Abstract
Childhood obesity and its comorbidities continue to accelerate across the globe. Two-thirds of pregnant women are obese/overweight, as are 20% of preschoolers. Gestational diabetes mellitus (GDM) is escalating, affecting up to 1 in 5 pregnant women. The field of developmental origins of health and disease has begun to move beyond associations to potential causal mechanisms for developmental programming. Evidence across species compellingly demonstrates that maternal obesity, diabetes, and Western-style diets create a long-lasting signature on multiple systems, including infant stem cells, the early immune system, and gut microbiota. Such exposures accelerate adipogenesis, disrupt mitochondrial metabolism, and impair energy sensing, affecting neurodevelopment, liver, pancreas, and skeletal muscle. Attempts to prevent developmental programming have met with very limited success. A challenging level of complexity is involved in how the host genome, metabolome, and microbiome throughout pregnancy and lactation increase the offspring’s risk of metabolic diseases across the life span. Considerable gaps in knowledge include the timing of exposure(s) and permanence or plasticity of the response, encompassing effects from both maternal and paternal dysmetabolism. Basic, translational, and human intervention studies targeting pathways that connect diet, microbiota, and metabolism in mothers with obesity/GDM and their infants are a critical unmet need and present new challenges for disease prevention in the next generation.
The concept of Developmental Origins of Health and Disease (DOHaD) has evolved rapidly since the National Institutes of Health–sponsored Intrauterine Environment Investigators’ Meeting and Workshop was held in Bethesda, MD, on 28 November 2007. This paradigm supports that the nidus for major chronic diseases, including diabetes, obesity, neuropsychiatric and immunologic disorders, and cardiovascular, gastrointestinal, and liver diseases begins in utero and in early development, thus creating a malicious cycle with enormous public health consequences. We are now aware that exposures during the first 1,000 days of life beginning at conception can herald early infant outcomes with persistent effects on metabolic health and an acquired susceptibility to disease later in life, possibly across generations. Half of childhood obesity occurs among children who are obese by age 5 (1), signaling that very early risk factors in the intrauterine and postnatal environment influence the genesis of childhood obesity. Alarmingly, recent models predict that a majority of today’s children (57%) will be obese by age 35 and roughly half of the projected prevalence will occur during childhood (2). A recent longitudinal study of 970 mother-child pairs reported that maternal hyperglycemia increased the risk of abnormal glucose tolerance, obesity, and higher blood pressure among offspring at 7 years of age. This increased risk was independent of maternal obesity, being born large for gestational age (≥90th percentile), and childhood BMI (3). These data emphasize the need for very early interventions in mothers with gestational diabetes mellitus (GDM) and their offspring who are at risk for reduced β-cell function and abnormal glucose tolerance. This is especially necessary in Asia, Africa, and the Middle East, where childhood obesity, young-onset type 2 diabetes, and chronic diseases are rampant (4). The fetal overnutrition hypothesis suggests that maternal fuels are in greater abundance in maternal obesity and GDM, leading to developmental programming, but these fuels are not limited to glucose and fats (5,6). Substantial evidence also exists for undernutrition causing DOHaD (7). Because of limitations in space, this Perspective will focus on emerging evidence concerning exposure to overnutrition and potential epigenetic, metabolic, endocrine, and molecular mechanisms underlying fetal programming and outcomes in humans, primates, and other models. The challenges and opportunities for future nutritional interventions are also discussed.
Evidence for Parental Environment and Transgenerational Epigenetic Susceptibility
Obesity and type 2 diabetes have a strong genetic component; however, the genetic basis for both disorders remains largely elusive. For example, a recent meta-analysis of BMI genome-wide association studies estimated that 97 loci account for only 2.7% of BMI variation, and common genetic variation accounted for up to 21% of BMI variation (8). Given this low frequency (<5%) and the relatively modest effect size estimates, today’s high prevalence of childhood obesity and type 2 diabetes cannot be easily explained by genetics alone. Transgenerational epigenetic inheritance has been proposed to contribute to developmental programming; however, the concept itself remains controversial (9,10). While supporting evidence is mounting in animal models, evidence in humans is scarce, in part because epidemiologic studies spanning multiple generations are challenging and exposures are often cumulative and difficult to isolate in time. Although whole genome sequencing and epigenetic analyses have given us a rapidly expanding list of genotypes and epigenotypes associated with metabolic disease risk in offspring, the expression of these variants depends on cumulative lifestyle and environmental factors acting together in a tissue-specific manner, likely at key points in development. Histone modifications, noncoding RNAs, and the methylation of cytosine within DNA allow the transmission of epigenetic signals, enabling the cell to “memorize” these encounters in a tissue-specific manner. Genetic variation, including single nucleotide polymorphisms located near methylation site(s), can also affect DNA methylation, which is often not considered in most epigenetic analyses (11).
In humans, small changes in DNA methylation in tissues consisting of heterogeneous cell types, such as cord blood, are often found (12). Whether DNA methylation (the most common DNA marker studied) is a cause or consequence of obesity and type 2 diabetes is an important consideration that currently impedes our ability to discern mechanism from association. To date, candidate gene studies have revealed persistent DNA methylation differences in the association of maternal obesity with adverse offspring outcomes at birth and in later life (12). Genome-wide methylation studies have shown differentially methylated regions in genes involved in cell differentiation, the immune system, and transcriptional regulation (12). These changes in methylation have been associative but might be better accounted for by genetic or lifestyle factors over a period of time rather than a causal intrauterine mechanism. However, additional analyses of newly identified single nucleotide polymorphisms might be necessary to explain where and how the sophisticated DNA methylation changes act in the genome and whether this extends to tissue-specific changes later in life. Further, methylation throughout the genome, including promoter, intergenic, and intragenic regions, can have very different correlations with levels of transcript expression (13).
In animal models of maternal and paternal overnutrition, specific methylation marks are found on individual genes within different tissues of the offspring. Maternal overnutrition in mice programmed hepatic DNA hypermethylation in male offspring (14) and altered fetal hepatic histone modifications that persisted up to 5 weeks of age, despite weaning to a low-fat diet (15). Animal studies have shown that epigenetic information carried in sperm is associated with phenotypes of the F2 generation (16–18). For example, high-fat feeding–induced paternal obesity in mice initiated intergenerational transmission of obesity and insulin resistance in two generations of offspring and was associated with altered sperm microRNA content and germ cell methylation status (19). Epigenetic effects of paternal genes can also occur in humans, as the DNA methylome in sperm showed differences between obese men before and following gastric bypass surgery (20). Further, distinct methylation patterns were observed between blood, adipose tissue, and spermatozoa, pointing toward tissue-specific remodeling after surgery (20). However, such studies are lacking causation for phenotype. In humans, epidemiologic studies are susceptible to confounding, reverse causation (i.e., when the disease process influences the exposure, rather than the other way around), and other biases (as reviewed [21]). Most parental DNA methylation marks found in embryos are erased during embryogenesis. True epigenetic transgenerational inheritance requires the presence of environmental cues resulting in permanent epigenetic changes that are exempt from erasure and persist across more than one generation (22). Interestingly, regions that escape DNA demethylation and are linked to metabolic disorders were found in human primordial germ cells, thus uncovering possible candidates for transgenerational epigenetic inheritance (23). Siklenka et al. (24) demonstrated that epigenetic inheritance of aberrant development can be initiated by histone demethylase activity in developing sperm without changes to DNA methylation at CpG-rich regions in mice. In addition to the nuclear genome, the effects of maternal obesity on germline mitochondria and mitochondrial dysfunction must be considered as a mechanism for transmission of metabolic diseases, since mitochondrial DNA is inherited from the mother. Saben et al. (25) found that consumption of a maternal high-fat, high-sugar diet programmed differential expression of electron transport chain proteins and altered mitochondrial dynamics in skeletal muscle via germline changes across three generations of mice. Epigenetic variation between individuals might hold the key to more accurate predictions of obesity risk, and better understanding of this variation could lead to new tools for fighting obesity. In addition to epigenetic biomarkers, such as metastable epialleles (26,27), epigenetic modifications established during early development and expressed in multiple tissue types might provide additional insight into intrauterine exposures and obesity risk in later life.
Programming in Primates and the Critical Role of Maternal Diet
Addressing causation and mechanisms of human developmental programming has been difficult because of tremendous variation in environment, genetics, nutrition, maternal behavior, and developmental programming of preceding generations. Despite the differences in maturation during fetal life, studies in sheep (28), rodents (29), flies (30), and even worms (31) have shown that overnutrition results in developmental programming of obesity and other disorders in the offspring. Since we do not understand the causal mechanisms for many of the disease outcomes in humans and therefore cannot institute prevention or treatment, we need to know more about the mechanisms involved, building on knowledge from animal models. Our group has spent the past decade, first in collaboration with Dr. Kevin Grove at the Oregon National Primate Research Center and later with Dr. Kjersti Aagaard at Baylor University (and others at other institutions), developing and studying a sophisticated nonhuman primate (NHP) model (the Japanese macaque) of chronic maternal high-fat, calorie-dense Western-style diet (WSD) consumption starting early in the reproductive years. Females are fed a WSD or chow diet for 3–8 years before mating; cesarean section is performed in the early third trimester for fetal studies or mothers are allowed to give birth and juveniles are followed for up to 3 years while either fed a WSD or switched to a healthy diet at weaning, allowing us to study the persistence of developmental programming. The importance of the NHP model is that the developmental changes in the placenta, islets, and brain are similar to those in humans, and it is the only natural model that develops the full spectrum of metabolic disease seen in humans, including complex psychosocial behaviors that can be studied longitudinally in the offspring. These qualities make the NHP model uniquely powerful and critically important.
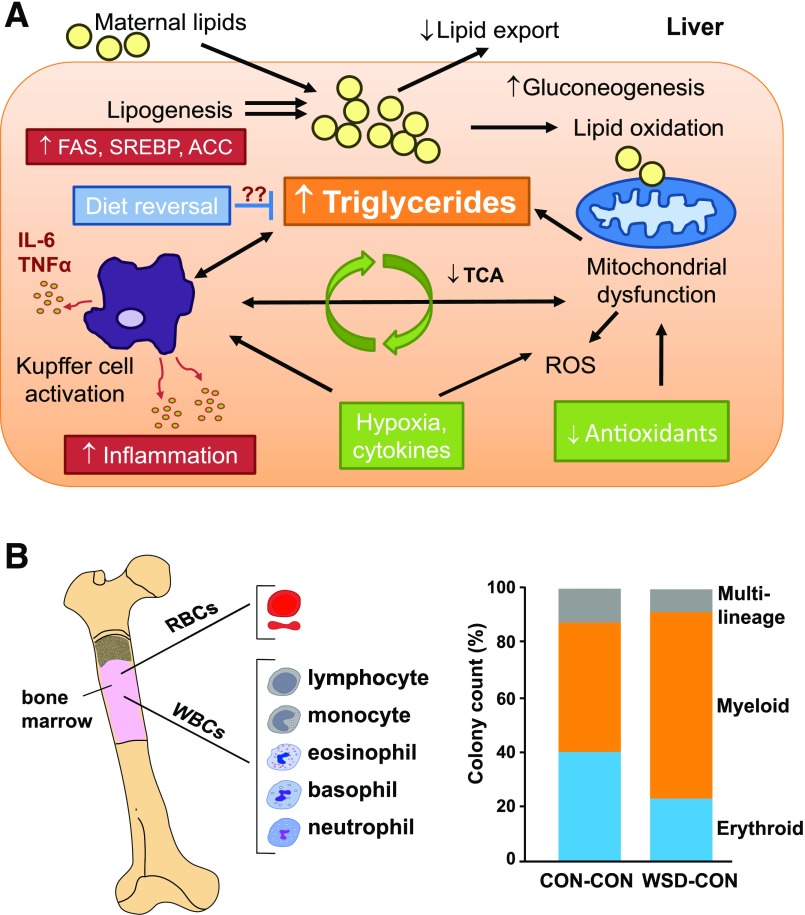
Notably, as in humans, we find that WSD consumption increases adiposity and insulin resistance in some, but not all, NHP dams. One striking example of developmental programming by maternal diet is our finding that all fetuses from mothers fed a WSD during gestation, independent of maternal obesity, had increased intrahepatic fat in the early third trimester (32). Remarkably, when combined with obesity, WSD consumption in NHP during gestation increased fetal hepatic oxidative stress and apoptosis, increased lipogenic gene expression, and altered chromatin structure in the early third trimester (33,34) (Fig. 1A and Table 1). Fetal hepatic metabolism is distinct in that the fetus develops in a low-oxygen environment and, compared with adult liver, the fetal liver has fewer mitochondria and little-to-no gluconeogenesis (35,36). Combined with low antioxidant activity (37), these conditions make the fetus uniquely vulnerable to hepatic oxidative stress and hypoxic injury prior to the development of obesity. In our human studies using MRI/MRS, we showed that infants born to mothers with obesity and GDM had a striking 68% more intrahepatic lipid at 2 weeks of life compared with those from normal-weight (NW) mothers and that maternal BMI correlated with newborn intrahepatocellular lipid storage (38), setting the stage for later development of nonalcoholic fatty liver disease (NAFLD) in offspring. NAFLD is a spectrum of conditions ranging from steatosis to nonalcoholic steatohepatitis (NASH) and a risk of progression to cirrhosis. NAFLD affects approximately 34% of obese children ages 3–18 years worldwide (39), and half have already progressed to the more severe NASH at time of diagnosis (40). Importantly, the severity of adolescent NAFLD and obesity in humans correlates with maternal obesity (41) and birth weight (42), even after adjusting for childhood BMI. In the 1-year-old juvenile offspring of obese, WSD-fed NHP dams switched to a normal diet at weaning, we found innate immune dysfunction and necro-inflammatory changes in the liver (43) (Fig. 1A), along with dysbiosis of the juvenile gut microbiome (44). Furthermore, we found that maternal WSD persistently altered hematopoietic bone marrow stem cell differentiation toward myeloid cells in both fetal and 3-year-old NHP (Fig. 1B) (J.E.F., unpublished results). As this population gives rise to the entire population of circulating blood cells and immune cells that are found in peripheral tissues, these results suggest that WSD exposure in utero drives myeloid cell precursors in fetal life (45) and contributes to unresolved inflammatory responses in obesity (46,47). Similarly, in rodent models we and others found that continued exposure to maternal WSD through postnatal life triggers hepatocyte injury and rapid progression to liver fibrosis by 20 weeks of age (48–50). These data reinforce the concept that multiple “first hits” occur in the liver of infants born to obese mothers prior to development of obesity (51). The disturbing consequences of fetal overnutrition in infants and animal models is clear; however, not all obese children get NAFLD. We know very little about the mechanisms driving NAFLD progression to advanced liver disease (NASH) in children. This is an important challenge as we move forward to more long-term studies.
Figure 1.
Programmed effects in the liver and bone marrow immune cells of NHP offspring exposed to maternal WSD. A: Livers from fetal offspring (early third trimester) of obese, WSD-fed NHP dams demonstrate increases in gluconeogenic genes, oxidative stress, and triglyceride accumulation. Diet reversal in obese mothers produces fetuses with lower lipogenic gene expression and normalized oxidative stress yet persistently higher triglycerides, demonstrating incomplete amelioration of the steatotic phenotype. Global metabolomic profiling of fetal liver and serum revealed decreased tricarboxylic acid (TCA) cycle intermediates, increased amino acid metabolism, and increased gluconeogenesis, indicating increased reliance on amino acid metabolism to meet energy needs in fetuses from obese, WSD-fed mothers. These fetuses have lower arterial oxygenation suggestive of mild hypoxia and exposure to higher plasma cytokine levels (shown in green). Incomplete mitochondrial and lipid oxidation and/or respiratory chain dysfunction, when combined with limited antioxidant activity, increases hepatic oxidative stress and liver injury prior to the development of obesity. Juvenile offspring from WSD-fed dams show innate immune (Kupffer cell) activation and inflammatory cytokine expression (interleukin-6 [IL-6], tumor necrosis factor-α [TNFα]) and a persistent increase in lipogenic gene expression (fatty acid synthase [FAS], sterol regulatory element binding protein [SREBP], acetyl-CoA carboxylase [ACC]) in vivo and in vitro (shown in red), even after weaning to a chow diet. B: Maternal WSD persistently alters bone marrow immune cell proportions in NHP offspring. Bone marrow from 3-year-old juvenile offspring exposed to maternal WSD, then shifted to a chow diet (CON) at weaning, was studied using colony-forming assays of plated bone marrow cells. A significant 34.5% (P < 0.05) relative increase in myeloid cell proliferation was observed at the expense of erythroid (−78.9%) and multilineage (−53.8%) progenitor cell types. RBCs, red blood cells; ROS, reactive oxygen species; WBCs, white blood cells.
Table 1.
Summary of findings from NHP cohorts studying the effects of maternal WSD and obesity on third-trimester fetuses and juvenile offspring
| Findings | First author, year (ref.) |
|---|---|
| Placenta | |
| Increased cytokine production and decreased function; reduced uterine volume blood flow | Frias, 2011 (145) |
| Reduced mRNA expression of AMPKα, plasma membrane fatty acid binding protein, and fatty acid transporter | O’Tierney-Ginn, 2015 (146) |
| Plasma | |
| Increased n-6:n-3 ratio in fetal plasma; increased fetal hepatic apoptosis; lower levels of EPA and DHA in breast milk | Grant, 2011 (34) |
| Impact of maternal diet on the fetal metabolome | Cox, 2009 (147) |
| Maternal diet | |
| WSD and genomic variants resistant to weight gain | Harris, 2016 (148) |
| Impact of maternal resveratrol supplementation on placenta and fetal outcomes | Roberts, 2014 (149) |
| Microbiome | |
| Maternal gut microbial dysbiosis and diminished abundance of Campylobacter in juveniles switched to healthy diet at weaning | Ma, 2014 (44) |
| Modulations in the offspring gut microbiome refractory to postnatal symbiotic supplementation among juveniles | Pace, 2018 (150) |
| Liver | |
| Increased fetal hepatic inflammation, oxidative stress, and triglyceride accumulation | McCurdy, 2009 (32) |
| Altered fetal chromatin structure and disrupted H3 acetylation | Aagaard-Tillery, 2008 (33) |
| Disruption of circadian gene expression in fetal and juvenile liver | Suter, 2011 (151) |
| Decreased fetal SIRT1 histone and protein deacetylase activity | Suter, 2012 (152) |
| Decreased juvenile hepatic innervation; increased apoptosis and inflammation | Grant, 2012 (153) |
| Increased inflammation, triglycerides, and de novo lipid synthesis; dysregulated juvenile hepatic immune system | Thorn, 2014 (43) |
| Skeletal muscle/vascular function | |
| Fetal mitochondrial dysfunction and fatty acid oxidation in skeletal muscle | McCurdy, 2016 (56) |
| Endothelial dysfunction, elevated expression levels of vascular inflammation, and fibrinolytic function in juvenile aorta | Fan, 2013 (154) |
| Pancreas | |
| Reduced fetal/juvenile α-cell mass and increased β-cell:α-cell ratio | Comstock, 2012 (52) |
| Increased inflammatory cytokines and islet-associated macrophages | Nicol, 2013 (155) |
| Reduced fetal/juvenile islet vascularization and impaired sympathetic islet innervation | Pound, 2014 (54) |
| Brain | |
| Abnormalities in the fetal melanocortin system | Grayson, 2010 (60) |
| Perturbations in the fetal serotonergic system; increased anxiety (female infants) and increase aggression (male infants) | Sullivan, 2010 (156) |
| Overconsumption of palatable energy-dense diet in juveniles; reduced dopamine signaling in juveniles | Rivera, 2015 (59) |
| Altered peripheral and central serotonergic system and persistent anxiety and aggressive behaviors | Aagaard, 2016 (157) |
| Increased anxiety in juveniles; modified cortisol stress response and decreased serotonergic immunoreactivity | Thompson, 2017 (57) |
| Disturbances in body weight regulation; impairments in central melanocortin signaling | Sullivan, 2017 (158) |
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
In the pancreas, a study by Comstock et al. (52) in NHP demonstrated that a maternal WSD leads to reduced fetal islet α-cell mass, with no change in β-cell mass, resulting in an increase in the β-cell:α-cell ratio. Furthermore, at 1 year of age, NHP offspring born to WSD-fed dams continue to display a significant reduction in α-cell area (52), indicating that an early insult to the development of the α-cell persists into the juvenile period. α-Cells contain a generating system that produces glucagon-like peptide 1 locally for paracrine actions within the islets, which likely promotes β-cell growth and survival and maintains β-cell mass (53); however, the significance of a reduced α-cell mass on diabetes risk remains to be tested. In NHP fetuses exposed to maternal WSD, impaired islet capillary density and decreased sympathetic islet innervation was observed in the early third trimester (54). Interestingly, reduced vascularity but not sympathetic innervation persisted into juvenile life (54). Switching an obese NHP dam to a healthy diet in the next pregnancy prevented the loss in islet vascularity but not sympathetic innervation in utero (54).
In skeletal muscle, reduced metabolic flexibility is linked to muscle insulin resistance (55), a key predictor for the development of metabolic diseases. McCurdy et al. (56) showed that maternal obesity in WSD-fed NHP leads to reduced mitochondrial content, oxidative capacity, and oxidative phosphorylation efficiency in fetal skeletal muscle. A reduction in maximal oxidative capacity in muscle fibers and in isolated primary fetal myotubes from offspring exposed to a maternal WSD was demonstrated. Interestingly, unlike the liver, fetal skeletal muscle adapts to the obesogenic environment by upregulating fatty acid oxidation and downregulating the ability to oxidize carbohydrates, promoted by increased carnitine palmitoyltransferase 1B and pyruvate dehydrogenase kinase 4 (56). Surprisingly, switching the obese mothers to a normal diet did not improve the decrease in oxidative metabolism in fetal skeletal muscle (56). Similar findings were observed in isolated soleus and gastrocnemius muscles from 1-year-old juveniles (C.E. McCurdy and J.E.F., unpublished results), suggesting that this condition precedes overt obesity in NHP offspring. Notably, mitochondrial dysfunction and altered mitochondrial dynamic and complex proteins in skeletal muscle have been demonstrated across three generations of mice in offspring exposed to F0 maternal high-fat/high-sugar diet (19).
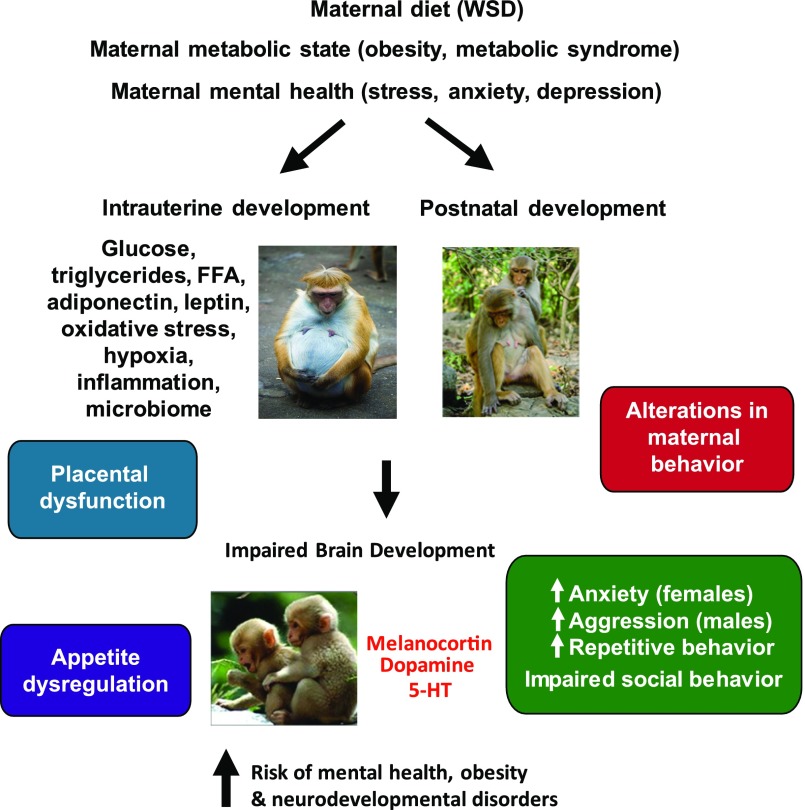
Alterations in brain structure and function are fundamental to understanding the impact of maternal diet and obesity on the next generation. In 1-year-old juvenile NHP exposed to maternal WSD through pregnancy and lactation, decreased brain serotonin and increased anxious, aggressive, and repetitive behaviors were observed, and reduced social behaviors demonstrated some sexual dimorphism (57,58). NHP offspring from WSD-fed dams displayed elevated body weight at 7.5 and 13 months of age, without a change in physical activity at 13 months (59). These offspring preferred a diet high in fat and sugar and demonstrated a reduction in dopamine signaling in the prefrontal cortex (59). NHP fetuses from WSD-fed dams had decreased expression of agouti-related peptide mRNA and protein in the third trimester but increased expression of proopiomelanocortin and melanocortin 4 receptor mRNA in the arcuate nucleus of the hypothalamus (60), supporting a change in appetite regulation. Maternal as well as postnatal WSD exposure reduced the level of agouti-related peptide fibers in the paraventricular nucleus of the hypothalamus in the NHP offspring (60). Exposure to maternal WSD also reduced tryptophan hydroxylase 2 mRNA expression in the dorsal and median raphe and reduced serotonin-positive fibers in the medial prefrontal cortex in 1-year-old juvenile NHP (57). These results suggest that the developmental timing of WSD exposure differentially impacts the melanocortin, dopamine, and serotonin systems and energy balance regulation and behavior. These effects extend past gestation into the juvenile period and suggest that the effects are additive after weaning. These data have important clinical implications as they suggest that, in NHP, exposure to maternal WSD increases the risk for early development of a number of behavioral disorders in offspring, and thus developmental changes in the central serotonergic circuitry could explain the comorbidity of obesity with anxiety, depression, and attention-deficit/hyperactivity disorder in humans (61,62). Together, these observations in NHP demonstrate that maternal-fetal nutritional overload during gestation and the perinatal period is a major driver of multiple neurologic and metabolic disease pathways that set the stage for development of metabolic syndrome (Fig. 2). An overall summary of novel findings in NHP is listed in Table 1.
Figure 2.
Maternal obesity in combination with WSD induces changes in fetal brain, offspring behavior, and the risk for neurocognitive developmental disorders in NHP offspring. Redrawn with permission from Rivera et al. The role of maternal obesity in the risk of neuropsychiatric disorders. Front Neurosci 2015;9:194.
Prenatal Factors That Predict Onset and Worsening of Obesity and Diabetes in Humans
A broad range of umbilical cord blood metabolites and hormones are associated with birth weight and adiposity in human infants (63,64). However, the hypothesis that these substances cause biochemical changes in newborn tissues remains largely untested. Maternal obesity is associated with multiple factors, including glucose, triglycerides, free fatty acids (FFAs), adiponectin, leptin, hypoxia, oxidative stress, inflammation, and the microbiome, that are linked to developmental programming (65). In women who are obese or insulin resistant prior to pregnancy, local inflammation and metabolic stressors in skeletal muscle and adipose tissue are already evident and likely become exacerbated with the increasing insulin resistance of pregnancy (66). Thus, an obese gravida exhibits elevated circulating lipids, glucose, insulin, leptin, and inflammatory cytokines when compared with NW pregnant women. My colleagues have demonstrated that even on a controlled, eucaloric diet, obese pregnant women have higher 24-h patterns of glycemia, fasting triglycerides, FFAs, and insulin resistance than NW women both early and late in gestation (5). These data suggest that obese pregnant women have intrinsic metabolic differences in hepatic and adipose tissue metabolism independent of maternal diet that increase early fuel availability to the placenta and fetus.
The developing fetus is an example of an organism that is metabolically inflexible, defined as having an intrinsically low capacity for lipid oxidative metabolism, and adverse metabolic consequences can result when it is challenged with excess fuels (67). Because the fetus develops in an extremely low-oxygen environment (68) and many of the enzymes for fatty acid oxidation are not highly expressed until late pregnancy, the fetus is particularly vulnerable to excess lipid exposure and oxidative stress early in pregnancy. Further, placental transfer of antioxidant micronutrients and endogenous antioxidant enzymes are limited until late in gestation in preparation for the oxidative stress upon exposure to higher oxygen following labor and birth (37). Activity levels of antioxidant enzymes such as superoxide dismutase and catalase are much lower in fetal tissues relative to adult values (69). As gestation progresses, the increased growth demands of the fetus are met by increases in placental glucose and FFA transfer, both of which are carried or transferred by specific transporters across the placenta. Notably, in the human fetus, limited capacity exists for de novo lipogenesis, and the precursors for fetal fat accretion are primarily supplied transplacentally and consist of maternal substrates derived from lipids rather than from glucose (70,71). However, in mothers with preexisting obesity or diabetes, greater concentrations of fasting maternal lipids and glucose early in pregnancy can have untoward effects on fetal development that create a predisposition for later adiposity and diabetes (72–74). Consistent with the hypothesis that fuels early in pregnancy are key drivers of developmental programming are epidemiologic observations that maternal insulin resistance in the first half of pregnancy is highly predictive of neonatal percent fat and that maternal glycemia, even within the normal range, is associated with increased neonatal adiposity, independent of prepregnancy BMI (75). Some have speculated that infants born to mothers with GDM have already developed insulin resistance in utero, presumably in response to fetal overnutrition (76). However, given that maternal glucose is transported down a concentration gradient to the fetus, elevated insulin and umbilical cord glucose levels, used to calculate HOMA-IR, might simply reflect elevated maternal glucose.
Evidence That Maternal Obesity Programs Adipogenesis and Mitochondrial Inflexibility in Human Umbilical Cord–Derived Mesenchymal Stem Cells
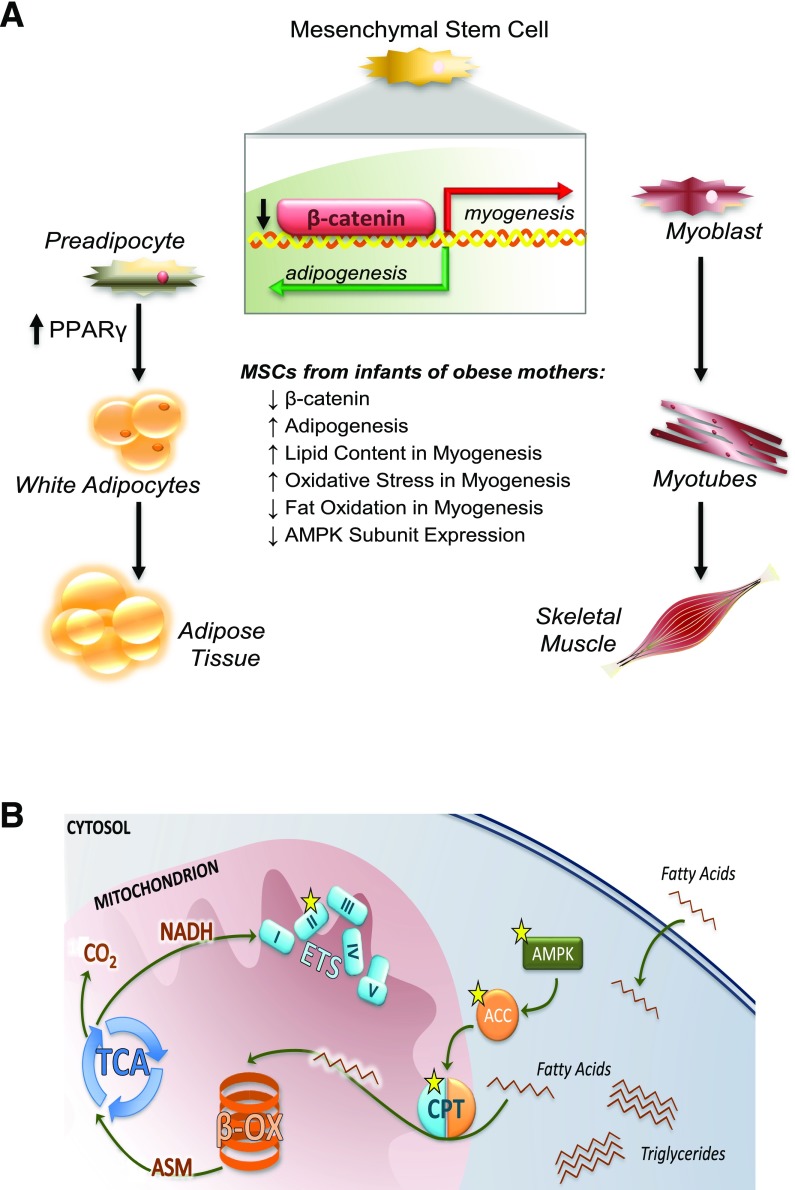
Emerging evidence in human stem cells from the umbilical cords of infants born to obese mothers suggests that mechanisms underlying an increase in infant adiposity might involve stem cell determination factors that increase adipogenesis (77). In utero, fetal muscle and adipose tissue develop from mesenchymal stem cells (MSCs). MSCs are progenitors to adult adipocytes, myocytes, osteocytes, chondrocytes, and hepatic stellate cells and reside in mature tissues where they initiate tissue repair and maintain tissue health (78). Although MSCs found in adult tissues retain cell surface markers and in vitro differentiation capacity consistent with fetal MSCs (79), adult MSCs might accumulate further changes beyond infancy based on postnatal exposures. Importantly, in rats, epigenetic markers in offspring of obese dams were present in adult tissue-resident MSCs, even when cells were subcultured for multiple passages or differentiated in vitro (80). This cell-autonomous phenotype suggests that early fetal programming events at the level of the MSC lineage not only alter the development of fetal tissue but can impact the health of tissues throughout the life span. Using umbilical cord–derived MSCs (uMSCs), we found evidence of greater adipogenesis and increased peroxisome proliferator–activated receptor γ protein in uMSCs from infants born to obese mothers compared with uMSCs from infants born to NW mothers (77) (Fig. 3A). In undifferentiated cells, glycogen synthase kinase 3β activation was higher, favoring β-catenin degradation, which might drive stem cell fate toward adipogenesis in uMSCs from infants born to obese mothers (77). When uMSCs were differentiated into myocytes, we found specific energy-sensing genes, including AMP kinase, carnitine palmitoyltransferase, and acetyl-CoA carboxylase, were downregulated and hypermethylation was identified, corresponding to reduced fatty acid oxidation in uMSCs from infants born to obese mothers (81) (Fig. 3B). Using RNA-Seq and metabolomics, we analyzed specific factors that might inform the changes in gene expression in uMSC-differentiated adipocytes and myocytes from infants born to mothers with normal versus obese prepregnancy BMIs (82). Incomplete β-oxidation, with a compensatory upregulation of ω-oxidation, was found in uMSC myocytes from offspring of obese mothers and was positively correlated with neonatal adiposity at birth and maternal lipid levels (82). The uMSC adipocytes showed more robust changes in transcriptomic analyses than myocytes. In uMSC adipocytes, infant percent fat mass correlated with a downregulation of genes in the AMPK, mTOR, PI3K, and calcium-dependent signaling pathways (82). Interestingly, a generalized upregulation of multiple genes in all respiratory chain complexes (I–V), mitochondrial ribosomal genes, and mitochondrial replication genes was shown in uMSC adipocytes relative to maternal FFA exposure (82). However, specific genes related to mitochondrial biogenesis, mitophagy, and fission/fusion were downregulated only in uMSCs from infants of obese mothers in relation to maternal FFAs, suggesting a lack of quality control in addition to reduced function and biogenesis. We went on to show that these changes in metabolism in uMSCs differentiated into myocytes and adipocytes were correlated with infant postnatal growth by determining adiposity at birth and 5 months of age (83). Strikingly, we found that higher long-chain acylcarnitine concentrations, lipid transport gene expression, and indicators of oxidative stress in uMSC adipocytes were correlated with accelerated adiposity gain (but not increased body weight) in infants from birth to 5 months of age. In uMSC myocytes, we found lower amino acid concentrations, amino acid biosynthesis, and oxidative stress in uMSCs from infants with accelerated adiposity gain, particularly if those infants were born to obese mothers (83). Overall, our findings suggest that cell-specific programmed differences in infant stem cell metabolism, and mitochondria function in particular, correspond with differences in body composition at birth and predict changes in adiposity gain in these infants. Importantly, this uMSC model can be used to identify specific metabolites as important biomarkers in infants, address efficacy of interventional studies in mothers, and tackle major gaps in our understanding of cell-specific biochemical and epigenetic developmental programming of childhood obesity risk in early life.
Figure 3.
A: Human MSCs from the umbilical cord of infants born to obese mothers exhibit greater potential for adipogenesis. In undifferentiated cells, glycogen synthase kinase 3β activation was higher, including less nuclear content of β-catenin and increased PPARγ protein. Greater lipid content was positively correlated with infant percent fat mass. B: Human umbilical cord–derived MSCs from infants born to obese mothers demonstrate decreases in energy-sensing pathways, AMP kinase (AMPK), acetyl-CoA carboxylase (ACC), and carnitine palmitoyltransferase (CPT) in differentiated myocytes. Increased methylation of specific genes (indicated by stars) was found, affecting mitochondrial transport and fatty acid oxidation in infants with increased percent fat mass at birth. ASM, acid-soluble metabolites; β-OX, β-oxidation; ETS, electron transport system. Reprinted with permission from Boyle et al. (81).
New Factors Associated With Developmental Programming: The Maternal/Infant Microbiomes and Legacy Effects on the Immune System
The establishment of the infant gut microbiome during the first days and weeks of life can have lifelong implications for disease risk. How the infant gut microbiome develops is a complex, poorly understood process influenced by maternal modifiers and early-life infant dietary exposures. During and after birth, infants are rapidly colonized by microbes from the mother and surrounding environment, suggesting that variations in the mothers’ microbiota result in variations in their infants’ gut microbes (84). At birth, the intestine of the newborn is filled with contents acquired in utero, which are passed postnatally as meconium. Evidence of intrauterine seeding in the fetal gut exists (85–87), but the role and importance of prenatal microbial colonization is still open to debate (86,88,89). In mice, the maternal microbiota was shown to drive early postnatal innate immune development and to increase the expression of large classes of genes in the newborn intestine, including those involved in metabolism, oxidative stress, and innate immunity (90). In the human neonate, the immature gut is first colonized by aerobes and facultative anaerobes, which are subsequently replaced by strict anaerobes (91). The sequence and timing of the colonization of each of these classes affect innate immune signaling (92,93), endotoxin tolerance (92), and T helper 1 cell immune responses (94).
Disruption of microbiome colonization, including by maternal obesity, during critical developmental windows has been shown to initiate or predispose offspring to a variety of immunologic disorders later in life, such as asthma, allergies, and type 1 diabetes, and to contribute to increased obesity risk and metabolic disease (92,95,96). Cesarean delivery is associated with higher offspring risk of immune and metabolic disorders (97,98) and obesity (99). Antibiotics are commonly prescribed for women undergoing cesarean section within 60 min prior to incision (100). Maternal exposure to antibiotics in the second and third trimesters of pregnancy or offspring’s exposure in early infancy is associated with an approximately 80% increased risk of childhood obesity (101). Perhaps not surprisingly, increased maternal BMI was associated with an altered intestinal microbial community structure of infants’ stool at 2 days, 2 weeks, and up to 2 years of age (96,102,103). In a birth cohort of 935 infants, fecal samples collected at 3.7 months of age had a lesser abundance of two families of Proteobacteria in infants born to overweight or obese versus NW mothers, whereas four bacterial families belonging to Actinobacteria and Firmicutes were more abundant; those infants born to overweight or obese mothers were 3.8 times more likely to become overweight or obese at 1 year of age compared with infants born to NW mothers (104). Evidence that a maternal high-fat diet has persistent effects on the composition of the infant microbiota comes from studies in NHP (44) and estimates of maternal diet composition in humans (105). In NHP, a maternal WSD, independent of obesity status, resulted in the loss of key bacteria in 1-year-old offspring, as well as decreased overall bacterial diversity when compared with control diet–fed offspring (44). This dysbiosis was not fully corrected by weaning offspring from WSD-fed dams onto a control diet at a time when the microbiome is thought to be stabilized (44). Mechanistic studies of disease pathways due to differences in infants’ microbiomes are difficult to perform in humans because of the tremendous variation in environment, nutrition, and maternal factors. Therefore, most of what is currently known about the infant microbiome stems from experiments in animal models. Our recent unpublished results using human infant stool samples from babies born to obese mothers inoculated into germ-free mice suggest that microbial shifts in the stool of 2-week-old infants of obese mothers alter critical functions in hematopoietic bone marrow–derived macrophages and increase gut permeability in germ-free mice. Colonization in germ-free mice with stool from infants born to obese mothers leads to hepatic infiltration of immune cells and susceptibility to obesity and NAFLD in the mice when fed a WSD compared with germ-free mice colonized with stool from infants born to NW mothers (106). This suggests that human infant microbes from babies born to obese mothers can be an initiating factor to inflammation in infancy that precedes childhood obesity.
In mice given antibiotics during pregnancy only in the peripartum period, even brief disruption induced a skewing of the host immune system and resulted in increased susceptibility to inflammatory disease that persisted into adulthood (107,108). On the other hand, a high-fiber/high-acetate diet in pregnant mice, acting on the maternal microbiome, prevented asthma in their adult offspring (109). Rodent studies have also reported a legacy effect of exposure to maternal WSD on postnatal innate immunity (110–112). These results indicate that a mother’s diet can preset immune cell fitness in utero and that age-sensitive contact with commensal microbes is critical for establishing immune tolerance to later environmental exposures (113).
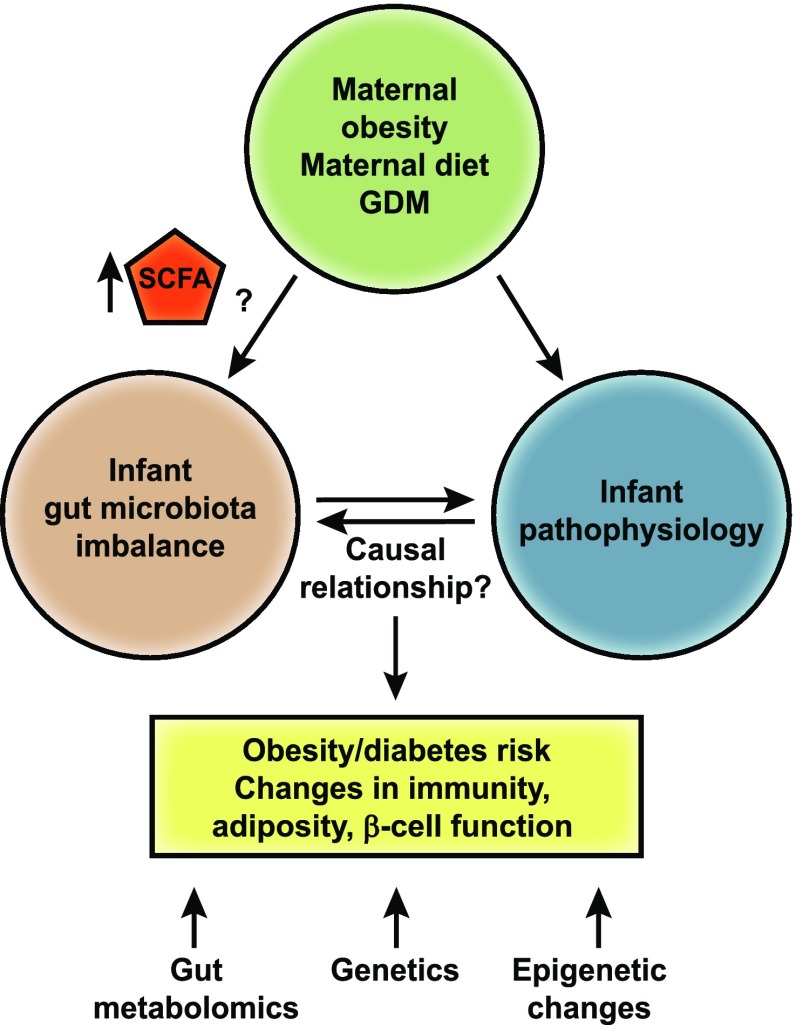
With an estimated 10 million genes encoded in the gut microbiome (114), compared with an estimated 19,000 in humans (115), the highly enhanced metabolic capabilities of microbes have yet to be solved. Microbiomes have the genetic capacity to generate an extensive array of structurally diverse metabolites; therefore, metabolomics is a valuable tool for understanding microbial community function. Very little is known regarding how maternal diet, obesity, or diabetes impacts blood or stool biomarkers derived from the gut of breast-fed or formula-fed infants. Short-chain fatty acids (SCFAs), primarily acetate, propionate, and butyrate, are the key metabolites produced during the catabolism of carbohydrates (e.g., dietary fiber) and proteins (116). Notably, butyrate, propionate, and acetate are elevated in the plasma of obese youth (117) and in the stool of infants born to obese mothers (102). Distinct SCFA receptors are present in gut epithelial cells, adipocytes, myocytes, and immune cells and might increase energy expenditure or gut hormone production, as well as improve insulin sensitivity (118). Recently, it was reported that a high-fiber diet in humans with diabetes was associated with increased SCFA-producing organisms and correlated with elevated glucagon-like peptide 1, a decline in acetylated hemoglobin, and improved blood glucose regulation (119). Butyrate is widely regarded as an important microbial metabolite for colonic mucosal homeostasis (120), and understanding the factors that regulate its production by intestinal microbes has broad-reaching implications. Butyrate acts as a histone deacetylase inhibitor, creating hyporesponsivity in human intestinal monocytes/macrophages (121) and regulatory T cells (122) and resulting in altered innate and adaptive immunity. However, a causal relationship between increased SCFAs, glucose/fat metabolism, and immunity in humans remains speculative. Integrative analyses of microbiomes and untargeted metabolomes in the future will yield a more detailed picture of when and how a deviation in microbial metabolites in infants of obese mothers can pattern immune and metabolic responses and will help us understand the early origins of metabolic and inflammatory diseases (Fig. 4).
Figure 4.
Relationship between maternal obesity, imbalanced gut microbiota, and host pathophysiology. Although maternal obesity is associated with significant alterations in the infant gut microbiome, the functional consequences of the early microbiome on changes in disease pathways have not been investigated. Animal models suggest that gut microbe disruption in early life profoundly alters development of the innate and adaptive immune system and offspring behavior. The increase in SCFA-producing bacteria found in infants born to obese mothers suggests that the initial microbes necessary for immune development and metabolic health are compromised.
Nutritional Countermeasures in Maternal Obesity and GDM: Is Disease Interception Possible?
Components of a typical WSD include excessive amounts of simple sugars, saturated fats, and cholesterol, which are particularly proinflammatory. A proinflammatory diet in pregnancy and early childhood, estimated by diet recall, was associated with development of adiposity in mid-childhood (123). As noted above, our studies in WSD-fed NHP dams showed that switching obese mothers to a healthy diet prior to conception is an effective countermeasure for some, but not all, of the critical pathways that result in dysmetabolism and disease risk in the offspring (Table 1). In transgenic mice, normalizing the high n-6 relative to n-3 polyunsaturated fatty acid ratio in obese dams on WSD prevented inflammation in the placenta and prevented NAFLD and obesity in the wild-type offspring exposed to a high-fat diet (124,125). Likewise, our recent studies demonstrated that the potent dietary antioxidant pyrroloquinoline quinone, found in high concentration in human breast milk (126), when administered to obese dams only during gestation and lactation prevented early microbial dysbiosis, hepatic macrophage accumulation, and NAFLD in adult offspring (50). In humans with GDM, high fasting glucose or high postprandial glucose are major contributors to fetal fat accretion, and when treatment is targeted with repeated glucose testing, fetal overgrowth is mitigated (127), although not normalized (128). A similar target and intervention to attenuate fetal overgrowth in maternal obesity without GDM in humans is sorely needed. Obesity in pregnancy accounts for the largest number of newborns with increased adiposity, a strong risk factor for childhood obesity. Despite over 40 randomized controlled trials implementing diet, physical activity, metformin, or probiotics in overweight and obese pregnancies, no targeted intervention effectively prevents fetal overgrowth (129). Our recently published study (130), along with other data (131,132), strongly support that maternal triglycerides are a clinically unrecognized but important substrate for fetal fat accretion. Specifically, we have shown that a 1- or 2-h postprandial triglyceride level (at 14–16 weeks of gestation) is highly correlated with newborn fat mass, accounting for 50% of newborn fat mass variability (130). Maternal lipids have not specifically been targeted for intervention. We are conducting the first randomized controlled trial to challenge the conventional low-carbohydrate, higher-fat diet prescribed for women with GDM with a higher–complex carbohydrate, lower-fat, isocaloric diet (termed CHOICE—Choosing Healthy Options in Carbohydrate Energy). All meals are provided from the time of diagnosis to delivery, maternal metabolism is closely monitored, and short- and long-term outcomes are measured in the offspring. Early pilot data showed that isocalorically reducing dietary fat and increasing complex carbohydrates resulted in no difference in gestational weight gain between diets and 24-h glycemic profiles that were within current treatment targets for both diets (133). However, the CHOICE diet, which was lower in fat, decreased gene expression of inflammatory markers in adipose tissue in women with GDM, resulting in a trend for reduced newborn adiposity (133,134).
Human milk is a dynamic and complex substance that delivers a milieu of hormones and other bioactive components that supports infant development and optimizes health but differs in mothers with increased BMI (135). Exclusive breastfeeding imparts a modest protective effect against later obesity and type 2 diabetes relative to formula (136,137). However, the biologic mechanisms for protection remain controversial and might differ depending on maternal overweight status, maternal insulin resistance, maternal dietary intake, and length of exposure. Bioactive components in human milk—for example, insulin—can contribute to the regulation of fat mass and the early infant microbiome in infants of NW versus overweight mothers (102,135). We demonstrated that a high ratio of n-6 relative to n-3 polyunsaturated fatty acids in human breast milk correlates with high adiposity gain in 4-month-old infants (138). Much more must be learned about the bioactive components in human milk, individually and in combination, that shape postnatal development of energy-sensing pathways, particularly in the infant of a mother with obesity or diabetes.
Conclusions and Future Directions
We are now facing major challenges in tackling the alarming increase and rising epidemic of childhood obesity and its consequences around the world. The considerable risk of progression to advanced diseases including NAFLD, diabetes, cardiovascular disease, and mental health disorders and the lack of pharmacologic approaches and suitable biomarkers for the early identification of disease risk are important roadblocks in child health. In many instances, the factors that trigger disease might not be the same as those that influence its progression. For example, while epigenetics might not be a cause of disease, it might be a marker or “passenger” important for identifying disease risk. Environmental exposures, particularly WSD, disrupt the equilibrium in susceptible individuals to tip the balance toward obesity and diabetes with a second hit that occurs after primary exposures in utero, potentiated by genetic susceptibility. Importantly, these “hits” occur throughout the body, including the placenta, and impact bone marrow and gut development, brain, muscle, liver, pancreas, and adipose tissue progenitors, as well as the nascent immune system. These effects are cumulative during critical periods and occur on a continuum rather than operating on a threshold effect. Changes that occur in the fetus during pregnancy or in the infant during lactation, in combination with genetics, likely cause exacerbation of a preexisting susceptibility or development of new pathways that impact postnatal metabolic risk.
Currently, behavioral changes aimed at limiting gestational weight gain or improving dietary choices in mothers with obesity or GDM have been found to have very little to no effect on infant or childhood outcomes, despite improved maternal metabolic control (139–141). Most of these trials have started later in pregnancy, suggesting that we need to rethink whether developmental programming is occurring much earlier and whether interventions focusing on diets in pregnant humans with obesity or GDM are missing the critical mix of nutrients. In our NHP model of maternal obesity, the offspring complications are not readily modifiable with an improved diet postweaning; however, mechanistic studies in this model suggest that returning obese mothers to a healthy diet prior to conception can modify many of the adverse consequences of an obese pregnancy. No new drugs have been introduced in the past 20 years for treatment of pregnancy disorders. Indeed, metformin use in pregnancy has been challenged recently based on potential negative longer-term outcomes (142). Although new mechanisms and pathways to developmental programming have been identified in animal models, the pathogenesis of these diseases is incompletely understood. We need better nutritional data and metabolic targets that can be translated in controlled studies of human pregnancy with longitudinal outcome data across the life span. The National Institutes of Health has launched a nationwide longitudinal cohort initiative of 83 observational cohorts, with an anticipated combined sample size exceeding 50,000 children from diverse populations across the U.S., termed Environmental Influences on Childhood Health Outcomes (ECHO), with the goal of identifying multiple exposure outcomes from pregnancy through childhood (143). This study is expected to generate massive amounts of data (e.g., genomics, metagenomics/transcriptomics, and metabolomics) and demands that biologists work with informatics scientists to generate meaningful biologic insights from these reams of data. A better understanding of clinical biomarkers (causal or noncausal) that can be revealed as “healthy” or unhealthy in infants and children will lead to improved insight for when and how to design sounder prevention strategies. As argued in a National Academy of Medicine initiative (144), preemptive intervention before disease begins not only will have a tremendous impact on lifelong health but is also the most cost-effective approach.
Article Information
Acknowledgments. The author is grateful to his valuable colleagues and collaborators, especially Drs. Linda A. Barbour and Teri L. Hernandez, for providing insightful and interactive discussions and recommendations in preparation of this review, to Rachel C. Janssen for editorial assistance and figure preparation, and to Drs. Kristen E. Boyle and Stephanie R. Wesolowski for figure designs (all from the University of Colorado Anschutz Medical Campus). He offers sincere apologies to those whose work was not cited in this review due to limitations in space and reference requirements.
Funding. J.E.F. is supported by National Institutes of Health grants R24DK090964, P30DK048520 (Nutrition Obesity Research Centers program), R01DK101659, 1UG3OD023248, and R01HL109517.
Duality of Interest. J.E.F. is a consultant to the scientific advisory board of Janssen Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
References
- 1.Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med 2014;370:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afshin A, Forouzanfar MH, Reitsma MB, et al.; GBD 2015 Obesity Collaborators . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam WH, Ma RCW, Ozaki R, et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care 2017;40:679–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes K, Bellis MA, Hardcastle KA, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health 2017;2:e356–e366 [DOI] [PubMed] [Google Scholar]
- 5.Harmon KA, Gerard L, Jensen DR, et al. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care 2011;34:2198–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro AL, Schmiege SJ, Brinton JT, et al. Testing the fuel-mediated hypothesis: maternal insulin resistance and glucose mediate the association between maternal and neonatal adiposity, the Healthy Start study. Diabetologia 2015;58:937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker DJ. The origins of the developmental origins theory. J Intern Med 2007;261:412–417 [DOI] [PubMed] [Google Scholar]
- 8.Locke AE, Kahali B, Berndt SI, et al.; LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium . Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiken CE, Ozanne SE. Transgenerational developmental programming. Hum Reprod Update 2014;20:63–75 [DOI] [PubMed] [Google Scholar]
- 10.Bansal A, Simmons RA. Epigenetics and developmental origins of diabetes: correlation or causation? Am J Physiol Endocrinol Metab 2018;315:E15–E28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Do C, Lang CF, Lin J, et al. Mechanisms and disease associations of haplotype-dependent allele-specific DNA methylation. Am J Hum Genet 2016;98:934–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharp GC, Salas LA, Monnereau C, et al. Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the Pregnancy and Childhood Epigenetics (PACE) consortium. Hum Mol Genet 2017;26:4067–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barua S, Kuizon S, Brown WT, Junaid MA. DNA methylation profiling at single-base resolution reveals gestational folic acid supplementation influences the epigenome of mouse offspring cerebellum. Front Neurosci 2016;10:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seki Y, Suzuki M, Guo X, et al. In utero exposure to a high-fat diet programs hepatic hypermethylation and gene dysregulation and development of metabolic syndrome in male mice. Endocrinology 2017;158:2860–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suter MA, Ma J, Vuguin PM, et al. In utero exposure to a maternal high-fat diet alters the epigenetic histone code in a murine model. Am J Obstet Gynecol 2014;210:463.e1–463.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng SF, Lin RC, Laybutt DR, Barres R, Owens JA, Morris MJ. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 2010;467:963–966 [DOI] [PubMed] [Google Scholar]
- 17.de Castro Barbosa T, Ingerslev LR, Alm PS, et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol Metab 2015;5:184–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huypens P, Sass S, Wu M, et al. Epigenetic germline inheritance of diet-induced obesity and insulin resistance. Nat Genet 2016;48:497–499 [DOI] [PubMed] [Google Scholar]
- 19.Fullston T, Ohlsson Teague EM, Palmer NO, et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J 2013;27:4226–4243 [DOI] [PubMed] [Google Scholar]
- 20.Donkin I, Versteyhe S, Ingerslev LR, et al. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab 2016;23:369–378 [DOI] [PubMed] [Google Scholar]
- 21.Smith GD, Ebrahim S. Data dredging, bias, or confounding. BMJ 2002;325:1437–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol 2008;25:2–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang WW, Dietmann S, Irie N, et al. A unique gene regulatory network resets the human germline epigenome for development. Cell 2015;161:1453–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siklenka K, Erkek S, Godmann M, et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science 2015;350:aab2006. [DOI] [PubMed] [Google Scholar]
- 25.Saben JL, Boudoures AL, Asghar Z, et al. Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Reports 2016;16:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waterland RA. Epigenetic mechanisms affecting regulation of energy balance: many questions, few answers. Annu Rev Nutr 2014;34:337–355 [DOI] [PubMed] [Google Scholar]
- 27.Kühnen P, Handke D, Waterland RA, et al. Interindividual variation in DNA methylation at a putative POMC metastable epiallele is associated with obesity. Cell Metab 2016;24:502–509 [DOI] [PubMed] [Google Scholar]
- 28.Jellyman JK, Martin-Gronert MS, Cripps RL, et al. Effects of cortisol and dexamethasone on insulin signalling pathways in skeletal muscle of the ovine fetus during late gestation. PLoS One 2012;7:e52363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carmody JS, Wan P, Accili D, Zeltser LM, Leibel RL. Respective contributions of maternal insulin resistance and diet to metabolic and hypothalamic phenotypes of progeny. Obesity (Silver Spring) 2011;19:492–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reis T. Effects of synthetic diets enriched in specific nutrients on Drosophila development, body fat, and lifespan. PLoS One 2016;11:e0146758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baugh LR. To grow or not to grow: nutritional control of development during Caenorhabditis elegans L1 arrest. Genetics 2013;194:539–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCurdy CE, Bishop JM, Williams SM, et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 2009;119:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aagaard-Tillery KM, Grove K, Bishop J, et al. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol 2008;41:91–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant WF, Gillingham MB, Batra AK, et al. Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates. PLoS One 2011;6:e17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalhan S, Parimi P. Gluconeogenesis in the fetus and neonate. Semin Perinatol 2000;24:94–106 [DOI] [PubMed] [Google Scholar]
- 36.Kim SR, Kubo T, Kuroda Y, et al. Comparative metabolome analysis of cultured fetal and adult hepatocytes in humans. J Toxicol Sci 2014;39:717–723 [DOI] [PubMed] [Google Scholar]
- 37.Qanungo S, Mukherjea M. Ontogenic profile of some antioxidants and lipid peroxidation in human placental and fetal tissues. Mol Cell Biochem 2000;215:11–19 [DOI] [PubMed] [Google Scholar]
- 38.Brumbaugh DE, Tearse P, Cree-Green M, et al. Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J Pediatr 2013;162:930–936.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PLoS One 2015;10:e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goyal NP, Schwimmer JB. The progression and natural history of pediatric nonalcoholic fatty liver disease. Clin Liver Dis 2016;20:325–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayonrinde OT, Oddy WH, Adams LA, et al. Infant nutrition and maternal obesity influence the risk of non-alcoholic fatty liver disease in adolescents. J Hepatol 2017;67:568–576 [DOI] [PubMed] [Google Scholar]
- 42.Newton KP, Feldman HS, Chambers CD, et al.; Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) . Low and high birth weights are risk factors for nonalcoholic fatty liver disease in children. J Pediatr 2017;187:141–146.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorn SR, Baquero KC, Newsom SA, et al. Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates. Diabetes 2014;63:2702–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma J, Prince AL, Bader D, et al. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun 2014;5:3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamimae-Lanning AN, Krasnow SM, Goloviznina NA, et al. Maternal high-fat diet and obesity compromise fetal hematopoiesis. Mol Metab 2014;4:25–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray PJ. Obesity corrupts myelopoiesis. Cell Metab 2014;19:735–736 [DOI] [PubMed] [Google Scholar]
- 47.Singer K, DelProposto J, Morris DL, et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab 2014;3:664–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruce KD, Cagampang FR, Argenton M, et al. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology 2009;50:1796–1808 [DOI] [PubMed] [Google Scholar]
- 49.Bayol SA, Simbi BH, Fowkes RC, Stickland NC. A maternal “junk food” diet in pregnancy and lactation promotes nonalcoholic fatty liver disease in rat offspring. Endocrinology 2010;151:1451–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman JE, Dobrinskikh E, Alfonso-Garcia A, et al. Pyrroloquinoline quinone prevents developmental programming of microbial dysbiosis and macrophage polarization to attenuate liver fibrosis in offspring of obese mice. Hepatol Commun 2018;2:313–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brumbaugh DE, Friedman JE. Developmental origins of nonalcoholic fatty liver disease. Pediatr Res 2014;75:140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Comstock SM, Pound LD, Bishop JM, et al. High-fat diet consumption during pregnancy and the early post-natal period leads to decreased α cell plasticity in the nonhuman primate. Mol Metab 2012;2:10–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stanojevic V, Habener JF. Evolving function and potential of pancreatic alpha cells. Best Pract Res Clin Endocrinol Metab 2015;29:859–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pound LD, Comstock SM, Grove KL. Consumption of a Western-style diet during pregnancy impairs offspring islet vascularization in a Japanese macaque model. Am J Physiol Endocrinol Metab 2014;307:E115–E123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muoio DM, Neufer PD. Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab 2012;15:595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCurdy CE, Schenk S, Hetrick B, et al. Maternal obesity reduces oxidative capacity in fetal skeletal muscle of Japanese macaques. JCI Insight 2016;1:e86612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson JR, Valleau JC, Barling AN, et al. Exposure to a high-fat diet during early development programs behavior and impairs the central serotonergic system in juvenile non-human primates. Front Endocrinol (Lausanne) 2017;8:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sullivan EL, Nousen EK, Chamlou KA, Grove KL. The impact of maternal high-fat diet consumption on neural development and behavior of offspring. Int J Obes Suppl 2012;2:S7–S13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rivera HM, Kievit P, Kirigiti MA, et al. Maternal high-fat diet and obesity impact palatable food intake and dopamine signaling in nonhuman primate offspring. Obesity (Silver Spring) 2015;23:2157–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 2010;151:1622–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics 2008;122:e1–e6 [DOI] [PubMed] [Google Scholar]
- 62.Rofey DL, Kolko RP, Iosif AM, et al. A longitudinal study of childhood depression and anxiety in relation to weight gain. Child Psychiatry Hum Dev 2009;40:517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lowe WL Jr, Bain JR, Nodzenski M, et al.; HAPO Study Cooperative Research Group . Maternal BMI and glycemia impact the fetal metabolome. Diabetes Care 2017;40:902–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perng W, Rifas-Shiman SL, McCulloch S, et al. Associations of cord blood metabolites with perinatal characteristics, newborn anthropometry, and cord blood hormones in project viva. Metabolism 2017;76:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wesolowski SR, Kasmi KC, Jonscher KR, Friedman JE. Developmental origins of NAFLD: a womb with a clue. Nat Rev Gastroenterol Hepatol 2017;14:81–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 2007;30(Suppl. 2):S112–S119 [DOI] [PubMed] [Google Scholar]
- 67.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 2000;49:677–683 [DOI] [PubMed] [Google Scholar]
- 68.Jauniaux E, Watson A, Burton G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am J Obstet Gynecol 2001;184:998–1003 [DOI] [PubMed] [Google Scholar]
- 69.Mackler B, Person RE, Nguyen TD, Fantel AG. Studies of the cellular distribution of superoxide dismutases in adult and fetal rat tissues. Free Radic Res 1998;28:125–129 [DOI] [PubMed] [Google Scholar]
- 70.Haggarty P, Allstaff S, Hoad G, Ashton J, Abramovich DR. Placental nutrient transfer capacity and fetal growth. Placenta 2002;23:86–92 [DOI] [PubMed] [Google Scholar]
- 71.Herrera E, Amusquivar E, López-Soldado I, Ortega H. Maternal lipid metabolism and placental lipid transfer. Horm Res 2006;65(Suppl. 3):59–64 [DOI] [PubMed] [Google Scholar]
- 72.Barrett HL, Dekker Nitert M, McIntyre HD, Callaway LK. Normalizing metabolism in diabetic pregnancy: is it time to target lipids? Diabetes Care 2014;37:1484–1493 [DOI] [PubMed] [Google Scholar]
- 73.Dutton H, Borengasser SJ, Gaudet LM, Barbour LA, Keely EJ. Obesity in pregnancy: optimizing outcomes for mom and baby. Med Clin North Am 2018;102:87–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Higgins L, Greenwood SL, Wareing M, Sibley CP, Mills TA. Obesity and the placenta: a consideration of nutrient exchange mechanisms in relation to aberrant fetal growth. Placenta 2011;32:1–7 [DOI] [PubMed] [Google Scholar]
- 75.Crume TL, Shapiro AL, Brinton JT, et al. Maternal fuels and metabolic measures during pregnancy and neonatal body composition: the Healthy Start Study. J Clin Endocrinol Metab 2015;100:1672–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009;32:1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boyle KE, Patinkin ZW, Shapiro ALB, Baker PR II, Dabelea D, Friedman JE. Mesenchymal stem cells from infants born to obese mothers exhibit greater potential for adipogenesis: the Healthy Start BabyBUMP Project. Diabetes 2016;65:647–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 2006;119:2204–2213 [DOI] [PubMed] [Google Scholar]
- 79.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant 2011;20:5–14 [DOI] [PubMed] [Google Scholar]
- 80.Borengasser SJ, Zhong Y, Kang P, et al. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. Endocrinology 2013;154:4113–4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boyle KE, Patinkin ZW, Shapiro ALB, et al. Maternal obesity alters fatty acid oxidation, AMPK activity, and associated DNA methylation in mesenchymal stem cells from human infants. Mol Metab 2017;6:1503–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baker PR II, Patinkin Z, Shapiro ALB, et al. Maternal obesity and increased neonatal adiposity correspond with altered infant mesenchymal stem cell metabolism. JCI Insight 2017;2:e94200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baker PR II, Patinkin ZW, Shapiro ALB, et al. Altered gene expression and metabolism in fetal umbilical cord mesenchymal stem cells correspond with differences in 5-month-old infant adiposity gain. Sci Rep 2017;7:18095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010;107:11971–11975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med 2014;6:237ra265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perez-Muñoz ME, Arrieta MC, Ramer-Tait AE, Walter J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 2017;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep 2016;6:23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiménez E, Marín ML, Martín R, et al. Is meconium from healthy newborns actually sterile? Res Microbiol 2008;159:187–193 [DOI] [PubMed] [Google Scholar]
- 89.Walker RW, Clemente JC, Peter I, Loos RJF. The prenatal gut microbiome: are we colonized with bacteria in utero? Pediatr Obes 2017;12(Suppl. 1):3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, et al. The maternal microbiota drives early postnatal innate immune development. Science 2016;351:1296–1302 [DOI] [PubMed] [Google Scholar]
- 91.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol 2007;5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vatanen T, Kostic AD, d’Hennezel E, et al.; DIABIMMUNE Study Group . Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 2016;165:842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nash MJ, Frank DN, Friedman JE. Early microbes modify immune system development and metabolic homeostasis—the “Restaurant” hypothesis revisited. Front Endocrinol (Lausanne) 2017;8:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014;63:559–566 [DOI] [PubMed] [Google Scholar]
- 95.Ling Z, Li Z, Liu X, et al. Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol 2014;80:2546–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bokulich NA, Chung J, Battaglia T, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med 2016;8:343ra82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mueller NT, Mao G, Bennet WL, et al. Does vaginal delivery mitigate or strengthen the intergenerational association of overweight and obesity? Findings from the Boston Birth Cohort. Int J Obes 2017;41:497–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rusconi F, Zugna D, Annesi-Maesano I, et al. Mode of delivery and asthma at school age in 9 European birth cohorts. Am J Epidemiol 2017;185:465–473 [DOI] [PubMed] [Google Scholar]
- 99.Yuan C, Gaskins AJ, Blaine AI, et al. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr 2016;170:e162385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.American College of Obstetricians and Gynecologists ACOG Practice Bulletin No. 120: Use of prophylactic antibiotics in labor and delivery. Obstet Gynecol 2011;117:1472–1483 [DOI] [PubMed] [Google Scholar]
- 101.Mueller NT, Whyatt R, Hoepner L, et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes 2015;39:665–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lemas DJ, Young BE, Baker PR II, et al. Alterations in human milk leptin and insulin are associated with early changes in the infant intestinal microbiome. Am J Clin Nutr 2016;103:1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Galley JD, Bailey M, Kamp Dush C, Schoppe-Sullivan S, Christian LM. Maternal obesity is associated with alterations in the gut microbiome in toddlers. PLoS One 2014;9:e113026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tun HM, Bridgman SL, Chari R, et al.; Canadian Healthy Infant Longitudinal Development (CHILD) Study Investigators . Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr 2018;172:368–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chu DM, Antony KM, Ma J, et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med 2016;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soderborg TK, Clark SE, Mulligan CE, et al. Maternal obesity confers metabolic disease and NAFLD promoting properties to infant gut microbiome. Nat Commun. In press [Google Scholar]
- 107.Miyoshi J, Bobe AM, Miyoshi S, et al. Peripartum antibiotics promote gut dysbiosis, loss of immune tolerance, and inflammatory bowel disease in genetically prone offspring. Cell Reports 2017;20:491–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schulfer AF, Battaglia T, Alvarez Y, et al. Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat Microbiol 2018;3:234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thorburn AN, McKenzie CI, Shen S, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 2015;6:7320. [DOI] [PubMed] [Google Scholar]
- 110.Odaka Y, Nakano M, Tanaka T, et al. The influence of a high-fat dietary environment in the fetal period on postnatal metabolic and immune function. Obesity (Silver Spring) 2010;18:1688–1694 [DOI] [PubMed] [Google Scholar]
- 111.Myles IA, Fontecilla NM, Janelsins BM, Vithayathil PJ, Segre JA, Datta SK. Parental dietary fat intake alters offspring microbiome and immunity. J Immunol 2013;191:3200–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van de Pavert SA, Ferreira M, Domingues RG, et al. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 2014;508:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012;336:489–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li J, Jia H, Cai X, et al.; MetaHIT Consortium; MetaHIT Consortium . An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol 2014;32:834–841 [DOI] [PubMed] [Google Scholar]
- 115.Ezkurdia I, Juan D, Rodriguez JM, et al. Multiple evidence strands suggest that there may be as few as 19,000 human protein-coding genes. Hum Mol Genet 2014;23:5866–5878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016;7:189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goffredo M, Mass K, Parks EJ, et al. Role of gut microbiota and short chain fatty acids in modulating energy harvest and fat partitioning in youth. J Clin Endocrinol Metab 2016;101:4367–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 2015;11:577–591 [DOI] [PubMed] [Google Scholar]
- 119.Zhao L, Zhang F, Ding X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018;359:1151–1156 [DOI] [PubMed] [Google Scholar]
- 120.Scheppach W, Luehrs H, Menzel T. Beneficial health effects of low-digestible carbohydrate consumption. Br J Nutr 2001;85(Suppl. 1):S23–S30 [DOI] [PubMed] [Google Scholar]
- 121.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A 2014;111:2247–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013;504:446–450 [DOI] [PubMed] [Google Scholar]
- 123.Sen S, Rifas-Shiman SL, Shivappa N, et al. Associations of prenatal and early life dietary inflammatory potential with childhood adiposity and cardiometabolic risk in Project Viva. Pediatr Obes 2018;13:292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heerwagen MJ, Stewart MS, de la Houssaye BA, Janssen RC, Friedman JE. Transgenic increase in n-3/n-6 fatty acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS One 2013;8:e67791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rudolph MC, Jackman MR, Presby DM, et al. Low neonatal plasma n-6/n-3 PUFA ratios regulate offspring adipogenic potential and condition adult obesity resistance. Diabetes 2018;67:651–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mitchell AE, Jones AD, Mercer RS, Rucker RB. Characterization of pyrroloquinoline quinone amino acid derivatives by electrospray ionization mass spectrometry and detection in human milk. Anal Biochem 1999;269:317–325 [DOI] [PubMed] [Google Scholar]
- 127.American Diabetes Association 13. Management of diabetes in pregnancy: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S137–S143 [DOI] [PubMed] [Google Scholar]
- 128.Hernandez TL. Glycemic targets in pregnancies affected by diabetes: historical perspective and future directions. Curr Diab Rep 2015;15:565. [DOI] [PubMed] [Google Scholar]
- 129.Yeo S, Walker JS, Caughey MC, Ferraro AM, Asafu-Adjei JK. What characteristics of nutrition and physical activity interventions are key to effectively reducing weight gain in obese or overweight pregnant women? A systematic review and meta-analysis. Obes Rev 2017;18:385–399 [DOI] [PubMed] [Google Scholar]
- 130.Barbour LA, Farabi SS, Friedman JE, et al. Postprandial triglycerides predict newborn fat more strongly than glucose in women with obesity in early pregnancy. Obesity (Silver Spring) 2018;26:1347–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008;31:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vrijkotte TG, Algera SJ, Brouwer IA, van Eijsden M, Twickler MB. Maternal triglyceride levels during early pregnancy are associated with birth weight and postnatal growth. J Pediatr 2011;159:736–742.e1 [DOI] [PubMed]
- 133.Hernandez TL, Van Pelt RE, Anderson MA, et al. A higher-complex carbohydrate diet in gestational diabetes mellitus achieves glucose targets and lowers postprandial lipids: a randomized crossover study. Diabetes Care 2014;37:1254–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hernandez TL, Van Pelt RE, Anderson MA, et al. Women with gestational diabetes randomized to a higher–complex carbohydrate/low-fat diet manifest lower adipose tissue insulin resistance, inflammation, glucose, and free fatty acids: a pilot study. Diabetes Care 2016;39:39–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Young BE, Patinkin Z, Palmer C, et al. Human milk insulin is related to maternal plasma insulin and BMI: but other components of human milk do not differ by BMI. Eur J Clin Nutr 2017;71:1094–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, Hu FB, Colditz GA, Gillman MW. Breast-feeding and risk for childhood obesity: does maternal diabetes or obesity status matter? Diabetes Care 2006;29:2231–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr 2006;84:1043–1054 [DOI] [PubMed] [Google Scholar]
- 138.Rudolph MC, Young BE, Lemas DJ, et al. Early infant adipose deposition is positively associated with the n-6 to n-3 fatty acid ratio in human milk independent of maternal BMI. Int J Obes 2017;41:510–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Flynn AC, Dalrymple K, Barr S, et al.; i-WIP (International Weight Management in Pregnancy) Collaborative Group . Dietary interventions in overweight and obese pregnant women: a systematic review of the content, delivery, and outcomes of randomized controlled trials. Nutr Rev 2016;74:312–328 [DOI] [PubMed] [Google Scholar]
- 140.Rogozińska E, Chamillard M, Hitman GA, Khan KS, Thangaratinam S. Nutritional manipulation for the primary prevention of gestational diabetes mellitus: a meta-analysis of randomised studies. PLoS One 2015;10:e0115526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Silva-Zolezzi I, Samuel TM, Spieldenner J. Maternal nutrition: opportunities in the prevention of gestational diabetes. Nutr Rev 2017;75(Suppl. 1):32–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hanem LGE, Stridsklev S, Júlíusson PB, et al. Metformin use in PCOS pregnancies increases the risk of offspring overweight at 4 years of age: follow-up of two RCTs. J Clin Endocrinol Metab 2018;103:1612–1621 [DOI] [PubMed] [Google Scholar]
- 143.Gillman MW, Blaisdell CJ. Environmental influences on Child Health Outcomes, a research program of the National Institutes of Health. Curr Opin Pediatr 2018;30:260–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dzau VJ, McClellan MB, McGinnis JM, et al. Vital directions for health and health care: priorities from a National Academy of Medicine initiative. JAMA 2017;317:1461–1470 [DOI] [PubMed] [Google Scholar]
- 145.Frias AE, Morgan TK, Evans AE, et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 2011;152:2456–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.O’Tierney-Ginn P, Roberts V, Gillingham M, et al. Influence of high fat diet and resveratrol supplementation on placental fatty acid uptake in the Japanese macaque. Placenta 2015;36:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cox J, Williams S, Grove K, Lane RH, Aagaard-Tillery KM. A maternal high-fat diet is accompanied by alterations in the fetal primate metabolome. Am J Obstet Gynecol 2009;201:281.e1–281.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Harris RA, Alcott CE, Sullivan EL, et al. Genomic variants associated with resistance to high fat diet induced obesity in a primate model. Sci Rep 2016;6:36123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Roberts VH, Pound LD, Thorn SR, et al. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J 2014;28:2466–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pace RM, Prince AL, Ma J, et al. Modulations in the offspring gut microbiome are refractory to postnatal synbiotic supplementation among juvenile primates. BMC Microbiol 2018;18:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Suter M, Bocock P, Showalter L, et al. Epigenomics: maternal high-fat diet exposure in utero disrupts peripheral circadian gene expression in nonhuman primates. FASEB J 2011;25:714–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Suter MA, Chen A, Burdine MS, et al. A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates. FASEB J 2012;26:5106–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Grant WF, Nicol LE, Thorn SR, Grove KL, Friedman JE, Marks DL. Perinatal exposure to a high-fat diet is associated with reduced hepatic sympathetic innervation in one-year old male Japanese macaques. PLoS One 2012;7:e48119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fan L, Lindsley SR, Comstock SM, et al. Maternal high-fat diet impacts endothelial function in nonhuman primate offspring. Int J Obes 2013;37:254–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nicol LE, Grant WF, Comstock SM, et al. Pancreatic inflammation and increased islet macrophages in insulin-resistant juvenile primates. J Endocrinol 2013;217:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sullivan EL, Grayson B, Takahashi D, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci 2010;30:3826–3830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aagaard K, Prince A, Oliva G, et al. 70: A high fat maternal diet contributes to microbiome-driven regulation of the offspring gut-brain axis & behavior in primates. Am J Obstet Gynecol 2016;214Suppl.:S50 [Google Scholar]
- 158.Sullivan EL, Rivera HM, True CA, et al. Maternal and postnatal high-fat diet consumption programs energy balance and hypothalamic melanocortin signaling in nonhuman primate offspring. Am J Physiol Regul Integr Comp Physiol 2017;313:R169–R179 [DOI] [PMC free article] [PubMed] [Google Scholar]