind of microtubulin polymerization agent, and has become an important treatment for lung, breast, and ovarian cancer. However, as with other antitumor drugs, the side effects and the emergence of resistance after administration limit the clinical use of microtubulin inhibitors. Paclitaxel cardiotoxicity can lead to AF, VT, ventricular fibrillation (VF), and even sudden mortality (Arbuck et al., 1993), and gradually increases with the time and dosage of the drug use (Brouty-Boye et al., 1995). There were 90 patients who were administered with paclitaxel as the second-line chemotherapeutic drug, and considering the cardiovascular events that occurred, the incidence rate of AF was 1%. In the perfused heart of guinea pigs, paclitaxel caused an abnormal conduction and consequently decreased coronary blood flow and LV systolic pressure (Alloatti et al., 1998). Meanwhile, in a study on frogs and rabbits, taxanes slowed the heart rate and generated auriculo-ventricular block, thereby leading to asystole (Bryan-Brown, 1932). In a randomized phase 3 trial, two studies employed gemcitabine and gemcitabine vinorelbine (Gridelli et al., 2001). Forty-nine patients participated in each group; in the gemcitabine vinorelbine combination group, four patients developed serious cardiotoxicity complications accompanied with atrial flutter or AF.
Histone deacetylase inhibitors
Depsipeptide is a histone deacetylase inhibitor, which can regulate gene expression and adjust cell cycle arrest and cell apoptosis. Studies have shown that it could validate cytotoxicity suppressing the human tumor cell lines (Ueda et al., 1994a,b). Based on a large amount of pre-clinical data, depsipeptide is likely to have conspicuous cardiotoxicity. There were 88 patients who received depsipeptide treatment in a clinical study (Sandor et al., 2002), and the DLT involved grade-4 arrhythmia in one patient (AF). Stadler et al. (2006) investigated some patients with refractory renal cell carcinoma, who participated in a phase II study. One patient developed grade 3 AF. Belinostat is also a new hydroxamic acid histone deacetylase inhibitor with potent antiproliferative activities (Plumb et al., 2003). Conversely, Steele et al. investigated (Steele et al., 2008) 46 patients who received treatment of belinostat, and the DLT involved grade 3 AF.
Antiestrogens
Some studies have shown that estrogen plays an important role in the occurrence and development of breast cancer. Approximately two-thirds of breast cancer cells contain a certain amount of estrogen receptors (Robertson et al., 1996). Tamoxifen is one of the most common estrogens blockers; it is often used to treat advanced breast and ovarian cancers. The effectiveness rate of clinical breast cancer treatment is generally 30%. There were 5,408 women who underwent hysterectomy and were distributed among the tamoxifen and placebo groups (Veronesi et al., 2007). AF occurred more often in the patients who received tamoxifen treatment.
Proteasome inhibitors
Proteasomes are a colossal protein composite existing in the cells, which can degrade other proteins, block cellular proliferation, and induce apoptosis in the tumor cells, particularly in multiple myelomas (MMs). The conditions of patients with MMs have changed prominently during the past few decades with the introduction of new drugs, such as thalidomide, bortezomib, and lenalidomide (Kumar et al., 2008). Dexamethasone is commonly used in combination with these drugs to treat cancer. The combination of lenalidomide and dexamethasone is perceived as a special treatment option in these patients. A critical aspect in the clinic application of lenalidomide is active monitoring for CAEs (Zangari et al., 2001, 2009; Neben et al., 2002; Weber et al., 2003; Zonder et al., 2006; Palumbo et al., 2008; Klein et al., 2009). Although these drugs have changed the therapeutic effect on MMs, substantially improving patient outcomes, their use easily induces cardiotoxicity. The primary CAE associated with lenalidomide use is AF, particularly when it is employed for a long period (Pérez Persona et al., 2011).
Immunotherapy
Interleukin refers to a lymphokine that interacts between leukocytes and immune cells, and is a cytokine of the same type as the hematopoietic growth factor, coordinating and interacting with each other to complete hematopoiesis and immune regulation. IL-2 can mediate tumor treatment in patients with renal cell carcinoma and metastatic melanoma (White et al., 1994). Considering the Food and Drug Administration approval, patients with renal cell carcinoma are treated with high-dose recombinant IL-2; however, its use has a high incidence rate of cardiotoxicity. ILs are found in various cancer-induced cardiotoxicities, including hypertension, HF, and AF (Aoyagi and Matsui, 2011; Guo et al., 2012a,b), revealing elevated concentrations of pro-inflammatory cytokines, such as IL-6 and IL-2 (Fildes et al., 2009; Guo et al., 2012a). In affected patients, IL-6 was highly expressed, which was closely associated with the AF duration (Issac et al., 2007). There were 199 patients who received 310 treatment courses. Cardiac arrhythmia occurred in 6% of the patients, with 11 of these patients retreated and two who showed AF recrudescence. Thompson et al. (1994) conducted a phase lb clinical test in sick patients receiving IL-2 combined with lymphokine-activated killer cell treatment for metastatic renal cell carcinoma; 18 patients were administered with IL-2, and AF occurred at a dose of 4.9 mg/kg.
Macrophage MIF plays an important role in the inflammatory pathways and is associated with the occurrence of many cancer phenotypes (O'Reilly et al., 2016). Some studies also found that inhibiting the function of MIF could significantly reduce the growth of cancer in vitro or in vivo systems, such as bladder cancer, lung cancer, and colon cancer (Choudhary et al., 2013; Kindt et al., 2013; Ioannou et al., 2014; Mawhinney et al., 2014; Varinelli et al., 2015). MIF also plays a crucial role in the pathophysiology of cardiovascular diseases (van der Vorst et al., 2015). A previous study also showed that MIF was related to electrical remodeling with AF, probably through falling ICa, L amplitudes and activating c-Src kinases in the atrial myocytes (Rao et al., 2009). In summary, these studies highlight the importance of controlling MIF expression in preventing atrial electrical remodeling in patients with cancer.
TNF-α is deemed as a primary moderator of immune responses and a vital participator in the cytokines (Balkwill, 2009). It is generally involved in the upregulation of all kinds of chemotherapeutic drug-induced cardiotoxicity (Karayiannakis et al., 2001; Balkwill, 2002; Szlosarek and Balkwill, 2003) and can affect patients' prognosis (Balkwill, 2006). A previous study deemed TNF-α as a pivotal regulator in colon cancer progression and proved that interdicting TNF-α in a mice model can lessen colonitis-related carcinoma of the colon (Popivanova et al., 2008). TNF-α yields apoptosis of various cells (Haudek et al., 2007) and enhances PV arrhythmogenicity and Ca2+ homeostasis maladjustment, thus resulting in the occurrence of AF (Lee et al., 2007).
Radiotherapy
With the application of radiotherapy technology, treatment of malignant tumors and some benign diseases by ionizing radiation has significantly reduced mortality. The cardiovascular toxicity of chest radiotherapy increases cardiovascular mortality, partially offsetting the improvement in survival rate for chest radiotherapy (Qi and Zhang, 2015). The main manifestations of radiation therapy for cardiotoxicity are ischemic heart disease, HF, and AF. Among patients who were treated for breast cancer between 1980 and 2000, the cardiotoxicity risk was highest in the patients treated with left breast radiotherapy (Hooning et al., 2007). Significant myocardial fibrosis is very common in the radiotherapy-induced cardiotoxicity (Jaworski et al., 2013). Although there is a lack of clear evidence, it is assumed that radiotherapy could also provoke AF by the occurrence and development of myocardial fibrosis and HF (Mery et al., 2017).
Postoperation
In recent years, the incidence of AF after thoracic surgery has increased, and postoperative AF (POAF) in cancer is closely associated with inflammation and sympathetic activation (Mc Cormack et al., 2014). AF is a common complication of postoperative lung cancer. Approximately 10–20% of patients develop AF, which occurs about 2–3 days after surgery (Vaporciyan et al., 2004; Roselli et al., 2005; Gómez-Caro et al., 2006). Meanwhile, many reports found POAF occurrence and proinflammatory cytokines activated (Bruins et al., 1997; Chung et al., 2001; Aviles et al., 2003; Gaudino et al., 2003; Anselmi et al., 2009). In some studies, for example, CRP and IL-6 increased in the lung cancer patients after surgery (Craig et al., 2001; Alifano et al., 2014). Moreover, there are also some studies that show atrial KCNE1 (potassium channel subunit) down-regulation, which indicated an increased outward current and shortened action potentials in POAF (Heerdt et al., 2012). In addition, POAF happened in 12.6% of the colorectal cancer patients receiving elective colectomy, and also occurred in 9.2% of the esophageal cancer patients after esophagectomy (Siu et al., 2005; Ojima et al., 2014). AF may be regulated by sympathovagal nerve injury following surgical trauma (Amar et al., 1996; Ma et al., 2006), which plays a crucial effect on the occurrence of AF.
Prevention and management
In view of the lack of evidence, there is no specific guideline for the treatment of AF in patients with malignant tumors. The prevention and treatment of AF are based on the current guidelines for the practical management of patients with and without cancer (Chelu et al., 2009; Anderson et al., 2013). The therapeutic management should be individualized, and the decisions regarding anti-arrhythmic drugs or instrumental treatments (Priori et al., 2015) should consider the contending risks of cancer, cardiac-related life expectancy, living quality, and risks of complications. The management of antitumor therapy-induced AF mainly has two aspects: (1) Rhythm control can prevent AF and ameliorate optimal rate control symptoms in patients who still have symptoms (Ferrari et al., 2016). The original method used in managing AF requires the usual decisions regarding rhythm management, particularly in terms of antithrombotic therapy for stroke prevention. So, in the future, more personalized rhythm control therapies could help ameliorate the therapeutic effect and security of therapy. (2) Some patients with cancers in the blood are prone to coagulation defects resulting in bleeding, which may be contraindicated for antithrombotic therapy. Some patients with cancer, such as lung cancer and primary liver cancer, have an increased risk of thromboembolism and therefore, need to be evaluated using risk assessment tools. An assessment tool for antithrombotic therapy in cancer-induced AF, according to cancer features, and established thromboembolic and bleeding risk assessment tools, such as the CHA2DS2-VASc and HAS-BLED scores, are used (Lee, 2005; Hu et al., 2013). Hence, the decision regarding the initiation of antithrombotic treatment in patients with cancer has to be austerely individualized, weighing modestly the benefits against the risks based on the characteristics of every specific patient.
Conclusions
In this review, the mechanisms of some chemotherapeutic drugs, post-surgery, radiation therapy, and cancer system immunity in inducing AF were summarized on the basis of existing data. We hope to attract more attention of cardiologists to this problem. As anticancer therapy-induced AF usually occurs in cancer centers, clinically relevant data on treatment, risk of embolic events, persistence period, and particularly ischemic strokes are not available in the literature. Moreover, the development of AF may impact the therapeutic effects of people with cancer. Therefore, it is necessary to understand the potential mechanism of AF occurrence in people with cancer, which can help increase the effectiveness of cancer treatments. As the field of oncocardiology expands, cardiac oncologists need to know the fundamental electrophysiology principles and management so as to offer proper care for people with cancer.
Author contributions
YX and HS confirmed the article theme. YY, TH, NL, and SH looked for related articles. CT, MY, XZ, YS, GC, and YG collated all related articles. XY wrote the manuscript. XL, XW, and DH modified this manuscript. All authors commented on the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work was supported by the National Key Research and Development Program of China (Grant No.2017YFC1700400), the National Natural Science Foundation of China (Grant Nos. 81725024, 81870244, and 81430098) and National high-level talent special support plan (No.W02020052), Clinical base project of State Administration of traditional Chinese medicine of China (JDZX2015007), and the outstanding project of Beijing University of Chinese Medicine (2015-JYBXJQ001).
References
- Abidi M. H., Agarwal R., Ayash L., Deol A., Al-Kadhimi Z., Abrams J., et al. (2012). Melphalan 180 mg/m2 can be safely administered as conditioning regimen before an autologous stem cell transplantation (ASCT) in multiple myeloma patients with creatinine clearance 60 mL/min/1.73 m2 or lower with use of palifermin for cytoprotection: results of a phase i trial. Biol. Blood Marrow Transplant. 18, 1455–1461. 10.1016/j.bbmt.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albini A., Pennesi G., Donatelli F., Cammarota R., De Flora S., Noonan D. M. (2010). Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J. Natl. Cancer Inst. 102, 14–25. 10.1093/jnci/djp440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alifano M., Mansuet-Lupo A, Lococo F., Roche N., Bobbio A., Canny E., et al. (2014). Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS ONE 9:e106914. 10.1371/journal.pone.0106914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloatti G., Penna C., Gallo M. P., Levi R. C., Bombardelli E., Appendino G. (1998). Differential effects of paclitaxel and derivatives on Guinea pig isolated heart and papillary muscle. J. Pharmacol. Exp. Ther. 284, 561–567. [PubMed] [Google Scholar]
- Amar D., Burt M. E., Bains M. S., Leung D. H. (1996). Symptomatic tachydysrhythmias after esophagectomy: incidence and outcome measures. Ann. Thorac. Surg. 61, 1506–1509. 10.1016/0003-4975(96)00111-7 [DOI] [PubMed] [Google Scholar]
- Anderson J. L., Halperin J. L., Albert N. M., Bozkurt B., Brindis R. G., Curtis L. H., et al. (2013). Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 61, 1935–1944. 10.1016/j.jacc.2013.02.001 [DOI] [PubMed] [Google Scholar]
- Anselmi A., Possati G., Gaudino M. (2009). Postoperative inflammatory reaction and atrial fibrillation: simple correlation or causation? Ann. Thorac. Surg. 88, 326–333. 10.1016/j.athoracsur.2009.01.031 [DOI] [PubMed] [Google Scholar]
- Aoyagi T., Matsui T. (2011). The cardiomyocyte as a source of cytokines in cardiac injury. J. Cell Sci. Ther. 2012, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai M., Tomaru K., Takizawa T., Sekiguchi K., Yokoyama T., Suzuki T., et al. (1998). Sarcoplasmic reticulum genes are selectively down-regulated in cardiomyopathy produced by doxorubicin in rabbits. J. Mol. Cell. Cardiol. 30, 243–254. 10.1006/jmcc.1997.0588 [DOI] [PubMed] [Google Scholar]
- Arai Y., Tadokoro J., Mitani K. (2005). Ventricular tachycardia associated with infusion of rituximab in mantle cell lymphoma. Am. J. Hematol. 78, 317–318. 10.1002/ajh.20303 [DOI] [PubMed] [Google Scholar]
- Arbuck S. G., Strauss H., Rowinsky E., Christian M., Suffness M., Adams J., et al. (1993). A reassessment of cardiac toxicity associated with Taxol. J. Natl. Cancer Inst. Monogr. 29, 117–130. [PubMed] [Google Scholar]
- Aviles R. J., Martin D. O., Apperson-Hansen C., Houghtaling P. L., Rautaharju P., Kronmal R. A., et al. (2003). Inflammation as a risk factor for atrial fibrillation. Circulation 108, 3006–3010. 10.1161/01.CIR.0000103131.70301.4F [DOI] [PubMed] [Google Scholar]
- Aziz S. A., Tramboo N. A., Mohi-ud-Din K., Iqbal K., Jalal S., Ahmad M. (1998). Supraventricular arrhythmia: a complication of 5-fluorouracil therapy. Clin. Oncol. 10, 377–378. 10.1016/S0936-6555(98)80033-2 [DOI] [PubMed] [Google Scholar]
- Balkwill F. (2002). Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 13, 135–141. 10.1016/S1359-6101(01)00020-X [DOI] [PubMed] [Google Scholar]
- Balkwill F. (2006). TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 25, 409–416. 10.1007/s10555-006-9005-3 [DOI] [PubMed] [Google Scholar]
- Balkwill F. (2009). Tumour necrosis factor and cancer. Nat. Rev. Cancer 9, 361–371. 10.1038/nrc2628 [DOI] [PubMed] [Google Scholar]
- Beck M. A. (1999). Selenium and host defence towards viruses. Proc. Nutr. Soc. 58, 707–11. 10.1017/S0029665199000920 [DOI] [PubMed] [Google Scholar]
- Berliner S., Rahima M., Sidi Y., Teplitsky Y., Zohar Y., Nussbaum B., et al. (1990). Acute coronary events following cisplatin-based chemotherapy. Cancer Invest. 8, 583–586. 10.3109/07357909009018924 [DOI] [PubMed] [Google Scholar]
- Bracci L., Schiavoni G., Sistigu A., Belardelli F. (2014). Belardelli, immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationalebased combined treatments against cancer. Cell Death Differ. 21, 15–25. 10.1038/cdd.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouty-Boye D., Kolonias D., Lampidis T. J. (1995). Antiproliferative activity of taxol on human tumor and normal breast cells vs. effects on cardiac cells. Int. J. Cancer. 60, 571–575. 10.1002/ijc.2910600424 [DOI] [PubMed] [Google Scholar]
- Bruins P., te Velthuis H., Yazdanbakhsh A. P., Jansen P. G., van Hardevelt F. W., de Beaumont E. M., et al. (1997). Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation 96, 3542–3548. 10.1161/01.CIR.96.10.3542 [DOI] [PubMed] [Google Scholar]
- Bryan-Brown T. (1932). The pharmacological actions of taxine. Quart. J. Pharmacol. 5, 205–219. [Google Scholar]
- Burger J. A., Tedeschi A., Barr P. M., Robak T., Owen C., Ghia P., et al. (2015). Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N. Engl. J. Med. 373, 2425–2437. 10.1056/NEJMoa1509388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J. C., Brown J. R., O'Brien S., Barrientos J. C., Kay N. E., Reddy N. M., et al. (2014). Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N. Engl. J. Med. 371, 213–223. 10.1056/NEJMoa1400376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd J. C., Furman R. R., Coutre S. E., Burger J. A., Blum K. A., Coleman M., et al. (2015). Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 125, 2497–2506. 10.1182/blood-2014-10-606038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camm A. J., Lip G. Y., De Caterina R., Savelieva I., Atar D., Hohnloser S. H., et al. (2012). 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Eur Heart. J. 33, 2719–2747. 10.1093/eurheartj/ehs253 [DOI] [PubMed] [Google Scholar]
- Carver J. R., Shapiro C. L., Ng A., Jacobs L., Schwartz C., Virgo K. S., et al. (2007). American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J. Clin. Oncol. 25, 3991–4008 10.1200/JCO.2007.10.9777 [DOI] [PubMed] [Google Scholar]
- Chelu M. G., Sarma S., Sood S., Wang S., van Oort R. J., Skapura D. G., et al. (2009). Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J. Clin. Invest. 119, 1940–1951. 10.1172/JCI37059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S., Hegde P., Pruitt J. R., Sielecki T. M., Choudhary D., Scarpato K., et al. (2013). Macrophage migratory inhibitory factor promotes bladder cancer progression via increasing proliferation and angiogenesis. Carcinogenesis 34, 2891–2899. 10.1093/carcin/bgt239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu T. F., Rupnick M. A., Kerkela R., Dallabrida S. M., Zurakowski D., Nguyen L. (2007) Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370 2011–2019. 10.1016/S0140-6736(07)61865-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M. K., Martin D. O., Sprecher D., Wazni O., Kanderian A., Carnes C. A., et al. (2001). Creactive protein elevation in patients with atrial arrhythmias: inflammatorymechanisms and persistence of atrial fibrillation Circulation 104, 2886–2891. 10.1161/hc4901.101760 [DOI] [PubMed] [Google Scholar]
- Coiffier B., Lepage E., Briere J., Herbrecht R., Tilly H., Bouabdallah R., et al. (2002). CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 346, 235–242. 10.1056/NEJMoa011795 [DOI] [PubMed] [Google Scholar]
- Conway D. S., Buggins P., Hughes E., Lip G. Y. (2004). Prognostic significance of raised plasma levels of interleukin-6 and C-reactive protein in atrial fibrillation. Am. Heart J. 148, 462–466. 10.1016/j.ahj.2004.01.026 [DOI] [PubMed] [Google Scholar]
- Craig S. R., Leaver H. A., Yap P. L., Pugh G. C., Walker W. S. (2001). Acute phase responses following minimal access and conventional thoracic surgery. Eur. J. Cardiothorac. Surg. 20, 455–463. 10.1016/S1010-7940(01)00841-7 [DOI] [PubMed] [Google Scholar]
- Curigliano G., Mayer E. L., Burstein H. J., Winer E. P., Goldhirsch A. (2010). Cardiac toxicity from systemic cancer therapy: a comprehensive review. Prog. Cardiovasc. Dis. 53, 94–104. 10.1016/j.pcad.2010.05.006 [DOI] [PubMed] [Google Scholar]
- de Forni M., Malet-Martino M. C., Jaillais P., Shubinski R. E., Bachaud J. M., Lemaire L., et al. (1992). Cardiotoxicity of high-dose continuous infusion fluorouracil: a prospective clinical study. J. Clin. Oncol. 10, 1795–1801. 10.1200/JCO.1992.10.11.1795 [DOI] [PubMed] [Google Scholar]
- Dobrev D., Voigt N., Wehrens X. H. (2011). The ryanodine receptor channel as a molecular motif in atrial fibrillation: pathophysiological and therapeutic implications. Cardiovasc. Res. 89, 734–743. 10.1093/cvr/cvq324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty K. R., Wappel R. L., Talbert D. R., Trusk P. B., Moran D. M., Kramer J. W., et al. (2013). Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes. Toxicol. Appl. Pharmacol. 272, 245–255. 10.1016/j.taap.2013.04.027 [DOI] [PubMed] [Google Scholar]
- Dorr F. A., Von Hoff D. D., Kuhn J. G., Schwartz R., Kisner D. L. (1986). Phase I clinical investigation of 7-con-O-methylnogaril, a new anthracycline antibiotic. Cancer Res. 46, 2562–2565. [PubMed] [Google Scholar]
- Duran J. M., Makarewich C. A., Trappanese D., Gross P., Husain S., Dunn J., et al. (2014). Sorafenib cardiotoxicity increases mortality aftermyocardial infarction. Circ. Res. 114, 1700–1712. 10.1161/CIRCRESAHA.114.303200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzarrad M. K., Mukhopadhyay P., Mohan N., Hao E., Dokmanovic M., Hirsch D. S., et al. (2013). Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. PLoS ONE 8:e79543. 10.1371/journal.pone.0079543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen R., Christiansen C. F., Mehnert F., Weiss N. S., Baron J. A., Sorensen H. T. (2012). Colorectal cancer and risk of atrial fibrillation and flutter: a population-based case–control study. Intern. Emerg. Med. 7, 431–438. 10.1007/s11739-011-0701-9 [DOI] [PubMed] [Google Scholar]
- Eskilsson J., Albertsson M., Mercke C. (1988). Adverse cardiac effects during induction chemotherapy treatment with cis-platin and 5-fluorouracil. Radiother. Oncol. 13, 41–46 10.1016/0167-8140(88)90296-4 [DOI] [PubMed] [Google Scholar]
- European Heart Rhythm Association European Association for Cardio-Thoracic Surgery, Camm, A. J., Kirchhof P., Lip G. Y., Schotten U., et al. (2010). Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 31, 2369–2429 10.1093/eurheartj/ehq278 [DOI] [PubMed] [Google Scholar]
- Farmakis D., Parissis J., Filippatos G. (2014). Insights into onco-cardiology: atrial fibrillation in cancer. J. Am. Coll. Cardiol. 63, 945–953. 10.1016/j.jacc.2013.11.026 [DOI] [PubMed] [Google Scholar]
- Feliz V., Saiyad S., Ramarao S. M., Khan H., Leonelli F., Guglin M. (2011). Melphalan-induced supraventricular tachycardia: incidence and risk factors. Clin. Cardiol. 34, 356–359. 10.1002/clc.20904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R., Bertini M., Blomstrom-Lundqvist C., Dobrev D., Kirchhof P., Pappone C., et al. (2016). An update on atrial fibrillation in 2014: from pathophysiology to treatment. Int. J. Cardiol. 203, 22–29. 10.1016/j.ijcard.2015.10.089 [DOI] [PubMed] [Google Scholar]
- Ferreira C., Providencia R., Ferreira M. J., Goncalves L. M. (2015). Atrial fibrillation and noncardiovascular diseases: a systematic review. Arq. Bras. Cardiol. 105, 519–526. 10.5935/abc.20150142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fildes J. E., Shaw S. M., Yonan N., Williams S. G. (2009). The immune system and chronic heart failure: is the heart in control? J. Am. Coll. Cardiol. 53, 1013–1020. 10.1016/j.jacc.2008.11.046 [DOI] [PubMed] [Google Scholar]
- Floyd J. D., Nguyen D. T., Lobins R. L., Bashir Q., Doll D. C., Perry M. C. (2005). Cardiotoxicity of cancer therapy. J. Clin. Oncol. 23, 7685–7696. 10.1200/JCO.2005.08.789 [DOI] [PubMed] [Google Scholar]
- Frickhofen N., Beck F. J., Jung B., Fuhr H. G., Andrasch H., Sigmund M. (2002). Capecitabine can induce acute coronary syndrome similar to 5-fluorouracil. Ann. Oncol. 13, 797–801. 10.1093/annonc/mdf035 [DOI] [PubMed] [Google Scholar]
- Fuster V., Rydén L. E., Cannom D. S., Crijns H. J., Curtis A. B., Ellenbogen K. A., et al. (2011). 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 123, e269–367 10.1161/CIR.0b013e318214876d [DOI] [PubMed] [Google Scholar]
- Gaudino M., Andreotti F., Zamparelli R., Di Castelnuovo A., Nasso G., Burzotta F., et al. (2003). The−174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation 108(Suppl. 1), II195–II199. 10.1161/01.cir.0000087441.48566.0d [DOI] [PubMed] [Google Scholar]
- Gen W., Tani M., Takeshita J., Ebihara Y., Tamaki K. (2001). Mechanisms of Ca2+ overioad induced by extracellular H2O2 in quiescent isoiated rat cardiomyocytes. Basic Res Cardioi. 96, 623–629. 10.1007/s003950170014 [DOI] [PubMed] [Google Scholar]
- Gertz M. A. (2017). Waldenström macroglobulinemia: 2017 update on diagnosis, risk stratification, and management. Am. J. Hematol. 92, 209–217. 10.1002/ajh.24557 [DOI] [PubMed] [Google Scholar]
- Gómez-Caro A., Moradiellos F. J., Ausfn P., Díaz-Hellín V., Larrú E., Pérez-Antón J. A., et al. (2006). Risk factors foratrial fibrillation after thoracic surgery. Arch. Bronconeumol. 42, 9–13. [DOI] [PubMed] [Google Scholar]
- Gorelik J., Vodyanoy I., Shevchuk A. I., Diakonov I. A., Lab M. J., Korchev Y. E. (2003). Esmolol is antiarrhythmic in doxorubicin-induced arrhythmia in cultured cardiomyocytes - determination by novel rapid cardiomyocyte assay. FEBS Lett. 548, 74–78. 10.1016/S0014-5793(03)00743-9 [DOI] [PubMed] [Google Scholar]
- Gridelli C., Cigolari S., Gallo C., Manzione L., Ianniello G. P., Frontini L., et al. (2001). Activity and toxicity of gemcitabine and gemcitabinevinorelbine in advanced non-small-cell lung cancer elderly patients Phase II data from the Multicenter Italian Lung Cancer in the Elderly Study (MILES) randomized trial. Lung Cancer. 31, 277–284. 10.1016/S0169-5002(00)00194-X [DOI] [PubMed] [Google Scholar]
- Gu J. F. (2015). The research progress on cardiac toxic mechanism of anthracyclines and prevention treatment measures. World Notes Antibiot. 6, 241–248. 10.13461/j.cnki.wna.004903 [DOI] [Google Scholar]
- Guglin M., Aljayeh M., Saiyad S., Ali R., Curtis A. B. (2009). Introducing a new entity: chemotherapy-induced arrhythmia. Europace 11, 1579–1586. 10.1093/europace/eup300 [DOI] [PubMed] [Google Scholar]
- Guo Y., Lip G. Y., Apostolakis S. (2012a). Inflammation in atrial fibrillation. J. Am. Coll. Cardiol. 60, 2263–2270. 10.1016/j.jacc.2012.04.063 [DOI] [PubMed] [Google Scholar]
- Guo Y., Tian Y., Wang H., Si Q.Y, Wang, Lip G. Y. (2015). Prevalence, incidence, and lifetime risk of atrial fibrillation in China: new insights into the global burden of atrial fibrillation. Chest 147, 109–119. 10.1378/chest.14-0321 [DOI] [PubMed] [Google Scholar]
- Guo Y., Xu F., Lu T., Duan Z., Zhang Z. (2012b). Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 38, 904–910. 10.1016/j.ctrv.2012.04.007 [DOI] [PubMed] [Google Scholar]
- Gupta A. K., Maheshwari A., Tresch D. D., Thakur R. K. (2002). Cardiac arrhythmias in the elderly. Cardiac. Electrophysiol. Rev. 6, 120–128. 10.1023/A:1017963928016 [DOI] [PubMed] [Google Scholar]
- Guzzetti S., Costantino G., Fundaro C. (2002). Systemic inflammation, atrial fibrillation, and cancer. Circulation 106:e40. [DOI] [PubMed] [Google Scholar]
- Haudek S. B., Taffet G. E., Schneider M. D., Mann D. L. (2007). TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J. Clin. Invest. 117, 2692–2701. 10.1172/JCI29134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerdt P. M., Kant R., Hu Z., Kanda V. A., Christini D. J., Malhotra J. K., et al. (2012). Transcriptomic analysis reveals atrial KCNE1 down-regulation following lung lobectomy. J. Mol. Cell. Cardiol. 53, 350–353. 10.1016/j.yjmcc.2012.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman S. E., Gordon A. L., Hertlein E., Ramanunni A., Zhang X., Jaglowski S., et al. (2011). Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 117, 6287–6296. 10.1182/blood-2011-01-328484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández M. A. (2006). C-reactive protein and atrial fibrillation. an old marker looking for a new target. Rev. Esp. Cardiol. 59, 94–98. 10.1016/S1885-5857(06)60116-3 [DOI] [PubMed] [Google Scholar]
- Hersey P., Sosman J., O'Day S., Richards J., Bedikian A., Gonzalez R., et al. (2010). A randomized phase 2 study of etaracizumab, a monoclonal antibody against integrin alpha(v)beta(3), + or - dacarbazine in patients with stage IV metastatic melanoma. Cancer 116, 1526–1534. 10.1002/cncr.24821 [DOI] [PubMed] [Google Scholar]
- Honigberg L. A., Smith A. M., Sirisawad M., Verner E., Loury D., Chang B., et al. (2010). The bruton tyrosine kinase inhibitor PCI-32765 blocks Bcell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. U. S. A. 107, 13075–13080 10.1073/pnas.1004594107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooning M. J., Botma A., Aleman B. M., Baaijens M. H., Bartelink H., Klijn J. G., et al. (2007). Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J. Natl. Cancer Inst. 99, 365–375. 10.1093/jnci/djk064 [DOI] [PubMed] [Google Scholar]
- Hove-Madsen L., Llach A., Bayes-Genis A., Roura S., Rodriguez Font E., Arís A., et al. (2004). Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes Circulation 110, 1358–1363. 10.1161/01.CIR.0000141296.59876.87 [DOI] [PubMed] [Google Scholar]
- Hrovatin E., Viel E., Lestuzzi C., Tartuferi L., Zardo F., Brieda M., et al. (2006). Severe ventricular dysrhythmias and silent ischemia during infusion of the antimetabolite 5-fluorouracil and cis-platin. J. Cardiovasc. Med. 7, 637–640. 10.2459/01.JCM.0000237914.12915.dd [DOI] [PubMed] [Google Scholar]
- Hu Y. F., Liu C. J., Chang P. M., Tsao H. M., Lin Y. J., Chang S. L., et al. (2013). Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int. J. Cardiol. 165, 355–357. 10.1016/j.ijcard.2012.08.036 [DOI] [PubMed] [Google Scholar]
- Ifran A., Kaptan K., Beyan C. (2005). High-dose cyclophosphamide and MESNA infusion can cause acute atrial fibrillation. Am. J. Hematol. 80:247 10.1002/ajh.20441 [DOI] [PubMed] [Google Scholar]
- Illiano A., Barletta E., De Marino V., Battiloro C., Barzelloni M., Scognamiglio F., et al. (2000). New triplet chemotherapy combination with carboplatin, paclitaxel and gemcitabine plus amifostine support in advanced non small cell lung cancer: a phase II study. Anticancer Res. 20, 3999–4003. [PubMed] [Google Scholar]
- Ioannou K., Cheng K. F., Crichlow G. V., Birmpilis A. I., Lolis E. J., Tsitsilonis O. E., et al. (2014). ISO-66, a novel inhibitor of macrophage migration, shows efficacy in melanoma and colon cancer models. Int. J. Oncol. 45, 1457–1468. 10.3892/ijo.2014.2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issac T. T., Dokainish H., Lakkis N. M. (2007). Role of inflammation in initiation and perpetuation of atrial fibrillation: a systematic review of the published data, J. Am. Coll. Cardiol. 50, 2021–2028. 10.1016/j.jacc.2007.06.054 [DOI] [PubMed] [Google Scholar]
- Iwasaki Y. K., Nishida K., Kato T., Nattel S. (2011). Atrial fibrillation pathophysiology: implications for management. Circulation 124, 2264–2274. 10.1161/CIRCULATIONAHA.111.019893 [DOI] [PubMed] [Google Scholar]
- Jaworski C., Mariani J. A., Wheeler G., Kaye D. M. (2013). Cardiac complications of thoracic irradiation. J. Am. Coll. Cardiol. 61, 2319–2328. 10.1016/j.jacc.2013.01.090 [DOI] [PubMed] [Google Scholar]
- Jones A. L., Barlow M., Barrett-Lee P. J., Canney P. A., Gilmour I. M., Robb S. D., et al. (2009). Management of cardiac health in trastuzumab-treated patients with breast cancer: updated United Kingdom National Cancer Research Institute recommendations for monitoring. Br. J. Cancer 100, 684–692. 10.1038/sj.bjc.6604909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiannakis A. J., Syrigos K. N., Polychronidis A., Pitiakoudis M., Bounovas A., Simopoulos K. (2001). Serum levels of tumor necrosis factor-alpha and nutritional status in pancreatic cancer patients. Anticancer Res. 21, 1355–1358. [PubMed] [Google Scholar]
- Kawabata M., Umemoto N., Shimada Y., Nishimura Y., Zhang B., Kuroyanagi J., et al. (2015). Downregulation of stanniocalcin 1 is responsible for sorafenib-induced cardiotoxicity. Toxicol. Sci. 143, 374–384. 10.1093/toxsci/kfu235 [DOI] [PubMed] [Google Scholar]
- Keefe D. (2001). Anthracyciine-Induced cardiomyopathy. Semin Oncol. 28(4 Supp12), 2–7. 10.1053/sonc.2001.26431 [DOI] [PubMed] [Google Scholar]
- Keefe D. L., Roistacher N., Pierri M. K. (1993). Clinical cardiotoxicity of 5-fluorouracil. J. Clin. Pharmacol. 33, 1060–1070. 10.1002/j.1552-4604.1993.tb01943.x [DOI] [PubMed] [Google Scholar]
- Khan M. A., Ahmed F., Neyses L., Mamas M. A. (2013). Atrial fibrillation in heart failure: the sword of Damocles revisited. World J. Cardiol. 5, 215–227. 10.4330/wjc.v5.i7.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilickap S., Barista I., Akgul E., Aytemir K., Aksoy S., Tekuzman G. (2007). Early and late arrhythmogenic effects of doxorubicin. South. Med. J. 100, 262–265. 10.1097/01.smj.0000257382.89910.fe [DOI] [PubMed] [Google Scholar]
- Kindt N., Laurent G., Nonclercq D., Journe F., Ghanem G., Duvillier H., et al. (2013). Pharmacological inhibition of macrophage migration inhibitory factor interferes with the proliferation and invasiveness of squamous carcinoma cells. Int. J. Oncol. 43, 185–193. 10.3892/ijo.2013.1944 [DOI] [PubMed] [Google Scholar]
- Klein U., Kosely F., Hillengass J., Hundemer M., Schmitt S., Neben K., et al. (2009). Effective prophylaxis with low molecular weight heparin in relapsed multiple myeloma patients treated with lenalidomide and dexamethasone. Ann. Hematol. 88, 67–71. 10.1007/s00277-008-0561-1 [DOI] [PubMed] [Google Scholar]
- Kluza J., Marchetti P., Gallego M. A., Lancel S., Fournier C., Loyens A., et al. (2004). Mitochondrial proliferation during apoptosis induced by anticancer agents: effects of doxorubicin and mitoxantrone on cancer and cardiac cells. Oncogene 23, 7018–7030. 10.1038/sj.onc.1207936 [DOI] [PubMed] [Google Scholar]
- Kumar S. K., Rajkumar S. V., Dispenzieri A., Lacy M. Q., Hayman S. R., Buadi F. K., et al. (2008). Improved survival in multiple myeloma and the impact of novel therapies. Blood. 111, 2516–2520. 10.1182/blood-2007-10-116129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupari M., Volin L., Suokas A., Timonen T., Hekali P., Ruutu T. (1990). Cardiac involvement in bone marrow transplantation: electrocardiographic changes, arrhythmias, heart failure and autopsy findings. Bone Marrow Transplant 5, 91–98 [PubMed] [Google Scholar]
- Lai H. C., Yeh Y. C., Wang L. C., Ting C. T., Lee W. L., Lee H. W., et al. (2011). Propofol ameliorates doxorubicin-induced oxidative stress and cellular apoptosis in rat cardiomyocytes. Toxicol. Appl. Pharmacol. 257, 437–448. 10.1016/j.taap.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Lara P. N., Mack P. C., Synold T., Frankel P., Longmate J., Gumerlock P. H., et al. (2005).The cyclin-dependent kinase inhibitor UCN-01 plus cisplatin in advanced solid tumors: a California cancer consortium phase I pharmacokinetic and molecular correlative trial. Clin. Cancer Res. 11, 4444–4450. 10.1158/1078-0432.CCR-04-2602 [DOI] [PubMed] [Google Scholar]
- Lau D. H., Psaltis P. J., Mackenzie L., Kelly D. J., Carbone A., Worthington M., et al. (2011). Atrial remodeling in an ovine model of anthracycline-induced nonischemic cardiomyopathy: remodeling of the same sort. J. Cardiovasc. Electrophysiol. 22, 175–182. 10.1111/j.1540-8167.2010.01851.x [DOI] [PubMed] [Google Scholar]
- Lebedinsky C., Gómez J., Park Y. C., Nieto A., Soto-Matos A., Parekh T., et al. (2011). Trabectedin has a low cardiac risk profile: a comprehensive cardiac safety analysis. Cancer Chemother. Pharmacol. 68, 1223–1231. 10.1007/s00280-011-1614-z [DOI] [PubMed] [Google Scholar]
- Lee A. Y. (2005). Deep vein thrombosis and cancer: survival, recurrence, and anticoagulant choices. Dis. Mon. 51, 150–157. 10.1016/j.disamonth.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Lee H. T., Shin W., Chae J., Choi J., Kim S. H., et al. (2016). Structural basis of checkpoint blockade by monoclonal antibodies in cancer immunotherapy. Nat. Commun. 7:13354. 10.1038/ncomms13354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Chen Y. C., Chen Y. J., Chang S. L., Tai C. T., Wongcharoen W., et al. (2007). Tumor necrosis factor-alpha alters calcium handling and increases arrhythmogenesis of pulmonary vein cardiomyocytes. Life Sci. 80, 1806–1815. 10.1016/j.lfs.2007.02.029 [DOI] [PubMed] [Google Scholar]
- Lenihan D. J., Alencar A. J., Yang D., Kurzrock R., Keating M. J., Duvic M. (2004). Cardiac toxicity of alemtuzumab in patients with mycosis fungoides/Sezary syndrome. Blood 104, 655–658. 10.1182/blood-2003-07-2345 [DOI] [PubMed] [Google Scholar]
- Leong D. P., Caron F., Hillis C., Duan A., Healey J. S., Fraser G., et al. (2016). The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 128, 138–140. 10.1182/blood-2016-05-712828 [DOI] [PubMed] [Google Scholar]
- Liu Y., Tan D., Shi L., Liu X., Zhang Y., Tong C., et al. (2015). Blueberry anthocyaninsenriched extracts attenuate cyclophosphamide-induced cardiac injury. PLoS ONE 10:e0127813. 10.1371/journal.pone.0127813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Jones K. R., Guo R., Xu P., Shen Y., Ren J. (2010). Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress, Clin. Exp. Pharmacol. Physiol. 37, 460–465. 10.1111/j.1440-1681.2009.05323.x [DOI] [PubMed] [Google Scholar]
- Ma J. Y., Wang Y., Zhao Y. F., Wu Z., Liu L. X., Kou Y. L., et al. (2006). Atrial fibrillation after surgery for esophageal carcinoma: clinical and prognostic significance. World J. Gastroenterol. 12, 449–452. 10.3748/wjg.v12.i3.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawhinney L., Armstrong M. E., OR C., Bucala R., Leng L., Fingerle-Rowson G, et al. (2014). Macrophage migration inhibitory factor (MIF) enzymatic activity and lung cancer. Mol. Med. 20, 729–735. 10.2119/molmed.2014.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Cormack O., Zaborowski A., King S., Healy L., Daly C., O'Farrell N., et al. (2014). Newonset atrial fibrillation post-surgery for esophageal and junctional cancer: incidence, management, and impact on short- and long-term outcomes. Ann. Surg. 260, 772–778, discussion. 8. 10.1097/SLA.0000000000000960 [DOI] [PubMed] [Google Scholar]
- McMullen J. R., Boey E. J., Ooi J. Y., Seymour J. F., Keating M. J., Tam C. S. (2014). Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood 124, 3829–3830. 10.1182/blood-2014-10-604272 [DOI] [PubMed] [Google Scholar]
- Mego M., Reckova M., Obertova J., Sycova-Mila Z., Brozmanova K., Mardiak J. (2007). Increased cardiotoxicity of sorafenib in sunitinib-pretreated patients with metastatic renal cell carcinoma. Ann Oncol. 18, 1906–1907. 10.1093/annonc/mdm489 [DOI] [PubMed] [Google Scholar]
- Menard O., Martinet Y., Lamy P. (1991). Cisplatin-induced atrial fibrillation. J. Clin. Oncol. 9, 192–193 10.1200/JCO.1991.9.1.192 [DOI] [PubMed] [Google Scholar]
- Mery B., Guichard J. B., Guy J. B., Vallard A., Barthelemy J. C., Da Costa A., et al. (2017). Atrial fibrillation in cancer patients: hindsight, insight and foresight. Int. J. Cardiol. 240, 196–202 10.1016/j.ijcard.2017.03.132 [DOI] [PubMed] [Google Scholar]
- Meydan N., Kundak I., Yavuzsen T., Oztop I., Barutca S., Yilmaz U., et al. (2005). Cardiotoxicity of de gramont's regimen: incidence, clinical characteristics and long-term follow-up. Jpn. J. Clin. Oncol. 35, 265–270. [DOI] [PubMed] [Google Scholar]
- Montella L., Caraglia M., Addeo R., Costanzo R., Faiola V., Abbruzzese A., et al. (2005). Atrial fibrillation following chemotherapy for stage IIIE diffuse large B-cell gastric lymphoma in a patient with myotonic dystrophy. Ann. Hematol. 84, 192–193. 10.1007/s00277-004-0867-6 [DOI] [PubMed] [Google Scholar]
- Moreau P., Milpied N., Mahé B., Juge-Morineau N., Rapp M. J., Bataille R., et al. (1999). Melphalan 220 mg/m2 followed by peripheral blood stem cell transplantation in 27 patients with advanced multiple myeloma. Bone Marrow Transplant. 23, 1003–1006. 10.1038/sj.bmt.1701763 [DOI] [PubMed] [Google Scholar]
- Moslehi J. J. (2016). Cardiovascular toxic effects of targeted cancer therapies. N. Engl. J. Med. 375, 1457–1467. 10.1056/NEJMra1100265 [DOI] [PubMed] [Google Scholar]
- Nattel S. (2002). New ideas about atrial fibrillation 50 years on. Nature 415, 219–226. 10.1038/415219a [DOI] [PubMed] [Google Scholar]
- Nattel S., Burstein B., Dobrev D. (2008). Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ. Arrhythm Electrophysiol. 1, 62–73. 10.1161/CIRCEP.107.754564 [DOI] [PubMed] [Google Scholar]
- Neben K., Moehler T., Benner A., Kraemer A., Egerer G., Ho A. D., et al. (2002). Dose-dependent effect of thalidomide on overall survival in relapsed multiple myeloma. Clin. Cancer Res. 8, 3377–3382. [PubMed] [Google Scholar]
- Neef S., Dybkova N., Sossalla S., Ort K. R., Fluschnik N., Neumann K., et al. (2010). CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 106, 1134–1144. 10.1161/CIRCRESAHA.109.203836 [DOI] [PubMed] [Google Scholar]
- Numico G., Castiglione F., Granetto C., Garrone O., Mariani G., Costanzo G. D., et al. (2002). Single-agent pegylated liposomal doxorubicin (Caelix®) in chemotherapy pretreated non-small cell lung cancer patients: a pilot trial. Lung Cancer. 35, 59–64. 10.1016/S0169-5002(01)00269-0 [DOI] [PubMed] [Google Scholar]
- Ojima T., Iwahashi M., Nakamori M., Nakamura M., Katsuda M., Iida T., et al. (2014). Atrial fibrillation after esophageal cancer surgery: an analysis of 207 consecutive patients. Surg. Today 44, 839–847. 10.1007/s00595-013-0616-3 [DOI] [PubMed] [Google Scholar]
- Olivieri A., Corvatta L., Montanari M., Brunori M., Offidani M., Ferretti G. F., et al. (1998). Paroxysmal atrial fibrillation after high-dose melphalan in five patients autotransplanted with blood progenitor cells. Bone Marrow Transplant 21, 1049–1053. 10.1038/sj.bmt.1701217 [DOI] [PubMed] [Google Scholar]
- O'Neal W. T., Lakoski S. G., Qureshi W., Judd S. E., Howard G., Howard V. J., et al. (2015). Relation between cancer and atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke Study). Am. J. Cardiol. 115, 1090–1094. 10.1016/j.amjcard.2015.01.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly C., Doroudian M., Mawhinney L., Donnelly S. C. (2016). Targeting MIF in cancer: therapeutic strategies, current developments, and future opportunities. Med. Res. Rev. 36, 440–460. 10.1002/med.21385 [DOI] [PubMed] [Google Scholar]
- Ott A., Breteler M. M., de Bruyne M. C., van Harskamp F., Grobbee D. E., Hofman A. (1997). Atrial fibrillation and dementia in a population-based study. the rotterdam study. Stroke 28, 316–321. 10.1161/01.STR.28.2.316 [DOI] [PubMed] [Google Scholar]
- Ozaki M., Deshpande S. S., Angkeow P., Bellan J., Lowenstein C. J., Dinauer M. C., et al. (2000). Inhibition of the Rac1 GTPase protects against nonlethal ischemia/reperfusion-induced necrosis and apoptosis in vivo. FASEB J. 14, 418–429. 10.1096/fasebj.14.2.418 [DOI] [PubMed] [Google Scholar]
- Palumbo A., Rajkumar S. V., Dimopoulos M. A., Richardson P. G., San Miguel J., Barlogie B., et al. (2008). Prevention of thalidomide and lenalidomide associated trombosis in myeloma. Leukemia. 22, 414–423. 10.1038/sj.leu.2405062 [DOI] [PubMed] [Google Scholar]
- Pérez Persona E., Mesa M. G., García Sánchez P. J., González Rodríguez A. P. (2011). Lenalidomide treatment for patients with multiple myeloma: diagnosis and management of most frequent adverse events. Adv. Ther. 28, 11–16. 10.1007/s12325-010-0102-x [DOI] [PubMed] [Google Scholar]
- Perez-Verdia A., Angulo F., Hardwicke F. L., Nugent K. M. (2005). Acute cardiac toxicity associated with high-dose intravenous methotrexate therapy: case report and review of the literature. Pharmacotherapy 25, 1271–1276. 10.1592/phco.2005.25.9.1271 [DOI] [PubMed] [Google Scholar]
- Petrella V., Alciato P., Cantone P. A., Fico D., Gagliardini R. (1989). High-frequency supraventricular arrhythmias induced by a cisplatin-etoposide combination. Minerva Med. 80, 305–307 [PubMed] [Google Scholar]
- Pfister D. G., Su Y. B., Kraus D. H., Wolden S. L., Lis E., Aliff T. B., et al. (2006). Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combinedmodality paradigm. J. Clin. Oncol. 24, 1072–1078. 10.1200/JCO.2004.00.1792 [DOI] [PubMed] [Google Scholar]
- Plumb J. A., Finn P. W., Williams R. J., Bandara M. J., Romero M. R., Watkins C. J., et al. (2003). Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol. Cancer Ther. 2, 721–728. [PubMed] [Google Scholar]
- Popivanova B. K., Kitamura K., Wu Y., Kondo T., Kagaya T., Kaneko S., et al. (2008). Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest. 118, 560–570. 10.1172/JCI32453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius L., Du X. J., Woodcock E. A., Kiriazis H., Lin R. C., Marasco S., et al. (2010). Reduced phosphoinositide 3-kinase (p110a) activation increases the susceptibility to atrial fibrillation. Am. J. Pathol. 175, 998–1009. 10.2353/ajpath.2009.090126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori S. G., Blomstrom-Lundqvist C., Mazzanti A., Blom N., Borggrefe M., Camm J., et al. (2015). 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patientswith ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 36, 2793–2867 10.1093/eurheartj/ehv316 [DOI] [PubMed] [Google Scholar]
- Qi H. T., Zhang X. T. (2015). Advance in research of mechanism, prevention and treatment of radiation-induced cardiac toxicity in cancer patients. Chin. Jo. Cancer Prevent. Treat. 10, 814–818. [Google Scholar]
- Qu Y. C., Du Y. M., Wu S. L., Chen Q. X., Wu H. L., Zhou S. F. (2009). Activated nuclear factor kappaB and increased tumor necrosis factor-alpha in atrial tissue of atrial fibrillation. Scand. Cardiovasc. J. 43, 292–297. 10.1080/14017430802651803 [DOI] [PubMed] [Google Scholar]
- Quezado Z. M., Wilson W. H., Cunnion R. E., Parker M. M., Reda D., Bryant G., et al. (1993). High-dose ifosfamide is associated with severe, reversible cardiac dysfunction. Ann. Intern. Med. 118, 31–36 10.7326/0003-4819-118-1-199301010-00006 [DOI] [PubMed] [Google Scholar]
- Rao F., Deng C. Y., Wu S. L., Xiao D. Z., Yu X. Y., Kuang S. J., et al. (2009). Involvement of Src in L-type Ca2+channel depression induced bymacrophage migration inhibitory factor in atrial myocytes. J. Mol. Cell. Cardiol. 47, 586–594. 10.1016/j.yjmcc.2009.08.030 [DOI] [PubMed] [Google Scholar]
- Richards W. G., Zellos L., Bueno R., Jaklitsch M. T., Jänne P. A., Chirieac L. R., et al. (2006). Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J. Clin. Oncol. 24, 1561–1567. 10.1200/JCO.2005.04.6813 [DOI] [PubMed] [Google Scholar]
- Robertson J. F. R., Cannon P. M., Nicholson R. I., Blamey R. W. (1996). Oestrogen and progesterone receptors as prognostic variables in hormonally treated breast cancer. Int. J. Biol. Mark. 11, 2. [DOI] [PubMed] [Google Scholar]
- Roselli E. E., Murthy S. S., Rice T. W., Houghtaling P. L., Pierce C. D., Karchmer D. P., et al. (2005). Atrial fibrillation complicating lung cancer resection. J. Thorac. Cardiovasc. Sur. 130, 438–444. 10.1016/j.jtcvs.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Russell R. R., Alexander J., Jain D., Poornima I. G., Srivastava A. V., Storozynsky E., et al. (2016). The role and clinical effectiveness of multimodality imaging in the management of cardiac complications of cancer and cancer therapy. J. Nucl. Cardiol. 23, 856–884. 10.1007/s12350-016-0538-8 [DOI] [PubMed] [Google Scholar]
- Ryder M., Callahan M., Postow M. A., Wolchok J., Fagin J. A. (2014). Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr. Relat. Cancer. 21, 371–381. 10.1530/ERC-13-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sag C. M., Köhler A. C., Anderson M. E., Backs J., Maier L. S. (2011). CaMKII-dependent SR Ca leak contributes to doxorubicin-induced impaired Ca handling in isolated cardiac myocytes. J. Mol. Cell Cardiol. 51, 749–759. 10.1016/j.yjmcc.2011.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif M. W., Shah M. M., Shah A. R. (2009). Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin. Drug Saf. 8, 191–202. 10.1517/14740330902733961 [DOI] [PubMed] [Google Scholar]
- Samman Tahhan A., Sandesara P. B., Hayek S. S., Alkhoder A., Chivukula K., Hammadah M., et al. (2017). Association between oxidative stress and atrial fibrillation. Heart Rhythm. 14, 1849–1855. 10.1016/j.hrthm.2017.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandor V., Bakke S., Robey R. W., Kang M. H., Blagosklonny M. V., Bender J., et al. (2002). Phase I trial of the histone deacetylase inhibitor, depsipeptide (FR901228, NSC 630176), in patients with refractory neoplasms. Clin. Cancer Res. 8, 718–728. [PubMed] [Google Scholar]
- Schmitt J., Duray G., Gersh B. J., Hohnloser S. H. (2009). Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur. Heart. J. 30, 1038–1045. 10.1093/eurheartj/ehn579 [DOI] [PubMed] [Google Scholar]
- Schwartz R. G., Jain D., Storozynsky E. (2013). Traditional and novel methods to assess and prevent chemotherapy-related cardiac dysfunction noninvasively. J. Nucl. Cardiol. 20, 443–464. 10.1007/s12350-013-9707-1 [DOI] [PubMed] [Google Scholar]
- Shanafelt T. D., Parikh S. A., Noseworthy P. A., Goede V., Chaffee K. G., Bahlo J., et al. (2017). Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL). Leuk. Lymphoma. 58, 1630–1639. 10.1080/10428194.2016.1257795 [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2016). Cancer statistics 2016. CA Cancer J. Clin. 66, 7–30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- Siemes C., Visser L. E., Coebergh J. W., Splinter T. A., Witteman J. C., Uitterlinden A. G., et al. (2006). C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J. Clin. Oncol. 24, 5216–5222. 10.1200/JCO.2006.07.1381 [DOI] [PubMed] [Google Scholar]
- Siu C. W., Tung H. M., Chu K. W., Jim M. H., Lau C. P., Tse H. F. (2005). Prevalence and predictors of new-onset atrial fibrillation after elective surgery for colorectal cancer. Pacing Clin. Electrophysiol. 28, S120–S123. 10.1111/j.1540-8159.2005.00024.x [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. (1987). Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235, 177–182 10.1126/science.3798106 [DOI] [PubMed] [Google Scholar]
- Stadler W. M., Margolin K., Ferber S., McCulloch W., Thompson J. A. (2006). A phase II study of depsipeptide in refractory metastatic renal cell cancer. Clin. Genitourin. Cancer. 5, 57–60. 10.3816/CGC.2006.n.018 [DOI] [PubMed] [Google Scholar]
- Steele N. L., Plumb J. A., Vidal L., Tjørnelund J., Knoblauch P., Rasmussen A., et al. (2008). A phase 1Pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin. Cancer Res. 14, 804–810. 10.1158/1078-0432.CCR-07-1786 [DOI] [PubMed] [Google Scholar]
- Sudharsan P. T., Mythili Y., Selvakumar E., Varalakshmi P. (2006). Lupeol and its ester ameliorate the cyclophosphamide provoked cardiac lysosomal damage studied in rat. Mol. Cell. Biochem. 282, 23–29. 10.1007/s11010-006-1169-1 [DOI] [PubMed] [Google Scholar]
- Szlosarek P.W, Balkwill F R. (2003). Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol. 4, 565–573. 10.1016/S1470-2045(03)01196-3 [DOI] [PubMed] [Google Scholar]
- Tamargo J., Caballero R., Delpon E. (2015). Cancer chemotherapy and cardiac arrhythmias: a review. Drug Saf. 38, 129–152. 10.1007/s40264-014-0258-4 [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Bianco J. A., Benyunes M. C., Neubauer M. A., Slattery J. T., Fefer A. (1994). Phase Ib trial of pentoxifylline and ciprofloxacin in patients treated with interleukin-2 and lymphokine-activated killer cell therapy for metastatic renal cell carcinoma. Cancer Res. 54, 3436–3441. [PubMed] [Google Scholar]
- Tilleman T. R., Richards W. G., Zellos L., Johnson B. E., Jaklitsch M. T., Mueller J., et al. (2009). Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J. Thorac. Cardiovasc. Surg. 138, 405–411. 10.1016/j.jtcvs.2009.02.046 [DOI] [PubMed] [Google Scholar]
- Tomkowski W. Z., Wiśniewska J., Szturmowicz M., Kuca P., Burakowski J., Kober J., et al. (2004). Evaluation of intrapericardial cisplatin administration in cases with recurrent malignant pericardial effusion and cardiac tamponade. Support Care Cancer 12, 53–57. 10.1007/s00520-003-0533-x [DOI] [PubMed] [Google Scholar]
- Treon S. P., Tripsas C. K., Meid K., Warren D., Varma G., Green R., et al. (2015). Ibrutinib in previously treated Waldenstr€om's macroglobulinemia. N. Engl. J. Med. 372, 1430–1440. 10.1056/NEJMoa1501548 [DOI] [PubMed] [Google Scholar]
- Ueda H., Nakajima H., Hori Y., Fujita T., Nishimura M., Goto T., et al. (1994a). FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. Taxonomy, I., fermentation, isolation, physicochemical and biological properties, and antitumor activity. J. Antibiot. 47, 301–310. 10.7164/antibiotics.47.301 [DOI] [PubMed] [Google Scholar]
- Ueda H., Nakajima H., Hori Y., Goto T., Okuhara M. (1994b). Action of FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum no. 968, on Ha-ras transformed NIH3T3 cells. Biosci. Biotechnol. Biochem. 58, 1579–1583. 10.1271/bbb.58.1579 [DOI] [PubMed] [Google Scholar]
- van der Vorst E. P., Doring Y., Weber C. (2015). MIF and CXCL12 in cardiovascular diseases: functional differences and similarities. Front. Immunol. 6:373 10.3389/fimmu.2015.00373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaporciyan A. A., Correa A. M., Rice D. C., Roth J. A., Smythe W. R., Swisher S. G., et al. (2004). Risk factors associated with atrial fibrillation after noneardiac thoracic surgery:analysis of 2588 patients. J. Thorac. Cardiovasc. Surg. 127, 779–786. 10.1016/j.jtcvs.2003.07.011 [DOI] [PubMed] [Google Scholar]
- Varga Z. V., Ferdinandy P., Liaudet L., Pacher P. (2015). Drug-induced mitochondrial dysfunction and cardiotoxicity. Am. J. Phys. Heart Circ. Phys. 309, H1453–H1467. 10.1152/ajpheart.00554.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varinelli L., Caccia D., Volpi C. C., Caccia C., De Bortoli M., Taverna E., et al. (2015). 4-IPP, a selective MIF inhibitor, causes mitotic catastrophe in thyroid carcinomas. Endocr. Relat. Cancer 22, 759–775. 10.1530/ERC-15-0299 [DOI] [PubMed] [Google Scholar]
- Veronesi U., Maisonneuve P., Rotmensz N., Bonanni B., Boyle P., Viale G., et al. (2007). Tamoxifen for the prevention of breast cancer: late results of the italian randomized tamoxifen prevention trial among women with hysterectomy. J. Natl. Cancer Inst. 99, 727–737. 10.1093/jnci/djk154 [DOI] [PubMed] [Google Scholar]
- Vest J. A., Wehrens X. H., Reiken S. R., Lehnart S. E., Dobrev D., Chandra P., et al. (2005). Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 111, 2025–2032. 10.1161/01.CIR.0000162461.67140.4C [DOI] [PubMed] [Google Scholar]
- Voigt N., Heijman J., Wang Q., Chiang D. Y., Li N., Karck M., et al. (2014). Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 129, 145–156. 10.1161/CIRCULATIONAHA.113.006641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt N., Li N., Wang Q., Wang W., Trafford A. W., Abu-Taha I., et al. (2012). Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 125, 2059–2070. 10.1161/CIRCULATIONAHA.111.067306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. L., Blum K. A., Martin P., Goy A., Auer R., Kahl B. S., et al. (2015). Long-term follow-up of MCL patients treated with single-agent ibrutinib:updated safety and efficacy results. Blood. 126, 739–745. 10.1182/blood-2015-03-635326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. X., Wang H. Q., Cheng J., et al. (2018). Research progress on pathogenesis of atrial fibrillation. China Medical Herald. 15, 26–29. [Google Scholar]
- Weber D., Rankin K., Gavion M., Dalasalle K., Alexanian R. (2003). Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J. Clin. Oncol. 21, 16–19. 10.1200/JCO.2003.03.139 [DOI] [PubMed] [Google Scholar]
- White R. L., Schwartzentruber D. J., Guleria A., MacFarlane M. P., White D. E., Tucker E., et al. (1994). Cardiopulmonary toxicity of treatment with high dose interleukin-2 in 199 consecutive patients with metastatic melanoma or renal cell carcinoma. Cancer. 74, 3212–3222. [DOI] [PubMed] [Google Scholar]
- Will Y., Dykens J. A., Nadanaciva S., Hirakawa B., Jamieson J., Marroquin L. D., et al. (2008). Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicol. Sci. 106, 153–161. 10.1093/toxsci/kfn157 [DOI] [PubMed] [Google Scholar]
- Wolchok J. D. (2015). PD-1 blockers. Cell. 162:937 10.1016/j.cell.2015.07.045 [DOI] [PubMed] [Google Scholar]
- Woolley P. V., Ayoob M. J., Levenson S. M., Smith F. P. (1982). A phase I clinical trial of aclacinomycin A administered on a five-consecutive- day sychedule. J. Clin. Pharmacol. 22, 359–365. 10.1002/j.1552-4604.1982.tb02686.x [DOI] [PubMed] [Google Scholar]
- Xin Y., Zhang S., Gu L., Liu S., Gao H., You Z., et al. (2011). Electrocardiographic and biochemical evidence for the cardioprotective effect of antioxidants in acute doxorubicin-induced cardiotoxicity in the beagle dogs. Biol. Pharm. Bull. 34, 1523–1526. 10.1248/bpb.34.1523 [DOI] [PubMed] [Google Scholar]
- Xing Y., Gao Y., Chen J., Zhu H., Wu A., Yang Q., et al. (2013). Wenxin-Keli regulates the calcium/calmodulin-dependent protein kinase ii signal transduction pathway and inhibits cardiac arrhythmia in rats with myocardial infarction. Evid. Based Complement. Alternat. Med, 2013:464508. 10.1155/2013/464508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T., Moleslehi J., Roden D. M. (2015). Proarrhythmic effects of ibrutinib, a clinically approved inhibitor of Bruton'S tyrosine kinase (BTK) used in cancer therapy. Circulation 132(Supp. 3):A14587. 10.1161/circ.132.suppl_3.14587 [DOI] [Google Scholar]
- Yang X., Li Y., Li Y., Ren X., Zhang X., Hu D., et al. (2017a). Oxidative stress-mediated atherosclerosis: mechanisms and therapies. Front. Physiol. 8:600. 10.3389/fphys.2017.00600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu C., Li Y., Ren X., Xing Y., Shang H. (2017b). Effects of Wenxin Keli on cardiac hypertrophy and arrhythmia via regulation of the calcium/calmodulin dependent kinase ii signaling pathway. Biomed. Res. Int. 2017:1569235 10.1155/2017/1569235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Chopra I. J., Ha E. (2015). A novel melanoma therapy stirs up a storm: ipilimumab-induced thyrotoxicosis. Endocrinol. Diabetes Metab. Case Rep. 2015:140092 10.1530/EDM-14-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun S., Vincelette N. D., Acharya U., Abraham I. (2017). Risk of atrial fibrillation and bleeding diathesis associated with ibrutinib treatment: a systematic review and pooled analysis of four randomized controlled trials. Clin. Lymphoma Myeloma Leuk. 17, 31–37. 10.1016/j.clml.2016.09.010 [DOI] [PubMed] [Google Scholar]
- Zangari M., Anaissie E., Barlogie B., Badros A., Desikan R., Gopal A. V., et al. (2001). Increased risk of deep-vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapy. Blood. 98, 1614–1615. 10.1182/blood.V98.5.1614 [DOI] [PubMed] [Google Scholar]
- Zangari M., Tricot G., Polavaram L., Zhan F., Finlayson A., Knight R., et al. (2009). Survival effect of venous thromboembolism in patients with multiple myeloma treated with lenalidomide and high dose dexamethasone. J. Clin. Oncol. 28, 132–135. 10.1200/JCO.2009.23.0169 [DOI] [PubMed] [Google Scholar]
- Zellos L., Richards W. G., Capalbo L., Jaklitsch M. T., Chirieac L. R., Johnson B. E., et al. (2009). A phase I study of extrapleural pneumonectomy and intracavitary intraoperative hyperthermic cisplatin with amifostine cytoprotection for malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 137, 453–458. 10.1016/j.jtcvs.2008.07.055 [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhang A., Guo C., Shi C., Zhang Y., Liu Q., et al. (2011). S-diclofenac protects against doxorubicin-induced cardiomyopathy in mice via ameliorating cardiac gap junction remodeling. PLoS ONE 6:e26441. 10.1371/journal.pone.0026441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang Y. T., Shan Z. L., Guo H. Y., Guan Y., Yuan H. T. (2015). Role of inflammation in the initiation andmaintenance of atrial fibrillation and the protective effect of atorvastatin in a goat model of aseptic pericarditis. Mol. Med. Rep. 11, 2615–2623. 10.3892/mmr.2014.3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonder J. A., Barlogie B., Durie B. G., McCoy J., Crowley J., Hussein M. A. (2006). Thrombotic complications in patients with newly diagnosed multiple treated with lenalidomide and dexamethasone: benefit of aspirin prophylaxis. Blood. 108:403 10.1182/blood-2006-01-0154 [DOI] [PubMed] [Google Scholar]