Abstract
Since the original discovery of stem cells, a new era of promising results has emerged in the clinical application of stem cells for the treatment of several important diseases, including cancer and autoimmune diseases. The plentiful research on stem cells during the past decades has provided significant information on the developmental, morphological, and physiological processes that govern tissue and organ formation, maintenance, and regeneration; cellular differentiation; molecular processes; and tissue homeostasis. In this review, we present the history of the use of stem cells in different clinical applications. Furthermore, we discuss the various therapeutic options for stem cells in cancer, followed by the role of stem cells in the treatment of autoimmune disorders. Additionally, we highlight the risks of and obstacles to the application of stem cells in clinical practice. Ultimately, we show future perspectives in stem cell use, with an aim to improve the clinical usefulness of stem cells.
Keywords: autoimmune disease, cancer, clinical advances, stem cells
Introduction
History of Human Embryonic Stem Cell Research
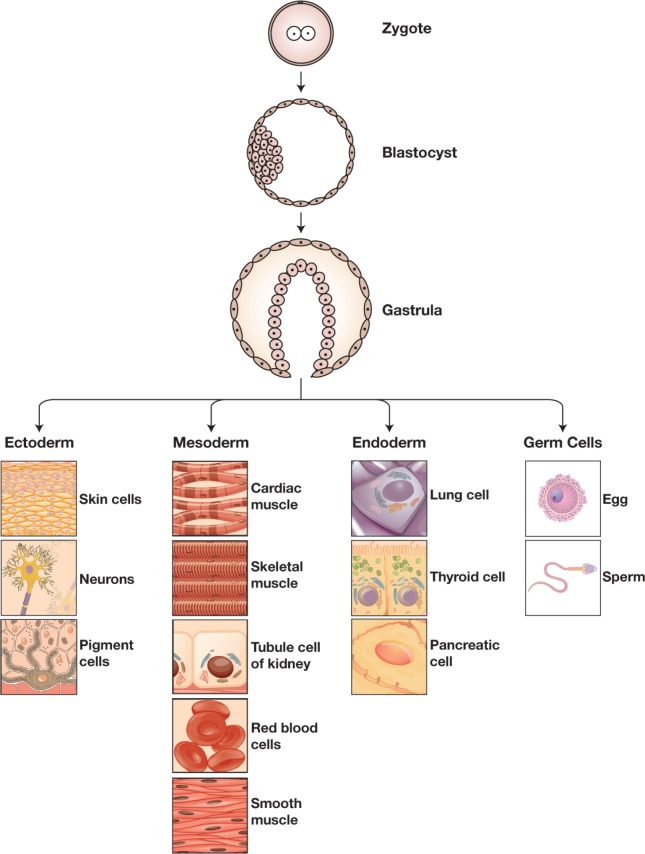
Embryonic stem cells (ESCs) are cells that originate from the inner cell mass of the blastocyst stage embryo during embryonic development. These cells have some extraordinary properties, such as self-replication and cell pluripotency, which give them the ability to differentiate into cells of all 3 germ layers that form all membranes in the body (Figure 1). Due to these features, the establishment of SC research has led to the beginning of a new era in cell biology. Despite the debate and controversy surrounding it, SC research started in the mid-1800s and is ongoing. The term “stem cell” appeared in the scientific literature as early as 1868 in the works of the eminent German biologist Ernst Haeckel (1834-1919). In 1878, the initial report on the first attempts to fertilize mammalian eggs outside the human body was published.1 After 9 decades, in 1959, rabbits were produced for the first time through in vitro fertilization (IVF) in the United States.2 In the mid-1960s, Friedrich and colleagues studied teratocarcinomas in the testes of some inbred strains of mice and showed that the teratocarcinomas originated from embryonic germ cells (EGCs), thus identifying embryonal carcinoma cells (ECCs) as a main type of stem cell (SC).3 Subsequently, in 1968, Edwards and Bavister fertilized the first human egg in vitro.4 In the early 1970s, ECCs were inoculated into mouse blastocysts to produce chimeric mice; cultured SCs were then used as the first models of embryonic development.5 In 1978, the first IVF infant (Louise Brown) was born in England. Two years later, in 1980, Candace Reed, Australia’s first IVF baby, was born in Melbourne. In 1981, Evans, Kaufman, and Martin collected mouse ESCs from the inner cell mass of blastocysts and developed culture conditions for growing pluripotent mouse ESCs in vitro; they found that infusing ESCs into mice induced the formation of teratomas.6,7 Between 1984 and 1988, Andrews and coworkers developed pluripotent ECCs from the Tera-2 human testicular teratocarcinoma cell line and showed that when teratoma cells get exposed to retinoic acid, they differentiate into neuron-like cells and other cell types. In 1989, Pera and colleagues isolated and characterized multipotent clones of human ECCs, which yielded tissues of all 3 primary germ layers.8 In 1994, human blastocysts donated by patients were established for reproductive use followed by isolation and culture of the inner cell mass.9 In 1995, the first nonhuman primate ESCs were derived and maintained in vitro; these cells were first isolated from the inner call mass of Rhesus monkeys and then from that of marmosets.10 The primate ESCs emulated human ECCs, which made obtaining and maintaining human ESCs cells in vitro possible. In 1998, a significant breakthrough was achieved by Thomson and colleagues when they acquired and maintained human ESCs from the inner cell mass of human blastocysts that were produced through IVF.10 Gearhart and coworkers derived human EGCs from the gonadal ridge and mesenchymal tissue of fetal material originating from abortions at 5 to 9 weeks of gestation.11 Since then, new cell lines have been consequently derived, and novel methods have been developed to direct the differentiation of the cells in vitro (Table 1).
Figure 1.
Differentiation of tissues.
Table 1.
Summary of the History of Stem Cell Research.
| Year | Research Performed | References |
|---|---|---|
| 1878 | First report of endeavors to fertilize mammalian eggs outside the body is published. | Trounson et al (2000) |
| 1959 | First report on animals produced through IVF is published. | Trounson et al (2000) |
| 1960 | Studies of teratocarcinomas in the testes of several inbred strains of mice indicate that the teratocarcinomas originated from EGCs. | Friedrich et al (1983), Kleinsmith and Pierce (1964)3 |
| 1968 | The first human egg in vitro fertilization is performed. | Trounson et al (2000) |
| 1970 | Cultured SCs are explored as models of embryonic development, although their complement of chromosomes is abnormal. | Martin (1980)5 |
| 1978 | Louise Brown, the first IVF baby, is born. | Trounson et al (2000) |
| 1980 | Australia’s first IVF baby, Candace Reed, is born in Melbourne. | Trounson et al (2000) |
| 1981 | Evans and colleagues derive mouse cells (ESCs) from the inner cell mass of blastocysts and develop culture conditions for growing pluripotent mouse ESCs in vitro; they find that infusing the ESCs into mice induced the formation of teratomas. The first IVF baby in the United States, Elizabeth Carr, is born. | Evans and Kaufman (1981),6 Martin (1981),7 Trounson et al (2000) |
| 1984-1988 | Andrews and coworkers develop pluripotent cells (ECCs) from the Tera-2 human testicular teratocarcinoma cell line. Thus, the teratoma cells exposed to retinoic acid differentiate into neuron-like cells and other cell types. | Andrews (1988), Thompson et al (1984) |
| 1989 | Pera and coworkers isolate and characterize multipotent clones of human embryonal carcinoma cells, which yield tissues of all 3 primary germ layers. | Pera et al (1989)8 |
| 1994 | Human blastocysts are established for reproductive purposes using IVF and are donated by patients for research. The inner cell mass is isolated and cultured. | Bongso et al (1994)9 |
| 1995-1996 | Nonhuman primate ESCs are derived and maintained in vitro; these cells were first isolated from the inner cell mass of rhesus monkeys and then from that of marmosets. The primate ESCs resemble human ECCs, indicating that it should be possible to derive and maintain human ESCs in vitro. | Thompson et al (1995, 1996) |
| 1998 | Thompson and coworkers acquire and maintain human ESCs from the inner cell mass of human blastocysts that were produced through in vitro fertilization and were donated for research purposes. Gearhart and colleagues derived human embryonic germ (EG) cells from the gonadal ridge and mesenchymal tissue of fetal material originating from abortions at 5 to 9 weeks of gestation. | Thompson et al (1998), Sharp et al (2000) |
| 2000 | Scientists in Singapore and Australia derive human ES cells from the inner cell mass of blastocysts donated by couples undergoing treatment for infertility. The ES cells proliferate for extended periods in vitro, maintain normal karyotypes, differentiate into somatic cell lineages derived from all 3 primary germ layers, and form teratomas when injected into immunodeficient mice. | Pera et al (1989)8 |
| 2001 | Human ES cell lines are shared and new lines are derived in vitro. Many methods are aimed at generating human tissues for transplantation purposes. |
Abbreviations: ECCs, embryonal carcinoma cells; EGCs, embryonic germ cells; ESCs, embryonic stem cells; IVF, in vitro fertilization; SC, stem cell.
Stem Cells
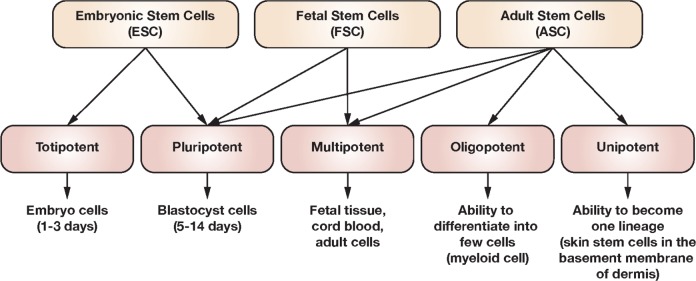
Over the past 2 decades, the use of SCs in research has developed rapidly due to their unique capabilities to self-renew as well as to differentiate into numerous specialized cells in the body.12 Therefore, more focus has been placed toward using these cells for the treatment of several important diseases, including cancer, leukemia, and autoimmune diseases. Currently, there are 5 different types of SCs: ESCs, ECCs, induced pluripotent stem cells (SCs), germinal SCs, and adult stem cells (ASCs; Figure 2). The ASCs are useful for tissue regeneration and tissue replacement after severe injuries, and in fact, they are the tool of choice for regenerative medicine.13 Intriguingly, ESCs have the ability to differentiate into different kinds of cells and thus have great applicability in the development of new cell-based therapies.14 In addition, recent studies have also associated dysregulated SCs with certain types of cancer.15
Figure 2.
Classification of stem cells.
Stem cells undergo mitosis, which results in 2 different daughter cells; mitosis starts with cell polarization, which is followed by the alignment of the mitotic spindle to the cell polarity axis.16,17 Morphologically, SCs usually have a circular shape with a low cytoplasm to nucleus ratio. Several specific markers of the general lineages have been previously described; however, alkaline phosphatase is common to most SC types.18
Physiologically, ASCs maintain tissue homeostasis and usually differentiate into a restricted range of progenitors and terminal cells that replace the local parenchyma; they are involved in injury repair in other areas19 or damaged cells or in sustaining cellular turnover.20 The ESCs, on the other hand, are pluripotent and can generate all active cell types.21 Fetal stem cells (FSCs) arise from the placenta, amniotic fluid, amniotic membranes, or fetal tissues. The FSCs have a higher ability for development and differentiation than do SCs from adult tissues.22,23
Accordingly, a major part of the scientific research focus on the use of SCs has been driven by the identification of the molecular behaviors of SCs and the cellular cross talk that involves SCs.
Despite the potential therapeutic applications, SCs also have some challenging side effects in clinical practice. For instance, self-renewal and plasticity are essential properties of cancer cells, and the probability of transplanted SCs losing control and inciting tumor development is a dangerous and unacceptable side effect.24 Additionally, allogeneic SC grafts can lead to immune rejection or graft-versus-host disease (GVHD),25 which makes immunosuppressive treatment necessary to avoid an immune response to the transplant and the resulting risk of infections. Additionally, ESC cultures need manipulation to generate embryos for scientific use. However, the Islamic Code of Medical Ethics agreed in 1981 that the human life has the full right to protect the embryo in all stages. Additionally, the Catholic Church has attributed rights to embryos even at this juncture of human development, which makes obtaining ethical approval for experimentation a challenge.26
Cancer Stem Cells
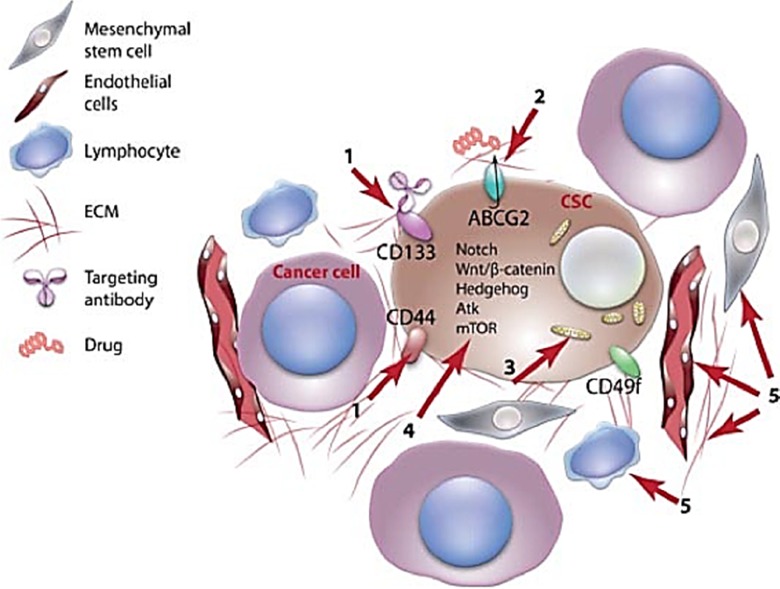
In 1997, Bonnet and Dick27 described a new model of hematological malignancies. This model hypothesized that only a minor subclass of cancer cells within a tumor has the capability to sustain tumorigenesis, thus resulting in a hierarchical organization of primary tumors.28 Later, these subgroups of tumor-propagating cells were officially named cancer stem cells (CSCs), since they share several characteristics, including self-renewal, with normal SCs.29 Importantly, such a novel model of cells is intriguing because it contributes to a better understanding of tumor recurrence and resistance to conventional cancer therapies.30 Therefore, the discovery of novel agents that can sensitize CSCs to conventional cancer treatments holds therapeutic promise (Figure 3).
Figure 3.
Possible therapeutic strategies for cancer stem cell elimination. (1) Targeting surface protein biomarkers such as CD133 with the aim to develop CSC-specific therapies. (2) Inhibiting the role of efflux transporters to reduce resistance to cancer drugs. (3) Reprogramming CSCs to a normal state by altering their metabolism and inducing them to differentiate. (4) Inhibiting pathways essential for CSC proliferation such as the Wnt/β-catenin signaling pathway. (5) Affecting the vascular niche to impair microenvironmental mechanisms that regulate GSC maintenance and function. CSC indicates cancer stem cell; GSC, germinal stem cell.
Clinical Uses of SCs in Cancer Treatment
Breast Cancer
Over the past decades, millions of women have died of breast cancer (BC) as a result of late diagnosis, disease relapse,31 and the development of resistance to endocrine and chemotherapeutic treatment regimens.32 Breast cancer remains the most common noncutaneous female malignancy and currently is responsible for 15% of all female deaths caused by cancer and is the second most common form of cancer in women after lung cancer (LC).33 Importantly, the vast majority of patients with BC die from metastases to distant sites or vital organs; these deaths account for over 90% of mortality of patients with cancer,34 and approximately 70% of patients develop a metastatic bone disease.35 Endocrine therapy is still the mainstay option for the treatment of estrogen receptor–positive patients with BC and significantly decreases the mortality rate.36 However, there have been cases of treated patients who experienced relapse and metastasis from the existence of BC SCs, which are a subset of tumor cells.37
Recent advances in medicine have produced several treatment options such as surgery, hormonal therapy, chemotherapy, and radiotherapy that are effective against nonmetastatic BC. However, a new strategy for BC management was improved by using high-dose chemotherapy (HDC) along with SC support; this combination improved disease-free survival in metastatic BC. Nevertheless, HDC has induced serious cytotoxicity, thus subjecting the method to considerable controversy.38 On the other hand, allogeneic hematopoietic stem cell transplantation (HSCT) becomes more efficient in persistent and progressive metastatic BC and decreases the relapse incidence after reduced-intensity conditioning transplantation. Moreover, allogeneic stem cell transplantation (SCT) along with myeloablative conditioning regimens results in cytoreduction, the suppression of immune-mediated graft-versus-tumor (GVT) effects, and a cancer-free graft as the treatment is mediated via the donor’s immune cells.39 Accordingly, the use of SCs in BC holds promise as a therapeutic option.
Prostate Cancer
Prostate cancer (PC) is the second most commonly diagnosed cancer in men worldwide. Localized PC with a low Gleason grade tumor can be managed with active surveillance. However, higher grade PC is effectively treated by radical prostatectomy or resection of the prostate in addition to radiation therapy.40 Prostate cancer can reach an advanced stage in which it metastasizes and becomes incurable.41,42 Androgen-deprivation therapy (ADT) is often the best treatment of choice for advanced stage PC. The majority of patients with metastatic PC treated with ADT eventually relapse to castration-resistant prostate cancer (CRPC) and are likely to die of the disease.41,42 Although CRPC can be managed with docetaxel, abiraterone plus prednisone, enzalutamide, and cabazitaxel also provide significant survival advantages; however, none of these is therapeutic.43 Additionally, there is still a need to develop curative choices to present to patients with advanced stage PC. The CSCs hold therapeutic promise as they can be targeted and provide a unique opportunity for novel therapeutic interventions. Additionally, metformin has been used to sensitize prostate CSCs to respond to conventional anticancer therapies and increase the cure efficacy. Intriguingly, prostate CSCs are a very promising treatment for preexisting ADT-resistant cells in patients, which give rise to CRPC because they exhibit self-renewal and tumor-propagating capabilities as well as a lack of or deficient androgen receptor expression.44 Furthermore, samples from recurrent PCs are enriched in CSCs, which explains the role and responsibility of CSCs in chemoresistance.45
Ovarian Cancer
Ovarian cancer (OC) is a malignant growth arising from different parts of the ovary in females. Frequently, OC begins within the outer lining of the ovary. However, the fallopian tube or egg cells have also been described as an origination point of OC.
Currently, there are several treatment options such as surgery, which is sufficient for malignant tumors that are well differentiated, and chemotherapy for the most aggressive tumors that are confined to the ovary. However, in 1995, Schilder and colleagues suggested the possibility of combining chemotherapy with SCT.46 Additionally, allogeneic HSCT combined with chemotherapy has induced variable effects in the therapy of advanced OC. When SC infusion triggers GVT effects, OC progression is likely to be suppressed.47 However, GVT effects do not occur frequently, and some serious side effects have been reported.48
Colorectal Cancer
Colorectal cancer (CRC) refers to cancerous growths in the rectum or appendix and can also arise from adenomatous polyps in the colon. Commonly, surgery followed by chemotherapy is the best therapeutic option.49 However, CRC can also be treated by allogeneic SCT via the targeting of GVHD in transplants. In 2004, Kojima et al studied 4 patients with metastatic CRC who were treated with reduced-intensity SC transplantation (RIST) and observed nonsignificant graft toxicity and decreased levels of CRC markers in 3 of the patients. Despite that fact that all 3 patients died due to cancer progression, the postmortem examination revealed that the macroscopic metastatic lesions had disappeared,50 thus demonstrating a tumor response. The generation of antineoplastic T cells is likely to have been triggered by the allogeneic SCT.51
Renal Cell Cancer
Renal cell cancer (RCC) is kidney cancer that originates from the lining of the proximal convoluted renal tubules. The first treatment option is usually radical or partial nephrectomy with alternative treatment strategies such as immunotherapy, hormonal therapy, and chemotherapy that have a slight impact on global survival.52 The HSCT, combined with immunosuppressive or donor lymphocyte infusion, has been used as an alternative regimen for RCC management, especially for metastatic forms. Allografting has also been used successfully in association with 3 factors, namely, C-reactive protein level, performance status, and lactate dehydrogenase level.53 The HSCT has been shown to stimulate the GVT response, thus decreasing metastasis and extending survival duration.54
Lung Cancer
Lung cancer is described by uncontrolled cell growth arising from epithelial cells within the lung tissue. The most common lung carcinoma is called small-cell lung carcinoma (SCLC). Chemotherapy and radiotherapy are the common treatment options.55 The SCT has been used, and it both improved the survival rate and prevented relapse. Autologous hematopoietic stem cell transplantation (AHSCT) has frequently been combined with chemotherapy for SCLC treatment. The reason for this combination is that HSCs drastically reduce the side effects of chemotherapy, in particular, myeloablation.56 Most likely, HSCs also induce therapeutic effects opposing the tumor directly.57 In SCLC, HSCs triggered GVT effects and increased the survival rate.
Leukemia
Leukemia is one of the primary blood diseases and is a condition where cells in the myeloid or lymphoid lineage undergo uncontrolled proliferation due to a mutation in genes involved in cell proliferation, which leads to a significant blast accumulation in the bone marrow (BM). Leukemia and lymphoma, which are 2 types of blood cancer that result from the uncontrolled proliferation of white blood cells, were the first to be clinically treated using HSCs. During these treatments, radiotherapy or chemotherapy was employed to destroy the cancerous hematopoietic cells that were further replaced by the transplantation of either BM or HSCs that were collected from the peripheral circulation of a matched donor, such as a patient’s sibling, with similar human leukocyte antigens on the surface of their cells.
Allogeneic RIST has been introduced as an important procedure to achieve complete remission in patients with leukemia, especially if a human leukocyte antigen-compatible donor is used.58-63 However, one of the main obstacles to successful transplantation is GVHD. Nonetheless, the frequency of GVHD is perceptibly reduced compared to that of the first treatment,64 with a significantly reduced mortality rate.65
Clinical Uses of SCs in Autoimmune Diseases
Rheumatoid Arthritis and Juvenile Idiopathic Arthritis
Rheumatoid arthritis (RA) has been described as the progressive, irreversible erosion of the cartilage tissue of joints that results in movement loss, pain, and quality of life reduction. This condition, along with juvenile idiopathic arthritis (JIA), occurs due to some failure of tolerance and the resulting immune response to common tissue antigens, in addition to the abundant release of inflammatory cytokines and autoantibodies.66 Nonsteroidal medications with the slow addition of traditional disease-modifying antirheumatic drugs or intra-articular corticosteroid injections are the best treatment options, with a remission rate of only 15%.67 The AHSCT has been conducted to treat RA and JIA; patients with severe RA who were resistant to conventional therapies and underwent AHSCT showed a significant response. Recurrent disease activity has been controlled with antirheumatic drugs.68 A successful HSCT protocol has been proposed to cure severe JIA; the protocol consists of harvesting BM, selecting positive SCs, depleting T cells, reinfusing the cells, and administering antiviral drugs and immunoglobulin to avoid frequent infection until the immune system returns to its full capability.69
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is characterized by multisystem inflammation due to dysfunctions in the BM microenvironment and the resulting severe decline in the proliferative capability of HSCs, in addition to the robust release of immune complexes with CD34+ cells undergoing apoptosis at a high rate.70 Typically, SLE is treated with nonsteroidal anti-inflammatory drugs, antimalarials, corticosteroids, and cytotoxic factors, but each drug has severe side effects, and relapses are frequent.71
The AHSCT reduced the number of apoptotic CD34+ cells pretreatment.72 In 2002, Traynor et al 73 performed AHSCT in 15 patients with severe SLE and found 2 patients who had a recurrence. Burt and colleagues reported a lower disease-free rate and high mortality.74 For more drug-sensitive types of disease, HSCT should be used for patients with organ-threatening SLE persisting despite standard aggressive therapy.75
Systemic Sclerosis
Systemic sclerosis (SSc) is a rare multisystem disorder characterized by T-cell activation that leads to autoantibody generation, cytokine release, and excessive collagen deposition. The 5-year mortality of patients with SSc is estimated to be 40% to 50%, with a poor outcome.76 The best therapy for SSc is attained via the combination of angiotensin and cyclophosphamide (Cy).77
The AHSCT improved skin flexibility and stabilized the pulmonary involvement.78,79 Farge and coauthors showed that AHSCT extended the short life expectancy of patients with severe SSc.80
Multiple Sclerosis
Multiple sclerosis (MS) is mediated by T cells activated against structural components of myelin and results in axon degeneration in the central nervous system. The inflammatory reaction plays an essential role in MS physiopathology. Therefore, the traditional cures aim to decrease the inflammatory response to treat or postpone the course of the disease.81 There are 2 kinds of MS, namely, primary progressive MS, which is resistant to treatment and improvement, and secondary progressive MS, which exhibits episodic relapse and improvement.82
The AHSCT remains the gold standard therapy; it competently delays MS progression in patients with no response to conventional treatments.83 Mezey and coauthors hypothesized a plastic conversion of HSC-derived cells to replace damaged neurons.84
Autoimmune Cytopenias
Immune thrombocytopenia purpura (ITP) is another autoimmune disorder where autoantibodies severely reduce the platelet level in the blood, consequently leading to thrombocytopenia and bleeding. Commonly, patients with ITP are responsive to high doses of immunosuppressors. However, this treatment exposes patients to myelosuppression risks. The HSCT has been successfully employed to improve the reestablishment of hematological parameters accompanied by a decrease in the number of autoimmune cells.85 Huhn and colleagues presented evidence for the advantages of combined AHSCT and CY therapy in the treatment of chronic refractory ITP. They concluded that SCs accelerated hematological rebalance compared with classic immunotherapy.86 Additionally, the European Bone Marrow Transplantation group performed a clinical study by treating 12 patients with ITP with AHSCT and noticed that the treatment responses varied from a transient response to continuous remission or transplantation-related death.87
(Auto)immune hemolytic anemia (IHA) refers to hematologic diseases where either autoreactive antibodies or complement against membrane proteins triggers the early destruction of erythrocytes.88 Unfortunately, very little is known regarding these conditions. However, the close association between AHSCT and immunosuppressive therapy suggests a potentially effective treatment for IHA89 that needs further investigation in order to decrease the currently high rates of failure and death.90
Diabetes Mellitus
One of the most important autoimmune diseases is type 1 diabetes mellitus (DM), which is caused by a cell-mediated autoimmune attack on insulin-secreting pancreatic β cells, thereby inducing glycemia as glucose accumulates in the blood.
The introduction of exogenous insulin is the standard therapy to restore glucose homeostasis, even though resistance to insulin therapy has been reported in several cases.91,92 The SCT can rehabilitate pancreatic islets and reintroduce the physiological secretion of human insulin. Moreover, AHSCT has shown to effect some improvement in β-cell function in addition to a robust reduction in the requirement for exogenous insulin.93 Additionally, AHSCT induced persistent insulin independence with normal glycemic control when performed in patients with type 1 DM.94 Moreover, an insulin-free period has been achieved through combining AHSCT with Cy,72 thus suggesting a synergistic interaction between AHSCT and Cy that leads to independence from exogenous insulin. This effect was shown in the first successful Polish attempt to achieve remission in the early phase of type 1 DM following immunosuppressive treatment and subsequent AHSCT. Furthermore, the successful transfusion of allotropic human adipose tissue-derived insulin-producing mesenchymal SCs with unfractionated cultured BM was documented in patients with insulinopenic DM; this treatment was free of side effects and resulted in a marked reduction in the requirement for insulin.95
Crohn Disease
Crohn disease (CD) is an autoimmune disease manifesting as the gastrointestinal loss of immune tolerance due to an overactive T helper 1 cell response. This disorder can be controlled through surgical intervention, immunosuppressive drugs, and antibody therapy.96 The AHSCT has been safely used with high efficiency to induce and maintain remission in refractory patients affected by CD.97 Additionally, the combination of AHSCT and Cy achieved a better clinical remission rate, with a reduction in abdominal pain and the disappearance of diarrhea.98 Despite advancement in medical science, an absolute cure for CD is still not available. Recently, SC therapy has been used in CD and has shown several advantages over conventional therapies. The main types of SCs being investigated for CD treatment are mesenchymal stem/stromal cells, adipose tissue-derived SCs, and hematopoietic SCs.
Neurological Disorders
Stem cells have also been used in the treatment of several neurological disorders, including amyotrophic lateral sclerosis,99 Parkinson disease,100 spinal cord lesions,101 Huntington disease,102 stroke,103 Duchenne muscular dystrophy,104 heart failure,105 ocular surface diseases,106 cartilage repair,107 and liver disease.108
Cancer Stem Cells Implications for Tumor Therapy
The isolation and characterization of SC from leukemia and solid tumors have become essential in the study of cancer therapy.109 A tumor consists of different clones that vary in their level of proliferation, differentiation, and tumor initiation ability. In human leukemia, some of the cells have the capacity to divide indefinitely and maintain the tumor clone. Leukemia also gives rise to other cells that possess vast proliferative and self-renewal potential, and we call those cells leukemia SCs.110 ABC transporters are responsible for a common mechanism of drug resistance used by SCs.111 The efficiency of cytotoxic chemotherapy is limited due to the resistance of CSCs to drugs and because of their limited replication.
Although those cells form a very small proportion of the tumor, their immortal phenotype is likely to be sufficient to allow tumor recurrence. Cancer may originate at some time after its initial treatment by chemotherapy or radiotherapy. It is proposed that if cancer cells arise from early SCs or progenitor cells, then metastases are formed readily and have extensive genetic heterogeneity. Metastases resulting from a later SC are more homogenous with more limited metastatic capability. A tumor’s heterogeneity and its growth in distant sites of the body under different environments may be derived from the differentiation and/or dedifferentiation of CSCs. Further identification of CSCs is required for differentiation therapy, which might become an improved therapeutic approach in the future. These improvements may foster the therapeutic potential of the CSC concept. Eventually, cancer treatment will involve the elimination of all cells within a tumor; consequently, combination therapies that target both CSCs and the tumor mass are likely to emerge as particularly effective clinical strategies.112 Current targeted approaches to kill CSCs via targeting their properties are summarized in Figure 1. Successful therapy also requires both a deep understanding of the relationship between CSCs and the innate tumor microenvironment (associated host tissue) and the capability to interfere with the role of this environment to disrupt the tumor.113
Complications and Challenges in SC Therapy
Rapid technological advances in SC research hold future promise. However, scientists are facing some significant problems and challenges along with ethical dilemmas. A major problem in using SCs as a therapeutic agent for cancer treatment is that they can foster tumor growth instead. Therefore, to overcome this obstacle, scientists need to find a balance between the growth replacement of cancer cells and the production of cancer cells. One of the major stumbling blocks that scientists continue to encounter is the identification of SCs in adult tissues and within an actual tissue culture. Due to the diversity of cell types, the SC population in tissues usually comprises thousands of different cells. Scientists need to collect precise cell populations to treat the target disease. Therefore, it is hard to isolate, process, and differentiate SCs into the desired cell type. In addition, there are some difficulties in growing SCs and maintaining them in a suitable medium. Moreover, the patient’s body has to accept the implemented SCs as native cells, which relies on effective integration. Implemented SCs must also first be screened for purity and for the presence of cancer cells. Another challenge in successful SCT is immunological rejection; therefore, recipients of SCs usually need immunosuppressive drugs, which leave them vulnerable to infection. The brain is the most challenging of the organs that can utilize SC-based therapeutics due to the complexity of neurons and the multiple interactions in the brain. Additionally, many degenerative neurological disorders such as Alzheimer disease involve more than one cell type, making them difficult targets for SC-based therapies.
The impressive initial outcome of SC-based therapies has motivated scientists to explore more techniques to treat several diseases. However, SC therapy remains a new discipline. Future advancement in SC therapies will rely on continuous close collaboration between clinicians, scientists, industry, academia, and regulatory authorities to overcome barriers and discover novel SC-based therapies to save patients with such devastating diseases.
Conclusion
This review aims to highlight the historical advances in the clinical use of SCs for the treatment of an array of cancers and autoimmune disorders. Despite the promising uses of SCs in clinical practice, there are still many questions regarding the risks of SC applications that need better answers. The amount of research on the use of SC therapy is increasing at the same rate as the risks associated with their clinical use. Due to the considerable variation among studies, it is challenging to compare results from different studies as well as to compare one SC-based medicinal product to another. Overall, SC therapy has a high potential to treat multiple diseases and degenerative conditions. However, a more detailed evaluation of the risk factors and potential hazards that are associated with these therapies is a prerequisite before their more extensive clinical application.
Acknowledgments
Y.H. & M.B. would like to thank King Faisal Specialist Hospital and Research Center (Gen. Org; KFSH & RC-Jed) and the Saudi Human Genome Program (SHGP). Also, the author would like to thank Department of Biology, Faculty of Sciences, Saudi Digital Library, and University Library for providing the facility for literature survey and collection.
Abbreviations
- ADT
androgen-deprivation therapy
- AHSCT
autologous hematopoietic stem cell transplantation
- ASCs
adult stem cells
- BC
breast cancer
- BM
bone marrow
- CD
Crohn disease
- CRC
colorectal cancer
- CRPC
castration-resistant prostate cancer
- CSCs
cancer stem cells
- Cy
cyclophosphamide
- DM
diabetes mellitus
- ECCs
embryonal carcinoma cells
- EGC
embryonic germ cells
- ESC
embryonic stem cells
- FSCs
fetal stem cells
- GVHD
graft-versus-host disease
- GVT
graft-versus-tumor
- HDC
high-dose chemotherapy
- HSCT
hematopoietic stem cell transplantation
- IHA
immune hemolytic anemia
- ITP
immune thrombocytopenia purpura
- IVF
in vitro fertilization
- JIA
juvenile idiopathic arthritis
- LC
lung cancer
- MS
multiple sclerosis
- OC
ovarian cancer
- PC
prostate cancer
- RA
rheumatoid arthritis
- RCC
renal cell cancer
- RIST
reduced-intensity SC transplantation
- SCs
stem cells
- SCLC
small-cell lung carcinoma
- SCT
stem cell transplantation
- SLE
systemic lupus erythematosus
- SSc
systemic sclerosis
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work received financial suuport from the Deanship of Scientific Research (DSR), University of Tabuk, Tabuk, Saudi Arabia, under grant no. S-1438-120.
ORCID iD: Shalini Saggu, MSc, PhD  http://orcid.org/0000-0002-4058-3760
http://orcid.org/0000-0002-4058-3760
References
- 1. Rock J, Menkin MF. In vitro fertilization and cleavage of human ovarian eggs. Science. 1944;100(2588):105–107. [DOI] [PubMed] [Google Scholar]
- 2. Austin CR. Fertilization of mammalian eggs in vitro. Int Rev Cytol. 1961;12:337–359. [PubMed] [Google Scholar]
- 3. Kleinsmith LJ, Pierce GB., Jr Multipotentiality of single embryonal carcinoma cells. Cancer Res. 1964;24(9):1544–1551. [PubMed] [Google Scholar]
- 4. Edwards RG, Bavister BD, Steptoe PC. Early stages of fertilization in vitro of human oocytes matured in vitro. Nature. 1969;221(5181):632–635. [DOI] [PubMed] [Google Scholar]
- 5. Martin GR. Teratocarcinomas and mammalian embryogenesis. Science. 1980;209(4458):768–776. [DOI] [PubMed] [Google Scholar]
- 6. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. [DOI] [PubMed] [Google Scholar]
- 7. Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78(12):7634–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pera MF, Cooper S, Mills J, Parrington JM. Isolation and characterization of a multipotent clone of human embryonal carcinoma-cells. Diff Res Biol Diver. 1989;42(1):10–23. [DOI] [PubMed] [Google Scholar]
- 9. Bongso TA, Fong CY, Ng CY, Ratnam SS. Blastocyst transfer in human in vitro fertilization: the use of embryo co-culture. Cell Biol Int. 1994;18(12):1181–1189. [DOI] [PubMed] [Google Scholar]
- 10. Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol Reprod. 1996;55(2):254–259. [DOI] [PubMed] [Google Scholar]
- 11. Shamblott MJ, Axelman J, Wang S, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci U S A. 1998;95(23):13726–13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100(1):157–168. [DOI] [PubMed] [Google Scholar]
- 13. Liu SP, Fu RH, Huang SJ, et al. Stem cell applications in regenerative medicine for neurological disorders. Cell Transpl. 2013;22(4):631–637. [DOI] [PubMed] [Google Scholar]
- 14. Daley GQ, Goodell MA, Snyder EY. Realistic prospects for stem cell therapeutics. Hematol Am Soc Hematol Educ Progr. 2003:398–418. [DOI] [PubMed] [Google Scholar]
- 15. Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. [DOI] [PubMed] [Google Scholar]
- 16. Zhong W. Timing cell-fate determination during asymmetric cell divisions. Curr Opin Neurobiol. 2008;18(5):472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132(4):583–597. [DOI] [PubMed] [Google Scholar]
- 18. Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8(3):301–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ladurner P, Rieger R, Baguna J. Spatial distribution and differentiation potential of stem cells in hatchlings and adults in the marine Platyhelminth macrostomum sp.: a bromodeoxyuridine analysis. Dev Biol. 2000;226(2):231–241. [DOI] [PubMed] [Google Scholar]
- 20. Fang TC, Alison MR, Wright NA, Poulsom R. Adult stem cell plasticity: will engineered tissues be rejected? Int J Exp Pathol. 2004;85(3):115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pessina A, Gribaldo L. The key role of adult stem cells: therapeutic perspectives. Curr Med Res Opin. 2006;22(11):2287–2300. [DOI] [PubMed] [Google Scholar]
- 22. Gucciardo L, Lories R, Ochsenbein-Kolble N, Done E, Zwijsen A, Deprest J. Fetal mesenchymal stem cells: isolation, properties and potential use in perinatology and regenerative medicine. BJOG. 2009;116(2):166–172. [DOI] [PubMed] [Google Scholar]
- 23. Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105(7):829–841. [DOI] [PubMed] [Google Scholar]
- 24. Vicente-Duenas C, Gutierrez de Diego J, Rodriguez FD, Jimenez R, Cobaleda C. The role of cellular plasticity in cancer development. Curr Med Chem. 2009;16(28):3676–3685. [DOI] [PubMed] [Google Scholar]
- 25. Reddy P, Arora M, Guimond M, Mackall CL. GVHD: a continuing barrier to the safety of allogeneic transplantation. Biol Blood Marrow Transplant. 2009;15(1 suppl):162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zarzeczny A, Caulfield T. Emerging ethical, legal and social issues associated with stem cell research & and the current role of the moral status of the embryo. Stem Cell Rev. 2009;5(2):96–101. [DOI] [PubMed] [Google Scholar]
- 27. Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. [DOI] [PubMed] [Google Scholar]
- 28. Chen X, Rycaj K, Liu X, Tang DG. New insights into prostate cancer stem cells. Cell Cycle. 2013;12(4):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bao B, Ahmad A, Azmi AS, Ali S, Sarkar FH. Overview of cancer stem cells (CSCs) and mechanisms of their regulation: implications for cancer therapy. Curr Protoc Pharmacol. 2013;Chapter 14:Unit 14 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10(6):717–728. [DOI] [PubMed] [Google Scholar]
- 31. Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–1126. [DOI] [PubMed] [Google Scholar]
- 32. Osborne CK. Tamoxifen in the treatment of breast cancer. New Engl J Med. 1998;339(22):1609–1618. [DOI] [PubMed] [Google Scholar]
- 33. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 34. Bendre M, Gaddy D, Nicholas RW, Suva LJ. Breast cancer metastasis to bone—it is not all about PTHrP. Clin Orthop Relat Res. 2003;(415):S39–S45. [DOI] [PubMed] [Google Scholar]
- 35. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20):6243s–6249s. [DOI] [PubMed] [Google Scholar]
- 36. Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. New Engl J Med. 2014;371(2):107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Biron P, Durand M, Roche H, et al. Pegase 03: a prospective randomized phase III trial of FEC with or without high-dose thiotepa, cyclophosphamide and autologous stem cell transplantation in first-line treatment of metastatic breast cancer. Bone Marrow Transpl. 2008;41(6):555–562. [DOI] [PubMed] [Google Scholar]
- 39. Ueno NT, Rizzo JD, Demirer T, et al. Allogeneic hematopoietic cell transplantation for metastatic breast cancer. Bone Marrow Transpl. 2008;41(6):537–545. [DOI] [PubMed] [Google Scholar]
- 40. Maitland NJ, Collins AT. Prostate cancer stem cells: a new target for therapy. J Clin Oncol. 2008;26(17):2862–2870. [DOI] [PubMed] [Google Scholar]
- 41. Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1(1):34–45. [DOI] [PubMed] [Google Scholar]
- 42. Li HW, Tang DG. Prostate cancer stem cells and their potential roles in metastasis. J Surg Oncol. 2011;103(6):558–562. [DOI] [PubMed] [Google Scholar]
- 43. Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(2):467–479. [DOI] [PubMed] [Google Scholar]
- 44. Zong Y, Goldstein AS. Adaptation or selection—mechanisms of castration-resistant prostate cancer. Nat Rev Urol. 2013;10(2):90–98. [DOI] [PubMed] [Google Scholar]
- 45. Guzel E, Karatas OF, Duz MB, Solak M, Ittmann M, Ozen M. Differential expression of stem cell markers and ABCG2 in recurrent prostate cancer. Prostate. 2014;74(15):1498–1505. [DOI] [PubMed] [Google Scholar]
- 46. Schilder RJ, Boente MP, Corn BW, Lanciano RM, Young RC, Ozols RF. The management of early ovarian cancer. Oncology. 1995;9(2):171–182; discussion 185-177. [PubMed] [Google Scholar]
- 47. Rini BI, Zimmerman T, Stadler WM, Gajewski TF, Vogelzang NJ. Allogeneic stem-cell transplantation of renal cell cancer after nonmyeloablative chemotherapy: feasibility, engraftment, and clinical results. J Clin Oncol. 2002;20(8):2017–2024. [DOI] [PubMed] [Google Scholar]
- 48. Sarosy GA, Reed E. Autologous stem-cell transplantation in ovarian cancer: is more better? Ann Intern Med. 2000;133(7):555–556. [DOI] [PubMed] [Google Scholar]
- 49. Nagy VM. Updating the management of rectal cancer. J Gastrointest Liver. 2008;17(1):69–74. [DOI] [PubMed] [Google Scholar]
- 50. Kojima R, Kami M, Hori A, et al. Reduced-intensity allogeneic hematopoietic stem-cell transplantation as an immunotherapy for metastatic colorectal cancer. Transplantation. 2004;78(12):1740–1746. [DOI] [PubMed] [Google Scholar]
- 51. Carnevale-Schianca F, Cignetti A, Capaldi A, et al. Allogeneic nonmyeloablative hematopoietic cell transplantation in metastatic colon cancer: tumor-specific T cells directed to a tumor-associated antigen are generated in vivo during GVHD. Blood. 2006;107(9):3795–3803. [DOI] [PubMed] [Google Scholar]
- 52. Oudard S, George D, Medioni J, Motzer R. Treatment options in renal cell carcinoma: past, present and future. Ann Oncol. 2007;18(suppl 10):x25–x31. [DOI] [PubMed] [Google Scholar]
- 53. Peccatori J, Barkholt L, Demirer T, et al. Prognostic factors for survival in patients with advanced renal cell carcinoma undergoing nonmyeloablative allogeneic stem cell transplantation. Cancer. 2005;104(10):2099–2103. [DOI] [PubMed] [Google Scholar]
- 54. Childs R, Chernoff A, Contentin N, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. New Engl J Med. 2000;343(11):750–758. [DOI] [PubMed] [Google Scholar]
- 55. Seidenfeld J, Samson DJ, Bonnell CJ, Ziegler KM, Aronson N. Management of small cell lung cancer. Evid Rep Technol Assess. 2006;(143):1–154. [PMC free article] [PubMed] [Google Scholar]
- 56. Humblet Y, Symann M, Bosly A, et al. Late intensification chemotherapy with autologous bone marrow transplantation in selected small-cell carcinoma of the lung: a randomized study. J Clin Oncol. 1987;5(12):1864–1873. [DOI] [PubMed] [Google Scholar]
- 57. Brugger W, Fetscher S, Hasse J, et al. Multimodality treatment including early high-dose chemotherapy with peripheral blood stem cell transplantation in limited-disease small cell lung cancer. Semin Oncol. 1998;25(1 suppl 2):42–48. [PubMed] [Google Scholar]
- 58. Fagioli F, Zecca M, Locatelli F, et al. Allogeneic stem cell transplantation for children with acute myeloid leukemia in second complete remission. J Pediatr Hematol Oncol. 2008;30(8):575–583. [DOI] [PubMed] [Google Scholar]
- 59. Frassoni F, Gualandi F, Podesta M, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008;9(9):831–839. [DOI] [PubMed] [Google Scholar]
- 60. Ruiz-Arguelles GJ, Gomez-Almaguer D, Morales-Toquero A, et al. The early referral for reduced-intensity stem cell transplantation in patients with Ph1 (+) chronic myelogenous leukemia in chronic phase in the imatinib era: results of the Latin American Cooperative Oncohematology Group (LACOHG) prospective, multicenter study. Bone Marrow Transpl. 2005;36(12):1043–1047. [DOI] [PubMed] [Google Scholar]
- 61. Oehler VG, Radich JP, Storer B, et al. Randomized trial of allogeneic related bone marrow transplantation versus peripheral blood stem cell transplantation for chronic myeloid leukemia. Biol Blood Marrow Transplant. 2005;11(2):85–92. [DOI] [PubMed] [Google Scholar]
- 62. Mohty M, Labopin M, Tabrizzi R, et al. Reduced intensity conditioning allogeneic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93(2):303–306. [DOI] [PubMed] [Google Scholar]
- 63. Isidori A, Motta MR, Tani M, et al. Positive selection and transplantation of autologous highly purified CD133(+) stem cells in resistant/relapsed chronic lymphocytic leukemia patients results in rapid hematopoietic reconstitution without an adequate leukemic cell purging. Biol Blood Marrow Transplant. 2007;13(10):1224–1232. [DOI] [PubMed] [Google Scholar]
- 64. Gutierrez-Aguirre CH, Gomez-Almaguer D, Cantu-Rodriguez OG, et al. Non-myeloablative stem cell transplantation in patients with relapsed acute lymphoblastic leukemia: results of a multicenter study. Bone Marrow Transpl. 2007;40(6):535–539. [DOI] [PubMed] [Google Scholar]
- 65. Dreger P, Brand R, Hansz J, et al. Treatment-related mortality and graft-versus-leukemia activity after allogeneic stem cell transplantation for chronic lymphocytic leukemia using intensity-reduced conditioning. Leukemia. 2003;17(5):841–848. [DOI] [PubMed] [Google Scholar]
- 66. Ringe J, Sittinger M. Tissue engineering in the rheumatic diseases. Arthritis Res Ther. 2009;11(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hayward K, Wallace CA. Recent developments in anti-rheumatic drugs in pediatrics: treatment of juvenile idiopathic arthritis. Arthritis Res Ther. 2009;11(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Snowden JA, Passweg J, Moore JJ, et al. Autologous hemopoietic stem cell transplantation in severe rheumatoid arthritis: a report from the EBMT and ABMTR. J Rheumatol. 2004;31(3):482–488. [PubMed] [Google Scholar]
- 69. De Kleer IM, Brinkman DM, Ferster A, et al. Autologous stem cell transplantation for refractory juvenile idiopathic arthritis: analysis of clinical effects, mortality, and transplant related morbidity. Ann Rheum Dis. 2004;63(10):1318–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jallouli M, Frigui M, Hmida MB, Marzouk S, Kaddour N, Bahloul Z. Clinical and immunological manifestations of systemic lupus erythematosus: study on 146 South Tunisian patients. Saudi J Kidney Dis Transpl. 2008;19(6):1001–1008. [PubMed] [Google Scholar]
- 71. Ioannou Y, Isenberg DA. Current concepts for the management of systemic lupus erythematosus in adults: a therapeutic challenge. Postgrad Med J. 2002;78(924):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rosa SB, Voltarelli JC, Chies JAB, Pranke P. The use of stem cells for the treatment of autoimmune diseases. Braz J Med Biol Res. 2007;40(12):1579–1597. [DOI] [PubMed] [Google Scholar]
- 73. Traynor AE, Barr WG, Rosa RM, et al. Hematopoietic stem cell transplantation for severe and refractory lupus. Analysis after five years and fifteen patients. Arthritis Rheum. 2002;46(11):2917–2923. [DOI] [PubMed] [Google Scholar]
- 74. Burt RK, Traynor A, Statkute L, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295(5):527–535. [DOI] [PubMed] [Google Scholar]
- 75. Goldblatt F, Isenberg DA. New therapies for systemic lupus erythematosus. Clin Exp Immunol. 2005;140(2):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lim IG, Schrieber L. Management of systemic sclerosis. Isr Med Assoc J. 2002;4(11 suppl):953–957. [PubMed] [Google Scholar]
- 77. Akerkar SM, Bichile LS. Therapeutic options for systemic sclerosis. Indian J Dermatol Venereol Leprol. 2004;70(2):67–75. [PubMed] [Google Scholar]
- 78. Martini A, Maccario R, Ravelli A, et al. Marked and sustained improvement two years after autologous stem cell transplantation in a girl with systemic sclerosis. Arthritis Rheum. 1999;42(4):807–811. [DOI] [PubMed] [Google Scholar]
- 79. Binks M, Passweg JR, Furst D, et al. Phase I/II trial of autologous stem cell transplantation in systemic sclerosis: procedure related mortality and impact on skin disease. Ann Rheum Dis. 2001;60(6):577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Farge D, Passweg J, van Laar JM, et al. Autologous stem cell transplantation in the treatment of systemic sclerosis: report from the EBMT/EULAR Registry. Ann Rheum Dis. 2004;63(8):974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. [DOI] [PubMed] [Google Scholar]
- 82. Ebers GC. Natural history of primary progressive multiple sclerosis. Mult Scler. 2004;10(suppl 1):S8–S13; discussion S13-S15. [DOI] [PubMed] [Google Scholar]
- 83. Saccardi R, Mancardi GL, Solari A, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: impact on disease activity and quality of life. Blood. 2005;105(6):2601–2607. [DOI] [PubMed] [Google Scholar]
- 84. Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290(5497):1779–1782. [DOI] [PubMed] [Google Scholar]
- 85. Stasi R, Provan D. Management of immune thrombocytopenic purpura in adults. Mayo Clinic Proc. 2004;79(4):504–522. [DOI] [PubMed] [Google Scholar]
- 86. Huhn RD, Fogarty PF, Nakamura R, et al. High-dose cyclophosphamide with autologous lymphocyte-depleted peripheral blood stem cell (PBSC) support for treatment of refractory chronic autoimmune thrombocytopenia. Blood. 2003;101(1):71–77. [DOI] [PubMed] [Google Scholar]
- 87. Urban C, Lackner H, Sovinz P, et al. Successful unrelated cord blood transplantation in a 7-year-old boy with Evans syndrome refractory to immunosuppression and double autologous stem cell transplantation. Eur J Haematol. 2006;76(6):526–530. [DOI] [PubMed] [Google Scholar]
- 88. Pratt G, Kinsey SE. Remission of severe, intractable autoimmune haemolytic anaemia following matched unrelated donor transplantation. Bone Marrow Transpl. 2001;28(8):791–793. [DOI] [PubMed] [Google Scholar]
- 89. Seeliger S, Baumann M, Mohr M, Jurgens H, Frosch M, Vormoor J. Autologous peripheral blood stem cell transplantation and anti-B-cell directed immunotherapy for refractory auto-immune haemolytic anaemia. Eur J Pediatr. 2001;160(8):492–496. [DOI] [PubMed] [Google Scholar]
- 90. Passweg JR, Rabusin M, Musso M, et al. Haematopoetic stem cell transplantation for refractory autoimmune cytopenia. Br J Haematol. 2004;125(6):749–755. [DOI] [PubMed] [Google Scholar]
- 91. Eisenbarth GS. Update in type 1 diabetes. J Clin Endocr Metab. 2007;92(7):2403–2407. [DOI] [PubMed] [Google Scholar]
- 92. Ingberg CM, Palmer M, Schvarcz E, Aman J. Prevalence of urinary tract symptoms in long-standing type 1 diabetes mellitus. Diabetes Metab. 1998;24(4):351–354. [PubMed] [Google Scholar]
- 93. Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297(14):1568–1576. [DOI] [PubMed] [Google Scholar]
- 94. Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301(15):1573–1579. [DOI] [PubMed] [Google Scholar]
- 95. Trivedi HL, Vanikar AV, Thakker U, et al. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transpl Proc. 2008;40(4):1135–1139. [DOI] [PubMed] [Google Scholar]
- 96. Rampton DS. Management of Crohn’s disease. BMJ. 1999;319(7223):1480–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Oyama Y, Craig RM, Traynor AE, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterol. 2005;128(3):552–563. [DOI] [PubMed] [Google Scholar]
- 98. Burt RK, Traynor A, Oyama Y, Craig R. High-dose immune suppression and autologous hematopoietic stem cell transplantation in refractory Crohn disease. Blood. 2003;101(5):2064–2066. [DOI] [PubMed] [Google Scholar]
- 99. Janson CG, Ramesh TM, During MJ, Leone P, Heywood J. Human intrathecal transplantation of peripheral blood stem cells in amyotrophic lateral sclerosis. J Hematother Stem Cell. 2001;10(6):913–915. [DOI] [PubMed] [Google Scholar]
- 100. Hagell P, Schrag A, Piccini P, et al. Sequential bilateral transplantation in Parkinson’s disease: effects of the second graft. Brain. 1999;122 (Pt 6):1121–1132. [DOI] [PubMed] [Google Scholar]
- 101. Mackay-Sim A, Feron F, Cochrane J, et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131(pt 9):2376–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Freeman TB, Cicchetti F, Hauser RA, et al. Transplanted fetal striatum in Huntington’s disease: phenotypic development and lack of pathology. Proc Natl Acad Sci U S A. 2000;97(25):13877–13882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rabinovich SS, Seledtsov VI, Banul NV, et al. Cell therapy of brain stroke. Bull Exp Biol Med. 2005;139(1):126–128. [DOI] [PubMed] [Google Scholar]
- 104. Torrente Y, Belicchi M, Marchesi C, et al. Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant. 2007;16(6):563–577. [DOI] [PubMed] [Google Scholar]
- 105. Menasche P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117(9):1189–1200. [DOI] [PubMed] [Google Scholar]
- 106. Daya SM, Ilari FA. Living related conjunctival limbal allograft for the treatment of stem cell deficiency. Ophthalmology. 2001;108(1):126–133; discussion 133-124. [DOI] [PubMed] [Google Scholar]
- 107. Centeno CJ, Busse D, Kisiday J, Keohan C, Freeman M, Karli D. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician. 2008;11(3):343–353. [PubMed] [Google Scholar]
- 108. Pai M, Zacharoulis D, Milicevic MN, et al. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103(8):1952–1958. [DOI] [PubMed] [Google Scholar]
- 109. Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17(3):313–319. [DOI] [PubMed] [Google Scholar]
- 110. Iwasaki H. Leukemia stem cell [in Japanese]. Gan Kagaku Ryoho. 2014;41(3):280–284. [PubMed] [Google Scholar]
- 111. Elliott A, Adams J, Al-Hajj M. The ABCs of cancer stem cell drug resistance. IDrugs. 2010;13(9):632–635. [PubMed] [Google Scholar]
- 112. Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120(1):41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Malanchi I. Tumour cells coerce host tissue to cancer spread. BoneKEy Rep. 2013;2:371. [DOI] [PMC free article] [PubMed] [Google Scholar]