Abstract
Objective:
Determine the incidence of ear infections in cochlear implant patients, evaluate the contribution of otitis media to complications, describe the bacteriology of otitis media in the cochlear implant population, the treatment provided at our center, and the long term outcome.
Methods:
Data collected included age at implantation, history of otitis media or ear tubes, etiology of hearing loss, inner ear anatomy, postoperative infections, time to infection, route of antibiotic administration, and interventions for infections. Categories of infection were acute otitis media, otitis media with effusion, tube otorrhea, meningitis, scalp cellulitis, and infection at the implant site.
Results:
Middle ear infections were diagnosed in 37% of implanted ears. Extension of middle ear infections into the implant site occurred in 2.8% of all implants (n = 16). Of the 16 infected devices, 10 were successfully treated with antibiotic therapy and did not require explantation. The retained implant group and explanted group both included some middle ear microbes such as Haemophilus influenzae and Streptococcus pneumoniae, as well as skin flora such as Staphylococcus aureus.
Conclusion:
Otitis media in pediatric cochlear implant patients is a common event and usually does not lead to complications of the cochlear implant. However, when the ear infection spreads to the scalp and the implant site, it is still possible to eliminate the infection using antibiotic therapy, particularly when treatment is directed to the specific organism that is recovered from the infected space and the duration and route of antibiotic treatment is carefully considered.
Keywords: Cochlear implant, Otitis media, Acute otitis media, Infectious complications, Tube otorrhea, Meningitis
1. Introduction
Cochlear implantation (CI) has provided hearing to deaf children and the opportunity to develop speech and language that is not possible with amplification alone [1–4]. The importance of hearing at an early age is known to be critical for development of speech and language; thus, cochlear implantation for congenitally deaf children is typically pursued at an early age. Because infants are at higher risk of otitis media (OM), there has been concern about the potential infectious complications of OM in young children with CI. Currently, we do not consider recurrent OM to be a contraindication for CI.
There is uncertainty and some degree of controversy regarding how best to manage OM in children with CIs and how to address an infection that has spread to the implant bed. An infection involving a CI that is not successfully treated with antibiotics often leads to explantation of the device. If the implanted ear is the better hearing ear, or the only hearing ear, this can lead to significant hardship for the child, with inability to communicate during the time without the device or loss of speech language progress during a critical period of their development. A previous study showed that speech performance worsened in nearly 10% of patients undergoing reimplantation [5]. Because infections are the second most common reason for explantation after device failure [6], further understanding of the etiology, management, and prevention of infectious complications are of great importance to this patient population.
OM has not always been viewed as an important risk factor for developing an infection of the CI site. While early studies did not report sequelae from postoperative OM in CI patients [7,8], Kempf et al. recommended that “antibiotics should be administered intravenously and a few days longer than for ears without implants,” [9] (p131) while Luntz et al. treated postoperative OM with oral antibiotics and did not report any adverse events [8]. The lack of adverse events after treatment with oral antibiotics was confirmed in a later prospective study, which found no OM-related complications [10]. The question of whether myringotomy tubes are safe in the setting of a new CI has also been debated, due to a perceived concern with exposing the middle ear space to the ear canal [11]. However, a number of studies have shown no increased risk of tube-related complications after CI [12–16], and others encourage this practice in children with recurrent acute otitis media (AOM) after CI [14,17].
2. Methods
This retrospective case series was performed at an urban, academic, tertiary care center in the Midwest. All children undergoing cochlear implantation from August 1999 through October 2013 were included for study. Six experienced CI surgeons performed the procedures. The Institutional Review Board at Washington University in St. Louis approved this study.
2.1. Data collection
Study data were collected (PV, KH) from inpatient and outpatient records, and entered into a Research Electronic Data Capture (REDCap) database, a secure, web-based application designed to support data capture for research studies [18]. In the case of questions regarding a specific episode of care, the surgeon caring for the patient was asked to clarify the record in the electronic chart. Data that were collected included age at implantation, implant manufacturer, history of preoperative ear tubes or ear infections, history of meningitis, known reasons for hearing loss, whether ear tubes were present at the time of surgery, abnormal anatomy, history of genetic syndromes, postoperative infections, time to infection, route of antibiotic administration, and surgical interventions used for management of postoperative infections. Recorded postoperative events included AOM, OM with effusion, tube otorrhea, meningitis, wound site infection, or implant infection. Device explantation was also included as an outcome.
2.2. Study definitions of infections
In our chart review, AOM was defined by history and physical exam with some of the following signs and symptoms: history of otalgia, poor sleep, fussiness, or fevers, accompanied by physical findings of erythema of the ear drum and the presence of middle ear fluid diagnosed by either the otolaryngologist or pediatrician. A wound infection was characterized by erythema, swelling, and sometimes pain at the incision without swelling or bogginess at the receiver-stimulator. Implant infection was defined as redness, swelling and/or pain with palpation over the receiver-stimulator, sometimes accompanied by purulent drainage from the incision, fever, or elevated white blood count.
2.3. Data analysis
A descriptive analysis of patient characteristics, types of infections, culture results, treatment and final outcome was performed. Infectious organisms and site of infection were analyzed in order to assess the frequency with which otitis led to infection at the CI site, how often such spread of OM resulted in a need for prolonged antibiotic therapy, and whether spread of organisms from OM to the implant resulted in explantation.
3. Results
During the study period, 568 ears were implanted in 421 patients. The median age at first implantation was 3.6 years (Range: 7 months–21 years), and for the second implant, median age was 4.6 years (Range: 7 months–20 years). The median length of follow up was 5.2 years (Range: < 1 month–15 years). Table 1 includes characteristics of the study population.
Table 1.
Characteristics of the study population (n = 568 implants, 421 patients).
| Patient characteristics | n | Percentages |
|---|---|---|
| Age (Years) | ||
| 0–2 | 147 | 26% |
| 2–6 | 194 | 34% |
| 6–21 | 227 | 40% |
| Gender | ||
| Male | 281 | 49% |
| Female | 287 | 51% |
| Type of Implantation | ||
| First implant | 514 | 90% |
| Explant-reimplant | 54 | 10% |
| Laterality | ||
| Unilateral | 383 | 68% |
| Bilateral sequential | 110 | 19% |
| Bilateral simultaneous | 74 | 13% |
| Manufacturer | ||
| Cochlear Corporation | 336 | 59% |
| Advanced Bionics | 200 | 35% |
| Med-El | 31 | 6% |
| Cause of Hearing Loss | ||
| Unknown | 382 | 67% |
| History of Bacterial Meningitis | 39 | 7% |
| Connexin 26/30 | 36 | 6% |
| History of Congenital CMV | 33 | 6% |
| Large vestibular aqueduct | 31 | 5% |
| Auditory neuropathy | 14 | 2% |
| Wolfram syndrome | 1 | <1% |
| Trauma: fracture through both otic capsules | 1 | <1% |
| Risk Factors for Hearing Loss | ||
| History of aminoglycoside antibiotic use | 21 | 4% |
| History of Measles | 2 | <1% |
| History of Rubella | 1 | <1% |
| Active neurosarcoidosis | 1 | <1% |
| Other Medical Conditions | ||
| Intracranial hemorrhage | 3 | <1% |
| Ventilator-dependent in NICU | 3 | <1% |
| Insulin-dependent Diabetes Mellitus | 2 | <1% |
| Lung transplant | 2 | <1% |
| Cerebral palsy | 2 | <1% |
| Cystic fibrosis | 1 | <1% |
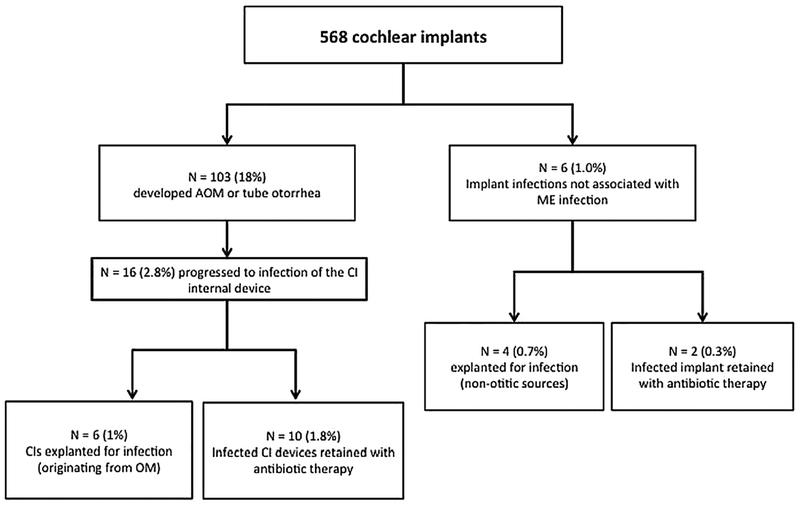
Middle ear infection was the most common postoperative infection, (n = 210 infections in 103 ears) and 18% of ears experienced OM at least once after implantation (See Fig. 1). The median interval from the time of implantation to the first episode of postoperative otitis was 6 months with a range of 1 weeke65 months. The next most common infection in implanted ears was otitis externa (n = 26, 4.6%). Infection of the internal device occurred in 22 ears (3.8%) with 16 of these implant infections originating from OM and 6 without evidence of middle ear infection. One patient developed fulminant pneumococcal meningitis accompanied by bilateral acute otomastoiditis. This patient was treated with device removal, surgical drainage of the mastoid cavities and intravenous antibiotics over a prolonged period. This patient initially presented with opacified middle ears and mastoids and mental status changes. She was developmentally delayed and was not known to have a malformation of the inner ear or any reason for immune compromise. The pathogen in this case was Streptococcus pneumoniae.
Fig. 1.
Summary of Cochlear Implant Infections.
(N = numbers of ears/implants).
Cultures were obtained in 40% of all ear infections. Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae were commonly recovered when middle ear samples were cultured in children with OM. Pseudomonas aeruginosa was observed in several children with tube otorrhea. A complete listing of cultured organisms is shown in Table 2 stratified by age.
Table 2.
Microorganisms cultured from children with postoperative otitic infections stratified by age at the time of infection. (n = 18 total, 11 single).
| Microorganisms | Total isolates | Single isolates | ||
|---|---|---|---|---|
| n | Percentages | n | Percentages | |
| Age 0–2 years (n = 18 total, 11 single) Skin pathogens | ||||
| Staphylococcus aureus, methicillin resistant (MRSA) | 4 | 22% | 1 | 9% |
| Coagulase negative Staphylococcus | 1 | 6% | 1 | 9% |
| Middle ear pathogens | ||||
| Haemophilus influenzae | 3 | 17% | 3 | 27% |
| Streptococcus pneumoniae | 3 | 17% | 3 | 27% |
| Ear canal/middle ear pathogens | ||||
| Pseudomonas aeruginosa | 1 | 6% | 1 | 9% |
| Other | ||||
| Corynebacterium spp. | 2 | 11% | ||
| Aspergillus flavus | 1 | 6% | 1 | 9% |
| Streptococcus viridans | 1 | 6% | ||
| Escherichia coli | 1 | 6% | 1 | 9% |
| Streptococcus pyogenes | 1 | 6% | ||
| Age 2–6 years (n = 54 total, 20 single) Skin pathogens | ||||
| Coagulase negative Staphylococcus | 8 | 15% | ||
| Staphylococcus aureus, methicillin resistant (MRSA) | 4 | 7% | 3 | 15% |
| Staphylococcus aureus, methicillin sensitive (MSSA) | 2 | 4% | 2 | 10% |
| Staphylococcus epidermidis | 2 | 4% | 1 | 5% |
| Middle ear pathogens | ||||
| Streptococcus pneumoniae | 6 | 11% | 3 | 15% |
| Turicella Otitidis | 4 | 7% | 1 | 5% |
| Haemophilus influenza | 2 | 4% | 3 | 15% |
| Streptococcus pyogenes | 1 | 2% | ||
| Ear canal/middle ear pathogens | ||||
| Pseudomonas aeruginosa | 8 | 15% | 4 | 20% |
| Serratia marcescens | 2 | 4% | ||
| Other | ||||
| Aspergillus flavus | 2 | 4% | 1 | 5% |
| Candida albicans | 2 | 4% | 1 | 5% |
| Candida parapsilopsis | 2 | 4% | ||
| Mixed microorganisms | 2 | 4% | ||
| Corynebacterium afermentans | 1 | 2% | 1 | 5% |
| Corynebacterium spp. | 1 | 2% | ||
| Achromobacter xylosoxidans | 1 | 2% | ||
| Citrobacter freundii complex | 1 | 2% | ||
| Group G Streptococcus | 1 | 2% | ||
| Leclercia adecarboxylata | 1 | 2% | ||
| Stenotrophomonas maltophilia | 1 | 2% | ||
| Age 6–21 years (n = 12 total, 6 single) Skin pathogens | ||||
| Staphylococcus aureus, methicillin sensitive (MSSA) | 3 | 25% | 1 | 17% |
| Coagulase negative Staphylococcus | 1 | 8% | ||
| Staphylococcus caprae | 1 | 8% | ||
| Ear canal/middle ear pathogens | ||||
| Pseudomonas aeruginosa | 5 | 42% | 5 | 83% |
| Other | ||||
| Corynebacterium coyleae | 1 | 8% | ||
| Corynebacterium amycolatum | 1 | 8% | ||
Extension of middle ear infections to the implant site occurred in 2.8% (n = 16). Of the 16 infected devices, 10 were successfully treated with antibiotic therapy without explantation. The retained implant group and explanted group had similar times to implant infection (retained implant group = 21.9 months, explanted group = 19.8 months) and age at implantation (retained implant group = 5.2 y, explanted group = 7.4 y). Because the numbers were small, we could not determine the effect of time between implantation and infection on device retention. Table 3 demonstrates other factors potentially relevant to implant infection, including the presence of ear tubes at the time of CI surgery. We found that 1–2% of our Cochlear and Advanced Bionics (AB) devices became infected: 7 of 336 (2%) Cochlear devices, 2 of 200 (1%) AB devices, and 1 of 31 (3%) Med El devices were infected and explanted. When the data were stratified by device manufacturer, no difference in implant infection resulted. Because implant infections were rare, it was difficult to identify specific factors to device infection. Many children had patent ear tubes at the time of CI surgery and did not develop an implant infection in subsequent months. Surgeons in our group did not routinely place or remove ear tubes at the time of implant surgery. Some patients had repeated ear tubes placed after implantation either due to recurrent OM or chronic tympanic membrane retraction from Eustachian tube dysfunction.
Table 3.
Implant Infections with descriptive data.
| Patient | Origin of implant infection | Manufacturer/Electrode | Age at implantation (yrs) | Age at infection (yrs) | Presence of myringotomy tube | Pathogen cultured |
|---|---|---|---|---|---|---|
| CI 1 | Otitic meningitis | Med-El | 5.8 | 6.6 | None at time of implant, placed for infection | Streptococcus pneumonia, Coagulase negative Staphylococcus |
| CI 2 | Acute and chronic OM | Advanced Bionic Clarion | 6.8 | 10.3 | Prior history of ear tubes and recurrent OM | Pseudomonas aeruginosa |
| CI 3 | Acute OM | Cochlear Nucleus CI24RE | 1 | 5.7 | None at time of implant | Hemophilus influenza |
| CI 4, first infection | Acute OM | Cochlear Nucleus CI24RE | 1.9 | 1.9 | Tubes in place at implant | MRSA |
| CI 4, second infection | Acute OM | Cochlear Nucleus CI24RE | 3.7 | 3.8 | Tubes in place at implant | MRSA |
| CI 5, first infection | Skin infection | Cochlear Nucleus CI24RE | 7.3 | 7.7 | None at time of implant | MSSA |
| CI 6, first infection | Acute OM | Cochlear Nucleus CI24RE | 15.2 | 16.1 | None at time of implant | Coagulase negative Staphylococcus Propionibacteria |
| CI 6, second infection | Skin infection | Cochlear Nucleus CI24RE | 3.4 | 18.2 | None at time of implant | No growth, antibiotic pretreatment suppressed cultures |
| CI 7 | Skin infection | Cochlear Nucleus CI24RE | 2.7 | 3.7 | None at time of implant | MSSA, Enterococcus |
| CI 8 | Acute OM | Advanced Bionics Clarion | 3.2 | 3.3 | None at time of implant, placed for infection | Pseudomonas aeruginosa, Coagulase negative Staphylococcus |
| CI 5, second infection | Acute OM | Cochlear Nucleus CI24RE | 7.9 | 7.9 | None at time of implant | Escherichia Coli |
| CI 9 | Skin Infection | Cochlear Nucleus CI24RE | 19.6 | 19.7 | None at time of implant | MRSA |
| CI 10 | Acute OM | Cochlear Nucleus CI24RE | 1.5 | 1.6 | None at time of implant | No growth, antibiotic pretreatment suppressed cultures |
| CI 11 | Tube otorrhea | Cochlear 512 | 1.8 | 1.8 | Tubes in place at implant | MRSA, Corynebacterium, Streptococcus viridans |
| CI 12 | Tube otorrhea | Cochlear Nucleus CI24RE | 3.8 | 3.8 | Tubes in place at implant, h/o MRSA otorrhea | MRSA |
| CI 13 | Bilateral OM | Cochlear 512 | 1.1 | 1.9 | None at time of implant, placed for infection | Streptococcus pneumoniae |
| CI 14 | Acute OM | Advanced Bionics Hi-Res 90K1J | 1 | 1.2 | None at time of implant, placed for infection | Streptococcus pyogenes, Corynebacterium |
| CI 15 | Acute OM | Med-El | 12.6 | 12.7 | None at time of implant | No culture obtained, presumptive antibiotic treatment |
| CI 16 | Acute OM | Advanced Bionics Hi-Res 90 K 1 J | 7.8 | 9.8 | None at time of implant, TM perforation postop | MSSA |
| CI 17 | Acute OM | Cochlear Nucleus CI24RE | 1.6 | 3.8 | None at time of implant, placed for infection | Streptococcus pneumoniae Coagulase negative Staphylococcus |
| CI 18 | Acute OM | Advanced Bionics Clarion | 1.4 | 4.7 | None at time of implant | Pseudo monas aeruginosa |
| CI 19 | Acute OM | Advanced Bionics Hi-Res 90 K 1 J | 1.4 | 1.6 | None at time of implant | Coagulase negative Staphylococcus |
The microbiology of implant infections was carefully reviewed with a pediatric infectious disease specialist to determine if there were specific organisms that provided a strong prognosis regarding implant loss or retention. In the explanted group, we observed methicillin sensitive staphylococcus aureus (MSSA) (n = 3) most commonly, and we observed infections with Methicillin-resistant Staphylococcus aureus (MRSA), Haemophilus influenzae, and Pseudomonas aeruginosa in three separate cases. Staphylococcal infections were thought to be the most difficult to eradicate without explantation, and pseudomonal infections were also considered particularly challenging to address medically. The bacteriology of the explanted patients appeared to support this impression. However, we did not find that all staphylococcal implant infections resulted in explantation. The retained implant group included 2 cases of MRSA that were successfully treated with antibiotic therapy. Thus, two of these implant site infections involving Staphylococcus aureus were eradicated using antibiotic therapy alone without removing the device.
In summary, we explanted 10 devices out of a total of 568 due to refractory infection resulting in a 1.8% rate of explantation for infection (see Fig. 1). Overall, we performed 81 device explants during the 14-year interval for all indications combined (14% total). The most common reason for explantation was device failure (n = 71), followed by implant infection from otitis (n = 6, 1%), implant infection not associated with otitis (n = 4), device extrusion (n = 1), and electrode migration (n = 1). The median time to explantation was 3.6 years (Range: < 1 month to 15 years).
4. Discussion
This retrospective study of over 500 pediatric CI performed at a large academic center demonstrates that postoperative ear infections are common, and can be serious, resulting in implant infections and in some cases, device removal. Though our sample was among the largest single-center study to report on infections in pediatric cochlear implantation, we cannot make a definitive recommendation about how best to treat these infections with respect to length of treatment, intravenous versus oral route, role of ear tubes or prognosis on the basis of the microbiology. However, we can conclude that in children, the pathogens causing implant infections are often organisms that cause OM. We observed that treatment of OM with culture-directed therapy could be associated with retention of the device and restriction of the infection to the middle ear space, and prevented progression of infection to the scalp and the receiver–stimulator site. Ten out of sixteen implant infections (62%) that originated from the middle ear were resolved with targeted or empiric antibiotic therapy; in contrast, only two out of six devices (33%) that were infected as wound or scalp infections, without a middle ear infection, were able to be retained using targeted antibiotic therapy. In addition, we find that the risk of device explantation is influenced by the infectious organism. Five out of seven devices (71%) that were infected with Staphylococcus aureus required explantation, whereas only four out of fourteen implants (29%) that were infected with other organisms, more commonly associated with OM, required explantation. These organisms included Streptococcus pneumoniae, Haemophilus influenzae and Streptococcus pyogenes (see Table 4).
Table 4.
Culture results from CI infections organized by implant outcome.
| Explanted | N | Retained implant | N |
|---|---|---|---|
| MRSA | 3 | No growth or no culture | 6 |
| MSSA | 2 | MRSA | 2 |
| Streptococcus pneumoniae | 1 | Streptococcus pneumoniae | 2 |
| Haemophilus influenzae | 1 | Streptococcus pyogenes | 1 |
| Pseudomonas aeruginosa | 1 | Steptococcus viridans | 1 |
| Propionibacterium acnes | 1 |
4.1. Managing otitis in recently implanted children
We routinely face the question of how best to treat a child with a recent CI who has an AOM in the implanted ear. Particularly in cases of OM where implant surgery occurred within two months, there is a concern for an increased risk of meningitis due to the spread of middle ear bacteria into the CSF via the cochlear perilymph. There is also a concern for implant colonization prior to the formation of a dense fibrous capsule around the device. In this study, 15 of the 19 device infections occurred within one year of implantation, and 11 of them occurred within 3 months of the original implant surgery. This observation is consistent with our general impression that an ear infection in the first few months after implantation is a concern because of the possibility of spread of the infection to the device site.
We observed that some cases of middle ear infections led to device infection and others did not. Ten children with overt signs of infection at the implant site, including symptoms of redness, swelling, tenderness, and inability to wear the external device, were successfully treated with intravenous antibiotic therapy, and were able to avoid explantation. In those children who successfully retained their implants, most underwent tympanocentesis or myringotomy and tube placement, with cultures taken from the middle ear space and antibiotic therapy directed to the specific organism. In those cases where no organisms were recovered, broad-spectrum antibiotics were given, such as vancomycin and cefepime for 14 days.
Our practice generally agrees with those outlined by Rubin, Papsin and the Committee on Infectious Diseases [17]. In this policy statement, the authors recommend broad-spectrum antibiotics including agents to cover MRSA for wound infections at CI site. They also recommend consideration of broad spectrum parenteral antibiotics for children with ear infections in the following groups: those who are less than two months post-surgery, children who have an inner ear malformations, children with a CI device with a positioner, or for children who appear systemically ill. They also recommend that an otolaryngologist evaluate the child early during the course of treatment for OM with or without implant site infection to confirm antibiotic efficacy. While our paper does not provide new recommendations with respect to this practice, the outcomes that we observed would support the liberal use of antibiotics for ear infections in children with CI and indicate that broad spectrum parenteral therapy is effective in preserving implant integrity in some cases even when there is infection around the device.
4.2. Managing Staphylococcus aureus infections
Infections with Staphylococcus aureus (S. aureus) deserve special consideration. In our cohort, we found that eradication of infection and retention of the device with antibiotic therapy alone is uncommon (<30% retention rate), but possible. In general, because S. aureus forms antibiotic-resistant biofilms, removal of infected prosthetic devices is often necessary to eradicate the infection [19]. However, the morbidity of device removal is high and patients are generally stable in these cases; thus, medical therapy for S. aureus device infections is appropriate to consider and has been shown to be safe and effective in other populations [20,21]. Thus, even if MSSA or MRSA is cultured from the CI site, we believe that it is reasonable, in a child that is otherwise healthy, to attempt medical therapy with an extended course of anti-staphylococcal antibiotic therapy, typically with a beta-lactam antibiotic, such as nafcillin, for MSSA in combination with rifampin, and vancomycin or linezolid for MRSA. Of note, most CI infections that are observed in the adult population appear to be associated with Staphylococcal organisms, suggesting a skin source of the infection. Middle ear pathogens and otitis media are rarely observed in these adult infections. In children, we observe that implant infections are more commonly associated with middle ear pathogens, and these organisms are associated with a better prognosis for retention of the CI with antimicrobial therapy.
4.3. Risk of meningitis
Children with CIs are at a higher risk than the average population for developing bacterial meningitis [22]. Children with inner ear anomalies are also at higher risk for bacterial meningitis. The etiology of the increased risk of meningitis in both these populations is likely due to facilitated transit of pathogens from the middle ear into the inner ear, which communicates with the CSF through the implant device and through the anomalous inner ear structures. Fortunately, meningitis was a rare event and occurred only once in our population. Although bacterial meningitis in CI patients is rare, we adhere to guidelines for pneumococcal vaccines closely and treat OM promptly and consistently in patients with CIs with hopes to use all preventative measures possible to avoid this potentially devastating complication.
4.4. Limitations
While this paper lends insight into approaches for treating OM in children with CIs, this study is limited by the fact that it is a retrospective analysis of observational data during routine clinical care. How best to manage postoperative OM after cochlear implantation is an important question, because it is a common event that can lead to devastating loss of function in children who are deaf without their cochlear implants. In this study, we observed various types of treatment with involvement of the pediatric infectious disease team in some cases. Some children were treated without cultures and without intravenous antibiotic therapy. In other patients, empiric broad-spectrum antibiotics were given until culture results returned from tympanocentesis and narrowing of the antibiotic choice occurred once cultures were obtained. Others were treated with empiric intravenous antibiotics without culture information. For these reasons, the organisms cultured from children with postoperative otitis (Table 2) and from implant infections (Table 4) must be interpreted with the knowledge that some children received pre-treatment antibiotics.
We observed that 18% of CI patients had AOM or tympanostomy tube otorrhea, but the incidence of ear infections was likely higher. We suspect that OM was frequently treated by the primary care provider without informing the CI team. There are times when empiric oral antibiotic therapy for AOM in the implant patient is sufficient: however, a child with a CI is at higher risk for complications such as meningitis or chronic infection at the implant site if therapy fails, which could lead to loss of the device. Careful use of antibiotics for these infections, using culture directed therapy, and advice from a pediatric infectious disease specialist can make a significant impact in the final outcome of these infections.
5. Conclusion
OM in pediatric CI patients is a common event and usually resolves without affecting the device. However, when the ear infection spreads outside of the middle ear space and contaminates the receiver stimulator and magnet site, the implant can be at risk of chronic infection and extrusion. It is possible to eliminate the infection using antibiotic therapy, particularly if directed by cultures, and the duration and route of antibiotic treatment is carefully considered. Obtaining specimens to determine the causative organism can enhance our ability to make good antibiotic choices and to optimize the chance of retaining the device after the infection is cleared.
Table of contents summary.
Pathogens causing otitis media can result in infectious complications of cochlear implants. Fortunately, may of these infections can be treated and the implant spared.
What’s known on this subject.
Children with cochlear implants have a higher risk of meningitis and can develop implant infections as a result of otitis media, and optimal management for these cases is unknown.
What this study adds.
18% of implanted ears developed OM. 2.8% developed an infection of the implant. Many pathogens from CI infections originated from a middle ear source. 62% of infected implants were successfully retained with medical management and 38% required explantation.
Acknowledgments
Peter M. Vila: gathered patient data, performed a portion of the analyses, initiated the writing of the manuscript, and approved the final manuscript as submitted.
Nsangou T. Ghogomu: participated in the original concept of the study, gathered patient data, performed the initial analyses, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Audrey R. Odom-John: advised the team on the design of the data collection, critically reviewed the microbiology data, and edited and approved the final manuscript.
Timothy E. Hullar: participated in the original concept of the study, edited the manuscript, and approved the final manuscript as submitted.
Keiko Hirose: participated in the original concept of the study, supervised the project, collected and interpreted patient data, finalized the figures and manuscript, incorporated revisions from the other authors, and edited and wrote the final manuscript submitted the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Abbreviations:
- CI
cochlear implant
- OM
otitis media
- MSSA
methicillin sensitive staphylococcus aureus
- MRSA
methicillin-resistant Staphylococcus aureus
- S. aureus
Staphylococcus aureus
- AOM
acute otitis media
Footnotes
Conflict of interest
Timothy Hullar is on the medical advisory board of Advanced Bionics Corporation and Med El. The other authors have no conflicts of interest to disclose.
References
- [1].Koch ME, Wyatt JR, Francis HW, Niparko JK, A model of educational resource use by children with cochlear implants, Otolaryngol. Head. Neck Surg 117 (3 Pt 1) (1997) 174–179. [DOI] [PubMed] [Google Scholar]
- [2].Francis HW, Koch ME, Wyatt JR, Niparko JK, Trends in educational placement and cost-benefit considerations in children with cochlear implants, Arch. Otolaryngol. Head. Neck Surg 125 (5) (1999) 499–505. [DOI] [PubMed] [Google Scholar]
- [3].Niparko JK, Tobey EA, Thal DJ, et al. , Spoken language development in children following cochlear implantation, JAMA 303 (15) (2010) 1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nicholas JG, Geers AE, Will they catch up? The role of age at cochlear implantation in the spoken language development of children with severe to profound hearing loss, J. Speech Lang. Hear Res 50 (4) (2007) 1048–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lassig AA, Zwolan TA, Telian SA, Cochlear implant failures and revision, Otol. Neurotol 26 (4) (2005) 624–634. [DOI] [PubMed] [Google Scholar]
- [6].Broomfield SJ, Murphy J, Wild DC, Emmett SR, O’Donoghue GM, Writing for the UK National Paediatric CI Surgical Audit Group, Results of a prospective surgical audit of bilateral paediatric cochlear implantation in the UK, Cochlear Implant. Int 15 (5) (2014) 246–253. [DOI] [PubMed] [Google Scholar]
- [7].Cohen NL, Hoffman RA, Complications of cochlear implant surgery in adults and children, Ann. Otol. Rhinol. Laryngol 100 (9 Pt 1) (1991) 708–711. [DOI] [PubMed] [Google Scholar]
- [8].Luntz M, Hodges AV, Balkany T, Dolan-Ash S, Schloffman J, Otitis media in children with cochlear implants, Laryngoscope 106 (11) (1996) 1403–1405. [DOI] [PubMed] [Google Scholar]
- [9].Kempf HG, Johann K, Lenarz T, Complications in pediatric cochlear implant surgery, Eur. Arch. Otorhinolaryngol 256 (3) (1999) 128–132. [DOI] [PubMed] [Google Scholar]
- [10].Luntz M, Teszler CB, Shpak T, Feiglin H, Farah-Sima’an A, Cochlear implantation in healthy and otitis-prone children: a prospective study, Laryngoscope 111 (9) (2001) 1614–1618. [DOI] [PubMed] [Google Scholar]
- [11].Preciado D, Choi S, Management of acute otitis media in cochlear implant recipients: to tube or not to tube? Laryngoscope 122 (4) (2012) 709–710. [DOI] [PubMed] [Google Scholar]
- [12].Barañano CF, Sweitzer RS, Mahalak ML, Alexander NS, Woolley AL, The management of myringotomy tubes in pediatric cochlear implant recipients, Arch. Otolaryngol. Head. Neck Surg 136 (6) (2010) 557–560. [DOI] [PubMed] [Google Scholar]
- [13].Kennedy RJ, Shelton C, Ventilation tubes and cochlear implants: what do we do? Otol. Neurotol 26 (3) (2005) 438–441. [DOI] [PubMed] [Google Scholar]
- [14].Luntz M, Teszler CB, Shpak T, Cochlear implantation in children with otitis media: second stage of a long-term prospective study, Int. J. Pediatr. Otorhinolaryngol 68 (3) (2004) 273–280. [DOI] [PubMed] [Google Scholar]
- [15].Fayad JN, Tabaee A, Micheletto JN, Parisier SC, Cochlear implantation in children with otitis media, Laryngoscope 113 (7) (2003) 1224–1227. [DOI] [PubMed] [Google Scholar]
- [16].Kulak JG, Brown K, Telischi F, Balkany T, Tympanostomy tubes in cochlear implant patients, Laryngoscope 119 (2009) 119. [Google Scholar]
- [17].Rubin LG, Papsin B, Committee on Infectious Diseases and Section on otolaryngology-Head and Neck Surgery, Cochlear implants in children: surgical site infections and prevention and treatment of acute otitis media and meningitis, Pediatrics 126 (2) (2010) 381–391. [DOI] [PubMed] [Google Scholar]
- [18].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inf 42 (2) (2009) 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu C, Bayer A, Cosgrove SE, et al. , Infectious Diseases Society of America, Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children, Clin. Infect. Dis 52 (3) (2011) e18–55. [DOI] [PubMed] [Google Scholar]
- [20].Barberan J, Aguilar L, Carroquino G, et al. , Conservative treatment of staphylococcal prosthetic joint infections in elderly patients, Am. J. Med 119 (11) (2006), 993.e7–10. [DOI] [PubMed] [Google Scholar]
- [21].Aboltins CA, Page MA, Buising KL, et al. , Treatment of staphylococcal prosthetic joint infections with debridement, prosthesis retention and oral rifampicin and fusidic acid, Clin. Microbiol. Infect 13 (6) (2007) 586–591. [DOI] [PubMed] [Google Scholar]
- [22].Reefhuis J, Honein MA, Whitney CG, et al. , Risk of bacterial meningitis in children with cochlear implants, N. Engl. J. Med 349 (5) (2003) 435–445. [DOI] [PubMed] [Google Scholar]