Summary
It has been proposed that sleep’s contribution to memory consolidation is to reactivate prior encoded information. To elucidate the neural mechanisms carrying reactivation-related mnemonic information, we investigated whether content-specific memory signatures associated with memory reactivation during wakefulness reoccur during subsequent sleep. We show that theta oscillations orchestrate the reactivation of memories during both wakefulness and sleep. Reactivation patterns during sleep autonomously re-emerged at a rate of ∼1 Hz, indicating a coordination by slow oscillatory activity.
Keywords: sleep, consolidation, memory reactivation, retrieval, oscillations, EEG, theta, episodic memory, phase similarity
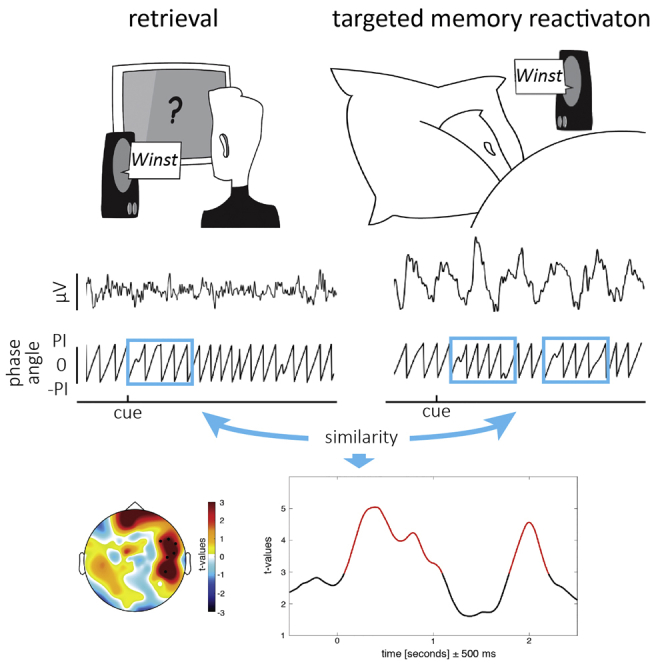
Graphical Abstract
Highlights
-
•
Theta orchestrates the reactivation of memories during both wakefulness and sleep
-
•
Reactivation patterns during sleep autonomously re-emerge at a rate of ∼1 Hz
-
•
Interrupting the reactivation diminishes the beneficial effects of consolidation
Schreiner et al. show that cue-triggered memory reactivation shares the same neural signatures during wakefulness and sleep. Theta oscillations orchestrate the reactivation of memories during both physiological states. During sleep, reactivation patterns autonomously re-emerge at a rate of ∼1 Hz, indicating a coordination by slow oscillations.
Introduction
The memory function of sleep relies on the reactivation of newly acquired information during non-rapid-eye movement (NREM) sleep (Rasch and Born, 2013). Rodent studies have consistently shown hippocampal reactivation of previous learning experiences during sleep (Chen and Wilson, 2017), and studies in humans have provided first hints indicating similar processes (Peigneux et al., 2004, Schönauer et al., 2017). Furthermore, triggering reactivation processes during sleep by re-exposure to associated memory cues (targeted memory reactivation [TMR]) has been shown to improve memory consolidation (Oudiette and Paller, 2013).
However, the neural mechanisms coordinating reactivation-related mnemonic information in humans remain poorly understood. Furthermore, it is essentially unknown whether memory trace reactivation during wakefulness and sleep is orchestrated by the same neural signatures. Here, we investigated whether memory reactivation during wakefulness and sleep shares oscillatory patterns that carry memory-representation-specific information using electroencephalography (EEG) and multivariate analysis methods. Building on previous findings (Schyns et al., 2011), we hypothesized that low-frequency oscillatory phase conveys a representation (i.e., content)-specific temporal code. We applied a newly developed method (Michelmann et al., 2016) that reveals the phase-related similarity between content-specific memory representations to recently published data (Schreiner et al., 2015; see Figure 1 for experimental design and behavioral results).
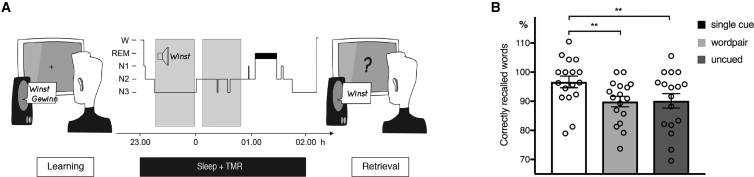
Figure 1.
Experimental Design and Behavioral Results
(A) Participants performed a vocabulary-learning task in the evening. They learned to associate Dutch words (cues) with German words (targets). After the initial learning phase, a cued recall, including feedback, was performed (recall1). Afterward, the cued recall was repeated without feedback (recall2). Subsequently, participants slept for 3 hr. During NREM sleep, 80 Dutch words (40 cued and 40 cued + feedback) were repeatedly presented. Memory performance was assessed in the final retrieval phase after sleep
(B) Presenting single Dutch word cues during NREM sleep enhanced memory performance as compared to word-pair TMR and uncued words. Retrieval performance is indicated as percentage of recalled words, with performance before sleep set to 100%.
Values are mean ± SEM. ∗∗p < 0.01.
We provide evidence for memory-reactivation processes during wakefulness and their reoccurrence during NREM sleep. Theta oscillations orchestrated the reactivation of memories when triggered by memory cues during both physiological states. Reactivation patterns during sleep autonomously re-emerged at a rate of ∼1 Hz, suggesting a supra-ordinate coordination by slow oscillatory activity.
Results
Word-Specific Phase Similarity at 5 Hz Indicates Memory Reactivation during Wake Retrieval
First, we aimed at identifying the content specificity of phase and its time course when retrieving the very same memory content during consecutive recall instances, indicating recall-related memory reactivation. The degree of phase similarity for retrieving the same memory content during consecutive recall instances (recall1, recall2) was assessed using the pairwise phase consistency (Vinck et al., 2010) and contrasted between remembered and non-remembered words for frequencies between 3 and 16 Hz (see Figures S1A–S1C for results on the content specificity of our approach). We found significantly higher phase similarity for remembered as compared to non-remembered words in the theta range (p = 0.006; corrected for multiple comparisons), peaking at 5 Hz (Figures 2A and 2B). The time course of the phase similarity at 5 Hz displayed an early significant difference between remembered and non-remembered words (p = 0.008; corrected for multiple comparisons; Figure 2C; Figures S2A–S2D for unmasked data). Additional analyses indicated that the phase similarity results were not biased by spectral power (see Figures S3L–S3P for details). It seems unlikely that results were driven by similarities in auditory stimulation. Still, we tested this possibility by assessing phase similarity between learning and both recall instances, with the learning data being segmented around the onset of the Dutch words (thus before any association was learned). Phase similarity was assessed for the very same words and contrasted between remembered and non-remembered words. No significant cluster was observed (both p’s > 0.3; see Figures S1F–S1M).
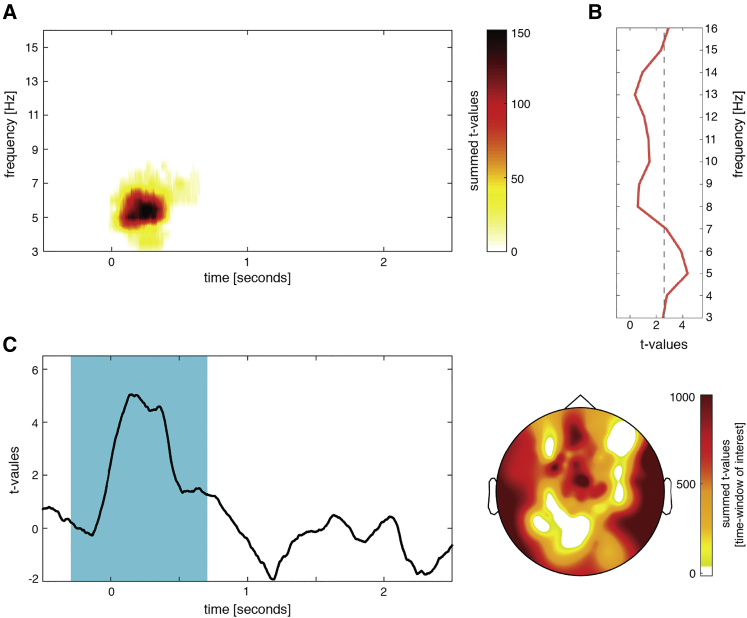
Figure 2.
Word-Specific Phase Similarity during Wake Retrieval
(A) Significantly enhanced phase similarity during successful subsequent retrieval was observed early after cue onset (t = 0 s) in the theta range. t-values were summed across electrodes in the significant cluster.
(B) t-statistics of similarity results averaged over time and electrodes indicate a peak at 5 Hz.
(C) Time course and topography of phase similarity at 5 Hz, indicating a rapid reactivation of memory content. The one-second time window around the center of the strongest cluster is highlighted. For the time course, t-values were averaged across all electrodes (n = 83), showing the content-specific phase-similarity effect. The topography displays summed t-values of the averaged difference between 0 and 2.5 s.
See also Figures S1, S2, and S3.
Theta Phase-Coordinated Memory Reactivation Reoccurs during NREM Sleep
The next crucial step was to test whether these content-specific features tracked by phase similarity at 5 Hz would be shared between reactivation processes during wakefulness (recall2) and sleep (TMR). Because memory reactivation during sleep could emerge at any point after TMR cue presentation, phase similarity between recall2 and TMR was examined with a sliding window approach (Michelmann et al., 2016) using the single-trial phase locking value (Lachaux et al., 2000).
Target words remembered after sleep were paired with their equivalent during recall2 and contrasted against non-remembered words. A one-second time window exhibiting the strongest content specificity from the pre-sleep retrieval (center: 0.193 ms; see Figures S3F and S3G for different window lengths) was used as sliding window. Test statistics on the averaged difference between remembered and non-remembered words revealed the reactivation of recall-related phase patterns at 5 Hz during TMR (p = 0.008; corrected for multiple comparisons) over right temporal electrodes (Figure 3A; see Figures S1D and S1E for results on the content specificity of our approach and Figures S2E–S2G for unmasked contrasts). No difference in spectral power biased the results (see Figures S3L–S3P for details). To assess the frequency specificity of the obtained results, the same analysis was performed for 3 Hz and 8 Hz (both p > 0.16). To test whether our measures were driven by similarity in auditory stimulation, we assessed phase similarity between learning and TMR. No significant cluster was observed (p > 0.3; see Figures S1N and S1O).
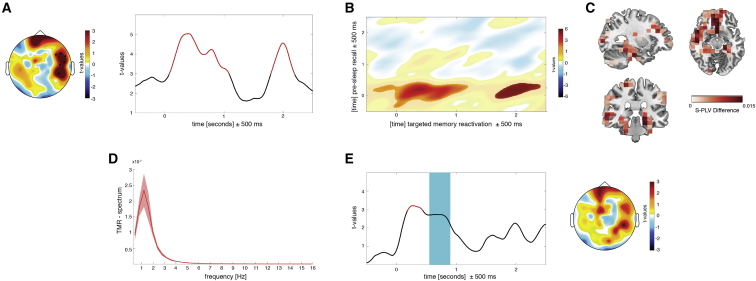
Figure 3.
Word-Specific Phase Similarity between recall2 and TMR
(A) Recurrent reactivation of recall-related phase patterns at 5 Hz during TMR emerged over right temporal electrodes. The topography displays the test statistics of the averaged difference in phase similarity between remembered and not-remembered words (0–2.5 s). The time course depicts t-values averaged across highlighted electrodes (n = 6). The phase similarity at a given time point reflects the similarity computed in a window of ±500 ms around this time point.
(B) Assessing phase similarity at 5 Hz between every time point of retrieval and TMR confirmed the re-occurring pattern of similarity.
(C) Source reconstruction. The difference in phase similarity for remembered and not-remembered items indicates effects in right (para)hippocampal regions and left frontal areas.
(D) Frequency spectrum of the TMR similarity measures showed a ∼1 Hz periodicity of reactivation processes. Shading denotes SEM.
(E) In line with behavioral predictions, providing a target stimulus after the TMR cue blocked associated reactivation processes. The time course depicts t-values averaged across highlighted electrodes in (A). Presentation of the target word is highlighted in petrol blue. Only a brief reactivation effect at 270 ms (before target word onset) emerged. The topography displays the test statistics of the averaged difference in phase similarity between remembered and not-remembered words (0–2.5 s). No significant cluster was found.
See also Figures S1, S2, and S3.
To examine the time course of the reactivation effect, similarity measures were averaged across significant electrodes and t-statistics were computed for every time point. Two distinct reactivation episodes emerged, peaking at 390 ms (t16 = 4.49; p = 0.0003) and 1,990 ms (t16 = 4.59; p = 0.0002). This pattern of results suggests that presenting a memory cue during sleep triggered re-occurring memory reactivation, fluctuating at a frequency of ∼1 Hz (Figure 3D; for analogous analyses using a longer time window, see Figures S3A–S3E).
To test whether slow oscillatory activity might underlie the 1 Hz periodicity found in the TMR similarity measures, we detected slow oscillations (SOs) in all TMR data segments (n = 581.7 ± 26.5). As expected, SOs appeared regularly in the sleep recordings (n = 416.58 ± 122.61), indicating that the fluctuation of phase similarity at a frequency of ∼1 Hz might be indeed driven by slow oscillatory activity (for details, see Figures S3H–S3K and Supplemental Experimental Procedures). Testing all combinations of recall and TMR time windows revealed that no additional recall episodes were reactivated during TMR (Figure 3B). To evaluate the sources of the scalp-level effects, phase similarity was assessed on virtual sensors by applying a Dynamic Imaging of Coherent Source (DICS) beamformer. Source-level contrasts exhibited differences in right (para)hippocampal regions as well as more widespread differences in left frontal areas, including the frontal gyrus and insula (Figure 3C).
Our analysis focused on similarity measures between recall and single-cue TMR, because presenting Dutch-German word pairs during sleep abolished the beneficial effects of TMR on later memory performance (Schreiner et al., 2015). Based on this behavioral outcome, we predicted that providing both the cue and target word during TMR should block functionally relevant memory-reactivation processes. There was no significant effect when comparing the averaged difference between subsequently remembered and non-remembered words (p > 0.17). As the topographical distribution resembled our main results (Figure 3E), the same electrode cluster was used to characterize the time course. We found an early reactivation episode peaking at 270 ms (t16 = 3.2; p = 0.005) for word pair TMR, thus before the onset of the second word. No later episode was observable, indicating that the presentation of a second stimulus may have blocked further memory reactivation.
Discussion
We show that memory-related reactivation processes during wakefulness and sleep triggered by memory cues share the same neural signature in humans. Theta oscillations at 5 Hz orchestrated the reactivation of memories during both physiological states. A growing number of TMR studies (Laventure et al., 2018, Lehmann et al., 2016, Oyarzún et al., 2017, Schreiner and Rasch, 2015) have already pointed toward a critical role of theta with regards to memory reactivation during sleep, but its exact contribution remained unknown. The current work closes this gap by directly relating memory-associated neural activity during wakefulness and sleep and providing evidence for a common role of theta activity: in both physiological states, the function of theta activity may be to coordinate the reactivation of memories, thus constituting a state-independent feature of memory reactivation.
To investigate the dynamics of reactivation processes in humans, we applied a recently developed method (Michelmann et al., 2016) that detects the phase-related similarity between content-specific memory representations and importantly is robust against variations in the onset of reactivation processes. Here, we show that this procedure constitutes a promising approach, in particular for future research on memory processes acting during offline periods (i.e., rest and sleep).
One of our core findings is that presenting memory cues during sleep triggered re-occurring memory reactivations. After providing a TMR cue, memory-related reactivation patterns re-emerged autonomously at a frequency of ∼1 Hz, indicating a coordination by slow oscillatory activity. Models of memory consolidation assume that cortical SOs coordinate reactivation processes as a time-giving pace maker by driving the repeated reactivation of memories in the hippocampus, together with sharp wave ripples and thalamo-cortical sleep spindles (Rasch and Born, 2013). The formation of these spindle-ripple events is thought to be essential for the integration of reactivated hippocampal memory information into neocortical long-term stores (Born and Wilhelm, 2012). Our result that theta-phase coordinated memory reactivation fluctuated at a frequency of ∼1 Hz is in line with previous findings, indicating a key role of SOs in guiding reactivation processes (e.g., Johnson et al., 2010). Crucially, our findings expand current models of memory consolidation, as theta activity has not yet been included in theoretical considerations of sleep-dependent memory processing.
Furthermore, our study revealed a re-occurring effect of experimentally induced memory reactivation, as TMR cues triggered a repeated cycling of reactivation patterns during sleep, and presenting a second stimulus abolished this fluctuation and diminished the beneficial effects of TMR. Interestingly, a recent TMR study (Cairney et al., 2018) demonstrated that the decodability of previously learned materials was maximal around 2 s following TMR cues, also hinting toward a perpetuation of the TMR-induced bias. In a similar vein, previous work in rodents has demonstrated that presenting auditory cues during sleep biases the content of associated memory reactivations with maintaining the biasing effect for multiple seconds (Bendor and Wilson, 2012, Rothschild et al., 2017). Although our results indicate that highly comparable processes take place in humans, it is still an open question how the maintenance of TMR induced activity is accomplished.
Our results are also in line with previous findings indicating a role for theta oscillations in mediating communication between the medial temporal lobe and neocortical regions (Fuentemilla et al., 2014), possibly conveying hippocampal-driven memory reactivation in the cortex during retrieval (Nyhus and Curran, 2010). Likewise, hippocampal reactivations are thought to drive consolidation processes during sleep, leading to the integration of newly acquired memories into cortical networks (Rasch and Born, 2013). Our source level results corroborate this assumption, as not only right (para)hippocampal areas, previously associated with successful TMR in humans (van Dongen et al., 2012), but also language-related regions in the left frontal cortex (Binder et al., 2009) showed the theta-driven phase similarity effects.
In sum, our results demonstrate that the same neural mechanisms guide memory-trace reactivation during both physiological states of wakefulness and sleep, with a cycling and spontaneous re-processing of memories during sleep when triggered by cueing.
Experimental Procedures
Participants
The data were taken from Schreiner et al. (2015). Thus, detailed information about participants, stimuli, task, data acquisition, and behavioral results can be found in the original article and the Supplemental Experimental Procedures. From the total of 20 participants (13 female; age: 22.45 ± 2.39) who entered the main EEG analyses in the original study (Schreiner et al., 2015), 3 datasets had to be excluded due to extensive artifacts in the pre-sleep EEG data (recall 1/2). Only those 17 participants were included in the illustration of the behavioral results in Figure 1B. The study was approved by the ethics committee of the Department of Psychology, University of Zurich. All subjects gave written informed consent prior to participating.
Word-Specific Phase Similarity during Awake Recall
To detect content (i.e., word) specificity of phase and its time course with regards to successful recall during wakefulness, a modified version of the pairwise phase consistency (PPC) was applied (Michelmann et al., 2016, Vinck et al., 2010). In a first step, oscillatory phase was extracted using complex Morlet wavelets of 6 cycles for all frequencies between 1 and 20 Hz in steps of 0.5 Hz and 1 ms, ranging from 1,000 ms pre-stimulus to 3,000 ms after stimulus onset.
We computed the pairwise phase consistency between the very same words retrieved during consecutive recall instances (i.e., similarity [worda, recall1, worda, recall2; wordb, recall1, wordb, recall2; …]). Phase similarity was computed separately per condition (similarityremembered and similaritynon-remembered) and contrasted. Retrieval success during recall2 determined the assignment of words to conditions (see Figures S2H–S2J for contrasts determined by memory performance in both recall1 and recall2). Thereby, the degree of phase similarity between identical words and associated memory content was assessed during consecutive recall instances (recall1 and recall2). We assumed that recall processes associated with remembering the very same items should exhibit a higher content-related similarity as compared to non-remembered ones. For each pair of trials, the cosine of the absolute angular distance was then computed and finally averaged across all (remembered and non-remembered, respectively) combinations (Michelmann et al., 2016). A value, representing the average similarity specifically for each set of combinations, was derived for every electrode, frequency, and time bin and subsequently used for statistics. As we assessed phase similarity for the same words presented during recall1 and recall2 and contrasted remembered pairs against an equal number of non-remembered pairs, potential confounding influences of similarity in auditory stimulation should be equal in both conditions and thus controlled for. To further strengthen this point, we assessed the phase similarity between learning and both recall instances. Importantly, data from learning were also segmented with regards to the onset of the Dutch words. As the German translations were presented with a delay of 3 s, no association-driven reactivation could have happened at this point and the recorded EEG activity primarily mirrors perceptual processing. As power differences can bias phase estimation, we tested whether there was a significant difference in power between conditions.
Word-Specific Phase Similarity between Recall and TMR
We focused our analysis on the single-cue TMR condition. Thus, we tested whether content-specific features of memories would be shared between recall before and single-word TMR during sleep, indicating that TMR during sleep leads to the reactivation of those properties. Phase similarity was assessed for the very same words between recall2 and TMR (i.e., similarity [worda, recall2, worda, TMR;…]), separately for each condition (remembered and non-remembered), and contrasted. Retrieval success after TMR determined the assignment of words to condition (see Figure S2K for contrasts being determined by retrieval success in both recall2 and TMR). Phase similarity was contrasted between pairs of successfully acquired memories against an equal number of pairs lacking a stable memory trace. The analysis was restricted to 5 Hz and those electrodes (83 electrodes) that showed the phase similarity effect during wake recall.
We determined phase similarity with the single-trial phase-locking value (S-PLV) (Lachaux et al., 2000; see Supplemental Experimental Procedures). To account for the fact that reactivation processes associated with TMR during sleep might be non-time-locked to the cue, a sliding window approach was utilized. Phase similarity was determined between the 1-s time window from recall2 and each 1-s time window around consecutive time point in the TMR interval (also see Supplemental Experimental Procedures).
The pre-stimulus interval between −500 ms and 0 ms was used as padding to slide the recall window into TMR episodes. This procedure resulted in a single value of phase similarity for every time point and electrode at TMR for any given trial combination. Similarity values thus characterize the similarity of the surrounding 1-s window during TMR to the 1-s time window from recall2. Due to the length of our TMR data segments (ranging to 3 s after stimulus onset) and the 1-s width of the sliding time window (from retrieval), robust similarity values could be obtained up to 2.5 s after cue presentation (for analogous analyses ranging from −500 ms to 3,500 ms, see Figures S3A–S3E).
Next, we explored the time course of reactivation processes during sleep. Electrodes derived from the 5 Hz cluster displaying the significant difference were averaged and subjected to a series of post hoc t tests between remembered and non-remembered combinations for every time point of sleep cueing. We repeated the sliding time window analysis using the same electrodes but varying time windows from the pre-sleep recall. This allowed us to evaluate similarity between every time point of recall and TMR, given an uncertainty of ±500 ms. Afterward, the differences of all combinations were averaged across electrodes. Two control frequencies (3 Hz and 8 Hz) were tested to estimate the frequency specificity.
As TMR during sleep seemed to have triggered reactivation processes in a recurrent fashion, we evaluated whether the similarity measures would fluctuate at a certain frequency (“TMR-spectrum”). We performed a spectral analysis of the time course of the phase similarity differences (for details, see Supplemental Experimental Procedures).
To evaluate whether our similarity measures between recall2 and TMR were influenced by the similarity in perceptual processing, we assessed the phase similarity between learning and TMR. To test whether power differences might have biased the phase estimation, the same control analyses as for the retrieval data were applied. Finally, we tested whether presenting an additional stimulus in the word-pair TMR condition might have interfered with ongoing reactivation processes (Schreiner et al., 2015). Phase similarity was assessed between recall and word-pair TMR in the same way as for the main analysis.
Source Estimation
To estimate the sources of the obtained effects of a virtual electrode approach, we applied the DICS beamforming method (Gross et al., 2001), as implemented in FieldTrip (Oostenveld et al., 2011; see Supplemental Experimental Procedures).
Statistics
Recall-Specific Phase Similarity
Statistical testing of differences in phase similarity between remembered and non-remembered words of recall1 and recall2 was accomplished using a cluster-based nonparametric permutation approach (Maris and Oostenveld, 2007; for details, see Supplemental Experimental Procedures). To estimate the time course and the topographical distribution of the peak frequency, effects at 5 Hz were tested specifically using the same procedure as above against a one-sided distribution (controlling for multiple comparisons in time and space).
Phase Similarity between Recall and TMR
Statistical quantification of the phase similarity between recall2 and TMR contrasting remembered and non-remembered words was accomplished using a cluster-based nonparametric permutation approach. Initially, S-PLV values were averaged over time (0–2.5 s), and differences were tested using paired sampled t tests (p < 0.05; two-tailed). To correct for multiple comparisons, 500 permutations were drawn, and the cluster with the largest summed t-value was tested against the permutation distribution. To quantify the temporal characteristics of the obtained effects, phase similarity measures were averaged across the electrodes within a given significant cluster. Paired sampled t tests were computed for every time point (p < 0.01; two-tailed).
Cluster-Specific Estimation of Memory Reactivation between Recall and TMR
To statistically test similarity differences between varying time windows from the pre-sleep recall2 and TMR, a series of post hoc t tests was accomplished within the cluster of significant electrodes. t-values, thresholded against a p value of 0.01 (one-sided), were summed up, and 500 permutations were drawn. Following Michelmann et al. (2016), a distribution comprising the strongest cluster, the second strongest cluster, etc. was formed. The obtained clusters were compared against a random cluster distribution. The cluster showing the highest sum of t-values was compared with the distribution of the maximum cluster, and the next cluster was compared to the second strongest cluster, etc. p values were divided by the number of clusters (Bonferroni correction).
Acknowledgments
T.S. is supported by a grant of the Swiss National Science Foundation (SNSF) (P300P1_174450). C.F.D.’s research is supported by the Max Planck Society; the Kavli Foundation; the European Research Council (ERC-CoG GEOCOG 724836); the Centre of Excellence scheme of the Research Council of Norway – Centre for Neural Computation (223262/F50); The Egil and Pauline Braathen and Fred Kavli Centre for Cortical Microcircuits; the National Infrastructure scheme of the Research Council of Norway – NORBRAIN (197467/F50); and the Netherlands Organisation for Scientific Research (NWO-Vidi 452-12-009; NWO-Gravitation 024-001-006; NWO-MaGW 406-14-114; NWO-MaGW 406-15-291). B.R. is supported by the SNSF (100014_162388) and the Clinical Research Priority Program (CRPP) “Sleep and Health” from the University of Zurich. O.J.’s research is funded by the Wellcome Trust (207550), the James. S. McDonnell Foundation (220020448), and the Royal Society Wolfson Research Merit Award. We want to thank Maurice Göldi for providing analytical tools used for SO detection.
Author Contributions
T. Schreiner and B.R. conceived the design. T. Schreiner collected the data. T. Schreiner and T. Staudigl analyzed the data. T. Schreiner and T. Staudigl wrote the manuscript. T.S., C.F.D., O.J., B.R., and T. Staudigl discussed the analyses and results and finalized the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: October 9, 2018
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and three figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.09.037.
Contributor Information
Thomas Schreiner, Email: t.schreiner@bham.ac.uk.
Tobias Staudigl, Email: tobias.staudigl@cshs.org.
Data and Software Availability
All data and analysis codes are available on reasonable request from the corresponding author.
Supplemental Information
References
- Bendor D., Wilson M.A. Biasing the content of hippocampal replay during sleep. Nat. Neurosci. 2012;15:1439–1444. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J., Wilhelm I. System consolidation of memory during sleep. Psychol. Res. 2012;76:192–203. doi: 10.1007/s00426-011-0335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairney S.A., Guttesen A.Á.V., El Marj N., Staresina B.P. Memory consolidation is linked to spindle-mediated information processing during sleep. Curr. Biol. 2018;28:948–954.e4. doi: 10.1016/j.cub.2018.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Wilson M.A. Deciphering neural codes of memory during sleep. Trends Neurosci. 2017;40:260–275. doi: 10.1016/j.tins.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dongen E.V., Takashima A., Barth M., Zapp J., Schad L.R., Paller K.A., Fernández G. Memory stabilization with targeted reactivation during human slow-wave sleep. Proc. Natl. Acad. Sci. USA. 2012;109:10575–10580. doi: 10.1073/pnas.1201072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentemilla L., Barnes G.R., Düzel E., Levine B. Theta oscillations orchestrate medial temporal lobe and neocortex in remembering autobiographical memories. Neuroimage. 2014;85:730–737. doi: 10.1016/j.neuroimage.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Gross J., Kujala J., Hamalainen M., Timmermann L., Schnitzler A., Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc. Natl. Acad. Sci. USA. 2001;98:694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L.A., Euston D.R., Tatsuno M., McNaughton B.L. Stored-trace reactivation in rat prefrontal cortex is correlated with down-to-up state fluctuation density. J. Neurosci. 2010;30:2650–2661. doi: 10.1523/JNEUROSCI.1617-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux J.-P., Rodriguez E., Le van Quejen M., Lutz A., Martinerie J., Varela F.J. Studying single-trials of phase synchronous activity in the brain. Int. J. Bifurcat. Chaos. 2000;10:2429–2439. [Google Scholar]
- Laventure S., Pinsard B., Lungu O., Carrier J., Fogel S., Benali H., Lina J.-M., Boutin A., Doyon J. Beyond spindles: interactions between sleep spindles and boundary frequencies during cued reactivation of motor memory representations. Sleep (Basel) 2018;41 doi: 10.1093/sleep/zsy142. zsy142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M., Schreiner T., Seifritz E., Rasch B. Emotional arousal modulates oscillatory correlates of targeted memory reactivation during NREM, but not REM sleep. Sci. Rep. 2016;6:39229. doi: 10.1038/srep39229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Michelmann S., Bowman H., Hanslmayr S. The temporal signature of memories: identification of a general mechanism for dynamic memory replay in humans. PLoS Biol. 2016;14:e1002528. doi: 10.1371/journal.pbio.1002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhus E., Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci. Biobehav. Rev. 2010;34:1023–1035. doi: 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudiette D., Paller K.A. Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn. Sci. 2013;17:142–149. doi: 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Oyarzún J.P., Morís J., Luque D., de Diego-Balaguer R., Fuentemilla L. Targeted memory reactivation during sleep adaptively promotes the strengthening or weakening of overlapping memories. J. Neurosci. 2017;37:7748–7758. doi: 10.1523/JNEUROSCI.3537-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P., Laureys S., Fuchs S., Collette F., Perrin F., Reggers J., Phillips C., Degueldre C., Del Fiore G., Aerts J. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rasch B., Born J. About sleep’s role in memory. Physiol. Rev. 2013;93:681–766. doi: 10.1152/physrev.00032.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild G., Eban E., Frank L.M. A cortical-hippocampal-cortical loop of information processing during memory consolidation. Nat. Neurosci. 2017;20:251–259. doi: 10.1038/nn.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönauer M., Alizadeh S., Jamalabadi H., Abraham A., Pawlizki A., Gais S. Decoding material-specific memory reprocessing during sleep in humans. Nat. Commun. 2017;8:15404. doi: 10.1038/ncomms15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner T., Rasch B. Boosting vocabulary learning by verbal cueing during sleep. Cereb. Cortex. 2015;25:4169–4179. doi: 10.1093/cercor/bhu139. [DOI] [PubMed] [Google Scholar]
- Schreiner T., Lehmann M., Rasch B. Auditory feedback blocks memory benefits of cueing during sleep. Nat. Commun. 2015;6:8729. doi: 10.1038/ncomms9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schyns P.G., Thut G., Gross J. Cracking the code of oscillatory activity. PLoS Biol. 2011;9:e1001064. doi: 10.1371/journal.pbio.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M., van Wingerden M., Womelsdorf T., Fries P., Pennartz C.M.A. The pairwise phase consistency: a bias-free measure of rhythmic neuronal synchronization. Neuroimage. 2010;51:112–122. doi: 10.1016/j.neuroimage.2010.01.073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.